Thermal Transformation of Coal Molecular Structures and Their Impact on Reactivity in Metallurgical-Grade Silicon Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
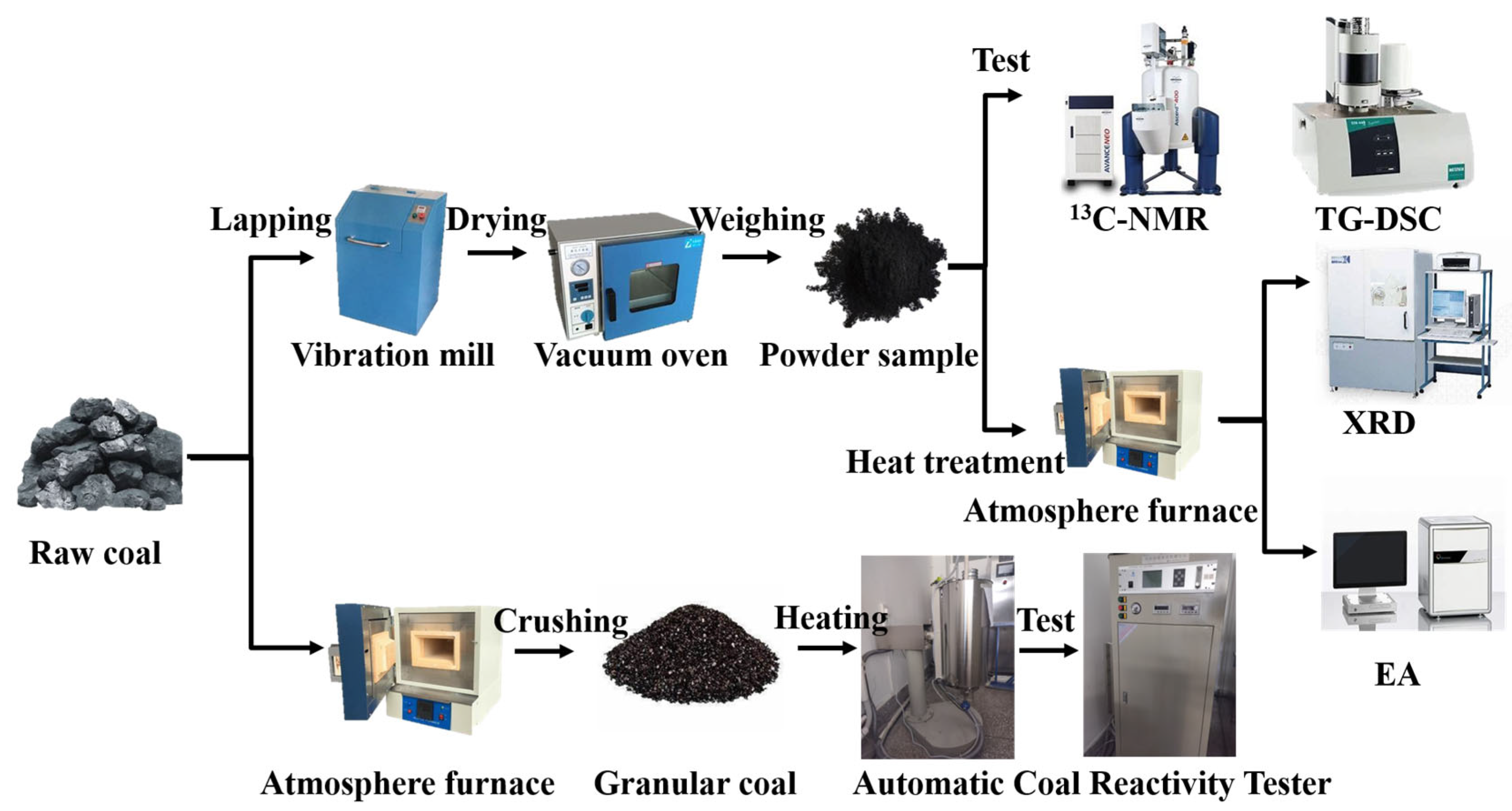
2.2. Experimental Procedure
2.3. Analysis and Characterization
2.3.1. 13C-NMR
2.3.2. TG–DSC
2.3.3. EA
2.3.4. XRD
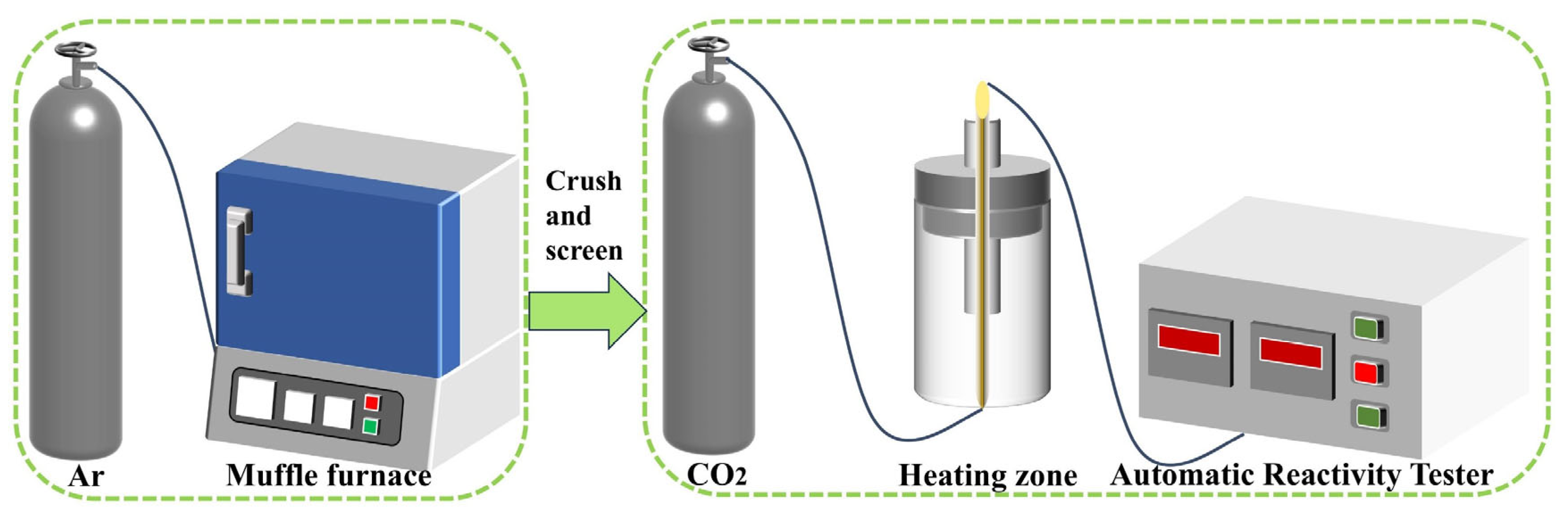
2.4. Experiments for Measuring the Reactivity of Coal to CO2
3. Results and Discussion
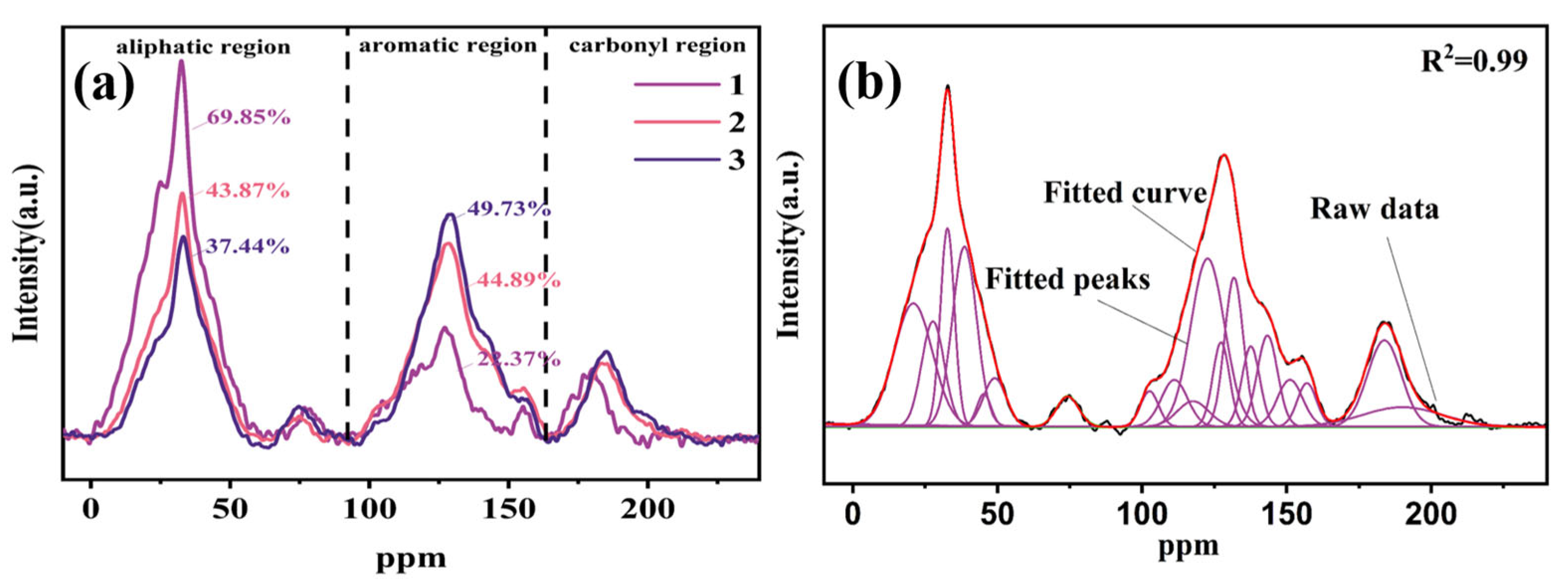
3.1. 13C-NMR Studies on the Molecular Structure of Coal
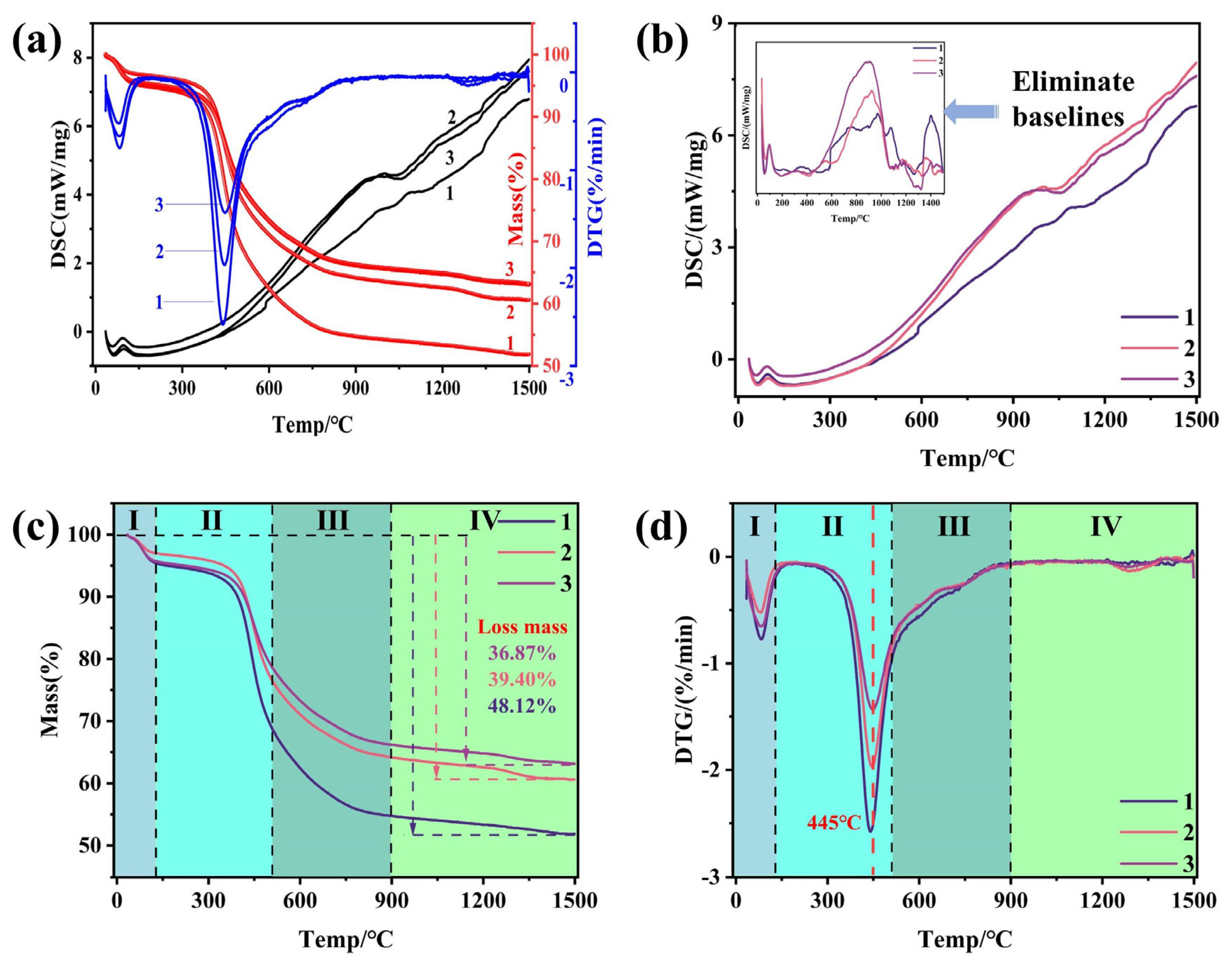
3.2. The Pyrolysis Process of Coal
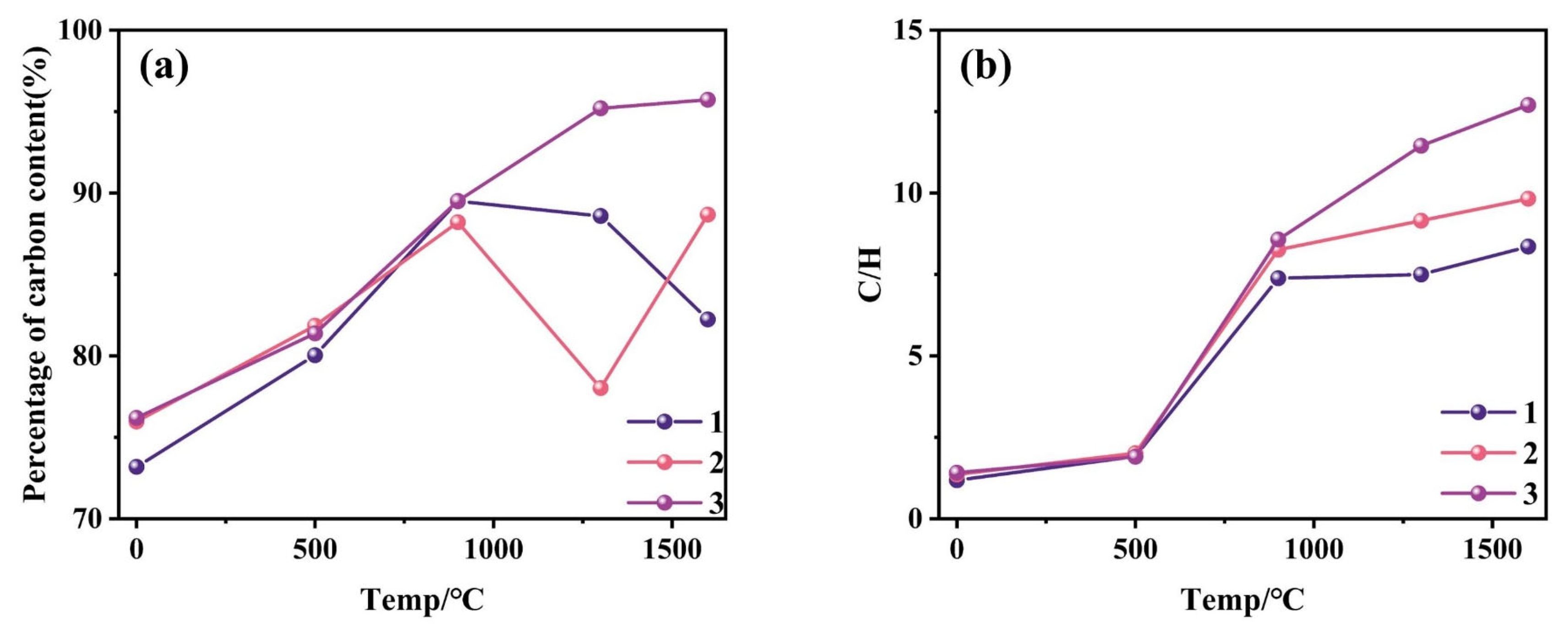
3.3. Organic Elemental Composition of Coal
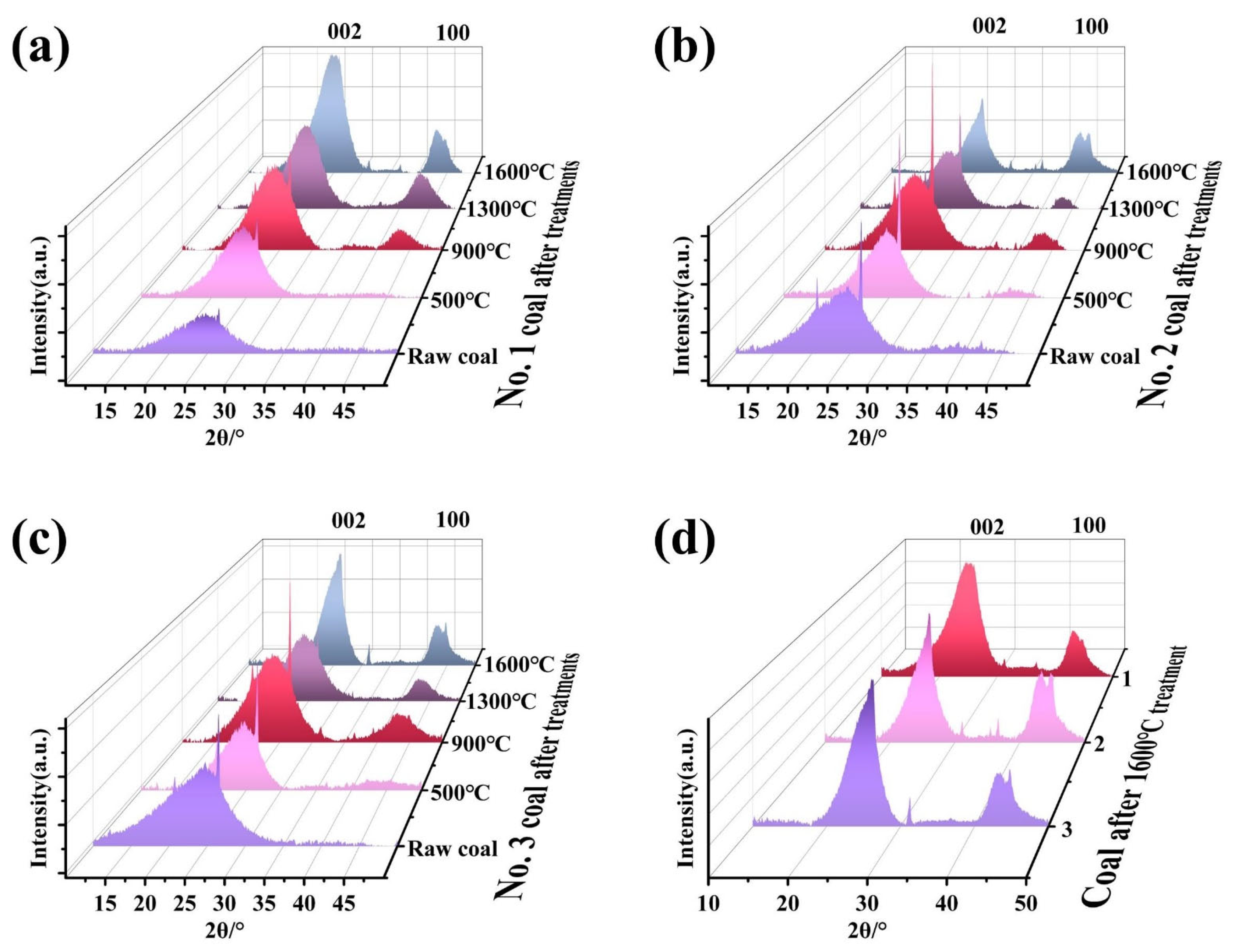
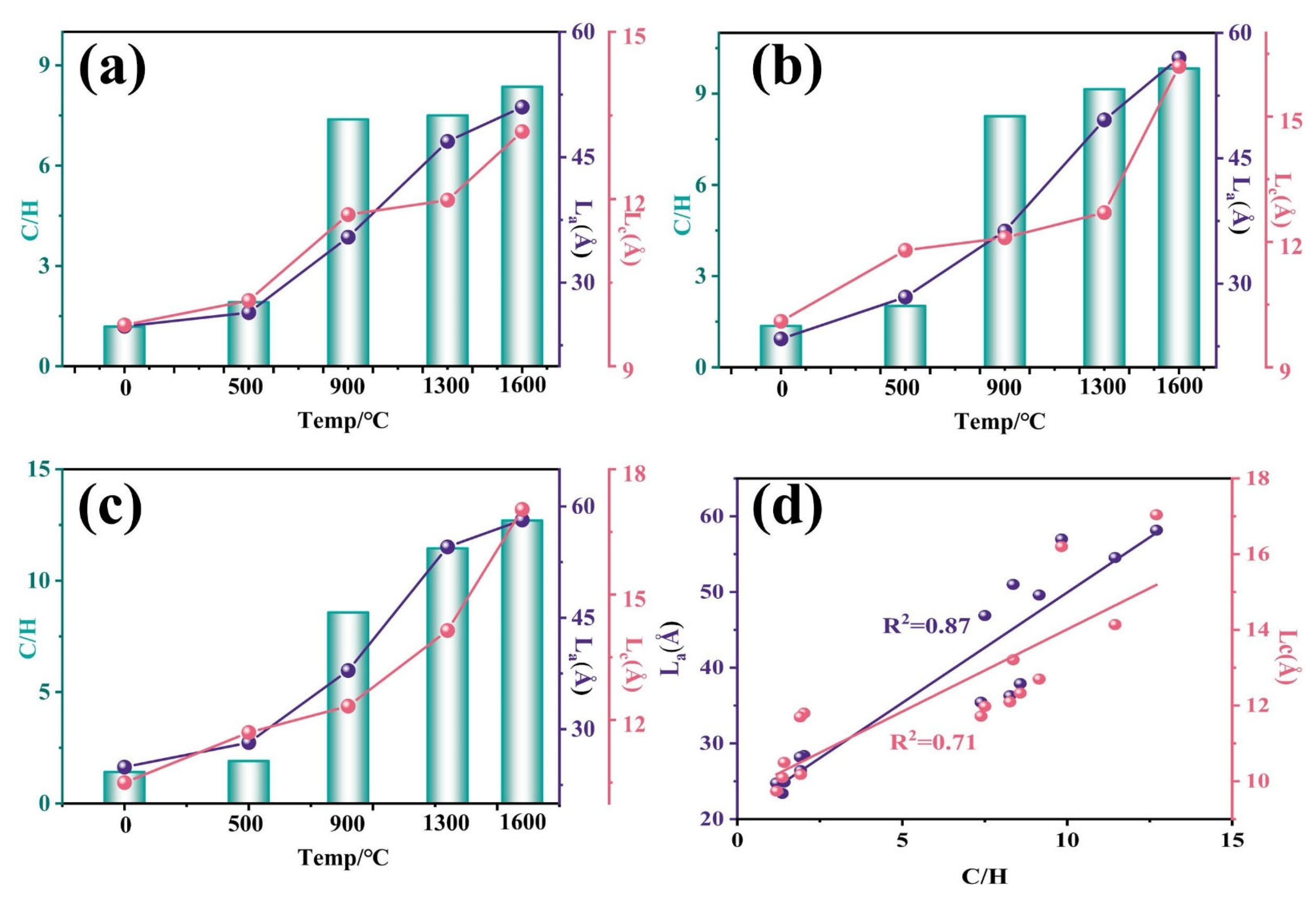
3.4. Thermal Transformation of Microcrystalline Structures
3.5. Coal Reactivity Verification Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, C.; Wei, K.; Du, Z.; Ma, W. Effect of particle size on the properties of biomass gasification residue pellets used as a MG-Si reducing agent. Powder Technol. 2024, 435, 119406. [Google Scholar] [CrossRef]
- Xie, R.; Chen, Z.; Ma, W.; Gan, X.; Wang, X.; Tao, C. Study on using waste biomass as carbon reducing agent in industrial silicon smelting. J. Clean. Prod. 2024, 472, 143468. [Google Scholar] [CrossRef]
- Tao, C.; Chen, Z.; Ma, W.; Wang, X. Comparative Study on the Graphitization Index of Carbonaceous Reducing Agents Used in Silicon Smelting. Silicon 2024, 16, 3121–3139. [Google Scholar] [CrossRef]
- Song, Y.; Wang, N. Exploring temporal and spatial evolution of global coal supply-demand and flow structure. Energy 2019, 168, 1073–1080. [Google Scholar] [CrossRef]
- Qu, Y.; Song, S.; Zou, Z.; Shao, L. Mathematical modeling for the process of smelting reduction ironmaking integrated with hydrogen-rich coal gasification. Processes 2024, 12, 370. [Google Scholar] [CrossRef]
- Hower, J.C.; Finkelman, R.B.; Eble, C.F.; Arnold, B.J. Understanding coal quality and the critical importance of comprehensive coal analyses. Int. J. Coal Geol. 2022, 263, 104120. [Google Scholar] [CrossRef]
- Zhang, C.; Vladislav, L.; Xu, R.; Sergey, G.; Jiao, K.; Zhang, J.; Li, T.; Aleksei, T.; Wang, C.; Wang, G. Blast furnace hydrogen-rich metallurgy-research on efficiency injection of natural gas and pulverized coal. Fuel 2022, 311, 122412. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Ma, W.; Wu, J. High efficient and clean utilization of coal for the carbothermic reduction of silica. Sustain. Energy Technol. Assess. 2022, 53, 102602. [Google Scholar] [CrossRef]
- Tao, C.; Chen, Z.; Ma, W. Analysis of Microstructural Change Mechanism During High-Temperature Pyrolysis of Carbonaceous Reducing Agents for Silicon Smelting. Silicon 2024, 16, 647–663. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Ding, L.; Gong, Y.; Yu, G. Studying effects of solid structure evolution on gasification reactivity of coal chars by in-situ Raman spectroscopy. Fuel 2020, 270, 117603. [Google Scholar] [CrossRef]
- Asadullah, M.; Zhang, S.; Min, Z.; Yimsiri, P.; Li, C.Z. Effects of biomass char structure on its gasification reactivity. Bioresour. Technol. 2010, 101, 7935–7943. [Google Scholar] [CrossRef]
- Lu, L.; Kong, C.; Sahajwalla, V.; Harris, D. Char structural ordering during pyrolysis and combustion and its influence on char reactivity. Fuel 2002, 819, 1215–1225. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Cheng, H.; Hu, M.; Zhang, S. Classification and carbon structural transformation from anthracite to natural coaly graphite by XRD, Raman spectroscopy, and HRTEM. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2021, 249, 119286. [Google Scholar] [CrossRef]
- Li, J.; Qin, Y.; Chen, Y.; Shen, J.; Song, Y.; Wang, Z. Structural characteristics and evolution of meta-anthracite to coaly graphite: A quantitative investigation using X-ray diffraction, Raman spectroscopy, and high-resolution transmission electron microscopy. Fuel 2023, 333, 126334. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, X.; Zhao, H.; Li, J.; He, J.; Yang, Y.; Yan, L. Comparative study of the composition and microstructural properties of semi-coke from microwave and conventional pyrolysis of low rank coal. J. Energy Inst. 2024, 116, 101752. [Google Scholar] [CrossRef]
- Appiah, J.; Tian, L.; Dou, J.; Chen, Y.; Chen, X.; Xu, X.; Yu, J. Investigation into the impact of coal blending on the carbon structure of chars obtained from Chinese coals during coking. J. Anal. Appl. Pyrolysis 2024, 179, 106504. [Google Scholar] [CrossRef]
- Yao, Q.; Ma, M.; Liu, Y.; Ma, D.; Chen, H.; Hao, Q.; Sun, M.; Ma, X. The structural and pyrolysis characteristics of vitrinite and inertinite from Shendong coal and the gasification performance of chars. J. Anal. Appl. Pyrolysis 2022, 164, 105519. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Liu, S.; Chen, S.; Li, W.; Wang, Y. Molecular structure controls on micropore evolution in coal vitrinite during coalification. Int. J. Coal Geol. 2018, 199, 19–30. [Google Scholar] [CrossRef]
- Kuang, Y.; Xie, W.; Wu, H.; Liu, X.; Sher, F.; Qiu, S.; Dang, J.; Zhang, S. Molecular structure of coal macerals and thermal response behavior of their chemical bonds obtained by structural characterizations and molecular dynamics simulations. Energy 2024, 301, 131735. [Google Scholar] [CrossRef]
- Cui, X.; Yan, H.; Zhao, P.; Yang, Y.; Xie, Y. Modeling of molecular and properties of anthracite base on structural accuracy identification methods. J. Mol. Struct. 2019, 1183, 313–323. [Google Scholar] [CrossRef]
- Ping, A.; Xia, W.; Peng, Y.; Xie, G. Construction of bituminous coal vitrinite and inertinite molecular assisted by 13C NMR, FTIR and XPS. J. Mol. Struct. 2020, 1222, 128959. [Google Scholar] [CrossRef]
- Li, Y.; Dai, C.; Meng, S.; Wu, J. Molecular structure characterization of coal with different coalification degrees: A combined study from FT-IR, Raman, 13C NMR spectroscopy and modelling. J. Mol. Struct. 2024, 1310, 138354. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Nassar, N.N.; Acevedo, S.A.; Cortés, F.B.; Franco, C.A. Thermo-oxidative decomposition behaviors of different sources of n-C7 Asphaltenes under high-pressure conditions. Energy Fuels 2020, 34, 8740–8758. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Pan, T.; Zhang, D.; Chen, L.; Sun, C. Accurately quantifying carbon structural types and predicting pyrolysis behavior of coal using solid 13C NMR Cp/MAS spectra. Fuel 2022, 327, 125092. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Mesroghli, S.; Hower, J.C. Simultaneous prediction of coal rank parameters based on ultimate analysis using regression and artificial neural network. Int. J. Coal Geol. 2010, 83, 31–34. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Hower, J.C.; Jorjani, E.; Mesroghli, S.; Bagherieh, A.H. Prediction of coal grindability based on petrography, proximate and ultimate analysis using multiple regression and artificial neural network models. Fuel Process. Technol. 2008, 89, 13–20. [Google Scholar] [CrossRef]
- Lu, L.; Sahajwalla, V.; Kong, C.; Harris, D. Quantitative X-ray diffraction analysis and its application to various coals. Carbon 2001, 39, 1821–1833. [Google Scholar] [CrossRef]
- Lampropoulos, A.; Binas, V.D.; Zouridi, L.; Athanasiou, C.; Montes-Morán, M.A.; Menéndez, J.A.; Konsolakis, M.; Marnellos, G.E. CO2 Gasification Reactivity and Syngas Production of Greek Lignite Coal and Ex-Situ Produced Chars under Non-Isothermal and Isothermal Conditions: Structure-Performance Relationships. Energies 2022, 1, 679. [Google Scholar] [CrossRef]
- Tian, J.; Wei, K.; Deng, X.; Ma, W. A Study of the Effect of Quartz-to-Cristobalite Transformation on SiC Generation in MG-Si Production. Silicon 2024, 16, 3155–3164. [Google Scholar] [CrossRef]
- Bailey, N.T.; Ikejiani, A.O.; Lawson, G.J.; Monsef-Mirzai, P. Measurement of hydroxyl groups in coals of different rank by acetylation with ketene and other reagents. Fuel 1992, 71, 45–52. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, Y.; Xie, Z.; Xi, L.; Li, Y.; Yang, J.; Hu, H. Quantitative characterization of coal structure by high-resolution CP/MAS 13C solid-state NMR spectroscopy. Proc. Combust. Inst. 2021, 38, 4161–4170. [Google Scholar] [CrossRef]
- Rosenvold, R.J.; Dubow, J.B.; Rajeshwar, K. Thermal analyses of Ohio bituminous coals. Thermochim. Acta 1982, 53, 321–332. [Google Scholar] [CrossRef]
- Zhu, Y.; Wen, W.; Li, Y.; Lu, L.; Yang, J.; Xu, M.; Pan, Y. Pyrolysis study of Huainan coal with different particle sizes using TG analysis and online Py-PI-TOF MS. J. Energy Inst. 2020, 93, 405–414. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Qiu, P.H.; Xie, X.; Chen, X.Y.; Lin, D.; Sun, S.Z. Impacts of chemical fractionation on Zhundong coal’s chemical structure and pyrolysis reactivity. Fuel Process. Technol. 2017, 155, 144–152. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, D.; Fu, X.; Lu, W.; Yuan, S. A novel dual-way inference modeling method for coal coking: Predicting H2 and CH4 concentrations in coke oven gas and inferring optimal reaction conditions. Fuel 2025, 381, 133325. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Zheng, F.; Lyu, W. Molecular structure model construction and pyrolysis mechanism study on low-rank coal by experiments and ReaxFF simulations. J. Anal. Appl. Pyrolysis 2024, 178, 106387. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Shekhawat, D.; Smith, M.W.; Link, D.; Stiegman, A.E. Microwave-assisted pyrolysis of Mississippi coal: A comparative study with conventional pyrolysis. Fuel 2018, 217, 656–667. [Google Scholar] [CrossRef]
- Huang, Y.B.; Zhao, Y.P.; Qiu, L.L.; Wu, F.P.; Liang, J.; Xiao, J.; Liu, F.J.; Cao, J.P. Pyrolysis behaviors of lump sub-bituminous coal. Part 1: Structural evolution of chars both at particle surface and center. Fuel 2024, 376, 132709. [Google Scholar] [CrossRef]


| Sample | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | C | H | O | N | |
| 1 | 8.68 | 4.45 | 38.76 | 48.11 | 73.19 | 5.15 | 20.53 | 1.13 |
| 2 | 7.39 | 4.12 | 32.37 | 56.12 | 75.98 | 4.65 | 18.62 | 0.75 |
| 3 | 7.40 | 3.42 | 30.13 | 59.05 | 76.20 | 4.48 | 18.52 | 0.80 |
| Type | Symbol | Chemical Shift (ppm) | 1 | 2 | 3 |
|---|---|---|---|---|---|
| Area Percent (%) | Area Percent (%) | Area Percent (%) | |||
| Aliphatic | fal | 0–100 | 69.85 | 43.87 | 37.44 |
| Aromatic | fα | 100–165 | 22.37 | 44.89 | 49.73 |
| C=O | fC=O | 165–220 | 7.77 | 11.24 | 12.79 |
| Sample | Raw Coal | 500 °C | 900 °C | 1300 °C | 1600 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | C/H | C | H | C/H | C | H | C/H | C | H | C/H | C | H | C/H | |
| 1 | 73.19 | 5.15 | 1.18 | 80.04 | 3.48 | 1.92 | 89.5 | 1.01 | 7.38 | 88.59 | 0.99 | 7.5 | 82.24 | 0.82 | 8.36 |
| 2 | 75.98 | 4.65 | 1.36 | 81.86 | 3.38 | 2.02 | 88.21 | 0.89 | 8.26 | 78.03 | 0.71 | 9.15 | 88.67 | 0.75 | 9.83 |
| 3 | 76.2 | 4.48 | 1.42 | 81.38 | 3.56 | 1.9 | 89.52 | 0.87 | 8.57 | 95.21 | 0.69 | 11.45 | 95.73 | 0.63 | 12.7 |
| Sample | d002 (Å) | La (Å) | Lc (Å) | d002 (Å) | La (Å) | Lc (Å) | d002 (Å) | La (Å) | Lc (Å) |
| 1 | 2 | 3 | |||||||
| Raw | 3.64 | 24.77 | 9.74 | 3.62 | 23.44 | 10.06 | 3.59 | 24.92 | 10.48 |
| 500 °C | 3.63 | 26.41 | 10.18 | 3.61 | 28.43 | 11.76 | 3.58 | 28.21 | 11.67 |
| 900 °C | 3.62 | 35.44 | 11.72 | 3.60 | 36.25 | 12.14 | 3.56 | 37.90 | 12.33 |
| 1300 °C | 3.59 | 46.89 | 11.98 | 3.56 | 49.60 | 12.67 | 3.56 | 54.55 | 14.14 |
| 1600 °C | 3.49 | 51.00 | 13.21 | 3.41 | 56.97 | 16.19 | 3.42 | 58.17 | 17.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Guo, W.; Han, L. Thermal Transformation of Coal Molecular Structures and Their Impact on Reactivity in Metallurgical-Grade Silicon Production. Processes 2025, 13, 1371. https://doi.org/10.3390/pr13051371
Du Z, Guo W, Han L. Thermal Transformation of Coal Molecular Structures and Their Impact on Reactivity in Metallurgical-Grade Silicon Production. Processes. 2025; 13(5):1371. https://doi.org/10.3390/pr13051371
Chicago/Turabian StyleDu, Zhouyuntian, Weifang Guo, and Linjun Han. 2025. "Thermal Transformation of Coal Molecular Structures and Their Impact on Reactivity in Metallurgical-Grade Silicon Production" Processes 13, no. 5: 1371. https://doi.org/10.3390/pr13051371
APA StyleDu, Z., Guo, W., & Han, L. (2025). Thermal Transformation of Coal Molecular Structures and Their Impact on Reactivity in Metallurgical-Grade Silicon Production. Processes, 13(5), 1371. https://doi.org/10.3390/pr13051371






