Development of Chitosan–Hydroxyapatite Membranes from Bone of Armoured Catfish (Pterygoplichthys spp.) for Applications in Guided Bone Regeneration (GBR)
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Hydroxyapatite Powder from Armoured Catfish (Pterygoplichthys spp.)
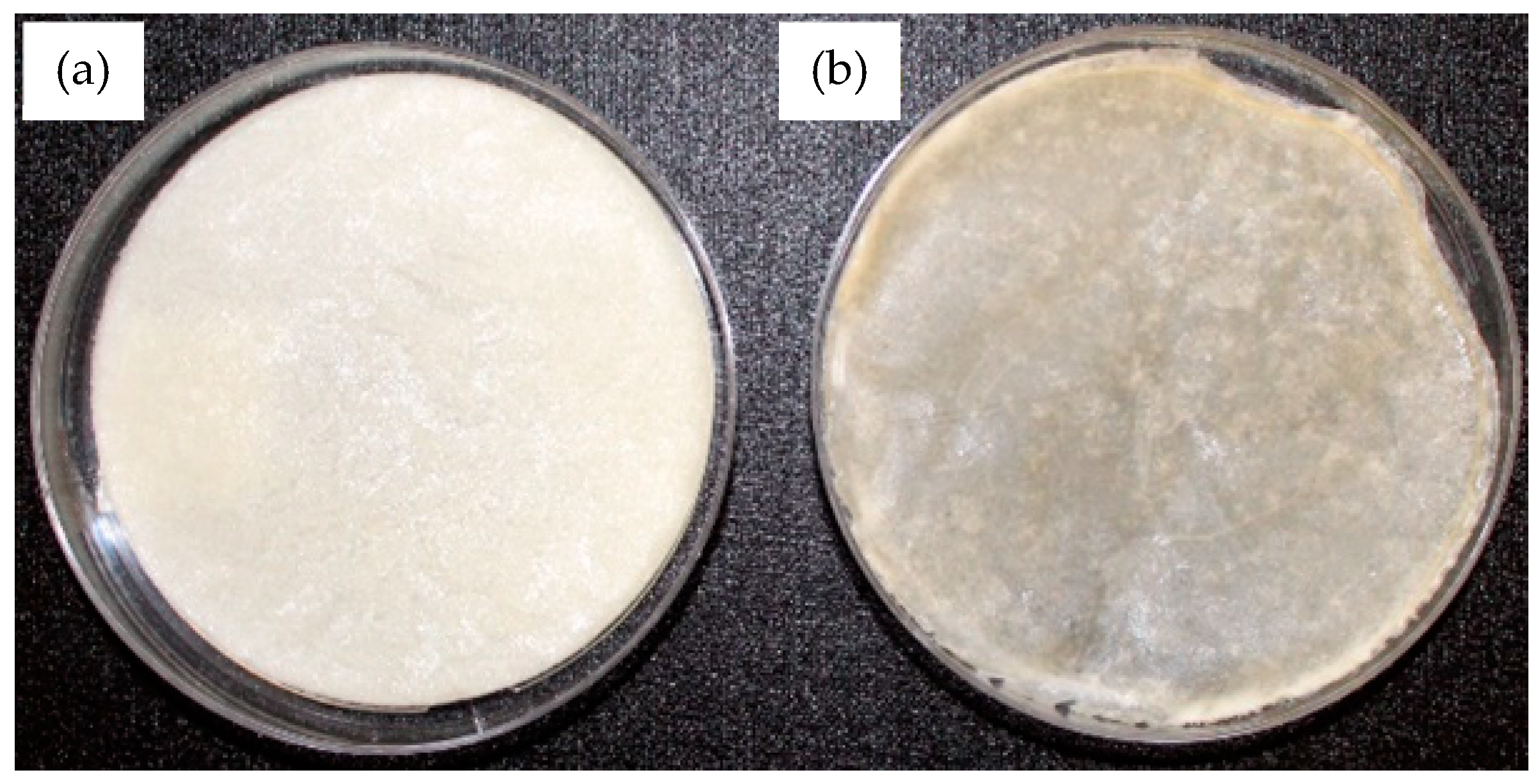
2.2. Fabrication of the Membranes of Chitosan and Chitosan–Hydroxyapatite
2.2.1. Preparation of the Chemical Solution for the Fabrication of the Membranes of Chitosan
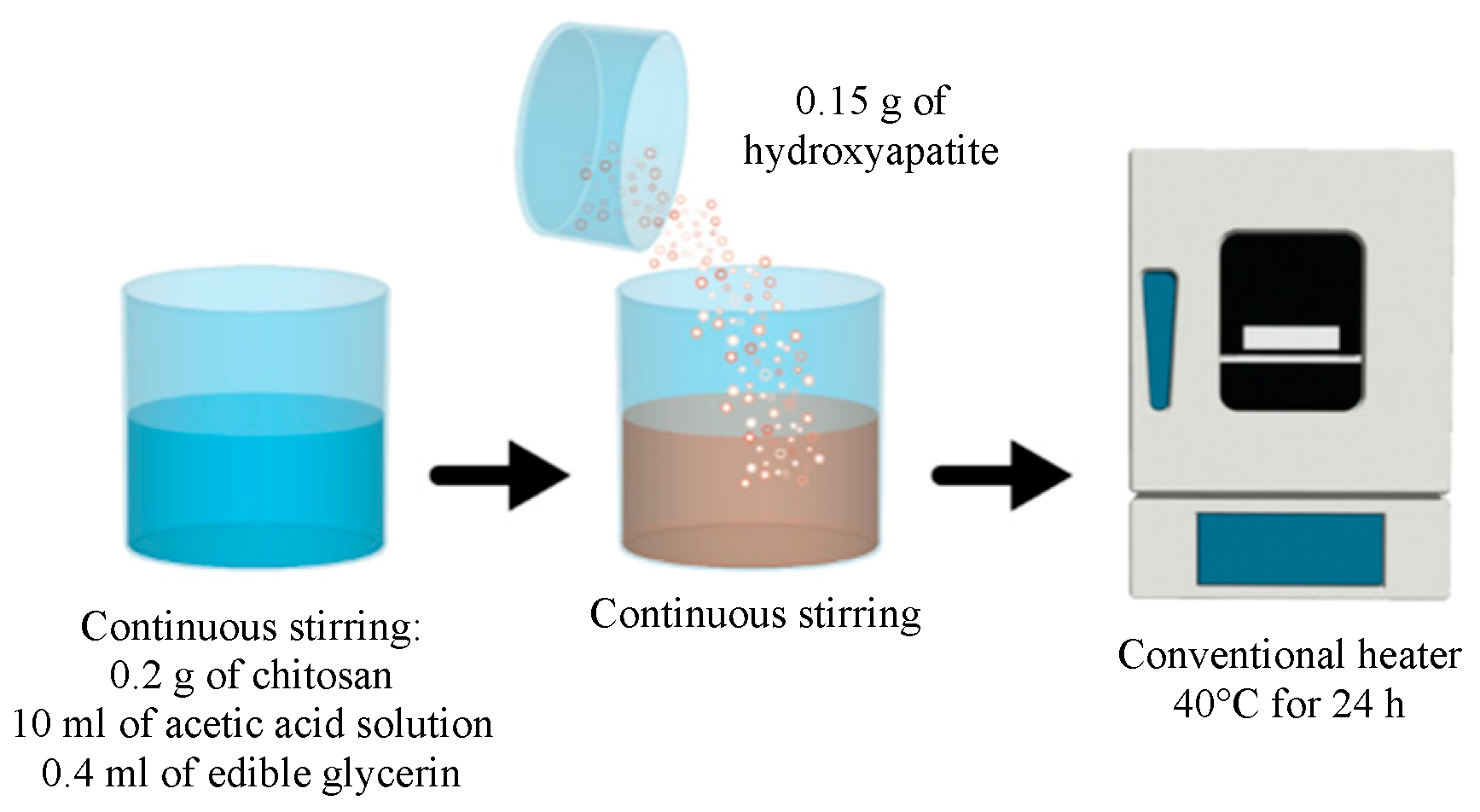
2.2.2. Preparation of the Chemical Solution for the Fabrication of the Membranes of Chitosan–Hydroxyapatite
2.3. Characterization of the Powder of Hydroxyapatite, the Membranes of Chitosan, and the Membranes of Chitosan–Hydroxyapatite
2.4. Mechanical and Superficial Evaluation of the Membranes of Chitosan and Chitosan–Hydroxyapatite
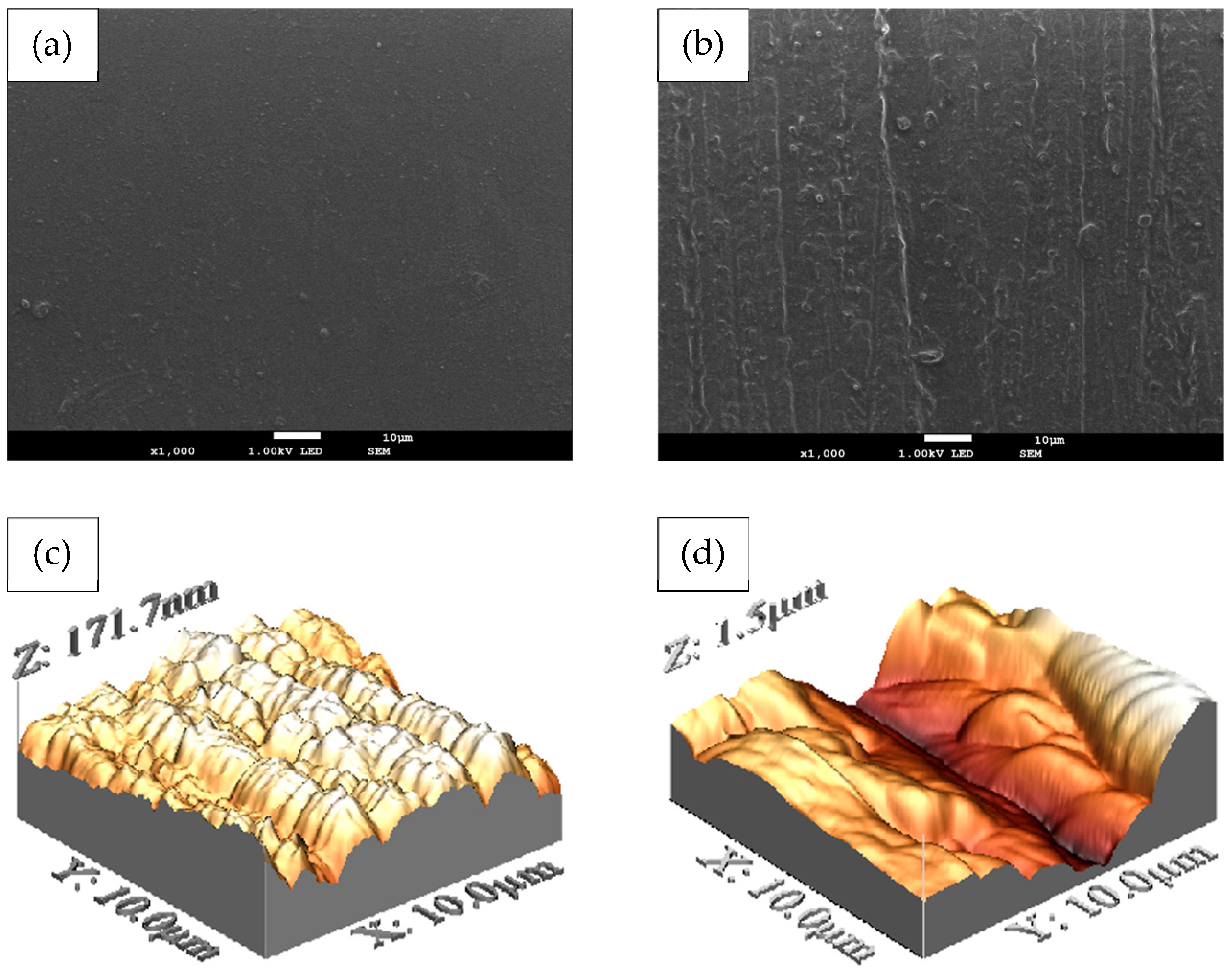
2.4.1. Evaluation of the Surface Roughness
2.4.2. Evaluation of the Tensile Mechanical Properties
2.5. In Vitro Membrane Degradation
2.6. Swelling Behaviour Analysis of the Membranes
2.7. Cell Culture
2.8. Cytotoxicity Assay
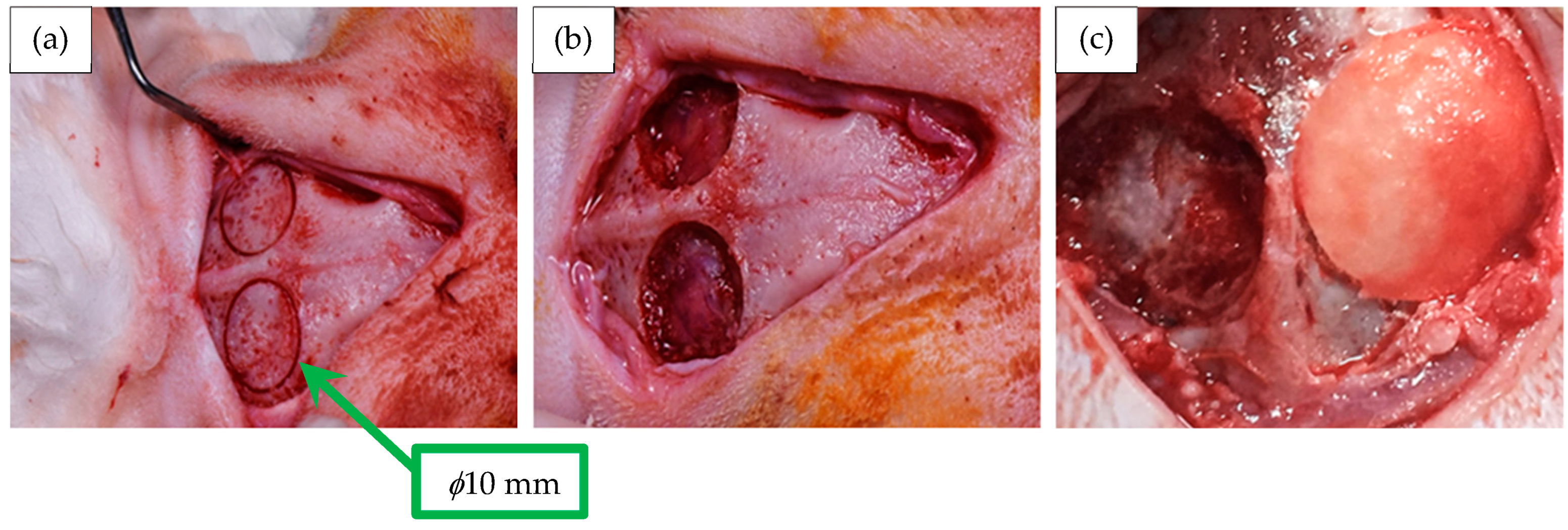
2.9. Membrane Implantation in the Animal Model
- Eight craniotomy defects (n = 8) were treated with membranes of chitosan;
- Eight craniotomy defects (n = 8) were treated with membranes of chitosan–hydroxyapatite;
- Eight craniotomy defects (n = 8) were treated without membranes, which were considered as the negative control.
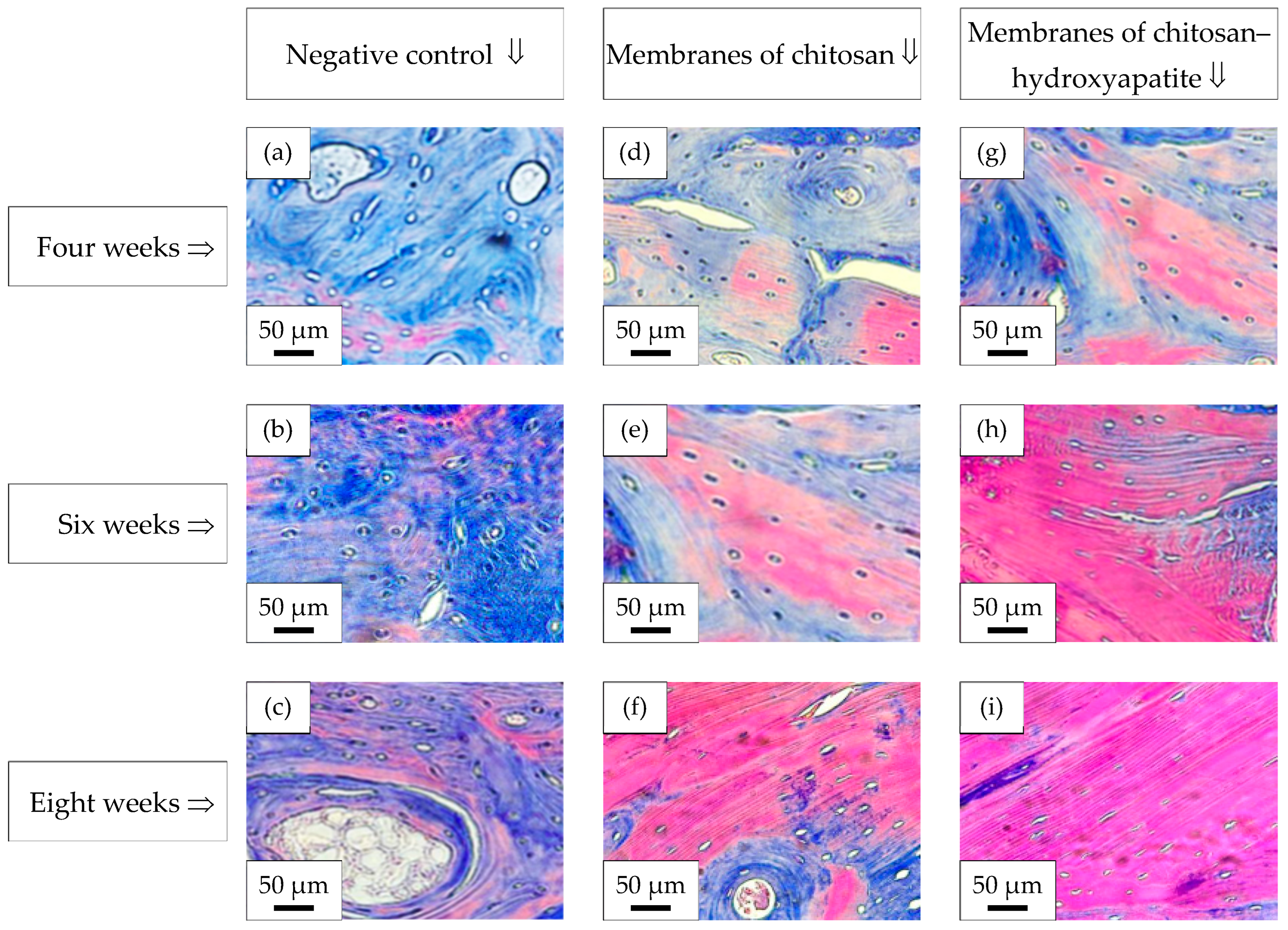
2.10. Histological Analysis—Masson’s Trichrome Stain
2.11. Statistical Analysis
3. Results and Discussion
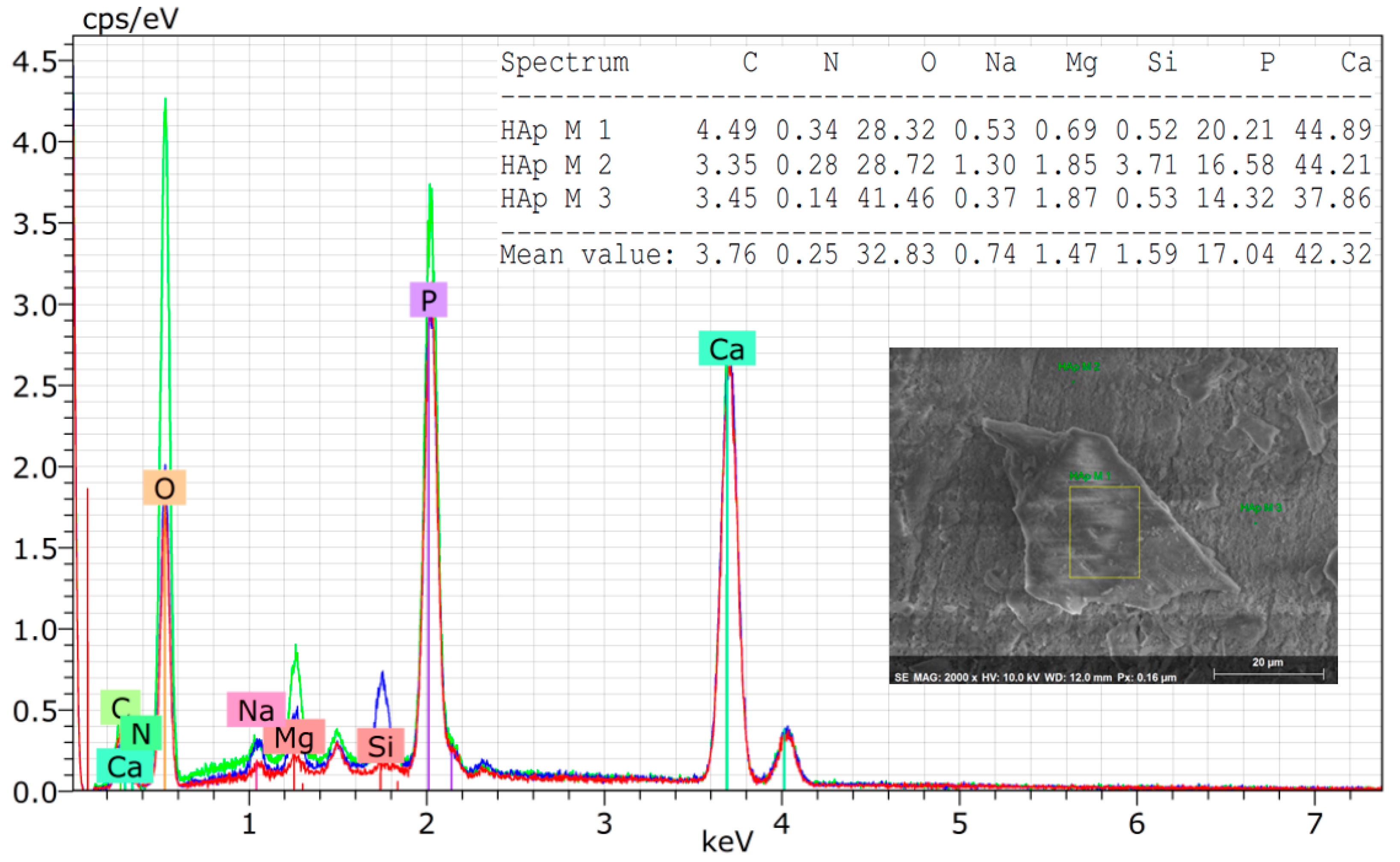
3.1. Characterization of the Hydroxyapatite Powder Isolated from Armoured Catfish
3.2. Superficial, Chemical, and Mechanical Characterizations of the Membranes of Chitosan and Chitosan–Hydroxyapatite
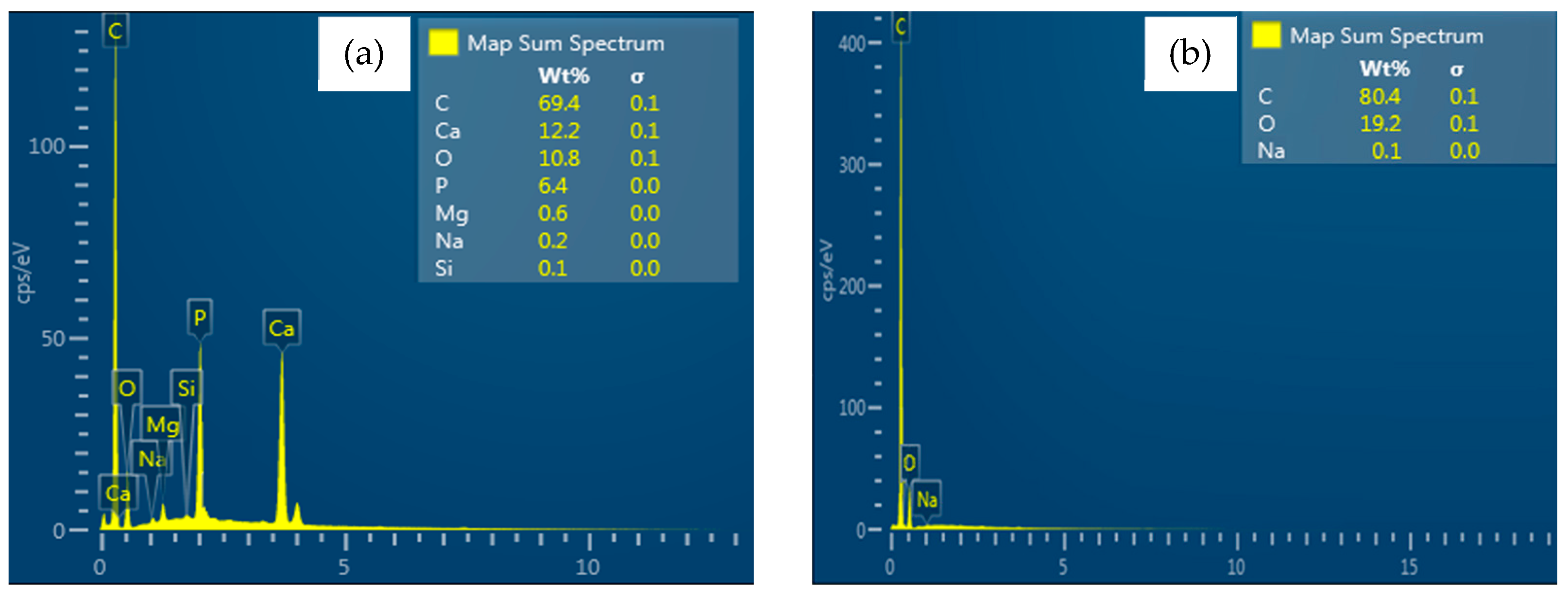
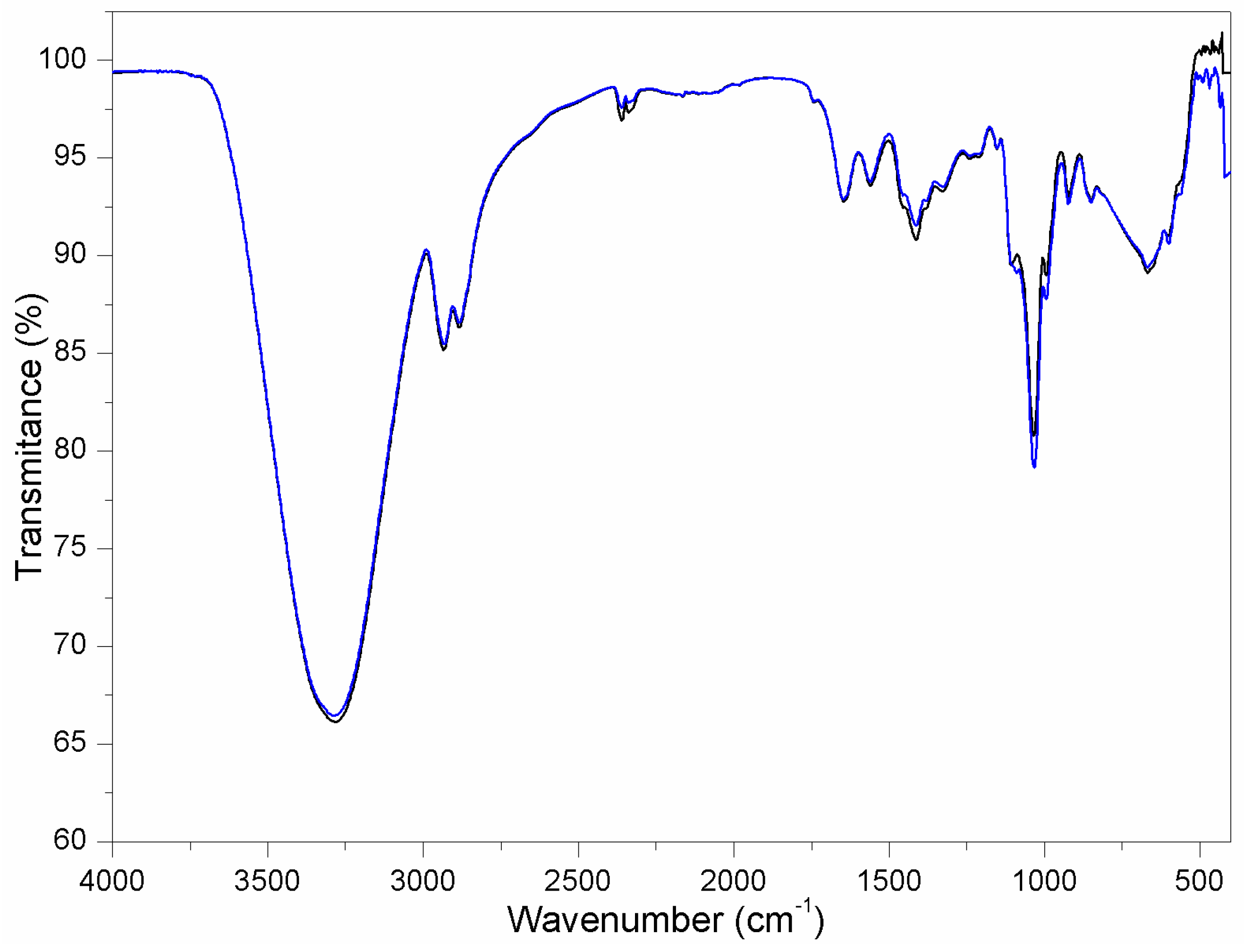
3.2.1. Chemical Characterization of the Membranes of Chitosan and Chitosan–Hydroxyapatite
3.2.2. Superficial Characterization of the Membranes of Chitosan and Chitosan–Hydroxyapatite
3.3. Mechanical Evaluation of the Membranes of Chitosan and Chitosan–Hydroxyapatite
3.4. In Vitro Membrane Degradation and Swelling Analysis
3.5. Cytotoxicity Assay
3.6. Histological Analysis—Masson’s Trichrome Stain
4. Conclusions
- From the analysis conducted by X-Ray Diffraction, a diffractogram corresponding to a hexagonal phase was observed.
- The FT-IR (Fourier Transform Infrared Spectroscopy) revealed the triple group absorption, confirming an apatite structure.
- The ratio Ca/P = 1.9±0.03 and the presence of trace chemical elements improve the biological performances of the powder of hydroxyapatite.
- The incorporation of particles of hydroxyapatite into a matrix of chitosan enhances the barrier function properties and the bone-promoting capacity.
- The surface of the membranes of chitosan–hydroxyapatite had sufficient roughness to increase the cell adhesion and the mechanical tissue integration.
- The chemical interactions between the particles of hydroxyapatite and the matrix of chitosan chains increase the mechanical tensile properties of the membranes of chitosan–hydroxyapatite.
- The membranes of chitosan–hydroxyapatite did not have a cytotoxic effect in direct contact with osteoblasts and human gingival fibroblasts.
- The defects treated with the membranes of chitosan–hydroxyapatite induced the acceleration of new bone formation in comparison with the defects treated with the membranes of chitosan or control defects.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambert, F.; Bacevic, M.; Layrolle, P.; Schüpbach, P.; Drion, P.; Rompen, E. Impact of biomaterial microtopography on bone regeneration: Comparison of three hydroxyapatites. Clin. Oral Implant. Res. 2016, 28, e201–e207. [Google Scholar] [CrossRef] [PubMed]
- Smolka, W.; Eggensperger, N.; Carollo, V.; Ozdoba, C.; Iizuka, T. Changes in the volume and density of calvarial split bone grafts after alveolar ridge augmentation. Clin. Oral Implant. Res. 2006, 17, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Delaine-Smith, R.M.; Fey, T.; Rawlinson, A.; Rehman, I.U. Freeze gelated porous membranes for periodontal tissue regeneration. Acta Biomater. 2015, 23, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Sima, C.; Glogauer, M. Bone Replacement Materials and Techniques Used for Achieving Vertical Alveolar Bone Augmentation. Materials 2015, 8, 2953–2993. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Irinakis, T. Rationale for Socket Preservation after Extraction of a Single-Rooted Tooth when Planning for Future Implant Placement. J. Can. Dent. Assoc. 2006, 72, 917–922. [Google Scholar]
- Fenbo, M.; Xingyu, X.; Bin, T. Strontium chondroitin sulfate/silk fibroin blend membrane containing microporous structure modulates macrophage responses for guided bone regeneration. Carbohydr. Polym. 2019, 213, 266–275. [Google Scholar] [CrossRef]
- Rocchietta, I.; Simion, M.; Hoffmann, M.; Trisciuoglio, D.; Benigni, M.; Dahlin, C. Vertical Bone Augmentation with an Autogenous Block or Particles in Combination with Guided Bone Regeneration: A Clinical and Histological Preliminary Study in Humans. Clin. Implant Dent. Relat. Res. 2016, 18, 19–29. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Hoover, J.J.; Killgore, K.J.; Cofrancesco, A.F. Suckermouth Catfishes: Threats to Aquatic Ecosystems of the United States? ANSRP Bulletin, Vol-04-1; US Army Corp of Engineers Research and Development Center: Vicksburg, MS, USA, 2004. [Google Scholar]
- Atrian, M.; Kharaziha, M.; Emadi, R.; Alihosseini, F. Silk-Laponite® fibrous membranes for bone tissue engineering. Appl. Clay Sci. 2019, 174, 90–99. [Google Scholar] [CrossRef]
- Stankovic, D.; Labudovic-Borovic, M.; Radosavljevic, R.; Marinkovic, M.; Isenovic, E. Use of acellular collagen matrix for the closure of the open oral wound in bone regeneration. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.M.; Khorasani, S.N.; Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Foroughi, M.R.; Kharaziha, M.; Saadatkish, N.; Ramakrishna, S. Fabrication and characterization of two-layered nanofibrous membrane for guided bone and tissue regeneration application. Mater. Sci. Eng. C 2017, 80, 75–87. [Google Scholar] [CrossRef]
- Cheng, H.; Chabok, R.; Guan, X.; Chawla, A.; Li, Y.; Khademhosseini, A.; Jang, H.L. Synergistic interplay between the two major bone minerals, hydroxyapatite and whitlockite nanoparticles, for osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 69, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, R.; Maturana, C.; Silva, D.; Tobar, N.; Tapia, C.; Salazar, J.C.; Martínez, J.; Smith, P.C. Effects of Chitosan Particles in Periodontal Pathogens and Gingival Fibroblasts. J. Dent. Res. 2013, 92, 740–745. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Chitosan Composites for Bone Tissue Engineering—An Overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef]
- Acevedo, C.A.; Olguín, Y.; Briceño, M.; Forero, J.C.; Osses, N.; Díaz-Calderón, P.; Jaques, A.; Ortiz, R. Design of a biodegradable UV-irradiated gelatin-chitosan/nanocomposed membrane with osteogenic ability for application in bone regeneration. Mater. Sci. Eng. C 2019, 99, 875–886. [Google Scholar] [CrossRef]
- Munhoz, M.A.S.; Hirata, H.H.; Plepis, A.M.G.; Martins, V.C.A.; Cunha, M.R. Use of collagen/chitosan sponges mineralized with hydroxyapatite for the repair of cranial defects in rats. Injury 2018, 49, 2154–2160. [Google Scholar] [CrossRef]
- Pereira, I.C.; Duarte, A.S.; Neto, A.S.; Ferreira, J. Chitosan and polyethylene glycol based membranes with antibacterial properties for tissue regeneration. Mater. Sci. Eng. C 2019, 96, 606–615. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, Z.; Zhao, Y.; Zou, Z.; Lu, S.; Zhang, B.; Li, S. Sponges of Carboxymethyl Chitosan Grafted with Collagen Peptides for Wound Healing. Int. J. Mol. Sci. 2019, 20, 3890. [Google Scholar] [CrossRef]
- Chen, P.; Liu, L.; Pan, J.; Mei, J.; Li, C.; Zheng, Y. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 325–335. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tai, H.-Y.; Fu, E.; Don, T.-M. Guided bone regeneration activity of different calcium phosphate/chitosan hybrid membranes. Int. J. Biol. Macromol. 2019, 126, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Baek, S.D.; Venkatesan, J.; Bhatnagar, I.; Chang, H.K.; Kim, H.T.; Kim, S.-K. In vivo study of chitosan-natural nano hydroxyapatite scaffolds for bone tissue regeneration. Int. J. Biol. Macromol. 2014, 67, 360–366. [Google Scholar] [CrossRef]
- Park, K.-W.; Yun, Y.-P.; Kim, S.E.; Song, H.-R. The Effect of Alendronate Loaded Biphasic Calcium Phosphate Scaffolds on Bone Regeneration in a Rat Tibial Defect Model. Int. J. Mol. Sci. 2015, 16, 26738–26753. [Google Scholar] [CrossRef] [PubMed]
- De Tullio, I.; Caputi, S.; Perfetti, G.; Mavriqi, L.; Wismeijer, D.; Traini, T. A Human Clinical and Histomorphometrical Study on Different Resorbable and Non-Resorbable Bone Substitutes Used in Post-Extractive Sites. Preliminary Results. Materials 2019, 12, 2408. [Google Scholar] [CrossRef] [PubMed]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Bee, S.-L.; Hamid, Z.A. Characterization of chicken bone waste-derived hydroxyapatite and its functionality on chitosan membrane for guided bone regeneration. Compos. Part B Eng. 2019, 163, 562–573. [Google Scholar] [CrossRef]
- Luna-Domínguez, J.H.; Téllez-Jiménez, H.; Hernández-Cocoletzi, H.; García-Hernández, M.; Melo-Banda, J.A.; Nygren, H. Development and in vivo response of hydroxyapatite/whitlockite from chicken bones as bone substitute using a chitosan membrane for guided bone regeneration. Ceram. Int. 2018, 44, 22583–22591. [Google Scholar] [CrossRef]
- Mondal, S.; Mondal, B.; Dey, A.; Mukhopadhyay, S.S. Studies on Processing and Characterization of Hydroxyapatite Biomaterials from Different Bio Wastes. J. Miner. Mater. Charact. Eng. 2012, 11, 55–67. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Ghonjizade-Samani, F.; Aghazadeh, J.; Sadeghi, A. Bioactivity evaluation of novel nanocomposite scaffolds for bone tissue engineering: The impact of hydroxyapatite. Ceram. Int. 2016, 42, 11055–11062. [Google Scholar] [CrossRef]
- Pon-On, W.; Suntornsaratoon, P.; Charoenphandhu, N.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. Hydroxyapatite from fish scale for potential use as bone scaffold or regenerative material. Mater. Sci. Eng. C 2016, 62, 183–189. [Google Scholar] [CrossRef]
- Kamalanathan, P.; Ramesh, S.; Bang, L.; Niakan, A.; Tan, C.; Purbolaksono, J.; Chandran, H.; Teng, W. Synthesis and sintering of hydroxyapatite derived from eggshells as a calcium precursor. Ceram. Int. 2014, 40, 16349–16359. [Google Scholar] [CrossRef]
- Wakida-Kusunoki, A.T.; Ruiz-Carus, R.; Amador-Del-Angel, E. Amazon sailfin catfish, Pterygoplichthys pardalis (Castelnau, 1855) (Loricariidae), Another Exotic Species Established in Southeastern Mexico. Southwest. Nat. 2007, 52, 141–144. [Google Scholar] [CrossRef]
- Wu, L.-W.; Liu, C.-C.; Lin, S.-M. Identification of Exotic Sailfin Catfish Species (Pterygoplichthys, Loricariidae) in Taiwan Based on Morphology and mtDNA Sequences. Zool. Stud. 2011, 50, 235–246. [Google Scholar]
- Capps, K.A.; Nico, L.G.; Mendoza-Carranza, M.; Arévalo-Frías, W.; Ropicki, A.J.; Heilpern, S.A.; Rodiles-Hernández, R. Salinity tolerance of non-native suckermouth armoured catfish (Loricariidae: Pterygoplichthys) in south-eastern Mexico: Implications for invasion and dispersal. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 528–540. [Google Scholar] [CrossRef]
- ASTM D 882-02; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2002.
- ISO 10993-5: 2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- NOM-062-ZOO-1999; Especificaciones Técnicas para la Producción, Cuidado y Uso de los Animales de Laboratorio; Miércoles 22 de Agosto de 2001. Diario Oficial (Primera Sección): Washington, DC, USA, 2001.
- Laney, R.W. Glossary of Oral and Maxillofacial Implants; Quintessence Publishing Co, Ltd.: Berlin, Germany, 2007; ISBN 978-3-938947-00-5. [Google Scholar]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Halbritter, S.; Harnisch, H.; Weber, H.-P.; Buser, D. A Retrospective Analysis of Patients Referred for Implant Placement to a Specialty Clinic: Indications, Surgical Procedures, and Early Failures. Int. J. Oral Maxillofac. Implant. 2008, 23, 1109–1116. [Google Scholar]
- Mohd Pu’Ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef]
- Shi, P.; Liu, M.; Fan, F.; Yu, C.; Lu, W.; Du, M. Characterization of natural hydroxyapatite originated from fish bone and its biocompatibility with osteoblasts. Mater. Sci. Eng. C 2018, 90, 706–712. [Google Scholar] [CrossRef]
- Poinern, G.E.; Brundavanam, R.; Le, T.; Djordjevic, S.; Prokic, M.; Fawcett, D.; Poinern, G. Thermal and ultrasonic influence in the formation of nanometer scale hydroxyapatite bio-ceramic. Int. J. Nanomed. 2011, 6, 2083–2095. [Google Scholar] [CrossRef]
- Camargo, N.H.A.; de Lima, E.G.S.A. Synthesis and Characterization of Hydroxyapatite/TiO2n Nanocomposites for Bone Tissue Regeneration. Am. J. Biomed. Eng. 2012, 2, 41–47. [Google Scholar] [CrossRef]
- Bee, S.-L.; Mariatti, M.; Ahmad, N.; Yahaya, B.; Hamid, Z.A. Effect of the calcination temperature on the properties of natural hydroxyapatite derived from chicken bone wastes. Mater. Today Proc. 2019, 16, 1876–1885. [Google Scholar] [CrossRef]
- Sunil, B.R.; Jagannatham, M. Producing hydroxyapatite from fish bones by heat treatment. Mater. Lett. 2016, 185, 411–414. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Manivasagan, P.; Kang, K.-H.; Chalisserry, E.P.; Anil, S.; Kim, D.G.; Kim, S.-K. Isolation and Characterization of Nano-Hydroxyapatite from Salmon Fish Bone. Materials 2015, 8, 5426–5439. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Zhu, Q.; Ablikim, Z.; Chen, T.; Cai, Q.; Xia, J.; Jiang, D.; Wang, S. The preparation and characterization of HA/β-TCP biphasic ceramics from fish bones. Ceram. Int. 2017, 43, 12213–12220. [Google Scholar] [CrossRef]
- Zainol, I.; Adenan, N.; Rahim, N.; Jaafar, C.A. Extraction of natural hydroxyapatite from tilapia fish scales using alkaline treatment. Mater. Today Proc. 2019, 16, 1942–1948. [Google Scholar] [CrossRef]
- Tai, H.-Y.; Fu, E.; Don, T.-M. Calcium phosphates synthesized by reverse emulsion method for the preparation of chitosan composite membranes. Carbohydr. Polym. 2012, 88, 904–911. [Google Scholar] [CrossRef]
- Matinfar, M.; Mesgar, A.S.; Mohammadi, Z. Evaluation of physicochemical, mechanical and biological properties of chitosan/carboxymethyl cellulose reinforced with multiphasic calcium phosphate whisker-like fibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 341–353. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Xiang, L.; Wu, Y.; Wei, X.; Qu, Y.; Man, Y. Evaluation of epigallocatechin-3-gallate (EGCG) cross-linked collagen membranes and concerns on osteoblasts. Mater. Sci. Eng. C 2016, 67, 386–394. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Effect of Temperature on Isolation and Characterization of Hydroxyapatite from Tuna (Thunnus obesus) Bone. Materials 2010, 3, 4761–4772. [Google Scholar] [CrossRef] [PubMed]
- Forero-Sossa, P.A.; Salazar-Martínez, J.D.; Giraldo-Betancur, A.L.; Segura-Giraldo, B.; Restrepo-Parra, E. Temperature effect in physicochemical and bioactive behavior of biogenic hydroxyapatite obtained from porcine bones. Sci. Rep. 2021, 11, 11069. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Fernando, A.; Martins, G.; Freitas, J.; Judas, F.; Figueiredo, H. Effect of the calcination temperature on the composition and microstructure of hydroxyapatite derived from human and animal bone. Ceram. Int. 2010, 36, 2383–2393. [Google Scholar] [CrossRef]
- Aarthy, S.; Thenmuhil, D.; Dharunya, G.; Manohar, P. Exploring the effect of sintering temperature on naturally derived hydroxyapatite for bio-medical applications. J. Mater. Sci. Mater. Med. 2019, 30, 21. [Google Scholar] [CrossRef]
- Lama-Odría, M.d.C.D.; del Valle, L.J.; Puiggalí, J. Lanthanides-Substituted Hydroxyapatite for Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 3446. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; LewisOscar, F.; Pugazhendhi, A.; Subashkumar, R. Biosynthesis and characterization of hydroxyapatite and its composite (hydroxyapatite-gelatin-chitosan-fibrin-bone ash) for bone tissue engineering applications. Int. J. Biol. Macromol. 2019, 129, 844–852. [Google Scholar] [CrossRef]
- Fraga, A.F.; Filho, E.d.A.; Rigo, E.C.d.S.; Boschi, A.O. Synthesis of chitosan/hydroxyapatite membranes coated with hydroxycarbonate apatite for guided tissue regeneration purposes. Appl. Surf. Sci. 2011, 257, 3888–3892. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Morganti, P.; Morganti, G.; Palombo, P.; Palombo, M.; Biagini, G.; Belmonte, M.M.; Giantomassi, F.; Orlandi, F.; Muzzarelli, C. Chitin nanofibrils/chitosan glycolate composites as wound medicaments. Carbohydr. Polym. 2007, 70, 274–284. [Google Scholar] [CrossRef]
- Banerjee, S.; Bagchi, B.; Bhandary, S.; Kool, A.; Hoque, N.A.; Biswas, P.; Pal, K.; Thakur, P.; Das, K.; Karmakar, P.; et al. Antimicrobial and biocompatible fluorescent hydroxyapatite-chitosan nanocomposite films for biomedical applications. Colloids Surf. B Biointerfaces 2018, 171, 300–307. [Google Scholar] [CrossRef]
- Ortolani, E.; Quadrini, F.; Bellisario, D.; Santo, L.; Polimeni, A.; Santarsiero, A. Mechanical qualification of collagen membranes used in dentistry. Ann. dell’Istituto Super. Sanita 2015, 51, 229–235. [Google Scholar] [CrossRef]
- Guo, M.; Chu, Z.; Yao, J.; Feng, W.; Wang, Y.; Wang, L.; Fan, Y. The effects of tensile stress on degradation of biodegradable PLGA membranes: A quantitative study. Polym. Degrad. Stab. 2016, 124, 95–100. [Google Scholar] [CrossRef]
- Kitayama, S.; Wong, L.O.; Ma, L.; Hao, J.; Kasugai, S.; Lang, N.P.; Mattheos, N. Regeneration of rabbit calvarial defects using biphasic calcium phosphate and a strontium hydroxyapatite-containing collagen membrane. Clin. Oral Implant. Res. 2016, 27, 206–214. [Google Scholar] [CrossRef]
- Gnaneshwar, P.V.; Sudakaran, S.V.; Abisegapriyan, S.; Sherine, J.; Ramakrishna, S.; Rahim, M.H.A.; Yusoff, M.M.; Jose, R.; Venugopal, J.R. Ramification of zinc oxide doped hydroxyapatite biocomposites for the mineralization of osteoblasts. Mater. Sci. Eng. C 2019, 96, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Qi, C.; Chen, Y.-X.; Zhu, Y.-J.; Sun, T.-W.; Chen, F.; Zhang, C.-Q. Comparative study of porous hydroxyapatite/chitosan and whitlockite/chitosan scaffolds for bone regeneration in calvarial defects. Int. J. Nanomed. 2017, 12, 2673–2687. [Google Scholar] [CrossRef] [PubMed]
- Nygren, H.; Bigdeli, N.; Ilver, L.; Malmberg, P. Mg-corrosion, hydroxyapatite, and bone healing. Biointerphases 2017, 12, 02C407. [Google Scholar] [CrossRef]
- Liao, S.; Watari, F.; Zhu, Y.; Uo, M.; Akasaka, T.; Wang, W.; Xu, G.; Cui, F. The degradation of the three layered nano-carbonated hydroxyapatite/collagen/PLGA composite membrane in vitro. Dent. Mater. 2007, 23, 1120–1128. [Google Scholar] [CrossRef]
- Wu, H.-D.; Ji, D.-Y.; Chang, W.-J.; Yang, J.-C.; Lee, S.-Y. Chitosan-based polyelectrolyte complex scaffolds with antibacterial properties for treating dental bone defects. Mater. Sci. Eng. C 2012, 32, 207–214. [Google Scholar] [CrossRef]
- Pawinska, M.; Paszynska, E.; Amaechi, B.T.; Meyer, F.; Enax, J.; Limeback, H. Clinical evidence of caries prevention by hydroxyapatite: An updated systematic review and meta-analysis. J. Dent. 2024, 151, 105429. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Ji, C.; Ma, J.; He, B. The impact of hydroxyapatite crystal structures and protein interactions on bone’s mechanical properties. Sci. Rep. 2024, 14, 9786. [Google Scholar] [CrossRef]
- Damiri, F.; Fatimi, A.; Musuc, A.M.; Santos, A.C.P.; Paszkiewicz, S.; Idumah, C.I.; Singh, S.; Varma, R.S.; Berrada, M. Nano-hydroxyapatite (nHAp) scaffolds for bone regeneration: Preparation, characterization and biological applications. J. Drug Deliv. Sci. Technol. 2024, 95, 105601. [Google Scholar] [CrossRef]



| Wavenumber—λ [cm−1] (from Figure 7) | Chemical Occurrence |
|---|---|
| 3420 | Stretching vibration of O–H of alkenes |
| 3255 | Stretching vibration of N–H of alkenes |
| 2879 | Stretching vibration of C–H of alkenes |
| 1654 | Stretching vibration of C=O—characteristic of Amide I |
| 1625 | Stretching vibration of C=O—characteristic of Amide II |
| 1570 | NH2 flexion—corresponds to the deformation in the CONH plane |
| 1423 | Stretching vibration of secondary absorption of CH2 alkenes |
| 1375 | Stretching vibration of C–CH amide |
| 1315 | Stretching vibration of C–N |
| 1157 | Stretching vibration of C–O–C bridge |
| 1079 | Stretching vibration of symmetric C–O–C in the ring |
| 1023 | Stretching vibration of C–O–C glycoside |
| 890 | Stretching vibration of C–O–C glycoside |
| 713 | Stretching vibration of C–O–C glycoside |
| Membrane | Roughness—Ra [μm] | Critical Value of Roughness—Ra [µm] |
|---|---|---|
| Chitosan | 0.097±0.003 | >0.2 |
| Chitosan–hydroxyapatite | 0.176±0.006 |
| Membrane | Ultimate Tensile Strength—UTS [MPa] | Elongation at Break—Δl [%] |
|---|---|---|
| Chitosan | 3.30±0.46 | 54.0±6.1 |
| Chitosan–hydroxyapatite | 5.40±0.62 | 92.4±5.2 |
| Membrane | Loss of Mass—Δm [%] (Level of Degradation) | Swelling Ratio—SR [%] (Capacity of Liquid Absorption) |
|---|---|---|
| Chitosan | 54.2±7.1 | 54.4±3.3 |
| Chitosan–hydroxyapatite | 43.7±5.9 | 62.5±3.0 |
| Time of Analysis | Contact with “Osteoblasts” | Viability [%]—Membranes of Chitosan | Viability [%]—Membranes of Chitosan–Hydroxyapatite |
|---|---|---|---|
| 24 h | 100±9.8 | 89±12.0 | 88±11.4 |
| 48 h | 100±8.3 | 85±7.7 | 84±8.1 |
| 72 h | 100±4.2 | 82±6.1 | 81±6.9 |
| 96 h | 100±1.3 | 81±4.2 | 78±4.7 |
| Time of analysis | Contact with “human gingival fibroblasts” | Viability [%]—Membranes of chitosan | Viability [%]—Membranes of chitosan–hydroxyapatite |
| 24 h | 100±4.0 | 87±7.1 | 89±7.8 |
| 48 h | 100±3.1 | 83±4.9 | 87±6.4 |
| 72 h | 100±1.6 | 81±3.7 | 83±4.1 |
| 96 h | 100±0.8 | 79±2.8 | 81±2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, R.d.J.F.; Luna-Domínguez, C.R.; Mendoza-Martínez, A.M.; Şahin, M.; Al-Anzi, B.S.; Cozza, R.C.; Luna-Domínguez, J.H. Development of Chitosan–Hydroxyapatite Membranes from Bone of Armoured Catfish (Pterygoplichthys spp.) for Applications in Guided Bone Regeneration (GBR). Processes 2025, 13, 1559. https://doi.org/10.3390/pr13051559
López RdJF, Luna-Domínguez CR, Mendoza-Martínez AM, Şahin M, Al-Anzi BS, Cozza RC, Luna-Domínguez JH. Development of Chitosan–Hydroxyapatite Membranes from Bone of Armoured Catfish (Pterygoplichthys spp.) for Applications in Guided Bone Regeneration (GBR). Processes. 2025; 13(5):1559. https://doi.org/10.3390/pr13051559
Chicago/Turabian StyleLópez, Ricardo de Jesús Figueroa, Carlos Roberto Luna-Domínguez, Ana María Mendoza-Martínez, Muradiye Şahin, Bader Shafaqa Al-Anzi, Ronaldo Câmara Cozza, and Jorge Humberto Luna-Domínguez. 2025. "Development of Chitosan–Hydroxyapatite Membranes from Bone of Armoured Catfish (Pterygoplichthys spp.) for Applications in Guided Bone Regeneration (GBR)" Processes 13, no. 5: 1559. https://doi.org/10.3390/pr13051559
APA StyleLópez, R. d. J. F., Luna-Domínguez, C. R., Mendoza-Martínez, A. M., Şahin, M., Al-Anzi, B. S., Cozza, R. C., & Luna-Domínguez, J. H. (2025). Development of Chitosan–Hydroxyapatite Membranes from Bone of Armoured Catfish (Pterygoplichthys spp.) for Applications in Guided Bone Regeneration (GBR). Processes, 13(5), 1559. https://doi.org/10.3390/pr13051559









