Formation of Electrode Materials in the Process of Carbothermic Flux Smelting of Ilmenite Concentrate and Hydrothermal Refining of Titanium Slag
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Main Equipment and Technological Conditions
2.3. Physical and Chemical Studies
2.4. Thermodynamic Analysis
3. Results
3.1. Characteristics of Ilmenite Concentrate
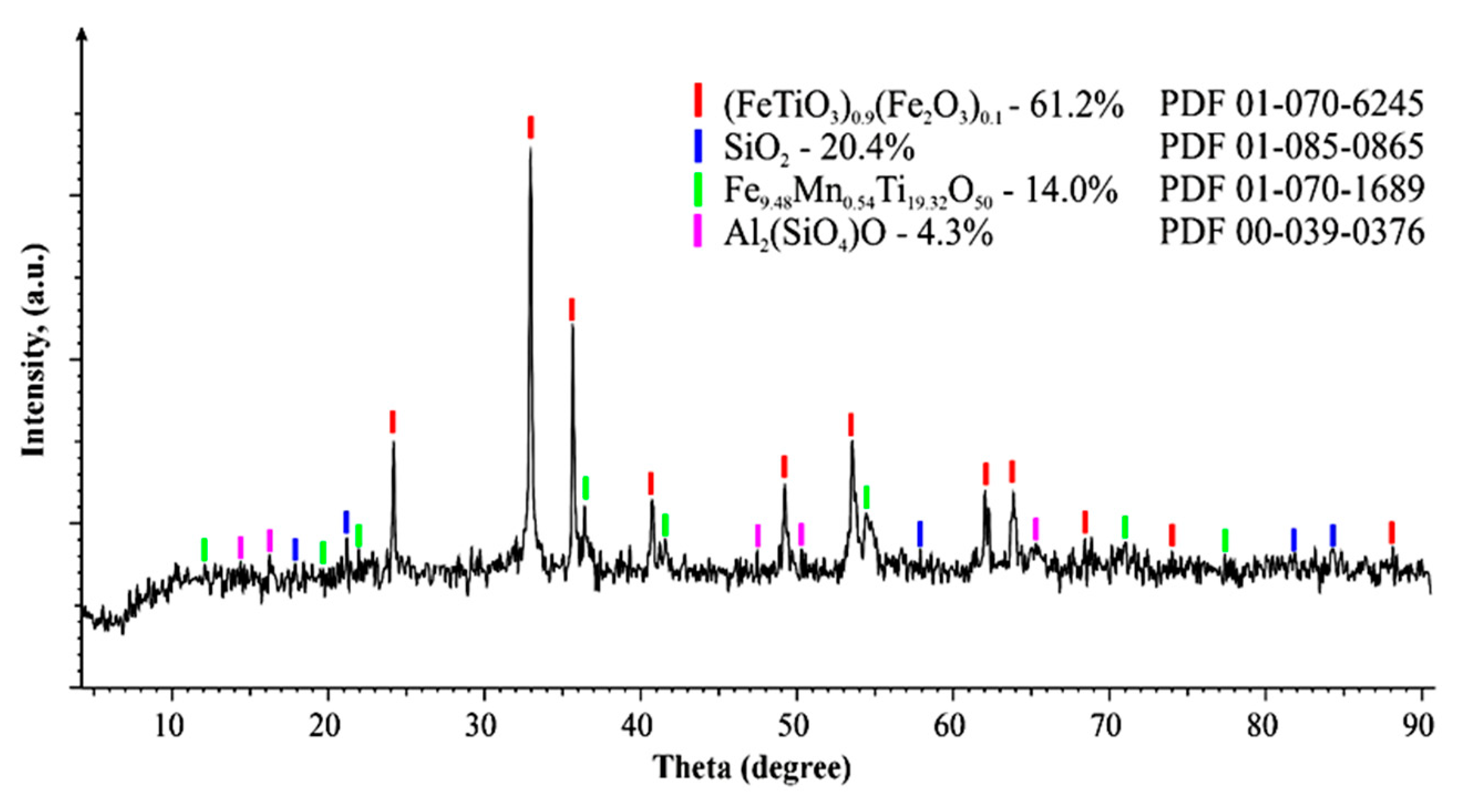
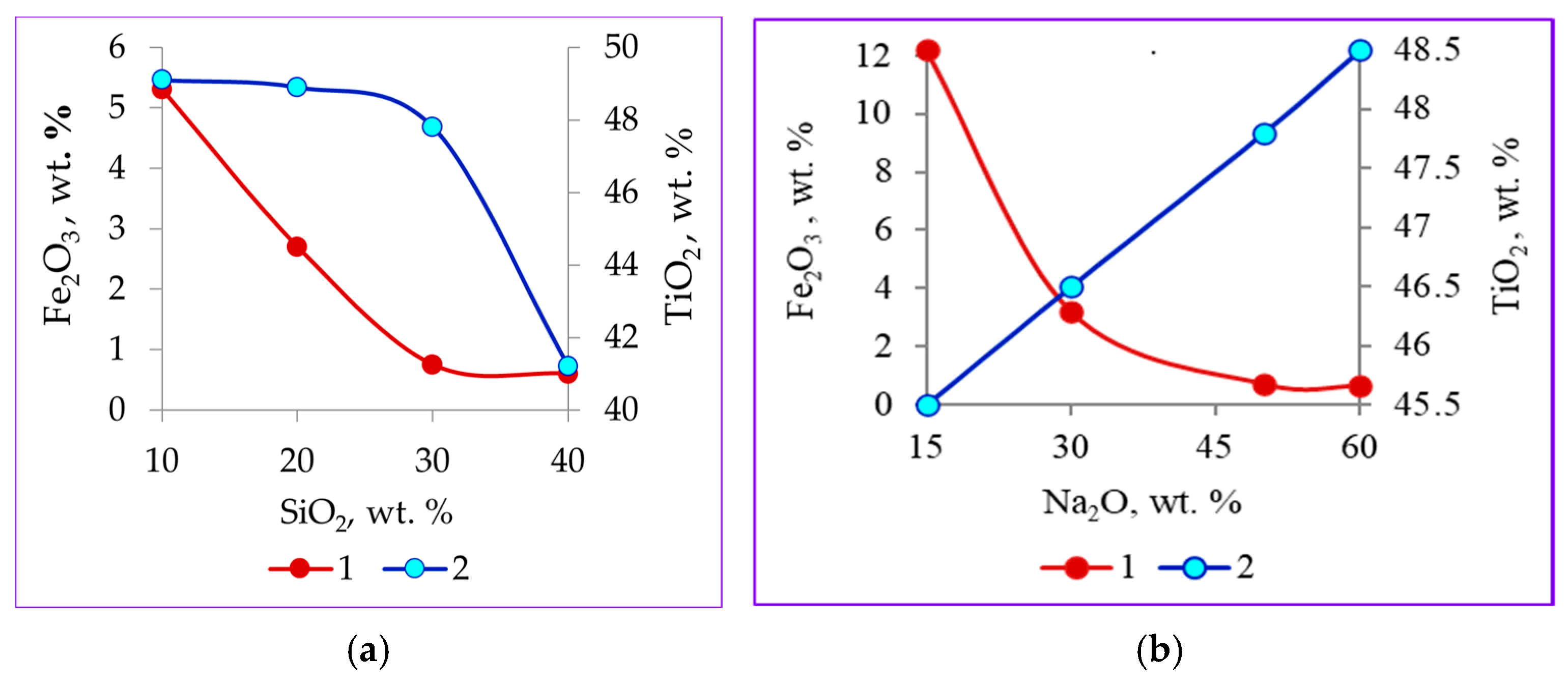
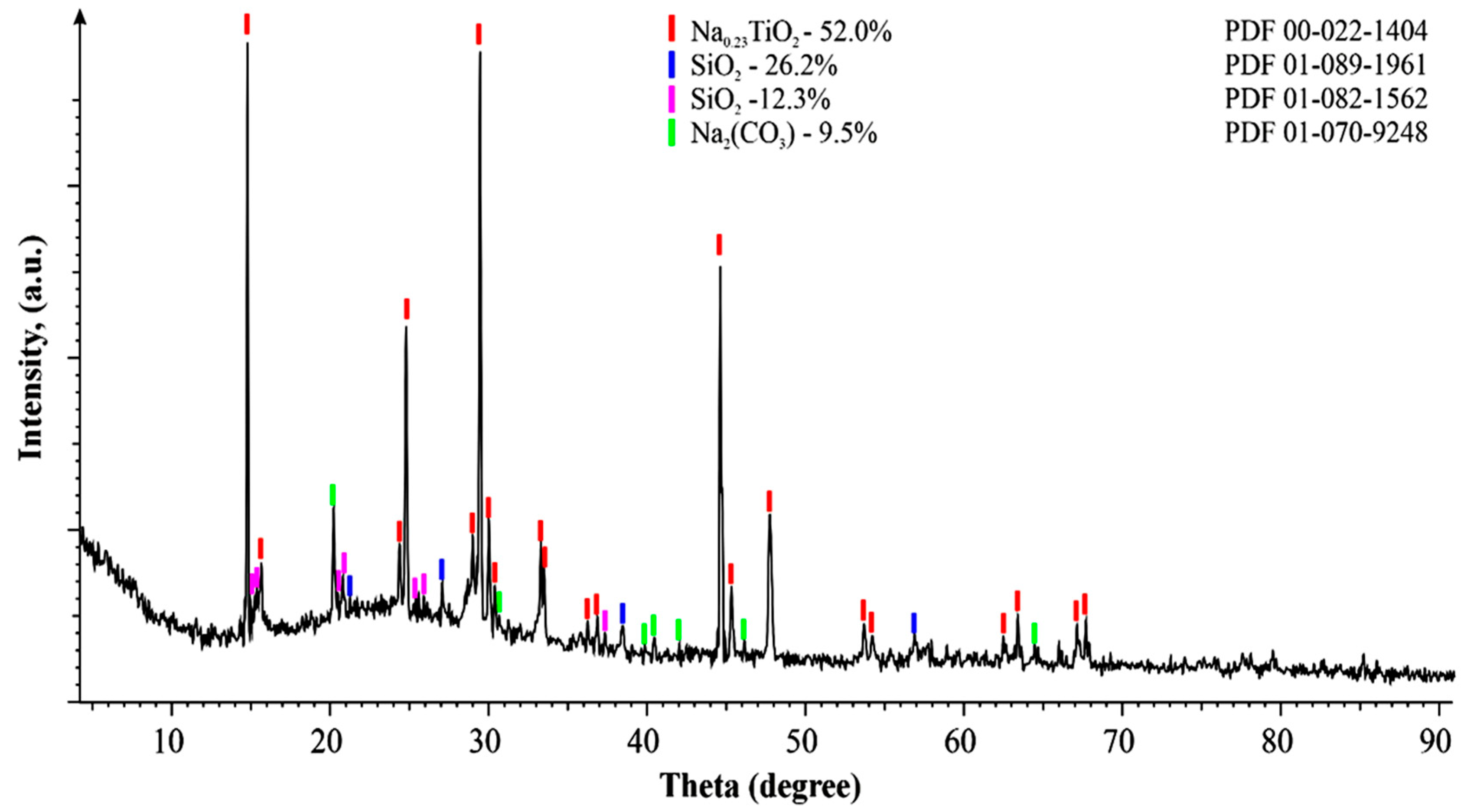
3.2. Material Composition of Primary and Modified Titanium Slag
4. Mechanism of Formation of Electrode Materials
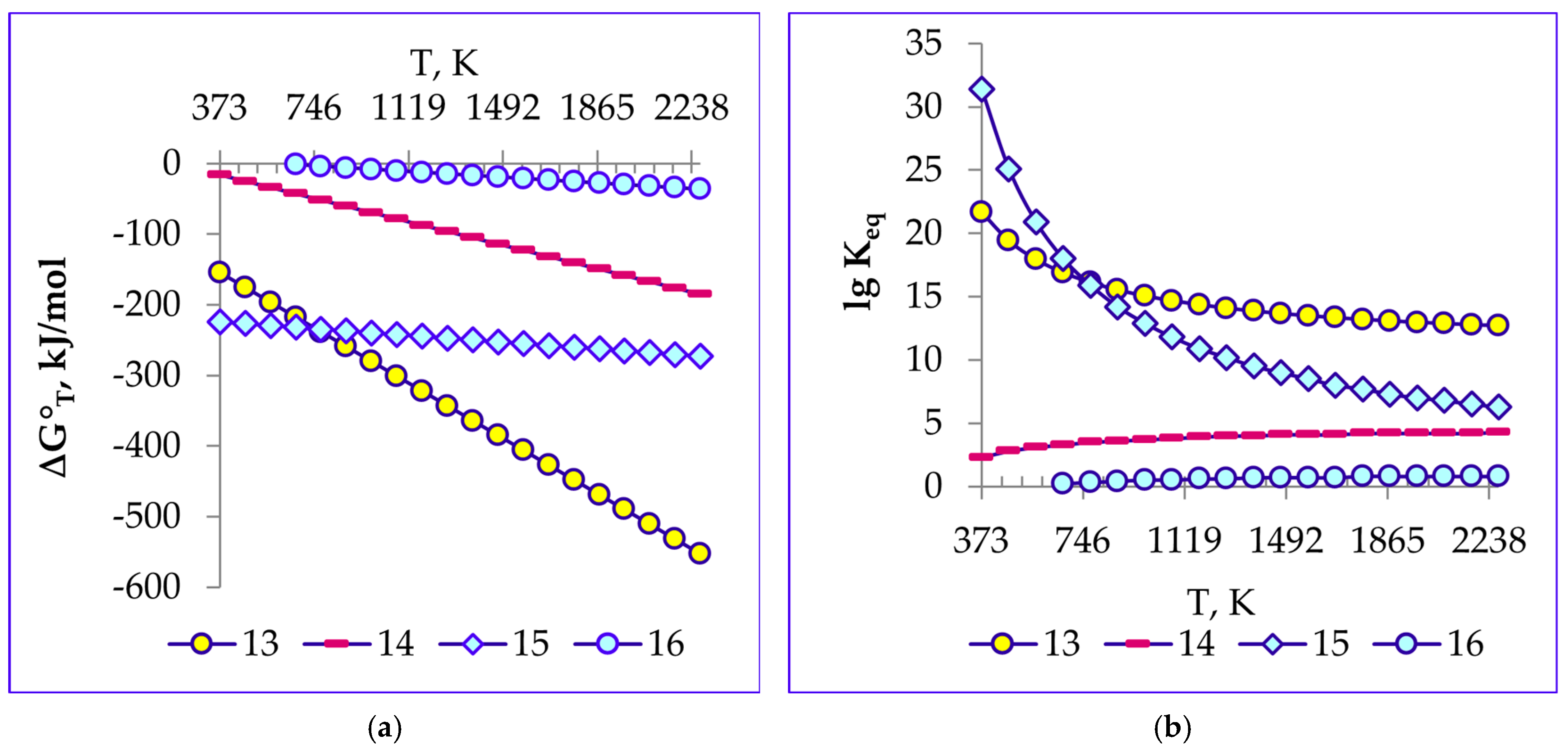
4.1. Thermodynamics of the Reactions of Calcined Soda Decomposition
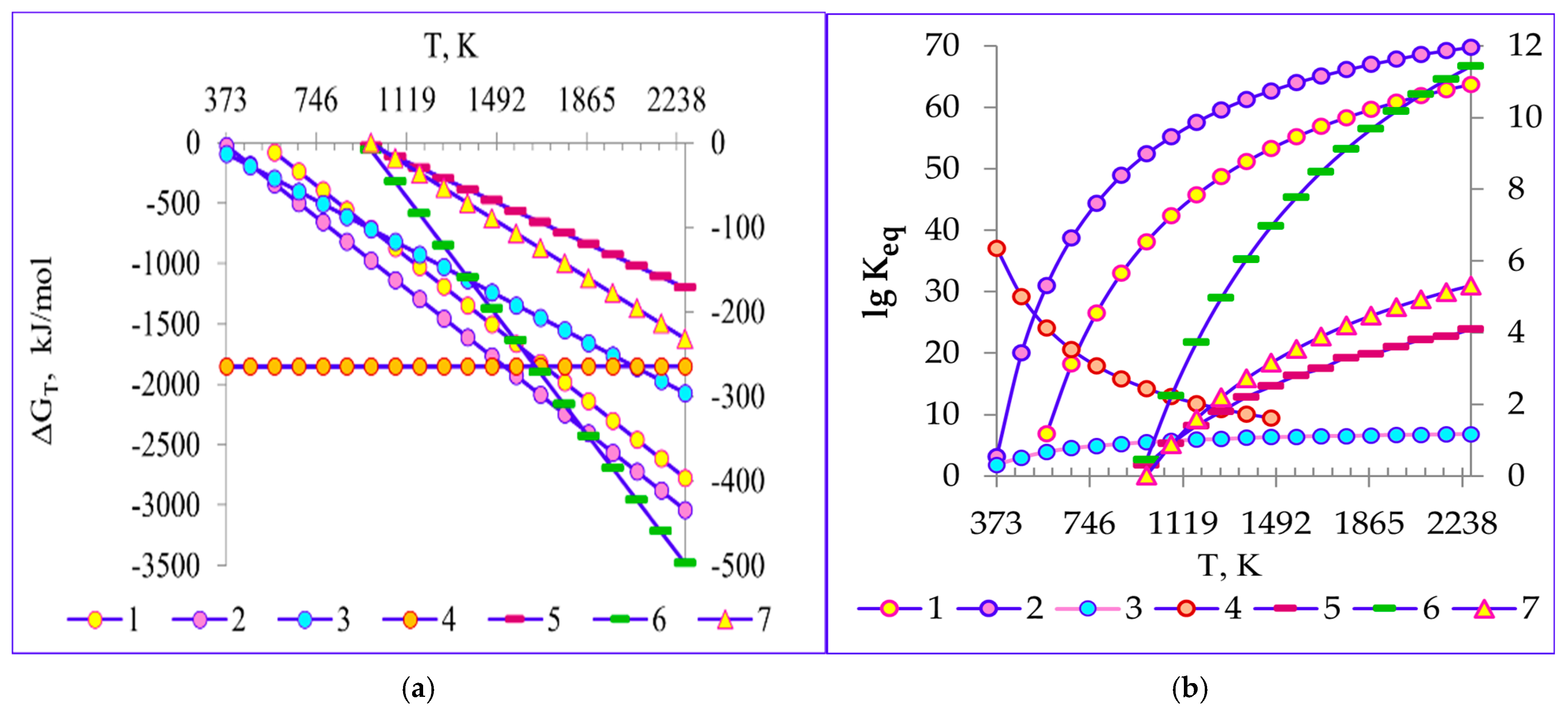
4.2. Thermodynamics of Decomposition Reactions of Ilmenite Concentrate
1.95SiO2 → 32.4036Fe + 22.634Na2Ti3O7 + 2Na0.23TiO2 + Na2TiSiO5 +
Na0.67Al0.1Mn0.9O2 + MnSiO3 + Fe1.966O2.963 + 40.173C
Na2TiSiO5 + 9CO2↑
0.54MnSiO3 + 5.41CO2↑
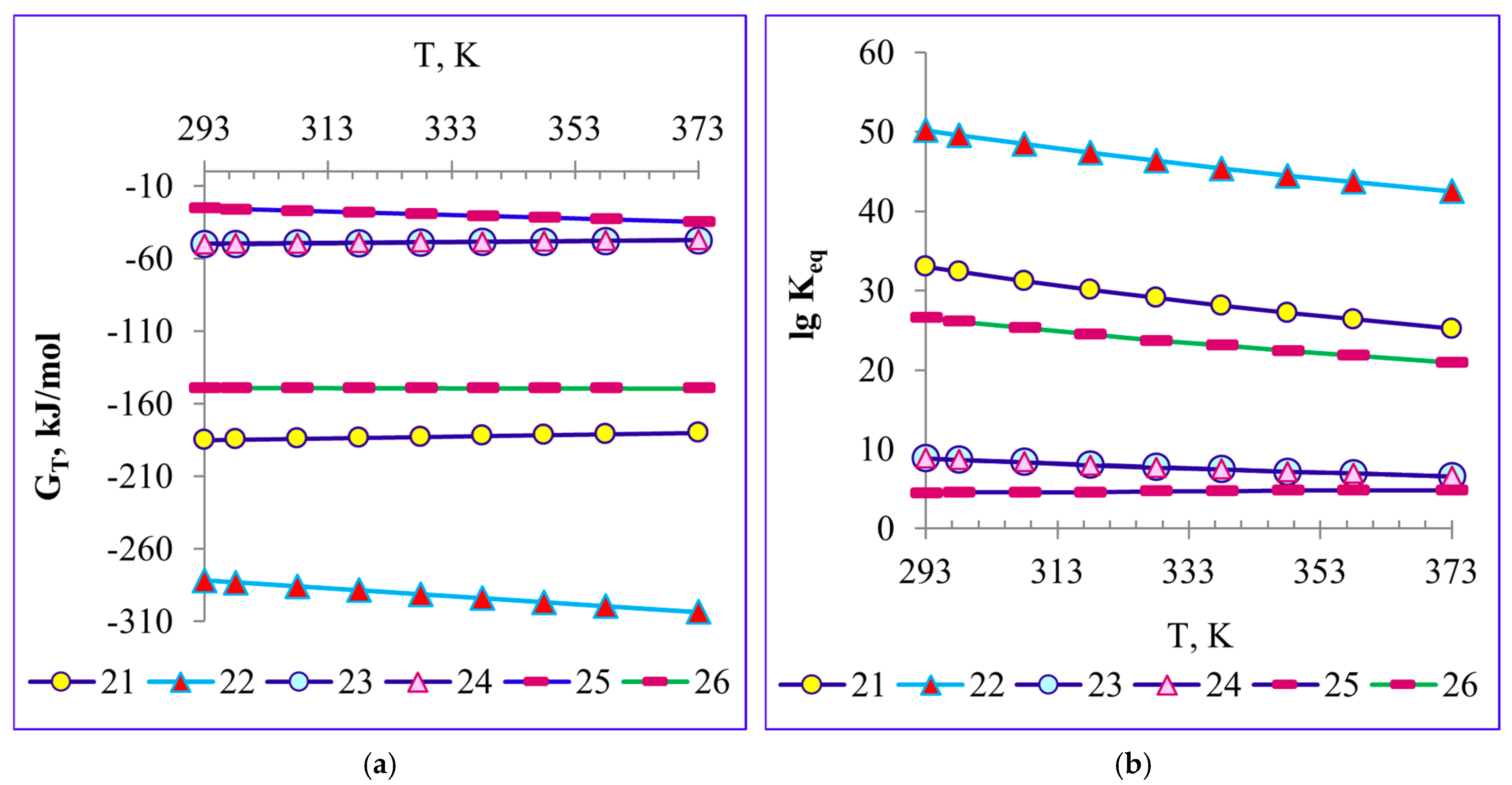
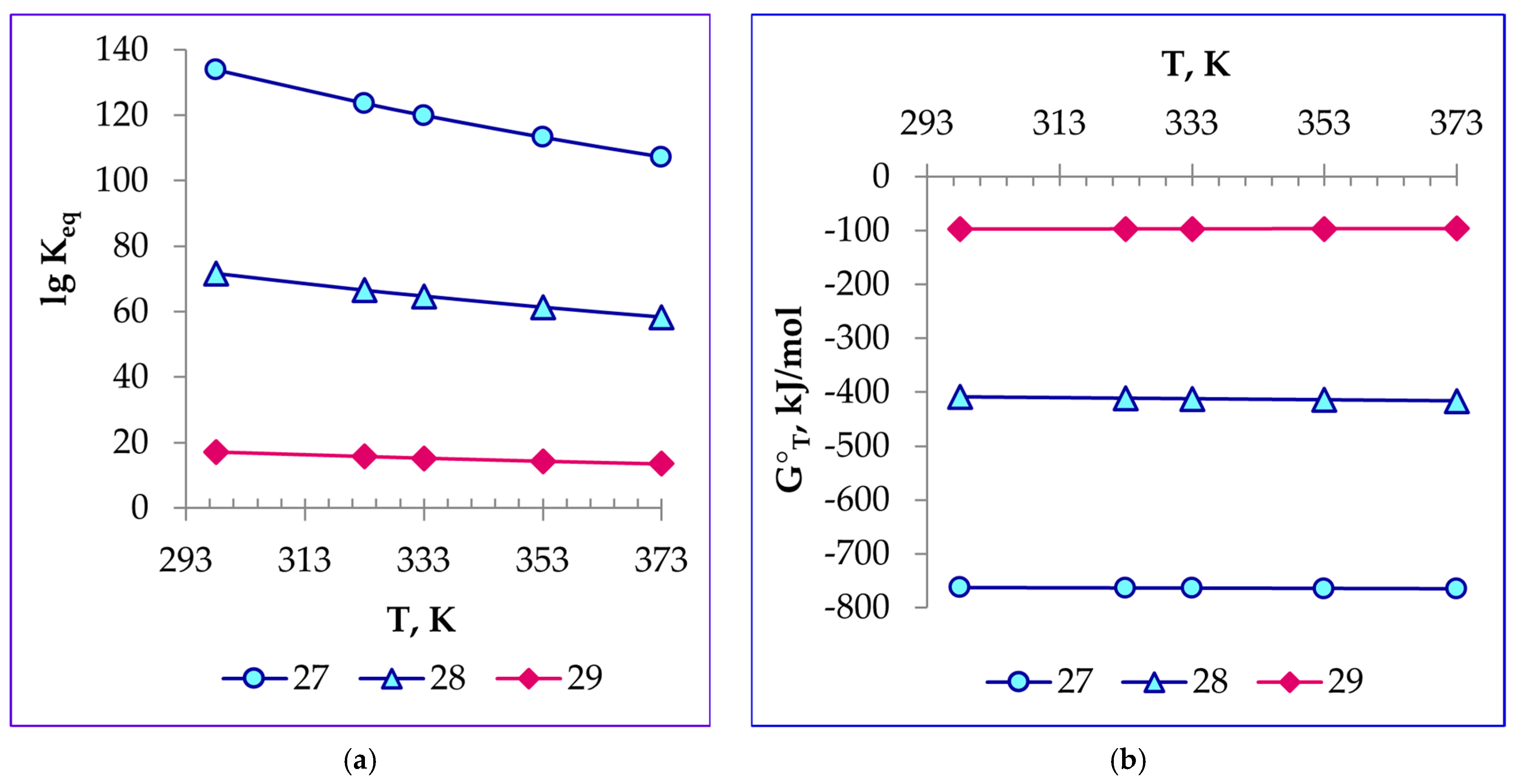
4.3. Thermodynamics of Titanium Slag-Refining Reactions with Water
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodiumion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, G.; Duan, Y.; Wu, Y.; Zhang, T.; Zhao, X.; Luo, M.; Liu, Y. The Research and Industrialization Progress and Prospects of Sodium Ion Battery. J. Alloys Compd. 2023, 958, 170486. [Google Scholar] [CrossRef]
- Rudola, A.; Saravanan, K.; Mason, C.W.; Balaya, P. Na2Ti3O7: An intercalation based anode for sodium-ion battery applications. J. Mater. Chem. A 2013, 1, 2653–2662. [Google Scholar] [CrossRef]
- Xu, Y.; Lotfabad, E.M.; Wang, H.; Farbod, B.; Xu, Z.; Kohandehghan, A.; Mitlin, D. Nanocrystalline anatase TiO2: A new anode material for rechargeable sodium ion batteries. Chem. Commun. 2013, 49, 8973–8975. [Google Scholar] [CrossRef]
- Wu, L.; Buchholz, D.; Bresser, D.; Chagas, L.G.; Passerini, S. Anatase TiO2 nanoparticles for high power sodium-ion anodes. J. Power Sources 2014, 251, 379–385. [Google Scholar] [CrossRef]
- Nava-Avendaño, J.; Morales-García, A.; Ponrouch, A.; Rousse, G.; Frontera, C.; Senguttuvan, P.; Tarascon, J.-M.; Dompablo, M.E.A.-D.; Palacín, M.R. Taking steps forward in understanding the electrochemical behavior of Na2Ti3O7. J. Mater. Chem. A 2015, 3, 22280–22286. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, K.; Lan, H.; Qian, S.; Yu, H.; Yan, L.; Long, N.; Shui, M.; Shu, J. Electrochemical kinetics of Na2Ti3O7 as anode material for lithium–ion batteries. J. Electroanal. Chem. 2017, 788, 203–209. [Google Scholar] [CrossRef]
- Yan, X.; Sun, D.; Jiang, J.; Yan, W.; Jin, Y. Self-assembled twine-like Na2Ti3O7 nanostructure as advanced anode for sodium-ion batteries. J. Alloys Compd. 2017, 697, 208–214. [Google Scholar] [CrossRef]
- Patent CN108455663; IPC C01G23/00, H01M10/054, H01M4/485. 28 August 2018. Available online: https://patentimages.storage.googleapis.com/5e/f6/1d/d3040ed58187a4/CN108455663A.pdf (accessed on 14 May 2025).
- Patent CN108134075; IPC H01M4/485. 8 June 2018. Available online: https://patentimages.storage.googleapis.com/51/71/e7/0cbd0f60faab6f/CN108134075A.pdf (accessed on 14 May 2025).
- Patent CN109148876; IPC C01G23/00, H01M10/54, H01M4/485, H01M4/58. 4 January 2019. Available online: https://patentimages.storage.googleapis.com/25/69/9b/7f383c2825c07a/CN109148876A.pdf (accessed on 14 May 2025).
- Patent CN109626415; IPC C01G23/00, H01M10/054, H01M4/485. 16 April 2019. Available online: https://patentimages.storage.googleapis.com/95/40/8c/501fb8e6d4da59/CN109626415A.pdf (accessed on 14 May 2025).
- Zakharova, G.S.; Fattakhova, Z.A. Method of Producing Sodium. Patent RU 2 716 186. Application No. 2019127022, 28 August 2019. [Google Scholar]
- Pan, J.; Wang, N.; Lv, D.; Dong, W.; Yang, J.; Huang, F. Layered Structure Na2Ti3O7 as a Promising Anode Material for Sodium-Ion Batteries. Adv. Energy Sustain. Res. 2021, 2, 2000095. [Google Scholar] [CrossRef]
- Piffet, C.; Eshraghi, N.; Mottet, G.; Hatert, F.; Światowska, J.; Cloots, R.; Boschini, F.; Mahmoud, A. Effect of the Calcination Duration on the Electrochemical Properties of Na2Ti3O7 as Anode Material for Na-Ion Batteries. Batteries 2023, 9, 495. [Google Scholar] [CrossRef]
- Jiang, Z.; Ke, H.; Zhang, Y.; Li, L.; Wang, F.; Li, J.; Huang, J.; Zhang, Y.; Jiang, Z.; Chen, B.; et al. Building a Stable Plateau—Type Na2Ti3O7 Anode Interface toward Advanced Sodium-Ion Batteries. Energy Fuels 2024, 38, 2472–2479. [Google Scholar] [CrossRef]
- Huang, J.; Meng, R.; Zu, L.; Wang, Z.; Feng, N.; Yang, Z.; Yu, Y.; Yang, J. Sandwich-like Na0.23TiO2 nanobelt/Ti3C2 MXene composites from a scalable in situ transformation reaction for long-life high-rate lithium/sodium-ion batteries. Nano Energy 2018, 46, 20–28. [Google Scholar] [CrossRef]
- Chandel, S.; Zulkifli; Singh, J.; Kim, J.; Rai, A.K. Facile synthesis of Na0.23TiO2 nanoparticles anode wrapped within full carbon network with high-rate capability for sodium ion battery application. Mater. Chem. Phys. 2022, 290, 126600. [Google Scholar] [CrossRef]
- Perovskiy, I.A. Synthesis of Titanosilicates from Leucoxene Ores. Bull. Tomsk. State Univ. 2014, 384, 182–188. [Google Scholar]
- Method for Obtaining Sodium–Containing Titanium Silicate. Patent RU2014126038/05A, 20 November 2015.
- Oleksiienko, O.; Meleshevych, S.; Strelko, V.; Wolkersdorfer, C.; Tsyba, M.M.; Kylivnyk, Y.M.; Levchuk, I.; Sitarz, M.; Sillanpää, M. Pore structure and sorption characterization of titanosilicates obtained from concentrated precursors by the sol–gel method. RSC Adv. 2015, 5, 72562–72571. [Google Scholar] [CrossRef]
- Liu, J.; Pang, W.K.; Zhou, T.; Chen, L.; Wang, Y.; Peterson, V.K.; Yang, Z.; Guo, Z.; Xia, Y. Li2TiSiO5: A Low Potential and Large Capacity Ti-Based Anode Material for Li-Ion Batteries. Energy Environ. Sci. 2017, 10, 1456–1464. [Google Scholar] [CrossRef]
- Gao, S.; Li, S.; Gao, J.; Liu, X.; Lin, C. Carbon coated “zero-strain” Li2TiSiO5 nanowires for high-performance lithium storage. Mater. Technol. 2023, 38, 2204292. [Google Scholar] [CrossRef]
- Baghramyan, V.V.; Sargsyan, A.A.; Knyazyan, N.B.; Gerasimova, L.G.; Leonelli, C. Synthesis of titaniumsilicate by microwave method. Tpyды Koльскoгo нayчнoгo цeнmpa PAH 2018, 219–223. [Google Scholar] [CrossRef]
- Kalashnikova, G.O.; Gryaznova, D.V.; Baranchikov, A.E.; Britvin, S.N.; Yakovenchuk, V.N.; Samburov, G.O.; Veselova, V.O.; Pulyalina, A.Y.; Pakhomovsky, Y.A.; Bazai, A.V.; et al. Microwave-Assisted Synthesis of Titanosilicates Using a Precursor Produced from Titanium Ore Concentrate. Chemengineering 2023, 7, 118. [Google Scholar] [CrossRef]
- Perovskiy, I.A.; Panikorovskii, T.L.; Shushkov, D.A. Effect of mineralizer and synthesis duration on sorption properties of sitinakite and ivanyukite. Vestn. Geosci. 2024, 3, 20–29. [Google Scholar] [CrossRef]
- GOST 5100-85; Calcinated Soda Technical. Technical Specifications. IPC Publishing Standards: Moscow, Russia, 2002.
- Lidin, L.A.; Andreeva, L.L.; Molochko, V.A. Constants of Inorganic Substances; Begell House: Danbury, CT, USA, 1995; 685p. [Google Scholar]
- Knunyantsa, I.L. (Ed.) Sodium Carbonate. In Chemical Encyclopedia in 5 Volumes; Great Russian Encyclopedia: Moscow, Russia, 1992; Volume 3, p. 639. ISBN 5-85270-039-8. [Google Scholar]
- Remy, G. Course of Inorganic Chemistry; MIR: Moscow, Russia, 1972; Volume 1, 824p. [Google Scholar]
- Guéguin, M.; Cardarelli, F. Chemistry and mineralogy of titania-rich slags. Part 1—Hemoilmenite, sulphate, and upgraded titania slags. Miner. Process. Extr. Metall. Rev. 2007, 28, 1–58. [Google Scholar] [CrossRef]
- Williams, G.E.; Steenkamp, J.D. Heavy Mineral Processing at Richards Bay Minerals. S. Afr. Pyrometallurgy 2006, 181–188. [Google Scholar]
- Schirmer, T.; Achimovičová, M.; Goldmann, D. Influence of chemical and phase composition in the hydrometallurgical processing of FeTi oxide phases. Hydrometallurgy 2020, 191, 105250. [Google Scholar] [CrossRef]
- Lidin, R.A.; Molochko, V.A.; Andreeva, L.L. Reactions of Inorganic Substances. In Handbook, 2nd ed.; Drofa: Moscow, Russia, 2007; 637p. [Google Scholar]
- Lidin, R.A.; Andreeva, L.L.; Molochko, V.A. Chemical Properties of Inorganic Substances. In A Textbook for Universities, 3rd ed.; Khimia: Moscow, Russia, 2000; 480p. [Google Scholar]




| Classes, mm | Mass Fraction, % |
|---|---|
| –0.5 + 0.315 | 3.80 |
| –0.315 + 0.2 | 27.86 |
| –0.2 + 0.074 | 58.32 |
| –0.074 + 0.044 | 7.22 |
| –0.044 + 0.0 | 2.80 |
| Total | 100 |
| Mass Fraction, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TiO2 | Fe2O3 | SiO2 | Al2O3 | Cr2O3 | MnO | CaO | MgO | P2O5 | SO3 |
| 52.3 | 39.55 | 4.83 | 0.95 | 1.43 | 1.74 | 0.08 | 0.16 | 0.014 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmetova, K.; Gladyshev, S.; Tussupbayev, N.; Kenzhaliev, B.; Imangaliyeva, L. Formation of Electrode Materials in the Process of Carbothermic Flux Smelting of Ilmenite Concentrate and Hydrothermal Refining of Titanium Slag. Processes 2025, 13, 1554. https://doi.org/10.3390/pr13051554
Akhmetova K, Gladyshev S, Tussupbayev N, Kenzhaliev B, Imangaliyeva L. Formation of Electrode Materials in the Process of Carbothermic Flux Smelting of Ilmenite Concentrate and Hydrothermal Refining of Titanium Slag. Processes. 2025; 13(5):1554. https://doi.org/10.3390/pr13051554
Chicago/Turabian StyleAkhmetova, Kuralai, Sergey Gladyshev, Nessipbay Tussupbayev, Bagdaulet Kenzhaliev, and Leila Imangaliyeva. 2025. "Formation of Electrode Materials in the Process of Carbothermic Flux Smelting of Ilmenite Concentrate and Hydrothermal Refining of Titanium Slag" Processes 13, no. 5: 1554. https://doi.org/10.3390/pr13051554
APA StyleAkhmetova, K., Gladyshev, S., Tussupbayev, N., Kenzhaliev, B., & Imangaliyeva, L. (2025). Formation of Electrode Materials in the Process of Carbothermic Flux Smelting of Ilmenite Concentrate and Hydrothermal Refining of Titanium Slag. Processes, 13(5), 1554. https://doi.org/10.3390/pr13051554








