Influence of 4,4′,6,6′-Tetra(azido)hydrazo-1,3,5-triazine on the Thermal Behavior of the Nitroguanidine-Base Propellant
Abstract
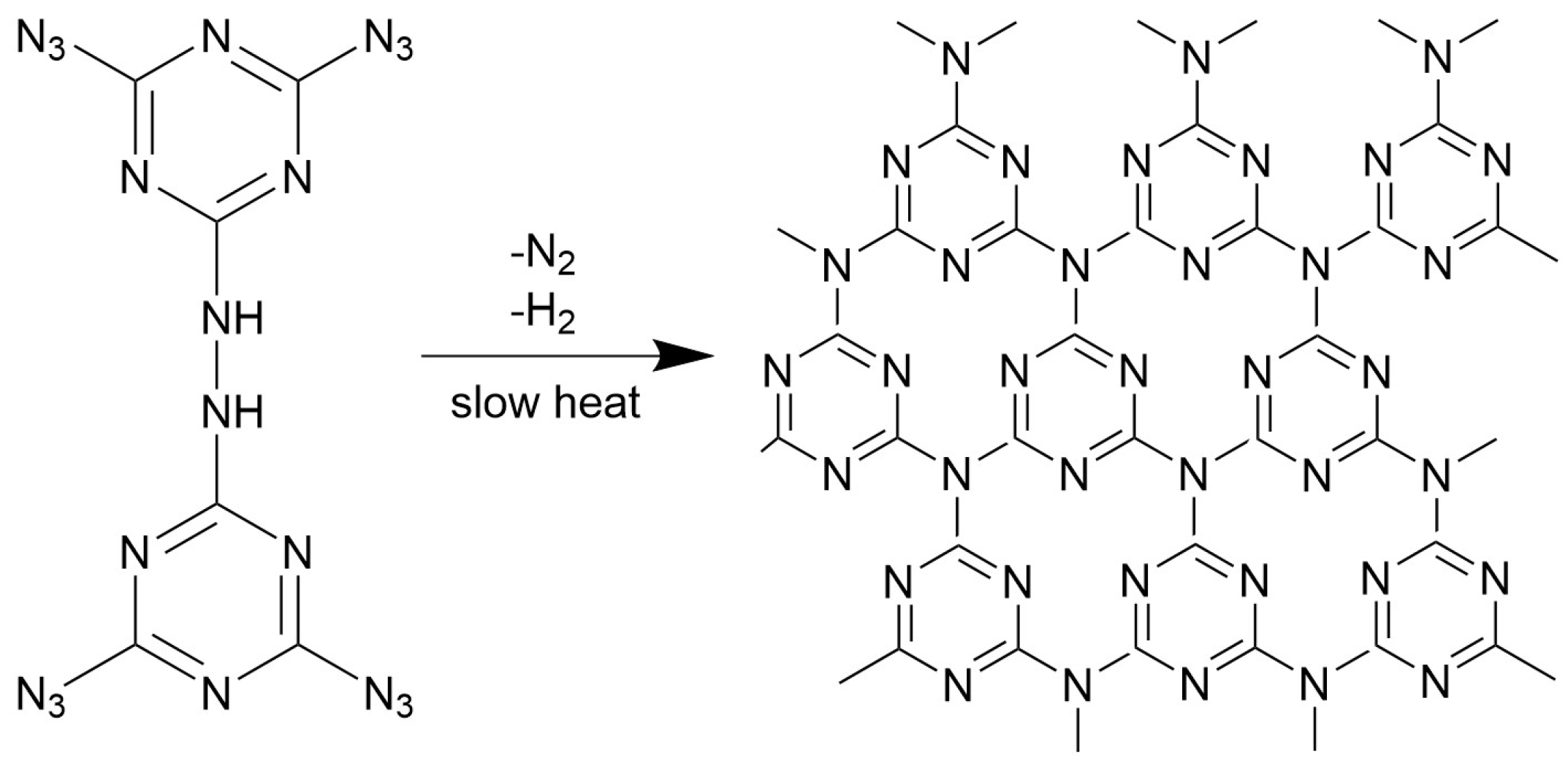
1. Introduction
2. Experimental
2.1. Reagents
2.2. Propellant Composition
2.3. Equipment and Conditions
3. Results and Discussion
3.1. Sensitivity Performance
3.2. Thermal Decomposition Reaction Characteristics of Propellant
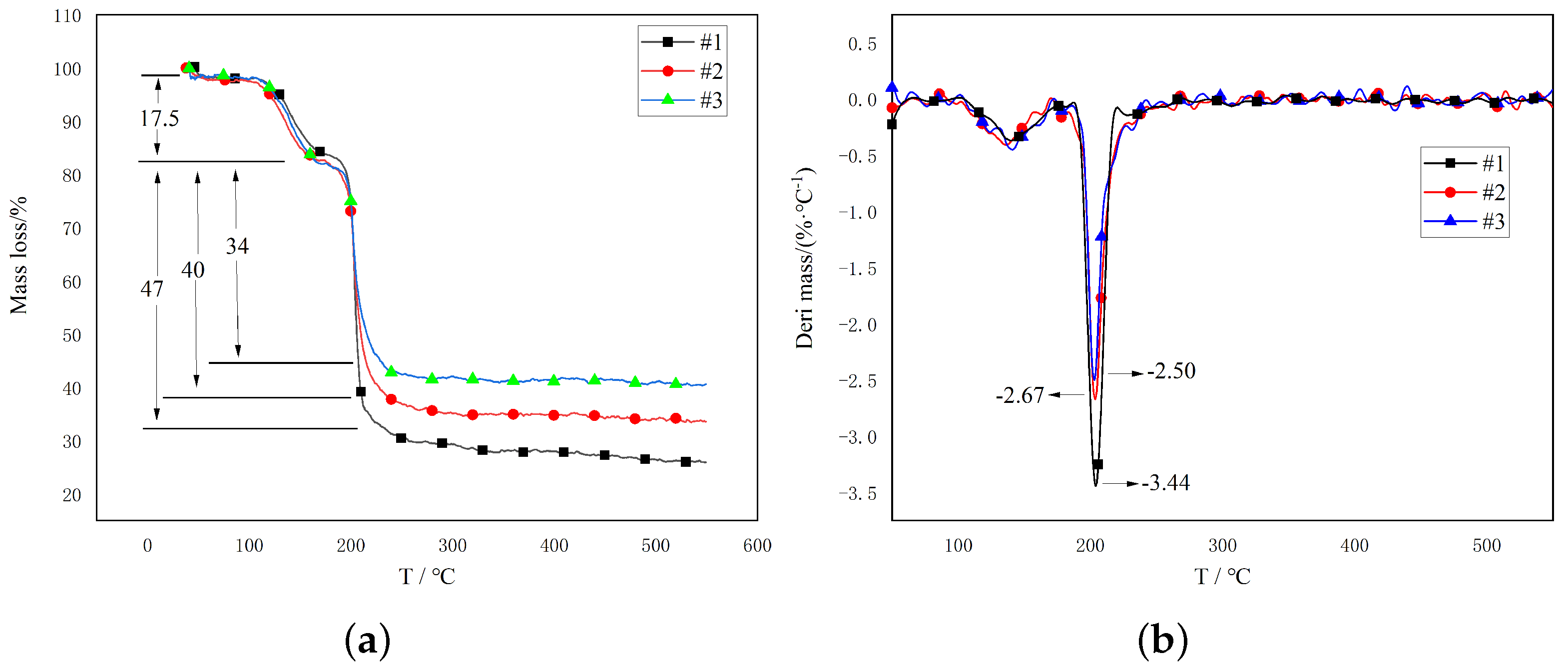
3.2.1. TG−DTG Analysis of Propellant Samples
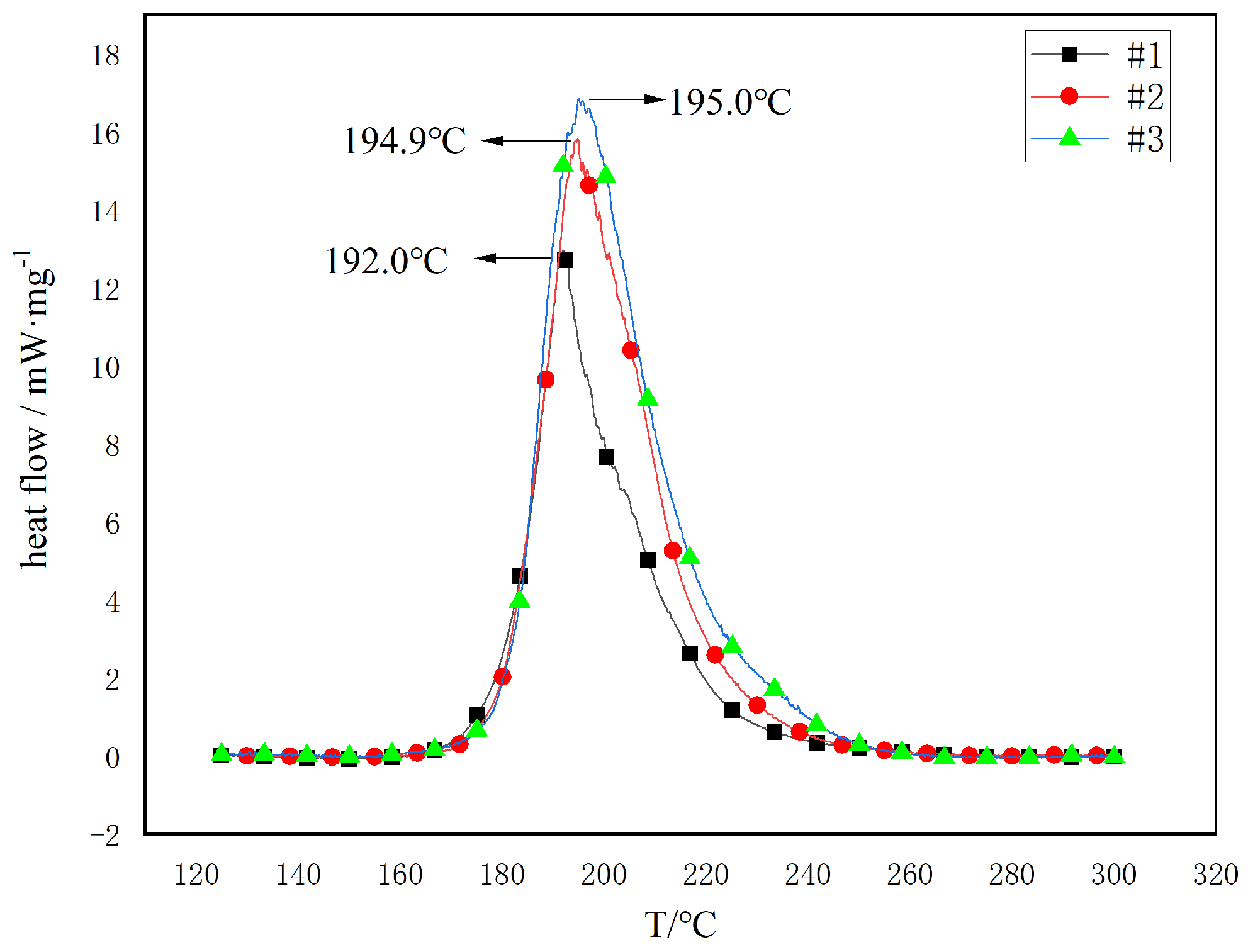
3.2.2. DSC Thermal Decomposition Characteristics of Propellants
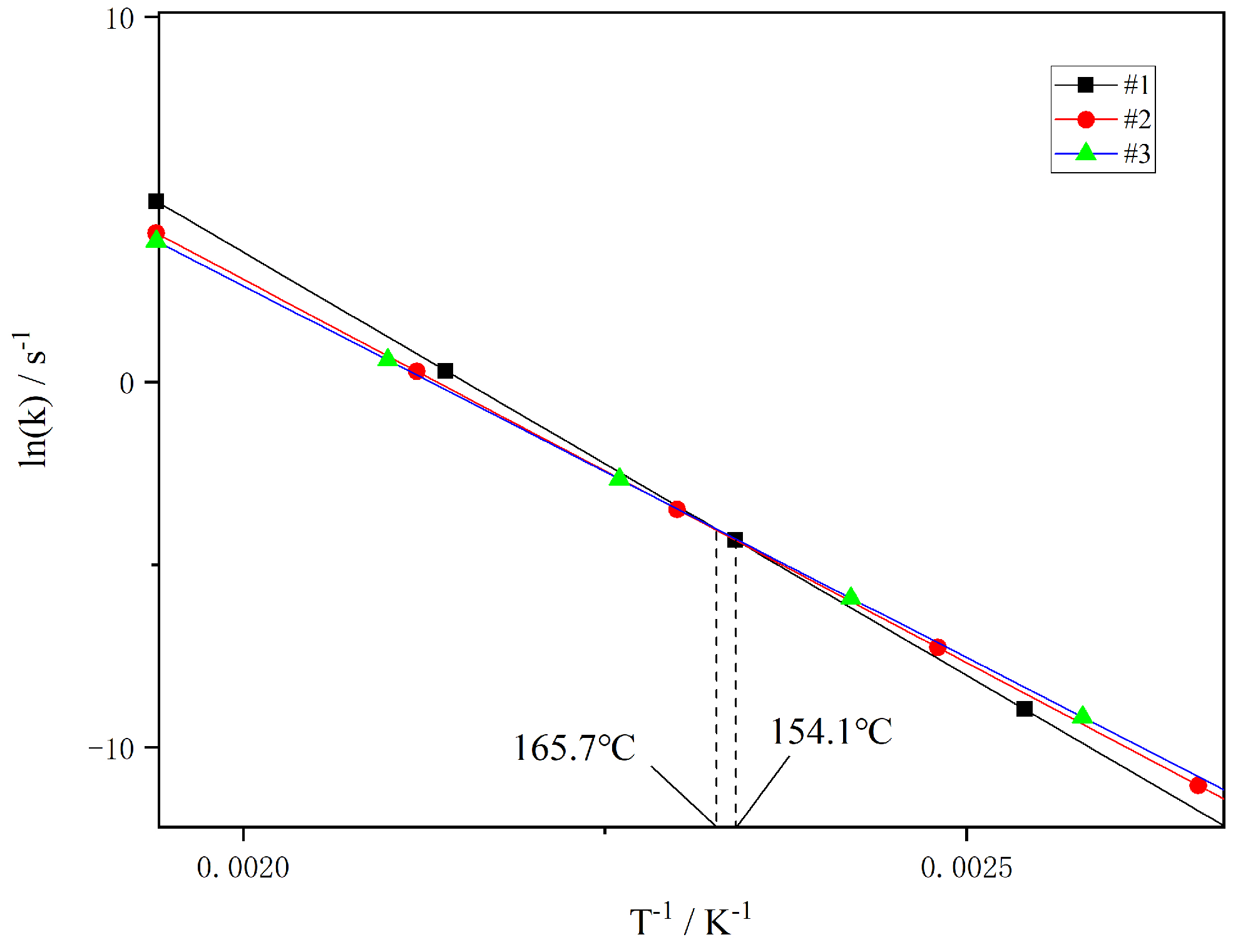
3.2.3. Kinetics of Thermal Breakdown in Propellants
3.3. Thermal Decomposition Characteristics of Pure TAHT
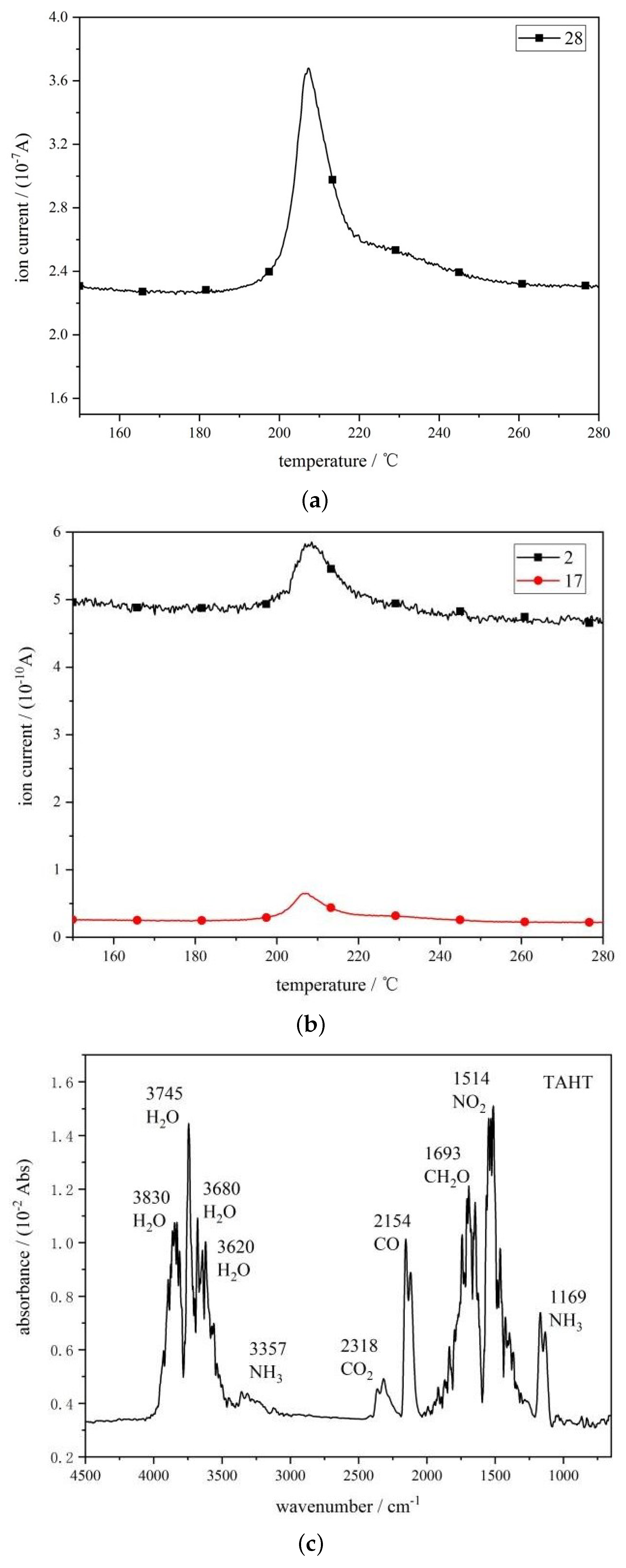
3.3.1. Differential Scanning Calorimetry (DSC) Analysis
3.3.2. Analysis of Pyrolysis Gas-Phase Products
4. Conclusions
- Thermogravimetric analysis indicated that adding TAHT to the nitroguanidine propellant can significantly reduce the mass loss rate of the propellant. When the TAHT content is 20%, the maximum mass loss rate is reduced by 27%.
- The DSC analysis shows that adding TAHT to the propellant raises the peak temperature of thermal decomposition. However, the apparent activation energy decreases. The activation energies for the three propellant samples were calculated using the Kissinger and Ozawa methods. The Kissinger method showed a decrease in activation energy from 192.8 kJ·mol−1 to 174.7 kJ·mol−1 and 169.4 kJ·mol−1, while the Ozawa method revealed a drop from 190.7 kJ·mol−1 to 173.5 kJ·mol−1 and 168.5 kJ·mol−1. The activation energies were reduced by about 9% and 12%, respectively. The isothermal decomposition temperatures for the three samples were 154.1 °C, 156.6 °C, and 165.7 °C. The addition of TAHT decreased the decomposition reaction rate constant of NGu propellant at high temperatures.
- The FTIR test results of TAHT show that a highly thermally stable azopolymer is formed during its thermal decomposition. This azopolymer accumulates instantaneously on the surface of the propellant, thereby preventing mass transfer from the condensed phase to the gas phase and affecting heat transfer from the gas phase to the condensed phase. Therefore, after being added to the propellant, TAHT has the potential to inhibit thermal decomposition and reduce temperature sensitivity.
Author Contributions
Funding
Data Availability Statement
DURC Statement
Conflicts of Interest
Abbreviations
| DIANP | Azidonitramine |
| DSC | Differential Scanning Calorimetry |
| DTG | Derivative Thermogravimetry |
| FTIR | Fourier Transform Infrared Spectroscopy |
| IR | Infrared Spectroscopy |
| MS | Mass Spectrometry |
| NC | Nitrocellulose |
| NGu | Nitroguanidine |
| NGy | Nitroglycerin |
| TAHT | 4,4′,6,6′-Tetraazido-1,3,5-Triazine |
| TATDO | 2,4,6-Triamino-1,3,5-Triazine-1,3-Dioxid |
| TG | Thermogravimetric Analysis |
References
- Yu, Q.; Singh, J.; Staples, R.J.; Shreeve, J.N. Assembling nitrogen-rich, thermally stable, and insensitive energetic materials by polycyclization. Chem. Eng. J. 2022, 431, 133235. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, W.; Wang, X.; Shen, F. Nitrification progress of nitrogen-rich heterocyclic energetic compounds: A review. Molecules 2022, 27, 1465. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lai, W.; Yu, T.; Liu, Y.; Wang, B. Can N-oxidation alleviate the energy-safety contradiction of energetic materials. FirePhysChem 2021, 1, 27–32. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Z.; Yang, R.; Li, H.; Liu, Y.; Ye, Z. Imino-bridged N-rich energetic materials: C4H3N17 and their derivatives assembled from the powerful combination of four tetrazoles. CrystEngComm 2021, 23, 5377–5384. [Google Scholar] [CrossRef]
- Zhang, X.G. Study on Synthesis and Thermal Decomposition of High-Nitrogen Compounds and Their Energetic Materials. Ph.D. Thesis, National University of Defense Technology, Changsha, China, 2005. [Google Scholar]
- Luo, Y.F.; Ge, Z.X.; Wang, B.Z.; Zhang, H.-H.; Xiong, C.-L. Synthesis and Characterization of 1H,4H-3,6-Dinitro-pyrazole[4,3-c]pyrazole Amine Salt. J. Propellants Explos. Pyrotech. 2008, 31, 98–101. [Google Scholar]
- Zhao, W.X.; Jiang, H.B.; Wang, H.S. Synthesis and Thermal Analysis of of nickel(II) complex of 5-Aminotetrazole. Appl. Chem. Ind. 2011, 40, 1868–1870. [Google Scholar]
- Yin, X. Synthesis and Properties of N-Trinitromethyl-1,2,3-Triazole Compounds. Ph.D. Thesis, Southwest University of Science and Technology, Mianyang, China, 2015. [Google Scholar]
- Huynh, M.H.; Hiskey, M.A.; Hartline, E.L.; Montoya, D.P.; Gilardi, R. Polyazido high-nitrogen compounds: Hydrazo- and azo-1,3,5-triazine. Angew. Chem. Int. Ed. 2004, 43, 4924–4928. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.H.; Hiskey, M.A.; Archuleta, J.G.; Roemer, E.L.; Gilardi, R. 3,6-di(azido)-1,2,4,5-tetrazine: A precursor for the preparation of carbon nanospheres and nitrogen-rich carbon nitrides. Angew. Chem. Int. Ed. 2004, 116, 5776–5779. [Google Scholar] [CrossRef]
- Ali, A.N.; Son, S.F.; Hiskey, M.A.; Naud, D.L. Novel high nitrogen propellant use in solid fuel micropropulsion. J. Propuls. Power. 2004, 20, 120–126. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Mayer, P.; Stierstorfer, J.; Weig, J.J. Bistetrazolylamines—Synthesis and characterization. J. Mater. Chem. 2008, 18, 5248–5258. [Google Scholar] [CrossRef]
- Myers, T.W.; Bjorgaard, J.A.; Brown, K.E.; Chavez, D.E.; Hanson, S.K.; Scharff, R.J.; Tretiak, S.; Veauthier, J.M. Energetic chromophores: Low-energy laser initiation in explosive Fe(II) tetrazine complexes. J. Am. Chem. Soc. 2016, 138, 4685–4692. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Wan, C.; Feng, Z.C.; Chapyshev, S.V.; Chukanov, N.V.; Yuantsze, S. Thermal Decomposition Characteristics of 2,4,6-Triamino-1,3,5-Triazine-1,3-Dioxide (TATDO). Chin. J. Explos. Propellants 2023, 46, 748–754. [Google Scholar]
- Cai, M. Synthesis, Characterization, and Properties of Five Triazine-Based Energetic Compounds. Ph.D. Thesis, Northwest University, Xi’an, China, 2021. [Google Scholar]
- Chen, X. Synthesis and Properties of Asymmetric Tetrazine-Based Energetic Compounds. Ph.D. Thesis, Northwest University, Xi’an, China, 2018. [Google Scholar]
- Jia, L.; Lu, H.L.; Han, F. Influence of Azidonitramine on the Thermal Behavior of the Nitroguanidine-Based Gun Propellant. Chin. J. Explos. Propellants 2015, 38, 90–93. [Google Scholar]
- GJB 772A-97; Explosive Test Methods. General Armaments Department: Beijing, China, 1997.
- WJ/T 9052.2-2006; Test Methods for Gunpowder and Pyrotechnics—Part 2: Thermal Analysis Method. Commission of Science, Technology and Industry for National Defense, China National Institute of Standardization of Military Industry: Beijing, China, 2006.
- Xie, D.F.; Huang, Z.Y.; Song, Y.P.; Zhang, C. Effect of Ethylenediamine Triethylenediamine Perchlorate on Thermal Decomposition Reaction of High Energy Gun Propellant. Chin. J. Explos. Propellants 2020, 43, 208–212. [Google Scholar]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Stoner, C.E.; Brill, T.B. Thermal Decomposition of Energetic Materials 46. The Formation of Melamin-like Cyclic Azines as a Mechanism for Ballistic Modification of Composite Propellants by DCD, DAG, and DAF. Combust. Flame 1991, 83, 302–308. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhang, X.J.; Fu, G.; Pang, S.; Zhao, C. Synthesis, Characterization, and Thermal Decomposition Study of 4,4′,6,6′-Tetraazido-Azo-1,3,5-Triazine (TAAT). J. Org. Chem. 2011, 31, 1484–1489. [Google Scholar]
- Steinhauser, G.; Klapötke, T.M. “Green” pyrotechnics: A chemists’ challenge. Angew. Chem. Int. Ed. 2008, 47, 3330–3347. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Moran, J.S. Decomposition of Azo- and Hydrazo-Linked Bis Triazines. J. Energetic Mater. 2009, 27, 63–93. [Google Scholar] [CrossRef]

| Samples | Basic Formula Composition | NGu (wt %) | TAHT (wt %) |
|---|---|---|---|
| #1 | NC/NGy/DIANP | 47 | 0 |
| #2 | 32 | 15 | |
| #3 | 27 | 20 |
| Samples | H50/cm | P/% |
|---|---|---|
| #1 | 58.62 | 29 |
| #2 | 67.93 | 22 |
| #3 | 71.49 | 19 |
| Samples | Tp/°C | |||
|---|---|---|---|---|
| 5 °C·min−1 | 10 °C·min−1 | 15 °C·min−1 | 20 °C·min−1 | |
| #1 | 185.6 | 192.0 | 195.5 | 197.9 |
| #2 | 188.5 | 194.9 | 198.0 | 202.8 |
| #3 | 189.2 | 195.0 | 198.6 | 203.8 |
| Samples | /(kJ·mol−1) | r2 | lnA/s−1 | ||
|---|---|---|---|---|---|
| Kissinger | Ozawa | Kissinger | Ozawa | Kissinger | |
| #1 | 192.8 | 190.7 | 0.9990 | 0.9991 | 49.95 |
| #2 | 174.7 | 173.5 | 0.9812 | 0.9828 | 44.84 |
| #3 | 169.4 | 168.5 | 0.9701 | 0.9727 | 43.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Yang, J.; Wu, R.; Gao, Y.; Yang, W.; Wei, D.; Zhang, Y. Influence of 4,4′,6,6′-Tetra(azido)hydrazo-1,3,5-triazine on the Thermal Behavior of the Nitroguanidine-Base Propellant. Processes 2025, 13, 1382. https://doi.org/10.3390/pr13051382
Xiao Y, Yang J, Wu R, Gao Y, Yang W, Wei D, Zhang Y. Influence of 4,4′,6,6′-Tetra(azido)hydrazo-1,3,5-triazine on the Thermal Behavior of the Nitroguanidine-Base Propellant. Processes. 2025; 13(5):1382. https://doi.org/10.3390/pr13051382
Chicago/Turabian StyleXiao, Yijie, Jianxing Yang, Rui Wu, Yuchen Gao, Weitao Yang, Ding Wei, and Yucheng Zhang. 2025. "Influence of 4,4′,6,6′-Tetra(azido)hydrazo-1,3,5-triazine on the Thermal Behavior of the Nitroguanidine-Base Propellant" Processes 13, no. 5: 1382. https://doi.org/10.3390/pr13051382
APA StyleXiao, Y., Yang, J., Wu, R., Gao, Y., Yang, W., Wei, D., & Zhang, Y. (2025). Influence of 4,4′,6,6′-Tetra(azido)hydrazo-1,3,5-triazine on the Thermal Behavior of the Nitroguanidine-Base Propellant. Processes, 13(5), 1382. https://doi.org/10.3390/pr13051382






