Temperature-Dependent Crystallization Optimization for Upcycling Purified Ash from the Calcium Carbide Industry: A Sustainable Approach for Mg(OH)2/Aragonite Coproduction
Abstract
1. Introduction
2. Experimental
2.1. Materials
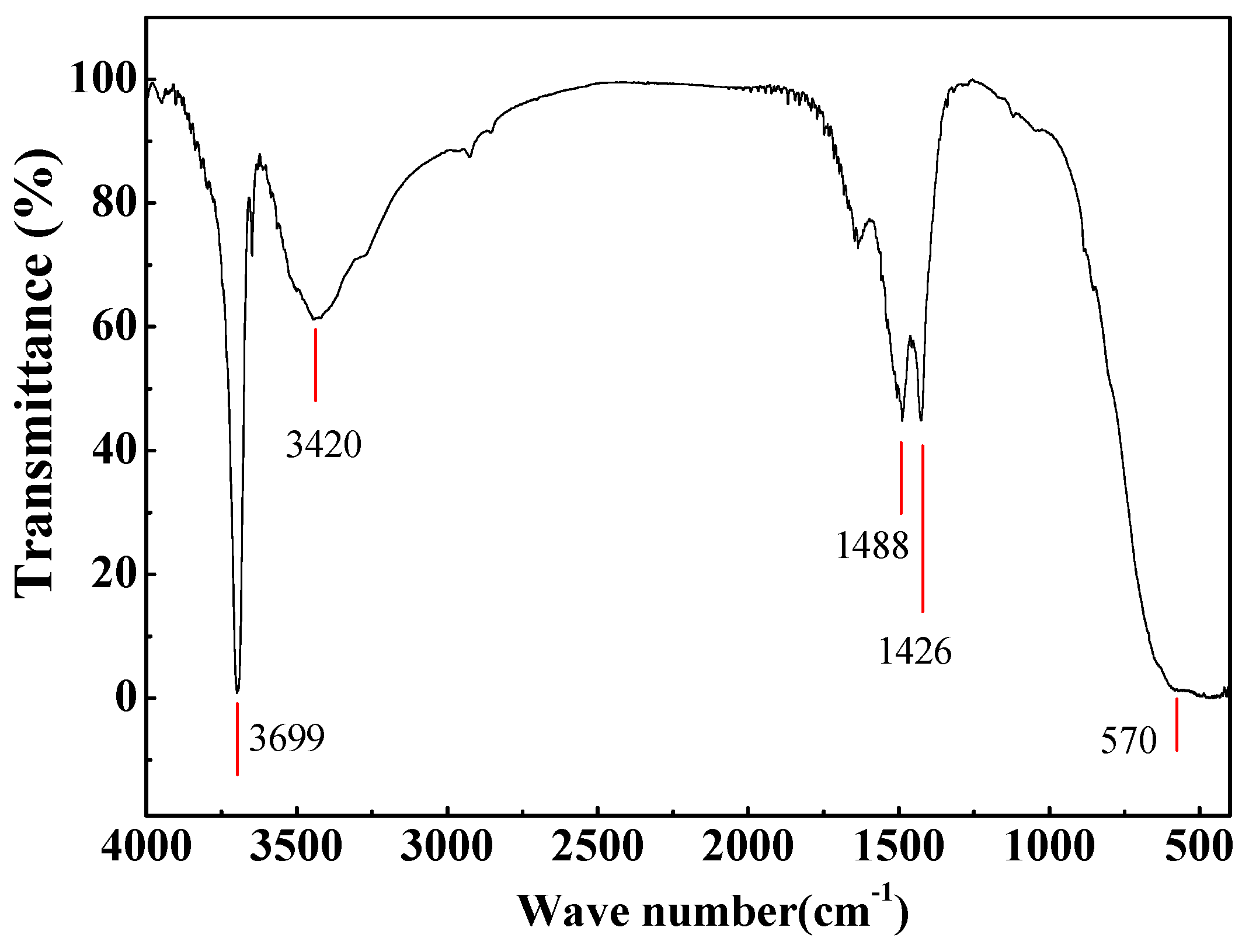
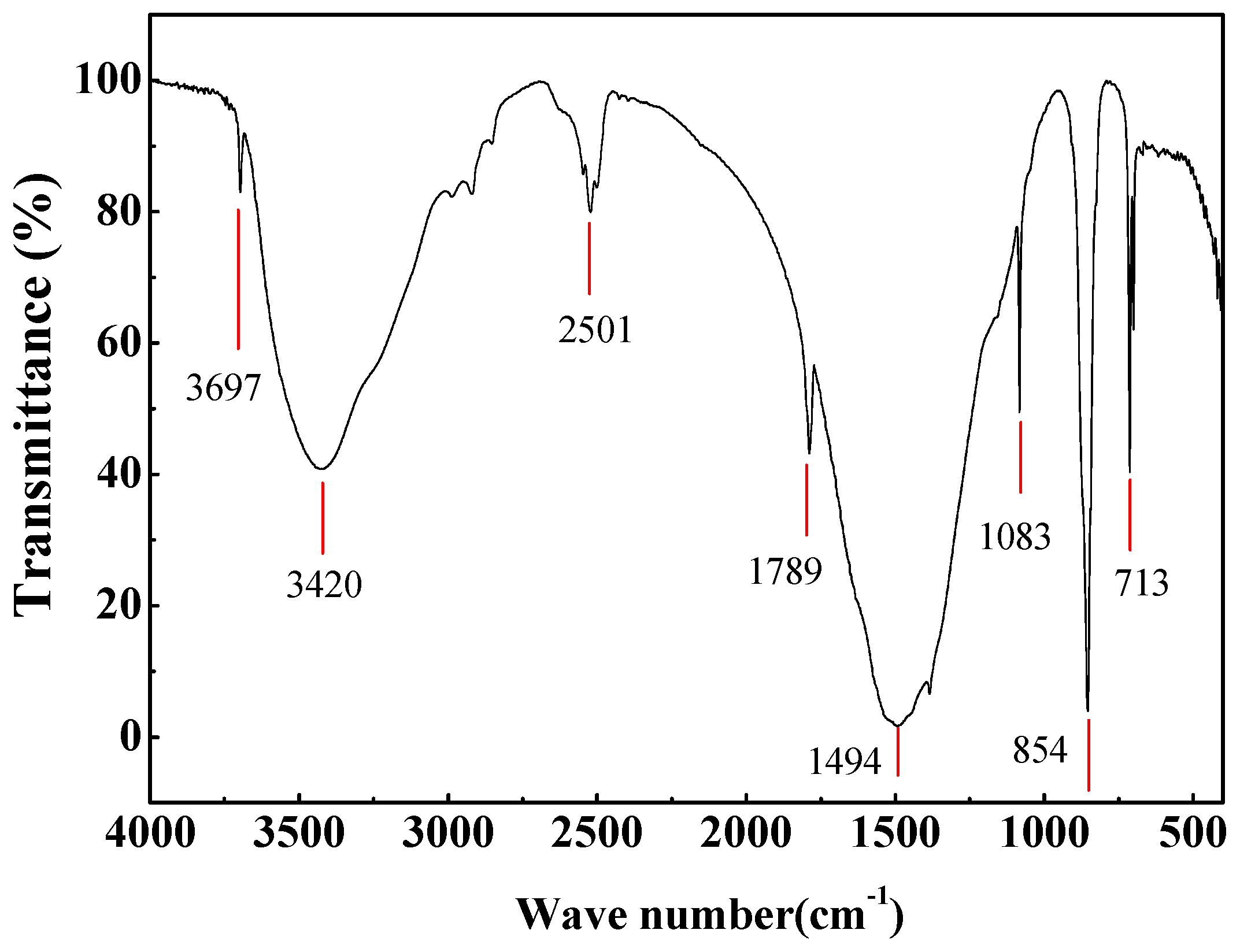
2.2. Characterization
2.3. Procedure
3. Results and Discussion
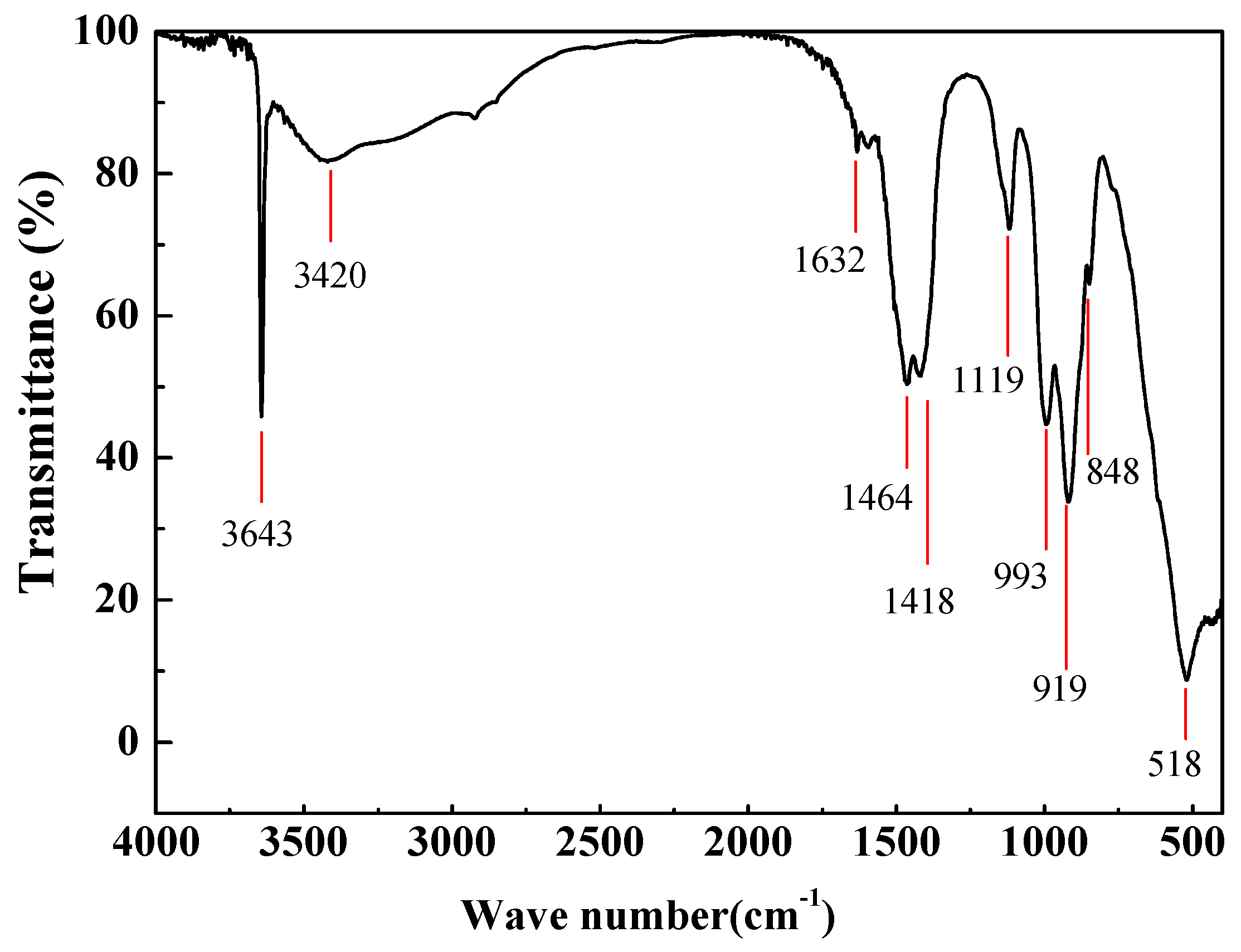
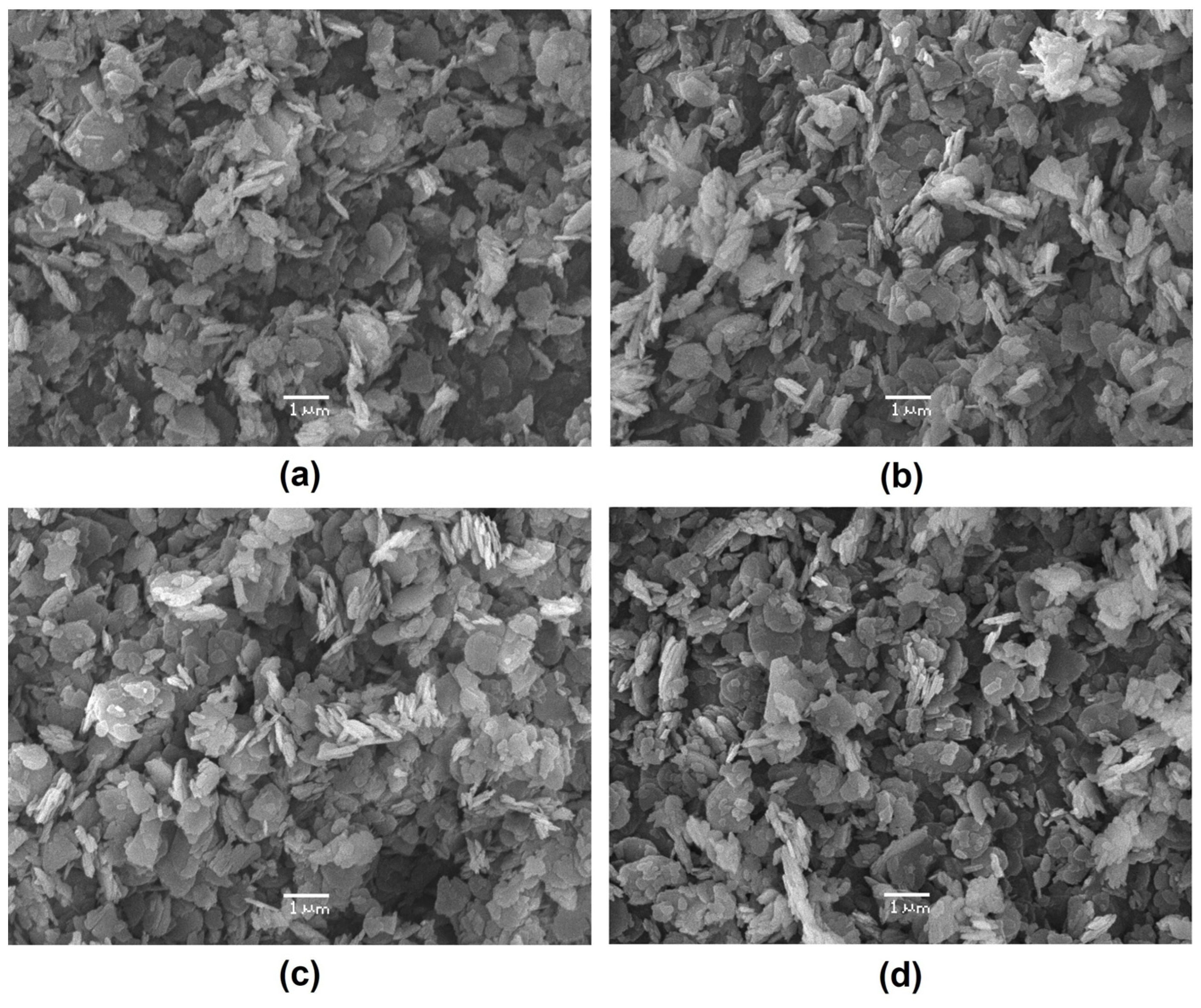
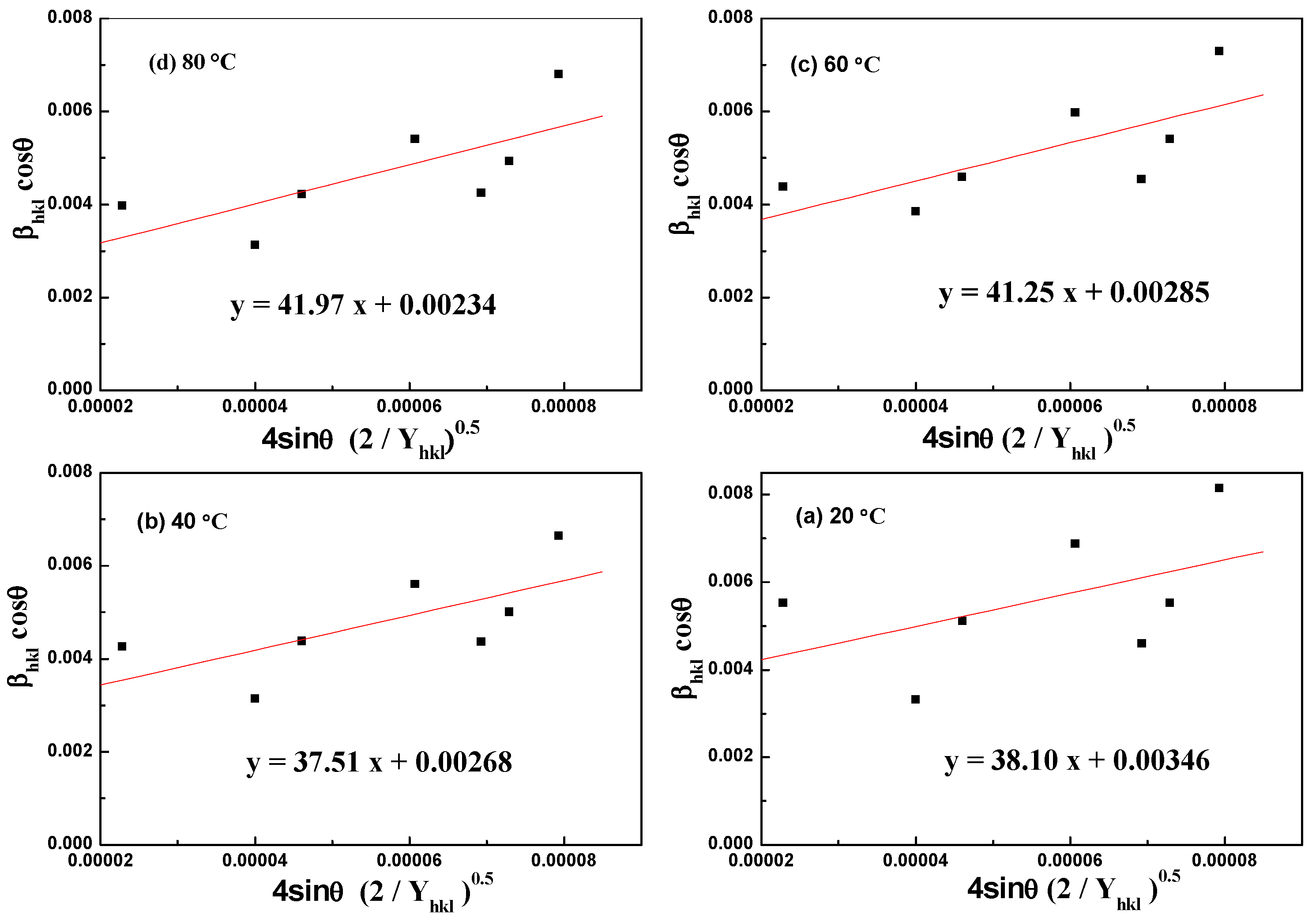
3.1. Thermogradient-Driven Crystallization Analysis of Mg(OH)2
- (1)
- UDM
- (2)
- USDM
- (3)
- UDEDM
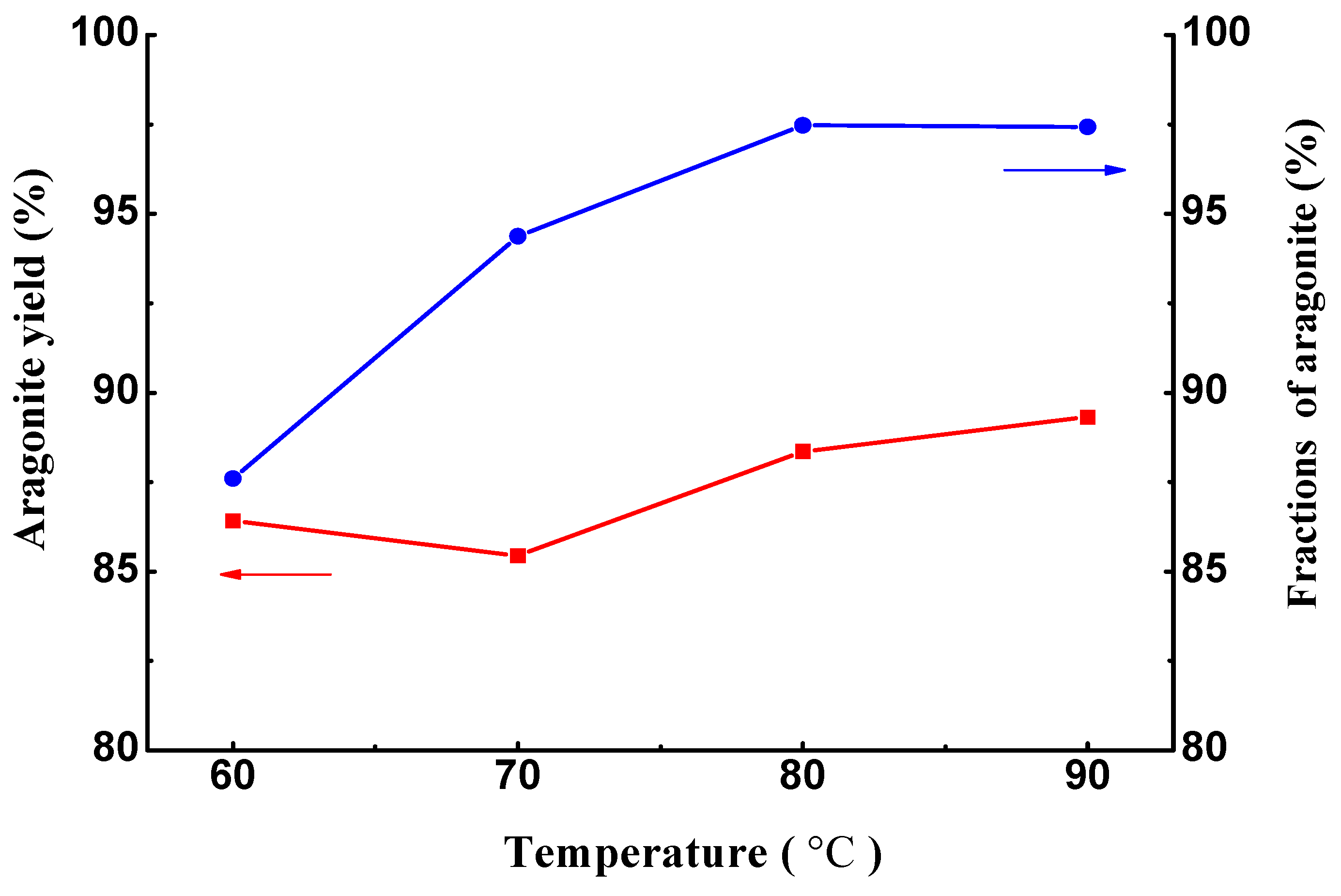
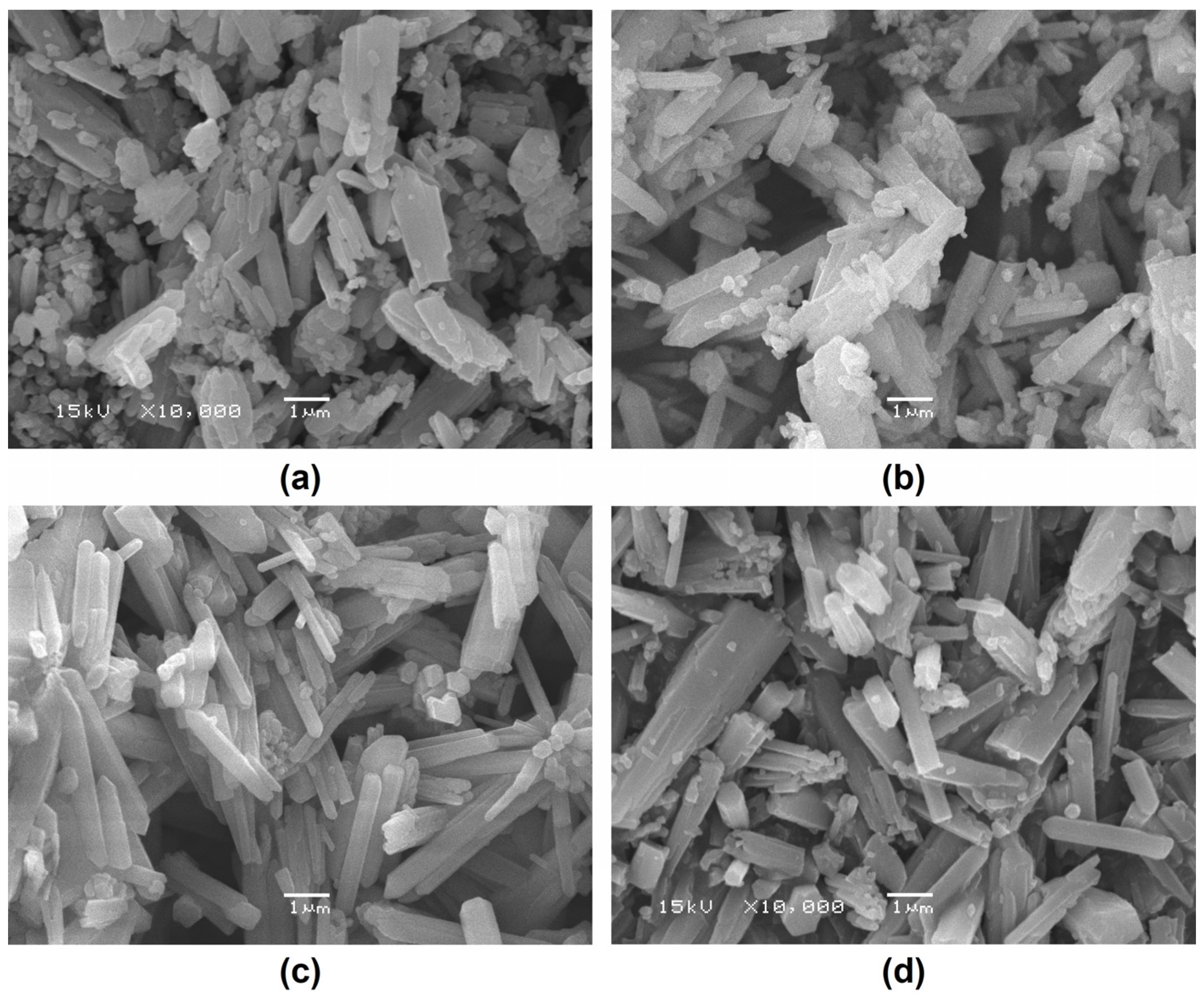
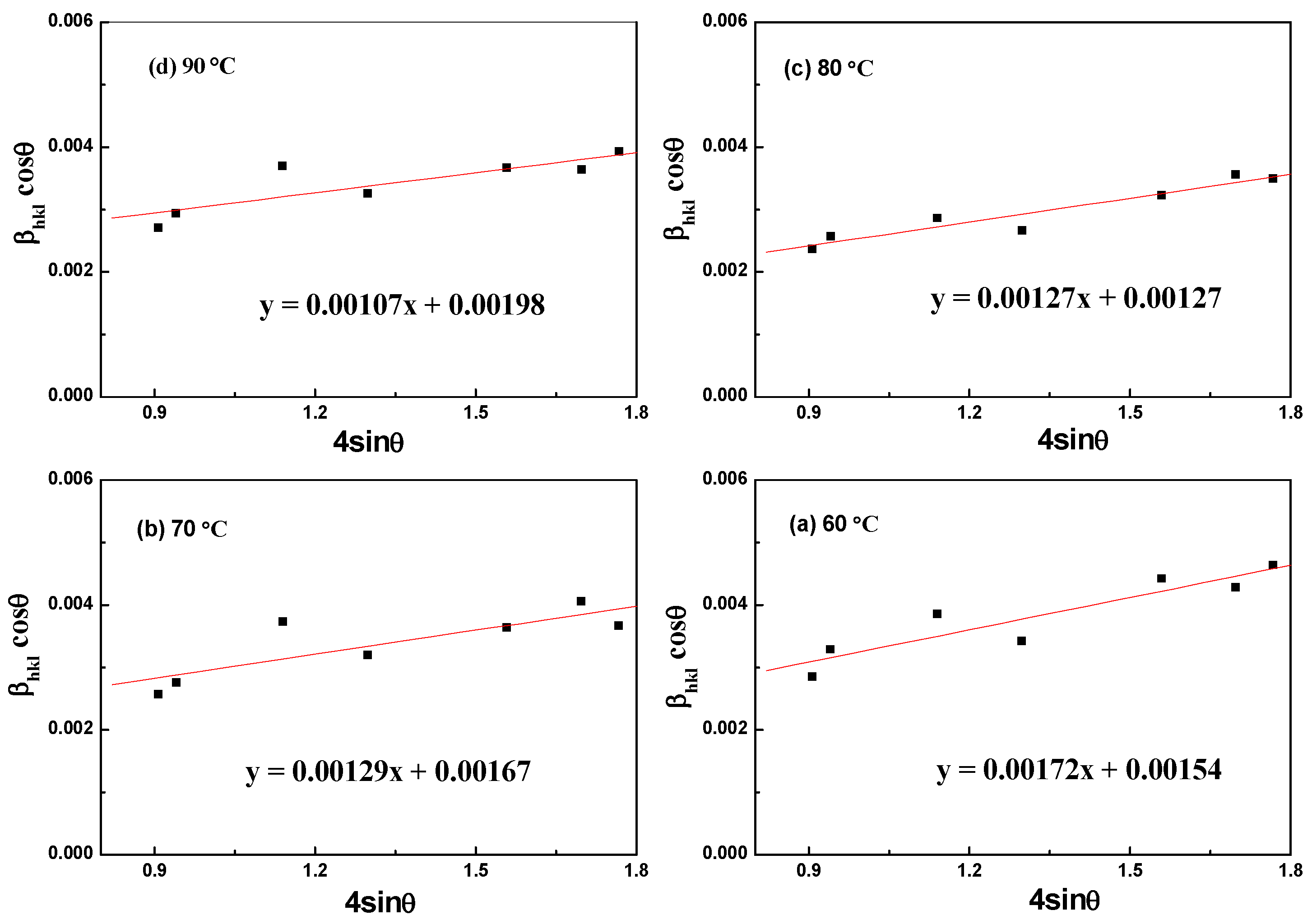
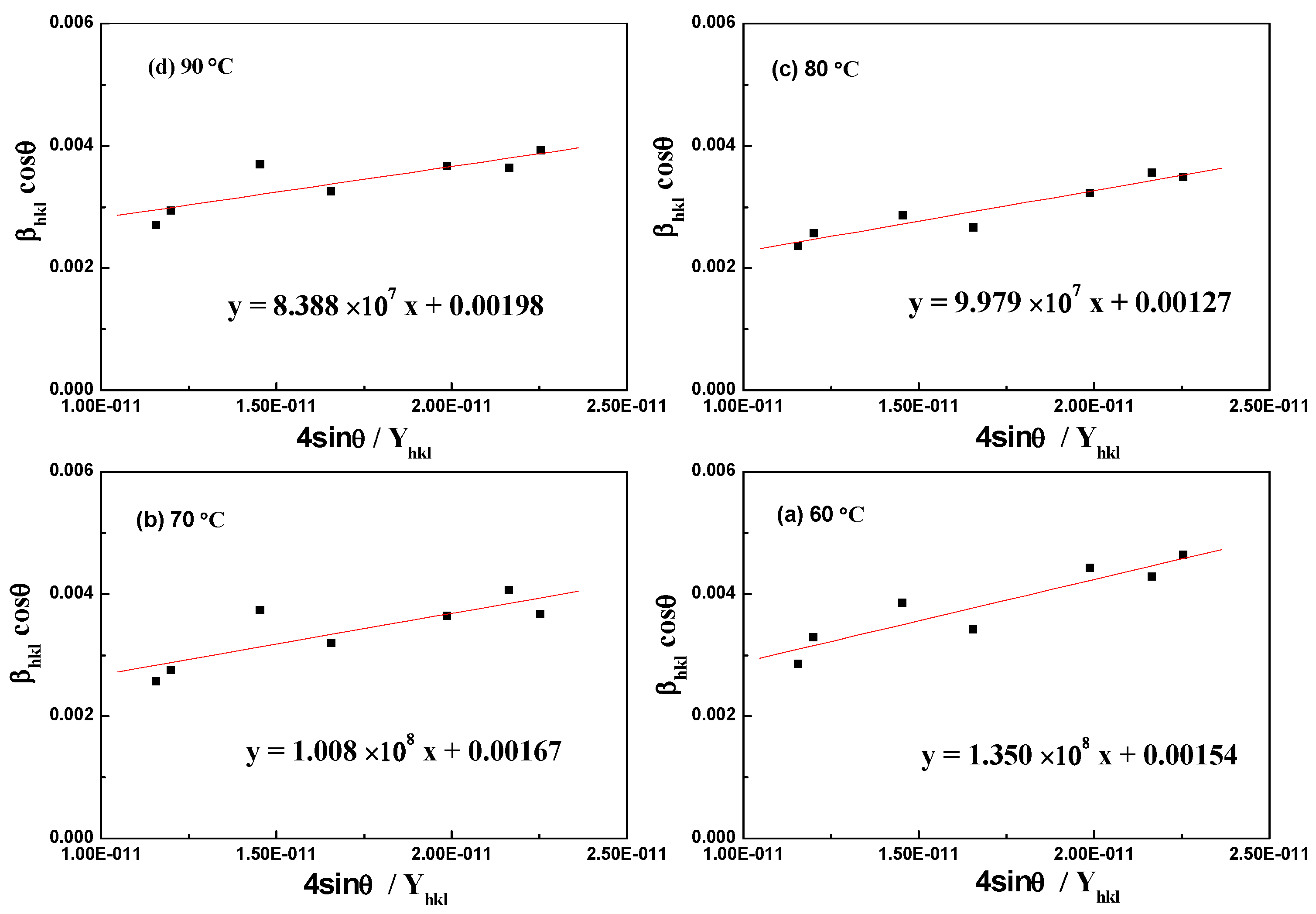
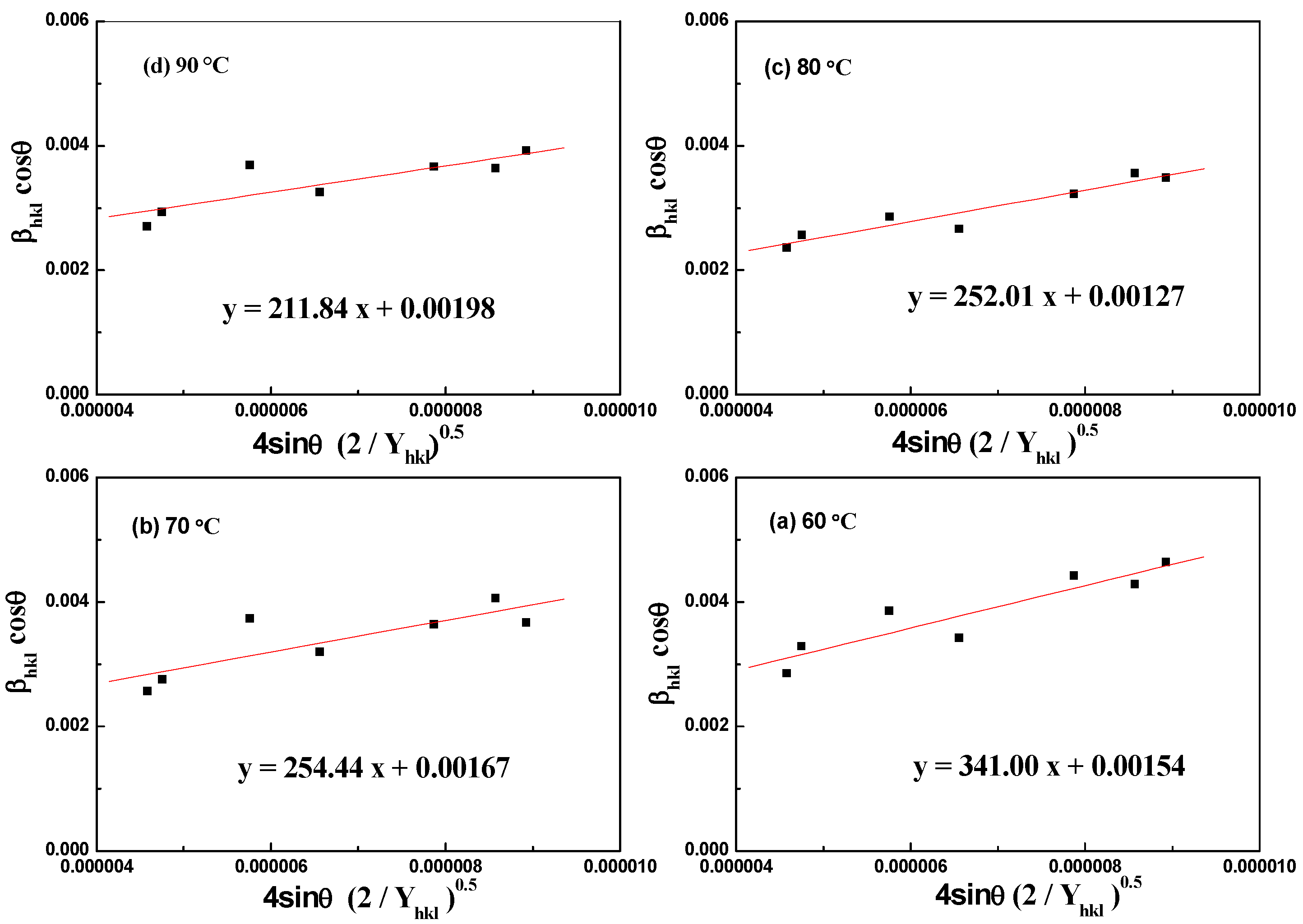
3.2. Temperature-Dependent Synthesis Analysis of Aragonite
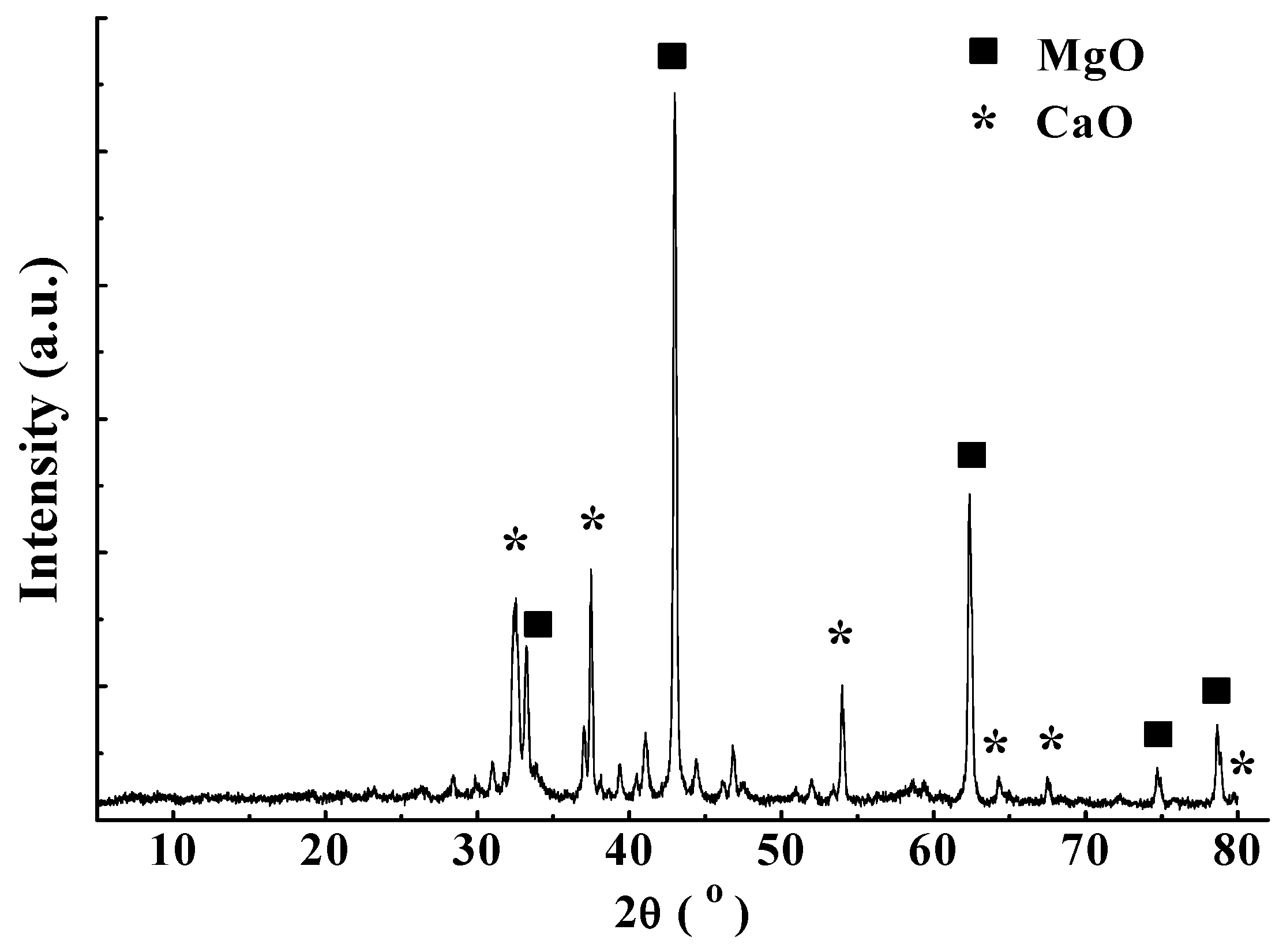
3.3. Component and Resource Efficiency Analysis of the Crystallization Product
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maalouf, A.; Mavropoulos, A. Re-assessing global municipal solid waste generation. Waste. Manag. Res. 2023, 41, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Xu, X.; Wang, W.; Jiang, J.; Gao, S. Do industrial solid waste recycling and technological innovation promote low-carbon development in China: New insights from the NARDL approach. Sci. Total Environ. 2024, 916, 170446. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Lopez-Maldonado, E.A.; Khan, N.A.; Villarreal-Gomez, L.J.; Munshi, F.M.; Alsabhan, A.H.; Perveen, K. Current solid waste management strategies and energy recovery in developing countries-state of art review. Chemosphere 2022, 291, 133088. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, J.; Dong, S.; Ma, L.; Dai, Q.; Guo, J. Zeolite preparation from industrial solid waste: Current status, applications, and prospects. Sep. Purif. Technol. 2025, 354, 128957. [Google Scholar] [CrossRef]
- Salman, S.; Bhattacharjee, P.; Rahman, M.; Nur, S.A.; Sindhwani, R.; Ali, S.M. Pathways to advancing sustainable practices in industrial solid waste management: Unveiling obstacles and implications. Next Res. 2025, 2, 100124. [Google Scholar] [CrossRef]
- Yıldız, T.D. Considering the development levels of countries, contributions of mineral recovery from mining tailings and urban mining wastes to sustainability criteria-A review. Res. Policy 2024, 99, 105399. [Google Scholar] [CrossRef]
- Tang, P.; Javadi, A.A.; Vinai, R. Sustainable utilization of calcium-rich industrial wastes in soil stabilization: Potential use of calcium carbide residue. J. Environ. Manag. 2024, 357, 120800. [Google Scholar] [CrossRef]
- Gupta, S. Industrial Calcium Byproduct Waste: Sustainable Construction Materials for Building Applications. ACS Sustain. Resour. Manag. 2025, 2, 212–218. [Google Scholar] [CrossRef]
- Man, L.; Zhao, Y. Mineral resource extraction and environmental sustainability for green recovery. Res. Policy 2024, 90, 104616. [Google Scholar]
- Qin, Z.L.; Xiao, J.H.; Zhang, J.H.; Zou, K. Practical experience to theoretical innovation: A model for recovering metal resources from industrial solid waste- A case study of copper smelting slag. Sep. Purif. Technol. 2025, 354, 129395. [Google Scholar] [CrossRef]
- Gu, J.; Liu, X.; Zhang, Z. Road base materials prepared by multi-industrial solid wastes in China: A review. Constr. Build. Mater. 2023, 373, 130860. [Google Scholar] [CrossRef]
- Bai, L.; Wang, M.; Liang, X.; Zhao, W.; Dai, S. Research on growth mechanism of magnesium hydroxide crystal thin films based on hydrothermal system. J. Mol. Struct. 2024, 643, 127810. [Google Scholar] [CrossRef]
- Zou, G.; Liu, R.; Chen, W.; Xu, Z. Preparation and characterization of lamellar-like Mg(OH)2 nanostructures via natural oxidation of Mg metal in formamide/water mixture. Mater. Res. Bull. 2007, 42, 1153–1158. [Google Scholar] [CrossRef]
- Battaglia, G.; Romano, S.; Raponi, A.; Marchisio, D.; Ciofalo, M.; Tamburini, A.; Cipollina, A.; Micale, G. Analysis of particles size distributions in Mg(OH)2 precipitation from highly concentrated MgCl2 solutions. Powder Technol. 2022, 398, 117106. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Klapiszewski, Ł.; Jesionowski, T. Recent development in the synthesis, modification and application of Mg(OH)2 and MgO: A review. Powder Technol. 2017, 319, 373–407. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, G.; Wu, H.; Hai, B.; Wang, L.; Qian, Y. Nanoscale magnesium hydroxide and magnesium oxide powders: Control over size, shape, and structure via hydrothermal synthesis. Chem. Mater. 2001, 13, 435–440. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, T.; Fang, H.; Li, S.; Yao, Y.; Fan, B.; Wang, J. A novel preparation of nanosized hexagonal Mg(OH)2 as a flame retardant. Particuology 2016, 24, 177–182. [Google Scholar] [CrossRef]
- Shen, P.; Jiang, Y.; Zhang, Y.; Liu, S.; Xuan, D.; Lu, J.; Zhang, S.; Poon, C.S. Production of aragonite whiskers by carbonation of fine recycled concrete wastes: An alternative pathway for efficient CO2 sequestration. Renew. Sustain. Energy Rev. 2023, 173, 113079. [Google Scholar] [CrossRef]
- Kotresh, M.G.; Patil, M.K.; Sunilkumar, A.; Sushilabai, A.; Inamdar, S.R. A study on the effect of reaction temperature on the synthesis of magnesium hydroxide nanoparticles: Comparative evaluation of microstructure parameters and optical properties. Results Opt. 2023, 10, 100336. [Google Scholar] [CrossRef]
- Kogo, M.; Suzuki, K.; Umegaki, T.; Kojima, Y. Control of aragonite formation and its crystal shape in CaCl2-Na2CO3-H2O reaction system. J. Cryst. Growth 2021, 559, 125964. [Google Scholar] [CrossRef]
- Jamil, N.H.; Abdullah, M.A.B.; Pa, F.C.; Mohamad, H.; Ibrahim, W.M.A.W.; Chaiprapa, J. Influences of SiO2, Al2O3, CaO and MgO in phase transformation of sintered kaolin-ground granulated blast furnace slag geopolymer. J. Mater. Res. Technol. 2020, 9, 14922–14932. [Google Scholar] [CrossRef]
- Alnehia, A.; Alnahari, H.; Al-Sharabi, A. Characterization and antibacterial activity of MgO/CuO/Cu2MgO3 nanocomposite synthesized by sol-gel technique. Results Chem. 2024, 8, 101620. [Google Scholar] [CrossRef]
- Liu, H.; Luo, C.G.; Zou, L.X. Effect of brine-ammonia reverse precipitation on crystal growth of magnesium hydroxide. Nonferrous Met. Sci. Eng. 2023, 14, 151–160. [Google Scholar]
- Li, N.; Li, Z.; Liu, Z.Q.; Yang, Y.X.; Jia, Y.C.; Li, J.S.; Wei, M.; Li, L.J.; Wang, D.Y. Magnesium hydroxide micro-whiskers as super-reinforcer to improve fire retardancy and mechanical property of epoxy resin. Poly. Compos. 2022, 43, 1996–2009. [Google Scholar] [CrossRef]
- Alam, M.K.; Hossain, M.S.; Bahadur, N.M.; Ahmed, S. A comparative study in estimating of crystallite sizes of synthesized and natural hydroxyapatites using Scherrer Method, Williamson-Hall model, Size-Strain Plot and Halder-Wagner Method. J. Mol. Struct. 2024, 1306, 137820. [Google Scholar] [CrossRef]
- Kawsar, M.; Hossain, M.S.; Bahadur, N.M.; Ahmed, S. Synthesis of nano-crystallite hydroxyapatites in different media and a comparative study for estimation of crystallite size using Scherrer method, Halder-Wagner method size-strain plot, and Williamson-Hall model. Heliyon 2024, 10, 25347. [Google Scholar] [CrossRef]
- Rajender, G.; Giri, P.K. Strain induced phase formation, microstructural evolution and bandgap narrowing in strained TiO2 nanocrystals grown by ball milling. J. Alloys Compd. 2016, 676, 591–600. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles- a comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Patel, K.; Patel, A.; Jethwa, V.P.; Patel, H.; Solanki, G.K. X-ray diffraction analysis of orthorhombic SnSe nanoparticles by Williamson–Hall, Halder–Wagner and Size–Strain plot methods. Chem. Phys. Impact 2024, 8, 100547. [Google Scholar] [CrossRef]
- Wan, C.; Zheng, Y.; Zhang, P.; Ma, M. Effect of hybrid basalt-brucite fiber on the fracture behavior of low-heat cement concrete. Constr. Build. Mater. 2024, 442, 137667. [Google Scholar] [CrossRef]
- Yogamalar, R.; Srinivasan, R.; Vinu, A.; Ariga, K.; Bose, A.C. X-ray peak broadening analysis in ZnO nanoparticles. Solid State Commun. 2009, 149, 1919–1923. [Google Scholar] [CrossRef]
- Park, W.K.; Ko, S.-J.; Lee, S.W.; Cho, K.-H.; Ahn, J.-W.; Han, C. Effects of magnesium chloride and organic additives on the synthesis of aragonite precipitated calcium carbonate. J. Cryst. Growth 2008, 310, 2593–2601. [Google Scholar] [CrossRef]
- Santos, R.M.; Ceulemans, P.; Van Gerven, T. Synthesis of pure aragonite by sonochemical mineral carbonation. Chem. Eng. Res. Des. 2012, 90, 715–725. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, K.; Luo, Y.; Qu, D.; Li, X.; Liu, T.; Zhang, J. Analyzing the dynamic failure process of coral reef limestone through digital core-based numerical trials. Comput. Geotech. 2024, 172, 106489. [Google Scholar] [CrossRef]
- Simm, T.H. Peak broadening anisotropy and the contrast factor in metal alloys. Crystals 2018, 8, 212. [Google Scholar] [CrossRef]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef]
- Yan, S.; Li, Y.; Cao, J.L.; Guo, H.F. Upgrading of Ca(NO3)2 waste slag to aragonite nanowhiskers via a facile precipitation-transformation method for uranium adsorption. Chem. Eng. Res. Des. 2025, 213, 221–229. [Google Scholar] [CrossRef]






| Oxide | MgO | CaO | SiO2 | K2O | Na2O | Al2O3 | SO3 | Fe2O3 | MnO | ZnO | Cr2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content(%) | 49.10 | 29.50 | 10.50 | 4.57 | 1.92 | 1.73 | 1.15 | 0.65 | 0.53 | 0.16 | 0.04 |
| Samples | Lattice Parameters (Å) | V (Å3) | |
|---|---|---|---|
| a = b | c | ||
| 20 °C | 3.1479 | 4.7796 | 41.02 |
| 40 °C | 3.1493 | 4.7814 | 41.07 |
| 60 °C | 3.1496 | 4.7814 | 41.08 |
| 80 °C | 3.1493 | 4.7801 | 41.06 |
| Reference data | 3.1450 | 4.7400 | 40.60 |
| Williamson–Hall Model | Crystallite Size, D (nm); Strain, ε (N/m2); Stress, σ (MPa); Energy Density, u (kJ/m3); | |||
|---|---|---|---|---|
| 80 °C | 60 °C | 40 °C | 20 °C | |
| UDM | ε = 0.00148 | ε = 0.00146 | ε = 0.00133 | ε = 0.00135 |
| D = 59.25 | D = 48.65 | D = 51.74 | D = 40.07 | |
| USDM | σ = 2.37 | σ = 2.33 | σ = 2.12 | σ = 2.16 |
| D = 59.25 | D = 48.65 | D = 51.74 | D = 40.07 | |
| UDEDM | u = 1.76 | u = 1.70 | u = 1.41 | u = 1.45 |
| D = 59.25 | D = 48.65 | D = 51.74 | D = 40.07 | |
| Samples | Lattice Parameters (Å) | V (Å3) | ||
|---|---|---|---|---|
| A | b | c | ||
| 60 °C | 4.9709 | 7.9905 | 5.7480 | 228.31 |
| 70 °C | 4.9698 | 7.9840 | 5.7487 | 228.10 |
| 80 °C | 4.9677 | 7.9817 | 5.7456 | 227.82 |
| 90 °C | 4.9698 | 7.9887 | 5.7328 | 227.61 |
| Reference data | 4.9614 | 7.9671 | 5.7404 | 226.91 |
| Williamson–Hall Model | Crystallite Size, D (nm); Strain, ε (N/m2); Stress, σ (MPa); Energy Density, u (kJ/m3); | |||
|---|---|---|---|---|
| 90 °C | 80 °C | 70 °C | 60 °C | |
| UDM | ε = 0.00107 | ε = 0.00127 | ε = 0.00129 | ε = 0.00172 |
| D = 70.03 | D = 109.18 | D = 83.03 | D = 90.04 | |
| USDM | σ = 83.88 | σ = 99.79 | σ = 100.80 | σ = 135.00 |
| D = 70.03 | D = 109.18 | D = 83.03 | D = 90.04 | |
| UDEDM | u = 44.88 | u = 63.51 | u = 64.74 | u = 116.28 |
| D = 70.03 | D = 109.18 | D = 83.03 | D = 90.04 | |
| Mg(OH)2 | Aragonite | ||
|---|---|---|---|
| Oxide | Content (%) | Oxide | Content (%) |
| MgO | 99.2826 | CaO | 98.7940 |
| SiO2 | 0.2993 | SO3 | 0.4439 |
| CaO | 0.1347 | Cl | 0.3106 |
| SO3 | 0.1343 | SrO | 0.1511 |
| MnO2 | 0.0981 | SiO2 | 0.1461 |
| Fe2O3 | 0.0252 | Cr2O3 | 0.1250 |
| P2O5 | 0.0187 | K2O | 0.0294 |
| MoO3 | 0.0072 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Wang, L.; Hong, Y.; Zhang, D.; Zhou, W.; Xie, L.; Zhao, W.; Huo, L.; Yan, S.; Ren, X. Temperature-Dependent Crystallization Optimization for Upcycling Purified Ash from the Calcium Carbide Industry: A Sustainable Approach for Mg(OH)2/Aragonite Coproduction. Processes 2025, 13, 1370. https://doi.org/10.3390/pr13051370
Duan Y, Wang L, Hong Y, Zhang D, Zhou W, Xie L, Zhao W, Huo L, Yan S, Ren X. Temperature-Dependent Crystallization Optimization for Upcycling Purified Ash from the Calcium Carbide Industry: A Sustainable Approach for Mg(OH)2/Aragonite Coproduction. Processes. 2025; 13(5):1370. https://doi.org/10.3390/pr13051370
Chicago/Turabian StyleDuan, Yingfeng, Lu Wang, Yanyun Hong, Deliang Zhang, Wenwu Zhou, Liangbin Xie, Weiqin Zhao, Lianjie Huo, Shaobang Yan, and Xiubin Ren. 2025. "Temperature-Dependent Crystallization Optimization for Upcycling Purified Ash from the Calcium Carbide Industry: A Sustainable Approach for Mg(OH)2/Aragonite Coproduction" Processes 13, no. 5: 1370. https://doi.org/10.3390/pr13051370
APA StyleDuan, Y., Wang, L., Hong, Y., Zhang, D., Zhou, W., Xie, L., Zhao, W., Huo, L., Yan, S., & Ren, X. (2025). Temperature-Dependent Crystallization Optimization for Upcycling Purified Ash from the Calcium Carbide Industry: A Sustainable Approach for Mg(OH)2/Aragonite Coproduction. Processes, 13(5), 1370. https://doi.org/10.3390/pr13051370






