Facilitation of CO2 Hydrogenation to Methanol by Spinel ZnGa2O4 in Cu-ZnO Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Catalyst Preparation
2.3. Characterization
2.4. Catalytic Activity Test
3. Results and Discussion
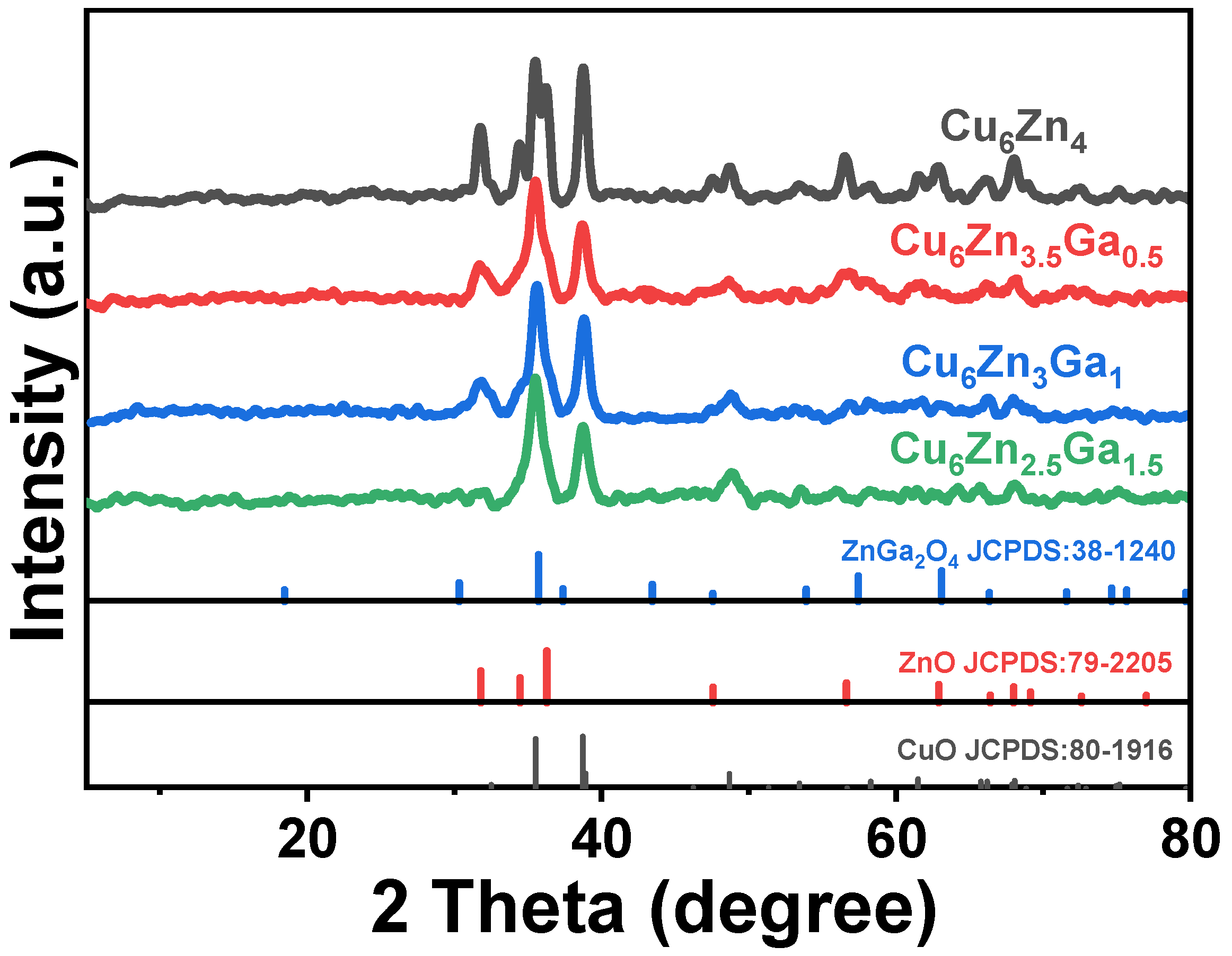
3.1. Phases of Catalyst Samples
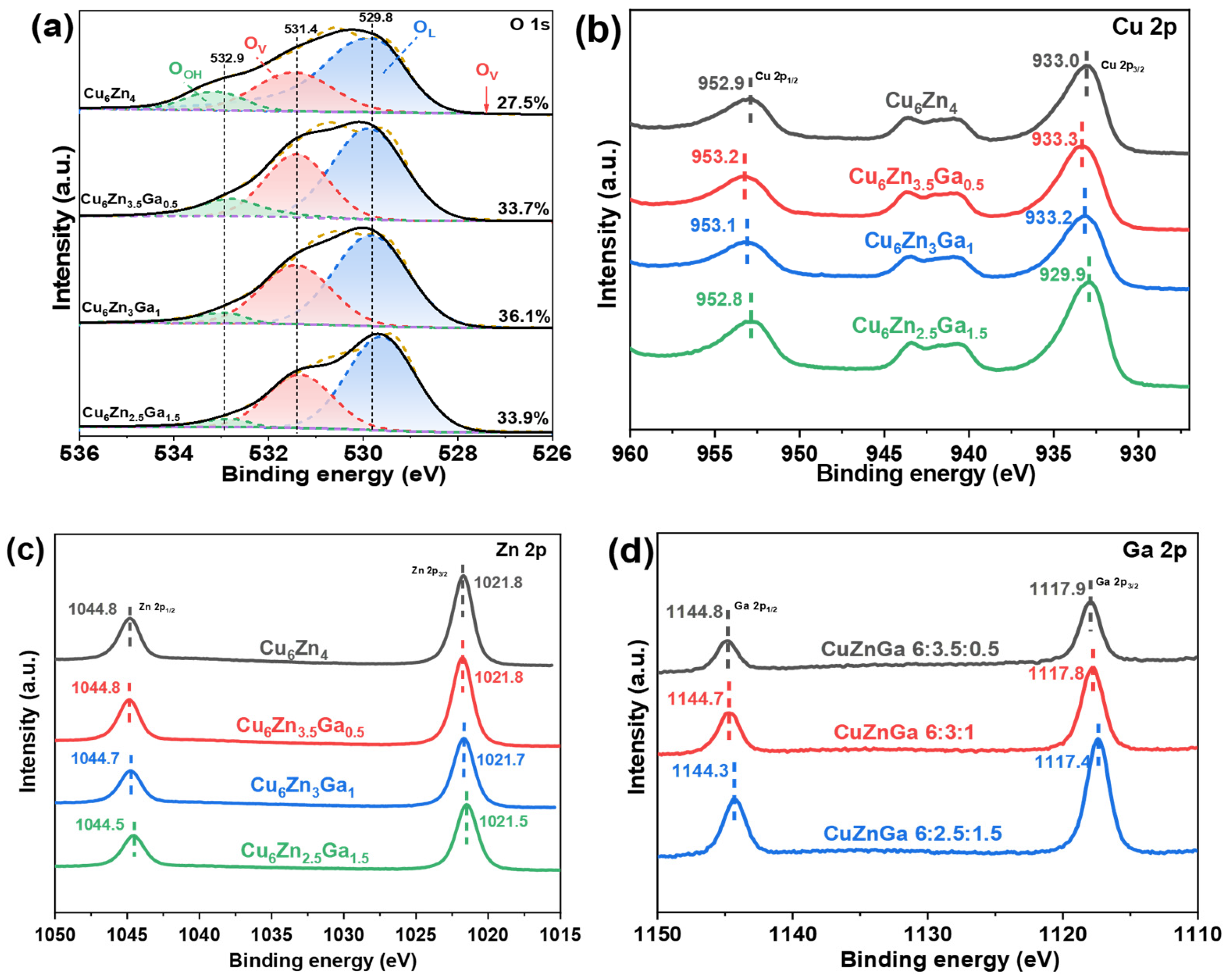
3.2. Electronic Structure of Catalysts
3.3. Adsorption and Reduction Behavior on Catalyst Surfaces
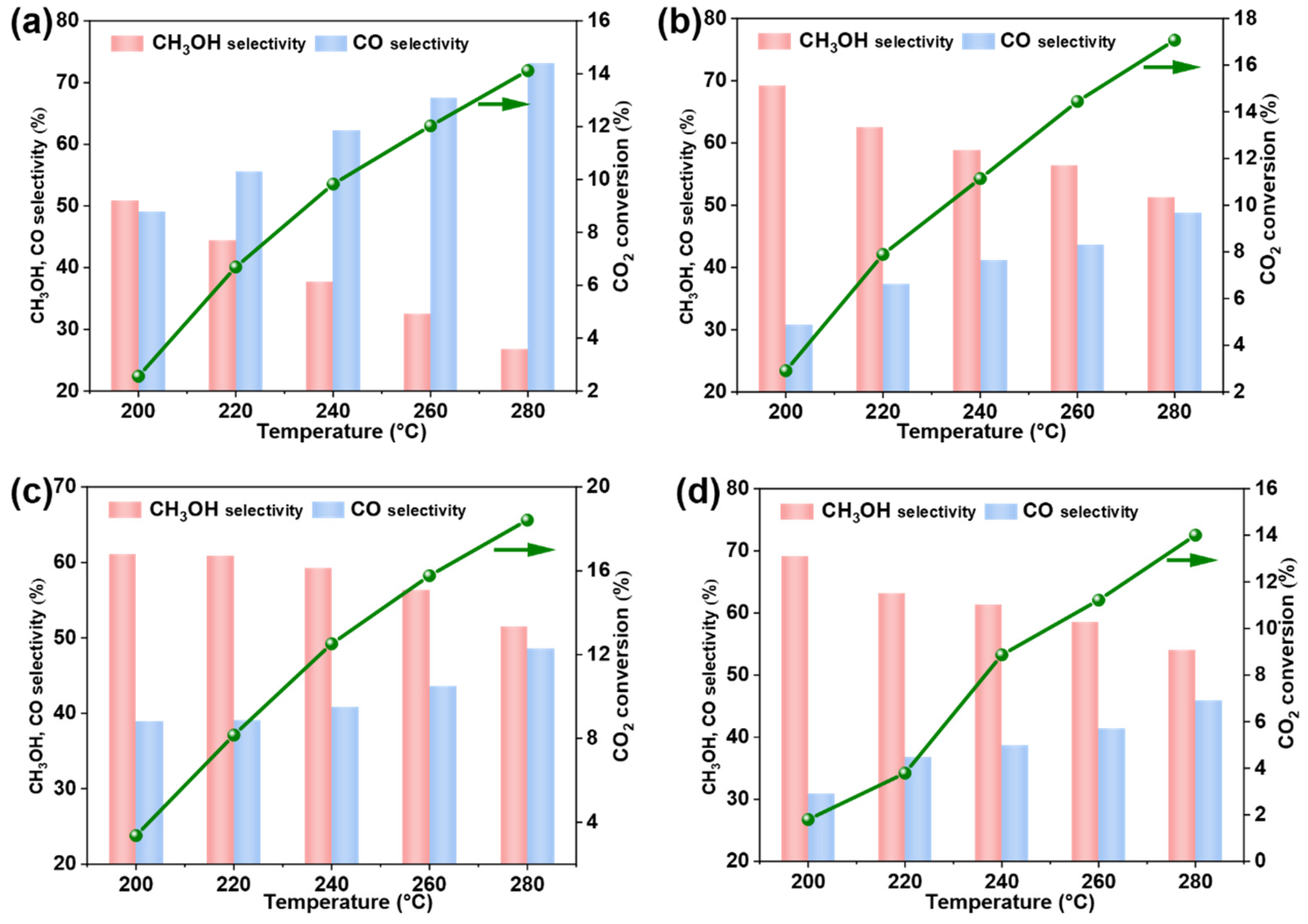
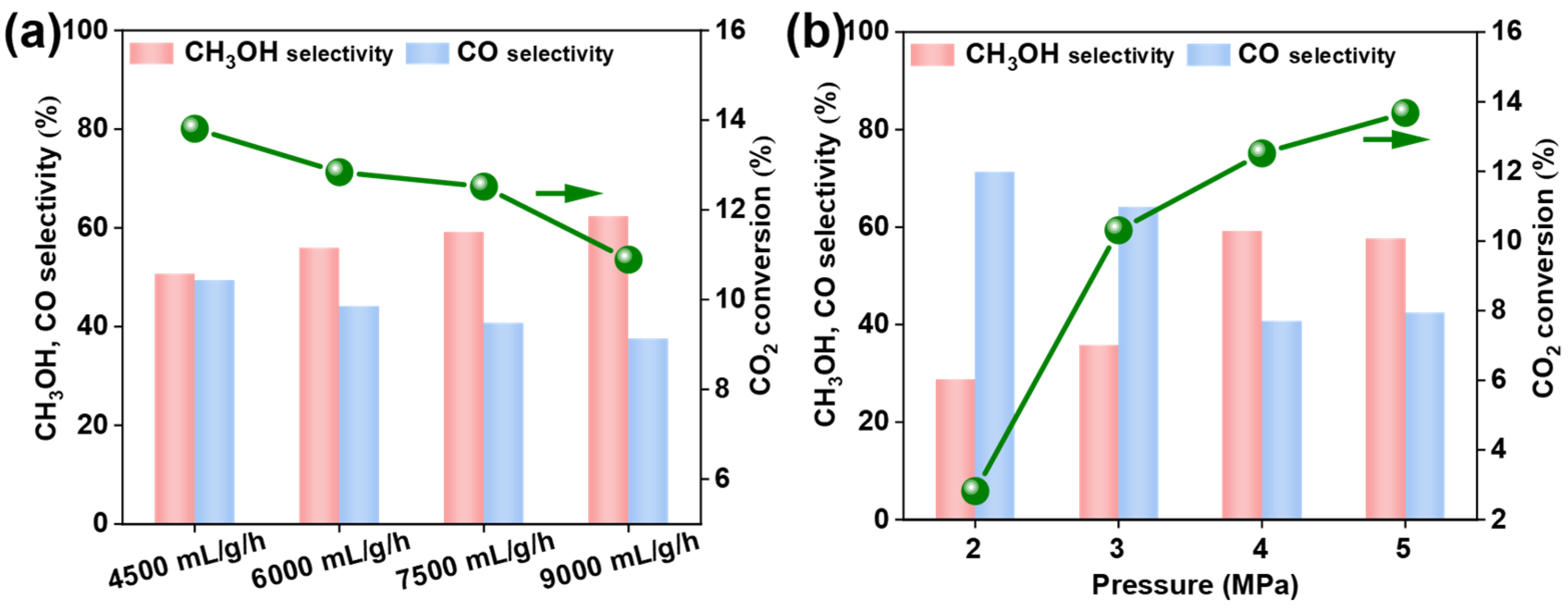
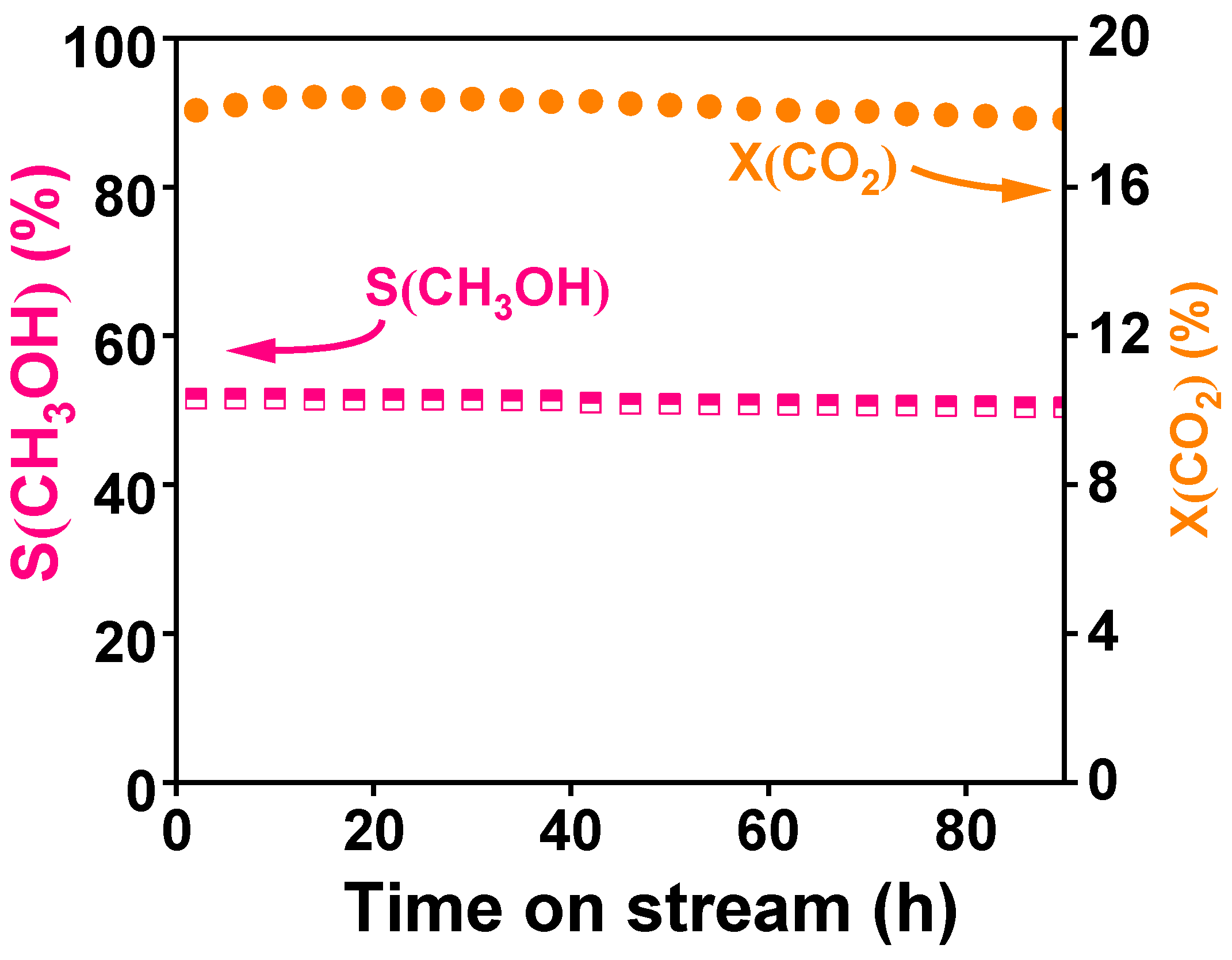
3.4. Catalytic Performance
3.5. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Q.; Wang, N.; Yu, J. Advances in Catalytic Applications of Zeolite-Supported Metal Catalysts. Adv. Mater. 2021, 33, 2104442. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qi, Y.; Wang, F.; Han, Z.; Jiang, Y.; Han, H.; Liu, J.; Zhang, X.; Ong, W.J. State-of-the-art advancements in photo-assisted CO2 hydrogenation: Recent progress in catalyst development and reaction mechanisms. J. Mater. Chem. A 2020, 8, 24868–24894. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Szima, S.; Cormos, C.-C. Improving methanol synthesis from carbon-free H2 and captured CO2: A techno-economic and environmental evaluation. J. CO2 Util. 2018, 24, 555–563. [Google Scholar] [CrossRef]
- Bai, S.-T.; De Smet, G.; Liao, Y.; Sun, R.; Zhou, C.; Beller, M.; Maes, B.U.W.; Sels, B.F. Homogeneous and heterogeneous catalysts for hydrogenation of CO2 to methanol under mild conditions. Chem. Soc. Rev. 2021, 50, 4259–4298. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Chemical Recycling of Carbon Dioxide to Methanol and Dimethyl Ether: From Greenhouse Gas to Renewable, Environmentally Carbon Neutral Fuels and Synthetic Hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef]
- Parastaev, A.; Muravev, V.; Osta, E.H.; van Hoof, A.J.F.; Kimpel, T.F.; Kosinov, N.; Hensen, E.J.M. Boosting CO2 hydrogenation via size-dependent metal–support interactions in cobalt/ceria-based catalysts. Nat. Catal. 2020, 3, 526–533. [Google Scholar] [CrossRef]
- Beck, A.; Zabilskiy, M.; Newton, M.A.; Safonova, O.; Willinger, M.G.; van Bokhoven, J.A. Following the structure of copper-zinc-alumina across the pressure gap in carbon dioxide hydrogenation. Nat. Catal. 2021, 4, 488–497. [Google Scholar] [CrossRef]
- Han, Z.; Tang, C.; Sha, F.; Tang, S.; Wang, J.; Li, C. CO2 hydrogenation to methanol on ZnO-ZrO2 solid solution catalysts with ordered mesoporous structure. J. Catal. 2021, 396, 242–250. [Google Scholar] [CrossRef]
- Yao, Y.; Chang, Y.; Huang, R.; Zhang, L.; Masanet, E. Environmental implications of the methanol economy in China: Well-to-wheel comparison of energy and environmental emissions for different methanol fuel production pathways. J. Clean. Prod. 2018, 172, 1381–1390. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Liu, X.; Shao, Z.; Xia, L.; Zhong, L.; Wang, H.; Sun, Y. Tuning the interaction between Na and Co2C to promote selective CO2 hydrogenation to ethanol. Appl. Catal. B Environ. 2021, 293, 120207. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Zhang, W.; Wang, P.; Qin, Z.; Yan, W.; Dong, M.; Li, J.; Wang, J.; He, L.; et al. Selective Conversion of CO2 into Propene and Butene. Chem 2020, 6, 3344–3363. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, L.; Tan, M.; Zhang, P.; Fang, Y.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Rationally Designing Bifunctional Catalysts as an Efficient Strategy to Boost CO2 Hydrogenation Producing Value-Added Aromatics. ACS Catal. 2019, 9, 895–901. [Google Scholar] [CrossRef]
- Frusteri, F.; Migliori, M.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Aloise, A.; Giordano, G.; Bonura, G. Direct CO2-to-DME hydrogenation reaction: New evidences of a superior behaviour of FER-based hybrid systems to obtain high DME yield. J. CO2 Util. 2017, 18, 353–361. [Google Scholar] [CrossRef]
- Bahruji, H.; Armstrong, R.D.; Esquius, J.R.; Jones, W.; Bowker, M.; Hutchings, G.J. Hydrogenation of CO2 to Dimethyl Ether over Brønsted Acidic PdZn Catalysts. Ind. Eng. Chem. Res. 2018, 57, 6821–6829. [Google Scholar] [CrossRef]
- Fang, X.; Xi, Y.; Jia, H.; Chen, C.; Wang, Y.; Song, Y.; Du, T. Tetragonal zirconia based ternary ZnO-ZrO2-MOx solid solution catalysts for highly selective conversion of CO2 to methanol at High reaction temperature. J. Ind. Eng. Chem. 2020, 88, 268–277. [Google Scholar] [CrossRef]
- Jiao, F.; Bai, B.; Li, G.; Pan, X.; Ye, Y.; Qu, S.; Xu, C.; Xiao, J.; Jia, Z.; Liu, W.; et al. Disentangling the activity-selectivity trade-off in catalytic conversion of syngas to light olefins. Science 2023, 380, 727–730. [Google Scholar] [CrossRef]
- Xiong, S.; Lian, Y.; Xie, H.; Liu, B. Hydrogenation of CO2 to methanol over Cu/ZnCr catalyst. Fuel 2019, 256, 115975. [Google Scholar] [CrossRef]
- Ruland, H.; Song, H.; Laudenschleger, D.; Stürmer, S.; Schmidt, S.; He, J.; Kähler, K.; Muhler, M.; Schlögl, R. CO2 Hydrogenation with Cu/ZnO/Al2O3: A Benchmark Study. ChemCatChem 2020, 12, 3216–3222. [Google Scholar] [CrossRef]
- Ortner, N.; Lund, H.; Armbruster, U.; Wohlrab, S.; Kondratenko, E.V. Factors affecting primary and secondary pathways in CO2 hydrogenation to methanol over CuZnIn/MZrOx (La, Ti or Y). Catal. Today 2022, 387, 47–53. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Phongamwong, T.; Donphai, W.; Boonyuen, C.; Warakulwit, C.; Chareonpanich, M.; Limtrakul, J. Enhanced activity, selectivity and stability of a CuO-ZnO-ZrO2 catalyst by adding graphene oxide for CO2 hydrogenation to methanol. Chem. Eng. J. 2018, 334, 1781–1791. [Google Scholar] [CrossRef]
- Martínez-Suárez, L.; Siemer, N.; Frenzel, J.; Marx, D. Reaction Network of Methanol Synthesis over Cu/ZnO Nanocatalysts. ACS Catal. 2015, 5, 4201–4218. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, J. The chemical modification seen in the Cu/ZnO methanol synthesis catalysts. Appl. Catal. A Gen. 2000, 191, 111–129. [Google Scholar] [CrossRef]
- Wu, J.; Saito, M.; Takeuchi, M.; Watanabe, T. The stability of Cu/ZnO-based catalysts in methanol synthesis from a CO2-rich feed and from a CO-rich feed. Appl. Catal. A Gen. 2001, 218, 235–240. [Google Scholar] [CrossRef]
- Orege, J.I.; Wei, J.; Ge, Q.; Sun, J. Spinel-structured nanocatalysts: New opportunities for CO2 hydrogenation to value-added chemicals. Nano Today 2023, 51, 101914. [Google Scholar] [CrossRef]
- Dasireddy, V.D.B.C.; Neja, S.Š.; Blaž, L. Correlation between synthesis pH, structure and Cu/MgO/Al2O3 heterogeneous catalyst activity and selectivity in CO2 hydrogenation to methanol. J. CO2 Util. 2018, 28, 189–199. [Google Scholar] [CrossRef]
- Liu, T.; Xu, D.; Wu, D.; Liu, G.; Hong, X. Spinel ZnFe2O4 Regulates Copper Sites for CO2 Hydrogenation to Methanol. ACS Sustain. Chem. Eng. 2021, 9, 4033–4041. [Google Scholar] [CrossRef]
- Wang, S.; Song, L.; Qu, Z. Cu/ZnAl2O4 catalysts prepared by ammonia evaporation method: Improving methanol selectivity in CO2 hydrogenation via regulation of metal-support interaction. Chem. Eng. J. 2023, 469, 144008. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Zhou, C.; Zhou, W.; Cheng, K.; Kang, J.; Zhang, Q.; Deng, W.; Wang, Y. Selective transformation of carbon dioxide into lower olefins with a bifunctional catalyst composed of ZnGa2O4 and SAPO-34. Chem. Commun. 2018, 54, 140–143. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Shi, D.; He, S.; Zhang, L.; Yan, W.; Qin, Z.; Li, J.; Dong, M.; Wang, J.; et al. Direct Conversion of Syngas into Light Olefins with Low CO2 Emission. ACS Catal. 2020, 10, 2046–2059. [Google Scholar] [CrossRef]
- He, H.; Lin, X.; Li, S.; Wu, Z.; Gao, J.; Wu, J.; Wen, W.; Ye, D.; Fu, M. The key surface species and oxygen vacancies in MnOx(0.4)-CeO2 toward repeated soot oxidation. Appl. Catal. B Environ. 2018, 223, 134–142. [Google Scholar] [CrossRef]
- Yang, S.-C.; Pang, S.H.; Sulmonetti, T.P.; Su, W.-N.; Lee, J.-F.; Hwang, B.-J.; Jones, C.W. Synergy between Ceria Oxygen Vacancies and Cu Nanoparticles Facilitates the Catalytic Conversion of CO2 to CO under Mild Conditions. ACS Catal. 2018, 8, 12056–12066. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Z.; Guo, X.; Yan, Z.; Ban, H.; Wang, P.; Yao, R.; Li, L.; Li, C. Modulating Electronic Interaction over Zr–ZnO Catalysts to Enhance CO2 Hydrogenation to Methanol. ACS Catal. 2024, 14, 508–521. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, S.; Liu, W.; Chen, B.; Gao, X.; Wang, P.; Gao, J.; Tan, Y.; Dang, S.; Tu, W. Unraveling the regulation of Mn in Cu-ZnOx formation during methanol synthesis from syngas over Cu/ZnO/Al2O3-Mn catalysts. Appl. Catal. B Environ. 2023, 338, 122985. [Google Scholar] [CrossRef]
- Mou, J.; Fan, X.; Liu, F.; Wang, X.; Zhao, T.; Chen, P.; Li, Z.; Yang, C.; Cao, J. CO2 hydrogenation to lower olefins over Mn2O3-ZnO/SAPO-34 tandem catalysts. Chem. Eng. J. 2021, 421, 129978. [Google Scholar] [CrossRef]


| Catalysts | CO2 Conversion (%) | CH3OH Selectivity (%) | TOFCu (10−3s−1) |
|---|---|---|---|
| Cu6Zn4 | 12.0 | 32.5 | 6.4 |
| Cu6Zn3.5Ga0.5 | 14.5 | 56.4 | 9.7 |
| Cu6Zn3Ga1 | 15.8 | 56.5 | 10.2 |
| Cu6Zn2.5Ga1.5 | 11.2 | 58.2 | 8.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zheng, Y.; Zhang, Y.; Qiu, J.; He, L.; Gu, B. Facilitation of CO2 Hydrogenation to Methanol by Spinel ZnGa2O4 in Cu-ZnO Catalysts. Processes 2025, 13, 1420. https://doi.org/10.3390/pr13051420
Wang X, Zheng Y, Zhang Y, Qiu J, He L, Gu B. Facilitation of CO2 Hydrogenation to Methanol by Spinel ZnGa2O4 in Cu-ZnO Catalysts. Processes. 2025; 13(5):1420. https://doi.org/10.3390/pr13051420
Chicago/Turabian StyleWang, Xiulin, Yuanshuang Zheng, Yu Zhang, Jiajun Qiu, Lun He, and Bang Gu. 2025. "Facilitation of CO2 Hydrogenation to Methanol by Spinel ZnGa2O4 in Cu-ZnO Catalysts" Processes 13, no. 5: 1420. https://doi.org/10.3390/pr13051420
APA StyleWang, X., Zheng, Y., Zhang, Y., Qiu, J., He, L., & Gu, B. (2025). Facilitation of CO2 Hydrogenation to Methanol by Spinel ZnGa2O4 in Cu-ZnO Catalysts. Processes, 13(5), 1420. https://doi.org/10.3390/pr13051420






