Abstract
The hydrogenation of CO2 to methanol is an effective approach for utilizing carbon resources. Cu-ZnO-based catalysts have attracted significant attention due to their ability to activate CO2; however, improving methanol selectivity remains a challenge. In this study, the incorporation of an appropriate amount of Ga into Cu-ZnO catalysts, resulting in the formation of spinel ZnGa2O4 crystals, significantly enhances the conversion of CO2 to methanol. Ternary CuZnGa composite oxides with varying Ga contents were synthesized, and their effects on CO2 hydrogenation were investigated. The optimal Cu6Zn3Ga1 catalyst achieved a CO2 conversion rate of 13% and a methanol selectivity of 59% under reaction conditions of 240 °C, 4 MPa, and a GHSV of 7500 mL⋅gcat−1⋅h−1. In contrast, the undoped Cu6Zn4 catalyst exhibited a lower CO2 conversion of 9.8% and a methanol selectivity of 38%. Characterization results indicate that the introduction of Ga promotes the formation of oxygen vacancies, enhances CO2 activation, and facilitates electronic interactions between spinel ZnGa2O4 and Cu sites, thereby improving methanol production. Furthermore, the spinel ZnGa2O4-modified Cu catalyst demonstrated excellent stability over 90 h of continuous operation. This study presents a novel approach to designing spinel ZnGa2O4-modified Cu-ZnO-based catalysts and offers a new strategy for enhancing CO2 hydrogenation to methanol.
1. Introduction
The burning of fossil fuels releases large amounts of CO2 into the atmosphere, and the rapid increase in CO2 has caused many environmental problems, such as ocean acidification, climate warming, and more frequent volcanic eruptions [1,2,3,4,5]. Many researchers are exploring ways to store and change CO2 into valuable chemicals [6,7]. CO2 is a safe and non-toxic C1 molecule that can be readily transformed into high-value chemicals, like CH4 [8], CH3OH [9,10,11], C2H5OH [12], olefins [13], aromatics [14], and others. One promising process is the catalytic hydrogenation of CO2 to methanol. Methanol serves as a crucial C1 platform molecular, facilitating the production of fuels and other valuable chemicals [15,16]. As an important bridge between CO2 and high-value-added chemicals, methanol is considered a key component in achieving “carbon neutrality” [17]. The hydrogenation of CO2 to methanol can help mitigate the conflict between economic development and sustainability, offering significant economic and energy value.
Since methanol synthesis and reverse water–gas shift (RWGS) reactions are competing processes, achieving a balance between high CO2 conversion and high methanol selectivity is particularly important [18]. CO2 hydrogenation to methanol is an exothermic reaction, while the competitive RWGS reaction is endothermic. Therefore, from a thermodynamic perspective, low temperature and high pressure favor methanol formation during CO2 hydrogenation [19]. Cu-ZnO-based catalysts are commonly used for methanol synthesis from CO2/H2 and have been extensively studied in the literature [20,21,22,23,24]. However, a significant challenge with these catalysts is that water produced during the reaction accelerates the sintering of Cu, the active component in the CO2 hydrogenation process, leading to poor stability [25]. Developing rational strategies for catalyst optimization remains a critical challenge.
The spinel structure has shown great potential in CO2 hydrogenation to methanol due to its unique properties [26]. It has been reported that surface-dissociative ZnO and ZnCr2O4 spinel structures promote methanol synthesis rates and inhibit CO formation from the RWGS reaction [19]. Furthermore, spinel CuAl2O4 and MgAl2O4 have also been found to increase the number of active basic sites for CO2 adsorption and activation, thereby enhancing methanol selectivity [27]. In addition, spinel ZnFe2O4-supported Cu catalysts with tunable Cu nanoparticle sizes have shown increased methanol selectivity due to the generation of additional Cu+ sites at Cu–spinel interfaces, which markedly inhibit the reverse water–gas shift reaction during CO2 hydrogenation [28]. More recently, Cu/ZnAl2O4 prepared by the ammonia evaporation method was also reported to improve methanol synthesis in CO2 hydrogenation by regulating the interaction between spinel ZnAl2O4 and Cu sites [29].
Encouraged by these findings, a series of Cu6ZnxGay (Cu: Zn: Ga = 6: x: y, x + y = 4) catalysts with varying Ga contents were synthesized using the co-precipitation method based on unmodified Cu-ZnO catalysts. All prepared catalysts were tested for their activity in CO₂ hydrogenation to methanol and compared with Cu-ZnO catalysts without Ga. It was discovered that the addition of Ga generated spinel ZnGa2O4 crystals, creating oxygen vacancies on the catalyst surfaces. The spinel ZnGa2O4 crystals also altered the electronic interactions with Cu sites to some extent. By correlating the physicochemical properties of the catalysts with their catalytic activities, it was determined that the formation of spinel ZnGa2O4 in CuZnGa ternary composite oxide catalysts effectively promotes CO2 hydrogenation to methanol. This work provides valuable insights for understanding and designing highly active catalysts for CO2 hydrogenation to methanol.
2. Materials and Methods
2.1. Material Preparation
All chemicals that were used in this study were used as received without further purification. Copper nitrate trihydrate (Cu(NO3)2⋅3H2O, 99.9 wt.%) was purchased from Thermo Fisher Scientific. Zinc nitrate hexahydrate (Zn(NO3)2⋅6H2O, 99.9 wt.%) was purchased from Alfa Aesar (Shanghai, China) Chemical Science Co. Gallium nitrate hydrate (Ga(NO3)3⋅xH2O, 99.9 wt.%) was purchased from Beijing Innochem Technology Co., Beijing China. Sodium carbonate (Na2CO3) was acquired from Sinopharm Chemical Reagent Co. Ltd., Shanghai, China. We made our own deionized water for the experiments.
2.2. Catalyst Preparation
All catalysts in this experiment were prepared by the co-precipitation method. The metal precursors were the corresponding metal nitrates: Cu(NO3)2⋅3H2O, Zn(NO3)2⋅6H2O, and Ga(NO3)3⋅xH2O. Sodium carbonate solution was used as a precipitant, and the concentration of the solution was controlled to be 0.1 mol/L. In the case of Cu6Zn3Ga1, 7.248 g of Cu(NO3)2⋅3H2O, 4.4621 g of Zn(NO3)2⋅6H2O, and 1.2787 g of Ga(NO3)3⋅xH2O were weighed and mixed with 200 mL of deionized water. A small amount of Na2CO3 was also weighed and mixed with 150 mL of deionized water to keep the solution’s concentration at 0.1 mol/L. The catalysts were prepared in accordance with the following conditions, which were controlled to be 0.1 mol/L. Using a dispensing funnel, the base and salt solutions were added at the same time to a beaker with 200 mL of deionized water. The pH was kept at 7 during the titration, and the mixture was stirred while it was being added. The entire co-precipitation process was carried out in a thermostatic water bath at 70 °C, and after the precipitation was completed, the resulting suspension continued to be aged with stirring at this temperature for 2 h. After aging, it was pump-filtered and washed with large amounts of deionized water to remove the impurity Na+ and finally dried at 110 °C overnight. The obtained precursor was calcined in a muffle furnace at a ramp rate of 2 °C/min for 3 h at 500 °C to obtain the sample, noted as Cu6Zn3Ga1. Cu6Zn4, Cu6Zn3.5Ga0.5, and Cu6Zn2.5Ga1.5 composite oxides were prepared by the same method.
2.3. Characterization
The crystal structure of various catalysts was tested by an X-ray powder diffractometer (XRD, Bruker D8 Advance). The scanning range was 5°–80°, and the scanning rate was 5 °/min. Scanning transmission electron microscopy (STEM) and energy-dispersive X-ray spectroscopy (EDS) analyses were carried out on a JEOL JEM-F200 filed-emission electron microscope with an acceleration voltage of 200 kV. X-ray photoelectron spectroscopy (XPS) was carried out on a Thermo Fisher Scientific (Waltham, MA, USA) K-Alpha+ apparatus equipped with a mono Al Kα radiation. The binding energy shift due to surface charging was adjusted to the standard of the C 1s line at 284.8 eV. Temperature-programmed reduction of H2 (H2-TPR) and temperature-programmed desorption of CO2 (CO2-TPD) were conducted on a Micromeritics Autochem II chemisorption analyzer. During TPR, the reduction profile of the catalyst was recorded from 50 to 800 °C (heating rate: 10 °C/min) in a 5% H2/Ar (in volume, 30 mL/min) mixed gas. For TPD, the catalyst was pretreated at 400 °C with 5% H2/Ar flow for 60 min and then cooled to 50 °C. Subsequently, it was switched to argon purge for 30 min, followed by the introduction of a 10% CO2/Ar gas mixture adsorption for 60 min. Then, argon was switched, and the gas-phase H2 was purged from the catalyst for 60 min. Finally, the desorption profile from 50 to 500 °C was recorded in the argon stream after waiting for the baseline to stabilize.
2.4. Catalytic Activity Test
All prepared catalysts were tested and evaluated for CO2 hydrogenation catalytic activity in a high-pressure fixed-bed reactor. Typically, 0.24 g of catalyst was loaded into a quartz tube prior to reaction testing, and pure H2 was introduced at a rate of 30 mL/min for 3 h at 400 °C for reduction. After the end of reduction and waiting for the reactor to cool down to room temperature, a reaction gas mixture with an H2/CO2 ratio of 3/1 was introduced into the reactor to start the experiment. The flow rate of all gases was controlled by a mass flow controller, and the line temperature was maintained at 150 °C to avoid condensation of the gas products. The products were analyzed using online gas chromatography (GC2060) equipped with a flame ionization detector (FID) and a thermal conductivity detector (TCD) for online detection. TCD used He as a carrier gas to analyze Ar, CO, and CO2, and FID used N2 as a carrier gas to analyze alcohols and other carbon-containing compounds, with 8% Ar as an internal standard. All test data were collected 2 h after the start of the reaction, and each catalyst sample was tested three times to ensure the reproducibility of the experiments.
3. Results and Discussion
3.1. Phases of Catalyst Samples
The XRD patterns of the catalyst phase structures prepared by the co-precipitation method are shown in Figure 1. The Cu₆Zn₄ catalyst exhibits characteristic peaks corresponding to ZnO (JCPDS 79-2205) and CuO (JCPDS 80-1916), with no additional crystalline phases detected. Due to the higher Cu content compared to Zn in this catalyst, the XRD pattern is dominated by the characteristic peaks of CuO. Upon the introduction of Ga, a ZnGa2O4 (JCPDS 38-1240) spinel structure appears [30], while some ZnO diffraction peaks disappear. The main diffraction peak of ZnGa2O4 is observed at 2θ = 35.7°, which partially overlaps with the primary diffraction peak of CuO. This may indicate the formation of a ZnGa2O4 spinel structure with a small amount of Ga and suggests that the ZnGa2O4 crystallite size is below the detection limit. The absence of ZnGa2O4 as a dominant diffraction peak also implies that the spinel phase is well-dispersed within the catalyst. Further increasing the Ga content results in no significant changes in the XRD patterns of Cu6Zn3Ga1 and Cu6Zn2.5Ga1.5, with CuO and ZnO remaining as the predominant phases. The simultaneous presence of CuO, ZnO, and ZnGa2O4 diffraction lines indicates the formation of a mixed crystalline phase, which is further confirmed by HRTEM analysis. However, regardless of the Ga content, no apparent structural changes to the Cu6Zn4 catalyst are observed in the XRD patterns.
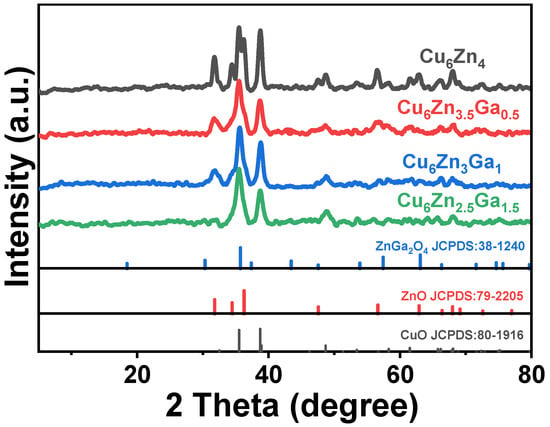
Figure 1.
XRD diffraction patterns of the samples.
To investigate the morphology and crystalline phase transition of the catalysts, TEM and HRTEM scanning were performed for Cu6Zn4 and Cu6Zn3Ga1 catalysts. As shown in Figure 2a,b,d,e, both Cu6Zn4 and Cu6Zn3Ga1 exhibit well-defined particle structures. In Cu6Zn4, the lattice spacing of 0.232 nm and 0.261 nm corresponds to the CuO (111) and ZnO (002) facets, respectively. The CuO (111) facet is also observed on Cu6Zn3Ga1, while ZnO is present in the form of the (111) facet with a lattice spacing of 0.246 nm. Additionally, the ZnGa2O4 (222) facet with a lattice spacing of 0.241 nm is identified. However, the (222) facet is not the strongest characteristic diffraction peak, which is typically observed at the (311) facet. This could be due to the small amount of Ga added, which results in the formation of a partial ZnGa2O4 spinel phase that deviates from the standard stoichiometric ratio. Therefore, the observed (222) peak is not the dominant diffraction peak. EDX mapping analysis of both catalysts, shown in Figure 2c,f, reveals that the elements are uniformly distributed, indicating the high stability of the catalysts and the effectiveness of the sample preparation method.
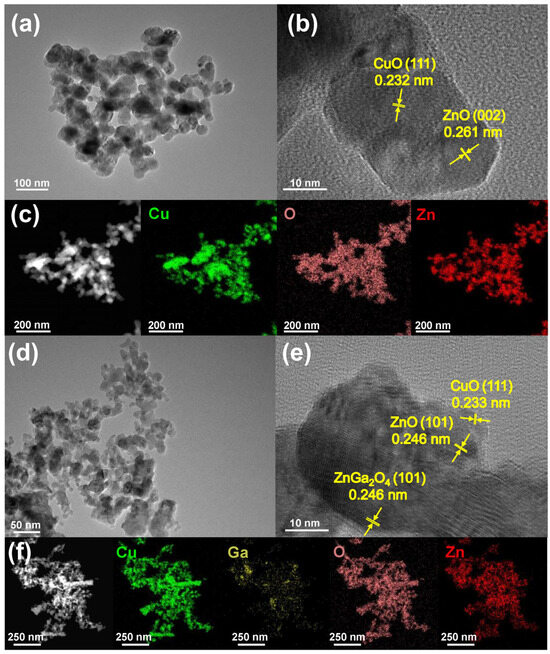
Figure 2.
(a) TEM, (b) HRTEM, and (c) HAADF-STEM images as well as corresponding element mapping of Cu6Zn4 catalyst. (d) TEM, (e) HRTEM, and (f) HAADF-STEM images as well as corresponding element mapping of Cu6Zn3Ga1 catalyst.
3.2. Electronic Structure of Catalysts
The surface chemical environments of four elements (O, Cu, Zn, and Ga) were investigated using XPS. As shown in Figure 3a, the surface oxygen vacancies of the catalysts were analyzed using O 1s XPS spectra. Oxygen vacancies were quantified by deconvoluting the O 1s XPS spectra into three components: lattice oxygen (~530.0 eV), oxygen near surface vacancies (~531.0 eV), and hydroxyl groups (~532.0 eV) [31,32]. The introduction of Ga significantly increased the oxygen vacancies, with the Cu6Zn3Ga1 catalyst showing the highest vacancy concentration (36.1%) at an optimal Ga content. However, the oxygen vacancy concentration decreased when excess Ga was added, suggesting that oxygen vacancies play a critical role in CO2 adsorption and activation, thereby promoting CO2 conversion [33]. Subsequently, XPS analysis of Cu, Zn, and Ga was performed. As shown in Figure 3b, the binding energies of Cu 2p1/2 and Cu 2p3/2 were approximately 953.22 eV and 933.3 eV, respectively. With increasing Ga content, the binding energies of Cu shifted first to higher and then to lower binding energies, indicating that Ga introduction alters the electronic state of Cu in the catalyst. Initially, Cu becomes electron-deficient, and later, it becomes electron-rich. In contrast, as shown in Figure 3c, Ga addition had minimal impact on the electronic structure of Zn, which remained in an electron-rich state. Figure 3d shows that with increasing Ga content, the electronic binding energy of Ga shifted to lower values, indicating that the surface became more electron-rich. These electronic interactions between Cu, Zn, and Ga significantly affect the adsorption and activation of CO2 and H2, thereby influencing CO2 hydrogenation to methanol [34]. Notably, rather than a single electronic interaction between two elements, the combined electronic interactions of all three elements (Cu, Zn, and Ga) govern the CO2 hydrogenation process. The changes in the electronic binding energies of these elements suggest that appropriate electronic interactions favor methanol production, whereas too-strong or too-weak interactions hinder the reaction.
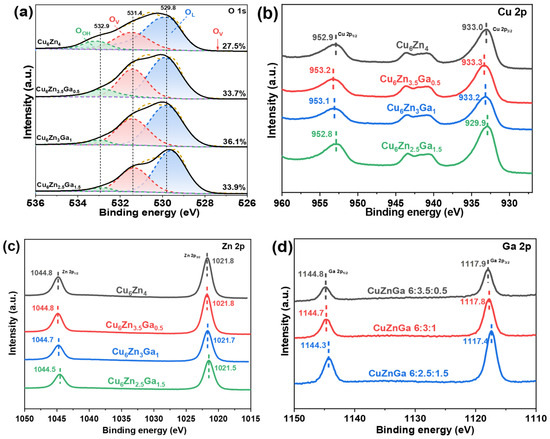
Figure 3.
(a) O 1s XPS spectra, (b) Cu 2p XPS spectra, (c) Zn 2p XPS spectra, and (d) Ga 2p XPS spectra of Cu6ZnxGay (x + y = 4) catalysts.
3.3. Adsorption and Reduction Behavior on Catalyst Surfaces
Surface adsorption and desorption of CO2 play a crucial role in CO2 hydrogenation to methanol [35]. As shown in Figure 4a, the Cu6ZnxGay catalysts, after the addition of Ga, all exhibit a low-temperature desorption peak around 105 °C, corresponding to physical desorption. There is no significant difference in the peak position across the catalysts, but the intensity and area of the peaks increase with the further addition of Ga. The Cu6Zn4 catalyst without the addition of Ga shows no low-temperature desorption peaks and only exhibits a large desorption peak near 300 °C, corresponding to chemical desorption. This suggests that modifying the Cu6Zn4 catalyst by adding Ga enhances the adsorption of CO2.
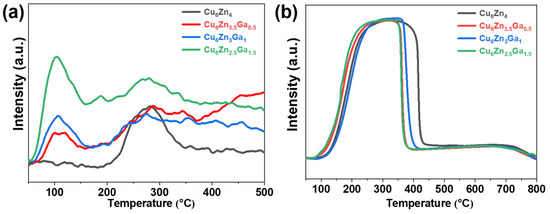
Figure 4.
(a) CO2-TPD profiles and (b) H2-TPR profiles of Cu6ZnxGay (x + y = 4) catalysts.
As shown in Figure 4b, H2-TPR was performed to study the reduction behavior of the catalysts. All the catalysts show a large reduction peak centered around 300 °C, with the largest peak area observed for Cu6Zn4. Upon the addition of Ga, the reduction peak temperature shifted to lower values, indicating that surface Ga reduces the reduction temperature and effectively promotes catalyst reduction.
3.4. Catalytic Performance
The methanol synthesis reaction (CO2 + 3H2 → CH3OH + H2O) and the RWGS reaction (CO2 + H2 → CO + H2O) are competing reactions that usually occur simultaneously [36]. Therefore, CO is considered a byproduct in activity evaluation. We conducted a detailed study on the Ga ratio, as shown in Figure 5a–d. The Cu6Zn4 catalyst without Ga catalyzed the generation of CO, which is undesired. However, upon adding Ga, the overall catalytic activity and selectivity followed a “volcano-type” trend with increasing Ga content, showing improved CO2 conversion and CH3OH selectivity compared to Cu6Zn4. The highest activity and selectivity were achieved when the Ga-to-Cu ratio formed Cu6Zn3Ga1, as shown in Tables S1–S4.
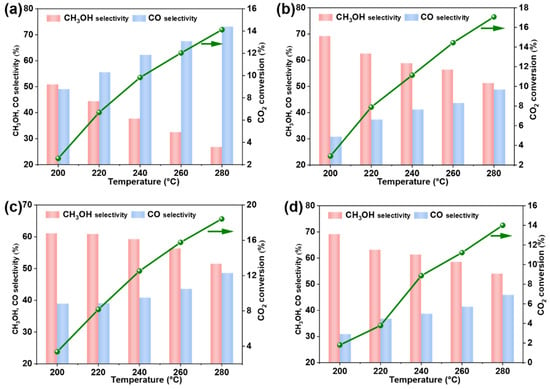
Figure 5.
(a) Cu6Zn4, (b) Cu6Zn3.5Ga0.5, (c) Cu6Zn3Ga1, and (d) Cu6Zn2.5Ga1.5 catalysts of CO2 conversion, methanol selectivity, and CO selectivity. Reaction conditions: 4 MPa, H2/CO2 = 3:1, and 7500 mL∙gcat−1∙h−1.
The activity of four catalysts with different Ga ratios at varying temperatures was tested. As expected, CO2 conversion increased with temperature, while CH3OH selectivity decreased to some extent. This behavior aligns with thermodynamic principles, where increased temperature promotes the forward reaction of CO2 hydrogenation to methanol. At 240 °C, we compared the catalytic activity of four catalysts. The Cu6Zn4 catalyst showed a CO2 conversion of 9.83%, with CO selectivity reaching 62.2%. When a small amount of Ga was added, Cu6Zn3.5Ga0.5 exhibited a CO2 conversion of 11.1% and a CH3OH selectivity of 58.8%. Increasing the Ga content further to form Cu6Zn3Ga1 led to a CO2 conversion of 12.5% and the highest CH3OH selectivity of 59.2%. However, when the Ga content exceeded this amount, as in Cu6Zn2.5Ga1.5, the catalytic performance decreased. Although CH3OH selectivity reached 61.3%, the CO2 conversion dropped significantly to 8.9%, far below that of Cu6Zn3Ga1. This indicates that an optimal Ga ratio is essential for maximizing CO2 conversion and CH3OH selectivity. Therefore, Cu6Zn3Ga1 demonstrates the best performance in terms of both CO2 conversion and CH3OH selectivity with optimal Ga incorporation.
The reaction process conditions were explored, as shown in Figure 6a,b. First, we investigated the effect of gas hourly space velocity (GHSV) on catalytic performance. GHSV is a crucial factor influencing the residence time of reactant gas molecules on the catalyst surface and the extent of the catalytic reaction. The catalytic activity of Cu6Zn3Ga1 catalysts varied under different GHSVs. As the GHSV increased from 4500 mL∙gcat−1∙h−1 to 9000 mL∙gcat−1∙h−1, CO2 conversion decreased. Similarly, CO selectivity declined with increasing GHSV, while CH3OH selectivity exhibited the opposite trend. Considering both CO2 conversion and CH3OH selectivity, the highest methanol yield was achieved at a GHSV of 7500 mL∙gcat−1∙h−1. Both higher and lower gas velocities resulted in reduced CH3OH yield. Therefore, the optimal GHSV for this experiment was determined to be 7500 mL∙gcat−1∙h−1.
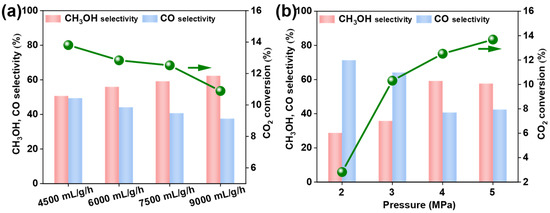
Figure 6.
The effects of gas hourly space velocity and reaction pressure on the performance of the Cu6Zn3Ga1 catalyst for CO2 hydrogenation to methanol were investigated. Reaction conditions (a): 240 °C, H2/CO2 = 3:1, and 4 MPa. Reaction conditions (b): 240 °C, H2/CO2 = 3:1, and 7500 mL∙gcat−1∙h−1.
Pressure, a key factor influencing reaction kinetics, also plays a significant role in catalytic performance [36]. In this study, the optimal reaction pressure was 4 MPa. When the pressure was lower than 4 MPa, both CO2 conversion and CH3OH selectivity were less favorable compared to the results at 4 MPa. Increasing the pressure beyond 4 MPa led to a slight decrease in CH3OH selectivity, even though CO2 conversion reached 13.7% at 5 MPa, where the CH3OH selectivity dropped to 57.6%. Given the potential impact of high pressure on reaction equipment and the limited improvement in catalytic performance with higher pressure, we selected 4 MPa as the optimal reaction pressure. At the same time, we summarized the performance of the four catalysts under the best test conditions and found that Cu6Zn3Ga1, the catalyst with the highest TOF, demonstrated the best performance (Table 1).

Table 1.
Catalytic properties of the Cu6ZnxGay catalyst.
After optimizing these conditions, a stability test under optimal conditions—240 °C, reaction pressure of 4 MPa, and air velocity of 7500 mL∙gcat−1∙h−1—was conducted, as shown in Figure 7. The catalytic performance showed only a slight decrease over the 90 h test, with the best performance occurring approximately 5 h after the reaction began. Overall, the Cu6Zn3Ga1 composite oxide catalyst demonstrated high stability.
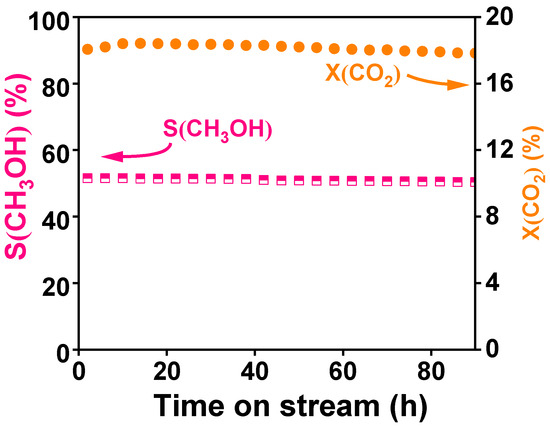
Figure 7.
Stability test for Cu6Zn3Ga1 at 240 °C, 4 MPa, and 7500 mL∙gcat−1∙h−1.
3.5. Discussion
The enhancement in catalytic activity observed upon Ga incorporation can be attributed to both structural and electronic modifications introduced by the spinel ZnGa2O4 phase. O 1s XPS analysis confirms an increase in surface oxygen vacancies, which facilitates CO2 adsorption and activation. At the same time, changes in the Cu 2p binding energies and the downward shift in H2-TPR reduction temperatures indicate electronic interactions between Ga species and Cu, promoting hydrogen activation and spillover. These dual effects—oxygen vacancy generation and electronic modulation—are likely to operate synergistically, although separating their individual contributions experimentally remains a challenge. Furthermore, the volcano-shaped trend in catalytic performance with increasing Ga content can be explained by an optimal balance between these factors. Excess Ga may lead to phase segregation or structural changes that disrupt the active Cu–Zn–Ga interface, as suggested by XRD and catalytic data, thereby lowering activity. Thus, integrating physical structure, electronic environment, and performance results supports the conclusion that Ga incorporation enhances CO2 hydrogenation to methanol through a combined mechanism.
4. Conclusions
In this work, we prepared spinel ZnGa2O4-modified Cu-ZnO catalysts using the co-precipitation method and evaluated their performance and stability for CO2 hydrogenation to methanol. The results demonstrated that the introduction of Ga facilitated the formation of the spinel crystal structure, enhancing CH3OH production and increasing CO2 conversion compared to the undoped Cu-ZnO catalyst. Characterization studies revealed that the incorporation of Ga promoted the formation of oxygen vacancies on the catalyst surface, which enhanced CO2 adsorption and the activity of carbon dioxide and increased the conversion of CO2. Additionally, spinel ZnGa2O4 altered the electron interactions, effectively driving the CO2 hydrogenation process to methanol, thereby further improving the selectivity of CH3OH. The catalysts prepared using this method exhibited excellent performance and stability during CO2 hydrogenation to methanol.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13051420/s1, Table S1: Catalytic hydrogenation of CO2 over different temperatures of Cu6Zn4 catalysts; Table S2: Catalytic hydrogenation of CO2 over different temperatures of Cu6Zn3.5Ga0.5 catalysts; Table S3: Catalytic hydrogenation of CO2 over different temperatures of Cu6Zn3Ga1 catalysts; Table S4. Catalytic hydrogenation of CO2 over different temperatures of Cu6Zn2.5Ga1.5 catalysts.
Author Contributions
X.W.: writing—review and editing, Y.Z. (Yuanshuang Zheng); writing—review and editing, Y.Z. (Yu Zhang); funding acquisition, J.Q.: methodology, L.H.; writing—original draft preparation, B.G.; resources, supervision and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (22362030, 22202173), Yunnan Fundamental Research Projects (202301AT070169, 202201AU070095), the Yunnan Revitalization Talent Support Program (XDYC-QNRC-2022-0656), and the Yunnan University Undergraduate Innovation Training Program Project (S202310673132).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
The authors would like to extend their thanks to the funding agencies for their financial backing and to Longchun Bian from Yunnan University for his assistance with TEM characterization.
Conflicts of Interest
Authors Xiulin Wang, Yuanshuang Zheng and Yu Zhang were employed by the company CNOOC Gas & Power Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Sun, Q.; Wang, N.; Yu, J. Advances in Catalytic Applications of Zeolite-Supported Metal Catalysts. Adv. Mater. 2021, 33, 2104442. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qi, Y.; Wang, F.; Han, Z.; Jiang, Y.; Han, H.; Liu, J.; Zhang, X.; Ong, W.J. State-of-the-art advancements in photo-assisted CO2 hydrogenation: Recent progress in catalyst development and reaction mechanisms. J. Mater. Chem. A 2020, 8, 24868–24894. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Szima, S.; Cormos, C.-C. Improving methanol synthesis from carbon-free H2 and captured CO2: A techno-economic and environmental evaluation. J. CO2 Util. 2018, 24, 555–563. [Google Scholar] [CrossRef]
- Bai, S.-T.; De Smet, G.; Liao, Y.; Sun, R.; Zhou, C.; Beller, M.; Maes, B.U.W.; Sels, B.F. Homogeneous and heterogeneous catalysts for hydrogenation of CO2 to methanol under mild conditions. Chem. Soc. Rev. 2021, 50, 4259–4298. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Chemical Recycling of Carbon Dioxide to Methanol and Dimethyl Ether: From Greenhouse Gas to Renewable, Environmentally Carbon Neutral Fuels and Synthetic Hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef]
- Parastaev, A.; Muravev, V.; Osta, E.H.; van Hoof, A.J.F.; Kimpel, T.F.; Kosinov, N.; Hensen, E.J.M. Boosting CO2 hydrogenation via size-dependent metal–support interactions in cobalt/ceria-based catalysts. Nat. Catal. 2020, 3, 526–533. [Google Scholar] [CrossRef]
- Beck, A.; Zabilskiy, M.; Newton, M.A.; Safonova, O.; Willinger, M.G.; van Bokhoven, J.A. Following the structure of copper-zinc-alumina across the pressure gap in carbon dioxide hydrogenation. Nat. Catal. 2021, 4, 488–497. [Google Scholar] [CrossRef]
- Han, Z.; Tang, C.; Sha, F.; Tang, S.; Wang, J.; Li, C. CO2 hydrogenation to methanol on ZnO-ZrO2 solid solution catalysts with ordered mesoporous structure. J. Catal. 2021, 396, 242–250. [Google Scholar] [CrossRef]
- Yao, Y.; Chang, Y.; Huang, R.; Zhang, L.; Masanet, E. Environmental implications of the methanol economy in China: Well-to-wheel comparison of energy and environmental emissions for different methanol fuel production pathways. J. Clean. Prod. 2018, 172, 1381–1390. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Liu, X.; Shao, Z.; Xia, L.; Zhong, L.; Wang, H.; Sun, Y. Tuning the interaction between Na and Co2C to promote selective CO2 hydrogenation to ethanol. Appl. Catal. B Environ. 2021, 293, 120207. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Zhang, W.; Wang, P.; Qin, Z.; Yan, W.; Dong, M.; Li, J.; Wang, J.; He, L.; et al. Selective Conversion of CO2 into Propene and Butene. Chem 2020, 6, 3344–3363. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, L.; Tan, M.; Zhang, P.; Fang, Y.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Rationally Designing Bifunctional Catalysts as an Efficient Strategy to Boost CO2 Hydrogenation Producing Value-Added Aromatics. ACS Catal. 2019, 9, 895–901. [Google Scholar] [CrossRef]
- Frusteri, F.; Migliori, M.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Aloise, A.; Giordano, G.; Bonura, G. Direct CO2-to-DME hydrogenation reaction: New evidences of a superior behaviour of FER-based hybrid systems to obtain high DME yield. J. CO2 Util. 2017, 18, 353–361. [Google Scholar] [CrossRef]
- Bahruji, H.; Armstrong, R.D.; Esquius, J.R.; Jones, W.; Bowker, M.; Hutchings, G.J. Hydrogenation of CO2 to Dimethyl Ether over Brønsted Acidic PdZn Catalysts. Ind. Eng. Chem. Res. 2018, 57, 6821–6829. [Google Scholar] [CrossRef]
- Fang, X.; Xi, Y.; Jia, H.; Chen, C.; Wang, Y.; Song, Y.; Du, T. Tetragonal zirconia based ternary ZnO-ZrO2-MOx solid solution catalysts for highly selective conversion of CO2 to methanol at High reaction temperature. J. Ind. Eng. Chem. 2020, 88, 268–277. [Google Scholar] [CrossRef]
- Jiao, F.; Bai, B.; Li, G.; Pan, X.; Ye, Y.; Qu, S.; Xu, C.; Xiao, J.; Jia, Z.; Liu, W.; et al. Disentangling the activity-selectivity trade-off in catalytic conversion of syngas to light olefins. Science 2023, 380, 727–730. [Google Scholar] [CrossRef]
- Xiong, S.; Lian, Y.; Xie, H.; Liu, B. Hydrogenation of CO2 to methanol over Cu/ZnCr catalyst. Fuel 2019, 256, 115975. [Google Scholar] [CrossRef]
- Ruland, H.; Song, H.; Laudenschleger, D.; Stürmer, S.; Schmidt, S.; He, J.; Kähler, K.; Muhler, M.; Schlögl, R. CO2 Hydrogenation with Cu/ZnO/Al2O3: A Benchmark Study. ChemCatChem 2020, 12, 3216–3222. [Google Scholar] [CrossRef]
- Ortner, N.; Lund, H.; Armbruster, U.; Wohlrab, S.; Kondratenko, E.V. Factors affecting primary and secondary pathways in CO2 hydrogenation to methanol over CuZnIn/MZrOx (La, Ti or Y). Catal. Today 2022, 387, 47–53. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Phongamwong, T.; Donphai, W.; Boonyuen, C.; Warakulwit, C.; Chareonpanich, M.; Limtrakul, J. Enhanced activity, selectivity and stability of a CuO-ZnO-ZrO2 catalyst by adding graphene oxide for CO2 hydrogenation to methanol. Chem. Eng. J. 2018, 334, 1781–1791. [Google Scholar] [CrossRef]
- Martínez-Suárez, L.; Siemer, N.; Frenzel, J.; Marx, D. Reaction Network of Methanol Synthesis over Cu/ZnO Nanocatalysts. ACS Catal. 2015, 5, 4201–4218. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, J. The chemical modification seen in the Cu/ZnO methanol synthesis catalysts. Appl. Catal. A Gen. 2000, 191, 111–129. [Google Scholar] [CrossRef]
- Wu, J.; Saito, M.; Takeuchi, M.; Watanabe, T. The stability of Cu/ZnO-based catalysts in methanol synthesis from a CO2-rich feed and from a CO-rich feed. Appl. Catal. A Gen. 2001, 218, 235–240. [Google Scholar] [CrossRef]
- Orege, J.I.; Wei, J.; Ge, Q.; Sun, J. Spinel-structured nanocatalysts: New opportunities for CO2 hydrogenation to value-added chemicals. Nano Today 2023, 51, 101914. [Google Scholar] [CrossRef]
- Dasireddy, V.D.B.C.; Neja, S.Š.; Blaž, L. Correlation between synthesis pH, structure and Cu/MgO/Al2O3 heterogeneous catalyst activity and selectivity in CO2 hydrogenation to methanol. J. CO2 Util. 2018, 28, 189–199. [Google Scholar] [CrossRef]
- Liu, T.; Xu, D.; Wu, D.; Liu, G.; Hong, X. Spinel ZnFe2O4 Regulates Copper Sites for CO2 Hydrogenation to Methanol. ACS Sustain. Chem. Eng. 2021, 9, 4033–4041. [Google Scholar] [CrossRef]
- Wang, S.; Song, L.; Qu, Z. Cu/ZnAl2O4 catalysts prepared by ammonia evaporation method: Improving methanol selectivity in CO2 hydrogenation via regulation of metal-support interaction. Chem. Eng. J. 2023, 469, 144008. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Zhou, C.; Zhou, W.; Cheng, K.; Kang, J.; Zhang, Q.; Deng, W.; Wang, Y. Selective transformation of carbon dioxide into lower olefins with a bifunctional catalyst composed of ZnGa2O4 and SAPO-34. Chem. Commun. 2018, 54, 140–143. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Shi, D.; He, S.; Zhang, L.; Yan, W.; Qin, Z.; Li, J.; Dong, M.; Wang, J.; et al. Direct Conversion of Syngas into Light Olefins with Low CO2 Emission. ACS Catal. 2020, 10, 2046–2059. [Google Scholar] [CrossRef]
- He, H.; Lin, X.; Li, S.; Wu, Z.; Gao, J.; Wu, J.; Wen, W.; Ye, D.; Fu, M. The key surface species and oxygen vacancies in MnOx(0.4)-CeO2 toward repeated soot oxidation. Appl. Catal. B Environ. 2018, 223, 134–142. [Google Scholar] [CrossRef]
- Yang, S.-C.; Pang, S.H.; Sulmonetti, T.P.; Su, W.-N.; Lee, J.-F.; Hwang, B.-J.; Jones, C.W. Synergy between Ceria Oxygen Vacancies and Cu Nanoparticles Facilitates the Catalytic Conversion of CO2 to CO under Mild Conditions. ACS Catal. 2018, 8, 12056–12066. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Z.; Guo, X.; Yan, Z.; Ban, H.; Wang, P.; Yao, R.; Li, L.; Li, C. Modulating Electronic Interaction over Zr–ZnO Catalysts to Enhance CO2 Hydrogenation to Methanol. ACS Catal. 2024, 14, 508–521. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, S.; Liu, W.; Chen, B.; Gao, X.; Wang, P.; Gao, J.; Tan, Y.; Dang, S.; Tu, W. Unraveling the regulation of Mn in Cu-ZnOx formation during methanol synthesis from syngas over Cu/ZnO/Al2O3-Mn catalysts. Appl. Catal. B Environ. 2023, 338, 122985. [Google Scholar] [CrossRef]
- Mou, J.; Fan, X.; Liu, F.; Wang, X.; Zhao, T.; Chen, P.; Li, Z.; Yang, C.; Cao, J. CO2 hydrogenation to lower olefins over Mn2O3-ZnO/SAPO-34 tandem catalysts. Chem. Eng. J. 2021, 421, 129978. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).