Abstract
Nostoc sphaeroides is a cyanobacterium known for its valuable health benefits, including antioxidant, anti-inflammatory, and immune-boosting properties, making it a promising addition to functional foods. However, large-scale cultivation has remained necessary due to economic value and decreased yield. This study focused on the effect of varying inoculation densities and seasonal conditions on the growth and quality of N. sphaeroides. The main results were as follows: The highest average fresh weight productivity per unit volume was observed in summer and autumn at an inoculation density of 10 g·L−1, with values of 0.26 g·L−1·d−1 and 0.31 g·L−1·d−1, respectively. In winter and spring, the highest productivity was achieved at an inoculation density of 15 g·L−1, with values of 0.52 g·L−1·d−1 and 1.40 g·L−1·d−1, respectively. Nutritional components varied seasonally, with chlorophyll and carotenoid contents peaking in spring and phycobiliproteins, including phycocyanin, allophycocyanin, and phycoerythrin, reaching their highest levels in summer. The dry weight was greatest in summer, while the total protein content was highest in autumn, with values of 40.87%, 39.66%, and 41.44% for 5 g·L−1, 10 g·L−1, and 15 g·L−1, respectively. In terms of texture, the hardness of N. sphaeroides was highest in autumn at inoculation densities of 5 g·L−1 and 15 g·L−1, with values of 153.96 g and 146.88 g, respectively. At 10 g·L−1, the highest hardness was observed in spring (109.67 g). The elasticity and chewiness of the algae were best in spring across all inoculation densities, with elasticity values of 2.86, 2.54, and 2.07, and chewiness values of 112.37, 120.67, and 75.96 for 5 g·L−1, 10 g·L−1, and 15 g·L−1, respectively. In conclusion, the optimal inoculation density for summer and autumn is 10 g·L−1, while for winter and spring, it is 15 g·L−1. N. sphaeroides exhibits better texture in spring. This study offers valuable information for the large-scale cultivation and functional food applications of N. sphaeroides.
1. Introduction
Nostoc sphaeroides belongs to the phylum Cyanophyta, class Cyanophyceae, and genus Nostoc. It has a long history of being consumed as food [1]. Not only is it rich in nutrients such as proteins, amino acids, fatty acids, polysaccharides, and vitamins [2,3], but it also possesses various bioactive functions, including anti-inflammatory, antibacterial, antioxidant, anticancer, and immunomodulatory properties, making it highly valuable for potentially medicinal purposes [4,5].
N. sphaeroides, a cyanobacterial species predominantly grown in rice paddies of Hefeng County, Hubei Province, exhibits an ordinary harvest season from March to late May. Environmental stressors have caused a dramatic decline in annual production from a historical peak of 25 tons to less than 0.5 tons in recent decades [6], with current market values reaching USD 300–800 per kilogram of dry product. This economic context underscores the necessity for developing cost-effective cultivation methodologies.
Substantial research efforts over the past decades have focused on commercial-scale production techniques. Indoor photobioreactor systems employing artificial illumination and aerated cultures achieved monthly yields of 38.30–293.70 kg fresh biomass per cubic meter [7]. However, the prohibitive operational costs and labor-intensive nature of these systems limit their commercial feasibility [8]. Raceway pond cultivation demonstrated peak productivity of 5.12 g dry weight·m−2·day−1 [9], yet practical implementation faces challenges, including significant biomass fragmentation, inconsistent yields, and failure to meet commercial size specifications (5–8 mm colony diameter) [10]. Traditional paddy field cultivation benefits from low management requirements and operational costs [11], but remains vulnerable to microbial contamination, pest infestations, and logistical constraints in harvesting and processing, compounded by strict thermal requirements (10–20 °C).
This study systematically investigates two critical optimization parameters: (1) long-term growth responses to varied inoculation densities, and (2) seasonal variations in the nutritional composition (protein, polysaccharide, and phycobiliprotein content) and textural properties (elasticity and hardness) of algal colonies. Our findings provide essential data for optimizing harvest timing based on target bioactive compounds while addressing the urgent need for standardized cultivation protocols [12]. The proposed methodology offers practical solutions to three persistent challenges in N. sphaeroides aquaculture: production cost reduction, yield stabilization, and quality control [13]. This research advances both fundamental understanding and commercial application potential, with particular relevance to regional agricultural development and specialized crop cultivation initiatives [14,15].
2. Materials and Methods
2.1. Materials
N. sphaeroides strain was collected from Hefeng County, Hubei Province, China (29°38′ N, 110°38′ E). Purification of N. sphaeroides was performed following the methodology described by Rippka (1988) [16]. After purification in the laboratory, the algal biomass was homogenized to obtain an algal slurry. The slurry was then spread onto solid BG-110 medium [17] and incubated in a light incubator of 50 μmol·m−2·s−1 until the filaments of N. sphaeroides formed visible macroscopic colonies [18]. All the other reagents used in this study were of analytical grade.
2.2. Culture Preparation
N. sphaeroides filaments were cultured on solid BG-110 medium until visible micro-colonies formed. The cultures were then transferred to 20-L bottles containing liquid BG-110 medium with aeration under the following conditions: surface light intensity of 150 μmol·m−2·s−1, aeration rate of 0.4 L·min−1·L−1 culture, and medium replacement every 10 days. This process continued until algal colonies reached a diameter of 0.5 mm. Subsequent cultivation was conducted in outdoor 500-L cylindrical white plastic barrels under standardized parameters: aeration rate of 0.5 L·min−1·L−1 culture, medium replacement every 15 days, and BG-110 medium prepared with municipal water (default condition unless specified). The outdoor phase proceeded until colonies attained a target diameter of 3 mm. All experimental procedures maintained the aforementioned baseline conditions (medium composition, aeration rate, and replacement frequency) unless explicitly modified.
2.3. Cultivation Conditions
N. sphaeroides colonies (≥3 mm in diameter) were cultivated in three cylindrical polyethylene vessels (500-L capacity each). The cultivation densities were set at 5 g·L−1, 10 g·L−1, and 15 g·L−1. The culture medium was replaced every 15 days, and the yield was recorded at each replacement. The harvested N. sphaeroides from each cycle was used as the inoculum for the next cycle, maintaining the same inoculation densities of 5 g·L−1, 10 g·L−1, and 15 g·L−1. After one quarter, the inoculum was replaced with new algal inoculum with a diameter of less than 3 mm, and the cultivation process was repeated for four consecutive quarters. The culture medium was prepared using BG-110 diluted with water. Aeration was provided daily from 7:30 to 17:30, with an aeration rate of 1700–1800 L·h−1 (Figure 1).
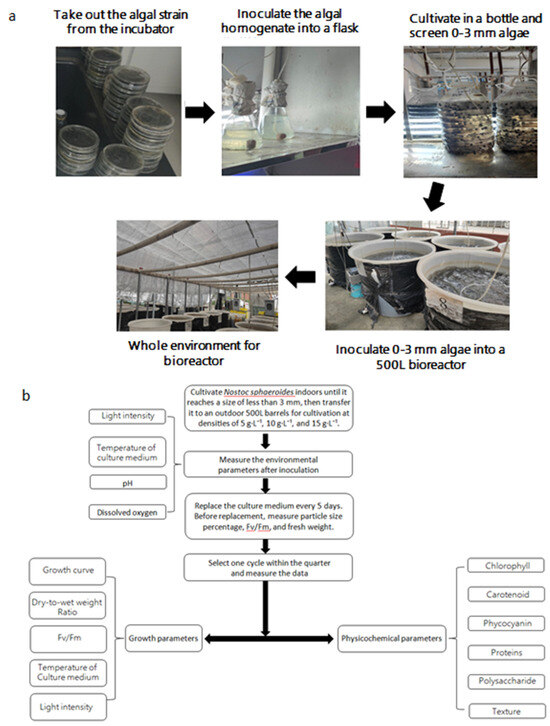
Figure 1.
Seed production and large-scale cultivation process (a) of Nostoc sphaeroides. (b) Cultivation and evaluation processes in this study.
2.4. Chlorophyll Determination
Approximately 0.5 g of the sample was homogenized with 100% methanol and subjected to a 60 °C water bath for 10 min. The extraction process was repeated 2–3 times until the extract became colorless. After cooling, the extract was adjusted to a final volume of 10 mL. Absorbance was measured at 665 nm using a spectrophotometer (Model UV Power, Beijing LabTech Instruments Co., Ltd., Beijing, China). Chlorophyll content was calculated as:
where A is the absorbance at 665 nm, ε is the extinction coefficient (74 cm−1·mg−1·mL), and 1 cm is the cuvette path length [19]
Cchla = A/(ε × l)
2.5. Calculation of Carotenoid Content
Fresh N. sphaeroides samples (1 g) were washed with distilled water, dried, and extracted with 10 mL of 95% ethanol in four steps: two 20-min extractions followed by two 10-min extractions at 60 °C. The combined extract was cooled, diluted to 10 mL, and analyzed by spectrophotometer (Model UV Power, Beijing LabTech Instruments Co., Ltd., Beijing, China) at 665 nm, 720 nm, and 470 nm [20]. Carotenoid content was calculated as:
where Ca represents the chlorophyll a content (mg·L−1), Cx.c represents the carotenoid content (mg·L−1).
Ca = 12.9447 × (A665 – A720)
Cx.c = [1000 × (A470 − A720)] − [(2.86 × Ca)/221]
2.6. Phycobiliprotein Quantification
Samples (1.0 g) were freeze-thawed, ground in phosphate buffer (pH 6.7), and centrifuged. The supernatant was collected after 3–4 cycles of freezing, thawing, and centrifugation, then diluted to 25 mL. Absorbance was measured using a spectrophotometer (Model UV Power, Beijing LabTech Instruments Co., Ltd., Beijing, China) at 562 nm, 615 nm, and 652 nm. Phycocyanin (PC), allophycocyanin (APC), and phycoerythrin (PE) concentrations were determined using established extinction coefficients [21]. The phosphate buffer was prepared by mixing equal volumes of 0.1 M KH2PO4 and K2HPO4, adjusted to 0.2 M NaCl. Carotenoid content was calculated as:
PC(mg/mL) = [OD615 − (0.474 × OD652)]/5.34
APC(mg/mL) = [OD652 − (0.208 × OD615)]/5.09
PE(mg/mL) = [OD652 − 2.41 × (PC) − 0.849(APC)]/9.62
2.7. Total Protein (TP) Content Measurement
Samples were digested using a catalyst mixture (0.23 g CuSO4, 3.77 g K2SO4, 10 mL H2SO4) under stepwise heating: 220 °C (1 h), 270 °C (30 min), and 400 °C (1 h). The digested solution was analyzed using an automatic Kjeldahl nitrogen analyzer (K1100, Jinan Hanon Instruments Co., Ltd., Jinan, China), with protein content calculated based on the dry-to-wet weight ratio [22].
2.8. Polysaccharide (PS) Extraction and Analysis
Ground samples (0.5 g) were boiled in 15–20 mL distilled water for 2–3 h, with repeated extractions. After centrifugation (8000 rpm, 15 min), the supernatant was adjusted to 50 mL. A 1 mL aliquot was mixed with 1 mL of 9% phenol and 5 mL of concentrated H2SO4, incubated for 30 min, and measured at 485 nm. Polysaccharide content was quantified against a standard curve [23].
2.9. Texture Profile Analysis
Samples were rinsed, dried, and analyzed using a TA.XT. Plus texture analyzer (Stable Micro Systems Ltd., Surrey, UK) with a P0.5 probe. The testing parameters included pre-test, test, and post-test speeds of 1.0 mm/s, 0.5 mm/s, and 1.0 mm/s, respectively, with 40% compression, a 5-s interval between compressions, and a 5.0 g trigger force [24].
2.10. Statistical Analysis
The obtained results were presented as mean and standard deviation. In addition, statistical analysis of all data was performed using the one-way analysis of variance (ANOVA) using SPSS 24.0 with p < 0.05 as statistical significance.
3. Results and Analysis
3.1. The Changes in Light Intensity at 12:00 PM, Temperature, and Dissolved Oxygen Levels During the Cultivation Period
As shown in Figure 2, the light intensity at 12:00 during outdoor cultivation varied significantly across seasons. In summer, it ranged from 9.9 to 876.3 μmol·m−2·s−1; in autumn, from 63.3 to 510.7 μmol·m−2·s−1; in winter, from 26.2 to 946.2 μmol·m−2·s−1; and in spring, from 68.7 to 1316.2 μmol·m−2·s−1. From August to October, shading nets were used, resulting in lower light intensity above the plastic bioreactors compared to spring. However, during cloudy or rainy days, the shading nets were removed to ensure sufficient light intensity [25]. In late February, the light intensity remained above 600 μmol·m−2·s−1 for most of the time. As illustrated in Figure 3, the temperature of the culture medium in the plastic bioreactors fluctuated with seasonal and diurnal variations [26]. The temperature ranged from 23.7 to 31.3 °C in summer, 7.1 to 19.9 °C in autumn, 3.7 to 23.8 °C in winter, and 15.2 to 29.6 °C in spring.
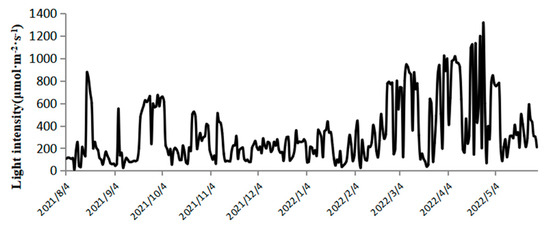
Figure 2.
Changes in Light Intensity from 1 August 2021 to 30 May 2022 at 12:00 PM per day.
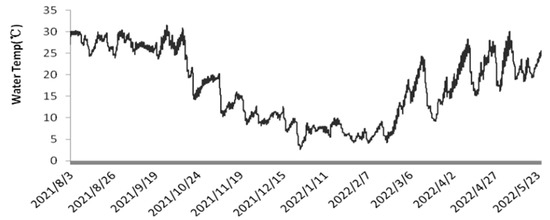
Figure 3.
Temperature Changes from1 August 2021 to 30 May 2022.
As shown in Figure 4, the dissolved oxygen levels ranged from 6.0 to 9.1 mg·L−1 during August to October, 6.7 to 13.3 mg·L−1 during November to December, 8.1 to 15.2 mg·L−1 during January to March, and 6.8 to 11.1 mg·L−1 during April to May. The relatively low dissolved oxygen values observed from August to October were due to high temperatures, with the lowest recorded at 6.0 mg·L−1. Similarly, the fluctuating light intensity and unstable temperatures in April to May also contributed to lower dissolved oxygen levels. As shown in Figure 5, the pH values during the cultivation of N. sphaeroides fluctuated between 7 and 10, with an average of approximately 8.5. This indicates that the outdoor cultivation environment for N. sphaeroides was slightly alkaline.
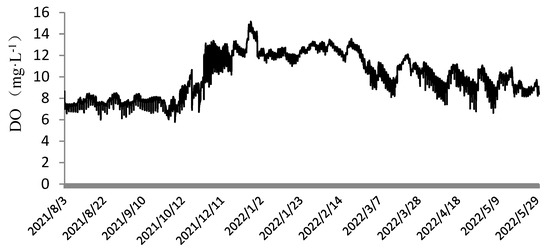
Figure 4.
Changes in the dissolved oxygen from 1 August 2021 to 30 May 2022.
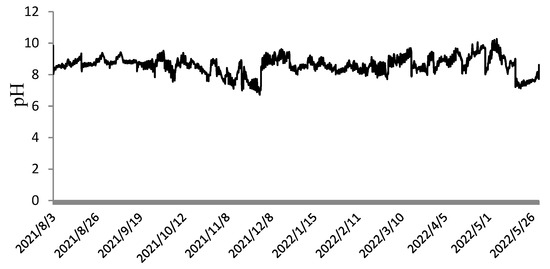
Figure 5.
Change in pH from 1 August 2021 to 30 May 2022.
3.2. Productivity Under Different Seasons and Inoculation Densities
As shown in Table 1, the highest yields in May were observed at inoculation densities of 5 g·L−1, 10 g·L−1, and 15 g·L−1, with values of 0.91 g·L−1·d−1, 1.28 g·L−1·d−1, and 1.28 g·L−1·d−1, respectively. From August to October and November to December, the highest average fresh weight productivity per unit volume was achieved at an inoculation density of 10 g·L−1, with values of 0.26 g·L−1·d−1 and 0.31 g·L−1·d−1, respectively. From January to March and April to May, the highest average fresh weight productivity was observed at an inoculation density of 15 g·L−1, with values of 0.52 g·L−1·d−1 and 1.40 g·L−1·d−1, respectively. Therefore, the optimal inoculation density for summer and autumn is 10 g·L−1, while for winter and spring, it is 15 g·L−1.

Table 1.
The productivity of Nostoc sphaeroides in different seasons.
3.3. The Changes in the Increase of Colony Diameter at Different Seasons and Inoculation Densities
As shown in Figure 6, from August to October, for the group with an inoculation density of 5 g·L−1, the largest proportion of colony size was 5–6 mm, accounting for 42.07%; for the group with an inoculation density of 10 g·L−1, the largest proportion was 3–4 mm, accounting for 50.92%; for the group with an inoculation density of 15 g·L−1, the largest proportions were 2–3 mm and 3–4 mm, both accounting for 45.18%.
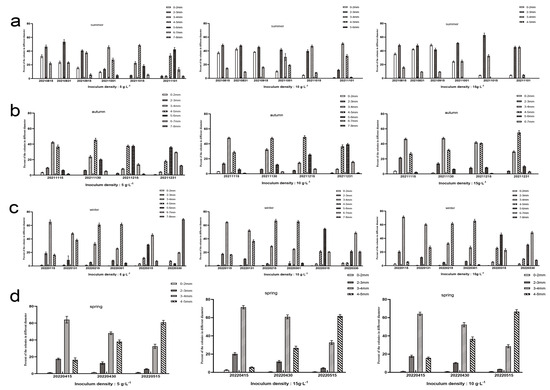
Figure 6.
Changes in (a) summer, (b) autumn, (c) winter, and (d) spring in the diameter of Nostoc sphaeroides colonies under different inoculation densities (n = 3).
From November to December, for the group with an inoculation density of 5 g·L−1, the largest proportion of colony size was 5–6 mm, accounting for 35.77%; for the group with an inoculation density of 10 g·L−1, the largest proportion was 5–6 mm, accounting for 39.71%; for the group with an inoculation density of 15 g·L−1, the largest proportion was 4–5 mm, accounting for 54.78%.
From January to March, for the group with an inoculation density of 5 g·L−1, the largest colony size was 7–8 mm, accounting for 69.58%; for the group with an inoculation density of 10 g·L−1, the largest colony size was 6–7 mm, accounting for 48.88%; for the group with an inoculation density of 15 g·L−1, the largest colony size was 6–7 mm, accounting for 48.87%.
From April to May, there was no significant difference in colony diameter growth between the groups with inoculation densities of 5 g·L−1, 10 g·L−1, and 15 g·L−1, and the largest proportion of colony size was 4–5 mm, accounting for 60.81%, 66.67%, and 61.82%, respectively.
The results demonstrate that the colony diameter of N. sphaeroides varies with both season and inoculation density. During the summer, winter, and spring, increasing inoculation density leads to smaller colony sizes, while in spring, the inoculation density does not significantly affect the growth rate of the colonies. These findings suggest that while inoculation density can influence colony size in most seasons, other environmental factors, such as temperature and light, play a critical role in determining growth patterns, especially in spring.
3.4. The Fv/Fm Values at the End of Each Cultivation Cycle
Fv/Fm is the chlorophyll fluorescence parameter used to assess the efficiency of photosynthesis. As shown in Figure 7, during the entire experimental period, different cultivation densities had varying degrees of impact on the Fv/Fm values of N. sphaeroides colonies. From August to October and from January to March, the highest Fv/Fm values were observed in the group with an inoculation density of 15 g·L−1, with values of 0.51 ± 0.03 and 0.50 ± 0.02, respectively. These values suggest that higher inoculation densities may promote optimal photosynthetic efficiency during these periods.
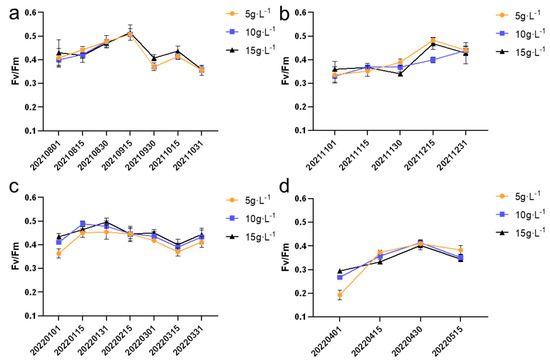
Figure 7.
Fv/Fm value in (a) summer, (b) autumn, (c) winter, and (d) spring (n = 8).
In contrast, from November to December and from April to May, the highest Fv/Fm values were observed in the group with an inoculation density of 5 g·L−1, with values of 0.48 ± 0.01 and 0.37 ± 0.01, respectively. These results indicate that lower inoculation densities may be more favorable for maintaining better photosynthetic efficiency during these colder months, possibly due to environmental factors such as lower light intensity and temperature fluctuations.
Overall, the findings highlight the influence of inoculation density on the photosynthetic performance of N. sphaeroides, with varying effects depending on the season. These observations underscore the importance of optimizing cultivation conditions to maximize the photosynthetic efficiency of the species under different environmental conditions. The Fv/Fm (maximum quantum yield of photosystem II) values exhibited statistically significant differences (p < 0.05) across three inoculation densities (5, 10, and 15 g·L−1) during six sampling periods: 30 November 2021; 1 January 2022; 15 January 2022; 1 March 2022; 1 April 2022; and 15 April 2022.
3.5. The Growth Curve and Fv/Fm Values of a Cycle in Different Seasons
As shown in Figure 8, during the summer, autumn, and winter seasons, the growth rates at different inoculation densities were relatively similar, with biomass increasing at a slow pace. In contrast, the growth rate during the spring season was the fastest. In spring, the growth rates of colonies at different inoculation densities remained steady during the first six days, but starting from the sixth day, the growth rate for the 5 g·L−1 and 15 g·L−1 groups began to increase rapidly. Meanwhile, for the 10 g·L−1 group, the growth rate started to accelerate around day nine. This suggests that spring provides an optimal environment for growth, particularly as the inoculation density increases, indicating a possible seasonal variation in the response to cultivation density. In Figure 8a, the summer inoculation density of 5 g·L−1 showed significant differences (p < 0.05) between samples 20210815 and 20210803. In Figure 8b, the autumn inoculation density of 5 g·L−1 exhibited significant differences (p < 0.05) in the growth curve of N. sphaeroides between 20211115 and 20211103. For the 10 g·L−1 inoculation density, significant differences (p < 0.05) were observed in the growth curve of N. sphaeroides at 20211103, 20211109, 20211112, and 20211115, while the 15 g·L−1 inoculation density showed significant differences (p < 0.05) at 20211103, 20211109, and 20211115. In Figure 8c, the autumn inoculation density of 5 g·L−1 displayed significant differences (p < 0.05) in the growth curve of N. sphaeroides at 20211115 compared to the preceding five time points. For the 10 g·L−1 inoculation density, significant differences (p < 0.05) were observed at 20211103 and 20211115, while the 15 g·L−1 inoculation density showed no significant differences. In Figure 8d, all three inoculation densities exhibited significant differences (p < 0.05) across the six time points.
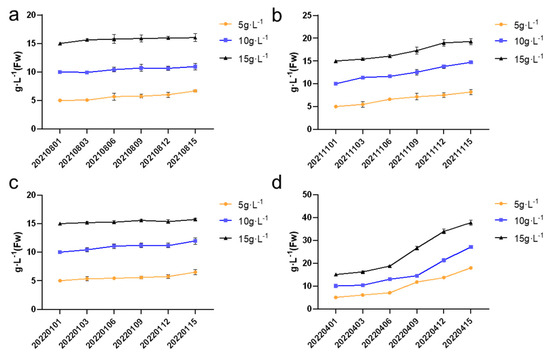
Figure 8.
The growth curve of a single cycle in (a) summer, (b) autumn, (c) winter, and (d) spring (n = 3).
As shown in Figure 9, the highest Fv/Fm values were observed in the summer, while the lowest values occurred in the spring. During the summer, due to higher light intensity and more stable temperatures, the Fv/Fm values peaked around 0.5, indicating optimal photosynthetic efficiency. In contrast, during the spring, fluctuations in light intensity and temperature instability led to a decrease in photosynthetic efficiency, with the lowest Fv/Fm values dropping to around 0.2. These findings highlight the significant seasonal variations in both the growth rate and photosynthetic efficiency of N. sphaeroides, emphasizing the importance of seasonal factors in optimizing cultivation conditions for improved productivity. The Fv/Fm values at the six time points (20220101, 20220103, 20220401, 20220403, 20220409, and 20220415) showed significant differences (p < 0.05) under three different inoculation densities.
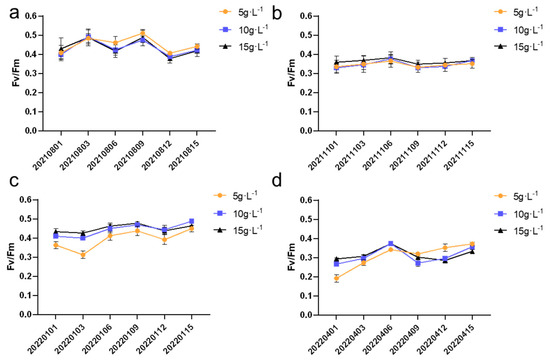
Figure 9.
The Fv/Fm values of a single cycle in (a) summer, (b) autumn, (c) winter, and (d) spring (n = 8).
3.6. Nutritional Analysis
3.6.1. The Dry-to-Wet Weight Ratio for Different Seasons and Inoculation Densities
As shown in Figure 10, during the summer (a), autumn (b), and winter (c) seasons, the highest dry-to-wet weight ratio of N. sphaeroides was observed at an inoculation density of 5 g·L−1, with values of 2.15%, 1.76%, and 1.54%, respectively. These results suggest that, in these seasons, a lower inoculation density favors a higher dry-to-wet weight ratio, indicating efficient biomass accumulation relative to the wet weight of the colonies.
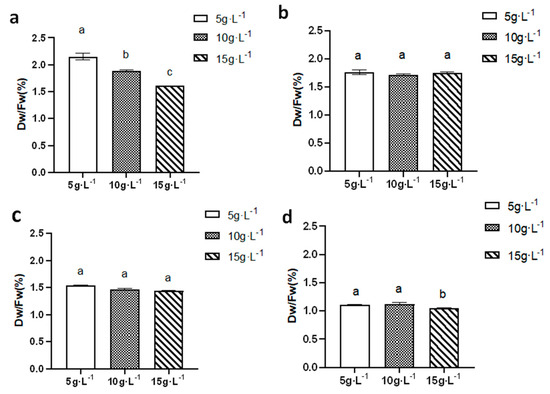
Figure 10.
The dry-to-wet weight ratio in (a) summer, (b) autumn, (c) winter, and (d) spring (n = 3). (a, b, and c are marked for significant difference analysis, and the mean values decrease successively from a to c).
In contrast, during the spring (d) season, the highest dry-to-wet weight ratio was observed at an inoculation density of 10 g·L−1, with a value of 1.13%. This implies that, in spring, a higher inoculation density leads to better biomass accumulation efficiency, possibly due to more favorable growth conditions for N. sphaeroides under these conditions, such as improved light intensity and temperature.
These findings highlight the seasonal variations in the dry-to-wet weight ratio of N. sphaeroides and the influence of inoculation density on biomass efficiency, suggesting that optimal inoculation densities may vary with the season to maximize the growth potential of N. sphaeroides under different environmental conditions. Statistical analysis demonstrated significant differences (p < 0.05) in dry weight content of N. sphaeroides among the three inoculation densities during summer cultivation. In spring, while distinct variations (p < 0.05) were observed between the 15 g·L−1 group and both the 5 g·L−1 and 10 g·L−1 inoculation densities, no statistically significant divergence (p > 0.05) was detected between the 5 g·L−1 and 10 g·L−1 treatment groups.
3.6.2. Comparison of Chlorophyll and Carotenoid Contents Across Seasons and Inoculation Densities
As depicted in Figure 11, the chlorophyll content in N. sphaeroides ranged from 0.40% to 0.60%, while the carotenoid content remained below 0.20%. The highest levels of both chlorophyll and carotenoids were observed during the spring season, suggesting that the environmental conditions during this period, including factors such as light intensity and temperature, are particularly favorable for the production of these pigments.
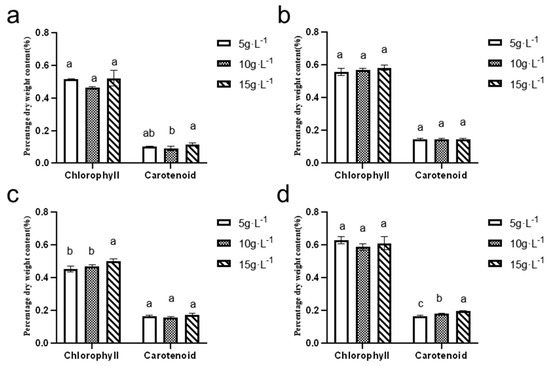
Figure 11.
Dry weight content of chlorophyll and carotenoids in (a) summer, (b) autumn, (c) winter, and (d) spring (n = 3). (a, b, and c are marked for significant difference analysis, and the mean values decrease successively from a to c).
The maximum chlorophyll content was recorded at an inoculation density of 5 g·L−1, with a value of 0.63 ± 0.02%. This indicates that, at this inoculation density, N. sphaeroides achieved optimal chlorophyll synthesis during spring. On the other hand, the highest carotenoid content was observed at an inoculation density of 15 g·L−1, with a value of −0.20 ± 0.61%. This suggests that at higher inoculation densities, the algae might have adapted to optimize carotenoid production, possibly as a protective mechanism against environmental stressors such as high light intensity.
These results emphasize the influence of both season and inoculation density on the production of key pigments in N. sphaeroides, with specific conditions favoring higher pigment synthesis. This information can help optimize cultivation conditions for enhanced pigment production, potentially benefiting applications such as natural colorants or bioactive compounds. Statistical analysis revealed significant seasonal variations in pigment accumulation of N. sphaeroides: Chlorophyll dry weight content exhibited statistically distinct differences (p < 0.05) between 15 and 5, or 10 g·L−1 during winter cultivation. Summer carotenoid content showed significant divergence (p < 0.05) between the 10 g·L−1 and 15 g·L−1 treatment groups, whereas spring cultivation demonstrated statistically significant variations (p < 0.05) in carotenoid dry weight content across all tested inoculation densities (5, 10, and 15 g·L−1).
3.6.3. Comparison of Phycobiliprotein Contents Across Seasons and Inoculation Densities
Figure 12 illustrates that the dry weight content of phycocyanin and phycoerythrin during the summer and autumn seasons ranged from 2% to 3%, while the allophycocyanin content varied between 1% and 2%. In contrast, during the winter and spring seasons, the dry weight content of phycocyanin and phycoerythrin ranged from 1% to 2.2%, and the allophycocyanin content remained below 1%.
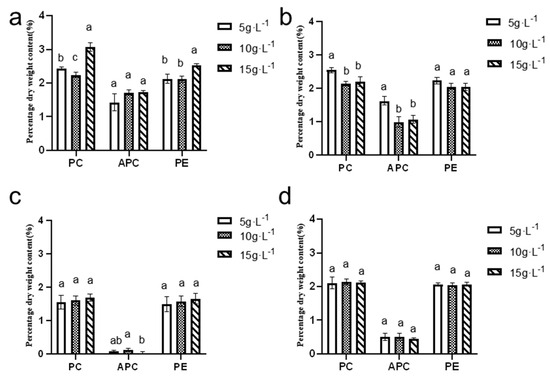
Figure 12.
Dry weight content of phycobilin in (a) summer, (b) autumn, (c) winter, and (d) spring (n = 3). (a, b, and c are marked for significant difference analysis, and the mean values decrease successively from a to c).
The highest levels of phycocyanin, allophycocyanin, and phycoerythrin were observed in the summer season, with maximum values recorded at an inoculation density of 15 g·L−1. Specifically, the phycocyanin content reached 3.07 ± 0.11%, the allophycocyanin content was 1.73 ± 0.03%, and the phycoerythrin content was 2.54 ± 0.03%.
These findings highlight the seasonal variation in pigment content and the significant influence of inoculation density on pigment production. Summer conditions, combined with a higher inoculation density, appear to favor the production of these valuable pigments, potentially enhancing the overall quality and yield of N. sphaeroides for various industrial and research applications. Statistical analysis indicated significant seasonal-dependent variations in pigment accumulation profiles: Summer phycocyanin content demonstrated statistically distinct differences (p < 0.05) across all three inoculation densities (5, 10, and 15 g·L−1), while autumn phycocyanin exhibited significant divergence (p < 0.05) among 5, 10, and 15 g·L−1 treatments, though no statistical significance (p > 0.05) was detected between the 10 and 15 g·L−1 groups. Autumn allophycocyanin accumulation showed significant differences (p < 0.05) between 5 and 10, or 15 g·L−1, contrasting with winter patterns where significant variations (p < 0.05) were restricted to the 10–15 g·L−1 density comparison. Summer carotenoid content displayed significant differentiation (p < 0.05), specifically between 10 and 15 g·L−1 treatments. Phycoerythrin accumulation during summer cultivation revealed significant differences (p < 0.05) among 5, 10, and 15 g·L−1 groups, though no statistical distinction (p > 0.05) emerged between the 5 and 10 g·L−1 inoculation densities.
3.6.4. Comparison of Total Protein and Polysaccharides Across Seasons and Inoculation Densities
As illustrated in Figure 13, the polysaccharide content of N. sphaeroides at inoculation densities of 5 g·L−1 and 15 g·L−1 was notably higher in the summer compared to the spring, autumn, and winter seasons, with recorded values of 42.83 ±1.68% and 48.23 ± 1.17%, respectively. In contrast, the highest polysaccharide content at an inoculation density of 10 g·L−1 was observed in autumn, with a value of 41.79 ± 0.29%.
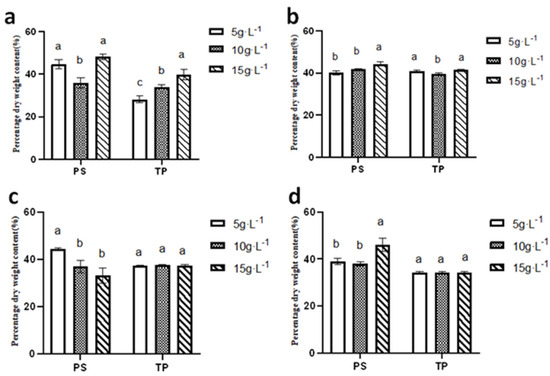
Figure 13.
Dry weight content of Polysaccharide and Total Protein in (a) summer, (b) autumn, (c) winter, and (d) spring (n = 3). (a, b, and c are marked for significant difference analysis, and the mean values decrease successively from a to c).
When considering protein content, the highest levels were observed in autumn across all inoculation densities, with values of 40.87 ± 0.41%, 39.66 ±−0.31%, and 41.44 ± 0.16% for inoculation densities of 5 g·L−1, 10 g·L−1, and 15 g·L−1, respectively. This indicates that autumn conditions are favorable for protein accumulation in N. sphaeroides.
These findings suggest that seasonal variations and inoculation densities significantly influence the biochemical composition of N. sphaeroides, with summer conditions favoring polysaccharide, while autumn conditions support higher protein content. This information is essential for optimizing cultivation conditions to enhance the desired biochemical yields in different applications. Statistical analysis demonstrated season-dependent variations in biochemical composition under different inoculation densities: summer polysaccharide content exhibited significant differences (p < 0.05) among all three densities (5, 10, and 15 g·L−1), while autumn and spring polysaccharides showed distinct divergence (p < 0.05) between the 15 g·L−1 group and lower-density treatments (5 and 10 g·L−1). Winter polysaccharides displayed significant differentiation (p < 0.05) between the 5 g·L−1 group and higher-density groups (10 and 15 g·L−1). Total protein accumulation in summer revealed statistically significant variations (p < 0.05) across all densities, whereas autumn total protein content exhibited significant differences (p < 0.05) between 5 g·L−1 and both 10 g·L−1 and 15 g·L−1 treatments.
3.6.5. Comparison of Texture Properties Across Inoculation Densities and Seasons
As shown in Table 2, the highest hardness of N. sphaeroides was observed in autumn at inoculation densities of 5 g·L−1 and 15 g·L−1, with values of 153.96 ± 6.02 g and 146.88 ± 3.87 g, respectively. At an inoculation density of 10 g·L−1, the highest hardness was recorded in spring, with a value of 109.67 ± 6.19 g.

Table 2.
Texture Data (n = 3). (a, b, c, and d are marked for significant difference analysis, and the mean values decrease successively from a to d).
Regarding texture properties, the elasticity and chewiness of N. sphaeroides was highest in spring across all inoculation densities. The elasticity values were 2.86 ± 0.50, 2.54 ± 0.48, and 2.09 ± 0.66 for inoculation densities of 5 g·L−1, 10 g·L−1, and 15 g·L−1, respectively. Similarly, the chewiness values were 112.36 ± 15.73, 120.67 ± 33.62, and 75.96 ± 29.98 for the same inoculation densities.
These findings indicate that seasonal factors, as well as inoculation density, play an important role in determining the texture characteristics of N. sphaeroides, with autumn promoting the highest hardness and spring enhancing elasticity and chewiness. This information is valuable for understanding how different environmental conditions and cultivation strategies influence the textural properties of this organism, potentially impacting its use in various applications. Texture analysis revealed significant seasonal and density-dependent variations in biomechanical properties: N. sphaeroides colonies at 5 and 10 g·L−1 inoculation densities exhibited significant differences (p < 0.05) in hardness across all four seasons, while the 10 g·L−1 group demonstrated distinct seasonal hardness patterns (summer/winter vs. autumn/spring; p < 0.05). Elasticity in spring cultivation showed significant divergence (p < 0.05) from other seasons across all three densities. Adhesiveness variations displayed density-specific seasonality: 5 g·L−1 colonies exhibited significant differences (p < 0.05) between summer/spring and autumn/winter, whereas 10 g·L−1 and 15 g·L−1 groups showed spring- and autumn-specific adhesiveness differentiation (p < 0.05), respectively. Chewiness profiles universally demonstrated significant spring-specific enhancement (p < 0.05) across all inoculation densities compared to other seasons.
3.7. Comparison of Different Methods on the Preservation of N. Sphaeroides
N. sphaeroides harvested in spring was stored for one week under several preservation conditions, including vacuum sealing with aluminum foil and plastic packaging, direct storage at 4 °C, and storage at 4 °C after a 10-min blanching treatment. Additionally, potassium sorbate was added to some of the samples as a preservative to assess its impact on the preservation process (Figure 14).

Figure 14.
Comparison of different methods for the preservation of Nostoc sphaeroides.
After the blanching treatment, the color of N. sphaeroides shifted from dark green to a lighter green. Under the 4 °C storage condition, N. sphaeroides that were directly stored or subjected to blanching showed a noticeable difference in water loss depending on the type of packaging. In both pre-blanching and post-blanching states, the samples packed in aluminum foil exhibited less water loss compared to those packed in plastic. However, when potassium sorbate was introduced, a shift was observed. In this case, the samples stored directly at 4 °C with plastic packaging had a reduced appearance of water loss on the surface, contrasting with the samples in aluminum foil packaging, which appeared to lose more moisture.
When comparing the two types of packaging after blanching, the exterior appearance of N. sphaeroides in both aluminum foil and plastic packaging was quite similar, regardless of whether the preservative was applied. However, a distinct difference in color was noted: the samples in plastic packaging appeared to retain a greener hue than those in aluminum foil. This suggests that the plastic packaging may have better preserved the visual quality of N. sphaeroides, especially in terms of color.
Overall, these findings indicate that the type of packaging material, the application of potassium sorbate, and the blanching treatment all play significant roles in influencing the water loss, color retention, and overall appearance of N. sphaeroides during preservation. These factors could potentially guide future storage strategies for enhancing the shelf life and quality of this organism.
4. Discussion
Throughout the experimental period, the light intensity at 12:00 ranged from 9.9 to 1316.2 μmol·m−2·s−1, and the culture temperature fluctuated between 3.7 and 31.3 °C. Wild N. sphaeroides typically grows between November and May of the following year, with an average temperature of approximately 12.2 °C during this period [27]. The temperature of the culture medium fluctuated between 3.7 °C and 31.3 °C, while the pH value varied between 7 and 10. During the summer and spring seasons, the dissolved oxygen levels were generally lower, which can be attributed to the higher temperatures in these seasons. The elevated temperatures led to a reduction in dissolved oxygen concentrations, particularly in the warmer months of spring and summer. Under periodic continuous cultivation, N. sphaeroides is more challenging to cultivate between August and October [28]. This is because the light intensity and temperature during this period are relatively high, with light intensity ranging from 9.9 to 876.3 μmol·m−2·s−1 and temperature ranging from 23.7 to 31.3 °C. Although shading nets can reduce light intensity, the high temperature may cause some colonies to rupture, and water evaporation can reduce the habitable environment. Therefore, it is essential to replenish the culture medium promptly during the cultivation period [29].
In summer and autumn, the highest average fresh weight productivity per unit volume was achieved at an inoculation density of 10 g·L−1, with values of 0.26 g·L−1·d−1 and 0.31 g·L−1·d−1, respectively. In winter and spring, the highest average fresh weight productivity was observed at an inoculation density of 15 g·L−1, with values of 0.52 g·L−1·d−1 and 1.40 g·L−1·d−1, respectively. Therefore, the optimal inoculation density for summer and autumn is 10 g·L−1, while for winter and spring, it is 15 g·L−1. N. sphaeroides can maintain photosynthetic activity at temperatures ranging from 5 to 45 °C, but cells will die once the temperature reaches 50 °C. Additionally, N. sphaeroides can adapt to low-temperature environments, with temperatures around 0 °C. In cultivation experiments, N. sphaeroides exhibited good growth when the culture temperature was between 10 and 30 °C [30]. High temperatures are unfavorable for the growth of N. sphaeroides, which explains why the highest average fresh weight productivity in summer (0.26 g·L−1·d−1 at 10 g·L−1) was the lowest among the four seasons, while the highest average fresh weight productivity in spring (1.40 g·L−1·d−1 at 15 g·L−1) was the highest. The ability of N. sphaeroides to adapt to a wide range of cultivation temperatures is an advantage for outdoor cultivation. The growth of N. sphaeroides varied with different inoculation densities. During the summer, winter, and spring seasons, the average colony size decreased with increasing inoculation density. However, in spring, the inoculation density did not lead to a significant increase in the slow growth of the colony diameter. The Fv/Fm values clearly demonstrated that both the inoculation density and the season of cultivation had a noticeable impact on the overall health of the colonies. In summer, the Fv/Fm values were the highest, while in spring, they were the lowest. The higher light intensity and stable temperature in summer contributed to better colony health, with the Fv/Fm value reaching approximately 0.5. In contrast, in spring, the unstable light intensity and temperature led to lower Fv/Fm values, which were as low as 0.2 in some cases.
During the continuous cultivation process, the dry weight content of chlorophyll and carotenoids in N. sphaeroides was highest in spring. The maximum chlorophyll content was recorded at an inoculation density of 5 g·L−1, with a value of 0.63 ± 0.02%, while the highest carotenoid content was observed at an inoculation density of 15 g·L−1, with a value of 0.20 ± 0.61%. This is because shading nets were used in summer, resulting in lower light intensity compared to spring. Light intensity significantly affects the photosynthetic efficiency of algae [31], thereby influencing the synthesis of photosynthetic products such as sugars and other organic compounds, including proteins and photosynthetic pigments [32,33]. The dry weight content of phycocyanin, allophycocyanin, and phycoerythrin was highest in summer, with the maximum values recorded at an inoculation density of 15 g·L−1, reaching 3.07 ± 0.11%, 1.73 ± 0.03%, and 2.54 ± 0.03%, respectively. Although the light intensity on sunny days in summer was reduced due to shading nets, the nets were opened on cloudy days, and the higher summer temperatures resulted in the highest Fv/Fm values, leading to the highest phycobiliprotein content [34,35].
In summer, the polysaccharide content of N. sphaeroides at inoculation densities of 5 g·L−1 and 15 g·L−1 was higher than in spring, autumn, and winter, with values of 42.83 ± 1.68% and 48.22 ± 1.17%, respectively. However, the polysaccharide content at an inoculation density of 10 g·L−1 in summer was lower than in the other three seasons, with a value of 35.98 ± 2.01%. The highest protein content was recorded in autumn across all inoculation densities, with values of 40.87 ± 0.41%, 39.66 ± 0.31%, and 41.44 ± 0.16% for 5 g·L−1, 10 g·L−1, and 15 g·L−1, respectively. The combined content of protein and polysaccharides in N. sphaeroides ranged between 70% and 80% across the four seasons, indicating that these are the primary components of its dry matter [36]. The highest hardness of N. sphaeroides was observed in autumn at inoculation densities of 5 g·L−1 and 15 g·L−1, with values of 153.96 ± 6.02 g and 146.88 ± 3.87 g, respectively. At an inoculation density of 10 g·L−1, the highest hardness was recorded in spring, with a value of 109.67 ± 6.19 g. The elasticity and chewiness of N. sphaeroides was highest in spring across all inoculation densities, with elasticity values of 2.86 ± 0.50, 2.54 ± 0.48, and 2.09 ± 0.66 and chewiness values of 112.36 ± 15.73, 120.67 ± 33.62, and 75.96 ± 29.98 for 5 g·L−1, 10 g·L−1, and 15 g·L−1, respectively. Overall, N. sphaeroides exhibits better texture in spring [37].
For the preservation of N. sphaeroides, direct storage at 4 °C showed that aluminum foil packaging performed better than plastic packaging. However, when preservatives were added, plastic packaging proved to be more effective than aluminum foil packaging. This indicates that the preservation method, as well as the type of packaging, can significantly impact the quality and moisture retention of the N. sphaeroides during storage, with the addition of preservatives altering the outcome in favor of plastic packaging.
5. Conclusions
The 10 g·L−1 inoculation density demonstrated superior performance in the summer/autumn cycle, while 15 g·L−1 exhibited the highest biomass yield in spring/summer cultivation. During summer, winter, and autumn cultivation, colony diameter gradually decreased with increasing inoculation density (5–15 g·L−1). Seasonal variations significantly influenced photosynthetic pigment content, particularly with allophycocyanin depletion in winter. Summer conditions favored polysaccharide, whereas autumn conditions enhanced protein content. Texture analysis revealed self-regulated sensory characteristics of N. sphaeroides, with spring-harvested colonies exhibiting optimal palatability.
Author Contributions
Conceptualization, B.W. and Z.D.; methodology, B.W.; software, B.W.; validation, B.W.; formal analysis, B.W.; investigation, B.W.; resources, Z.D.; data curation, B.W.; writing—original draft preparation, B.W.; writing—review and editing, B.W.; visualization, B.W.; supervision, B.W.; project administration, B.W.; funding acquisition, Z.D. All authors have read and agreed to the published version of the manuscript.
Funding
Project supported by the Key Research and Development Program of Hubei Provincial, China (2022BBA006).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xu, J.; Zhang, M.; An, Y.; Roknul, A.S.; Adhikari, B. Effects of radio frequency and high pressure steam sterilisation on the colour and flavour of prepared Nostoc sphaeroides. J. Sci. Food Agric. 2017, 98, 1719–1724. [Google Scholar] [CrossRef]
- Wang, X.P.; Xie, B.J.; Mo, K.J.; Cheng, C.; Chen, D. Study on Nutrtients Comparsion and Discusson between the Wild and Cultivated-indoor Species of the Nostoc sphaeroides. Food Sci. 2005, 26, 238–241. [Google Scholar]
- Huang, S.; Zhang, M.; Huang, J.; Chen, H. Impregnated Nostoc sphaeroids Kützing crisp grain conditioning processing technologies. J. Food Sci. Biotechnol. 2018, 37, 495–501. [Google Scholar]
- Kaga, Y.; Kuda, T.; Taniguchi, M.; Yamaguchi, Y.; Takenaka, H.; Takahashi, H.; Kimura, B. The effects of fermentation with lactic acid bacteria on the antioxidant and anti-glycation properties of edible cyanobacteria and microalgae. LWT 2021, 135, 110029. [Google Scholar] [CrossRef]
- Sahoo, C.R.; Swain, S.; Luke, A.M.; Paidesetty, S.K.; Padhy, R.N. Biogenic synthesis of silver-nanoparticles with the brackish water cyanobacterium Nostoc sphaeroides and assessment of antibacterial activity against urinary tract infecting bacteria. J. Taibah Univ. Sci. 2021, 15, 805–813. [Google Scholar] [CrossRef]
- Li, F.; Liu, R.; Qin, S.; Deng, Z.; Li, W. Progress in culture technology and active substance research on Nostoc sphaeroides Kützing. J. Sci. Food Agric. 2025, 105, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.-Y.; Kuang, Q.-J.; Hu, Z.-Y. Mass culture indoors, the structure of colony and analysis of nutrient composition of Nostoc sphaeroides. Wuhan Zhi Wu Xue Yan Jiu = Wuhan Bot. Res. 2006, 24, 481–484. [Google Scholar]
- Chen, Z.; Shang, J.-L.; Hou, S.; Li, T.; Li, Q.; Yang, Y.-W.; Hess, W.R.; Qiu, B.-S. Genomic and transcriptomic insights into the habitat adaptation of the diazotrophic paddy-field cyanobacterium Nostoc sphaeroides. Environ. Microbiol. 2021, 23, 5802–5822. [Google Scholar] [CrossRef]
- Yi, S.F.; Tian, Y. Raceway pond large-scale production technique of Nostoc sphaeroids Kützing. Mod. Agric. Sci. Technol. 2019, 16, 79–82. [Google Scholar]
- Qin, H.; Lu, J.; Wang, Z.; Li, D. The influence of soil and water physicochemical properties on the distribution of Nostoc sphaeroides (Cyanophyceae) in paddy fields and biochemical comparison with indoor cultured biomass. J. Appl. Phycol. 2013, 25, 1737–1745. [Google Scholar] [CrossRef]
- Li, L. Study on the Feature of n-alkane from the Soil of Growing Nostoc sphaeroides Area in Zouma Township of Hefeng County. J. Hubei Univ. Natl. (Nat. Sci. Ed.) 2012, 12, 428–431. [Google Scholar]
- Román, J.R.; Chamizo, S.; Roncero-Ramos, B.; Adessi, A.; De Philippis, R.; Cantón, Y. Overcoming field barriers to restore dryland soils by cyanobacteria inoculation. Soil Tillage Res. 2021, 207, 104799. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Chen, J.; Yuan, Z.-W.; Chen, X.-W. Effects of calcium ion on the colony formation, growth, and photosynthesis in the edible cyanobacterium Nostoc sphaeroides. J. Appl. Phycol. 2021, 33, 1409–1417. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Ma, S.; Zhang, R.; Wang, Y. Effects of Phycobiliprotein and Phycocyanin from Nostoc sphaeroides Kützing on the Growth of S180 Tumor-bearing Mice and Its Mechanism. Sci. Technol. Food Ind. 2022, 43, 399–405. [Google Scholar]
- Yang, Y.; Liu, S.; Li, H.; Liu, Y.; Ren, P.; Liu, Y.; Liu, S.; Guan, L. The protective effect of Nostoc commune Vauch. polysaccharide on alcohol-induced acute alcoholic liver disease and gut microbiota disturbance in mice. J. Gastroenterol. Hepatol. 2023, 38, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R. Isolated and purification of cyanobacteria. Methods Enzymol. 1988, 167, 3–27. [Google Scholar]
- Rippa, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar]
- Deng, Z.; Yan, C.; Lu, F.; Hu, Q.; Hu, Z. Growth kinetics of 1–2 mm and 3–4 mm colonies of Nostoc sphaeroides (Cyanophyta) in outdoor culture. Biotechnol. Lett. 2008, 30, 1741–1746. [Google Scholar] [CrossRef]
- Amin, M.; Chetpattananondh, P.; Khan, M.N.; Mushtaq, F.; Sami, S.K. Extraction and Quantification of Chlorophyll from Microalgae chlorella sp. IOP Conf. Ser. Mater. Sci. Eng. 2018, 414, 012025. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.; Fang, K.; Weng, Z.; Xu, J.; Chen, H.; Chen, J. Sequential Extraction and Purification of 50 Distinct Carotenoids from Aquatic Organisms. Food Anal. Methods 2021, 14, 1600–1610. [Google Scholar] [CrossRef]
- Ma, R.; Lu, F.; Bi, Y.; Hu, Z. Effects of light intensity and quality on phycobiliprotein accumulation in the cyanobacterium Nostoc sphaeroides Kutzing. Biotechnol. Lett. 2015, 37, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Syrpas, M.; Bukauskaitė, J.; Ramanauskienė, K.; Karosienė, J.R.; Majienė, D.; Bašinskienė, L.; Venskutonis, P.R. Ultrasound-Assisted Extraction and Assessment of Biological Activity of Phycobiliprotein-Rich Aqueous Extracts from Wild Cyanobacteria (Aphanizomenon flosaquae). J. Agric. Food Chem. 2020, 68, 1896–1909. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B. Protective Mechanism of Nostoc sphaeroides Kiitz. Polysaccharide on Liver Fibrosis by HFD-Induced Liver Fat Synthesis and Oxidative Stress. Evid.-Based Complement. Altern. Med. eCAM 2022, 2022, 1745244. [Google Scholar]
- Yu, Y.; Wang, H.; Yang, Y.; Pei, G.; Qin, Z.; Ji, J. Study on Preparation of Nutritional Nostoc sphaeroids Jelly. Amino Acids Biot. Resour. 2016, 38, 30–35. [Google Scholar] [CrossRef]
- Burlacot, A.; Dao, O.; Auroy, P.; Cuiné, S.; Li-Beisson, Y.; Peltier, G. Alternative photosynthesis pathways drive the algal CO2-concentrating mechanism. Nature 2022, 605, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Al-Harrasi, A.; Behl, T.; Anwer, M.K.; Ahmed, M.M.; Mittal, V.; Kaushik, D.; Chigurupati, S.; Kabir, M.T.; Sharma, P.B.; et al. Unravelling the photoprotective effects of freshwater alga Nostoc commune Vaucher ex Bornet et Flahault against ultraviolet radiations. Environ. Sci. Pollut. Res. Int. 2021, 29, 14380–14392. [Google Scholar] [CrossRef]
- Wu, X.-F.; Zhang, M.; Sun, Y.; Bhandari, B.; Fan, D. Effects of cryoprotectants on Nostoc sphaeroides superchilled at low temperature (−3.0 °C) and their action mechanisms. J. Food Process Eng. 2020, 43, e13488. [Google Scholar] [CrossRef]
- Duan, Z.; Feng, G.; Tan, X.; Zhu, R. Intra-colony light regulates morphology diversity of colonial cyanobacteria. Algal Res. 2025, 85, 103829. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Liu, Y.; Wang, X.; Zhang, H.; Shang, R.; Laba, C.; Wujin, C.; Hao, B.; Wang, S. Structural characteristic of polysaccharide isolated from Nostoc commune, and their potential as radical scavenging and antidiabetic activities. Sci. Rep. 2022, 12, 22155. [Google Scholar] [CrossRef]
- Sakamoto, T.; Wei, Y.; Yuasa, K.; Nishiyama, Y. Recovery of photosynthesis after long-term storage in the terrestrial cyanobacterium Nostoc commune. J. Gen. Appl. Microbiol. 2022, 68, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Możdżeń, K.; Leśnicka, P.Z.; Burnecki, T.; Śliwińska-Wilczewska, S.; Skoczowski, A.; Greczek-Stachura, M. Photosynthetic efficiency of endosymbiotic algae of Paramecium bursaria originating from locations with cold and warm climates. Oceanol. Hydrobiol. Stud. 2018, 47, 456–463. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Gong, Q.; Gao, X. Symbiotic bacteria diversity and metabolome analyses of Nostoc sphaeroides grown under different organic carbon sources. Algal Res. 2024, 81, 103592. [Google Scholar] [CrossRef]
- Matthes, L. Primary Productivity of the Helgolandic Kelp Forest. Light Response Curves of Biomass Dominant Brwon Algae (Phaeophyceae) as Basis for Productivity Estimates. Master’s Thesis, University of Rostock, Rostock, Germany, 2015. [Google Scholar]
- Chen, Z.; Yuan, Z.-W.; Luo, W.-X.; Wu, X.; Pan, J.-L.; Yin, Y.-Q.; Shao, H.-C.; Xu, K.; Li, W.-Z.; Hu, Y.-L.; et al. UV-A radiation increases biomass yield by enhancing energy flow and carbon assimilation in the edible cyanobacterium Nostoc sphaeroides. Appl. Environ. Microbiol. 2024, 90, e0211023. [Google Scholar] [CrossRef]
- Prabha, S.; Vijay, A.K.; Mathew, D.E.; George, B. Light sensitive orange carotenoid proteins (OCPs) in cyanobacterial photoprotection: Evolutionary insights, structural–functional dynamics and biotechnological prospects. Arch. Microbiol. 2025, 207, 32. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, X.; Liu, Z.; He, Z.; Yue, H.H.; Li, F.F.; Xu, K.; Shao, H.C.; Li, W.Z.; Chen, X.W. Proteomic insight into growth and defense strategies under low ultraviolet-B acclimation in the cyanobacterium Nostoc sphaeroides. J. Photochem. Photobiol. B Biol. 2025, 264, 113101. [Google Scholar] [CrossRef]
- Meng, X.; Huang, Y.; Xiong, J.; Cheng, Z.; Yang, T.; Li, Z.; Tuo, R.; Zhang, Z.; Wang, G.; Gu, Q.; et al. Lactiplantibacillus plantarum ZFM55 improves texture and flavor of yogurt, increases beneficial metabolites, and the co-fermented yogurt promotes human gut microbiota health. LWT 2024, 198, 115929. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).