Abstract
Peanut straw, a lignocellulosic agricultural residue rich in cellulose, hemicellulose, and lignin, has significant potential for biochar production, which may enhance the anaerobic digestion (AD) process. The addition of biochar can effectively enhance the AD process. However, the role of peanut straw biochar in anaerobic co-digestion of mixed substrate remains underexplored. This study innovatively employs peanut straw biochar as an exogenous additive in anaerobic co-digestion of cow manure and corn straw. The results demonstrate that the optimal methane yield was achieved at a biochar dosage of 8%, with a significant increase of 19.1% compared to the control group (832.23 mL). Various doses of biochar also facilitated the degradation of the digestion substrate to varying extents, with the highest substrate removal rate of 54.1% achieved when biochar was added at 8%. Furthermore, the addition of peanut straw biochar enhanced the microbial community structure. Specifically, the inclusion of 8% biochar increased the relative abundance of Methanosarcina by 2.8%, while the addition of 6% biochar elevated the relative abundance of Chloroflexota by 1.4%. This study contributes to the sustainable use of agricultural waste and supports the development of biochar-enhanced AD for improved waste management and energy recovery.
1. Introduction
China produces approximately 1.4 billion tons of cattle manure (Cm) and 0.9 billion tons of corn stover (Cs) annually, which, when improperly managed, pose significant environmental risks, including water eutrophication and resource waste. To mitigate environmental risks, enhance the sustainable management of agricultural resources, and facilitate the steady achievement of the United Nations Sustainable Development Goals (SDGs) by 2030, it is crucial to investigate the utilization of agricultural waste resources. Anaerobic digestion (AD) has emerged as a preferred method for converting agricultural waste into clean energy [1,2,3]. However, AD faces challenges, including an unstable digestion process and low methane production efficiency [4]. Current mitigation strategies primarily include anaerobic co-digestion, supplementation with exogenous additives, and feedstock pre-treatment. Anaerobic co-digestion, which simultaneously utilizes livestock manure and crop residues, has proven effective and is widely applied in practice [1]. Pre-treatment methods, categorized as physical, chemical, biological, and combined, are typically performed prior to AD [5,6,7]. In comparison, the introduction of exogenous additives offers reduced environmental impacts, improved cost-effectiveness, and immediate process enhancement, presenting considerable potential for industrial-scale applications [8].
Exogenous additives used in AD can be categorized into metallic elements, carbon-based additives, bio-additives, and alkaline additives [8]. Among these, biochar, a porous carbon-based additive, is produced through the pyrolysis of biomass. Biochar is characterized by its well-developed pore structure, large specific surface area, small particle size, and abundance of redox functional groups, all of which facilitate electron transfer. These properties enhance the attachment and proliferation of microbial communities during AD, thereby improving microbial community structure and boosting digestion efficiency [9,10]. Wang et al. added biochar to the mesophilic anaerobic digester, achieving a significant reduction in the lag phase, ranging from 27.5% to 64.4%, and a notable enhancement in the maximum methane production rate, ranging from 22.4% to 40.3% [11]. Marzieh et al. investigated the impact of two distinct biochar ratios on the AD of paint sludge. Their results demonstrated that both ratios significantly augmented methane production by 26.7% and 76.7%, respectively [12]. In a related study, Jun et al. incorporated biochar at dosages of 3%, 5%, and 7% into the AD of pig manure. This addition correspondingly augmented methane production by 26.7%, 23.0%, and 26.4%, respectively [13]. These findings support our hypothesis that biochar can similarly improve methane production during the co-digestion of cattle manure and corn stover. Moreover, biochar offers several advantages, including the fact that it is cost-effective, environmentally safe, easy to achieve, and available from a wide range of raw materials [14,15,16,17]. Utilizing agricultural solid waste as feedstock for biochar production not only improves AD performance but also aligns with the “waste for waste” concept, which involves using agricultural residues to produce biochar that enhances the digestion of other agricultural residues in the AD process, thereby promoting resource recovery and sustainable waste management practices.
According to the China National Bureau of Statistics, total peanut production in 2024 is projected to reach 18.983 million tons. Typically, 4.5 tons of peanut straw are obtained for every 4.5 tons of peanuts produced per hectare. However, a large amount of peanut straw remains ineffectively utilized, resulting in significant resource waste. Peanut straw is rich in cellulose, hemicellulose, and lignin, and it is an ideal feedstock for biochar production [18,19,20]. Despite the promising effects of biochar in AD, limited studies have focused on the use of peanut straw biochar in co-digestion systems. Given its unique chemical composition and potential benefits, exploring its role in anaerobic co-digestion is of practical significance. This study aims to fill this gap by evaluating the effects of peanut straw biochar on methane yield, substrate degradation, and microbial community dynamics during the anaerobic co-digestion of cattle manure and corn stover.
This study investigated the potential of peanut straw biochar as an additive to enhance the co-digestion of Cm and Cs. The objectives were (1) to evaluate methane production and process stability and (2) to explore changes in the abundance and diversity of the microbial community.
2. Materials and Methods
2.1. Materials and Reagents
Preparation of Biochar: Peanut straw was air-dried, pulverized, and sieved through a 20-mesh screen. The ground straw was placed into ceramic crucibles and covered with close-fitting lids. Then, the peanut straw in the ceramic crucibles was pyrolyzed under a limited oxygen supply in a muffle furnace (12D-16, Shanghai Yi Zhong Electricity Furnace Inc., Shanghai, China) [20]. The heating rate of the muffle furnace was set to 10 °C per minute. Once the temperature reached 400 °C, it was maintained for a duration of 2 h. Subsequently, the resulting product was washed with 3 L of ultrapure water until the pH of the supernatant reached neutrality. The washed material was then centrifuged and filtered through a 0.45 μm membrane. The residue collected after filtration was dried in an oven at 65 °C, yielding peanut straw biochar.
Digestive Substrate: Cm and Cs were air-dried, ground in a mill, sieved through a 20-mesh screen to remove stones and debris, washed thoroughly, and air-dried again. Through previous research, the ratio of cattle manure to corn straw was established as 60% to 40% (based on the dry matter portion that is biodegradable) [21], and the mixture was diluted with distilled water to achieve a solids content of approximately 10%, which was used as the AD substrate.
The inoculum for this experiment was obtained from the digester sludge at the Qingdao Haipo River Wastewater Treatment Plant in China. Peanut straw was sourced from a farm in Yantai City, Shandong Province; Cs was obtained from a farm in Qingdao City, Shandong Province; and Cm was collected from Cangshan State Farm in Linyi City, Shandong Province. The main physicochemical properties of the biochar raw material, inoculated sludge, and digestion substrate are presented in Table 1.

Table 1.
Characteristics of raw materials and inoculum *.
2.2. Experimental Setup
AD Batch Test Equipment: The AD batch test was conducted using the AMPTS II system manufactured by BPC Instruments AB. The AMPTS II system is a biogas potential analysis device that accurately measures and records methane production from the digestion substrate [22]. This system primarily consists of a constant-temperature water bath, an AD reaction tank, an electric stirrer, an alkali absorption bottle, and a gas collection bag. The water bath temperature is maintained at 37 °C. Each reactor is a serum vial with a 400 mL effective volume and a sampling port. A 140 rpm motorized stirrer is mounted above each reactor. The reactor flasks are purged with nitrogen for 3 min before sealing to remove any air. Biogas produced in the reactor is directed through a 4 mm silicone tube into a 100 mL alkaline flask containing a 2 mol/L NaOH solution. This solution absorbs CO2, H2S, and other gaseous components, allowing only methane to pass into the gas collection bag. Gas production is recorded daily.
Previous studies have demonstrated that the addition of 10% (w/w) biochar significantly enhances the co-digestion performance of cattle manure and straw [23]. Additionally, research on the AD of agricultural waste has shown that biochar concentrations of 1%, 2%, and 3% also yield positive effects [24]. Consequently, in this study, the addition of peanut straw biochar was categorized into several mass ratios: 0%, 2%, 4%, 6%, 8%, and 10% [25]. These were added to each reactor and labeled control, PS1, PS2, PS3, PS4, and PS5 (Figure 1).
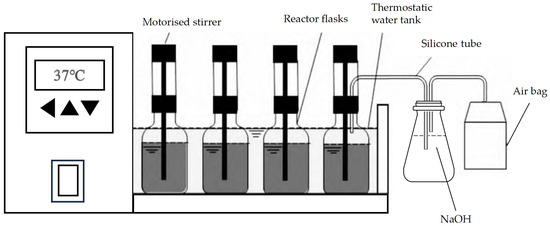
Figure 1.
Anaerobic digestion (AD) experimental setup.
2.3. Analytical Methods
2.3.1. Methods for the Measurement of Conventional Indicators
Total solids (TS), volatile solids (VS), and pH were measured according to standard methods through ALPHA [26]. The SCOD was determined using a fast digestion–spectrophotometric method [27], with measurements conducted using a UV-Vis spectrometer (HACH DR5000, HACH, Shanghai, China). The fast digestion–spectrophotometric method presents less environmental harm compared to the dichromate method [27]. This approach is rapid, precise, and appropriate for measuring SCOD concentrations in diluted samples ranging from 15 to 1000 mg/L. Dissolved carbohydrates were measured using the sulfuric acid–anthrone method (converted to a SCOD factor of 1.06) [28,29], while dissolved proteins were quantified using the BCA kit (converted to a SCOD factor of 1.50) [29,30]. The sulfuric acid–anthrone method is widely used for determining carbohydrates in dark fermentation. Volatile fatty acids (VFAs) were analyzed through gas chromatography (GC-7820A, Agilent, Santa Clara, CA, USA) [30]. The conversion factors for short-chain fatty acids (acetic, propionic, isobutyric, n-butyric, isovaleric, and n-valeric acids) to SCOD equivalents were 1.07, 1.51, 1.82, 1.82, 2.04, and 2.04, respectively [31]. The volume of methane produced was determined by measuring the amount of gas extracted from the collection bag using a syringe.
2.3.2. Microbial Analysis
The changes in the microbial community structure were analyzed on day 32 through high-throughput sequencing technology, and the analyses were conducted by Shanghai Meggie Biomedical Technology Co., Ltd., Shanghai, China. PCR amplification of the V3–V4 region of bacterial 16S rRNA genes was carried out using the specific primers 515F (5′-GTGCCAGCMGCCGCGG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Microbial data were finally processed using the Majorbio Cloud Platform (https://www.majorbio.com, accessed on 26 February 2025).
2.3.3. Data Analysis
The modified Gompertz model of gas production kinetics is an effective tool for modeling methane production and assessing the methane production potential of AD reactors under various experimental conditions. The corresponding Gompertz fitting equation is presented in Equation (1) below.
where P(t) represents the actual measurement of cumulative methane production (mL); Pm denotes the potential methane production (mL); Rm refers to the maximum rate of biogas production; λ is the AD lag time (d); and t is the AD time (d).
OriginPro 2023 (OriginLab Corporation, Northampton, MA, USA) fitted the results using non-linear regression analysis to determine the kinetic parameters and estimate the prediction accuracy through the coefficient of determination (R2). T-tests were used to detect statistically significant differences using Graphpad prism 10.1.2. (GraphPad Software, Boston, MA, USA).
2.3.4. Biochar Characterization
Scanning electron microscopy (SEM, Hitachi SU 8010, Tokyo, Japan) was operated to observe surface morphologies of materials. The specific surface area (SSA) and pore size distributions of LA-4 were analyzed through BET (Micromeritics ASAP 2460, Norcross, GA, USA). Fourier transform infrared spectroscopy (FTIR, Thermo Scientific Nicolet iS20, Waltham, MA, USA) was applied to detect the chemical bonds and functional groups on material surfaces.
3. Results and Discussion
3.1. Characterization of Biochar
3.1.1. BET and SEM of Biochar
The SEM images of biochar at magnifications of 2000× and 5000× are presented in Figure 2. The biochar exhibits a honeycomb-like pore structure, characterized by distinct holes and depressions within the pores, which are marked in blue. The regions highlighted in red appear rough and uneven. This porous structure of biochar facilitates the attachment and growth of microorganisms while also capturing nutrients from the digestate, thereby promoting microbial proliferation. Furthermore, the abundant porosity aids in the even distribution of the digestate matrix throughout the liquid [11]. Peanut straw biochar displays a more developed pore structure compared to the SEM image of wood biochar [32].
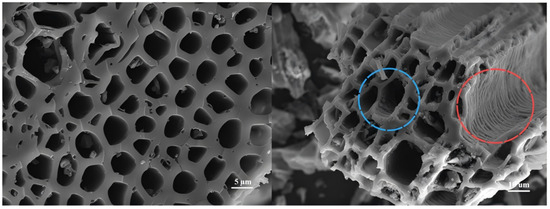
Figure 2.
SEM of peanut straw biochar at magnifications of 2000× and 5000×.
Observations of the morphology indicate that the biochar possesses a highly developed pore structure, which enhances the reaction rate and stability of anaerobic digestion.
The isothermal adsorption and desorption curves, along with the pore size distribution of the biochar, are illustrated in Figure 3. The adsorption–desorption curves of the biochar conform to the IUPAC type IV isotherm, exhibiting H3 hysteresis loops, which are characteristic of mesoporous materials [33]. These loops indicate the presence of irregular pore structures, including cracks and wedge-like formations with slit pores created by the accumulation of lamellar particles [34]. The mesopores in biochar enhance its ion exchange capacity and conductivity, facilitating electron transfer between the microbial community and the biochar. This process improves the efficiency of AD [35,36]. The specific surface area of the biochar is 28.29 m2/g, with a total pore volume of 0.024 cm3/g and an average pore size of 3.36 nm. Saif et al. utilized magnetic chicken bone biochar in their anaerobic digestion (AD) studies. This material is a mesoporous substance similar to peanut straw biochar, but it has a larger specific surface area and pore size, resulting in enhanced adsorption and electrochemical properties. Therefore, future research should focus on modifying peanut straw biochar to improve its application in the field of anaerobic digestion [37].
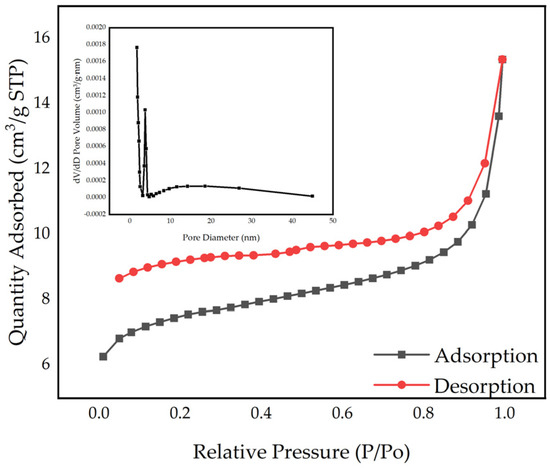
Figure 3.
N2 adsorption–desorption isotherms and pore size distribution curves of biochar.
3.1.2. FTIR of Biochar
Infrared scanning was performed on peanut straw biochar, and the results are illustrated in Figure 4. The characteristic peak at 1576 cm−1 corresponds to the stretching vibration of C=C and the symmetric or asymmetric vibration of C=O. The peak at 3376 cm−1 is associated with the hydroxyl functional group [38]. This peak is broad, indicating a high abundance of hydroxyl groups, which results in intensified vibrations related to these groups in the biochar samples. The protonation of hydroxyl groups can increase the pH of the external environment. In the context of an AD system, this rise in pH helps to mitigate acidification, thereby enhancing the stability of the system [19]. The peak at 875 cm−1 is attributed to CaCO3 [39,40], while the vibration at 1423 cm−1 indicates the presence of a significant carbonate component [41]. Trace metal elements can regulate the structure of microbial communities and, as cofactors or components of enzymes involved in methane production, enhance the reaction rate of AD [42]. These results suggest that peanut straw biochar contains a rich array of functional groups, which enhance its adsorption and ion exchange capacities, thereby promoting the adsorption of microorganisms and nutrients and improving the AD process.
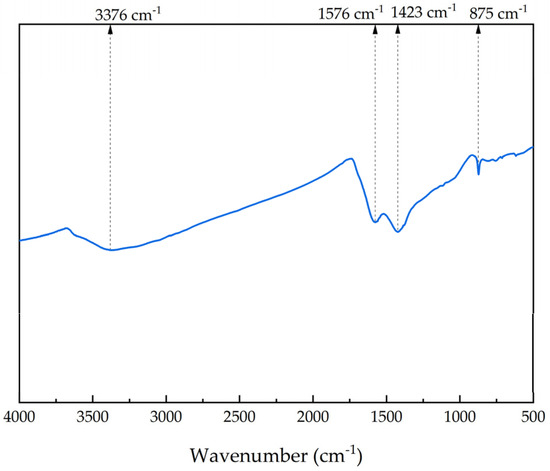
Figure 4.
FTIR of biochar.
Previous studies have demonstrated that peanut straw biochar enhances the pH buffering capacity of soils. The mechanism through which peanut straw biochar increases the system’s pH is attributed to the protonation of carboxyl and hydroxyl groups [17]. In this study, we showed that peanut straw biochar, when used as an additive, improved the pH buffering capacity of the AD system, thereby enhancing the stability of the process. However, our findings revealed no significant carboxylate peaks in the FTIR spectra, which could be due to the pretreatment method of the feedstock, the heating rate during pyrolysis, and the ambient temperature and humidity during the cooling phase in the preparation of peanut straw biochar [16,17]. This study did not investigate the effects of pyrolysis time, temperature, humidity, and other factors on the structural components and their impact on AD performance. Future research should focus on optimizing biochar preparation conditions. Additionally, because laboratory-scale AD experiments are primarily conducted in batch mode, further investigation into the operation of continuous AD reactors is recommended to enhance the applicability of these findings in real-world settings.
3.2. Co-Digestive Methanogenic Properties
3.2.1. Cumulative Methane Production and Daily Methane Production
As illustrated in Figure 5, the methane production rates among all groups exhibited statistically significant differences (p < 0.05). The cumulative and daily methane production for each group is presented in Figure 5. As shown in Figure 6a, the cumulative methane production over a 32-day period for the six groups was as follows: 832.23 mL (control), 855.88 mL (PS1), 909.93 mL (PS2), 984.52 mL (PS3), 991.19 mL (PS4), and 956.77 mL (PS5). Cumulative methane production in the control group was lower than that in the reactor with added biochar. The groups supplemented with peanut straw biochar exhibited increases in cumulative methane production of 2.8% (PS1), 9.3% (PS2), 18.3% (PS3), 19.1% (PS4), and 15.0% (PS5) compared to the control group.
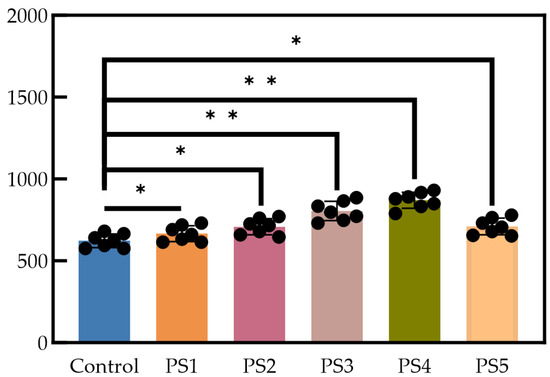
Figure 5.
Significant difference analysis, where * represents p ≤ 0.05 and ** represents p ≤ 0.01.
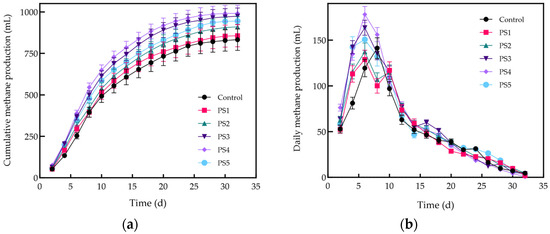
Figure 6.
Cumulative methane production (a) and changes in daily methane production (b).
These results suggest that peanut straw biochar can effectively enhance the co-digestion of Cm and Cs for methane production, further supporting the feasibility of reusing peanut stover through pyrolytic carbonization. The PS4 group demonstrated the highest cumulative methane production, indicating that the addition of 8% peanut straw biochar most effectively promoted co-digestion. In contrast, cumulative methane production decreased with the addition of 10% biochar, likely due to an excess of biochar occupying the reaction space, thereby reducing the effective contact area between the reactants and the microorganisms. This limitation in substrate availability for microorganisms may hinder microbial activity and decrease methane production efficiency [43,44,45].
During the initial phase of AD, the intermediate products generated during the hydrolysis stage provided substrates for the methanogenic phase. The methane production curves for all groups exhibit similar trends, characterized by a rapid increase followed by a gradual decline (Figure 6b). This pattern occurs because during anaerobic digestion, easily degradable organic matter is swiftly decomposed by the metabolic activity of anaerobic microorganisms, resulting in a peak in daily methane production. As the readily degradable organic matter diminishes over time, the degradation rate of more resistant organic matter decreases, leading to a gradual reduction in daily methane production. Daily peak methane production with the addition of biochar reached 178.08 mL on day 6, while the control group recorded a peak of 141.36 mL on day 8. The porous structure of biochar offers attachment sites for microorganisms, while its adsorption properties regulate nutrient availability and reduce the concentration of harmful substances in the AD system. Consequently, biochar enhances the activity of methanogens, increases the peak daily methane production, and shortens the reaction cycle. Among all of the groups, the PS4 group exhibited the highest peak in daily methane production, demonstrating a 26.0% improvement compared to the control group. The results indicate that an appropriate addition of biochar can effectively enhance peak daily methane production. However, excessive biochar addition can hinder the stability of methane production and reduce the peak daily methane yield.
These results indicate that peanut straw biochar, as an exogenous additive, effectively enhances the AD process by accelerating the decomposition of organic matter and increasing methane production efficiency. This effect can be primarily attributed to the porous structure of biochar, which promotes microbial growth and retains nutrients within the reactor, thereby creating a favorable environment for microbial proliferation. Furthermore, biochar, serving as a conductive material, facilitates the formation of microbial aggregates involved in direct interspecies electron transfer (DIET), which supports the development of electron transfer networks and promotes the growth of methanogenic bacteria that metabolize co-nutrients [46].
3.2.2. Modified Gompertz Kinetics of Methane Production
The relative parameters of the modified Gompertz model, including the maximum methane production rate (Rm) and the lag time (λ), are presented in Table 2. The correlation coefficient R2 of all groups is larger than 0.99, indicating that the modified Gompertz model accurately describes methanogenic performance. The Rm values for PS1, PS2, PS3, PS4, and PS5 increased by 4.4%, 7.5%, 28.4%, 36.0%, and 14.2%, respectively, suggesting that methane production from Cm and Cs can be enhanced with the addition of peanut straw biochar. The maximum methane potential for the groups treated with peanut straw biochar (PS1–PS5) increased by 2.5%, 9.4%, 18.0%, 19.2%, and 14.7%, respectively, compared to the control group. PS4 exhibited the highest values for both Pm and Rm, and these results were consistent with the actual methane yield. The λ value represents the adaptation of microorganisms to the digestive system; generally, lower λ values indicate better adaptation of the microorganisms to the substrate [47]. With the supplementation of peanut straw biochar, the λ value was lower than that of the control group. The modeling results demonstrated that the addition of peanut straw biochar significantly increases methane yield and reduces retention time. Saif et al. incorporated chicken bone biochar, and its methane production performance was comparable to the results of the present study. However, reactors containing magnetic chicken bone biochar demonstrated enhanced methane production [37]. This improvement may be attributed to the increased adsorption capacity and electrochemical properties of the magnetic biochar, which, in turn, enhanced methane production efficiency in the AD system.

Table 2.
Modified Gompertz model fitting parameters.
3.3. Changes in the Composition of VFAs
VFAs are essential intermediates in the AD process, including acetic acid, propionic acid, n-butyric acid, isobutyric acid, n-valeric acid, and isovaleric acid. Research has demonstrated that methanogenic bacteria primarily utilize H2, CO2, formic acid, methanol, methylamine, and acetic acid as substrates [48,49]. Acetic acid is the primary product of substrate digestion through the acetyl–CoA pathway. However, when the AD system operates at a very low pH, the substrate is converted into a significant amount of reduced products, such as propionic and butyric acids. These reduced products must be further oxidized by acetic-acid-producing bacteria to generate acetic acid, which considerably slows the rate of acetic acid production and, consequently, affects methane production efficiency [50]. Therefore, the rate of change in acetic acid concentration within the VFAs’ composition directly influences methane production efficiency. As illustrated in Figure 7, the initial concentrations of VFAs were similar across all groups. As acid-producing bacteria utilize dissolved organic matter to produce VFAs, their concentrations increase. In the biochar-amended reactors, the concentration of acetic acid peaked on day 2, two days earlier than in the control group, indicating that biochar, as an additive, helped regulate the pH of the AD system and accelerated the rate of acetic acid production.
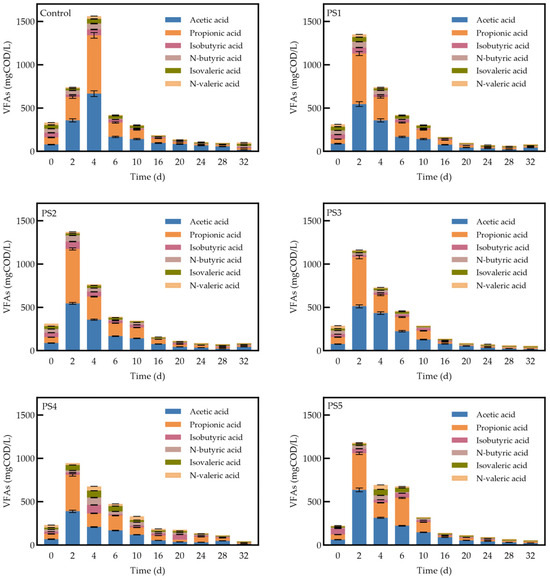
Figure 7.
Changes in the fraction of VFAs by reactor.
Acetic acid and propionic acid were the predominant components in each reactor, with acetic acid levels consistently exceeding those of propionic acid. This phenomenon is attributed to the conversion of hydrogen and carbon dioxide into acetic acid by homoacetogenic bacteria. Concurrently, methanogenic bacteria utilize acetic acid, hydrogen, and carbon dioxide to produce methane, which further reduces the hydrogen partial pressure in the system. This reduction in pressure allows acetic acid to become the primary substrate [51,52]. In groups supplemented with biochar, the concentrations of acetic acid were lower than those in the control group, indicating that peanut straw biochar enhanced the methanation of acetic acid. The overall VFA concentrations in the biochar-amended groups were lower than those in the control group, indicating that peanut straw biochar also facilitated VFAs’ consumption by methanogenic bacteria, thereby improving the efficiency of the methanogenic phase. At the conclusion of the AD process, the residual VFAs’ concentration in the reactors treated with peanut straw biochar was lower than that in the control group, further supporting the notion that biochar enhances VFAs’ utilization by methanogenic bacteria. Among the groups tested, the PS4 group exhibited the highest VFA removal rate, achieving 80.6% removal efficiency. Previous studies have shown that the concentration of VFA in AD reactors with biochar addition increased rapidly by day 2, with peak VFA concentrations occurring earlier and reaching lower levels than in the control group [10]. It appears that the addition of peanut straw biochar was beneficial for alleviating VFAs’ accumulation and shortening the duration of accumulation.
3.4. pH Change
The pH alterations for each reactor are illustrated in Figure 8. The initial pH was higher in the biochar addition groups, with this value being proportional to the amount of biochar added. During the reaction, the pH of all groups decreased rapidly. By day six, the pH in each reactor had reached its lowest point. However, the pH in the biochar-added groups remained higher than that in the control group, with values increasing in proportion to the amount of biochar added. The pH in the biochar addition groups fluctuated between 7.0 and 7.9, maintaining a range conducive to the proliferation of methanogenic microorganisms. The alkaline metal oxides in peanut straw biochar elevate the pH during the early stages of AD [53], promoting the conversion of CO2 to methane [54]. An increase in pH also accelerates the production rate of acetic acid in the AD system, promoting the enrichment of methane-producing bacteria that utilize acetic acid [50]. Additionally, the abundant functional groups enhance the conversion of intermediates and improve the acid–base buffering capacity of the system [55]. This study confirmed the pH buffering capacity of peanut straw biochar; however, it was insufficient to completely prevent acidification. Saif et al. introduced magnetic chicken-bone-derived biochar into the AD process, adjusting the pH to a range of 7.4 to 7.9. This adjustment had a significant effect, preventing acidification during the AD process [37]. Magnetic biochar is produced by loading iron or other magnetic materials onto the surface of biochar, which not only preserves the biochar’s basic properties but also imparts magnetic characteristics. This modification further enhances the adsorption capacity and metal content of the biochar, improving its pH-regulating ability [56]. Because even a slight decline in pH within the AD system can reduce methane production efficiency, future research should concentrate on optimizing the properties of biochar, particularly by developing metal-ion-loaded peanut straw biochar to improve its pH-regulating capacity.
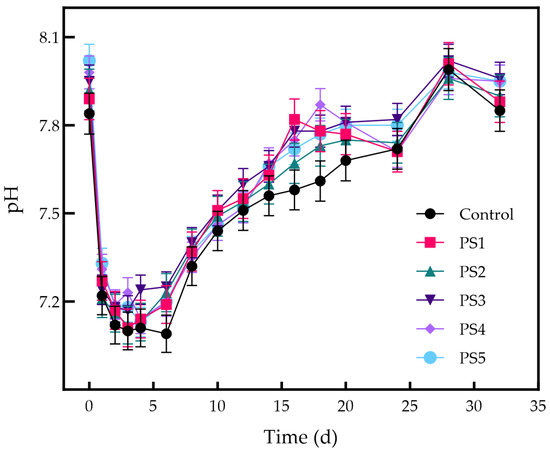
Figure 8.
Changes in pH for each reactor.
3.5. Changes in Dissolved Organic Matter Concentrations
Figure 9 illustrates the variations in the concentrations of various dissolved organic compounds in the reactor, including dissolved proteins, polysaccharides, VFAs, and others. In all groups, the concentration of soluble proteins was the highest, while the concentration of soluble polysaccharides was the lowest. This phenomenon can be attributed to the more complex structure of proteins, which makes microorganisms more inclined to utilize soluble polysaccharides as metabolic substrates [57]. Consequently, the accumulation of soluble proteins was more pronounced, whereas the consumption rate of soluble polysaccharides was faster, resulting in their lower concentration. Compared to the control group, all biochar-amended groups exhibited earlier peaks in VFA concentrations, with the PS4 group reaching its peak on day 2. This suggests that peanut straw biochar accelerates the acid production process of hydrolytic-acid-producing bacteria.
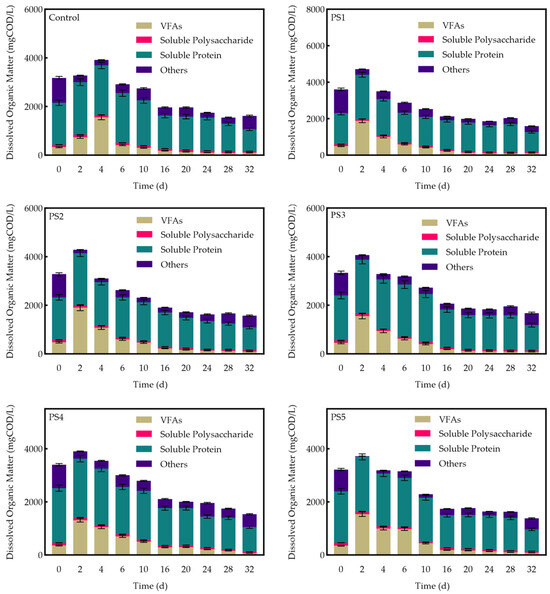
Figure 9.
Changes in dissolved organic matter concentration by reactor.
The PS1 group exhibited a 12.1% increase in peak dissolved organic matter concentration and a 12.0% increase in peak dissolved protein concentration. In contrast, the other groups demonstrated a decrease in peak dissolved organic matter, with PS2 showing a 2.8% reduction, PS3 a 7.8% reduction, PS4 a 13.4% reduction, and PS5 a 13.7% reduction. Additionally, all groups, except PS1, experienced a slight decrease in peak dissolved protein concentrations. These changes can be attributed to variations in the activity or abundance of anaerobic flora, such as Bacillus spp., which are involved in protein hydrolysis. Consequently, these variations result in changes in soluble protein concentrations. As dissolved organic matter is gradually consumed for methanation, its concentration decreases. Furthermore, the removal of SCOD was higher in all experimental groups compared to the control group, with the PS4 group demonstrating the most significant SCOD removal at 63.3%.
The changes in SCOD also corroborated the shifts in VFAs in relation to pH (Figure 7 and Figure 8). During the early stages of AD, the SCOD concentration continued to rise because the rate of hydrolytic acidification exceeded that of SCOD methanation, resulting in a decrease in pH due to the substantial production of VFAs during the hydrolytic acidification phase. As the AD process progressed, the rate of SCOD methanation increased relative to the rate of hydrolytic acidification. Consequently, the SCOD concentration decreased, which corresponded with the increased utilization of VFAs by methanogenic bacteria. This led to a reduction in VFAs’ accumulation and a gradual recovery of pH. The addition of biochar regulated the pH of the AD system. During the AD process, the pH fluctuated between 7.1 and 7.9, a range that is optimal for methane-producing microorganisms and enhances the conversion rate of the digestion substrate into intermediate products, such as acetate. Consequently, the concentrations of SCOD and VFAs in the reactors with added biochar reached their peak values earlier. Furthermore, biochar facilitates the formation of microbial aggregates involved in DIET, which supports the development of electron transfer networks and promotes the growth of methanogenic bacteria that metabolize co-nutrients [46]. Thus, biochar accelerated the conversion rate of intermediate products to methane in the AD system. This observation aligns with the findings that reactors with added biochar produced higher cumulative methane yields, achieved greater daily methane peaks, and shortened the reaction cycle (Figure 6).
3.6. Microbial Community Analysis
3.6.1. Alpha Diversity Analysis
Alpha diversity typically reflects the richness and diversity of microbial communities. As shown in Table 3 and Table 4, the Shannon and Simpson indices were utilized for analysis in this study. The Coverage Index for each group exceeded 0.99, indicating that the sequencing results captured the majority of bacterial diversity.

Table 3.
Alpha diversity index of bacterial communities.

Table 4.
Alpha diversity index of archaeal communities.
Compared to the control group, the Shannon index increased in the peanut straw biochar-amended group, while the Simpson index decreased. This indicates that peanut straw biochar increased the diversity of the bacterial community. For instance, the addition of an appropriate amount of biochar enhances the enrichment of Chloroflexota (Figure 10). The proliferation of Chloroflexota enhanced the efficiency of the hydrolysis and acidogenesis stages, thereby shortening the reaction cycle and improving methane production efficiency (Figure 6b).
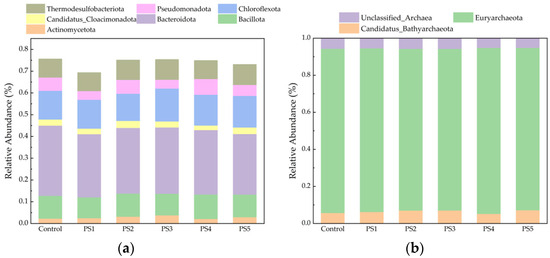
Figure 10.
Plot of relative abundance changes at the bacterial (a) and archaeal (b) phylum levels.
Compared to the control group, the Shannon index decreased, while the Simpson index increased in the peanut seedling biochar group. This indicates that the addition of peanut seedling biochar reduced the diversity of the archaeal community. This reduction is attributed to the promotion of acetic acid production and the conversion of other organic acids to acetic acid by peanut straw biochar, which led to the dominance of acetic-acid-type methanogens. Consequently, the activity of hydrogenotrophic and other nutrient-type methanogenic flora was diminished, further decreasing the archaeal diversity in the reactor with added peanut straw biochar. For instance, Methanospirillum is a hydrogenotrophic methanogen. With the exception of the PS4 and PS2 group, the addition of biochar led to a decrease in its relative abundance across all other groups [58]; Methanoregula is a strictly anaerobic H2/CO2-utilizing methan H2/CO2-utilizing methanogen. The population abundance of Methanoregula decreased in all reactor groups following the addition of biochar (Figure 11) [59].
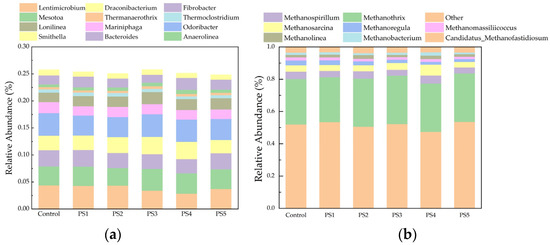
Figure 11.
Plot of relative abundance changes at the genus level for bacteria (a) and archaea (b).
3.6.2. Microbial Population Abundance Analysis
As shown in Figure 10a, the predominant bacterial phyla included Bacteroidota, Chloroflexota, Bacillota, Thermodesulfobacteriota, and Pseudomonadota. The total relative abundance of these five dominant taxa exceeded 60% across all groups, underscoring their role as key microorganisms involved in the hydrolysis of insoluble organic matter. This observation indicates the successful domestication of co-digesting hydrolytic bacteria. Among the groups receiving biochar supplementation, the relative abundance of Chloroflexota notably increased, with the exception of the PS2 group, which exhibited a relative abundance of 11.8%. In contrast, the PS1, PS3, PS4, and PS5 groups displayed relative abundances of 13.2%, 14.1%, 13.5%, and 13.8%, respectively. The pH regulation and adsorption capacity of biochar contribute to the enrichment of Chloroflexota. Chloroflexota has been primarily associated with acetic acid synthesis, a process that creates conditions favorable for the utilization of acetic acid by other microorganisms [60,61]. This finding aligns with the observed shifts in VFA concentrations. As shown in Figure 10b, the relative abundance of Euryarchaeota was the highest in each group, with values of 0.89% (control), 0.88% (PS1), 0.87% (PS2), 0.87% (PS3), 0.89% (PS4), and 0.88% (PS5), respectively. The addition of peanut straw biochar slightly decreased the relative abundance of Euryarchaeota by the end of AD. This decrease was likely due to the enhanced degradation efficiency of the digestion substrate facilitated by peanut straw biochar, which reduced the amount of substrate and available intermediates in the reactors of each group by day 32, thereby leading to a corresponding decrease in Euryarchaeota abundance.
At the genus level, the following bacterial genera were identified: Odoribacter, Lentimicrobium, Anaerolinea, Bacteroides, Smithella, Longilinea, and Mariniphaga (Figure 11a). With the exception of the PS1 group, the abundance of Bacteroides in the biochar-amended groups was slightly lower than that observed in the control group. Bacteroides, a group of anaerobic bacteria capable of hydrolyzing proteins, has been shown to facilitate the breakdown of proteins into soluble peptides, with a decrease in its abundance correlating with changes in soluble protein concentrations (Figure 9). AD continued until day 32, by which point the concentration of dissolved polysaccharides in the reactor was low. At this time, the relative abundance of Lentimicrobium decreased in the PS3, PS4, and PS5 groups compared to the control group. Lentimicrobium belongs to the Bacteroidetes phylum, which is known for its capacity to degrade carbohydrates under anaerobic conditions, the hydrolysis phase of AD. This finding suggests enhanced carbohydrate degradation in the PS3, PS4, and PS5 groups, accompanied by a decline in Lentimicrobium abundance due to the reduced availability of carbohydrates [62].
The archaeal population is primarily composed of Methanothrix, a specialized acetate-type methanogen capable of functioning as an electron acceptor (Figure 11b). This organism is involved in direct interspecies electron transfer, which enhances methanogenic performance [57,63]. Other methanogenic genera, including Methanospirillum, Methanomassiliicoccus, Methanosarcina, Methanolinea, and Methanoregula, are also present within the system. The PS4 group exhibited the highest relative abundance of Methanosarcina, a genus known for utilizing acetic acid in methane production [64]. This observation aligns with cumulative methane production and variations in acetic acid concentrations within this group. The lower abundance of Methanothrix in the PS4 group was attributed to the reduced concentration of VFAs at the end of AD, which limited the nutrients available to Methanothrix and resulted in decreased activity. Overall, the biochar-amended groups displayed only minor differences in species composition compared to the control group. However, moderate biochar supplementation led to significant alterations in bacterial community abundance.
4. Conclusions
Peanut straw biochar has been shown to enhance the performance of AD, thereby increasing methane production efficiency. Incorporating peanut straw biochar at an 8% inclusion rate (based on the mass of the digested substrate) resulted in the highest cumulative methane production, exhibiting a 19.1% increase compared to the control group. Analysis using the modified Gompertz model revealed that the addition of peanut straw biochar improves methane production performance, increases the maximum methane production potential and rate, and reduces the reaction cycle time. Furthermore, peanut straw biochar has been shown to accelerate the production and consumption of acetic acid, enhance the utilization efficiency of VFAs by methanogenic bacteria, and decrease the residual VFA content at the end of the AD process. Additionally, the inclusion of 8% peanut straw biochar increased the relative abundance of Methanosarcina by 2.8%, while the addition of 6% peanut straw biochar elevated the relative abundance of Chloroflexota by 1.4%. These changes contribute to the strengthening of the hydrolysis, acidification, and methanogenesis phases of AD. However, because this study was conducted under laboratory conditions using batch experiments, the results cannot be directly applied to real-world engineering scenarios. To enhance the applicability of the findings to practical engineering, future research will focus on the operation of continuous AD reactors.
Author Contributions
Conceptualization, J.H., Y.X. and W.X.; validation, J.H., H.Y., W.X. and Z.W.; formal analysis, J.H., H.Y. and Z.W.; investigation, X.K. and H.L.; resources, X.K.; data curation, C.L.; writing—original draft preparation, J.H. and Y.X.; writing—review and editing, H.L. and C.L.; visualization, Y.X.; supervision, H.L.; project administration, H.L. and X.K.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project of the Research and Demonstration Application of Key Technologies for Water Environment Management of Zhangcun River (No. 23-2-7-zdfn-2-nsh).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Xingsheng Kang was employed by the company Shandong Environmental Protection Science Research and Design Institute Co., Ltd. Author Hao Liu was employed by the company Qingdao Water Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kadam, R.; Jo, S.; Lee, J.; Khanthong, K.; Jang, H.; Park, J. A Review on the Anaerobic Co-Digestion of Livestock Manures in the Context of Sustainable Waste Management. Energies 2024, 17, 546. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Sun, C. A review of methane production from agricultural residues in China. Renew. Sustain. Energy Rev. 2016, 54, 857–865. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, Y.; Liu, Y. State of the art of straw treatment technology: Challenges and solutions forward. Bioresour. Technol. 2020, 313, 123656. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Du, X.; Gao, T.; Cheng, Z.; Fu, W.; Wang, S. Ammonia inhibition in anaerobic digestion of organic waste: A review. Int. J. Environ. Sci. Technol. 2025, 22, 3927–3942. [Google Scholar] [CrossRef]
- Akshaya, K.; Selvasembian, R. Insights into the recent advances of chemical pretreatment of waste activated sludge to enhance biomethane production. J. Environ. Chem. Eng. 2024, 12, 113999. [Google Scholar] [CrossRef]
- Aklilu, E.G.; Waday, Y.A. Optimizing the process parameters to maximize biogas yield from anaerobic co-digestion of alkali-treated corn stover and poultry manure using artificial neural network and response surface methodology. Biomass Convers. Biorefin. 2023, 13, 12527–12540. [Google Scholar] [CrossRef]
- Guan, S.; He, C.; Li, P.; Li, P.; Hou, T.; Gao, Z.; Li, G.; Jiao, Y. Enhancement of Anaerobic Digestion of Corn Straw: Effect of Biological Pretreatment and Heating with Bio-Heat Recovery from Pretreatment. Fermentation 2024, 10, 160. [Google Scholar] [CrossRef]
- Liu, M.R.; Wei, Y.Q.; Leng, X.Y. Improving biogas production using additives in anaerobic digestion: A review. J. Clean. Prod. 2021, 297, 126666. [Google Scholar] [CrossRef]
- Bhujbal, S.K.; Ghosh, P.; Vijay, V.K.; Kumar, M. Ruminal content biochar supplementation for enhanced biomethanation of rice straw: Focusing on biochar characterization and dose optimization. Sci. Total Environ. 2023, 905, 167250. [Google Scholar] [CrossRef]
- Chen, B.; Zeng, H.; Yang, F.; Yang, Y.F.; Qiao, Z.; Zhao, X.L.; Wang, L.; Wu, F.C. Functional biochar as sustainable precursors to boost the anaerobic digestion of waste activated sludge from a circular economy perspective: A review. Biochar 2024, 6, 60. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Sadani, M.; Shahsavani, A.; Bakhshoodeh, R.; Alavi, N. Enhancing anaerobic digestion of automotive paint sludge through biochar addition. Heliyon 2023, 9, e17640. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, X.; Liu, Z.; Guo, Z.; Zhu, L.; Xiong, B.; Jiang, D.; Shen, L.; Li, M.; Kang, B.; et al. Biochar improves heavy metal passivation during wet anaerobic digestion of pig manure. Environ. Sci. Pollut. Res. 2021, 28, 635–644. [Google Scholar] [CrossRef]
- He, Z.W.; Li, A.H.; Tang, C.C.; Zhou, A.J.; Liu, W.Z.; Ren, Y.X.; Li, Z.; Wang, A.J. Biochar regulates anaerobic digestion: Insights to the roles of pore size. Chem. Eng. J. 2024, 480, 148219. [Google Scholar] [CrossRef]
- Xiao, F.; Cheng, J.; Cao, W.; Yang, C.; Chen, J.; Luo, Z. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019, 540, 579–584. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of adsorption and functionalization of biochar for pesticides: A review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef]
- Novais, S.V.; Zenero, M.D.O.; Tronto, J.; Conz, R.F.; Cerri, C.E.P. Poultry manure and sugarcane straw biochars modified with MgCl2 for phosphorus adsorption. J. Environ. Manag. 2018, 214, 36–44. [Google Scholar] [CrossRef]
- Cao, Y.; Jing, Y.; Hao, H.; Wang, X. Changes in the physicochemical characteristics of peanut straw biochar after freeze-thaw and dry-wet aging treatments of the biomass. BioResources 2019, 14, 4329–4343. [Google Scholar] [CrossRef]
- Shi, R.-Y.; Hong, Z.-N.; Li, J.-Y.; Jiang, J.; Kamran, M.A.; Xu, R.-K.; Qian, W. Peanut straw biochar increases the resistance of two Ultisols derived from different parent materials to acidification: A mechanism study. J. Environ. Manag. 2018, 210, 171–179. [Google Scholar] [CrossRef]
- Pan, J.-J.; Jiang, J.; Xu, R.-K. Removal of Cr (VI) from aqueous solutions by Na2SO3/FeSO4 combined with peanut straw biochar. Chemosphere. 2014, 101, 71–76. [Google Scholar] [CrossRef]
- Zhang, W.L.; Wang, X.; Xing, W.L.; Li, R.D.; Yang, T.H.; Yao, N.; Lv, D. Links between synergistic effects and microbial community characteristics of anaerobic co-digestion of food waste, cattle manure and corn straw. Bioresour. Technol. 2021, 329, 124919. [Google Scholar] [CrossRef] [PubMed]
- Joseph, G.; Zhang, B.; Rahman, Q.M.; Wang, L.J.; Shahbazi, A. Two-stage thermophilic anaerobic co-digestion of corn stover and cattle manure to enhance biomethane production. J. Environ. Sci. Health Part A 2019, 54, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Chaher, N.E.H.; Nassour, A.; Hamdi, M.; Nelles, M. Monitoring of Food Waste Anaerobic Digestion Performance: Conventional Co-Substrates vs. Unmarketable Biochar Additions. Foods 2021, 10, 2353. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Zang, S.Y.; Xu, J.L.; Sheng, L.X. Dynamic simulation analysis of city tail water treatment by constructed wetland with biochar substrate. Environ. Sci. Pollut. Res. 2023, 30, 108582–108595. [Google Scholar] [CrossRef]
- Liang, X.F.; Zhou, W.L.; Yang, R.; Zhang, D.D.; Wang, H.; Li, Q.Z.; Li, Y.; Lin, W. Microbial mechanism of biochar addition to reduce N2O emissions from soilless substrate systems. J. Environ. Manag. 2023, 348, 119326. [Google Scholar] [CrossRef]
- Vayena, G.; Ghofrani-Isfahani, P.; Ziomas, A.; Grimalt-Alemany, A.; Lin, M.; Ravenni, G.; Angelidaki, I. Impact of biochar on anaerobic digestion process and microbiome composition; focusing on pyrolysis conditions for biochar formation. Renew. Energy 2024, 237, 121569. [Google Scholar] [CrossRef]
- Han, Y.H.; Li, C.; Wang, J.L.; Liu, L.N.; Ma, X.J.; Deng, Y.F. Assessing environmental impacts and carbon emissions of promising electrochemical and traditional methods of COD detection. J. Water Process Eng. 2025, 72, 107596. [Google Scholar] [CrossRef]
- Boshagh, F. Measurement methods of carbohydrates in dark fermentative hydrogen production- A review. Int. J. Hydrogen Energy 2021, 46, 24028–24050. [Google Scholar] [CrossRef]
- Li, J.B.; Lu, S.Y.; Wu, S.Q.; Zhang, W.M.; Hua, M.; Pan, B.C. The breakdown of protein hydrogen bonding networks facilitates biotransformation of protein wastewaters during anaerobic digestion methanogenesis: Focus on protein structure and conformation. Environ. Res. 2022, 208, 112735. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Fan, X.; Sangeetha, T.; Pan, K.L.; Bi, X.J.; Liu, W.; Lin, X.; Wang, X.; Wang, A.; et al. The dual role of potassium ferrate in promoting primary sludge hydrolysis and acidogenesis in anaerobic fermentation. Chem. Eng. J. 2023, 477, 147023. [Google Scholar] [CrossRef]
- Liu, X.L.; Liu, H.; Chen, Y.Y.; Dul, G.C.; Chen, J. Effects of organic matter and initial carbon-nitrogen ratio on the bioconversion of volatile fatty acids from sewage sludge. J. Chem. Technol. Biotechnol. 2008, 83, 1049–1055. [Google Scholar] [CrossRef]
- Lebrun, M.; Nandillon, R.; Miard, F.; Bourgerie, S.; Visser, R.; Morabito, D. Biochar application modifies soil properties of a former mine technosol: SEM/EDS study to investigate Pb and As speciation. Biomass Convers. Biorefin. 2024, 14, 5877–5887. [Google Scholar] [CrossRef]
- Shahrashoub, M.; Bakhtiari, S. The efficiency of activated carbon/magnetite nanoparticles composites in copper removal: Industrial waste recovery, green synthesis, characterization, and adsorption-desorption studies. Microporous Mesoporous Mater. 2021, 311, 110692. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef] [PubMed]
- Danhassan, U.A.; Zhang, X.; Qi, R.; Ali, M.M.; Sheng, K.; Lin, H. Insight into synthesis and catalytic performance of mesoporous electroactive biochar for aqueous sulfide adsorptive oxidation. J. Environ. Chem. Eng. 2023, 11, 110619. [Google Scholar] [CrossRef]
- Tarimo, D.J.; Oyedotun, K.O.; Sylla, N.F.; Mirghni, A.A.; Ndiaye, N.M.; Manyala, N. Waste chicken bone-derived porous carbon materials as high performance electrode for supercapacitor applications. J. Energy Storage 2022, 51, 104378. [Google Scholar] [CrossRef]
- Saif, I.; Alsaiari, M.; Jalalah, M.; Harraz, F.A.; Su, S.C.; Salama, E.; Li, X.K. Magnetic chicken bone biochar mediated anaerobic co-digestion of lignocellulosic biomass for energy enhancement and microbial synergism. Fuel 2024, 362, 130794. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, Z.; Feng, R.; Zhang, Y. Characterization of corncob-derived biochar and pyrolysis kinetics in comparison with corn stalk and sawdust. Bioresour. Technol. 2014, 170, 76–82. [Google Scholar] [CrossRef]
- Guo, X.; Li, C.; Zhu, Q.; Huang, T.; Cai, Y.; Li, N.; Liu, J.; Tan, X. Characterization of dissolved organic matter from biogas residue composting using spectroscopic techniques. Waste Manag. 2018, 78, 301–309. [Google Scholar] [CrossRef]
- Huang, H.-J.; Yang, T.; Lai, F.-Y.; Wu, G.-Q. Co-pyrolysis of sewage sludge and sawdust/rice straw for the production of biochar. J. Anal. Appl. Pyrolysis 2017, 125, 61–68. [Google Scholar] [CrossRef]
- Carvalho, J.; Araujo, J.; Castro, F. Alternative Low-cost Adsorbent for Water and Wastewater Decontamination Derived from Eggshell Waste: An Overview. Waste Biomass Valorization 2011, 2, 157–167. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of trace element supplementation on the performance of anaerobic digestion process: A critical review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Jing, Y.; Feng, J.; Luo, J.; Yu, J.; Zhao, L. Performance of enhanced anaerobic digestion with different pyrolysis biochars and microbial communities. Bioresour. Technol. 2020, 296, 122354. [Google Scholar] [CrossRef]
- Cimon, C.; Kadota, P.; Eskicioglu, C. Effect of biochar and wood ash amendment on biochemical methane production of wastewater sludge from a temperature phase anaerobic digestion process. Bioresour. Technol. 2020, 297, 122440. [Google Scholar] [CrossRef]
- Başar, İ.A.; Eskicioglu, C.; Perendeci, N.A. Biochar and wood ash amended anaerobic digestion of hydrothermally pretreated lignocellulosic biomass for biorefinery applications. Waste Manag. 2022, 154, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cao, H.; Luo, H.; Chen, W.; Chen, J. Enhanced MFC sensor performances and extracellular electron transport efficiency mediated by biochar and underlying biochemical mechanisms. J. Environ. Manag. 2023, 332, 117282. [Google Scholar] [CrossRef]
- Xu, F.; Mu, L.; Wang, Y.; Peng, H.; Tao, J.; Chen, G. Pretreatment with rumen fluid improves methane production in the anaerobic digestion of corn straw. Fuel 2024, 363, 130831. [Google Scholar] [CrossRef]
- Liu, Y.C.; Whitman, W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. In Incredible Anaerobes: From Physiology to Genomics to Fuels; Wiegel, J., Maier, R.J., Adams, M.W.W., Eds.; New York Academy of Sciences: New York, NY, USA, 2008; Volume 1125, pp. 171–189. [Google Scholar]
- Conrad, R. Microbial Ecology of Methanogens and Methanotrophs. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2007; Volume 96, pp. 1–63. [Google Scholar]
- Pan, X.F.; Zhao, L.; Li, C.X.; Angelidaki, I.; Lv, N.; Ning, J.; Cai, G.; Zhu, G.F. Deep insights into the network of acetate metabolism in anaerobic digestion: Focusing on syntrophic acetate oxidation and homoacetogenesis. Water Res. 2021, 190, 116774. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, N.; Liu, X.; Dong, X. Methanogenesis from Methanol at Low Temperatures by a Novel Psychrophilic Methanogen, “Methanolobus psychrophilus” sp. nov.; Prevalent in Zoige Wetland of the Tibetan Plateau. Appl. Environ. Microbiol. 2008, 74, 6114–6120. [Google Scholar] [CrossRef]
- Duan, X.; Chen, Y.; Feng, L.; Zhou, Q. Metagenomic analysis reveals nonylphenol-shaped acidification and methanogenesis during sludge anaerobic digestion. Water Res. 2021, 196, 117004. [Google Scholar] [CrossRef]
- Geng, H.; Xu, Y.; Dai, X.; Yang, D. Abiotic and biotic roles of metals in the anaerobic digestion of sewage sludge: A review. Sci. Total Environ. 2024, 912, 169313. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, Y.; Sima, J.; Zhao, L.; Mašek, O.; Cao, X. Indispensable role of biochar-inherent mineral constituents in its environmental applications: A review. Bioresour. Technol. 2017, 241, 887–899. [Google Scholar] [CrossRef]
- Codignole Luz, F.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biochar characteristics and early applications in anaerobic digestion-a review. J. Environ. Chem. Eng. 2018, 6, 2892–2909. [Google Scholar] [CrossRef]
- Zhou, W.N.; Mazarji, M.; Li, M.T.; Li, A.H.; Wang, Y.J.; Yang, Y.D.; Lee, J.T.E.; Rene, E.R.; Yuan, X.; Pan, J.T. Exploring magnetic nanomaterials with a focus on magnetic biochar in anaerobic digestion: From synthesis to application. Biochar 2024, 6, 63. [Google Scholar] [CrossRef]
- Cao, J.S.; Zhang, Q.; Wu, S.; Luo, J.Y.; Wu, Y.; Zhang, L.L.; Feng, Q.; Fang, F.; Xue, Z.X. Enhancing the anaerobic bioconversion of complex organics in food wastes for volatile fatty acids production by zero-valent iron and persulfate stimulation. Sci. Total Environ. 2019, 669, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Iino, T.; Mori, K.; Suzuki, K. Methanospirillum lacunae sp. nov.; a methane-producing archaeon isolated from a puddly soil, and emended descriptions of the genus Methanospirillum and Methanospirillum hungatei. Int. J. Syst. Evol. Microbiol. 2010, 60, 2563–2566. [Google Scholar] [CrossRef][Green Version]
- Yashiro, Y.; Sakai, S.; Ehara, M.; Miyazaki, M.; Yamaguchi, T.; Imachi, H. Methanoregula formicica sp. nov.; a methane-producing archaeon isolated from methanogenic sludge. Int. J. Syst. Evol. Microbiol. 2011, 61, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, T.; Yin, J.; Shen, D. Effect of nano-magnetite on the propionic acid degradation in anaerobic digestion system with acclimated sludge. Bioresour. Technol. 2021, 334, 125143. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Yuan, X.; Wang, X.; Zhu, W.; Yang, F.; Cui, Z. Effect of dairy manure to switchgrass co-digestion ratio on methane production and the bacterial community in batch anaerobic digestion. Appl. Energy 2015, 151, 249–257. [Google Scholar] [CrossRef]
- Qi, Y.; Evans, P.N.; Li, Y.; Rao, Y.; Qu, Y.; Tan, S.; Jiao, J.; Chen, Y.; Hedlund, B.P.; Shu, W.; et al. Comparative Genomics Reveals Thermal Adaptation and a High Metabolic Diversity in “Candidatus Bathyarchaeia”. mSystems 2021, 6, e0025221. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Z.; Yang, Y.; Zhang, Y. Dual roles of zero-valent iron in dry anaerobic digestion: Enhancing interspecies hydrogen transfer and direct interspecies electron transfer. Waste Manag. 2020, 118, 481–490. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).