Molecular-Level Regulation of Nitrogen-Doped Ordered Mesoporous Carbon Materials via Ligand Exchange Strategy
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Fe3O4 Nanocrystal Preparation
2.2.2. Ligand Exchange
2.2.3. Mesoporous Carbon Preparation
2.3. Sample Characterizations
3. Results and Discussion
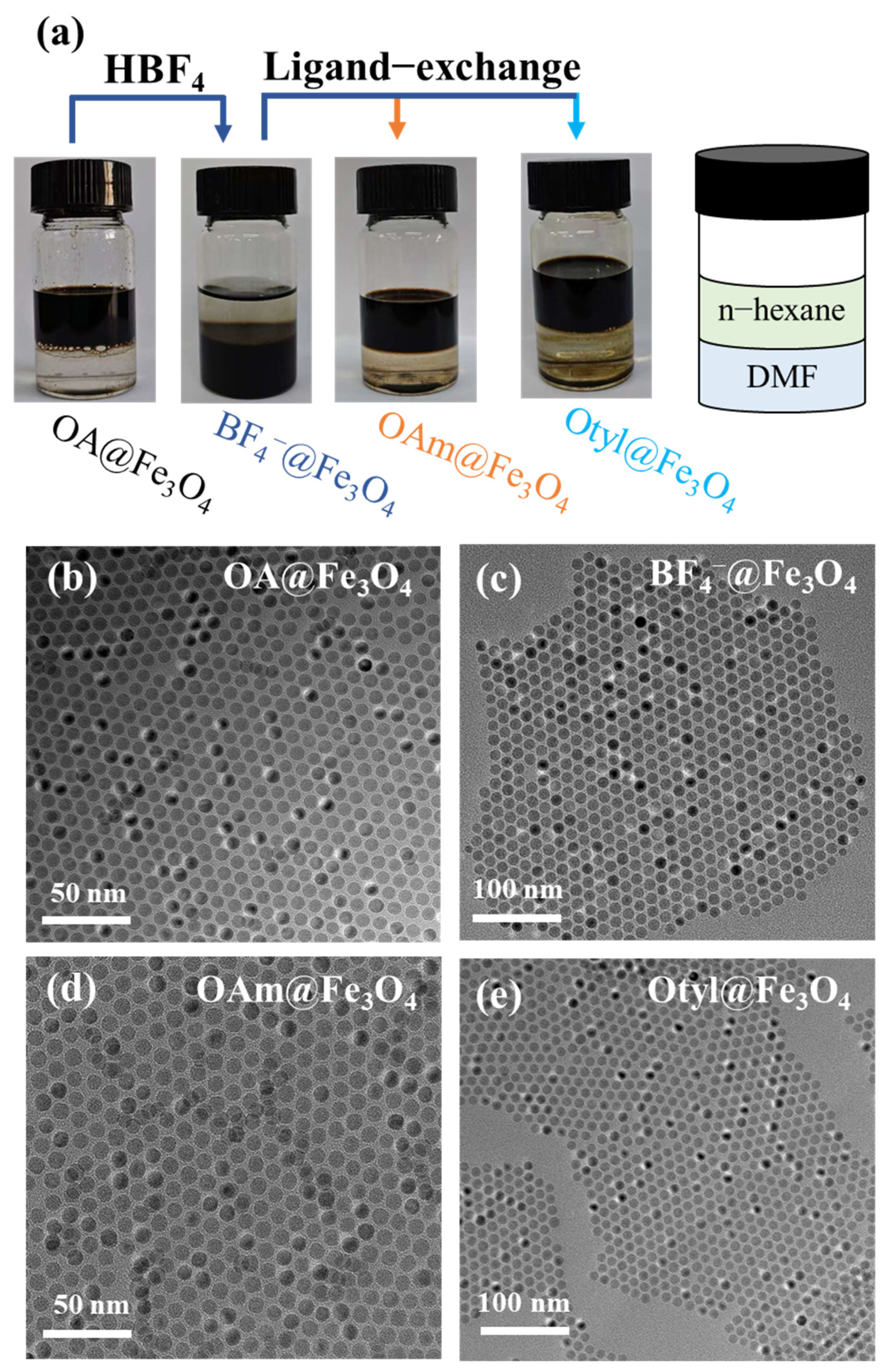
3.1. Synthesis of Fe3O4 Nanocrystals and Ligand Exchange
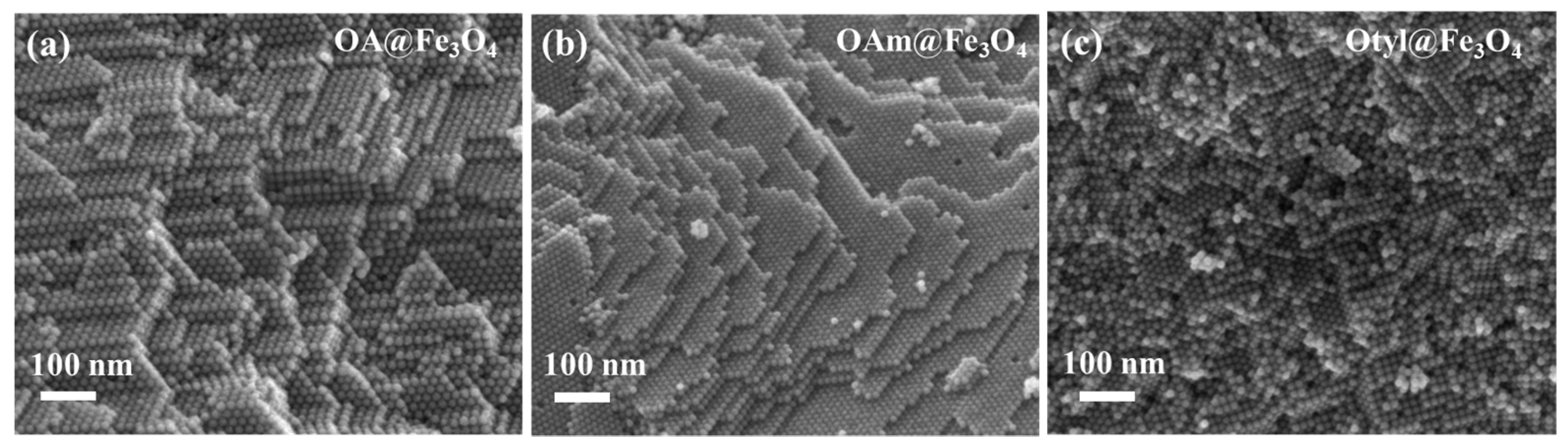
3.2. Assembly and Carbonization of Fe3O4 Nanocrystals
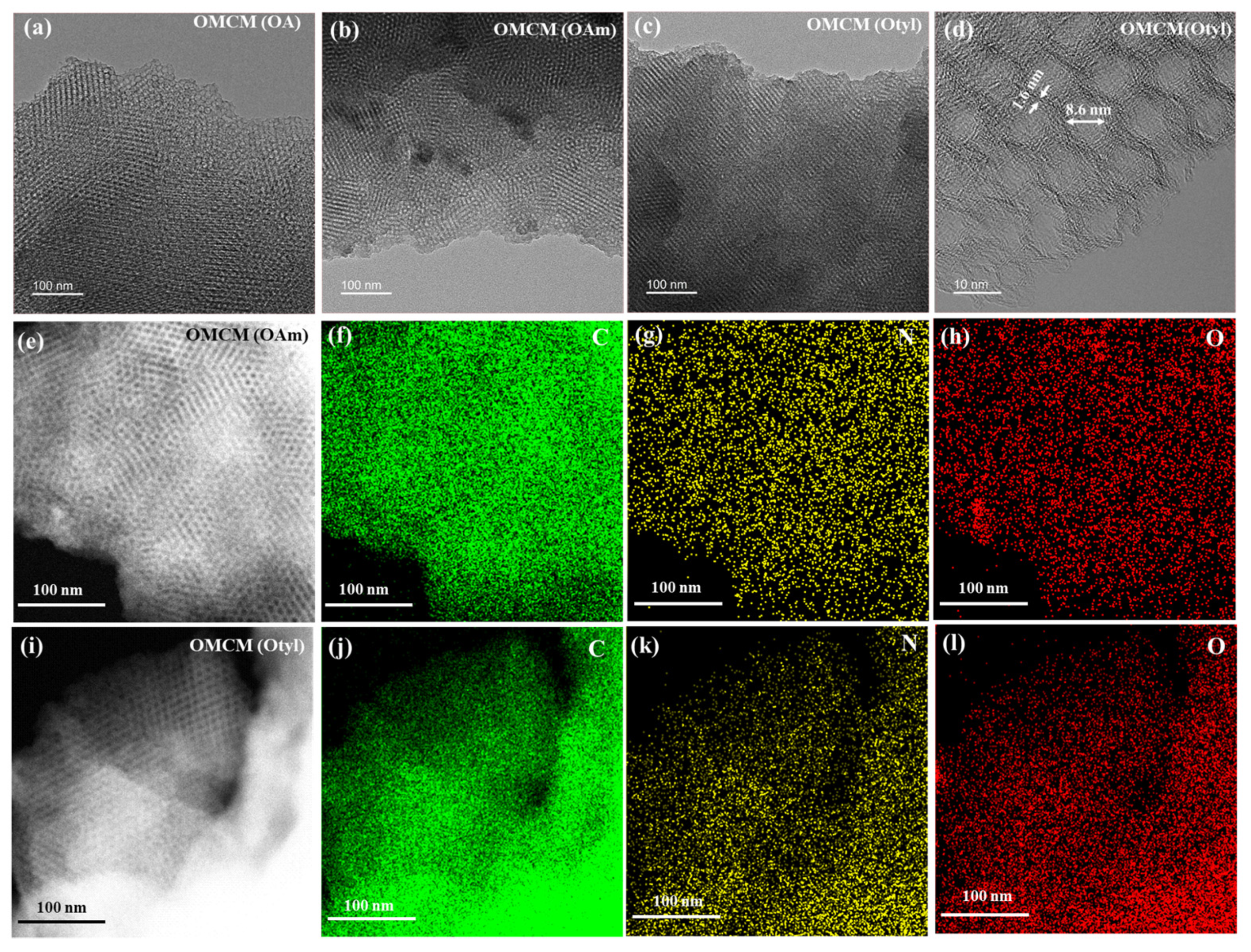
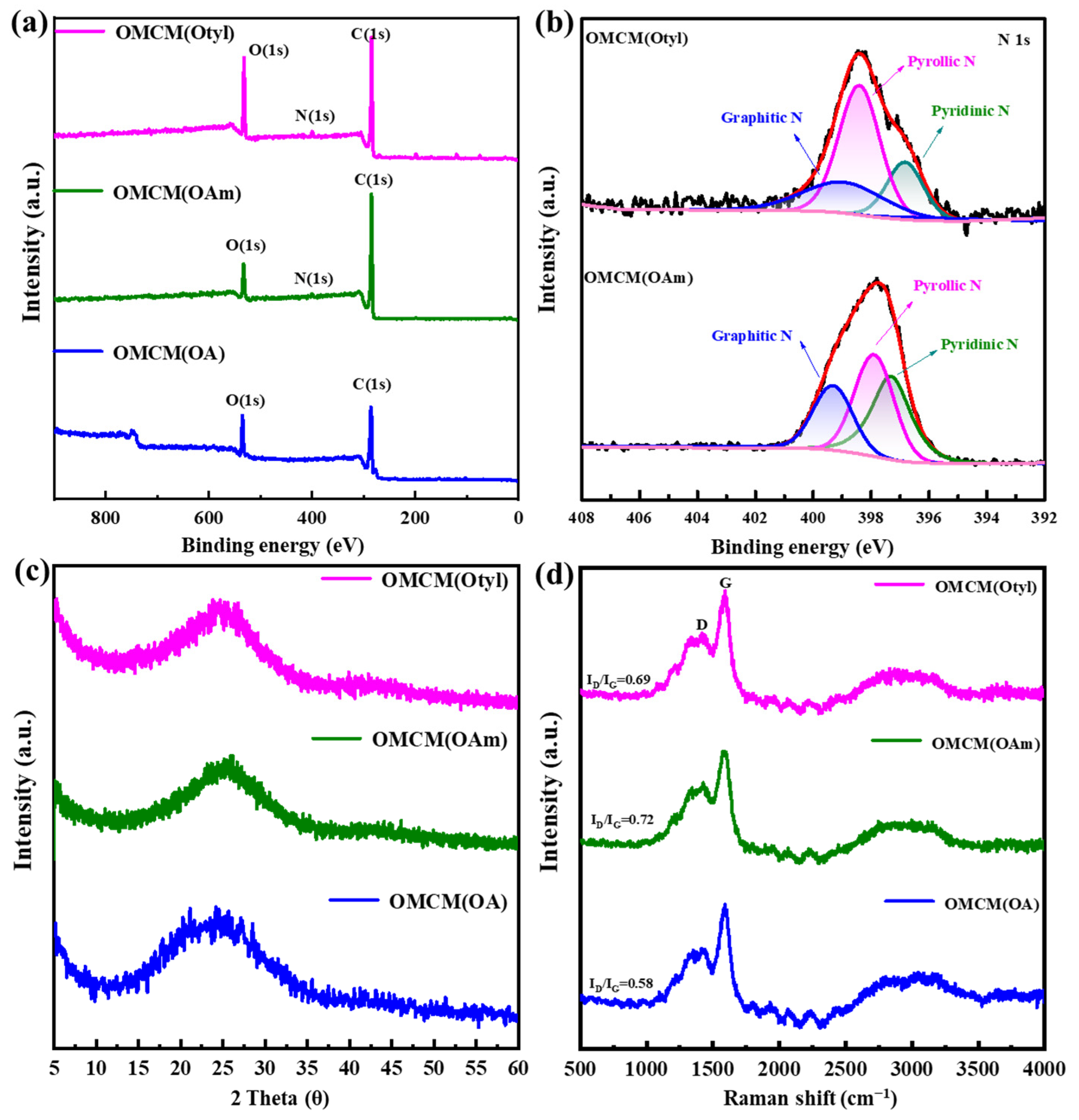
3.3. Preparation and Characterization of OMCMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Dai, Z.; Wu, J.; Liu, W.; Di, T.; Jiang, R.; Zheng, X.; Wang, W.; Ji, X.; Li, P.; et al. Fabrication of ordered macro-microporous single-crystalline MOF and its derivative carbon material for supercapacitor. Adv. Energy Mater. 2020, 10, 1903750. [Google Scholar] [CrossRef]
- Owen, R.; Brennan, G.; Lyon, F. Enabling investment for the transition to a low carbon economy: Government policy to finance early stage green innovation. Curr. Opin. Environ. Sustain. 2018, 31, 137–145. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Song, M.; Xu, C.; Liu, Z.; Jia, S.; Li, X.; Liu, J.; Meng, L.; Wang, Z.; et al. Enabling directional ion transport over graphene electrode surfaces for kilohertz electrochemical capacitors. Electrochim. Acta 2021, 368, 137561. [Google Scholar] [CrossRef]
- Ghosh, S.; Barg, S.; Jeong, S.M.; Ostrikov, K. Heteroatom-doped and oxygen-functionalized nanocarbons for high-performance supercapacitors. Adv. Energy Mater. 2020, 10, 2001239. [Google Scholar] [CrossRef]
- Ko, Y.; Kwon, C.H.; Lee, S.W.; Cho, J. Nanoparticle-Based Electrodes with High Charge Transfer Efficiency through Ligand Exchange Layer-by-Layer Assembly. Adv. Mater. 2020, 32, 2001924. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.; Wan, H.; Li, C.; An, W.; Sheng, X.; Liang, X.; Wang, X.; Ren, Y.; Zheng, X.; et al. Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction. Chin. Chem. Lett. 2022, 33, 2259–2269. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Wu, M.; Tong, S.; Jiang, L.; Hou, B.; Li, X.; Zhang, Y.; Yue, J.; Jiang, M.; Sheng, L. Nitrogen-doped porous carbon composite with three-dimensional conducting network for high rate supercapacitors. J. Alloys Compd. 2020, 844, 156217. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Pillai, S.C. MXenes-based nanocomposites for supercapacitor applications. Curr. Opin. Chem. Eng. 2021, 33, 100710. [Google Scholar] [CrossRef]
- Sheng, X.; Li, Y.; Yang, T.; Timmer, B.J.; Willhammar, T.; Cheung, O.; Li, L.; Brett, C.J.; Roth, S.V.; Zhang, B.; et al. Hierarchical micro-reactor as electrodes for water splitting by metal rod tipped carbon nanocapsule self-assembly in carbonized wood. Appl. Catal. B Environ. 2020, 264, 118536. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, S.; Hibino, M.; Honma, I.; Ichihara, M. Lithium storage in ordered mesoporous carbon (CMK-3) with high reversible specific energy capacity and good cycling performance. Adv. Mater. 2003, 15, 2107–2111. [Google Scholar] [CrossRef]
- Calcagno, G.; Agostini, M.; Xiong, S.; Matic, A.; Palmqvist, A.E.C.; Cavallo, C. Effect of nitrogen doping on the performance of mesoporous CMK-8 carbon anodes for Li-ion batteries. Energies 2020, 13, 4998. [Google Scholar] [CrossRef]
- Zhang, X.; Han, G.; Zhu, S. Flash Nitrogen-Doped Carbon Nanotubes for Energy Storage and Conversion. Small 2024, 20, 2305406. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, W.; Gu, Y.; Liao, T.; Sun, Z. Metal-nitrogen-doped carbon materials as highly efficient catalysts: Progress and rational design. Adv. Sci. 2020, 7, 2001069. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; You, W.; Yu, J. Core–shell nitrogen-doped carbon hollow spheres/Co3O4 nanosheets as advanced electrode for high-performance supercapacitor. Small 2018, 14, 1702407. [Google Scholar] [CrossRef]
- Ke, P.; Zeng, D.; Xing, H.; Cui, J.; Li, X.; Qin, R. Research progress of nitrogen-doped modified carbon materials. Appl. Chem. Ind. 2020, 49, 1818–1822. [Google Scholar]
- Wang, R.; Jang, W.Y.; Zhang, W.; Reddy, C.V.; Kakarla, R.R.; Li, C.; Gupta, V.K.; Shim, J.; Aminabhavi, T.M. Emerging two-dimensional (2D) MXene-based nanostructured materials: Synthesis strategies, properties, and applications as efficient pseudo-supercapacitors. Chem. Eng. J. 2023, 472, 144913. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhang, L.; Yoon, Y.; Weber, P.K.; Wang, H.; Guo, J.; Dai, H. N-doping of graphene through electrothermal reactions with ammonia. Science 2009, 324, 768–771. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, M.; Zhang, Y.; Zhao, W.; Wang, C. A biomass-derived nitrogen-doped porous carbon for high-energy supercapacitor. Carbon 2018, 140, 404–412. [Google Scholar] [CrossRef]
- Liu, K.; Wang, J.-A.; Zheng, H.; Sun, X.; Yang, Z.; Man, J.; Wang, X.; Sun, J. Direct synthesis of tin spheres/nitrogen-doped porous carbon composite by self-formed template method for enhanced lithium storage. J. Mater. Sci. Technol. 2022, 104, 88–97. [Google Scholar] [CrossRef]
- Han, D.; Jiao, Y.; Han, W.; Wu, G.; Li, T.; Yang, D.; Dong, A. A molecular-based approach for the direct synthesis of highly-ordered, homogeneously-doped mesoporous carbon frameworks. Carbon 2018, 140, 265–275. [Google Scholar] [CrossRef]
- Ragavan, R.; Pandurangan, A. Exploration on magnetic and electrochemical properties of nitrogen and phosphorus Co-doped ordered mesoporous carbon for supercapacitor applications. Microporous Mesoporous Mater. 2022, 338, 111959. [Google Scholar] [CrossRef]
- Ma, X.; Du, J.; Sun, H.; Ye, F.; Wang, X.; Xu, P.; Hu, C.; Zhang, L.; Liu, D. Boron, nitrogen co-doped carbon with abundant mesopores for efficient CO2 electroreduction. Appl. Catal. B Environ. 2021, 298, 120543. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Liu, H.; Ye, Y.; Wei, Z. Nitrogen-doped mesoporous carbon supported Pt nanoparticles as a highly efficient catalyst for decarboxylation of saturated and unsaturated fatty acids to alkanes. Appl. Catal. B Environ. 2017, 218, 679–689. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, R.; Lv, Y.; Deng, Y.; Elzatahrya, A.A.; Zhao, D. Nitrogen-doped ordered mesoporous carbons based on cyanamide as the dopant for supercapacitor. Carbon 2015, 84, 335–346. [Google Scholar] [CrossRef]
- Tian, S.; Wu, J.; Zhang, X.; Ostrikov, K.K.; Zhang, Z. Capacitive deionization with nitrogen-doped highly ordered mesoporous carbon electrodes. Chem. Eng. J. 2020, 380, 122514. [Google Scholar] [CrossRef]
- Ma, T.; Yang, W.; Wu, Z.; Lei, K.; Lin, J.; Zou, H.; Chen, S. Rich nitrogen-doped ordered mesoporous carbon synthesized by copolymerization of PMDA and ODA with SBA-15 as a template for high-performance supercapacitors. J. Porous Mater. 2020, 27, 525–535. [Google Scholar] [CrossRef]
- Chen, H.; Sun, F.; Wang, J.; Li, W.; Qiao, W.; Ling, L.; Long, D. Nitrogen doping effects on the physical and chemical properties of mesoporous carbons. J. Phys. Chem. C 2013, 117, 8318–8328. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef]
- Han, D.; Guo, G.; Yan, Y.; Li, T.; Wang, B.; Dong, A. Pomegranate-like, carbon-coated Fe3O4 nanoparticle superparticles for high-performance lithium storage. Energy Storage Mater. 2018, 10, 32–39. [Google Scholar] [CrossRef]
- Han, D.; Zhou, Q.; Xia, Y.; Huang, D.; Qin, J.; Wang, L.; Wang, X.; Zheng, X.; Wu, D. A general confinement co-assembly strategy enabling cross-dimensional supraspheres for boosting electrochemical performance. Carbon 2022, 200, 296–306. [Google Scholar] [CrossRef]
- Han, D.; Yan, Y.; Wei, J.; Wang, B.; Li, T.; Guo, G.; Yang, D.; Xie, S.; Dong, A. Fine-tuning the wall thickness of ordered mesoporous graphene by exploiting ligand exchange of colloidal nanocrystals. Front. Chem. 2017, 5, 117. [Google Scholar] [CrossRef]
- Wu, D.; Han, D.; Zhou, W.; Streiff, S.; Khodakov, A.Y.; Ordomsky, V.V. Surface modification of metallic catalysts for the design of selective processes. Catal. Rev. 2024, 66, 640–686. [Google Scholar] [CrossRef]
- Wu, D.; Baaziz, W.; Gu, B.; Marinova, M.; Hernández, W.Y.; Zhou, W.; Vovk, E.I.; Ersen, O.; Safonova, O.V.; Addad, A.; et al. Surface molecular imprinting over supported metal catalysts for size-dependent selective hydrogenation reactions. Nat. Catal. 2021, 4, 595–606. [Google Scholar] [CrossRef]
- Marshall, S.T.; O’brien, M.; Oetter, B.; Corpuz, A.; Richards, R.M.; Schwartz, D.K.; Medlin, J.W. Controlled selectivity for palladium catalysts using self-assembled monolayers. Nat. Mater. 2010, 9, 853–858. [Google Scholar] [CrossRef]
- Rahmawati, R.; Taufiq, A.; Sunaryono, S.; Fuad, A.; Yuliarto, B.; Suytman, S.; Kurniadi, D. Synthesis of magnetite (Fe3O4) Nanoparticles from iron sands by Co-precipitation-ultrasonic irradiation methods. J. Mater. Environ. Sci. 2018, 9, 155–160. [Google Scholar]
- Jiao, Y.; Han, D.; Liu, L.; Ji, L.; Guo, G.; Hu, J.; Yang, D.; Dong, A. Highly ordered mesoporous few-layer graphene frameworks enabled by Fe3O4 nanocrystal superlattices. Angew. Chem. 2015, 127, 5819–5823. [Google Scholar] [CrossRef]
- Jiao, Y.; Han, D.; Ding, Y.; Zhang, X.; Guo, G.; Hu, J.; Yang, D.; Dong, A. Fabrication of three-dimensionally interconnected nanoparticle superlattices and their lithium-ion storage properties. Nat. Commun. 2015, 6, 6420. [Google Scholar] [CrossRef]
- Liu, X.; Kang, J.; Dai, Y.; Dong, C.; Guo, X.; Jia, X. Graphene-Like Nitrogen-Doped Carbon Nanosheet Prepared from Direct Calcination of Dopamine Confined by g-C3N4 for Oxygen Reduction. Adv. Mater. Interfaces 2018, 5, 1800303. [Google Scholar] [CrossRef]
- Abe, C.; Tsumura, T.; Toyoda, M. Rapid preparation of nitrogen-doped carbon and its use in electrochemical capacitors. Carbon 2021, 176, 656. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, S.; Shen, G.; Zhao, Y.; Zhu, T.; Gao, Q.; Sun, N.; Wei, W. Controllable and rapid synthesis of nitrogen-doped ordered mesoporous carbon single crystals for CO2 capture. J. CO2 Util. 2022, 56, 101851. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, C.; Xie, Y.; Kang, Y.; Que, W.; Henzie, J.; Yamauchi, Y. Tailored MXene nano architectonics: MXene with mesoporous nitrogen-doped carbon confined ultrafine molybdenum carbide nanodots for efficient electrocatalytic hydrogen evolution. ACS Sustain. Chem. Eng. 2022, 11, 168–176. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Mi, J.; Fan, C.; Wang, Z.; Chen, X.; Wang, G.; Huang, Z.; Li, J. Efficient and simple strategy to obtain ordered mesoporous carbons with abundant structural base N sites toward CO2 selective capture and catalytic conversion. ACS Sustain. Chem. Eng. 2022, 10, 5175–5182. [Google Scholar] [CrossRef]
- Barat-Abtahi, S.; Jafari-Hafshejani, F.; Varmaghani, F.; Karimi, B.; Veisi, H.H. Robust interaction of cobalt phthalocyanine and nitrogen-doped ordered mesoporous carbon for CO2 reduction paired with the electro-oxidative synthesis of sulfonamide derivatives. Green Chem. 2024, 26, 362–374. [Google Scholar] [CrossRef]
- Mohan, T.V.; Nallagangula, M.; Kala, K.; Hernandez-Tamargo, C.E.; De Leeuw, N.H.; Namitharan, K.; Bhat, V.T.; Sasidharan, M.; Selvam, P. Pyridinic-nitrogen on ordered mesoporous carbon: A versatile NAD (P) H mimic for borrowing-hydrogen reactions. J. Catal. 2023, 419, 80–98. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Wang, Y.; Yang, G.; Zhu, Y.; Jiang, C.; Pan, Z.; Liu, X.; Xing, B. Degradation of Sulfamethoxazole by Activation of Persulfate Based on Nitrogen-Doped Mesoporous Carbon. Water Air Soil Pollut. 2024, 235, 162. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, C.; Wang, Z.; Fan, X.; Yan, C. Synergistic effects of N-doping and mesoporous structures in block copolymer-derived three-dimensionally ordered mesoporous carbon for PEMFC. Int. J. Hydrog. Energy 2024, 51, 747–757. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Wang, C.L.; Liu, Y.; Tao, D.J. Solvent-free self-assembly synthesis of N-doped ordered mesoporous carbons as effective and bifunctional materials for CO2 capture and oxygen reduction reaction. Chem. Eng. J. 2022, 427, 130878. [Google Scholar] [CrossRef]

| Ligand | BET Surface Area (m2/g) | N Content in LIGANDS (N/C + N) (%) | N Content Measured by EDS (%) | N Content Measured by XPS (%) |
|---|---|---|---|---|
| Oleic acid | 969.6 | 0 | 0 | 0 |
| Oleyl amine | 683.5 | 5.2 | 0.6 | 0.6 |
| Octylamine | 602.8 | 10.8 | 1.6 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Quan, Z.; Hu, C.; Wang, X.; Wang, L.; Li, R.; Sheng, X.; Liu, Y.; Song, M.; Zheng, X. Molecular-Level Regulation of Nitrogen-Doped Ordered Mesoporous Carbon Materials via Ligand Exchange Strategy. Processes 2025, 13, 1558. https://doi.org/10.3390/pr13051558
Han D, Quan Z, Hu C, Wang X, Wang L, Li R, Sheng X, Liu Y, Song M, Zheng X. Molecular-Level Regulation of Nitrogen-Doped Ordered Mesoporous Carbon Materials via Ligand Exchange Strategy. Processes. 2025; 13(5):1558. https://doi.org/10.3390/pr13051558
Chicago/Turabian StyleHan, Dandan, Zhen Quan, Congyuan Hu, Xiaopeng Wang, Lixia Wang, Ruige Li, Xia Sheng, Yanyan Liu, Meirong Song, and Xianfu Zheng. 2025. "Molecular-Level Regulation of Nitrogen-Doped Ordered Mesoporous Carbon Materials via Ligand Exchange Strategy" Processes 13, no. 5: 1558. https://doi.org/10.3390/pr13051558
APA StyleHan, D., Quan, Z., Hu, C., Wang, X., Wang, L., Li, R., Sheng, X., Liu, Y., Song, M., & Zheng, X. (2025). Molecular-Level Regulation of Nitrogen-Doped Ordered Mesoporous Carbon Materials via Ligand Exchange Strategy. Processes, 13(5), 1558. https://doi.org/10.3390/pr13051558








