An Early Study on the Synthesis of Lignin-Graft-(Net-Poly(acrylamide-co-N,N′methylenebisacrylamide)), Characterization of the Produced Copolymer, and Evaluation of Its Performance as Adsorbent for Lead Removal from Wastewater Purposes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Graft Copolymerization
2.2.1. Conventional Heating
2.2.2. Microwave Heating
2.2.3. Characterization
2.2.4. Adsorption of Pb2+
3. Results and Discussion
3.1. Lignin Modifications
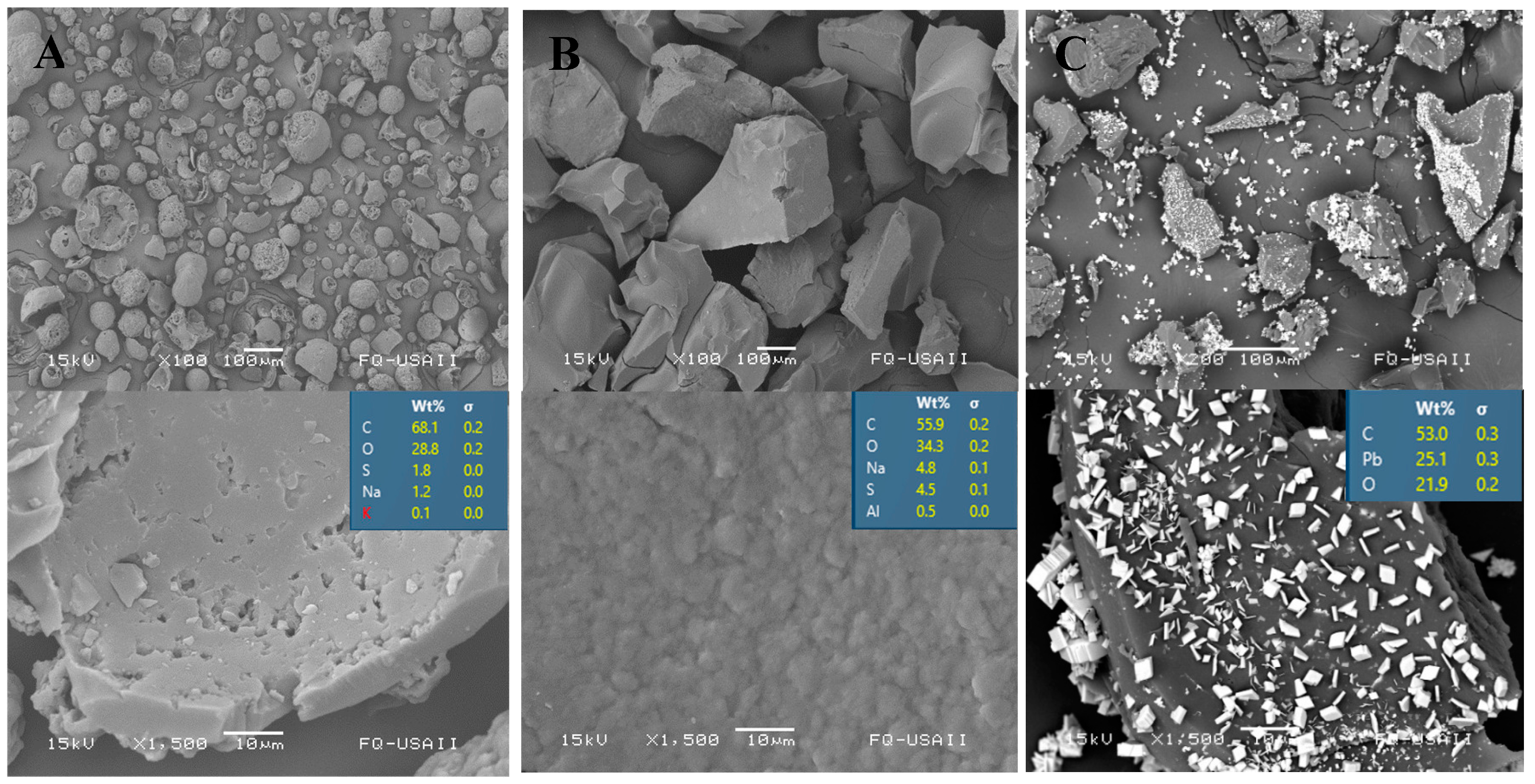
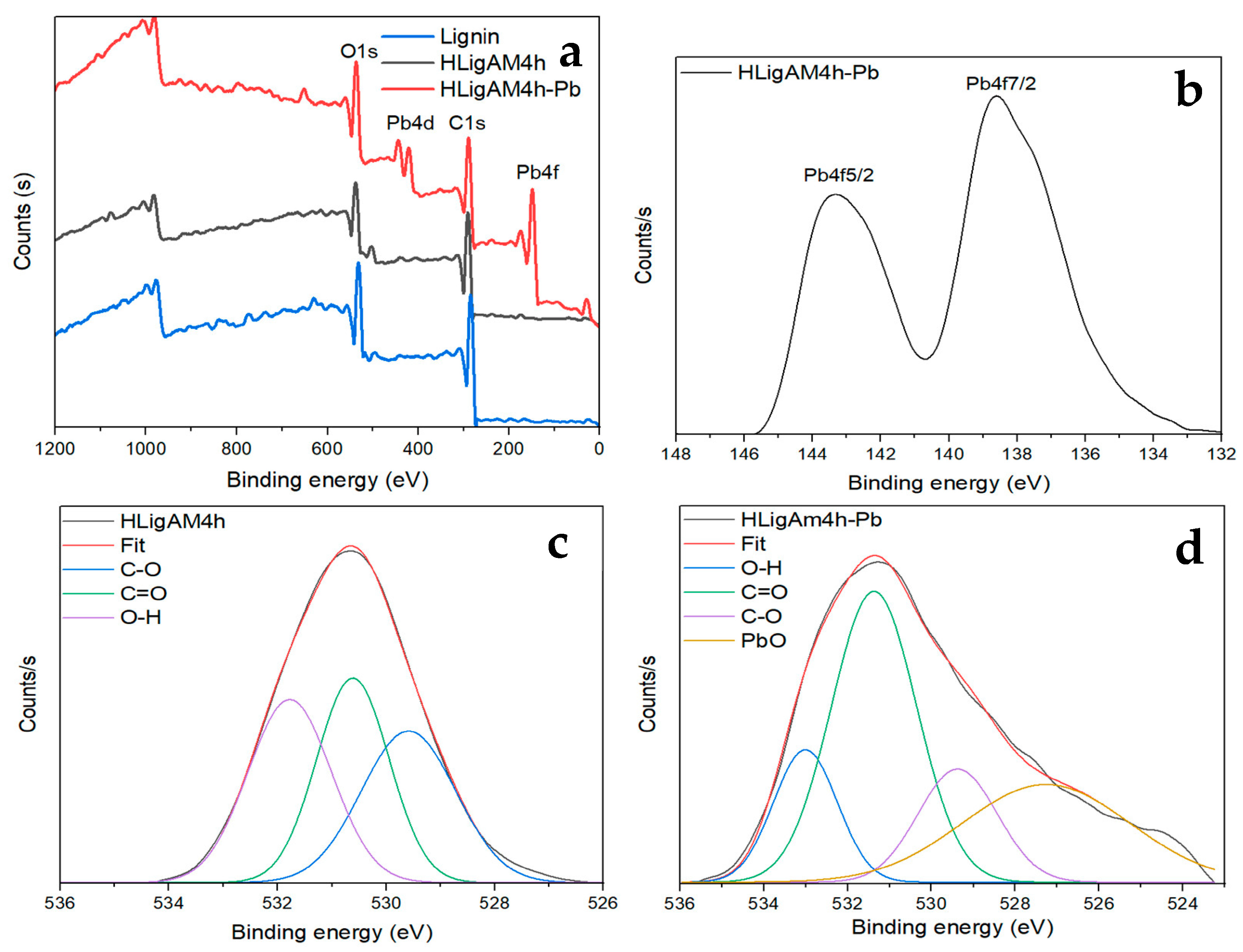
3.2. Characterization of Starting and Modifed Lignin Copolymers
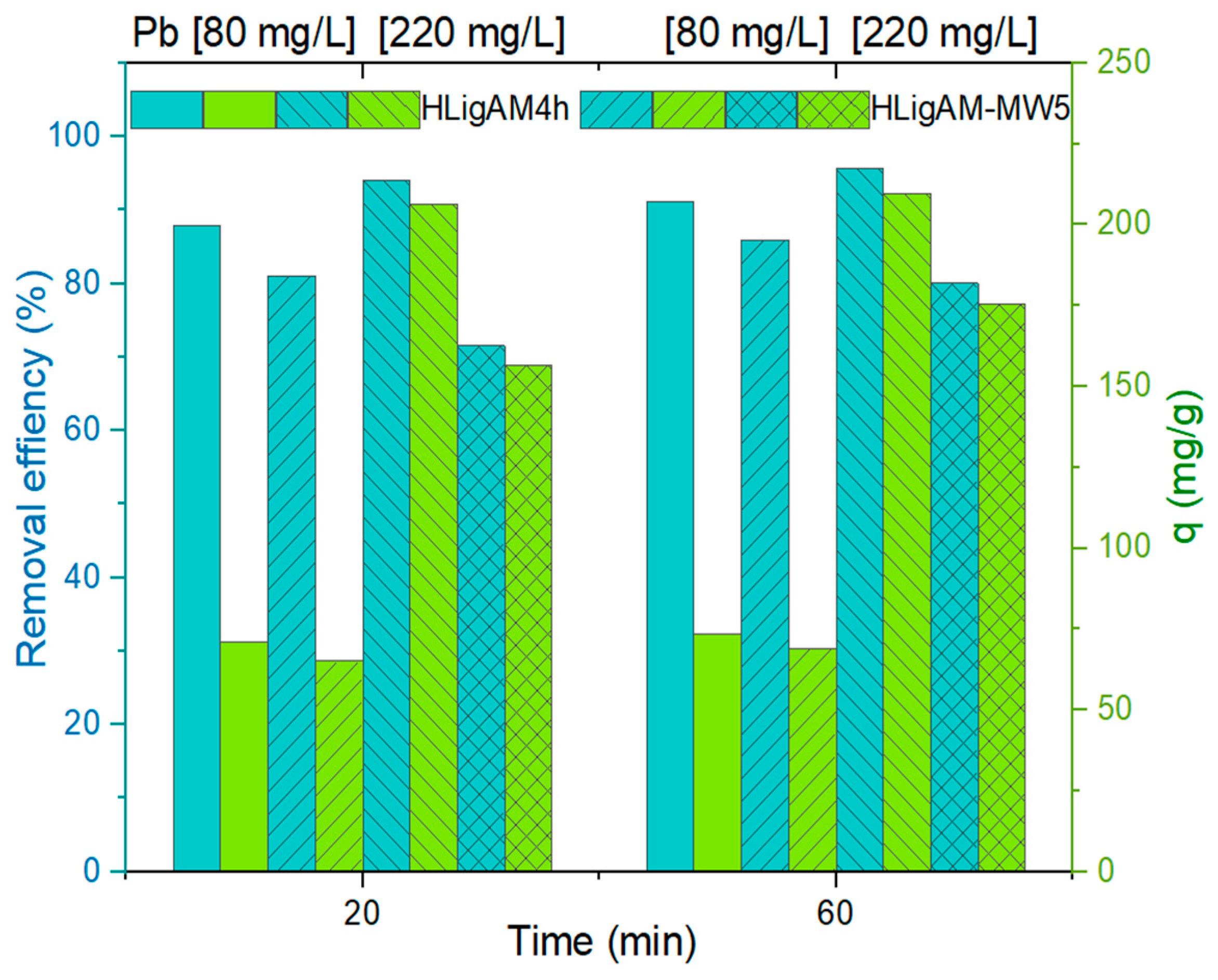
3.3. Pb2+ Adsorption Experiments
3.3.1. Effect of Material and Likely Removal Mechanism
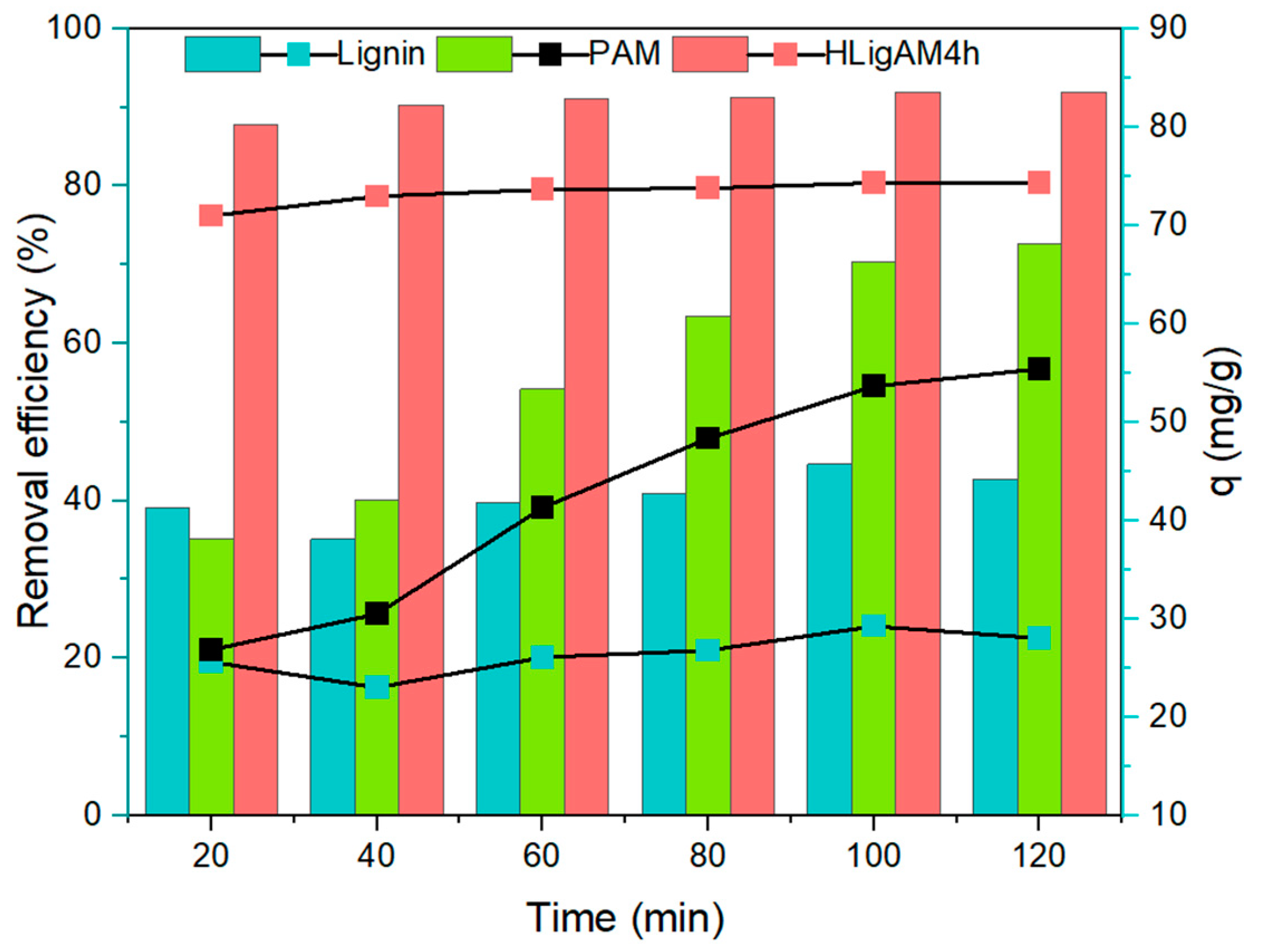
3.3.2. Effect of Lignin Modification Time
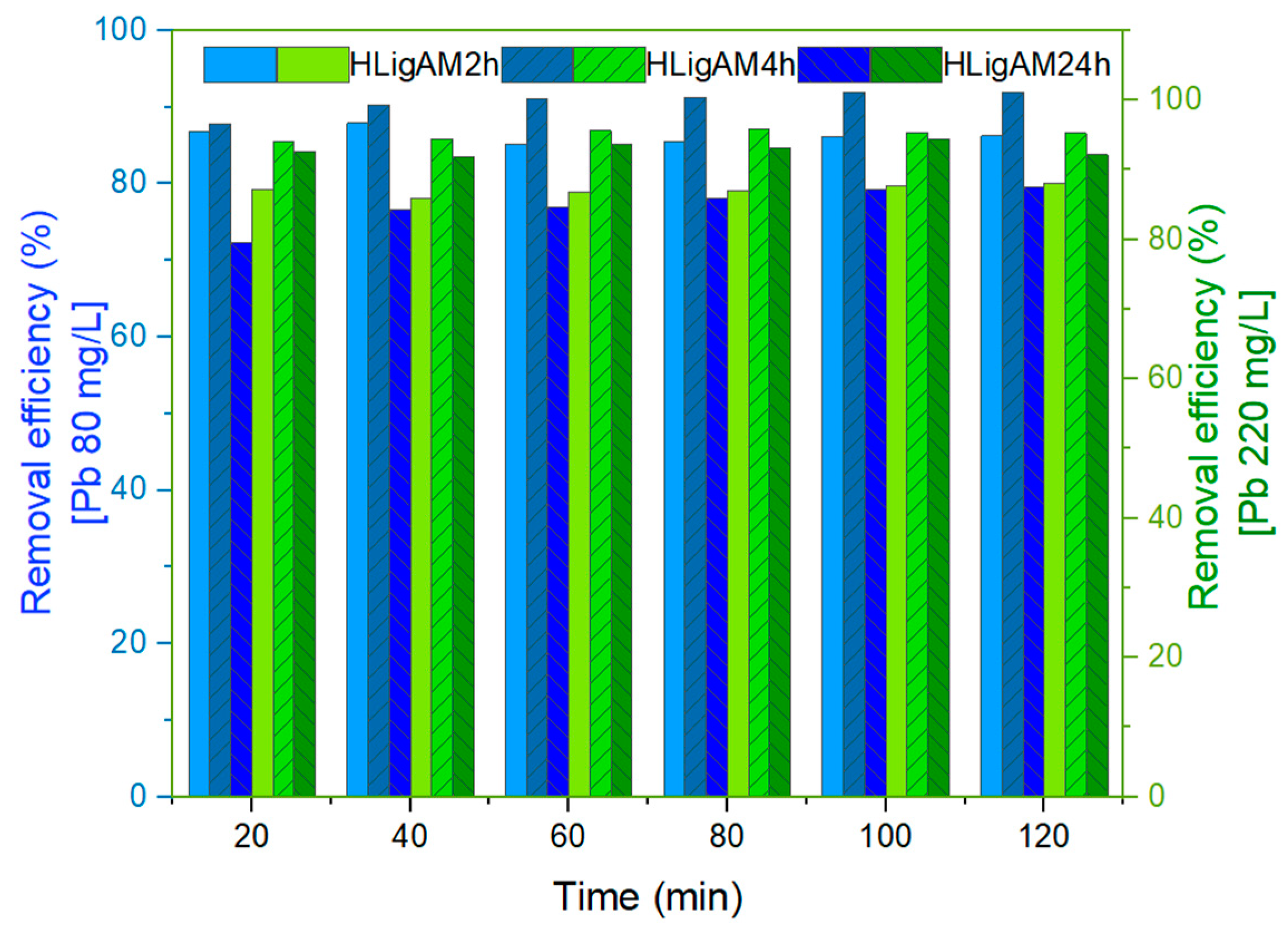
3.3.3. Effect of Heating Source
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sternberg, J.; Sequerth, O.; Pilla, S. Green chemistry design in polymers derived from lignin: Review and perspective. Prog. Polym. Sci. 2021, 113, 101344. [Google Scholar] [CrossRef]
- Araújo, D.M.F.; Filho, I.J.D.C.; Santos, T.; Pereira, D.T.M.; Marques, D.S.C.; Lima, A.d.C.A.d.; de Aquino, T.M.; Rocha, G.J.D.M.; de Lima, M.D.C.A.; Nogueira, F. Biological activities and physicochemical characterization of alkaline lignins obtained from branches and leaves of Buchenavia viridiflora with potential pharmaceutical and biomedical applications. Int. J. Biol. Macromol. 2022, 219, 224–245. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Hernández-Meléndez, O.; Garcés-Sandoval, F.I.; Montiel, C.; Hernández-Luna, M.G.; Manero, O.; Bárzana, E.; Vivaldo-Lima, E. Modeling of Pretreatment and Combined Alkaline and Enzymatic Hydrolyses of Blue Agave Bagasse in Corotating Twin-Screw Extruders. Macromol. React. Eng. 2022, 16, 2100059. [Google Scholar] [CrossRef]
- An, L.; Si, C.; Bae, J.H.; Jeong, H.; Kim, Y.S. One-step silanization and amination of lignin and its adsorption of Congo red and Cu(II) ions in aqueous solution. Int. J. Biol. Macromol. 2020, 159, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Fan, X.; Yu, Y.; Wang, Q.; Wang, P.; Yuan, J. Graft modification of lignin-based cellulose via enzyme-initiated reversible addition-fragmentation chain transfer (RAFT) polymerization and free-radical coupling. Int. J. Biol. Macromol. 2019, 144, 267–278. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin Biopolymers in the Age of Controlled Polymerization. Polymers 2019, 11, 1176. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z. Application of Lignin and Its Derivatives in Adsorption of Heavy Metal Ions in Water: A Review. ACS Sustain Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Kong, F.; Wang, S.; Price, J.T.; Konduri, M.K.; Fatehi, P. Water soluble kraft lignin–acrylic acid copolymer: Synthesis and characterization. Green Chem. 2015, 17, 4355–4366. [Google Scholar] [CrossRef]
- Xu, Q.; Bai, Y.; Zhao, X.; Ren, M.; Wang, S.; Kong, F. Synthesis and characterization of an amphiphilic lignin-based cationic surfactant. Ind. Crops Prod. 2021, 164, 113376. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Yuan, Z.; Fang, J.; Chang, L.; Zhang, H.; Li, C. Role of sulfonation in lignin-based material for adsorption removal of cationic dyes. Int. J. Biol. Macromol. 2019, 135, 1171–1181. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Miao, Y.; Mai, Y.; Li, H.; Chen, X.; Chen, J. A lignin-biochar with high oxygen-containing groups for adsorbing lead ion prepared by simultaneous oxidization and carbonization. Bioresour. Technol. 2020, 307, 123165. [Google Scholar] [CrossRef]
- Luo, S.; Cao, J.; McDonald, A.G. Esterification of industrial lignin and its effect on the resulting poly(3-hydroxybutyrate-co-3-hydroxyvalerate) or polypropylene blends. Ind. Crops Prod. 2017, 97, 281–291. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, X.; Wu, W.; An, X.; Tian, Y.; Qiao, Y. Facile preparation of lignosulfonate/N-methylaniline composite and its application in efficient removal of Cr(VI) from aqueous solutions. Int. J. Biol. Macromol. 2020, 154, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Yu, J.; Li, S.; Wang, J.; Wang, C.; Chu, F. Integration of lignin and acrylic monomers towards grafted copolymers by free radical polymerization. Int. J. Biol. Macromol. 2014, 67, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Vega-hernández, M.Á.; Cano-díaz, G.S.; Vivaldo-lima, E.; Rosas-aburto, A.; Hernández-luna, M.G.; Martinez, A.; Palacios-alquisira, J.; Mohammadi, Y.; Penlidis, A. A Review on the Synthesis, Characterization and Modeling of Polymer Grafting. Processes 2021, 9, 375. [Google Scholar] [CrossRef]
- Pourmahdi, M.; Abdollahi, M.; Nasiri, A. Effect of lignin source and initiation conditions on graft copolymerization of lignin with acrylamide and performance of graft copolymer as additive in water-based drilling fluid. J. Pet. Sci. Eng. 2023, 220, 111253. [Google Scholar] [CrossRef]
- Aldajani, M.; Alipoormazandarani, N.; Kong, F.; Fatehi, P. Acid hydrolysis of kraft lignin-acrylamide polymer to improve its flocculation affinity. Sep. Purif. Technol. 2020, 258, 117964. [Google Scholar] [CrossRef]
- Wu, W.; Qi, J.; Fang, J.; Lyu, G.; Yuan, Z.; Wang, Y.; Li, H. One-pot preparation of lignin-based cationic flocculant and its application in dye wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130082. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Shen, J.; Zhang, S.; Liu, X.; Chen, X.; Ma, Y.; Ren, S.; Fang, G.; Li, S.; et al. Simultaneous removal of Pb2+, Cu2+ and Cd2+ ions from wastewater using hierarchical porous polyacrylic acid grafted with lignin. J. Hazard. Mater. 2020, 392, 122208. [Google Scholar] [CrossRef]
- Lee, K.E.; Morad, N.; Teng, T.T.; Poh, B.T. Development, Characterization and the Application of Hybrid Ma-terials in Coagulation/Flocculation of Wastewater: A Review. Chem. Eng. J. 2012, 203, 370–386. [Google Scholar] [CrossRef]
- Guo, K.; Gao, B.; Yue, Q.; Xu, X.; Li, R.; Shen, X. Characterization and performance of a novel lignin-based flocculant for the treatment of dye wastewater. Int. Biodeterior. Biodegrad. 2018, 133, 99–107. [Google Scholar] [CrossRef]
- Feng, X.; Wan, J.; Deng, J.; Qin, W.; Zhao, N.; Luo, X.; He, M.; Chen, X. Preparation of acrylamide and carboxymethyl cellulose graft copolymers and the effect of molecular weight on the flocculation properties in simulated dyeing wastewater under different pH conditions. Int. J. Biol. Macromol. 2019, 155, 1142–1156. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Yang, X.; Shao, L.; Zhang, X.; Yao, J. Polyacrylamide grafted cellulose as an eco-friendly flocculant: Key factors optimization of flocculation to surfactant effluent. Carbohydr. Polym. 2016, 135, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, F.; Tang, Q.; Du, B.; Ma, D.; Zhao, Z.; Fan, L.; Luo, H.; Zhao, Z.; Huang, X.; et al. Evaluating the performance of bridging-assembly chelating flocculant for heavy metals removal: Role of branched architectures. Chemosphere 2021, 289, 133260. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wen, J.-L.; Sun, S.-L.; Wang, H.-M.; Wang, S.-F.; Liu, Q.-Y.; Charlton, A.; Sun, R.-C. Chemosynthesis and structural characterization of a novel lignin-based bio-sorbent and its strong adsorption for Pb (II). Ind. Crops Prod. 2017, 108, 72–80. [Google Scholar] [CrossRef]
- Shi, X.; Qiao, Y.; An, X.; Tian, Y.; Zhou, H. High-capacity adsorption of Cr(VI) by lignin-based composite: Characterization, performance and mechanism. Int. J. Biol. Macromol. 2020, 159, 839–849. [Google Scholar] [CrossRef]
- Popovic, A.L.; Rusmirovic, J.D.; Velickovic, Z.; Radovanovic, Z.; Ristic, M.; Pavlovic, V.P.; Marinkovic, A.D. Novel amino-functionalized lignin microspheres: High performance biosorbent with enhanced capacity for heavy metal ion removal. Int. J. Biol. Macromol. 2020, 156, 1160–1173. [Google Scholar] [CrossRef]
- Ge, Y.; Qin, L.; Li, Z. Lignin microspheres: An effective and recyclable natural polymer-based adsorbent for lead ion removal. Mater. Des. 2016, 95, 141–147. [Google Scholar] [CrossRef]
- Jiao, G.-J.; Ma, J.; Li, Y.; Jin, D.; Zhou, J.; Sun, R. Removed heavy metal ions from wastewater reuse for chemiluminescence: Successive application of lignin-based composite hydrogels. J. Hazard. Mater. 2021, 421, 126722. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; Jie, P.; Zhu, J.; Cheng, F. Preparation of chitosan/lignosulfonate for effectively removing Pb(II) in water. Polymer 2021, 228, 123878. [Google Scholar] [CrossRef]
- Fang, R.; Cheng, X.-S.; Fu, J.; Zheng, Z.-B. Research on the Graft Copolymerization of EH-lignin with acrylamide. Nat. Sci. 2009, 01, 17–22. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Kong, F.; Yang, G.; Fatehi, P. Preparation and Characterization of Lignin-Acrylamide Copolymer as a Paper Strength Additive. Bioresources 2015, 11, 1765–1783. [Google Scholar] [CrossRef]
- Li, Z.; Kong, Y.; Ge, Y. Synthesis of porous lignin xanthate resin for Pb2+ removal from aqueous solution. Chem. Eng. J. 2015, 270, 229–234. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Pang, Y.; Qiu, X.; Yang, D. Microwave-assisted synthesis of high carboxyl content of lignin for enhancing adsorption of lead. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 553, 187–194. [Google Scholar] [CrossRef]
- Du, B.; Chai, L.; Zheng, Q.; Liu, Y.; Wang, X.; Chen, X.; Zhai, S.; Zhou, J.; Sun, R.-C. Designed synthesis of multifunctional lignin-based adsorbent for efficient heavy metal ions removal and electromagnetic wave absorption. Int. J. Biol. Macromol. 2023, 234, 123668. [Google Scholar] [CrossRef]
- Dlamini, D.S.; Mishra, A.K.; Mamba, B.B. Kinetic and equilibrium studies of the removal of Pb2+ from aqueous solutions using Na2SO4-EVA/Cloisite® 20A composite. Mater. Chem. Phys. 2012, 133, 369–375. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, Z.; Xu, X.; Liu, X.; Liu, L.; Huang, G.; Liu, L.; Wang, H.; Song, P. Grafting Lignin with Bioderived Polyacrylates for Low-Cost, Ductile, and Fully Biobased Poly(lactic acid) Composites. ACS Sustain. Chem. Eng. 2020, 8, 2267–2276. [Google Scholar] [CrossRef]
- Sinnwell, S.; Ritter, H. Microwave assisted hydroxyalkylamidation of poly(ethylene-co-acrylic acid) and formation of grafted poly(ε-caprolactone) side chains. J. Polym. Sci. A Polym. Chem. 2007, 45, 3659–3667. [Google Scholar] [CrossRef]
- Sincari, V.; Petrova, S.L.; Konefał, R.; Hruby, M.; Jäger, E. Microwave-assisted RAFT polymerization of N-(2-hydroxypropyl) methacrylamide and its relevant copolymers. React. Funct. Polym. 2021, 162, 104875. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Hou, Y. Preparation of magnetic polyethylenimine lignin and its adsorption of Pb(II). Int. J. Biol. Macromol. 2019, 141, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, J.; Yan, M.; Li, J.; Yang, J.; Huang, M.; Zhang, R. Waste lignin-based cationic flocculants treating dyeing wastewater: Fabrication, performance, and mechanism. Sci. Total Environ. 2023, 874, 162383. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Wang, X.; Xun, J.; Lou, T. Microwave assisted synthesis and characterization of a ternary flocculant from chitosan, acrylamide and lignin. Int. Biodeterior. Biodegrad. 2017, 123, 269–275. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Hao, C.; Dai, X.; Zhou, Z.; Si, N. Ultrasonic-assisted synthesis of aminated lignin by a Mannich reaction and its decolorizing properties for anionic azo-dyes. RSC Adv. 2014, 4, 28156–28164. [Google Scholar] [CrossRef]
- Liu, X.; Gao, C.; Fu, C.; Xi, Y.; Fatehi, P.; Zhao, J.R.; Wang, S.; Gibril, M.E.; Kong, F. Preparation and Performance of Lignin-Based Multifunctional Superhydrophobic Coating. Molecules 2022, 27, 1440. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hong, S.; Sun, Q.; Cao, X.; Yu, S.; Sun, Z.; Yuan, T.-Q. Performance regulation of lignin-based flocculant at the practical molecular level by fractionation. Sep. Purif. Technol. 2022, 299, 121670. [Google Scholar] [CrossRef]
- Vilén, E.M.; Sandström, C. NMR Study on the Interaction of Trehalose with Lactose and Its Effect on the Hydrogen Bond Interaction in Lactose. Molecules 2013, 18, 9735–9754. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.H.; Zhou, M.S.; Qiu, X.Q. Preparation of Water-Soluble Carboxymethylated Lignin from Wheat Straw Alkali Lignin. Adv. Mater. Res. 2012, 550–553, 1293–1298. [Google Scholar] [CrossRef]
- Konduri, M.K.; Kong, F.; Fatehi, P. Production of carboxymethylated lignin and its application as a dispersant. Eur. Polym. J. 2015, 70, 371–383. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Yu, Z.; Zeng, G.; Luo, Y.; Jiang, L.; Yang, Z.; Qian, Y.; Wu, H. Amorphous MnO2 Modified Biochar Derived from Aerobically Composted Swine Manure for Adsorption of Pb(II) and Cd(II). ACS Sustain. Chem. Eng. 2017, 5, 5049–5058. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, S.; Wang, X.; Zhang, W.; Lagerquist, L.; Qin, M.; Willför, S.; Xu, C.; Fatehi, P. Ultrafast adsorption of heavy metal ions onto functionalized lignin-based hybrid magnetic nanoparticles. Chem. Eng. J. 2019, 372, 82–91. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, L.; Wang, R.; Li, G.; Rao, P.; Ju, M.; Jian, L.; Guo, X.; Che, L. Recyclable nitrogen-doped biochar via low-temperature pyrolysis for enhanced lead(II) removal. Chemosphere 2022, 286, 131666. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Hou, Y. Preparation of a Novel Lignin Nanosphere Adsorbent for Enhancing Adsorption of Lead. Molecules 2019, 24, 2704. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, H.; Deng, L.; Wang, Y.; Kang, D.; Li, C.; Chen, H. Enhanced adsorption of Pb(II) by nitrogen and phosphorus co-doped biochar derived from Camellia oleifera shells. Environ. Res. 2020, 191, 110030. [Google Scholar] [CrossRef]
- Abdelwahab, N.A.; Ammar, N.S.; Ibrahim, H.S. Graft copolymerization of cellulose acetate for removal and recovery of lead ions from wastewater. Int. J. Biol. Macromol. 2015, 79, 913–922. [Google Scholar] [CrossRef]
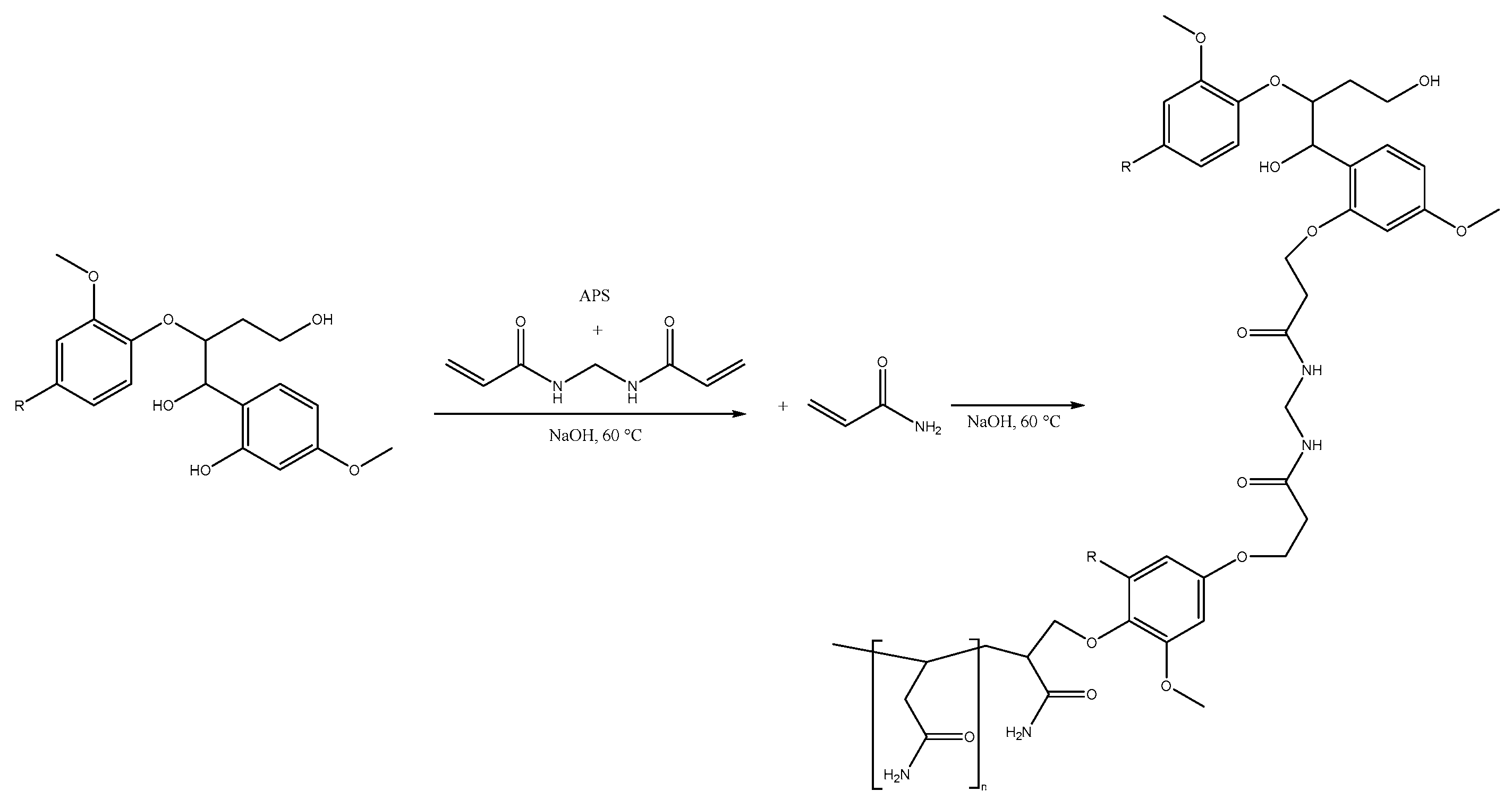

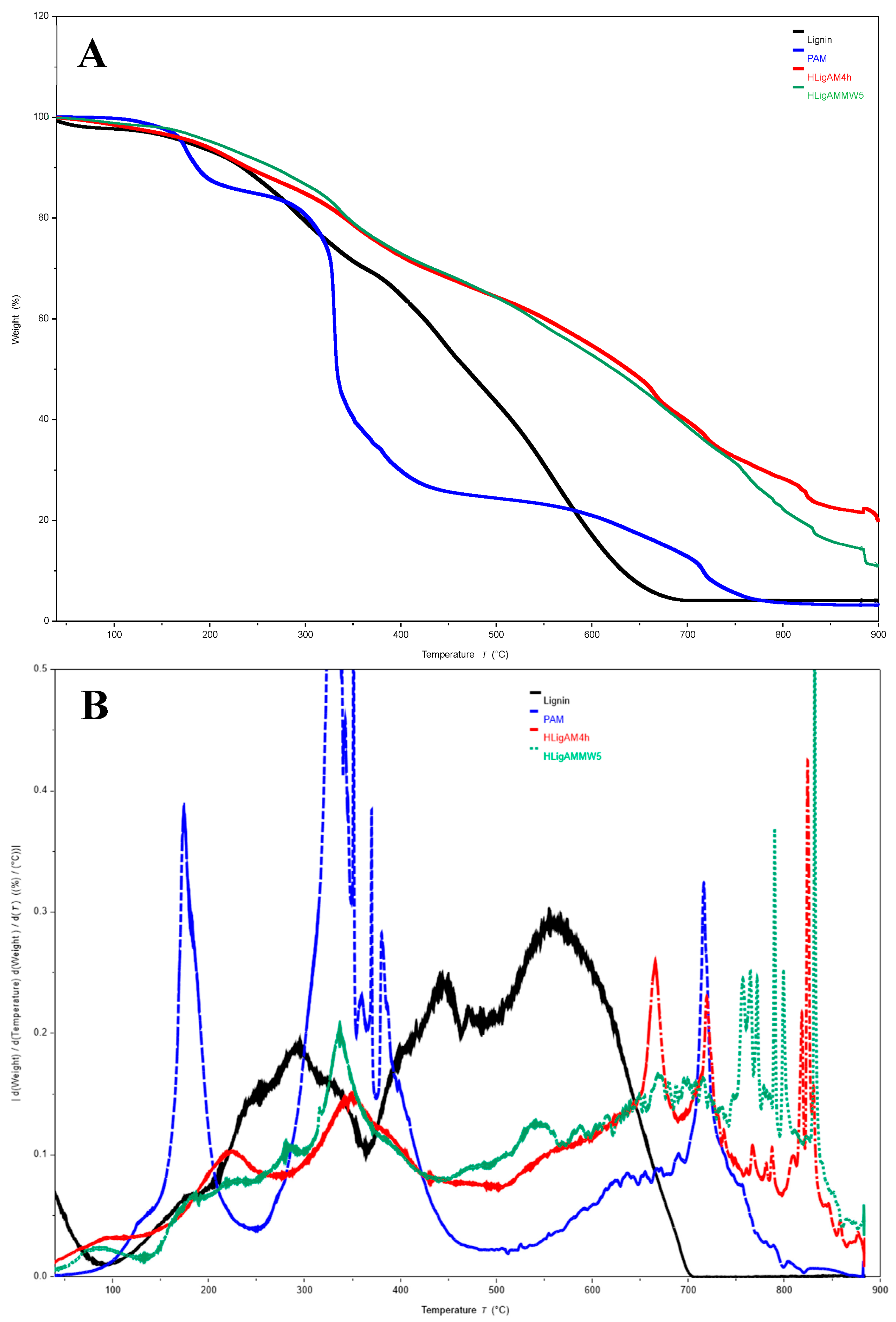
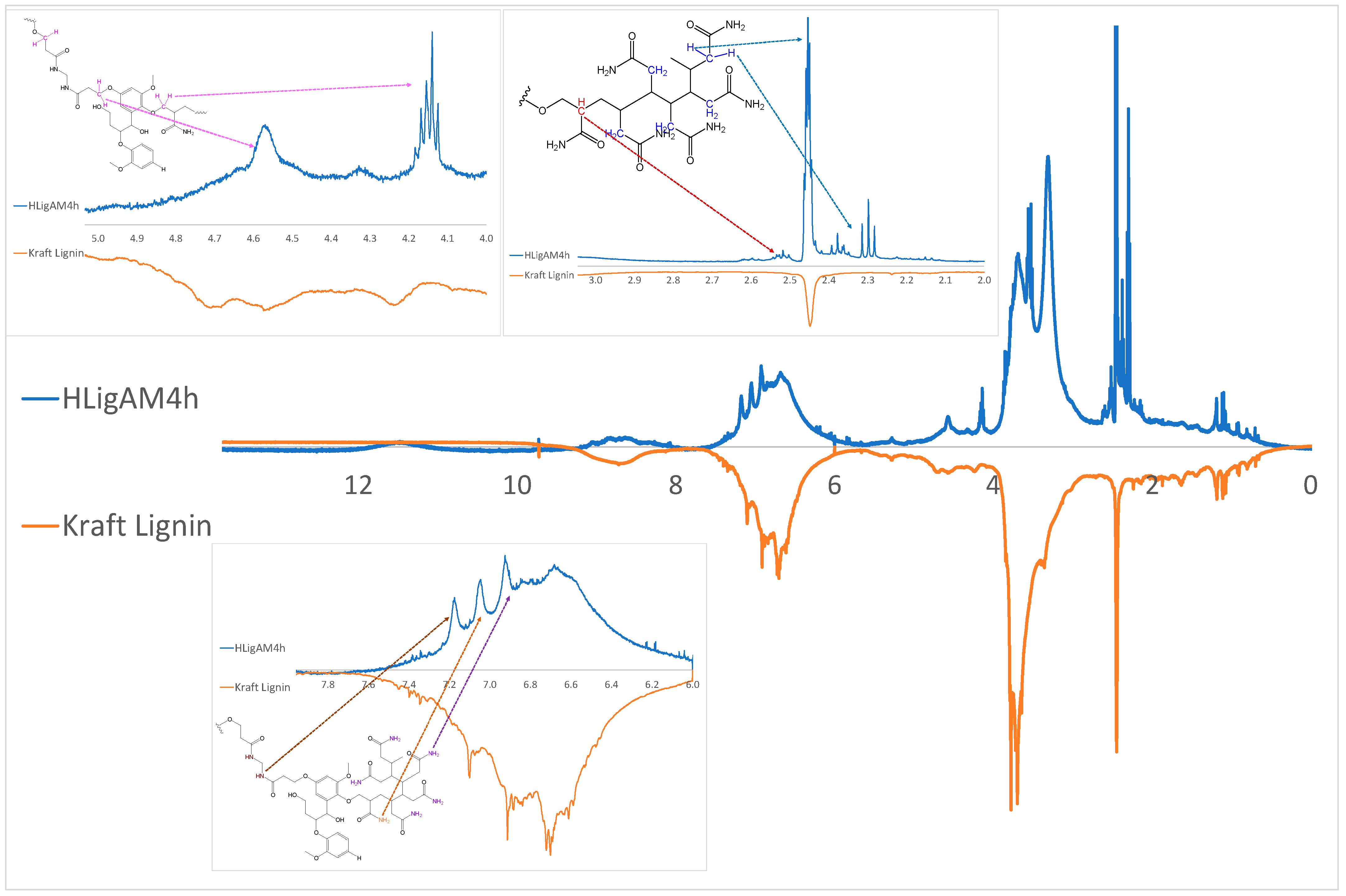
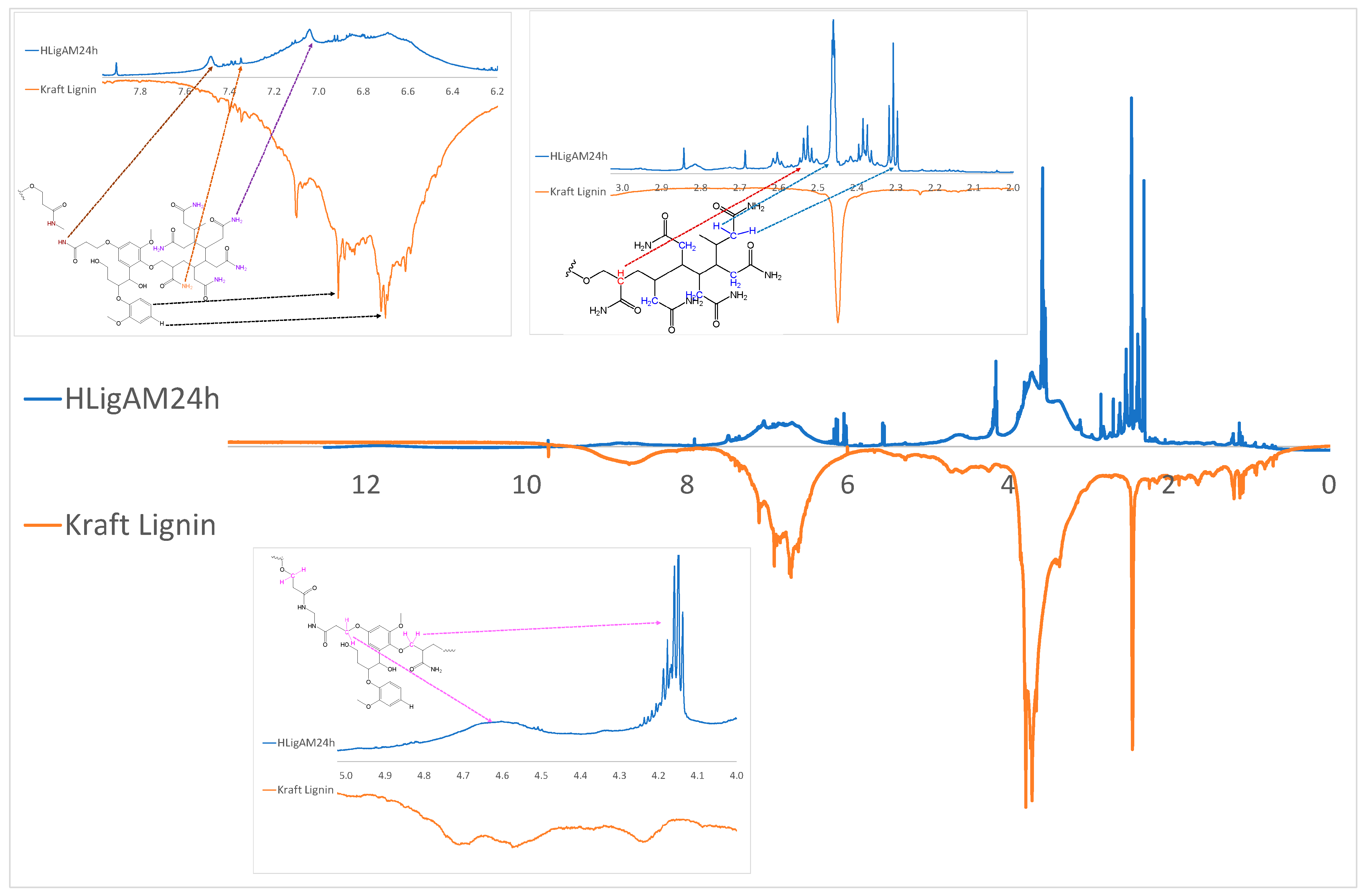


| Material | H (%) | O (%) | H (%) | N (%) |
|---|---|---|---|---|
| Lignin | 62.06 ± 0.105 | 27.10 ± 0.138 | 5.45 ± 0.018 | 1.71 ± 0.067 |
| HLigAM4h | 43.90 ± 0.169 | 35.19 ± 0.025 | 4.33 ± 0.075 | 2.57 ± 0.134 |
| HLigAMMW5 | 53.12 ± 4.108 | 30.43 ± 0.172 | 4.99 ± 0.237 | 2.47 ± 0.061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munguía-Quintero, M.F.; Vega-Hernández, M.Á.; Rosas-Aburto, A.; Hernández-Luna, M.G.; López-Ramírez, S.; Barragán-Aroche, J.F.; Vivaldo-Lima, E. An Early Study on the Synthesis of Lignin-Graft-(Net-Poly(acrylamide-co-N,N′methylenebisacrylamide)), Characterization of the Produced Copolymer, and Evaluation of Its Performance as Adsorbent for Lead Removal from Wastewater Purposes. Processes 2023, 11, 2309. https://doi.org/10.3390/pr11082309
Munguía-Quintero MF, Vega-Hernández MÁ, Rosas-Aburto A, Hernández-Luna MG, López-Ramírez S, Barragán-Aroche JF, Vivaldo-Lima E. An Early Study on the Synthesis of Lignin-Graft-(Net-Poly(acrylamide-co-N,N′methylenebisacrylamide)), Characterization of the Produced Copolymer, and Evaluation of Its Performance as Adsorbent for Lead Removal from Wastewater Purposes. Processes. 2023; 11(8):2309. https://doi.org/10.3390/pr11082309
Chicago/Turabian StyleMunguía-Quintero, María Fernanda, Miguel Ángel Vega-Hernández, Alberto Rosas-Aburto, Martín Guillermo Hernández-Luna, Simón López-Ramírez, José Fernando Barragán-Aroche, and Eduardo Vivaldo-Lima. 2023. "An Early Study on the Synthesis of Lignin-Graft-(Net-Poly(acrylamide-co-N,N′methylenebisacrylamide)), Characterization of the Produced Copolymer, and Evaluation of Its Performance as Adsorbent for Lead Removal from Wastewater Purposes" Processes 11, no. 8: 2309. https://doi.org/10.3390/pr11082309
APA StyleMunguía-Quintero, M. F., Vega-Hernández, M. Á., Rosas-Aburto, A., Hernández-Luna, M. G., López-Ramírez, S., Barragán-Aroche, J. F., & Vivaldo-Lima, E. (2023). An Early Study on the Synthesis of Lignin-Graft-(Net-Poly(acrylamide-co-N,N′methylenebisacrylamide)), Characterization of the Produced Copolymer, and Evaluation of Its Performance as Adsorbent for Lead Removal from Wastewater Purposes. Processes, 11(8), 2309. https://doi.org/10.3390/pr11082309







