A Review on Anatomical and Physiological Traits of Aquatic Macrophytes Coupled to a Bioelectrochemical System: Comparative Wastewater Treatment Performance
Abstract
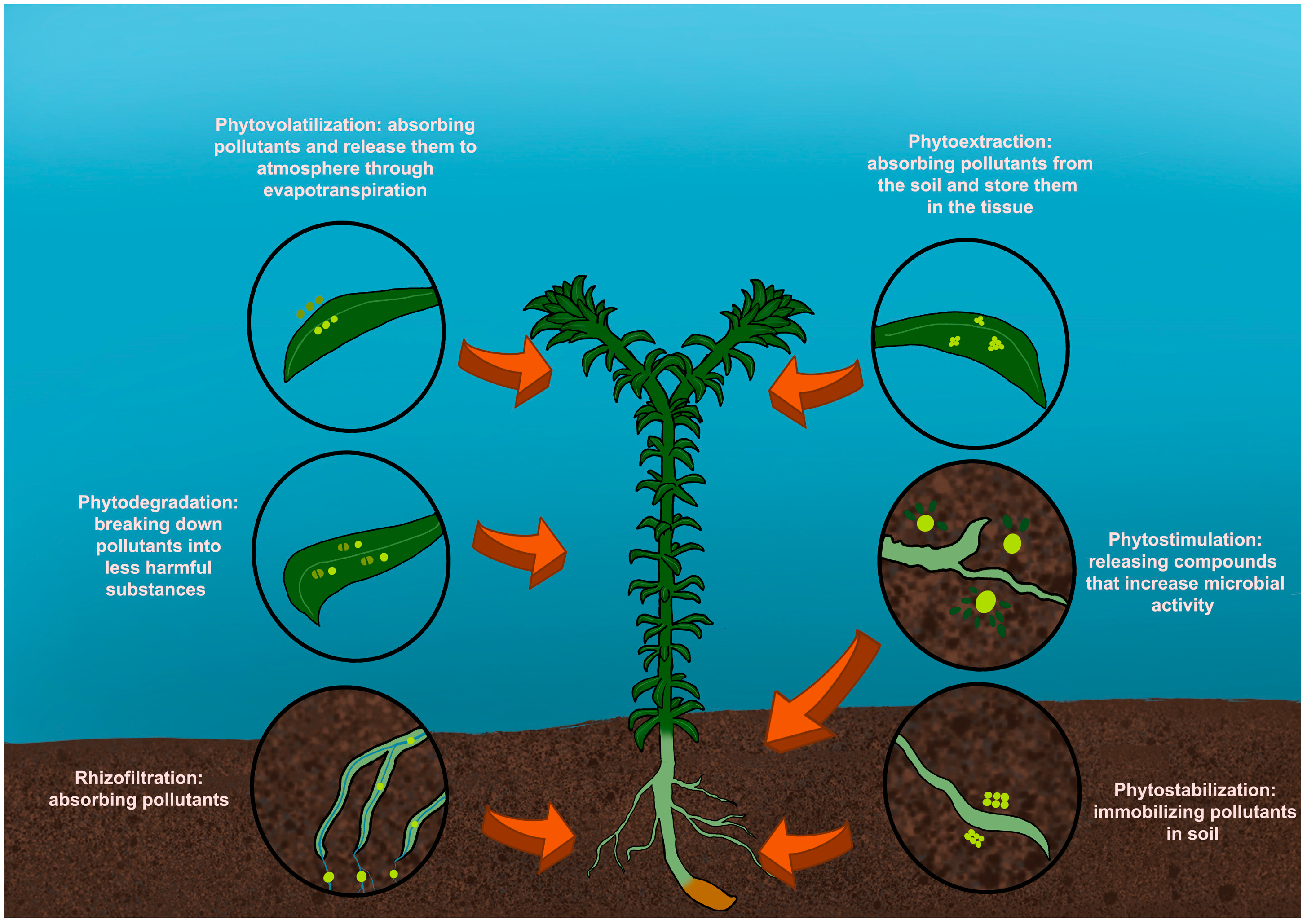
1. Introduction
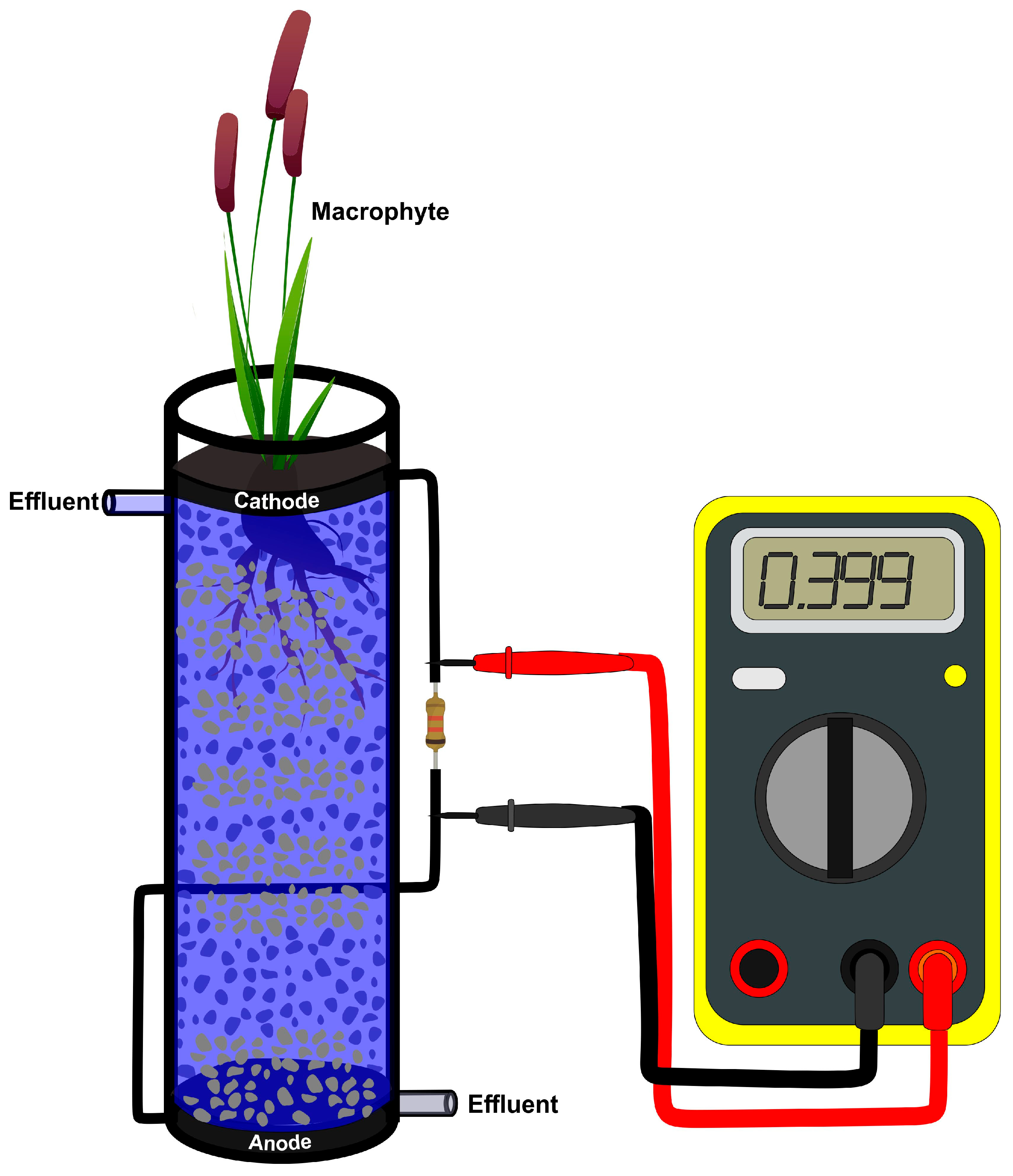
2. Plant Microbial Fuel Cells (PMFCs)
3. Anatomical Traits
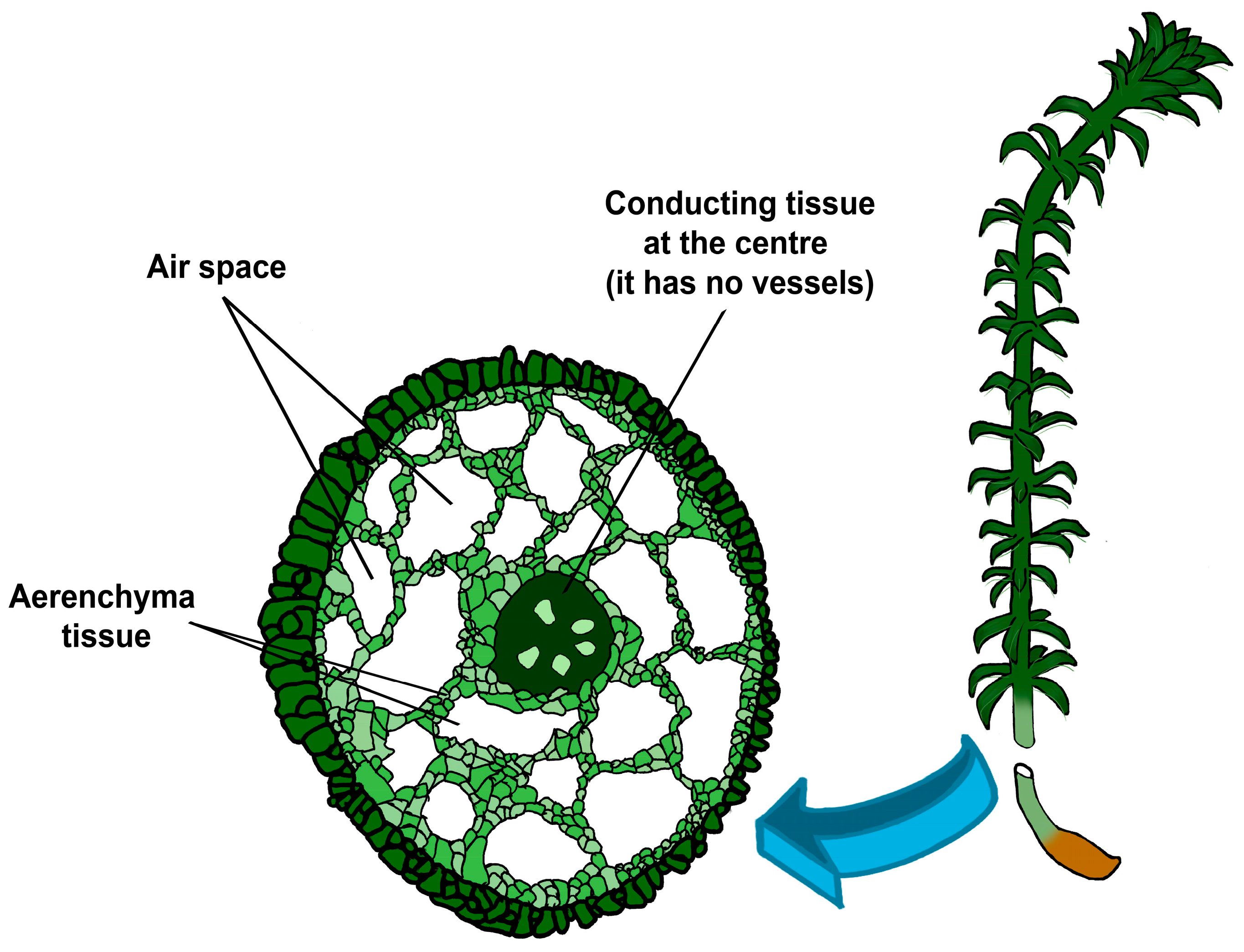
3.1. Aerenchyma
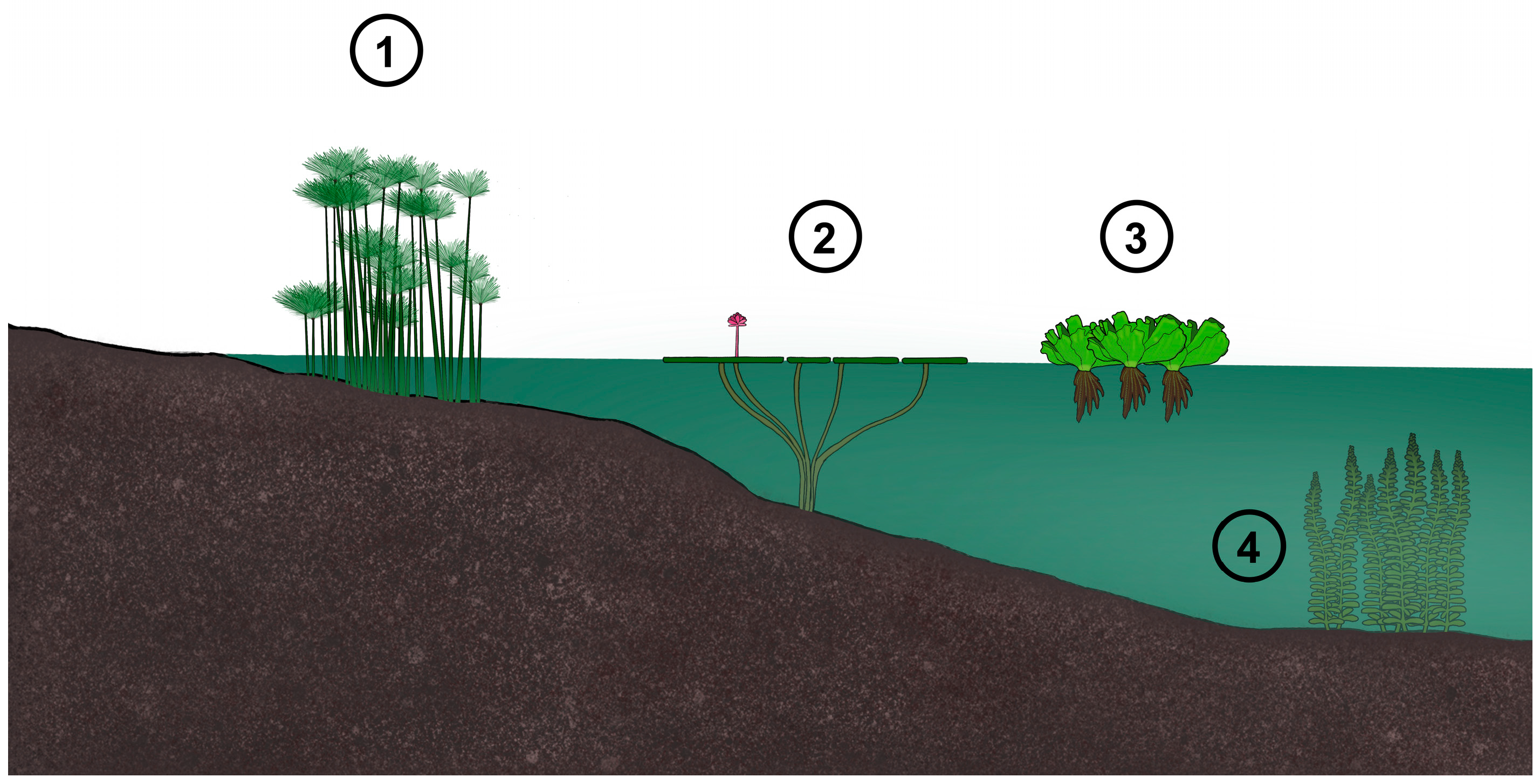
3.2. Root Morphological Features and Biomass
4. Physiological Features
4.1. Oxygen Release
4.2. Radial Oxygen Loss
4.3. Photosynthetic Rate
5. Bioelectricity Generation
6. Macrophyte Biotypes and Wastewater Treatment Efficiency
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lloyd, J.R.; Macaskie, L.E. Bioremediation of radionuclide-containing wastewaters. In Environmental Microbe-Metal Interactions; Wiley: Hoboken, NJ, USA, 2000; pp. 277–327. [Google Scholar] [CrossRef]
- Kumar, P.; Ramalingam, S.; Sathyaselvabala, V.; Kirupha, S.; Murugesan, A.; Sivanesan, S. Removal of Cd (II) from aqueous solution by agricultural waste cashew nut shell. Korean J. Chem. Eng. 2012, 29, 756–768. [Google Scholar] [CrossRef]
- Loix, C.; Huybrechts, M.; Vangronsveld, J.; Gielen, M.; Keunen, E.; Cuypers, A. Reciprocal interactions between cadmium-induced cell wall responses and oxidative stress in plants. Front. Plant Sci. 2017, 8, 1867. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Lang, T.; Ding, D.; Hu, J.; Li, C.; Zhang, H.; Li, G. Enhancement of repeated applications of chelates on phytoremediation of uranium contaminated soil by Macleay acordata. J. Environ. Radioact. 2019, 199, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Shen, Z. Leaching and uptake of heavy metals by ten different species of plants during an EDTA-assisted phytoextraction process. Chemosphere 2004, 57, 187–196. [Google Scholar] [CrossRef]
- Akhtar, A.B.T.; Yasar, A.; Ali, R.; Irfan, R. Phytoremediation using aquatic macrophytes. Phytoremediat. Manag. Environ. Contam. 2017, 5, 259–276. [Google Scholar] [CrossRef]
- Almaamary, E.A.; Abdullah, S.R.S.; Hasan, H.A.; Ismail, N.I.; Ab Rahim, R.A.; Idris, M. Plant-Assisted remediation of wastewater contaminated with methyl orange using Scirpus grossus. J. Environ. Biol. 2019, 40, 515–523. [Google Scholar] [CrossRef]
- Maranho, L.T.; Juneau, P.; Gomes, M.P. Aquatic macrophytes in constructed wetlands: A fight against water pollution. Sustainability 2020, 12, 9202. [Google Scholar] [CrossRef]
- Mohan, S.V.; Mohanakrishna, G.; Chiranjeevi, P. Sustainable power generation from floating macrophytes based ecological microenvironment through embedded fuel cells along with simultaneous wastewater treatment. Bioresour. Technol. 2011, 102, 7036–7042. [Google Scholar] [CrossRef]
- Akinbile, C.O.; Yusoff, M.S. Assessing water hyacinth (Eichhornia crassopes) and lettuce (Pistia stratiotes) effectiveness in aquaculture wastewater treatment. Int. J. Phytoremediation 2012, 14, 201–211. [Google Scholar] [CrossRef]
- Nizam, M.; Mohd, N.U.; Hanafiah, M.; Mohd, N.I.; Abd Karim, H.I. Efficiency of five selected aquatic plants in phytoremediation of aquaculture wastewater. Appl. Sci. 2020, 10, 2712. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Tam, N.F.Y.; Yang, L.; Mei, X.Q.; Yang, F.J. Growth characteristics of six wetland plants and their influences on domestic wastewater treatment efficiency. Ecol. Eng. 2013, 60, 382–392. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Rubio, A.V.; Valerio, G.A.; Ferrufino, L. Anatomía caulinar y foliar de tres especies de plantas acuáticas. Portal Cienc. 2015, 8, 31–44. [Google Scholar] [CrossRef]
- Srivastava, P.; Abbassi, R.; Garaniya, V.; Lewis, T.; Yadav, A.K. Performance of pilot-scale horizontal subsurface flow constructed wetland coupled with a microbial fuel cell for treating wastewater. J. Water Proc. Eng. 2020, 33, 100994. [Google Scholar] [CrossRef]
- Thakur, S.; Das, B. Bio-Electrochemical evaluation of two-stage constructed wetland microbial fuel cells with high strength raw domestic wastewater and simultaneous energy recovery. Water Environ. J. 2021, 35, 1239–1248. [Google Scholar] [CrossRef]
- Kalin-Seidenfaden, M. The Biofilm Generation Tool for the Reduction of Sulfate Oxidation. In Mine Wastes and Water, Ecological Engineering and Metals Extraction: Sustainability and Circular Economy; Springer International Publishing: Cham, Switzerland, 2022; pp. 105–119. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Electricity-Producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef]
- Lovley, D.R. The microbe electric: Conversion of organic matter to electricity. Curr. Opin. Biotechnol. 2008, 19, 564–571. [Google Scholar] [CrossRef]
- Yadav, A.K.; Dash, P.; Mohanty, A.; Abbassi, R.; Mishra, B.K. Performance assessment of innovative constructed wetland-microbial fuel cell for electricity production and dye removal. Ecol. Eng. 2012, 47, 126–131. [Google Scholar] [CrossRef]
- Liu, S.; Song, H.; Li, X.; Yang, F. Power generation enhancement by utilizing plant photosynthate in microbial fuel cell coupled constructed wetland system. Int. J. Photoenergy 2013, 2013, 172010. [Google Scholar] [CrossRef]
- Liu, S.; Song, H.; Wei, S.; Yang, F.; Li, X. Bio-Cathode materials evaluation and configuration optimization for power output of vertical subsurface flow constructed wetland—Microbial fuel cell systems. Bioresour. Technol. 2014, 166, 575–583. [Google Scholar] [CrossRef]
- Doherty, L.; Zhao, Y.; Zhao, X.; Wang, W. Nutrient and organics removal from swine slurry with simultaneous electricity generation in an alum sludge-based constructed wetland incorporating microbial fuel cell technology. Chem. Eng. J. 2015, 266, 74–81. [Google Scholar] [CrossRef]
- Doherty, L.; Zhao, X.; Zhao, Y.; Wang, W. The effects of electrode spacing and flow direction on the performance of microbial fuel cell-constructed wetland. Ecol. Eng. 2015, 79, 8–14. [Google Scholar] [CrossRef]
- Fang, Z.; Song, H.L.; Cang, N.; Li, X.N. Electricity production from Azo dye wastewater using a microbial fuel cell coupled constructed wetland operating under different operating conditions. Biosens. Bioelectron. 2015, 68, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Oon, Y.L.; Ong, S.A.; Ho, L.N.; Wong, Y.S.; Dahalan, F.A.; Oon, Y.S.; Thung, W.E. Synergistic effect of up-flow constructed wetland and microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour. Technol. 2016, 203, 190–197. [Google Scholar] [CrossRef]
- Kim, M.; Song, Y.E.; Li, S.; Kim, J.R. Microwave-Treated expandable graphite granule for enhancing the eelectricity generation of microbial fuel cell. J. Electrochem. Sci. Technol. 2021, 12, 297–301. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Tang, C.; Doherty, L. Influence of glass wool as separator on bioelectricity generation in a constructed wetland-microbial fuel cell. J. Environ. Manag. 2018, 207, 116–123. [Google Scholar] [CrossRef]
- Helder, M.; Strik, D.P.; Hamelers, H.V.M.; Kuhn, A.J.; Blok, C.; Buisman, C.J.N. Concurrent bio-electricity and biomass production in three Plant-Microbial Fuel Cells using Spartina anglica, Arundinella anomala and Arundo donax. Bioresour. Technol. 2010, 101, 3541–3547. [Google Scholar] [CrossRef]
- Liu, F.; Sun, L.; Wan, J.; Shen, L.; Yu, Y.; Hu, L.; Zhou, Y. Performance of different macrophytes in the decontamination of and electricity generation from swine wastewater via an integrated constructed wetland-microbial fuel cell process. J. Environ. Sci. 2020, 89, 252–263. [Google Scholar] [CrossRef]
- Aswad, Z.S.; Ali, A.H.; Al-Mhana, N.M. Energy production and wastewater treatment using Juncus, S. triqueter, P. australis, T. latifolia, and C. alternifolius plants in sediment microbial fuel cell. Desalination Water Treat. 2020, 205, 153–160. [Google Scholar] [CrossRef]
- Strik, D.P.; Hamelers, H.V.M.; Snel, J.F.; Buisman, C.J. Green electricity production with living plants and bacteria in a fuel cell. Int. J. Energy Res. 2008, 32, 870–876. [Google Scholar] [CrossRef]
- Kuleshova, T.; Rao, A.; Bhadra, S.; Garlapati, V.K.; Sharma, S.; Kaushik, A.; Sevda, S. Plant microbial fuel cells as an innovative, versatile agro-technology for green energy generation combined with wastewater treatment and food production. Biomass Bioenergy 2022, 167, 106629. [Google Scholar] [CrossRef]
- Harshitha, G.; Sahoo, A.; Sethy, R. Bioelectricity generation from different biomass feed at anode chamber and to study process parameters in microbial fuel cells. Biocatal. Agric. Biotechnol. 2019, 20, 101191. [Google Scholar] [CrossRef]
- Helder, M.; Strik, D.P.; Timmers, R.A.; Raes, S.M.T.; Hamelers, H.V.M.; Buisman, C.J.N. Resilience of roof-top plant-microbial fuel cells during Dutch winter. Biomass Bioenergy 2013, 1, 5–11. [Google Scholar] [CrossRef]
- Schievano, A.; Colombo, A.; Grattieri, M.; Trasatti, S.P.; Liberale, A.; Tremolada, P. Floating microbial fuel cells as energy harvesters for signal transmission from natural water bodies. J. Power Sources 2017, 340, 80–88. [Google Scholar] [CrossRef]
- Wang, J.; Song, X.; Wang, Y.; Bai, J.; Bai, H.; Yan, D.; Cao, Y.; Li, Y.; Yu, Z.; Dong, G. Bioelectricity generation, contaminant removal and bacterial community distribution as affected by substrate material size and aquatic macrophyte in constructed wetland-microbial fuel cell. Bioresour. Technol. 2017, 245, 372–378. [Google Scholar] [CrossRef]
- Regmi, R.; Nitisoravut, R.; Charoenroongtavee, S.; Yimkhaophong, W.; Phanthurat, E.O. Pot-Plant microbial fuel cell powered by vetiver for bioelectricity production and wastewater treatment. Clean Soil Air Water 2018, 46, 1700193. [Google Scholar] [CrossRef]
- Azri, Y.; Tou, M.; Sadi, I.; Benhabyles, L. Bioelectricity generation from three ornamental plants: Chlorophytum comosum, Chasmanthe floribunda and Papyrus diffusus. Int. J. Green Energy 2018, 15, 254–263. [Google Scholar] [CrossRef]
- De Schamphelaire, L.; Rabaey, K.; Boeckx, P.; Boon, N.; Verstraete, W. Outlook for benefits of sediment microbial fuel cells with two bio-electrodes. Microb. Biotechnol. 2008, 1, 446–462. [Google Scholar] [CrossRef]
- Oon, Y.L.; Ong, S.A.; Ho, L.N.; Wong, Y.S.; Dahalan, F.A.; Oon, Y.S.; Harvinder, K.L.; Thung, W.E.; Nordin, N. Role of macrophyte and effect of supplementary aeration in up-flow constructed wetland-microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour. Technol. 2017, 224, 265–275. [Google Scholar] [CrossRef]
- Timmers, R.A.; Strik, D.P.; Hamelers, H.V.M.; Buisman, C.J.N. Long-Term performance of a plant microbial fuel cell with Spartina anglica. Appl. Microbiol. Biotechnol. 2010, 86, 973–981. [Google Scholar] [CrossRef]
- Timmers, R.A.; Rothballer, M.; Strik, D.P.; Engel, M.; Schulz, S.; Schloter, M. Microbial community structure elucidates performance of Glyceria maxima plant microbial fuel cell. Appl. Microbiol. Biotechnol. 2012, 94, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Hubenova, Y.; Mitov, M.M. Conversion of solar energy into electricity by using duckweed in direct photosynthetic plant fuel cell. Bioelectrochemistry 2012, 87, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xing, D.; Jason, Z. Microbial community structure accompanied with electricity production in a constructed wetland plant microbial fuel cell. Bioresour. Technol. 2015, 195, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Lee, S.C.; Choi, H.K. Anatomical patterns of aerenchyma in aquatic and wetland plants. J. Plant Biol. 2008, 51, 428–439. [Google Scholar] [CrossRef]
- Martínez, M.; Gómez- Sánchez, M. Descripción anatómica vegetativa de dos especies de Nymphoides (Menyanthaceae). Rev. Mex. Biodivers. 2006, 77, 81–87. [Google Scholar] [CrossRef]
- Drew, M.C.; He, C.J.; Morgan, P.W. Programmed cell death and aerenchyma formation in roots. Trends Plants Sci. 2000, 5, 123–127. [Google Scholar] [CrossRef]
- Kawase, M.; Whitmoyer, R.E. Aerenchyma development in waterlogged plants. Am. J. Bot. 1980, 67, 18–22. [Google Scholar] [CrossRef]
- Guittonny-Philippe, A.; Masotti, V.; Combroux, I.; Malleret, L.; Boudenne, J.-L.; Petit, M.-E.; Monnier, Y.; Coulomb, B.; Viglione, J.; Laffont-Schwob, I. Proposal of a New Ecotoxicity Evaluation Tool Based on Morphological Responses of Five Helophytes to Mixtures of Pollutants: The Helophyte Development Index. Ecol. Eng. 2015, 77, 180–188. [Google Scholar] [CrossRef]
- Lai, W.L.; Zhang, Y.; Chen, Z.H. Radial oxygen loss, photosynthesis, and nutrient removal of 35 wetland plants. Ecol. Eng. 2012, 39, 24–30. [Google Scholar] [CrossRef]
- Visser, E.J.W.; Bögemann, G.M.; Van de Steeg, H.M.; Pierik, R.; Blom, C.W.P.M. Flooding tolerance of Carex species in relation to field distribution and aerenchyma formation. New Phytol. 2000, 148, 93–103. [Google Scholar] [CrossRef]
- Bezbaruah, A.N.; Zhang, T.C. pH, redox, and oxygen microprofiles in rhizosphere of bulrush (Scirpus validus) in a constructed wetland treating municipal wastewater. Biotechnol. Bioeng. 2004, 88, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann. Bot. 2002, 91, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.L.; Wang, S.Q.; Peng, C.L.; Chen, Z.H. Root features related to plant growth and nutrient removal of 35 wetland plants. Water Res. 2011, 45, 3941–3950. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, J.; Xie, H.; Li, C.; Bao, N.; Zhang, C.; Shi, Q. Physiological responses of Phragmites australis to wastewater with different chemical oxygen demands. Ecol. Eng. 2010, 36, 1341–1347. [Google Scholar] [CrossRef]
- Stevovic, S.; Mikovilovic, V.S.; Dragosavac, D.C. Environmental study of heavy metals influence on soil and Tansy (Tanacetum vulgare L.). Afr. J. Biotechnol. 2010, 9, 2413–2421. [Google Scholar]
- Küpper, H.; Küpper, F.; Spiller, M. In situ detection of heavy metal substituted chlorophylls in water plants. Photosynth. Res. 1998, 58, 123–133. [Google Scholar] [CrossRef]
- Zengin, F.K.; Munzuroglu, O. Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol. Cracoviensia Ser. Bot. 2005, 47, 157–164. [Google Scholar]
- Mei, X.Q.; Wong, M.H.; Yang, Y.; Dong, H.Y.; Qiu, R.L.; Ye, Z.H. The effects of radial oxygen loss on arsenic tolerance and uptake in rice and on its rhizosphere. Environ. Pollut. 2012, 165, 109–117. [Google Scholar] [CrossRef]
- Mei, X.; Li, Q.; Wang, H.; Fang, H.; Chen, H.; Chen, X.; Ye, Z. Effects of cultivars, water regimes, and growth stages on cadmium accumulation in rice with different radial oxygen loss. Plant Soil 2020, 453, 529–543. [Google Scholar] [CrossRef]
- Kemp, W.M.; Lewis, M.R.; Jones, T.W. Comparison of methods for measuring production by the submersed macrophyte, Potamogeton perfoliatus L. 1, 2. Limnol. Oceanogr. 1986, 31, 1322–1334. [Google Scholar] [CrossRef]
- Sorrell, B.K.; Brix, H. Gas transport and exchange through wetland plant aerenchyma. Methods Biogeochem. Wetl. 2013, 10, 177–196. [Google Scholar] [CrossRef]
- Dacey, J.W.H.; Klug, M.J. Tracer transport in Nuphar: 18O2 and 14CO2 transport. Physiol. Plant. 1982, 56, 361–366. [Google Scholar] [CrossRef]
- Bedford, B.L.; Bouldin, D.R.; Beliveau, B.D. Net oxygen and carbon-dioxide balances in solutions bathing roots of wetland plants. J. Ecol. 1991, 79, 943–959. [Google Scholar] [CrossRef]
- Reddy, K.R.; Patrick, W.H., Jr.; Lindau, C.W. Nitrification-denitrification at the plant root-sediment interface in wetlands. Limnol. Oceanogr. 1989, 34, 1004–1013. [Google Scholar] [CrossRef]
- Dong, Y.; Wiliński, P.R.; Dzakpasu, M.; Scholz, M. Impact of hydraulic loading rate and season on water contaminant reductions within integrated constructed wetlands. Wetlands 2011, 31, 499–509. [Google Scholar] [CrossRef]
- Wiessner, A.; Kappelmeyer, U.; Kaestner, M.; Schultze-Nobre, L.; Kuschk, P. Response of ammonium removal to growth and transpiration of Juncus effusus during the treatment of artificial sewage in laboratory-scale wetlands. Water Res. 2013, 47, 4265–4273. [Google Scholar] [CrossRef]
- McDonald, M.P.; Galwey, N.W.; Colmer, T.D. Waterlogging tolerance in the tribe Triticeae: The adventitious roots of Critesion marinum have a relatively high porosity and a barrier to radial oxygen loss. Plant Cell Environ. 2001, 24, 585–596. [Google Scholar] [CrossRef]
- Yang, J.; Ma, Z.; Ye, Z.; Guo, X.; Qiu, R. Heavy metal (Pb, Zn) uptake and chemical changes in rhizosphere soils of four wetland plants with different radial oxygen loss. J. Environ. Sci. 2010, 22, 696–702. [Google Scholar] [CrossRef]
- Rehman, F.; Pervez, A.; Mahmood, Q.; Nawab, B. Wastewater remediation by optimum dissolve oxygen enhanced by macrophytes in constructed wetlands. Ecol. Eng. 2017, 102, 112–126. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.H.; Yan, L.; Zhong, Q.S. Plant photosynthesis and its influence on removal efficiencies in constructed wetlands. Ecol. Eng. 2010, 36, 1037–1043. [Google Scholar] [CrossRef]
- Xin, J.; Tang, J.; Yao, Y.L.; Zhang, R.T. Pre-aeration of the rhizosphere offers potential for phytoremediation of heavy metal-contaminated wetlands. J. Hazard. Mater. 2019, 374, 437–446. [Google Scholar] [CrossRef]
- Liu, S.; Yan, B.; Wang, L. The layer effect in nutrient removal by two indigenous plant species in horizontal flow constructed wetlands. Ecol. Eng. 2011, 37, 2101–2104. [Google Scholar] [CrossRef]
- Coleman, J.; Hench, K.; Garbutt, K.; Sexstone, A.; Bissonnelte, G.; Skousen, J. Treatment of domestic wastewater by three plant species in constructed wetland. Water Air Soil Pollut. 2001, 128, 283–295. [Google Scholar] [CrossRef]
- Mayo, A.W.; Hanai, E.E. Modeling phytoremediation of nitrogen-polluted water using water hyacinth (Eichhornia crassipes). Phys. Chem. Earth Parts a/b/c 2017, 100, 170–180. [Google Scholar] [CrossRef]
- Vasanthi, D.; Karuppasamy, P.K.; Santhanam, P.; Kumar, S.D.; Malarvannan, G. Phytoremediation to remove nutrients and textile dye effluent using seagrass (Cymodocea rotundata). Adv. Biol. Res. 2015, 9, 405–412. [Google Scholar] [CrossRef]
- Białowiec, A.; Davies, L.; Albuquerque, A.; Randerson, P.F. Nitrogen removal from landfill leachate in constructed wetlands with reed and willow: Redox potential in the root zone. J. Environ. Manag. 2012, 97, 22–27. [Google Scholar] [CrossRef]
- Singh, O.V.; Jain, R.K. Phytoremediation of toxic aromatic pollutants from soil. Appl. Microbiol. Biotechnol. 2003, 63, 128–135. [Google Scholar] [CrossRef]
- Al-Saadi, M.; Al-Asaadi, M.; Al-Waheeb, H. The effect of some heavy metals accumulation on physiological and anatomical characteristic of some Potamogeton L. plant. J. Ecol. Environ. Sci. 2013, 4, 100–108. [Google Scholar] [CrossRef]
- Zhao, Y.; Collum, S.; Phelan, M.; Goodbody, T.; Doherty, L.; Hu, Y. Preliminary investigation of constructed wetland incorporating microbial fuel cell: Batch and continuous flow trials. Chem. Eng. J. 2013, 229, 364–370. [Google Scholar] [CrossRef]
- Ji, B.; Zhao, Y.; Li, Q.; Yang, Y.; Ting, W.; Tang, C.; Zhang, J.; Ruan, W.; Tai, Y. Interrelation between macrophytes roots and cathode in constructed wetland-microbial fuel cells: Further evidence. Sci. Total Environ. 2022, 838, 156071. [Google Scholar] [CrossRef]
- Saz, C.; Ture, C.; Turker, O.C.; Yakar, A. Effect of vegetation type on treatment performance and bioelectric production of constructed wetland modules combined with microbial fuel cell (CW-MFC) treating synthetic wastewater. Environ. Sci. Pollut. Res. Int. 2018, 25, 8777–8792. [Google Scholar] [CrossRef] [PubMed]
- González, T.; Puigagut, J.; Vidal, G. Organic matter removal and nitrogen transformation by a constructed wetland-microbial fuel cell system with simultaneous bioelectricity generation. Sci. Total Environ. 2021, 753, 142075. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, D.; Hu, Z.; Liu, H. Enhance performance of microbial fuel cell coupled surface flow constructed wetland by using submerged plants and enclosed anodes. Chem. Eng. J. 2018, 351, 312–318. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Tang, C.; Xu, L.; Morgan, D.; Liu, R. Role of macrophyte species in constructed wetland-microbial fuel cell for simultaneous wastewater treatment and bioenergy generation. Chem. Eng. J. 2020, 392, 123708. [Google Scholar] [CrossRef]
- Zang, G.L.; Sheng, G.P.; Tong, Z.H.; Liu, X.W.; Teng, S.X.; Li, W.W.; Yu, H.Q. Direct electricity recovery from Canna indica by an air-cathode microbial fuel cell inoculated with rumen microorganisms. Environ. Sci. Technol. 2010, 44, 2715–2720. [Google Scholar] [CrossRef]
- Xu, J.Y.; Xu, H.; Yang, X.L.; Singh, R.P.; Li, T.; Wu, Y.; Song, H.L. Simultaneous bioelectricity generation and pollutants removal of sediment microbial fuel cell combined with submerged macrophyte. Int. J. Hydrogen Energy 2021, 46, 11378–11388. [Google Scholar] [CrossRef]
- Villaseñor Camacho, J.; Rodríguez Romero, L.; Fernández Marchante, C.M.; Fernández Morales, F.J.; Rodrigo Rodrigo, M.A. The salinity effects on the performance of a constructed wetland-microbial fuel cell. Ecol. Eng. 2017, 107, 1–7. [Google Scholar] [CrossRef]
| System | Species | Biotype | COD (%) | Nitrogen (%) | Phosphorus (%) | Maximum Power Density | Coulombic Efficiency (%) | References |
|---|---|---|---|---|---|---|---|---|
| PCW-MFC | Iris pseudacorus | emergent | 46.9 | NO3− 51.6 NH4+ 66.2 | TP 57.6 PO43− 71.5 | 25.14 mWm−2 | -- | Yang et al. (2020) [86] |
| CW-MFC | Hydrilla verticillata | submerged | 64.02 | NO3− 77.9 | TP 93.54 | 20.70 mWm−2 | -- | Oon et al. (2017) [41] Yang et al. (2020) [86] Xu et al. (2021) [88] |
| CW-MFC | Elodea nuttallii | floating | 31.5 | NH4+ 66.0 | TP 97.5 | 6.37 mWm−2 41.46 mWm−2 | 10.28 | Zhao et al. (2013) [81] Shen et al. (2018) [85] Yang et al. (2020) [86] |
| PCW-MFC SMFC | Phragmites australis | emergent | 86.6 | -- | PO43− 81.0 | 0.856 mWm−3 | 0.6 | Oon et al. (2017) [41] Doherty et al. (2015) [24] Villaseñor et al. (2017) [89] Zhao et al. (2013) [81] Aswad et al. (2020) [31] |
| CW-MFC | Ipomoea aquatica | floating rooted | 94.8 | TN 90.8 NH4+ 68.47 | -- | 12.42 mWm−2 | 1.71 | Liu et al. (2013) [21] Liu et al. (2020) [30] Liu et al. (2013) [21] |
| PMFC CW-MFC | Canna indica | emergent | 88.67 | NH4+ 73.02 NO3− 57.82 | PO43− 88.81 | 320.8 mWm−2 0.4136 Wm−3 15.73 mVm−2 1.12 V | 1.86 | Yadav et al. (2012) [20] Wang et al. (2017) [37] Srivastava et al. (2020) [15] Liu et al. (2020) [30] |
| PCW-MFC | Schaenoplectis californicus | emergent | 87.0 | TN 98.0 | -- | 8.6 mWm−2 | 2.4 | González et al. (2021) [84] |
| CW-MFC | Eichhornia crassipes | floating | 77.22 | NO3− 45.23 | -- | 80.08 mWm−2 | 2.15 | Mohan et al. (2011) [9] Oon et al. (2017) [41] |
| CW-MFC | Diffenbachia seguine | emergent | 94.00 ± 0.05 | NO3− 50.02 NH4+ 64.31 | PO43− 42.05 | 7.75 mWm−3 | -- | Kim et al. (2021) [27] |
| SP-MFC | Ceratophyllum demersum | submerged | 81.16 | TN 65.27 | PO43− 79.10 | 24.5 mWm−2 | -- | Xu et al. (2021) [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Méndez, L.M.; Martínez-Amador, S.Y.; Ríos-González, L.J.; Pérez-Rodríguez, P.; Perez-Rodríguez, M.A.; Reyes-Acosta, A.V.; Rodríguez-De la Garza, J.A. A Review on Anatomical and Physiological Traits of Aquatic Macrophytes Coupled to a Bioelectrochemical System: Comparative Wastewater Treatment Performance. Processes 2025, 13, 1545. https://doi.org/10.3390/pr13051545
González-Méndez LM, Martínez-Amador SY, Ríos-González LJ, Pérez-Rodríguez P, Perez-Rodríguez MA, Reyes-Acosta AV, Rodríguez-De la Garza JA. A Review on Anatomical and Physiological Traits of Aquatic Macrophytes Coupled to a Bioelectrochemical System: Comparative Wastewater Treatment Performance. Processes. 2025; 13(5):1545. https://doi.org/10.3390/pr13051545
Chicago/Turabian StyleGonzález-Méndez, Laura M., Silvia Y. Martínez-Amador, Leopoldo J. Ríos-González, Pedro Pérez-Rodríguez, Miguel A. Perez-Rodríguez, Alfredo V. Reyes-Acosta, and José A. Rodríguez-De la Garza. 2025. "A Review on Anatomical and Physiological Traits of Aquatic Macrophytes Coupled to a Bioelectrochemical System: Comparative Wastewater Treatment Performance" Processes 13, no. 5: 1545. https://doi.org/10.3390/pr13051545
APA StyleGonzález-Méndez, L. M., Martínez-Amador, S. Y., Ríos-González, L. J., Pérez-Rodríguez, P., Perez-Rodríguez, M. A., Reyes-Acosta, A. V., & Rodríguez-De la Garza, J. A. (2025). A Review on Anatomical and Physiological Traits of Aquatic Macrophytes Coupled to a Bioelectrochemical System: Comparative Wastewater Treatment Performance. Processes, 13(5), 1545. https://doi.org/10.3390/pr13051545









