Pyrolyzed Biochar from Agricultural Byproducts: Synthesis, Characterization, and Application in Water Pollutants Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Collection and Pre-Processing of Biomass
2.1.2. FITC Dye and Oil
2.1.3. Preparation of Adsorbent (Beads)
2.1.4. Preparation of Cu2+ Solution
2.2. Characterization of Biochar
2.2.1. Physicochemical Property
2.2.2. Surface Chemistry
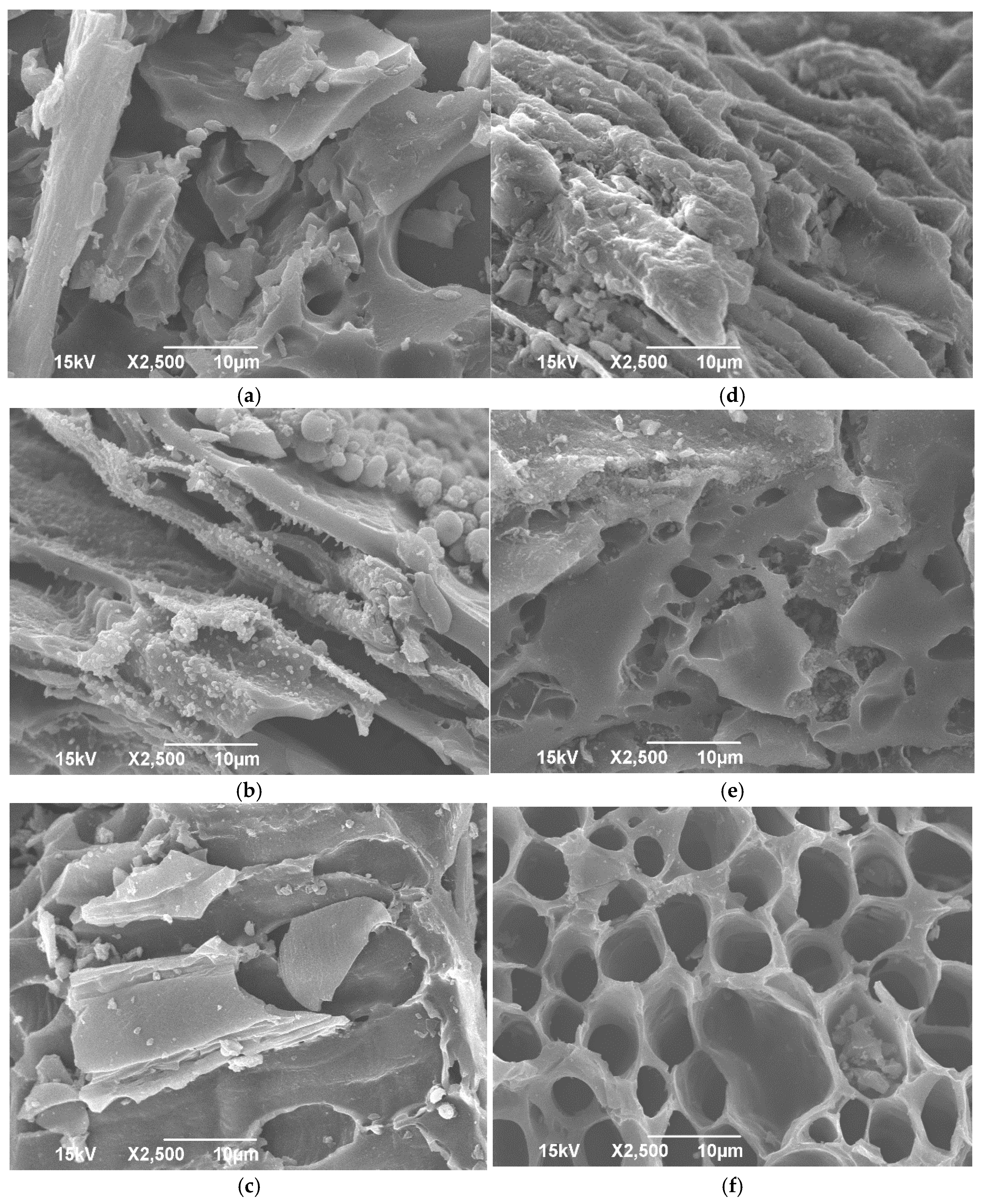
2.2.3. Surface Morphology
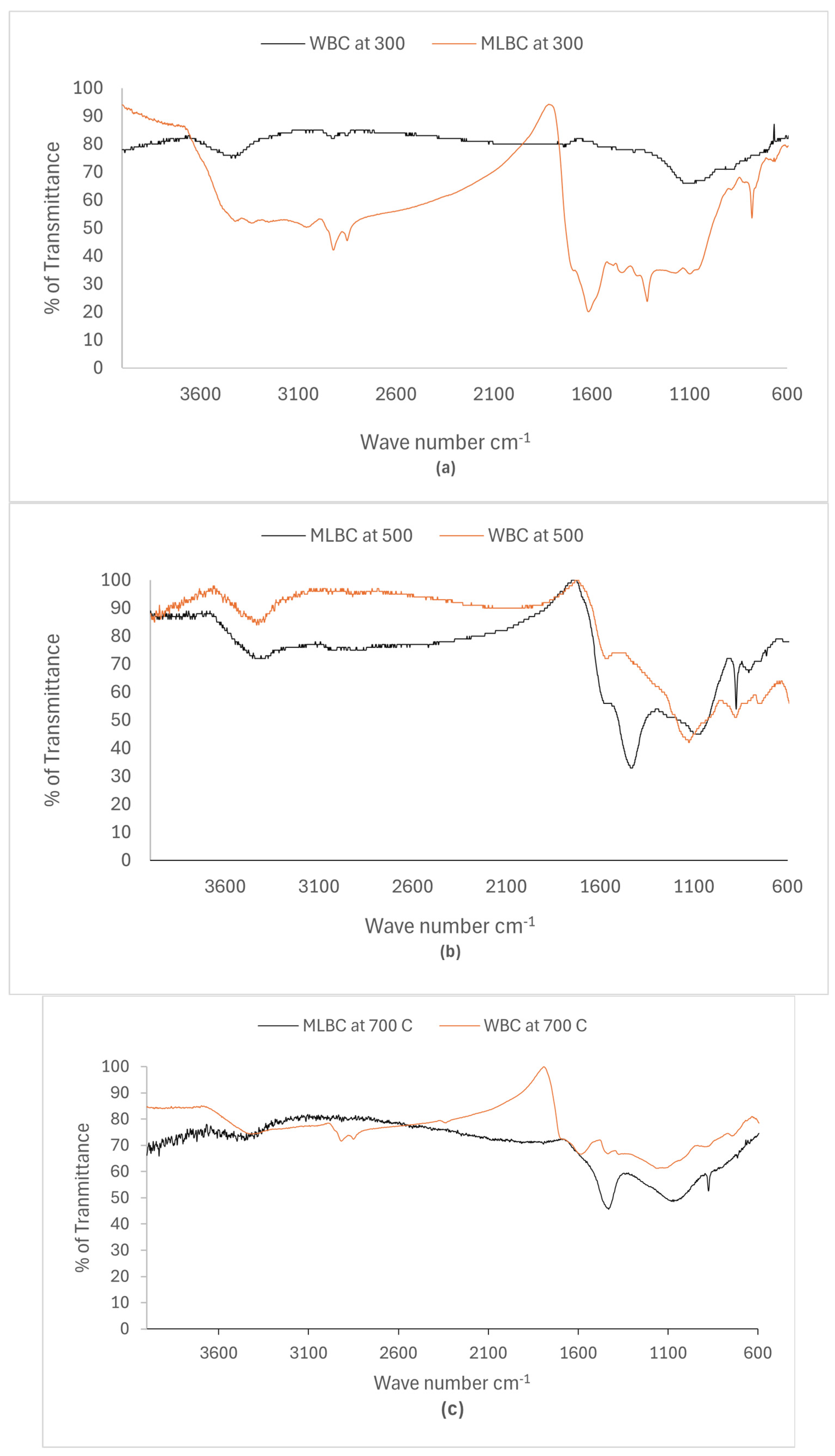
2.2.4. FT-IR Analysis for Biochar Beads
2.2.5. Batch Adsorption Study
2.2.6. Oil Adsorption
2.2.7. Adsorption Study for Biochar Beads
3. Results
3.1. Biochar pH, EC, Bulk Density, and Water Holding Capacity
3.2. Biochar Surface Chemistry
3.3. Surface Morphology of the Biochar
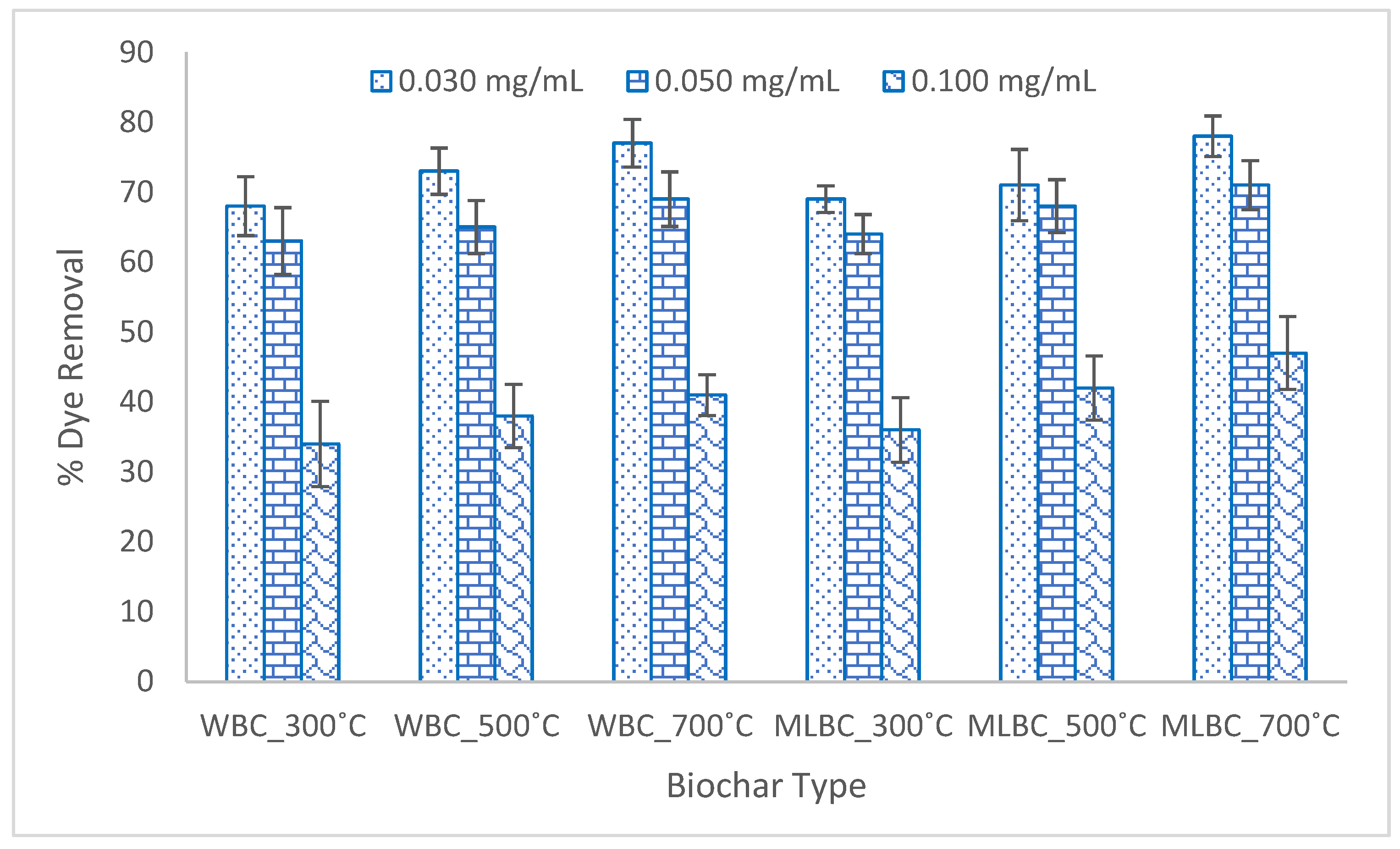
3.4. Dye Adsorption Potentiality of the Biochar
3.4.1. Effect of pH
3.4.2. Effect of Time
3.4.3. Effect of Adsorption Dose
3.4.4. Effect of Initial Dye Concentration
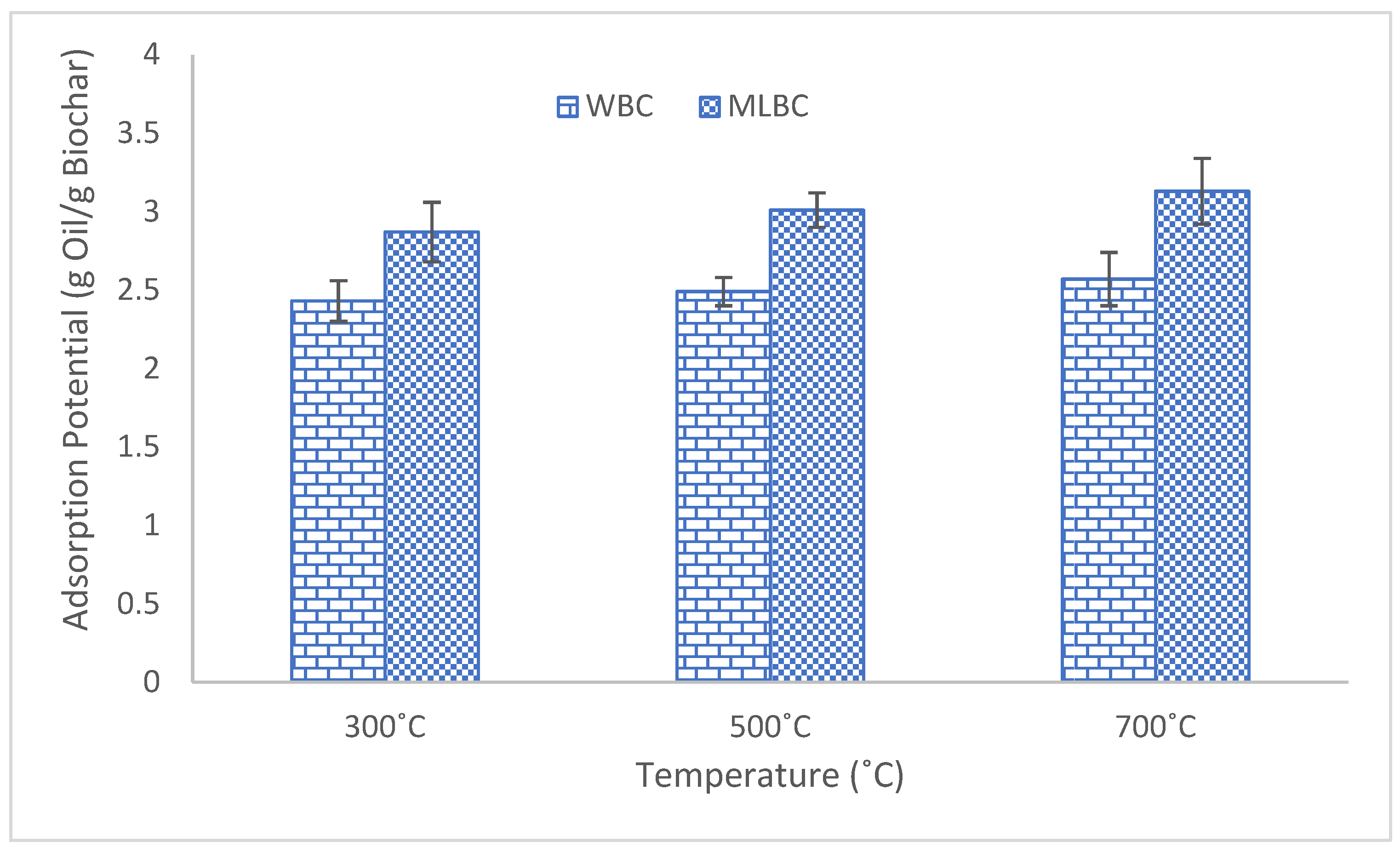
3.5. Oil Adsorption Potential
3.6. Dye Adsorption Potentiality of the Biochar Beads
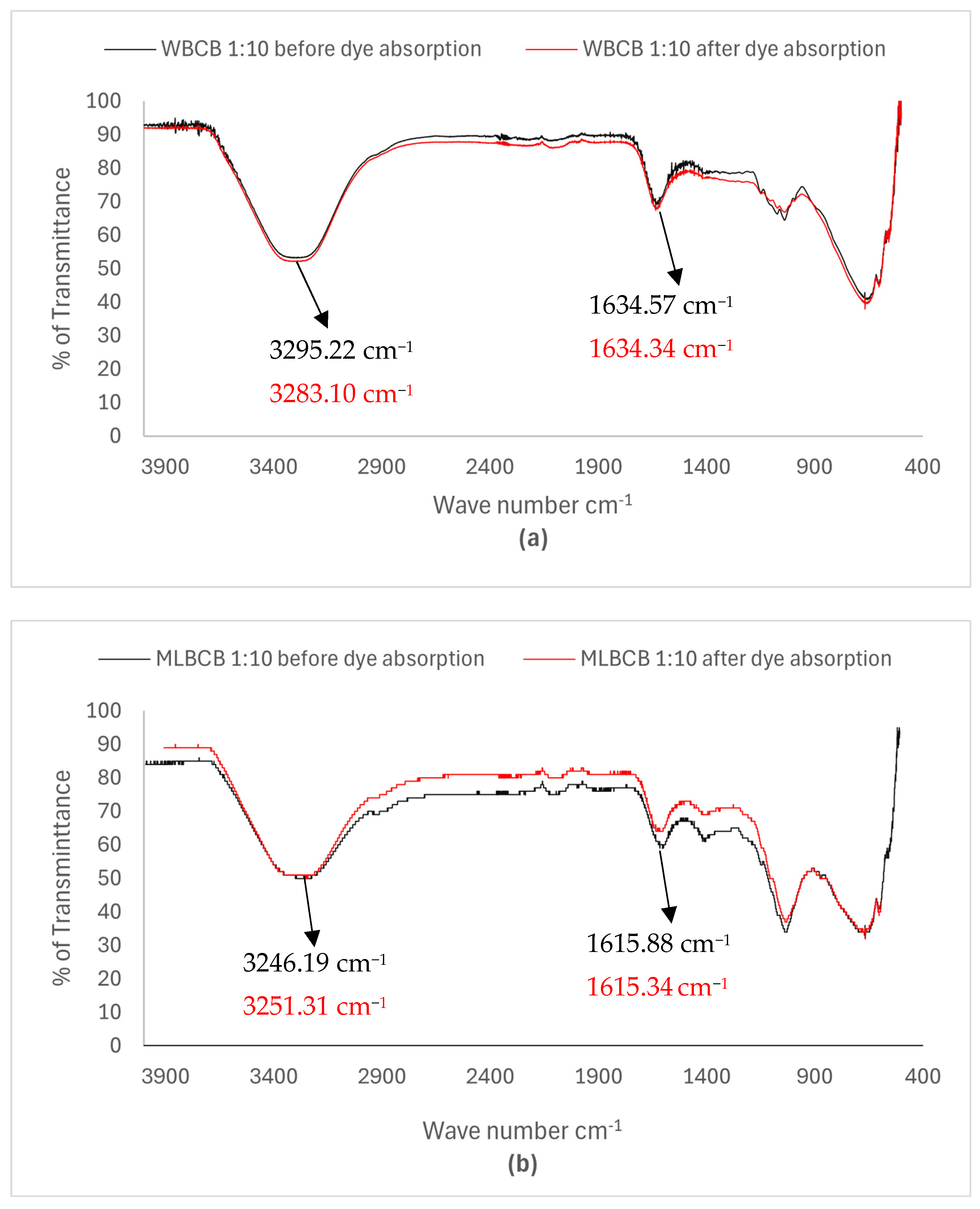
3.6.1. FT-IR Analysis of Biochar Beads
3.6.2. UV-VIS Spectroscopy Analysis of WBC and MLBC Beads
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Bulk capacity |
| BD | Bulk density |
| Ca | Absorption capacity |
| EC | Electrical conductivity |
| FITC | Fluorescein isothiocyanate |
| IR | Infrared |
| FT-IR | Fourier transform infrared |
| ML | Maple leaf |
| MLBC | Maple leaf biochar |
| MLBCB | Maple leaf biochar bead |
| ND | North Dakota |
| rpm | Rotation per minute |
| SEM | Scanning electron microscopy |
| TC | Total carbon content |
| UV-VIS | Ultraviolet–visible |
| WCM | Weight of the conjugated mass |
| WBC | Weight of the biochar |
| WB | Wheat bran |
| WBC | Wheat bran biochar |
| WBCB | Where bran biochar bead |
| WHC | Water holding capacity |
References
- Lehmann, J.; Joseph, S. Biochar for Environmental Management; Routledge: London, UK, 2012. [Google Scholar]
- Zhao, X.; Wang, J.; Wang, S.; Xing, G. Successive Straw Biochar Application as a Strategy to Sequester Carbon and Improve Fertility: A Pot Experiment with Two Rice/Wheat Rotations in Paddy Soil. Plant Soil 2014, 378, 279–294. [Google Scholar] [CrossRef]
- Trakal, L.; Bingöl, D.; Pohořelý, M.; Hruška, M.; Komárek, M. Geochemical and Spectroscopic Investigations of Cd and Pb Sorption Mechanisms on Contrasting Biochars: Engineering Implications. Bioresour. Technol. 2014, 171, 442–451. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Shen, J.; Robinson, B.; Huang, H.; Liu, D.; Bolan, N.; Pei, J.; Wang, H. Effect of Bamboo and Rice Straw Biochars on the Bioavailability of Cd, Cu, Pb and Zn to Sedum Plumbizincicola. Agric. Ecosyst. Environ. 2014, 191, 124–132. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, K.-R.; Kim, H.-J.; Yoon, J.-H.; Yang, J.E.; Ok, Y.S.; Owens, G.; Kim, K.-H. Effect of Biochar on Heavy Metal Immobilization and Uptake by Lettuce (Lactuca sativa L.) in Agricultural Soil. Environ. Earth Sci. 2015, 74, 1249–1259. [Google Scholar] [CrossRef]
- Puga, A.P.; Abreu, C.A.; Melo, L.C.A.; Beesley, L. Biochar Application to a Contaminated Soil Reduces the Availability and Plant Uptake of Zinc, Lead and Cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, B.; Song, Z.; Wang, H.; He, F.; Han, X. Wheat Straw Biochar Amendments on the Removal of Polycyclic Aromatic Hydrocarbons (PAHs) in Contaminated Soil. Ecotoxicol. Environ. Saf. 2016, 130, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, B.; Wester, A.E.; Chen, J.; He, F.; Chen, H.; Gao, B. Reclaiming Phosphorus from Secondary Treated Municipal Wastewater with Engineered Biochar. Chem. Eng. J. 2019, 362, 460–468. [Google Scholar] [CrossRef]
- Chaukura, N.; Murimba, E.C.; Gwenzi, W. Sorptive Removal of Methylene Blue from Simulated Wastewater Using Biochars Derived from Pulp and Paper Sludge. Environ. Technol. Innov. 2017, 8, 132–140. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G. Adsorptive Removal of Dye Using Biochar Derived from Residual Algae after In-Situ Transesterification: Alternate Use of Waste of Biodiesel Industry. J. Environ. Manag. 2016, 182, 187–197. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of Green Waste Compost and Biochar Soil Amendments for Reducing Lead and Copper Mobility and Uptake to Ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Kasozi, G.N.; Zimmerman, A.R.; Nkedi-Kizza, P.; Gao, B. Catechol and Humic Acid Sorption onto a Range of Laboratory-Produced Black Carbons (Biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.C.A.; Puga, A.P.; Coscione, A.R.; Beesley, L.; Abreu, C.A.; Camargo, O.A. Sorption and Desorption of Cadmium and Zinc in Two Tropical Soils Amended with Sugarcane-Straw-Derived Biochar. J. Soils Sediments 2015, 16, 226–234. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, Z.; Cai, C.; Wang, X.; Huang, Y.; Xiao, B.; Sun, G. Effect of biochars from rice husk, bran, and straw on heavy metal uptake by pot-grown wheat seedling in a historically contaminated soil. BioResources 2013, 8, 5965–5982. [Google Scholar] [CrossRef]
- Jamieson, T.; Sager, E.; Guéguen, C. Characterization of Biochar-Derived Dissolved Organic Matter Using UV–Visible Absorption and Excitation–Emission Fluorescence Spectroscopies. Chemosphere 2014, 103, 197–204. [Google Scholar] [CrossRef]
- Awika, J.M.; Piironen, V.; Bean, S.; American Chemical Society; Division of Agricultural and Food Chemistry. Advances in Cereal Science: Implications to Food Processing and Health Promotion; American Chemical Society: Washington, DC, USA, 2011. [Google Scholar]
- Hensel, G.; Kastner, C.; Oleszczuk, S.; Riechen, J.; Kumlehn, J. Agrobacterium-Mediated Gene Transfer to Cereal Crop Plants: Current Protocols for Barley, Wheat, Triticale, and Maize. Int. J. Plant Genom. 2009, 2009, 835608. [Google Scholar] [CrossRef]
- Hemery, Y.; Rouau, X.; Lullien-Pellerin, V.; Barron, C.; Abecassis, J. Dry Processes to Develop Wheat Fractions and Products with Enhanced Nutritional Quality. J. Cereal Sci. 2007, 46, 327–347. [Google Scholar] [CrossRef]
- Apprich, S.; Tirpanalan, Ö.; Hell, J.; Reisinger, M.; Böhmdorfer, S.; Siebenhandl-Ehn, S.; Novalin, S.; Kneifel, W. Wheat Bran-Based Biorefinery 2: Valorization of Products. LWT-Food Sci. Technol. 2014, 56, 222–231. [Google Scholar] [CrossRef]
- Curti, E.; Carini, E.; Bonacini, G.; Tribuzio, G.; Vittadini, E. Effect of the Addition of Bran Fractions on Bread Properties. J. Cereal Sci. 2013, 57, 325–332. [Google Scholar] [CrossRef]
- Reisinger, M.; Tirpanalan, Ö.; Prückler, M.; Huber, F.; Kneifel, W.; Novalin, S. Wheat Bran Biorefinery—A Detailed Investigation on Hydrothermal and Enzymatic Treatment. Bioresour. Technol. 2013, 144, 179–185. [Google Scholar] [CrossRef]
- Kim, J.E.; Bhatia, S.K.; Song, H.J.; Yoo, E.; Jeon, H.J.; Yoon, J.Y.; Yang, Y.; Gurav, R.; Yang, Y.H.; Kim, H.J.; et al. Adsorptive removal of tetracycline from aqueous solution by maple leaf-derived biochar. Bioresour. Technol. 2020, 306, 1230–1292. [Google Scholar] [CrossRef]
- Roy, D.; Neogi, S.; De, S. Adsorptive Removal of Heavy Metals from Battery Industry Effluent Using MOF Incorporated Polymeric Beads: A Combined Experimental and Modeling Approach. J. Hazard. Mater. 2021, 403, 123624. [Google Scholar] [CrossRef]
- Kesenci, K.; Say, R.; Denizli, A. Removal of Heavy Metal Ions from Water by Using Poly(Ethyleneglycol Dimethacrylate-Co-Acrylamide) Beads. Eur. Polym. J. 2002, 38, 1443–1448. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Ahmedna, M.; Clarke, S.J.; Rao, R.M.; Marshall, W.E.; Johns, M.M. Use of Filtration and Buffers in Raw Sugar Colour Measurements. J. Sci. Food Agric. 1997, 75, 109–116. [Google Scholar] [CrossRef]
- Borhan, M.S.; Rahman, S.; Sarker, N.C. Characterizing Corn and Cattle Manure Derived Biochars Relevant to Their Use as Soil Additives. Trans. ASABE 2018, 61, 1335–1349. [Google Scholar] [CrossRef]
- Stella Mary, G.; Sugumaran, P.; Niveditha, S.; Ramalakshmi, B.; Ravichandran, P.; Seshadri, S. Production, Characterization and Evaluation of Biochar from Pod (Pisum sativum), Leaf (Brassica oleracea) and Peel (Citrus sinensis) Wastes. Int. J. Recycl. Org. Waste Agric. 2016, 5, 43–53. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar Addition to Agricultural Soil Increased CH4 Uptake and Water Holding Capacity—Results from a Short-Term Pilot Field Study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption of Ammonium and Nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of Low-Temperature Pyrolysis Conditions on Biochar for Agricultural Use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Stewart, C.E.; Cantrell, K.B.; Uchimiya, M.; DuSaire, M.G.; Ro, K.S. Qualitative Analysis of Volatile Organic Compounds on Biochar. Chemosphere 2011, 85, 869–882. [Google Scholar] [CrossRef]
- Lopez-Capel, E.; Sohi, S.P.; Gaunt, J.L.; Manning, D.A.C. Use of Thermogravimetry-Differential Scanning Calorimetry to Characterize Modelable Soil Organic Matter Fractions. Soil Sci. Soc. Am. J. 2005, 69, 930. [Google Scholar] [CrossRef]
- Garg, U.; Kaur, M.P.; Jawa, G.K.; Sud, D.; Garg, V.K. Removal of Cadmium (II) from Aqueous Solutions by Adsorption on Agricultural Waste Biomass. J. Hazard. Mater. 2008, 154, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bai, R. Highly Enhanced Adsorption of Lead Ions on Chitosan Granules Functionalized with Poly(Acrylic Acid). Ind. Eng. Chem. Res. 2006, 45, 7897–7904. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Harzandi, A.M.; Hosseinzadeh, H. Modified Carrageenan 3. Synthesis of a Novel Polysaccharide-Based Superabsorbent Hydrogel via Graft Copolymerization of Acrylic Acid onto Kappa-Carrageenan in Air. Eur. Polym. J. 2004, 40, 1363–1370. [Google Scholar] [CrossRef]



| Parameter | Pyrolyzing Temperature | Biochar Type | |
|---|---|---|---|
| Wheat Bran Biochar | Maple Leaf Biochar | ||
| Yield (%) | 300 °C | 27.1% ± 2.17% | 29.3% ± 3.63% |
| 500 °C | 23.4% ± 1.78% | 24.5% ± 2.97% | |
| 700 °C | 19.7% ± 2.41% | 22.8% ± 4.29% | |
| pH | 300 °C | 8.32 ± 0.05 | 8.79 ± 0.09 |
| 500 °C | 8.29 ± 0.03 | 8.91 ± 0.06 | |
| 700 °C | 8.27 ± 0.02 | 8.78 ± 0.04 | |
| Electric conductivity (EC, µS cm−1) | 300 °C | 428 ± 8.00 | 613 ± 12.3 |
| 500 °C | 437 ± 11.0 | 647 ± 23.5 | |
| 700 °C | 417 ± 6.00 | 629 ± 14.8 | |
| Bulk density (BD, g mL−1) | 300 °C | 0.47 ± 0.08 | 0.29 ± 0.03 |
| 500 °C | 0.43 ± 0.05 | 0.27 ± 0.07 | |
| 700 °C | 0.46 ± 0.03 | 0.32 ± 0.04 | |
| Water holding capacity (WHC, %) | 300 °C | 72 ± 4.62 | 82 ± 4.32 |
| 500 °C | 76 ± 3.12 | 83 ± 7.12 | |
| 700 °C | 73 ± 5.34 | 86 ± 2.58 | |
| Total carbon (TC, %) | 300 °C | 52 ± 3.21 | 63 ± 4.93 |
| 500 °C | 54 ± 4.54 | 66 ± 5.67 | |
| 700 °C | 55 ± 6.12 | 61 ± 7.77 | |
| Title | Absorbance at 650 nm | Concentration of Dye Solution After Absorbance (mg/L) | Dye Absorbed by the Beads (mg) |
|---|---|---|---|
| Stock solution | 0.0065 | 500 | - |
| Diluted solution-1 | 0.0024 | 250 | - |
| Diluted solution-2 | 0.0018 | 125 | - |
| Diluted solution-3 | 0.0013 | 62.5 | - |
| Phytagel–WBC = 1:1 | 0.0039 | 290 | 10.5 |
| Phytagel–WBC = 1:2 | 0.0036 | 250 | 12.5 |
| Phytagel–WBC = 1:5 | 0.0030 | 190 | 15.5 |
| Phytagel–WBC = 1:10 | 0.0021 | 100 | 20.0 |
| Phytogel Beads | 0.0058 | 470 | 1.50 |
| Phytagel–MLBC = 1:1 | 0.0061 | 500 | 0.00 |
| Phytagel–MLBC = 1:2 | 0.0048 | 370 | 6.50 |
| Phytagel–MLBC = 1:5 | 0.0046 | 350 | 7.50 |
| Phytagel–MLBC = 1:10 | 0.0014 | 230 | 13.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarker, N.C.; Badsha, M.A.R.; Hillukka, G.; Holter, B.; Kjelland, M.; Hossain, K. Pyrolyzed Biochar from Agricultural Byproducts: Synthesis, Characterization, and Application in Water Pollutants Removal. Processes 2025, 13, 1358. https://doi.org/10.3390/pr13051358
Sarker NC, Badsha MAR, Hillukka G, Holter B, Kjelland M, Hossain K. Pyrolyzed Biochar from Agricultural Byproducts: Synthesis, Characterization, and Application in Water Pollutants Removal. Processes. 2025; 13(5):1358. https://doi.org/10.3390/pr13051358
Chicago/Turabian StyleSarker, Niloy Chandra, Md Abdur Rahim Badsha, Greta Hillukka, Bethany Holter, Michael Kjelland, and Khwaja Hossain. 2025. "Pyrolyzed Biochar from Agricultural Byproducts: Synthesis, Characterization, and Application in Water Pollutants Removal" Processes 13, no. 5: 1358. https://doi.org/10.3390/pr13051358
APA StyleSarker, N. C., Badsha, M. A. R., Hillukka, G., Holter, B., Kjelland, M., & Hossain, K. (2025). Pyrolyzed Biochar from Agricultural Byproducts: Synthesis, Characterization, and Application in Water Pollutants Removal. Processes, 13(5), 1358. https://doi.org/10.3390/pr13051358







