Calcium Precipitates as Novel Agents for Controlling Steam Channeling in Steam Injection Processes for Heavy Oil Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Calcium Precipitate Plugging Agent Formulation
2.3. Factors Influencing Calcium Precipitate Plugging Systems
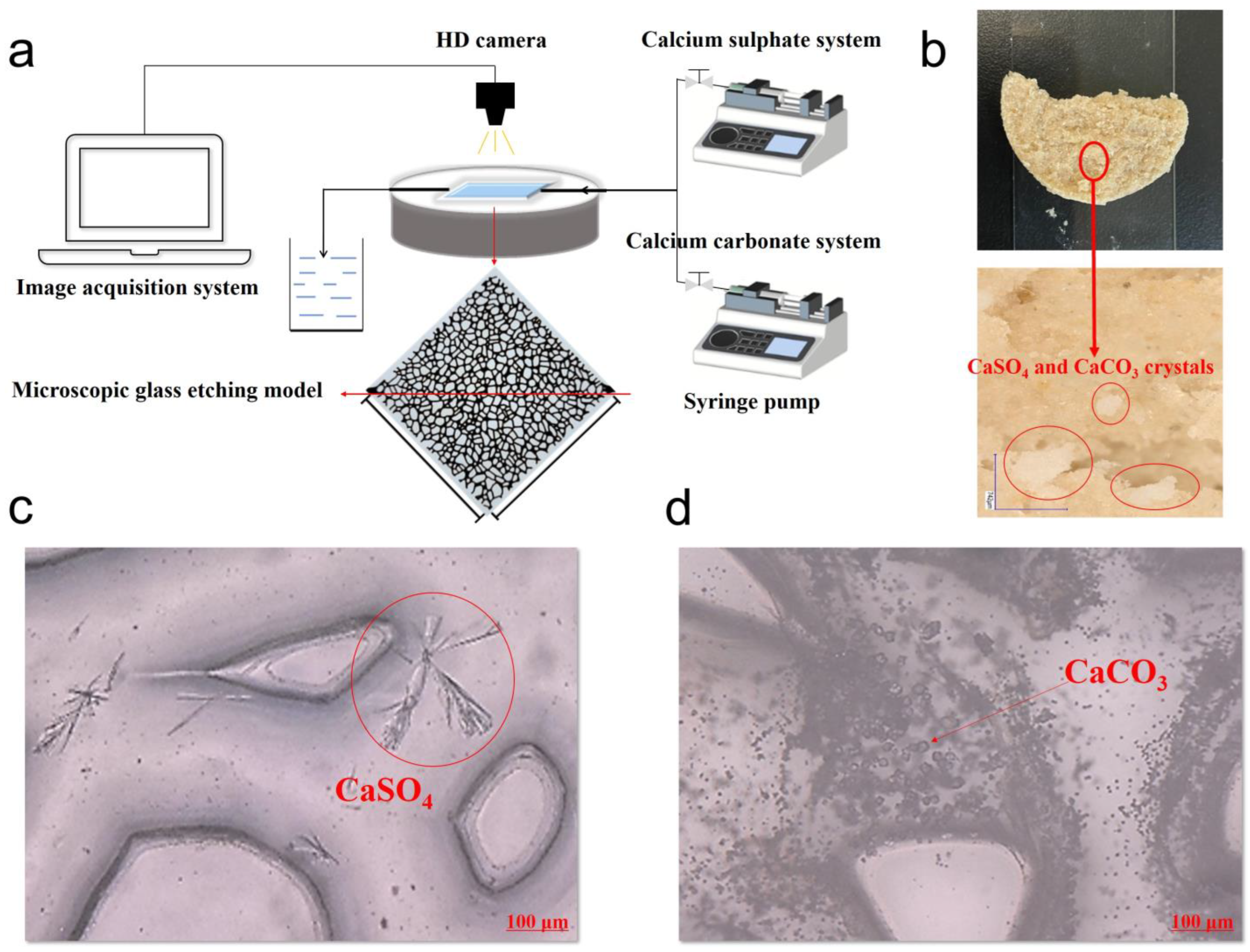
2.4. Micro-Visualization Experiments
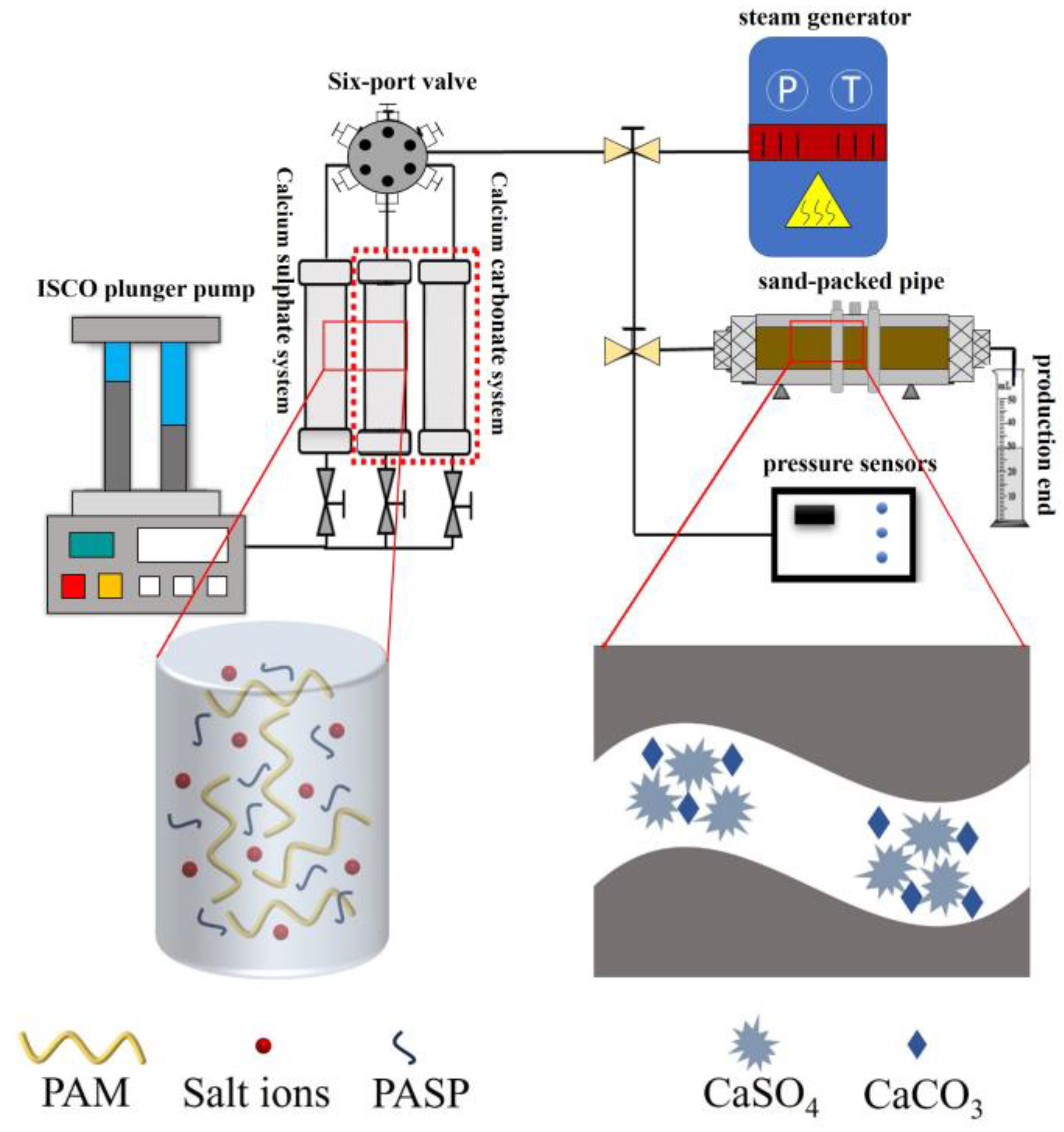
2.5. Single-Core Displacement Experiment
3. Results
3.1. Screening the Calcium Salt Blocking Agent Systems
3.1.1. Effect of Temperature on the Morphology of Calcium Salt Blocking Agent Systems
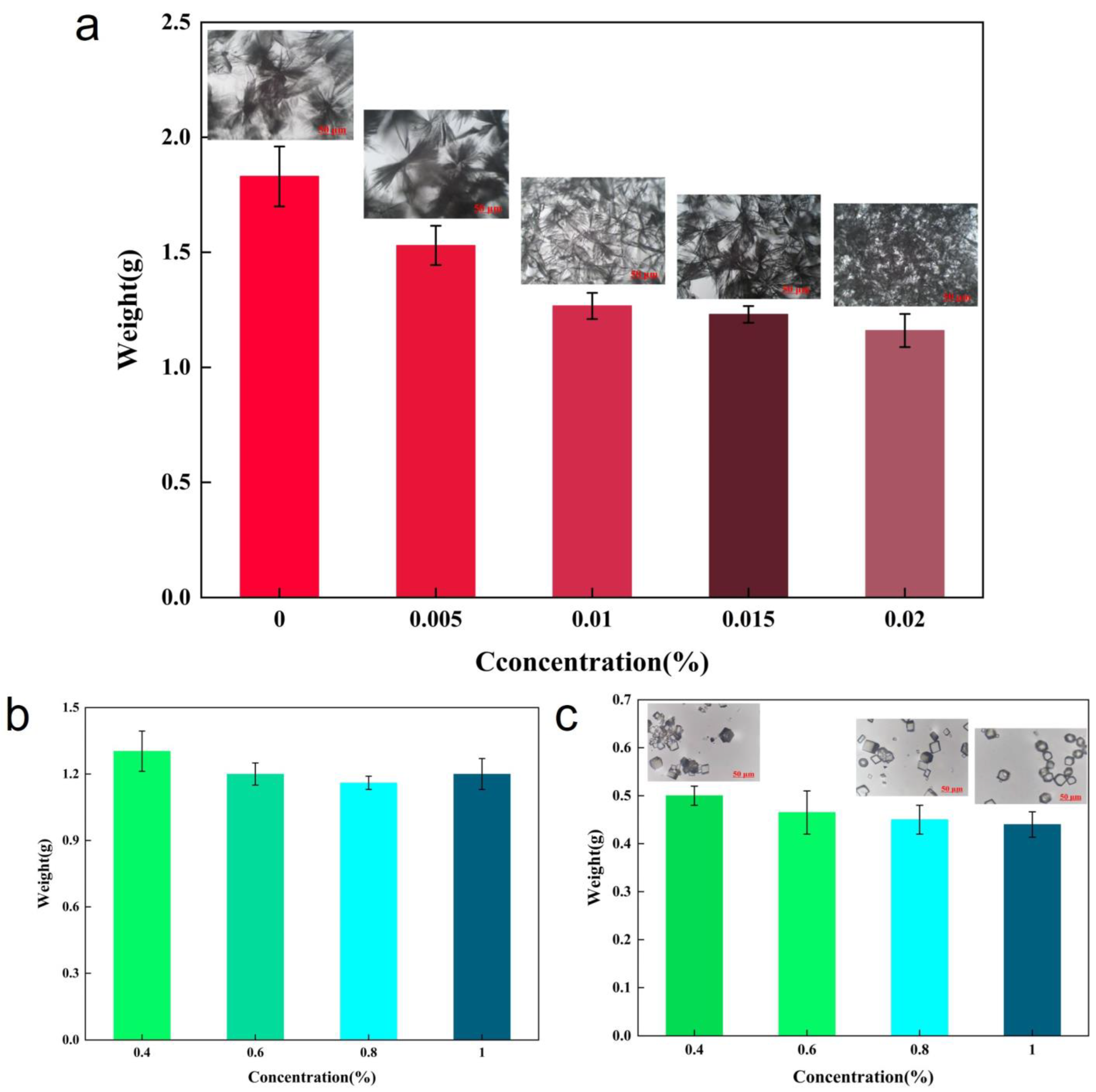
3.1.2. Selection of Suitable Concentrations of PAM and PASP
3.2. Studies of the Thermal Stability of Calcium Salt Systems
3.2.1. Effect of Temperature on the Precipitation Rate of Calcium Salts
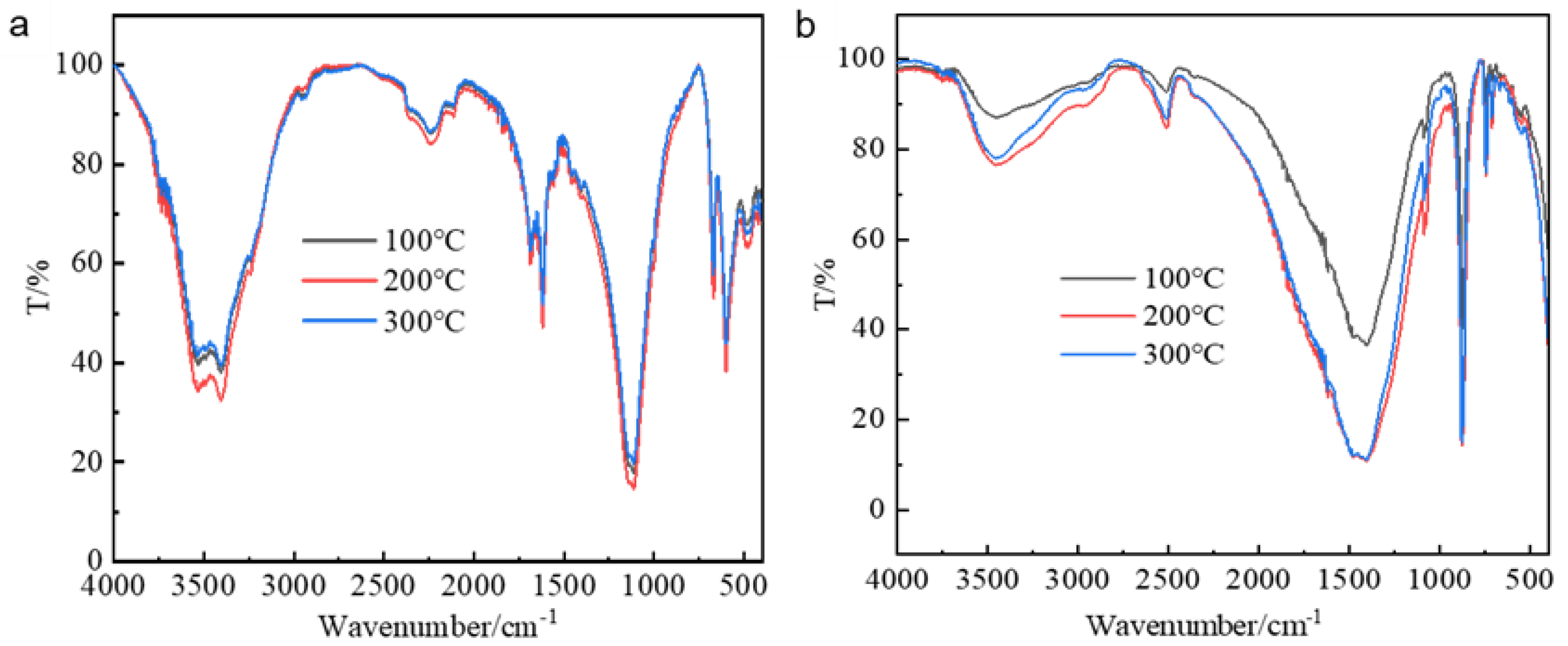
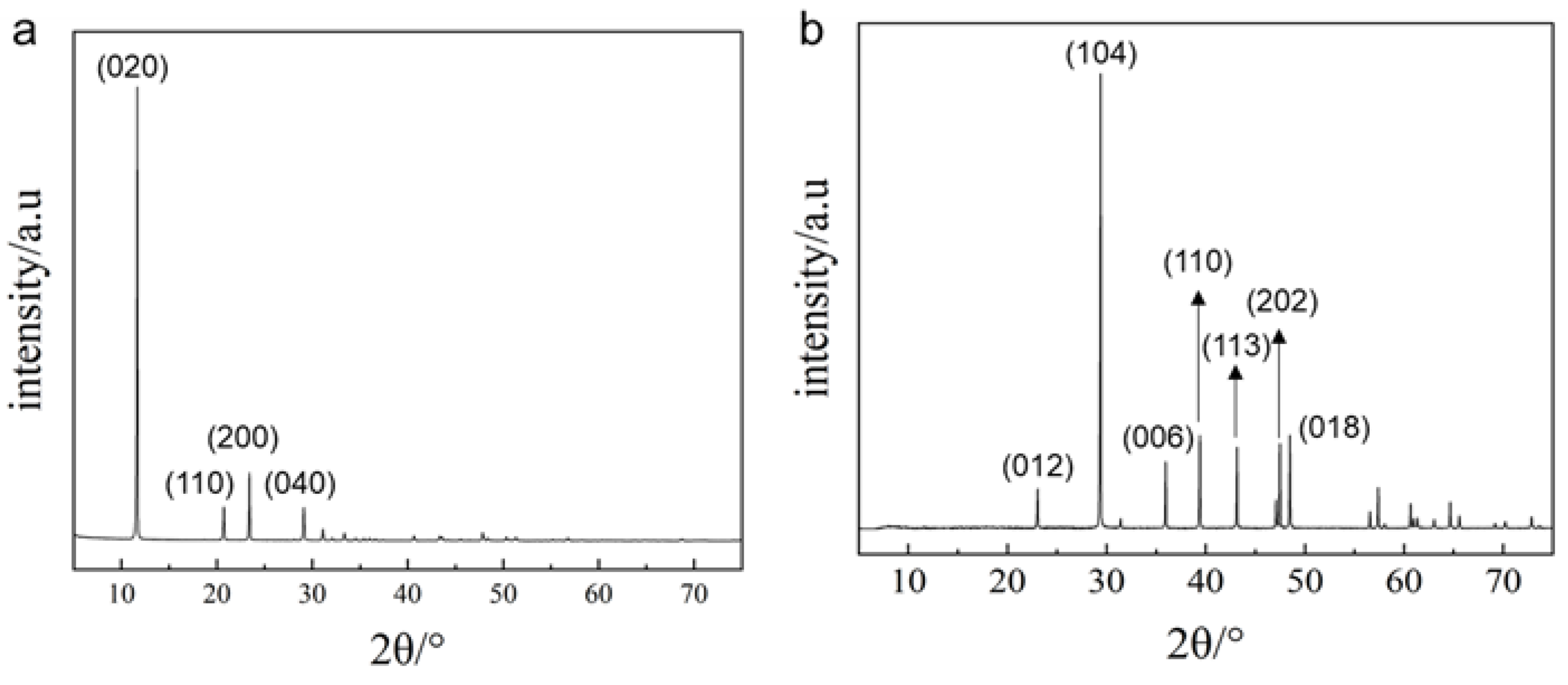
3.2.2. Effect of Temperature on the Crystal Structure of Calcium Salts
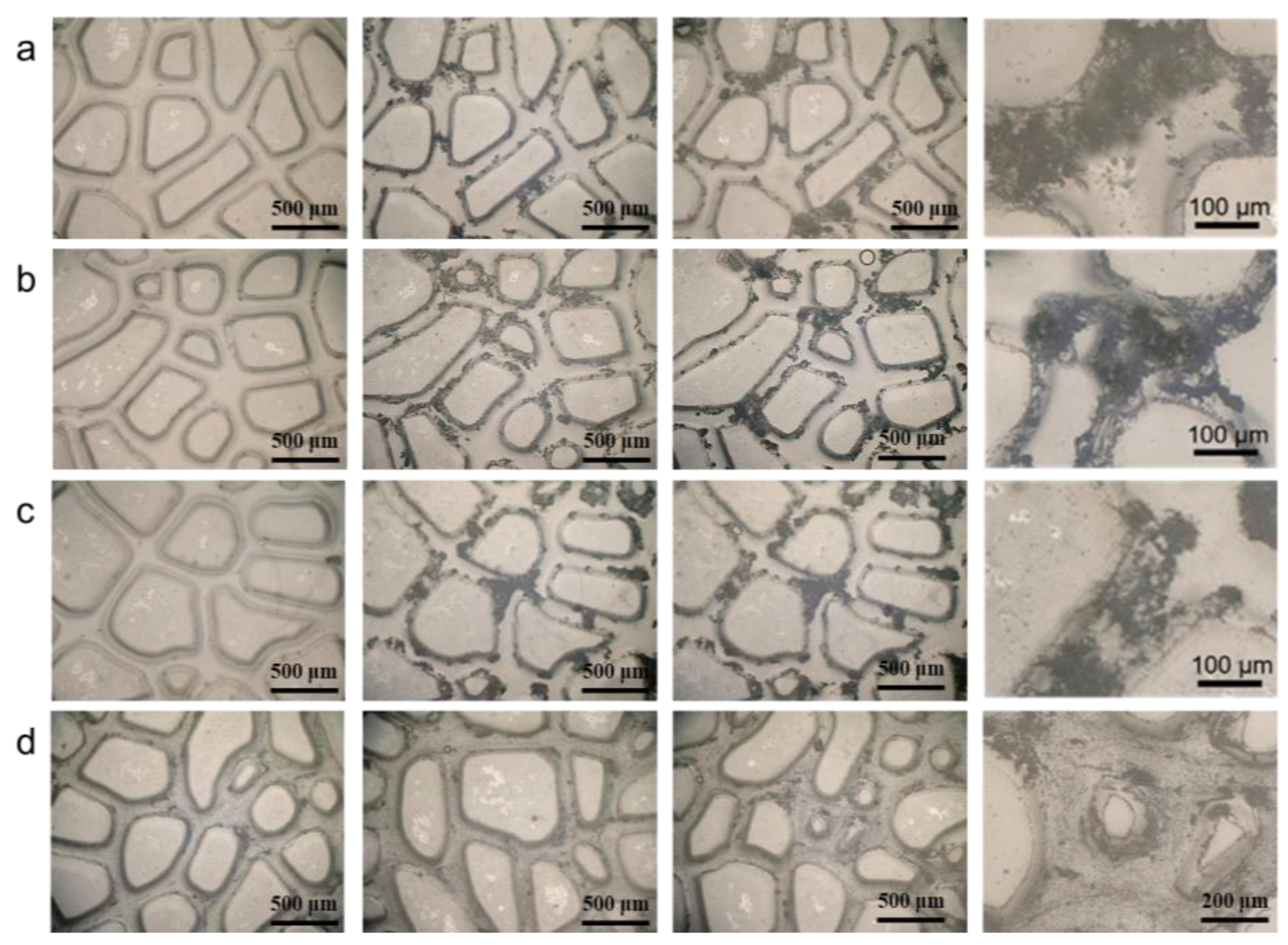
3.3. Analysis of Calcium Salt Crystal Growth in Core Pore Throat
3.4. Analysis of the Plugging Performance of Calcium Salt Blocking Agent Systems
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomaszewski, R. A comparative study of citations to chemical encyclopedias in scholarly articles: Kirk-Othmer Encyclopedia of Chemical Technology and Ullmann’s Encyclopedia of Industrial Chemistry. Scientometrics 2018, 117, 175–189. [Google Scholar] [CrossRef]
- Dou, L.R.; Li, D.W.; Wen, Z.X.; Wang, Z.M.; Mi, S.Y.; Zhang, Q. History and outlook of global oil and gas resources evaluation. Acta Pet. Sin. 2022, 43, 1035–1048. [Google Scholar]
- Jia, C.Z.; Pang, X.Q.; Song, Y. The mechanism of unconventional hydrocarbon formation: Hydrocarbon self-containment and intermolecular forces. Pet. Explor. Dev. 2021, 48, 437–452. [Google Scholar] [CrossRef]
- Xue, L.; Liu, P.C.; Zhang, Y. Development and research status of heavy oil enhanced oil recovery. Geofluids 2022, 1, 5015045. [Google Scholar] [CrossRef]
- Xiong, R.Y.; Guo, J.X.; Kiyingi, W.; Gao, C.H.; Wang, L.; Luo, J.J.; Song, H.X.; Wang, X.W. Technical transformation of heavy/ultra-heavy oil production in China driven by low carbon goals: A review. J. Clean. Prod. 2024, 458, 142531. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.L.; Yu, Z.X. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Malozyomov, B.V.; Martyushev, N.V.; Kukartsev, V.V.; Tynchenko, V.S.; Bukhtoyarov, V.V.; Wu, X.G.; Tyncheko, Y.A.; Kukartsev, V.A. Overview of Methods for Enhanced Oil Recovery from Conventional and Unconventional Reservoirs. Energies 2023, 16, 4907. [Google Scholar] [CrossRef]
- Pan, Y.; Qiao, W.; Chi, D.X.; Li, Z.X.; Shu, Y.J. Research of steam injection in-situ production technology to enhance unconventional oil and gas recovery: A review. J. Anal. Appl. Pyrolysis 2024, 177, 106332. [Google Scholar] [CrossRef]
- Qiao, J.; Zeng, J.; Jiang, S.; Feng, S.; Feng, X.; Guo, Z.; Teng, J. Heterogeneity of reservoir quality and gas accumulation in tight sandstone reservoirs revealed by pore structure characterization and physical simulation. Fuel 2019, 253, 1300–1316. [Google Scholar] [CrossRef]
- Dianshi, X.; Shuangfang, L.; Jinxiu, Y.; Luchuan, Z.; Bo, L. Classifying Multiscale Pores and Investigating Their Relationship with Porosity and Permeability in Tight Sandstone Gas Reservoirs. Energy Fuels 2017, 31, 9188–9200. [Google Scholar]
- Yuan, B.; Wood, D.A. A comprehensive review of formation damage during enhanced oil recovery. J. Pet. Sci. Eng. 2018, 167, 287–299. [Google Scholar] [CrossRef]
- Zhao, P.C.; Zhao, H.T.; Bai, B.J.; Yang, X.M. Improve injection profile by combining plugging agent treatment and acid stimulation. SPE Improv. Oil Recovery Conf. 2004, SPE-89394-MS. [Google Scholar]
- Gomaa, S.; Salem, K.G.; El-hoshoudy, A.N. Enhanced heavy and extra heavy oil recovery: Current status and new trends. Petroleum 2024, 3, 399–410. [Google Scholar] [CrossRef]
- Alvarez, J.; Han, S. Current overview of cyclic steam injection process. J. Pet. Sci. Res. 2023, 2, 116–127. [Google Scholar]
- Zhao, G.; Dai, C.L.; Gu, C.L.; You, Q.; Sun, Y.P. Expandable graphite particles as a novel in-depth steam channeling control agent in heavy oil reservoirs. Chem. Eng. J. 2019, 368, 668–677. [Google Scholar] [CrossRef]
- Li, B.L.; Li, B.F.; Zang, Y.N.; Zhu, D.; Li, Z.M.; Ruan, L.W. Experimental study of the distillation mechanism during co-injection of flue gas and steam for heavy oil development. Sep. Purif. Technol. 2023, 324, 124553. [Google Scholar] [CrossRef]
- Liu, P.; Shi, L.; Liu, P.; Li, L.; Hua, D. Experimental study of high-temperature CO2 foam flooding after hot-water injection in developing heavy oil reservoirs. J. Pet. Sci. Eng. 2020, 185, 106597. [Google Scholar] [CrossRef]
- Zhu, D.; Li, B.F.; Chen, L.K.; Zhang, C.B.; Zheng, L.; Chen, W.Q.; Li, Z.M. Experimental investigation of CO2 foam flooding-enhanced oil recovery in fractured low-permeability reservoirs: Core-scale to pore-scale. Fuel 2024, 362, 130792. [Google Scholar] [CrossRef]
- Li, S.P.; Yao, Z.Y.; Shang, F.H.; Li, M.H.; Wei, Y.H.; Li, S.Y. Improving CO2 storage efficiency in saline aquifers through wettability-optimized nanoparticle foam. Phys. Fluids 2025, 37, 013351. [Google Scholar] [CrossRef]
- Islam, M.; Ali, S.F. Use of silica gel for improving waterflooding performance of bottom-water reservoirs. J. Pet. Sci. Eng. 1993, 8, 303–313. [Google Scholar] [CrossRef]
- Xu, L. Chemical water shutoff profile research status and development trends. IOP Conf. Ser. Earth Environ. Sci. 2017, 82, 012061. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Wang, C.; Li, S.; Yuan, X.; Liu, Y.; Meng, X.; Zou, J.; Wang, Q. Investigation of a high temperature gel system for application in saline oil and gas reservoirs for profile modification. J. Pet. Sci. Eng. 2020, 195, 107852. [Google Scholar] [CrossRef]
- Wang, B.; Lin, M.; Guo, J.; Wang, D.; Xu, F.; Li, M. Plugging properties and profile control effects of crosslinked polyacrylamide microspheres. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Xie, K.; Su, C.; Liu, C.L.; Cao, W.J.; He, X.; Ding, H.N.; Mei, J.; Yan, K.; Cheng, Q.; Lu, X.G. Synthesis and Performance Evaluation of an Organic/Inorganic Composite Gel Plugging System for Offshore Oilfields. Acs Omega 2022, 7, 12870–12878. [Google Scholar] [CrossRef] [PubMed]
- Jaripatke, O.; Dalrymple, D. Water-control management technologies: A review of successful chemical technologies in the last two decades. SPE Int. Conf. Exhib. Form. Damage Control 2010, SPE-127806-MS. [Google Scholar] [CrossRef]
- Caili, D.; Qing, Y.; Fulin, Z. In-depth profile control technologies in China—A review of the state of the art. Pet. Sci. Technol. 2010, 28, 1307–1315. [Google Scholar] [CrossRef]
- Zhai, K.; Yi, H.; Liu, Y.; Geng, Y.; Fan, S.; Zhu, D. Experimental evaluation of the shielded temporary plugging system composed of calcium carbonate and acid-soluble preformed particle gels (ASPPG) for petroleum drilling. Energy Fuels 2020, 34, 14023–14033. [Google Scholar] [CrossRef]
- Cui, G.; Ren, S.; Dou, B.; Ning, F. Geothermal energy exploitation from depleted high-temperature gas reservoirs by recycling CO2: The superiority and existing problems. Geosci. Front. 2021, 12, 101078. [Google Scholar] [CrossRef]
- Barbosa, M.V.; Teixeira, L.A.C.; Martins, A.R.F.D.; Santos, B.F.D. Process Optimization for the Production of Ferric Sulfate Coagulant by the Oxidation of Ferrous sulfate with Hydrogen Peroxide. Chem. Prod. Process Model. 2020, 15, 20190091. [Google Scholar] [CrossRef]
- Erofeev, V.I.; Xiangguo, L. Optimization of parameters of inorganic profile control agent on the basis of sodium silicate for effective oil displacement from high salinity reservoir. Тoмскoгo Пoлитехническoгo Университета Инжиниринг Геoресурсoв 2019, 54, 67. [Google Scholar]
- Acock, A.M.; Reis, J.C. Oil recovery improvement through profile modification by thermal precipitation. SPE Improv. Oil Recovery Conf. 1994, SPE-27831-MS. [Google Scholar]
- Zhu, T.; Tiab, D. Improved sweep efficiency by selective plugging of highly watered out zones by alcohol induced precipitation. J. Can. Pet. Technol. 1993, 32, PETSOC-93-09-05. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.J.; Hormozi, F.; Mokhtari, R. Controlling the gelation time of sodium silicate gelants for fluid management in hydrocarbon reservoirs. Fuel 2023, 341, 127645. [Google Scholar] [CrossRef]
- Trukhina, M.; Tkachenko, S.; Ryabova, A.; Oshchepkov, M.; Redchuk, A.; Popov, K. Calcium sulfate crystallization in presence of fluorecent-tagged polyacrylate and some refinement of scale inhibition mechanism. Minerals 2023, 13, 559. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, B.F.; Shi, Z.; Shao, G.L.; Li, B.L.; Kang, N.; Wang, X.Q. Preparation and plugging characteristics investigation of a high temperature induced calcium salt precipitation system for profile control in high temperature reservoirs. Colloids Surf. A: Physicochem. Eng. Asp. 2025, 711, 136413. [Google Scholar] [CrossRef]
- Zhu, G.; Li, H.; Li, S.; Hou, X.; Xu, D.; Lin, R.; Tang, Q. Crystallization behavior and kinetics of calcium carbonate in highly alkaline and supersaturated system. J. Cryst. Growth 2015, 428, 16–23. [Google Scholar] [CrossRef]
- Liu, Y.; Xian, W.; He, J.; Li, Y. Interplay between entanglement and crosslinking in determining mechanical behaviors of polymer networks. Int. J. Smart Nano Mater. 2023, 14, 474–495. [Google Scholar] [CrossRef]
- Adelnia, H.; Sirous, F.; Blakey, I.; Ta, H.T. Metal ion chelation of poly (aspartic acid): From scale inhibition to therapeutic potentials. Int. J. Biol. Macromol. 2023, 229, 974–993. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, B.; Chen, J.; Yan, G. Research on plugging characteristics of microorganism induced calcite precipitation in sandstone environment. J. Pet. Sci. Eng. 2022, 218, 111040. [Google Scholar] [CrossRef]
- Morris, R. Infrared spectrophotometric analysis of calcium sulfate hydrates using internally standardized mineral oil mulls. Anal. Chem. 1963, 35, 1489–1492. [Google Scholar] [CrossRef]
- Vagenas, N.V.; Gatsouli, A.; Kontoyannis, C.G. Quantitative analysis of synthetic calcium carbonate polymorphs using FT-IR spectroscopy. Talanta 2003, 59, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Garside, J.; Mersmann, A.; Nývlt, J. Measurement of Crystal Growth and Nucleation Rates; IChemE: Birmingham, UK, 2002. [Google Scholar]
- Moghadasi, J.; Jamialahmadi, M.; Müller-Steinhagen, H.; Sharif, A. Scale formation in oil reservoir and production equipment during water injection kinetics of CaSO4 and CaCO3 crystal growth and effect on formation damage. SPE Eur. Form. Damage Conf. Exhib. 2003, SPE-82233-MS. [Google Scholar]
- Tang, X.F.; Shao, L.M.; Tan, H.; Liu, S.J.; Niu, L.W.; Ye, Y.Z. Improvement of Water Flooding Efficiency by Using the Reservoir Forming Ion Source to Realize the Adjustment of the Dominant Channel. Oilfield Chem. 2018, 35, 257–262. [Google Scholar]
- Niu, L.W.; Lu, X.G.; Xiong, C.M.; Tang, X.F.; Wu, X.C.; Jia, X.; Zhang, S. Experimental study on gelling property and plugging effect of inorganic gel system (OMGL). Pet. Explor. Dev. 2013, 40, 728–732. [Google Scholar] [CrossRef]



| Injection Agent Ratio/PV | Resistance Factor | Maximum Residual Resistance Factor | Stable Residual Resistance Factor | Plugging Rate/% |
|---|---|---|---|---|
| 0.3 + 0.7 | 48.00 | 45.95 | 40.00 | 97.50 |
| 0.4 + 0.6 | 55.13 | 278.00 | 244.50 | 99.59 |
| 0.5 + 0.5 | 52.10 | 256.92 | 212.82 | 99.53 |
| 0.6 + 0.4 | 51.79 | 160.53 | 101.05 | 99.01 |
| 0.7 + 0.3 | 49.72 | 79.46 | 71.35 | 98.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, G.; Shi, Z.; Jia, Y.; Cheng, Q.; Kang, N.; Wang, X. Calcium Precipitates as Novel Agents for Controlling Steam Channeling in Steam Injection Processes for Heavy Oil Recovery. Processes 2025, 13, 1319. https://doi.org/10.3390/pr13051319
Shao G, Shi Z, Jia Y, Cheng Q, Kang N, Wang X. Calcium Precipitates as Novel Agents for Controlling Steam Channeling in Steam Injection Processes for Heavy Oil Recovery. Processes. 2025; 13(5):1319. https://doi.org/10.3390/pr13051319
Chicago/Turabian StyleShao, Guolin, Zhuang Shi, Yunfei Jia, Qian Cheng, Ning Kang, and Xiaoqiang Wang. 2025. "Calcium Precipitates as Novel Agents for Controlling Steam Channeling in Steam Injection Processes for Heavy Oil Recovery" Processes 13, no. 5: 1319. https://doi.org/10.3390/pr13051319
APA StyleShao, G., Shi, Z., Jia, Y., Cheng, Q., Kang, N., & Wang, X. (2025). Calcium Precipitates as Novel Agents for Controlling Steam Channeling in Steam Injection Processes for Heavy Oil Recovery. Processes, 13(5), 1319. https://doi.org/10.3390/pr13051319







