Electrolyte Optimization for Anthraquinone-Based Slurry Batteries
Abstract
1. Introduction
2. Materials and Methods
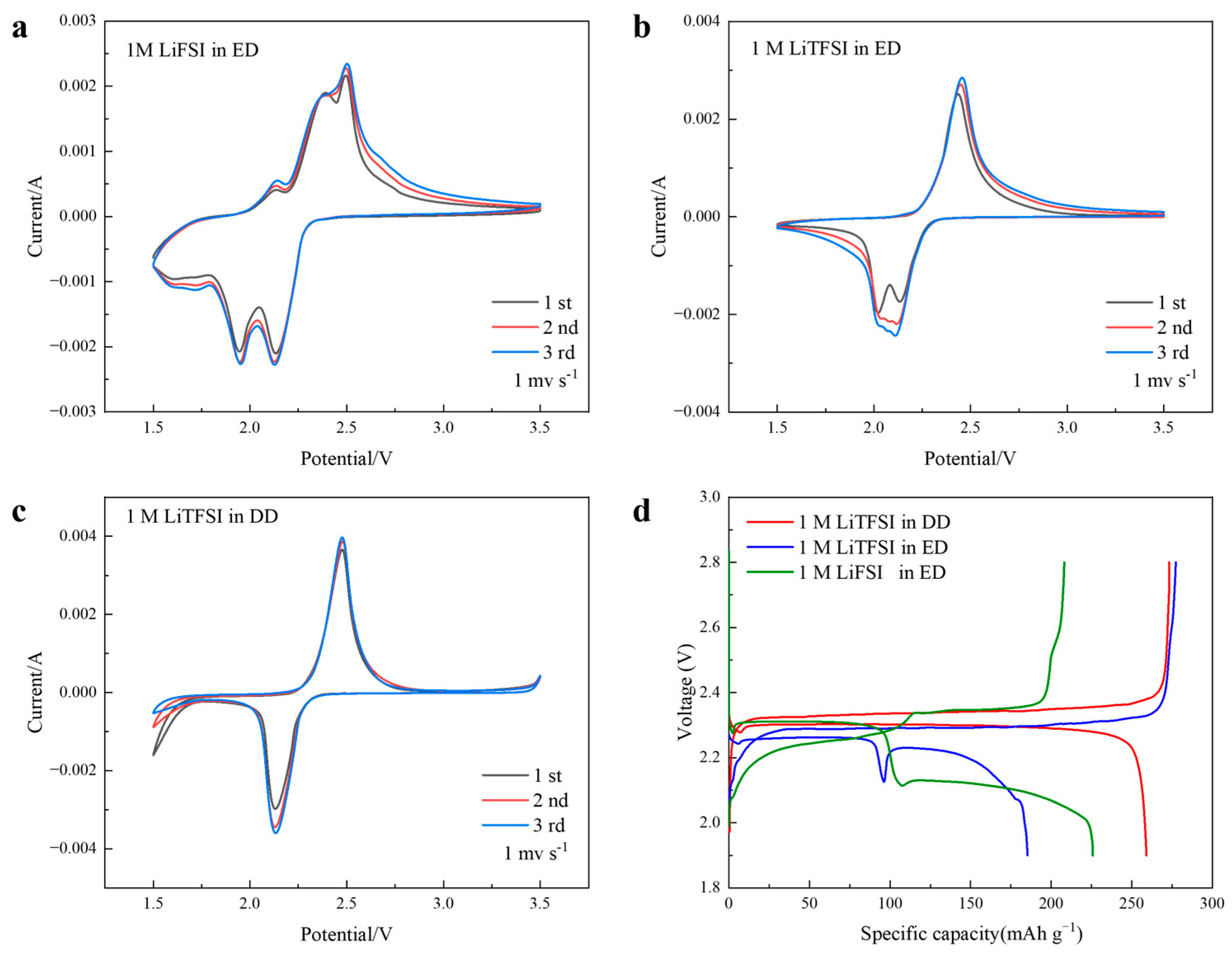
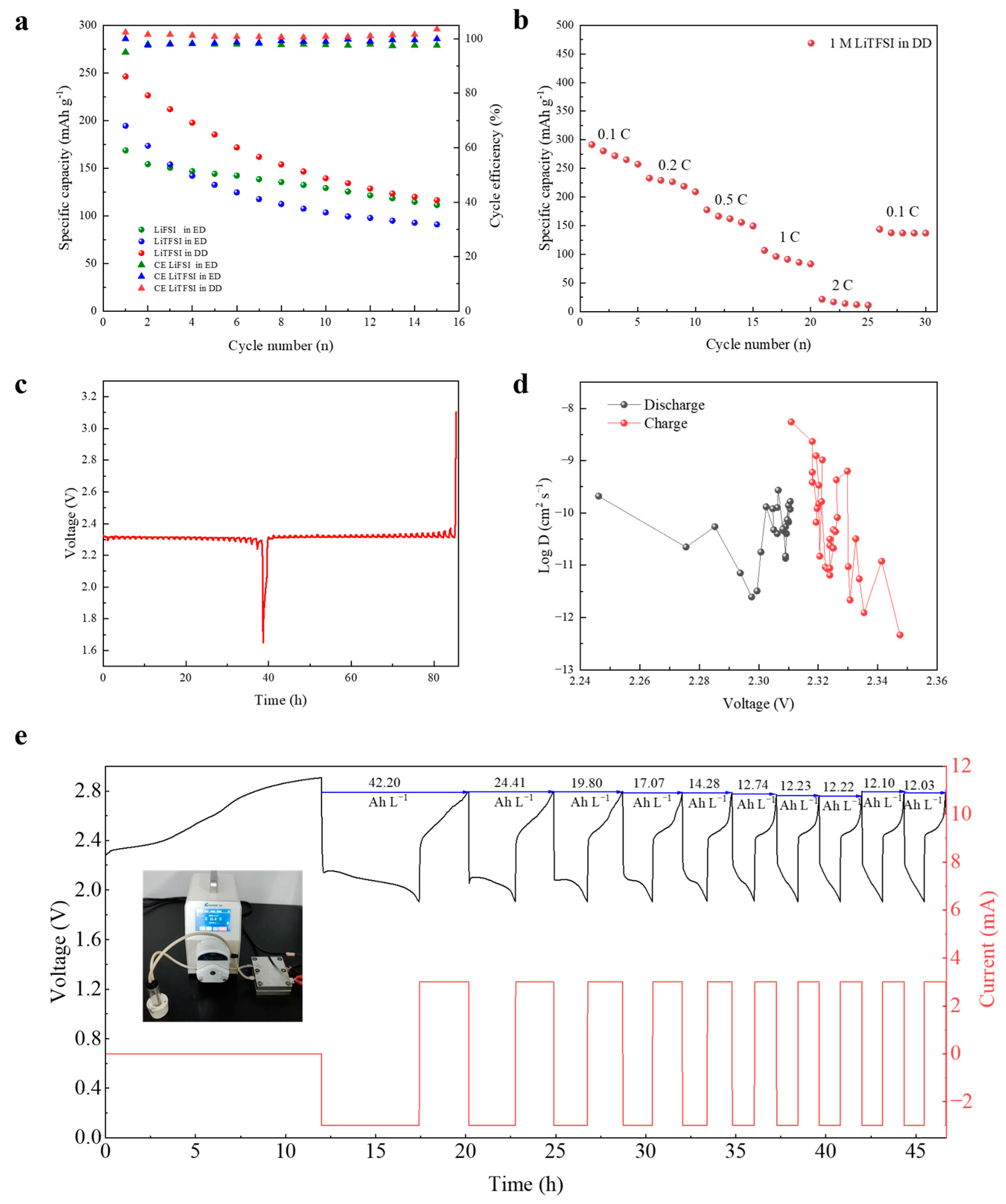
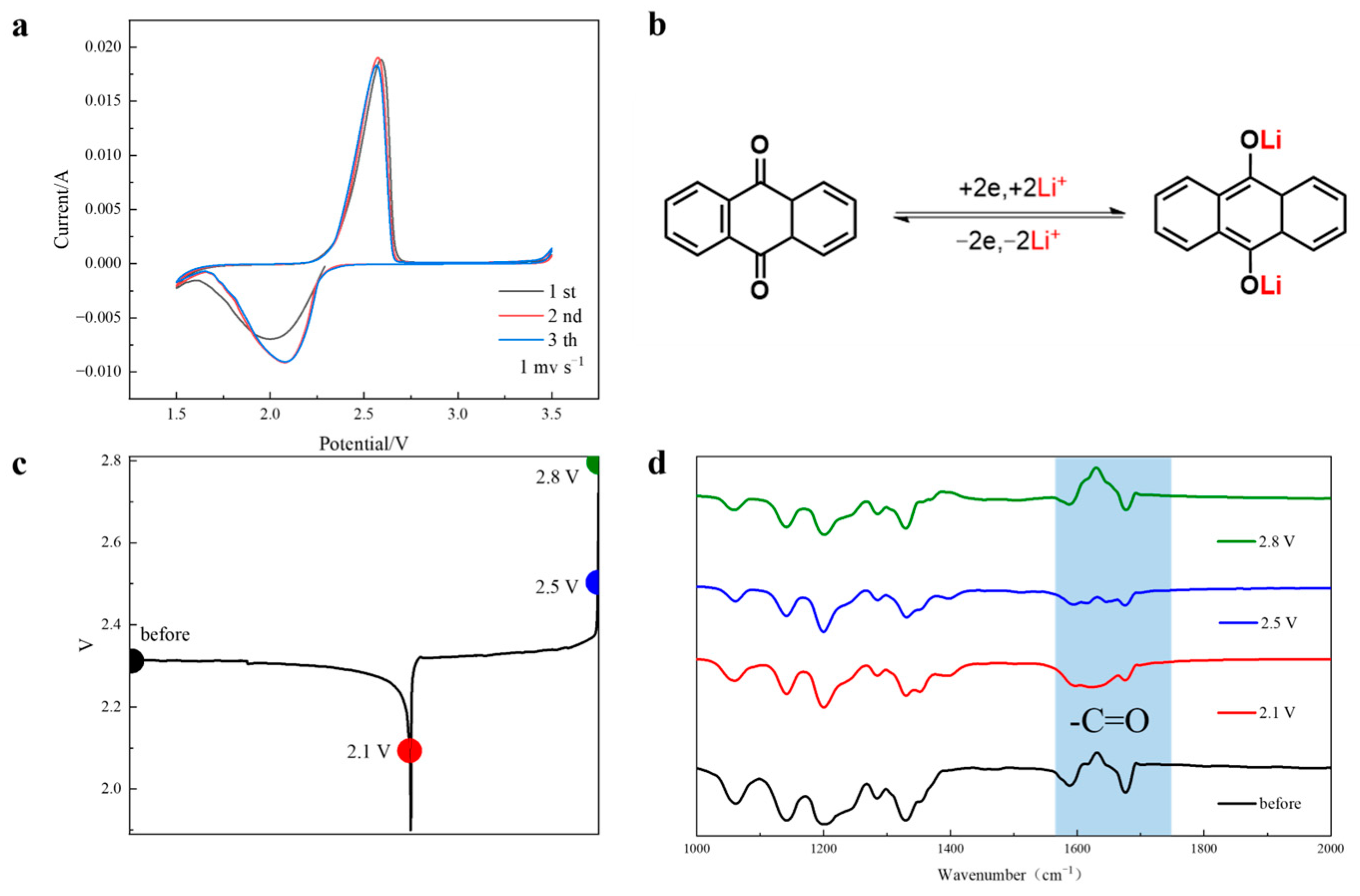
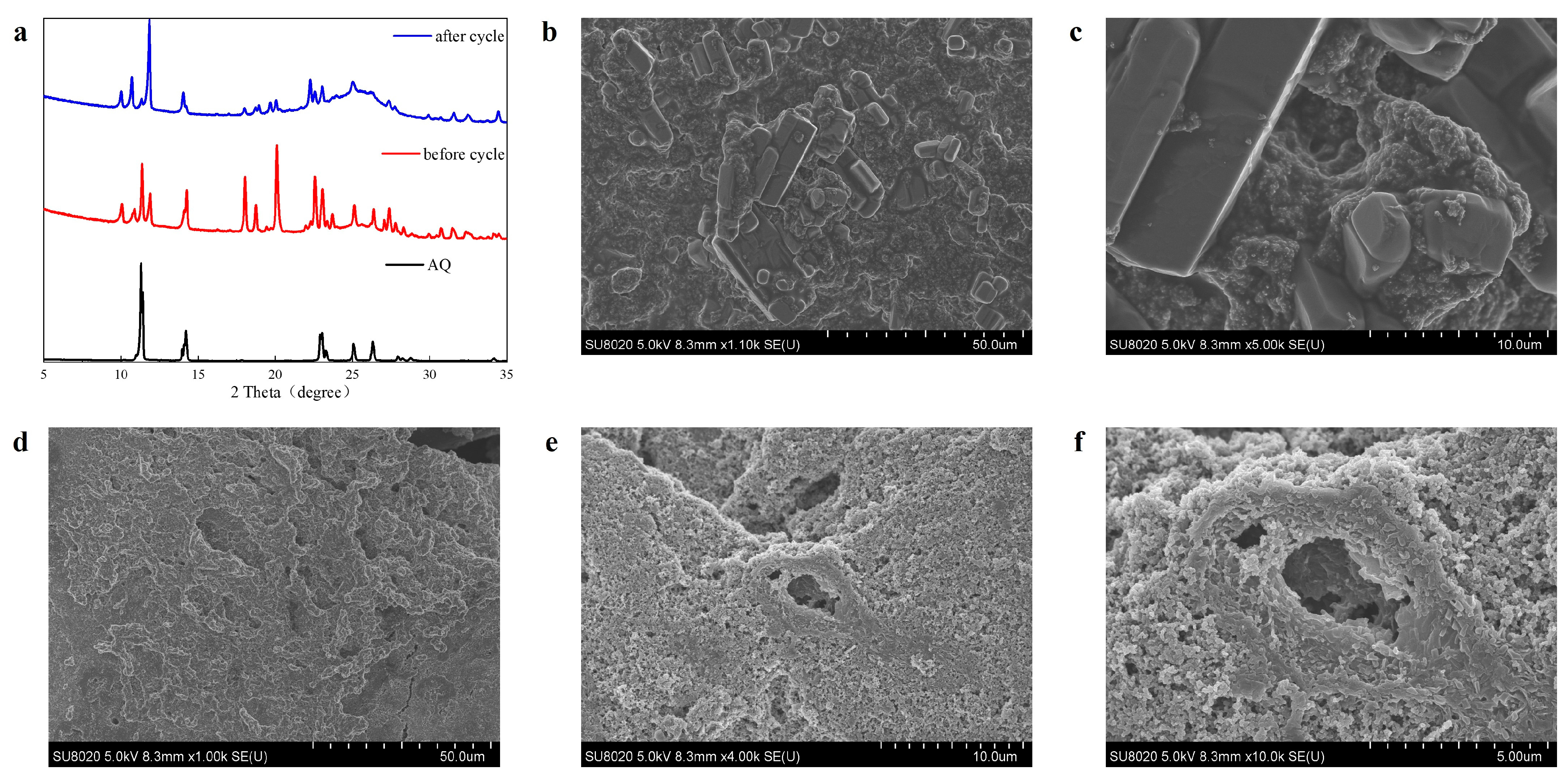
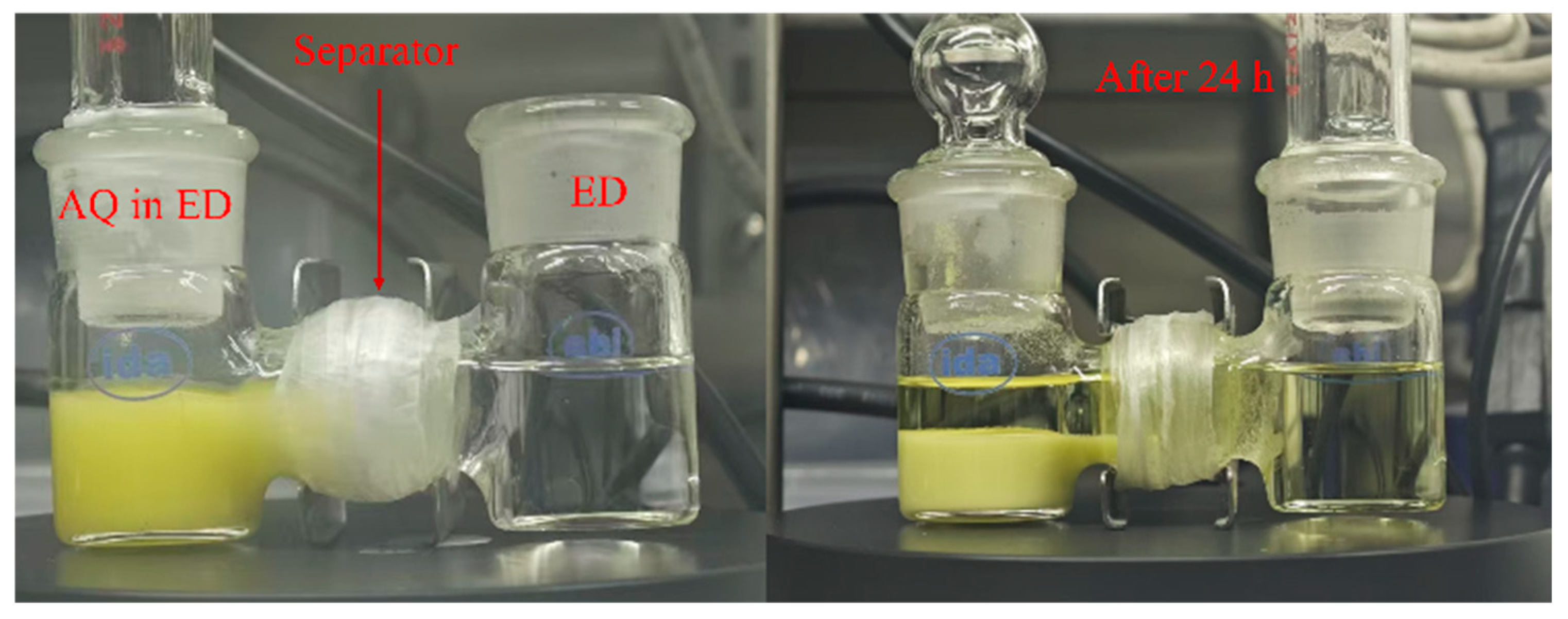
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mousaei, A.; Naderi, Y.; Bayram, I.S. Advancing State of Charge Management in Electric Vehicles With Machine Learning: A Technological Review. IEEE Access 2024, 12, 43255–43283. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, Y.; Zhou, X.; Sun, H.; Wan, W.; Li, Y.; Yao, R.; Bi, F.; Zhao, L.; Wang, L.; et al. Hierarchical Phosphide-Based Hybrid Anodes for High-Performance Lithium-Ion Batteries. Nano Lett. 2025, 25, 3532–3540. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, Z.; Ji, Y.; Rao, X.; Xin, M.; Liu, B.; Xie, Z. A novel strategy toward high energy density: Liquid–solid two-phase chemical reaction in flow battery environment. Chem. Eng. Sci. 2025, 305, 121126. [Google Scholar] [CrossRef]
- Han, L.; Wang, L.; Chen, Z.; Kan, Y.; Hu, Y.; Zhang, H.; He, X. Incombustible Polymer Electrolyte Boosting Safety of Solid-State Lithium Batteries: A Review. Adv. Funct. Mater. 2023, 33, 2300892. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, Y.; Hu, Y.; Pan, S.; Tang, S.; Luo, Y.; Liang, Y.; Liao, Y.; Lin, Y.; Zhang, K.; et al. Quantitative Analysis of Aging and Rollover Failure Mechanisms of Lithium-Ion Batteries at Accelerated Aging Conditions. Adv. Energy Mater. 2025, 2404997. [Google Scholar] [CrossRef]
- Wan, G.; Pollard, T.P.; Ma, L.; Schroeder, M.A.; Chen, C.-C.; Zhu, Z.; Zhang, Z.; Sun, C.-J.; Cai, J.; Thaman, H.L.; et al. Solvent-mediated oxide hydrogenation in layered cathodes. Science 2024, 385, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Kirkaldy, N.D.; Oehler, F.F.; Marinescu, M.; Offer, G.J.; O’Kane, S.E.J. The importance of degradation mode analysis in parameterising lifetime prediction models of lithium-ion battery degradation. Nat. Commun. 2025, 16, 2776. [Google Scholar] [CrossRef]
- Cheng, S.; Jiang, L.; Wei, Z.; Meng, X.; Duan, Q.; Xiao, H.; Sun, J.; Wang, Q. Elucidating in-situ heat generation of LiFePO4 semi-solid lithium slurry battery under specific cycling protocols. Electrochim. Acta 2024, 475, 143674. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, F.; Bai, H.; Wang, C.; Wang, Y.; Zhao, K.; Yan, J.; Zhang, Y.; Ding, J.; Wang, S.; et al. Multi-carbonyl naphthalene diimide polymer as a high-performance cathode for stable lithium-ion storage. Nano Res. Energy 2025, e9120159. [Google Scholar] [CrossRef]
- Zheng, M.; You, Y.; Lu, J. Understanding materials failure mechanisms for the optimization of lithium-ion battery recycling. Nat. Rev. Mater. 2025. [Google Scholar] [CrossRef]
- Bai, Q.; Huang, J.; Tang, K.; Zhu, Y.; Wu, D. Arylamine-Linked Porous Organic Polymers with Abundant Redox-Active Sites as High-Capacity and High-Rate Organic Cathodes for Lithium-Ion Batteries. Adv. Mater. 2025, 37, 2416661. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ye, H.; Li, Y. Molecular Engineering of Organic Electrode Materials for Beyond Lithium-Ion Batteries. Adv. Funct. Mater. 2025, 2424329. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Chen, Z.; Yang, X.; Zhang, R.; Wang, H.; Yang, Y. Molecule and Microstructure Modulations of Cyano-Containing Electrodes for High-Performance Fully Organic Batteries. Angew. Chem. Int. Ed. 2024, 63, e202401253. [Google Scholar] [CrossRef]
- Gao, H.; Zhu, Q.; Neale, A.R.; Bahri, M.; Wang, X.; Yang, H.; Liu, L.; Clowes, R.; Browning, N.D.; Sprick, R.S.; et al. Integrated Covalent Organic Framework/Carbon Nanotube Composite as Li-Ion Positive Electrode with Ultra-High Rate Performance. Adv. Energy Mater. 2021, 11, 2101880. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, L.; Wang, Y.; Qiu, S.; Luo, Y.; Zou, Y.; Xu, F.; Sun, L.; Chu, H. Recent progress on construction and applications of metal-organic frameworks-based materials for lithium-ion batteries and supercapacitors. Carbon Neutralization 2024, 3, 396–414. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Lu, Y.-C. Lithium–Organic Nanocomposite Suspension for High-Energy-Density Redox Flow Batteries. ACS Energy Lett. 2018, 3, 1991–1997. [Google Scholar] [CrossRef]
- Zhao, Y.; Si, S.; Liao, C. A single flow zinc//polyaniline suspension rechargeable battery. J. Power Sources 2013, 241, 449–453. [Google Scholar] [CrossRef]
- Gautam, R.K.; McGrath, J.J.; Wang, X.; Jiang, J.J. Nonaqueous Organic Slurry Battery over 4 V. ACS Energy Lett. 2024, 9, 4408–4413. [Google Scholar] [CrossRef]
- Gan, X.; Song, Z. Small-molecule organic electrode materials for rechargeable batteries. Sci. China Chem. 2023, 66, 3070–3104. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.-D.; Poon Ho, G.S.H.; Zhang, Z.; Huang, J.; Bang, K.-T.; Lau, C.Y.; Leu, S.-Y.; Wang, Y.; Kim, Y. Anthraquinone-Based Silicate Covalent Organic Frameworks as Solid Electrolyte Interphase for High-Performance Lithium–Metal Batteries. J. Am. Chem. Soc. 2023, 145, 24603–24614. [Google Scholar] [CrossRef]
- Wang, H.; Liu, G.; Zhou, W.; Wang, Y.; Dong, X. High-Potential and Stable Organic Cathode for Rechargeable Batteries with Fast-Charging and Wide-Temperature Adaptability. Angew. Chem. Int. Ed. 2025, 64, e202416874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Guo, C.; Zhao, Q.; Niu, Z.; Chen, J. High-Performance Organic Lithium Batteries with an Ether-Based Electrolyte and 9,10-Anthraquinone (AQ)/CMK-3 Cathode. Adv. Sci. 2015, 2, 1500018. [Google Scholar] [CrossRef]
- Wang, C.; Yu, B.; Liu, Y.; Wang, H.; Zhang, Z.; Xie, C.; Li, X.; Zhang, H.; Jin, Z. N-alkyl-carboxylate-functionalized anthraquinone for long-cycling aqueous redox flow batteries. Energy Storage Mater. 2021, 36, 417–426. [Google Scholar] [CrossRef]
- Pan, S.; Yang, L.; Su, P.; Zhang, H.; Zhang, S. Robust Multiscale Electron/Ion Transport and Enhanced Structural Stability in SiO Semi-Solid Anolytes Enabled by Trifunctional Artificial Interfaces for High-Performance Li-Ion Slurry Flow Batteries. Small 2022, 18, 2202139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, X.; Qian, W.; Zhang, H.; Zhang, S. Lithium slurry flow cell, a promising device for the future energy storage. Green Energy Environ. 2021, 6, 5–8. [Google Scholar] [CrossRef]
- Xin, Y.; Ge, Y.; Li, Z.; Zhang, Q.; Tian, H. Research Progress on Modification Strategies of Organic Electrode Materials for Energy Storage Batteries. Acta Phys.-Chim. Sin. 2024, 40, 2303060. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, Y.; Zhang, Q.; Chen, J. Insights into Redox Processes and Correlated Performance of Organic Carbonyl Electrode Materials in Rechargeable Batteries. Adv. Mater. 2022, 34, 2104150. [Google Scholar] [CrossRef]
- Gui, B.; Yang, X.; Fu, H.; Peng, P.; Lyu, W.; Rao, A.M.; Zhou, J.; Fan, L.; Lu, B. Synergistic Electrode and Electrolyte Polarities Lead to Outstanding Organic Potassium-based Batteries. Angew. Chem. Int. Ed. 2025, 64, e202421928. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, W.; Shi, M.; Li, D.; Sun, Q.; Cheng, M.; Tao, Z. Intramolecular Hydrogen Bonds Weaken Interaction Between Solvents and Small Organic Molecules Towards Superior Lithium-Organic Batteries. Angew. Chem. Int. Ed. 2025, 64, e202416845. [Google Scholar] [CrossRef]
- Sun, T.; Feng, X.-L.; Sun, Q.-Q.; Yu, Y.; Yuan, G.-B.; Xiong, Q.; Liu, D.-P.; Zhang, X.-B.; Zhang, Y. Solvation Effect on the Improved Sodium Storage Performance of N-Heteropentacenequinone for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 26806–26812. [Google Scholar] [CrossRef]
- Cai, T.; Han, Y.; Lan, Q.; Wang, F.; Chu, J.; Zhan, H.; Song, Z. Stable cycling of small molecular organic electrode materials enabled by high concentration electrolytes. Energy Storage Mater. 2020, 31, 318–327. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Ni, Y.; Zheng, S.; Yan, Z.; Zhang, K.; Zhao, Q.; Chen, J. Quinone Electrodes for Alkali–Acid Hybrid Batteries. J. Am. Chem. Soc. 2022, 144, 8066–8072. [Google Scholar] [CrossRef]
- Yao, Y.-X.; Chen, X.; Yan, C.; Zhang, X.-Q.; Cai, W.-L.; Huang, J.-Q.; Zhang, Q. Regulating Interfacial Chemistry in Lithium-Ion Batteries by a Weakly Solvating Electrolyte. Angew. Chem. Int. Ed. 2021, 60, 4090–4097. [Google Scholar] [CrossRef]
- Zhang, Y.; Katayama, Y.; Tatara, R.; Giordano, L.; Yu, Y.; Fraggedakis, D.; Sun, J.G.; Maglia, F.; Jung, R.; Bazant, M.Z.; et al. Revealing electrolyte oxidation via carbonate dehydrogenation on Ni-based oxides in Li-ion batteries by in situ Fourier transform infrared spectroscopy. Energy Environ. Sci. 2020, 13, 183–199. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Wu, H.; Yu, X.; Wu, X.; Li, Z.; Luo, H.; Zhang, H.; Hong, Y.; Zou, Y.; et al. Visualizing and Regulating Dynamic Evolution of Interfacial Electrolyte Configuration during De-solvation Process on Lithium-Metal Anode. Angew. Chem. Int. Ed. 2024, 63, e202400254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, Z.; Ma, Z.; Liu, G.; Cheng, H.; Zou, Y.; Cavallo, L.; Li, Q.; Ming, J. Weak Solvent–Solvent Interaction Enables High Stability of Battery Electrolyte. ACS Energy Lett. 2023, 8, 1477–1484. [Google Scholar] [CrossRef]
- Piao, Z.; Gao, R.; Liu, Y.; Zhou, G.; Cheng, H.-M. A Review on Regulating Li+ Solvation Structures in Carbonate Electrolytes for Lithium Metal Batteries. Adv. Mater. 2023, 35, 2206009. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, A.; Wang, W.; Yang, B.; Wang, J. A Review of Capacity Decay Studies of All-vanadium Redox Flow Batteries: Mechanism and State Estimation. ChemSusChem 2024, 17, e202301787. [Google Scholar] [CrossRef]

| Abbreviations | Terminology |
|---|---|
| RFBs | redox flow batteries |
| LIBs | lithium-ion batteries |
| SLBs | semi-solid lithium slurry batteries |
| OEMs | organic electrode materials |
| AQ | anthraquinone |
| LiFSI | lithium bisfluorosulfonimide |
| LiTFSI | lithium bis(trifluoromethanesulphonyl)imide |
| EC | ethylene carbonate |
| DMC | dimethyl carbonate |
| ED | EC/DMC; 3:7 by mass |
| DME | glycol dimethyl ether |
| DOL | 1,3-dioxolane |
| DD | DME/DOL; 1:1 by volume |
| KB | Ketjenblack |
| PVDF | polyvinylidene fluoride |
| NMP | N-methylpyrrolidone |
| TX-100 | Triton X-100 |
| CC | carbon cloth |
| GITT | Galvanostatic Intermittent Titration Technique |
| CV | cyclic voltammetry |
| H-cell | H-type diffusion cell |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Hu, T. Electrolyte Optimization for Anthraquinone-Based Slurry Batteries. Processes 2025, 13, 1403. https://doi.org/10.3390/pr13051403
Zhao C, Hu T. Electrolyte Optimization for Anthraquinone-Based Slurry Batteries. Processes. 2025; 13(5):1403. https://doi.org/10.3390/pr13051403
Chicago/Turabian StyleZhao, Cunhang, and Tu Hu. 2025. "Electrolyte Optimization for Anthraquinone-Based Slurry Batteries" Processes 13, no. 5: 1403. https://doi.org/10.3390/pr13051403
APA StyleZhao, C., & Hu, T. (2025). Electrolyte Optimization for Anthraquinone-Based Slurry Batteries. Processes, 13(5), 1403. https://doi.org/10.3390/pr13051403







