Spent Lithium Battery Recycling: Traditional and Innovative Approaches
Abstract
1. Introduction
2. Lithium-Based Batteries
| Cathode: | LiMO2 | Li1−nMO2 + nLi+ + ne− | (1) | |
| Anode: | C + nLi+ + ne− | LinC | ||
| Overall: | LiMO2 + C | LinC + Li1−nMO2 |
3. Battery Recycling Methods
3.1. Traditional Approaches
3.1.1. Pyrometallurgy
3.1.2. Hydrometallurgy
3.2. Innovative Approaches
3.2.1. Bioleaching
3.2.2. Mechanochemistry
3.2.3. Direct Recycling
4. Metal Recovery Methods
5. Scaling up of Recycling Methods
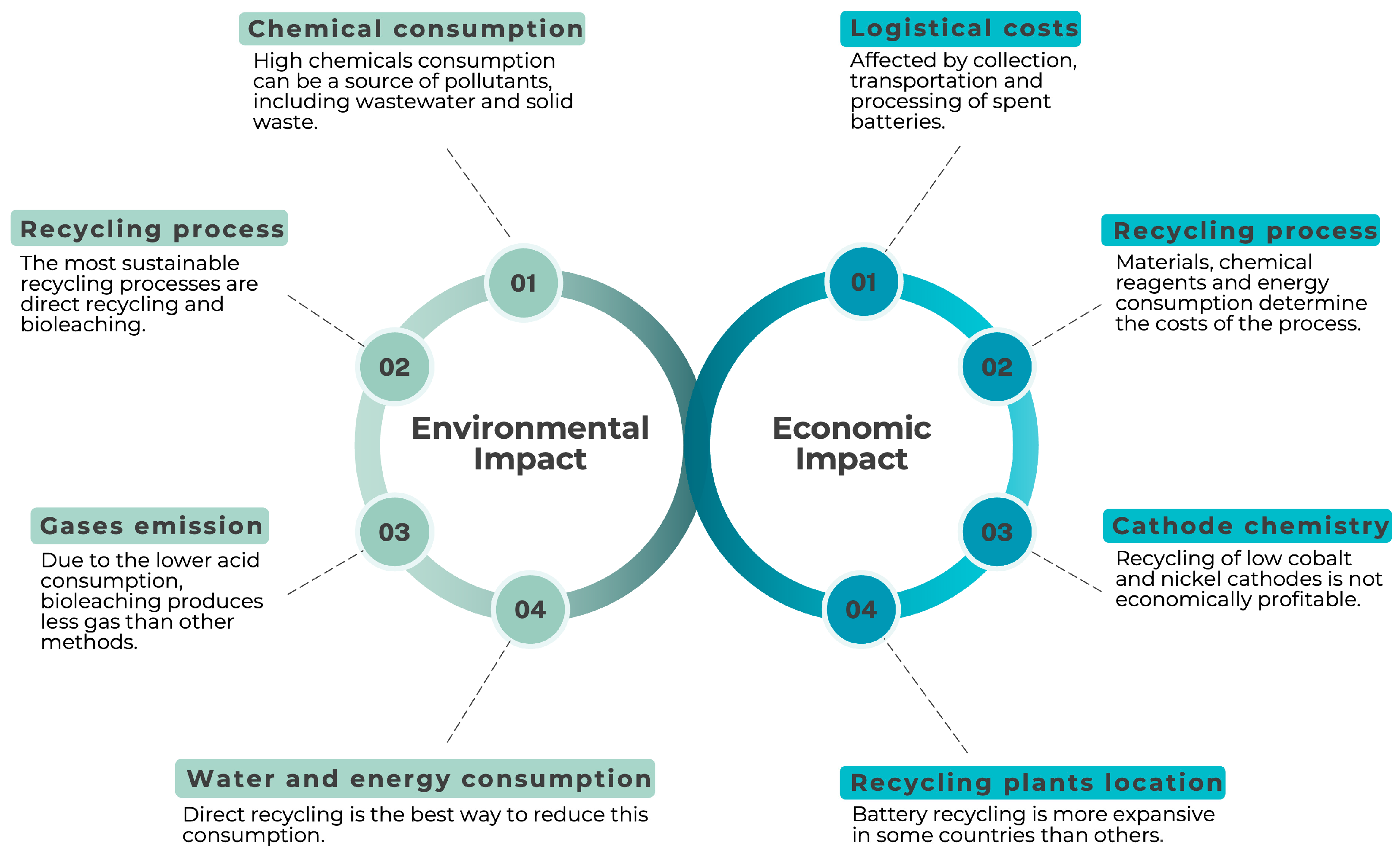
6. Economic and Environmental Issues of Recycling Methods
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jena, K.K.; AlFantazi, A.; Mayyas, A.T. Comprehensive Review on Concept and Recycling Evolution of Lithium-Ion Batteries (LIBs). Energy Fuels 2021, 35, 18257–18284. [Google Scholar] [CrossRef]
- Arshad, F.; Li, L.; Amin, K.; Fan, E.; Manurkar, N.; Ahmad, A.; Yang, J.; Wu, F.; Chen, R. A Comprehensive Review of the Advancement in Recycling the Anode and Electrolyte from Spent Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 13527–13554. [Google Scholar] [CrossRef]
- Sarma, D.D.; Shukla, A.K. Building Better Batteries: A Travel Back in Time. ACS Energy Lett. 2018, 3, 2841–2845. [Google Scholar] [CrossRef]
- Bagotsky, V.S. Fundamentals of Electrochemistry, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 349–350. [Google Scholar]
- Palacín, M.R. Recent Advances in Rechargeable Battery Materials: A Chemist’s Perspective. Chem. Soc. Rev. 2009, 38, 2565. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. 18–19. [Google Scholar]
- Zhang, J.; Chen, M.; Wu, S.; Chen, D.; Zhao, Y.; Zhou, X. Characteristics of Waste Dry Battery Powder and Its Enhancement Effect on the Physicochemical Properties of Asphalt Binder. J. Clean. Prod. 2023, 426, 139090. [Google Scholar] [CrossRef]
- Le, P.-A.; Nguyen, N.T.; Nguyen, P.L.; Phung, T.V.B. Minireview on Cathodic and Anodic Exfoliation for Recycling Spent Zinc–Carbon Batteries To Prepare Graphene Material: Advances and Outlook of Interesting Strategies. Energy Fuels 2023, 37, 7062–7070. [Google Scholar] [CrossRef]
- Lenhart, B.; Kathan, D.; Hull, M.; Omasta, T.; Gibbons, D.; Zuraw, M.; Mustain, W. Engineering Zinc Slurry Anodes for High-Performance Primary Alkaline Batteries. J. Power Sources 2024, 612, 234818. [Google Scholar] [CrossRef]
- Sabbaghi, M.; Behdad, S. Estimating Energy Left in Discarded Alkaline Batteries: Evaluating Consumption and Recovery Opportunities. Waste Manag. 2024, 189, 58–67. [Google Scholar] [CrossRef]
- Beck, F.; Rüetschi, P. Rechargeable Batteries with Aqueous Electrolytes. Electrochim. Acta 2000, 45, 2467–2482. [Google Scholar] [CrossRef]
- Yang, J.; Hu, C.; Wang, H.; Yang, K.; Liu, J.B.; Yan, H. Review on the Research of Failure Modes and Mechanism for Lead-Acid Batteries: Review on the Research of Failure Modes for Lead-Acid Batteries. Int. J. Energy Res. 2017, 41, 336–352. [Google Scholar] [CrossRef]
- Jiang, S.; Song, Z. A Review on the State of Health Estimation Methods of Lead-Acid Batteries. J. Power Sources 2022, 517, 230710. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, T.; Ali, M.; Meng, Y.; Jin, Y.; Cui, Y.; Chen, W. Rechargeable Batteries for Grid Scale Energy Storage. Chem. Rev. 2022, 122, 16610–16751. [Google Scholar] [CrossRef]
- Shukla, A.; Venugopalan, S.; Hariprakash, B. Nickel-Based Rechargeable Batteries. J. Power Sources 2001, 100, 125–148. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Wang, L.; Zhang, M.; Wang, R.; Zhan, C. A Critical Review on Nickel-Based Cathodes in Rechargeable Batteries. Int. J. Miner. Metall. Mater. 2022, 29, 925–941. [Google Scholar] [CrossRef]
- Scrosati, B. History of Lithium Batteries. J. Solid State Electrochem. 2011, 15, 1623–1630. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chem, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Zanoletti, A.; Carena, E.; Ferrara, C.; Bontempi, E. A Review of Lithium-Ion Battery Recycling: Technologies, Sustainability, and Open Issues. Batteries 2024, 10, 38. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of Lithium-Ion Batteries: Recent Advances and Perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Pražanová, A.; Knap, V.; Stroe, D.-I. Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part I: Recycling Technology. Energies 2022, 15, 1086. [Google Scholar] [CrossRef]
- Duan, X.; Zhu, W.; Ruan, Z.; Xie, M.; Chen, J.; Ren, X. Recycling of Lithium Batteries—A Review. Energies 2022, 15, 1611. [Google Scholar] [CrossRef]
- Wang, Y.; An, N.; Wen, L.; Wang, L.; Jiang, X.; Hou, F.; Yin, Y.; Liang, J. Recent Progress on the Recycling Technology of Li-Ion Batteries. J. Energy Chem. 2021, 55, 391–419. [Google Scholar] [CrossRef]
- Saju, D.; Ebenezer, J.; Chandran, N.; Chandrasekaran, N. Recycling of Lithium Iron Phosphate Cathode Materials from Spent Lithium-Ion Batteries: A Mini-Review. Ind. Eng. Chem. Res. 2023, 62, 11768–11783. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Costa, C.M.; Barbosa, J.C.; Gonçalves, R.; Castro, H.; Campo, F.J.D.; Lanceros-Méndez, S. Recycling and Environmental Issues of Lithium-Ion Batteries: Advances, Challenges and Opportunities. Energy Storage Mater. 2021, 37, 433–465. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, L.; Zhang, Y.; Tan, Q.; Li, J. An Overview of Global Power Lithium-Ion Batteries and Associated Critical Metal Recycling. J. Hazard. Mater. 2022, 425, 127900. [Google Scholar] [CrossRef]
- Speirs, J.; Contestabile, M.; Houari, Y.; Gross, R. The Future of Lithium Availability for Electric Vehicle Batteries. Renew. Sust. Energ. Rev. 2014, 35, 183–193. [Google Scholar] [CrossRef]
- Zanoletti, A.; Bresolin, B.M.; Bontempi, E. Building a Circular Economy for Lithium: Addressing Global Challenges. Glob. Chall. 2024, 8, 2400250. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling—Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of Lithium from Primary and Secondary Sources by Pre-Treatment, Leaching and Separation: A Comprehensive Review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Rentier, E.S.; Hoorn, C.; Seijmonsbergen, A.C. Lithium Brine Mining Affects Geodiversity and Sustainable Development Goals. Renew. Sust. Energ. Rev. 2024, 202, 114642. [Google Scholar] [CrossRef]
- Tan, J.; Keiding, J.K. Mapping the Cobalt and Lithium Supply Chains for E-Mobility Transition: Significance of Overseas Investments and Vertical Integration in Evaluating Mineral Supply Risks. Resour. Conserv. Recycl. 2024, 209, 107788. [Google Scholar] [CrossRef]
- Alera, A.C.; Benitez, J.P.; Fernandez, R.J.; Pascual, C.K.; Policarpio, F.; Lopez, E.C.R. Recent Advances in Lithium Extraction. Eng. Proc. 2024, 67, 52. [Google Scholar] [CrossRef]
- Lee, S.; Manthiram, A. Can Cobalt Be Eliminated from Lithium-Ion Batteries? ACS Energy Lett. 2022, 7, 3058–3063. [Google Scholar] [CrossRef]
- Fu, X.; Beatty, D.N.; Gaustad, G.G.; Ceder, G.; Roth, R.; Kirchain, R.E.; Bustamante, M.; Babbitt, C.; Olivetti, E.A. Perspectives on Cobalt Supply through 2030 in the Face of Changing Demand. Environ. Sci. Technol. 2020, 54, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Luo, S.; Zhang, L.; Liu, Q.; Wang, Y.; Lin, Y.; Xu, C.; Guo, J.; Cheali, P.; Xia, X. Progress, Challenges, and Prospects of Spent Lithium-Ion Batteries Recycling: A Review. J. Energy Chem. 2024, 89, 144–171. [Google Scholar] [CrossRef]
- Boyden, A.; Soo, V.K.; Doolan, M. The Environmental Impacts of Recycling Portable Lithium-Ion Batteries. Procedia CIRP 2016, 48, 188–193. [Google Scholar] [CrossRef]
- Piątek, J.; Afyon, S.; Budnyak, T.M.; Budnyk, S.; Sipponen, M.H. Sustainable Li-Ion Batteries: Chemistry and Recycling. Adv. Energy Mater. 2021, 11, 2003456. [Google Scholar] [CrossRef]
- Toro, L.; Moscardini, E.; Baldassari, L.; Forte, F.; Falcone, I.; Coletta, J.; Toro, L. A Systematic Review of Battery Recycling Technologies: Advances, Challenges, and Future Prospects. Energies 2023, 16, 6571. [Google Scholar] [CrossRef]
- Treptow, R.S. Lithium Batteries: A Practical Application of Chemical Principles. J. Chem. Educ. 2003, 80, 1015. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Li, Q.; Cartmell, S.; Ferrara, S.; Deng, Z.D.; Xiao, J. Lithium and Lithium Ion Batteries for Applications in Microelectronic Devices: A Review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef]
- Osiak, M.; Geaney, H.; Armstrong, E.; O’Dwyer, C. Structuring Materials for Lithium-Ion Batteries: Advancements in Nanomaterial Structure, Composition, and Defined Assembly on Cell Performance. J. Mater. Chem. A 2014, 2, 9433. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T.B. Lithium Battery. In Handbook of Batteries, 3rd ed.; Linden, D., Reddy, T.B., Eds.; McGraw-Hill: New York, NY, USA, 2002; pp. 14.1–14.85. [Google Scholar]
- Bae, H.; Kim, Y. Technologies of Lithium Recycling from Waste Lithium Ion Batteries: A Review. Mater. Adv. 2021, 2, 3234–3250. [Google Scholar] [CrossRef]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Pérez, J.M.; Colledani, M. Lithium-Ion Batteries towards Circular Economy: A Literature Review of Opportunities and Issues of Recycling Treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The Lithium-Ion Battery: State of the Art and Future Perspectives. Renew. Sust. Energ. Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Hossain, M.H.; Chowdhury, M.A.; Hossain, N.; Islam, M.A.; Mobarak, M.H. Advances of Lithium-Ion Batteries Anode Materials—A Review. Chem. Eng. J. Adv. 2023, 16, 100569. [Google Scholar] [CrossRef]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E. A Review of Current Collectors for Lithium-Ion Batteries. J. Power Sources 2021, 485, 229321. [Google Scholar] [CrossRef]
- Ehrlich, G.M. Lithium-ion Batteries. In Handbook of Batteries, 3rd ed.; Linden, D., Reddy, T.B., Eds.; McGraw-Hill: New York, NY, USA, 2002; pp. 35.4–35.37. [Google Scholar]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-Ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Wei, Q.; Wu, Y.; Li, S.; Chen, R.; Ding, J.; Zhang, C. Spent Lithium Ion Battery (LIB) Recycle from Electric Vehicles: A Mini-Review. Sci. Total Environ. 2023, 866, 161380. [Google Scholar] [CrossRef]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries —Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Gaines, L.; Zhang, J.; He, X.; Bouchard, J.; Melin, H.E. Tracking Flows of End-of-Life Battery Materials and Manufacturing Scrap. Batteries 2023, 9, 360. [Google Scholar] [CrossRef]
- Heelan, J.; Gratz, E.; Zheng, Z.; Wang, Q.; Chen, M.; Apelian, D.; Wang, Y. Current and Prospective Li-Ion Battery Recycling and Recovery Processes. JOM 2016, 68, 2632–2638. [Google Scholar] [CrossRef]
- Azimi, G.; Chan, K.H. A Review of Contemporary and Emerging Recycling Methods for Lithium-Ion Batteries with a Focus on NMC Cathodes. Resour. Conserv. Recycl. 2024, 209, 107825. [Google Scholar] [CrossRef]
- Davis, K.; Demopoulos, G.P. Hydrometallurgical Recycling Technologies for NMC Li-Ion Battery Cathodes: Current Industrial Practice and New R&D Trends. RSC Sustain. 2023, 1, 1932–1951. [Google Scholar] [CrossRef]
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical Recycling of Li-Ion, Ni–Cd and Ni–MH Batteries: A Minireview. Curr. Opin. Green Sustain. Chem. 2020, 24, 26–31. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Aidan, A.; Allawi, T.; Hammoud, F.; Al Ali, H.; Al Khamiri, M. Treatment and Recycling of Spent Lithium-Based Batteries: A Review. J. Mater. Cycles Waste Manag. 2024, 26, 76–95. [Google Scholar] [CrossRef]
- Mondal, A.; Fu, Y.; Gao, W.; Mi, C.C. Pretreatment of Lithium Ion Batteries for Safe Recycling with High-Temperature Discharging Approach. Batteries 2024, 10, 37. [Google Scholar] [CrossRef]
- Rautela, R.; Yadav, B.R.; Kumar, S. A Review on Technologies for Recovery of Metals from Waste Lithium-Ion Batteries. J. Power Sources 2023, 580, 233428. [Google Scholar] [CrossRef]
- Torabian, M.M.; Jafari, M.; Bazargan, A. Discharge of Lithium-Ion Batteries in Salt Solutions for Safer Storage, Transport, and Resource Recovery. Waste Manag. Res. 2022, 40, 402–409. [Google Scholar] [CrossRef]
- Ojanen, S.; Lundström, M.; Santasalo-Aarnio, A.; Serna-Guerrero, R. Challenging the Concept of Electrochemical Discharge Using Salt Solutions for Lithium-Ion Batteries Recycling. Waste Manag. Res 2018, 76, 242–249. [Google Scholar] [CrossRef]
- Zhou, M.; Li, B.; Li, J.; Xu, Z. Pyrometallurgical Technology in the Recycling of a Spent Lithium Ion Battery: Evolution and the Challenge. ACS EST Eng. 2021, 1, 1369–1382. [Google Scholar] [CrossRef]
- Tembo, P.M.; Dyer, C.; Subramanian, V. Lithium-Ion Battery Recycling—A Review of the Material Supply and Policy Infrastructure. NPG Asia Mater. 2024, 16, 43. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical Options for Recycling Spent Lithium-Ion Batteries: A Comprehensive Review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Liu, P.; Mi, X.; Zhao, H.; Cai, L.; Luo, F.; Liu, C.; Wang, Z.; Deng, C.; He, J.; Zeng, G.; et al. Effects of Incineration and Pyrolysis on Removal of Organics and Liberation of Cathode Active Materials Derived from Spent Ternary Lithium-Ion Batteries. Waste Manag. 2023, 169, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.; Ebin, B.; Steenari, B.-M.; Alemrajabi, M.; Karlsson, I.; Petranikova, M. Comparison of the Effects of Incineration, Vacuum Pyrolysis and Dynamic Pyrolysis on the Composition of NMC-Lithium Battery Cathode-Material Production Scraps and Separation of the Current Collector. Resour. Conserv. Recycl. 2021, 164, 105142. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Moorthy, T.; Friedrich, B. Degradation Mechanism of Nickel-Cobalt-Aluminum (NCA) Cathode Material from Spent Lithium-Ion Batteries in Microwave-Assisted Pyrolysis. Metals 2018, 8, 565. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, W.; Han, J.; Jiao, F.; Qin, W.; Liu, T.; Zhao, C. Pyrolysis and Physical Separation for the Recovery of Spent LiFePO4 Batteries. Waste Manag. 2019, 89, 83–93. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, M. Development of a Two-Stage Pyrolysis Process for the End-Of-Life Nickel Cobalt Manganese Lithium Battery Recycling from Electric Vehicles. Sustainability 2020, 12, 9164. [Google Scholar] [CrossRef]
- Cornelio, A.; Zanoletti, A.; Bontempi, E. Recent Progress in Pyrometallurgy for the Recovery of Spent Lithium-Ion Batteries: A Review of State-of-the-Art Developments. Curr. Opin. Green Sustain. Chem. 2024, 46, 100881. [Google Scholar] [CrossRef]
- Ahn, Y.; Koo, W.; Yoo, K.; Alorro, R.D. Carbothermic Reduction Roasting of Cathode Active Materials Using Activated Carbon and Graphite to Enhance the Sulfuric-Acid-Leaching Efficiency of Nickel and Cobalt. Minerals 2022, 12, 1021. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of Spent Lithium-Ion Batteries in View of Lithium Recovery: A Critical Review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Nuraeni, B.A.; Avarmaa, K.; Prentice, L.H.; Rankin, W.J.; Rhamdhani, M.A. Carbothermic Reduction of LiCoO2 Cathode Material: Thermodynamic Analysis, Microstructure and Mechanisms. Sustain. Mater. Technol. 2022, 34, e00526. [Google Scholar] [CrossRef]
- Yan, Z.; Sattar, A.; Li, Z. Priority Lithium Recovery from Spent Li-Ion Batteries via Carbothermal Reduction with Water Leaching. Resour. Conserv. Recycl. 2023, 192, 106937. [Google Scholar] [CrossRef]
- Pindar, S.; Dhawan, N. Rapid Recycling of Spent Lithium-Ion Batteries Using Microwave Route. Process Saf. Environ. Prot. 2021, 147, 226–233. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Zhang, L.; Guo, S. Microwave-Absorbing Properties of Cathode Material during Reduction Roasting for Spent Lithium-Ion Battery Recycling. J. Hazard. Mater. 2020, 384, 121487. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Yang, Y.; Qu, L.; Li, J.; Zhou, R. Microwave Reduction Enhanced Leaching of Valuable Metals from Spent Lithium-Ion Batteries. J. Alloys Compd. 2020, 832, 154920. [Google Scholar] [CrossRef]
- Cornelio, A.; Zanoletti, A.; Scaglia, M.; Galli, E.; La Corte, D.; Biava, G.; Bontempi, E. Thermal Approaches Based on Microwaves to Recover Lithium from Spent Lithium-Ion Batteries. RSC Sustain. 2024, 2, 2505–2514. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Lin, J.; Wu, J.; Yang, J.; Wu, F.; Chen, R. Low-Temperature Molten-Salt-Assisted Recovery of Valuable Metals from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 16144–16150. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, B.; Zhao, J.; Qiu, B.; Chen, X.; Zhou, F.; Li, X.; Gao, S.; Wang, D.; Yin, H. Salt-Thermal Methods for Recycling and Regenerating Spent Lithium-Ion Batteries: A Review. Green Chem. 2023, 25, 2992–3015. [Google Scholar] [CrossRef]
- Mao, J.; Ye, C.; Zhang, S.; Xie, F.; Zeng, R.; Davey, K.; Guo, Z.; Qiao, S. Toward Practical Lithium-Ion Battery Recycling: Adding Value, Tackling Circularity and Recycling-Oriented Design. Energy Environ. Sci. 2022, 15, 2732–2752. [Google Scholar] [CrossRef]
- Ren, G.; Xiao, S.; Xie, M.; Pan, B.; Chen, J.; Wang, F.; Xia, X. Recovery of Valuable Metals from Spent Lithium Ion Batteries by Smelting Reduction Process Based on FeO–SiO2–Al2O3 Slag System. Trans. Nonferrous Met. Soc. China 2017, 27, 450–456. [Google Scholar] [CrossRef]
- Ahmed, S.; Haleem, N.; Jamal, Y.; Khan, S.J.; Yang, X. Recovery of Lithium and Cobalt from Used Lithium-Ion Cell Phone Batteries through a Pyro-Hydrometallurgical Hybrid Extraction Process and Chemical Precipitation. J. Mater. Cycles Waste Manag. 2024, 27, 925–936. [Google Scholar] [CrossRef]
- Zhang, B.; Qu, X.; Chen, X.; Liu, D.; Zhao, Z.; Xie, H.; Wang, D.; Yin, H. A Sodium Salt-Assisted Roasting Approach Followed by Leaching for Recovering Spent LiFePO4 Batteries. J. Hazard. Mater. 2022, 424, 127586. [Google Scholar] [CrossRef]
- Feng, S.; Li, D.; Deng, J.; Yang, Z.; Zhang, J.; Zhou, Y. Closed-Loop Recovery of Spent Lithium-Ion Batteries Based on Preferentially Selective Extraction of Lithium Strategy. Sep. Purif. Technol. 2025, 354, 128953. [Google Scholar] [CrossRef]
- Shi, J.; Hou, C.; Dong, J.; Chen, D.; Li, J. Low-Temperature Chlorination Roasting Technology for the Simultaneous Recovery of Valuable Metals from Spent LiCoO2 Cathode Material. Int. J. Miner. Metall. Mater. 2025, 32, 80–91. [Google Scholar] [CrossRef]
- Habashi, F. A Short History of Hydrometallurgy. Hydrometallurgy 2005, 79, 15–22. [Google Scholar] [CrossRef]
- Liang, Z.; Cai, C.; Peng, G.; Hu, J.; Hou, H.; Liu, B.; Liang, S.; Xiao, K.; Yuan, S.; Yang, J. Hydrometallurgical Recovery of Spent Lithium Ion Batteries: Environmental Strategies and Sustainability Evaluation. ACS Sustain. Chem. Eng. 2021, 9, 5750–5767. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A Brief Review on Hydrometallurgical Technologies for Recycling Spent Lithium-ion Batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Asadi Dalini, E.; Karimi, G.; Zandevakili, S.; Goodarzi, M. A Review on Environmental, Economic and Hydrometallurgical Processes of Recycling Spent Lithium-Ion Batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 451–472. [Google Scholar] [CrossRef]
- Meshram, P.; Abhilash; Pandey, B.D.; Mankhand, T.R.; Deveci, H. Comparision of Different Reductants in Leaching of Spent Lithium Ion Batteries. JOM 2016, 68, 2613–2623. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical Processing of Spent Lithium Ion Batteries (LIBs) in the Presence of a Reducing Agent with Emphasis on Kinetics of Leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Windisch-Kern, S.; Gerold, E.; Nigl, T.; Jandric, A.; Altendorfer, M.; Rutrecht, B.; Scherhaufer, S.; Raupenstrauch, H.; Pomberger, R.; Antrekowitsch, H.; et al. Recycling Chains for Lithium-Ion Batteries: A Critical Examination of Current Challenges, Opportunities and Process Dependencies. Waste Manag. 2022, 138, 125–139. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of Lithium and Cobalt from Spent Lithium Ion Batteries (LIBs) Using Organic Acids as Leaching Reagents: A Review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Ferreira, D.A.; Prados, L.M.Z.; Majuste, D.; Mansur, M.B. Hydrometallurgical Separation of Aluminium, Cobalt, Copper and Lithium from Spent Li-Ion Batteries. J. Power Sources 2009, 187, 238–246. [Google Scholar] [CrossRef]
- Punt, T.; Bradshaw, S.M.; Van Wyk, P.; Akdogan, G. The Efficiency of Black Mass Preparation by Discharge and Alkaline Leaching for LIB Recycling. Minerals 2022, 12, 753. [Google Scholar] [CrossRef]
- Yu, J.; Ma, B.; Qiu, Z.; Wang, C.; Chen, Y. Separation and Recovery of Valuable Metals from Ammonia Leaching Solution of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2023, 11, 9738–9750. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Lai, F.; Yan, F.; Zhang, Z. Reduction-Ammoniacal Leaching to Recycle Lithium, Cobalt, and Nickel from Spent Lithium-Ion Batteries with a Hydrothermal Method: Effect of Reductants and Ammonium Salts. Waste Manag. 2020, 102, 122–130. [Google Scholar] [CrossRef]
- Zheng, X.; Gao, W.; Zhang, X.; He, M.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Spent Lithium-Ion Battery Recycling—Reductive Ammonia Leaching of Metals from Cathode Scrap by Sodium Sulphite. Waste Manag. 2017, 60, 680–688. [Google Scholar] [CrossRef]
- Guzolu, J.S.; Gharabaghi, M.; Mobin, M.; Alilo, H. Extraction of Li and Co from Li-Ion Batteries by Chemical Methods. J. Inst. Eng. India Ser. D 2017, 98, 43–48. [Google Scholar] [CrossRef]
- Su, F.; Zhou, X.; Liu, X.; Yang, J.; Tang, J.; Yang, W.; Li, Z.; Wang, H.; Ma, Y. Efficient Recovery of Valuable Metals from Spent Lithium-Ion Batteries by Pyrite Method with Hydrometallurgy Process. Chem. Eng. J. 2023, 455, 140914. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.-I. Reductive Leaching of Cathodic Active Materials from Lithium Ion Battery Wastes. Hydrometallurgy 2003, 68, 5–10. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, X.; Zhou, X.; He, Y.; Tang, J.; Su, F.; Yang, W.; Fan, S.; Wang, J.; Li, Z.; et al. Selective Extraction of Lithium from Spent LiNixCoyMnzO2 Cathode via In-Situ Conversion of Ethylene Glycol in Subcritical Water System. Chem. Eng. J. 2023, 451, 138535. [Google Scholar] [CrossRef]
- Almeida, J.R.; Moura, M.N.; Barrada, R.V.; Barbieri, E.M.S.; Carneiro, M.T.W.D.; Ferreira, S.A.D.; Lelis, M.D.F.F.; De Freitas, M.B.J.G.; Brandão, G.P. Composition Analysis of the Cathode Active Material of Spent Li-Ion Batteries Leached in Citric Acid Solution: A Study to Monitor and Assist Recycling Processes. Sci. Total Environ. 2019, 685, 589–595. [Google Scholar] [CrossRef]
- Tembo, P.M.; Werner, R.N.; Subramanian, V. Application of a Simple Organic Acid as a Green Alternative for the Recovery of Cathode Metals from Lithium-Ion Battery Cathode Materials. Green Technol. Sustain. 2025, 3, 100135. [Google Scholar] [CrossRef]
- Tanong, K.; Coudert, L.; Mercier, G.; Blais, J.-F. Recovery of Metals from a Mixture of Various Spent Batteries by a Hydrometallurgical Process. J. Environ. Manag. 2016, 181, 95–107. [Google Scholar] [CrossRef]
- Jain, S.; Hoseyni, S.M.; Cordiner, J. Safety Considerations for Hydrometallurgical Metal Recovery from Lithium-ion Batteries. Process Saf. Prog. 2024, 43, 542–549. [Google Scholar] [CrossRef]
- Roy, J.J.; Madhavi, S.; Cao, B. Metal Extraction from Spent Lithium-Ion Batteries (LIBs) at High Pulp Density by Environmentally Friendly Bioleaching Process. J. Clean. Prod. 2021, 280, 124242. [Google Scholar] [CrossRef]
- Roy, J.J.; Cao, B.; Madhavi, S. A Review on the Recycling of Spent Lithium-Ion Batteries (LIBs) by the Bioleaching Approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef]
- Do, M.P.; Jegan Roy, J.; Cao, B.; Srinivasan, M. Green Closed-Loop Cathode Regeneration from Spent NMC-Based Lithium-Ion Batteries through Bioleaching. ACS Sustain. Chem. Eng. 2022, 10, 2634–2644. [Google Scholar] [CrossRef]
- Biswal, B.K.; Jadhav, U.U.; Madhaiyan, M.; Ji, L.; Yang, E.-H.; Cao, B. Biological Leaching and Chemical Precipitation Methods for Recovery of Co and Li from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 12343–12352. [Google Scholar] [CrossRef]
- Kim, J.; Nwe, H.H.; Yoon, C.S. Enhanced Bioleaching of Spent Li-Ion Batteries Using A. Ferrooxidans by Application of External Magnetic Field. J. Environ. Manag. 2024, 367, 122012. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Kim, D.-J.; Ralph, D.E.; Ahn, J.-G.; Rhee, Y.-H. Bioleaching of Metals from Spent Lithium Ion Secondary Batteries Using Acidithiobacillus Ferrooxidans. Waste Manag. 2008, 28, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Naseri, T.; Mousavi, S.M. Improvement of Li and Mn Bioleaching from Spent Lithium-Ion Batteries, Using Step-Wise Addition of Biogenic Sulfuric Acid by Acidithiobacillus Thiooxidans. Heliyon 2024, 10, e37447. [Google Scholar] [CrossRef]
- Moazzam, P.; Boroumand, Y.; Rabiei, P.; Baghbaderani, S.S.; Mokarian, P.; Mohagheghian, F.; Mohammed, L.J.; Razmjou, A. Lithium Bioleaching: An Emerging Approach for the Recovery of Li from Spent Lithium Ion Batteries. Chemosphere 2021, 277, 130196. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Wu, W.; Zhang, X.; Gu, T.; Zhu, M.; Tan, W. Oxidative Stress Induced by Metal Ions in Bioleaching of LiCoO2 by an Acidophilic Microbial Consortium. Front. Microbiol. 2020, 10, 3058. [Google Scholar] [CrossRef]
- Chandakhiaw, T.; Teaumroong, N.; Piromyou, P.; Songwattana, P.; Tanthanuch, W.; Tancharakorn, S.; Khumkoa, S. Efficiency of Penicillium sp. and Aspergillus sp. for Bioleaching Lithium Cobalt Oxide from Battery Wastes in Potato Dextrose Broth and Sucrose Medium. Results Eng. 2024, 24, 103170. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Wang, H.; Zhang, B. Research Progress on Bioleaching Recovery Technology of Spent Lithium-Ion Batteries. Environ. Res. 2023, 238, 117145. [Google Scholar] [CrossRef]
- Sethurajan, M.; Gaydardzhiev, S. Bioprocessing of Spent Lithium Ion Batteries for Critical Metals Recovery—A Review. Resour. Conserv. Recycl. 2021, 165, 105225. [Google Scholar] [CrossRef]
- Biswal, B.K.; Balasubramanian, R. Recovery of Valuable Metals from Spent Lithium-Ion Batteries Using Microbial Agents for Bioleaching: A Review. Front. Microbiol. 2023, 14, 1197081. [Google Scholar] [CrossRef]
- Jegan Roy, J.; Srinivasan, M.; Cao, B. Bioleaching as an Eco-Friendly Approach for Metal Recovery from Spent NMC-Based Lithium-Ion Batteries at a High Pulp Density. ACS Sustain. Chem. Eng. 2021, 9, 3060–3069. [Google Scholar] [CrossRef]
- Liao, X.; Ye, M.; Liang, J.; Li, S.; Liu, Z.; Deng, Y.; Guan, Z.; Gan, Q.; Fang, X.; Sun, S. Synergistic Enhancement of Metal Extraction from Spent Li-Ion Batteries by Mixed Culture Bioleaching Process Mediated by Ascorbic Acid: Performance and Mechanism. J. Clean. Prod. 2022, 380, 134991. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M.; Baniasadi, M. Use of Adapted Metal Tolerant Aspergillus Niger to Enhance Bioleaching Efficiency of Valuable Metals from Spent Lithium-Ion Mobile Phone Batteries. J. Clean. Prod. 2018, 197, 1546–1557. [Google Scholar] [CrossRef]
- Wang, M.; Liu, K.; Yu, J.; Zhang, C.-C.; Zhang, Z.; Tan, Q. Recycling Spent Lithium-Ion Batteries Using a Mechanochemical Approach. Circ. Econ. 2022, 1, 100012. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Q.; Liu, L.; Li, J. Selective Regeneration of Lithium from Spent Lithium-Ion Batteries Using Ionic Substitution Stimulated by Mechanochemistry. J. Clean. Prod. 2021, 279, 123612. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Zhang, X.; Ma, E. A Mechanochemical Method for One-Step Leaching of Metals from Spent LIBs. Waste Manag. 2023, 161, 245–253. [Google Scholar] [CrossRef]
- Wang, M.-M.; Zhang, C.-C.; Zhang, F.-S. An Environmental Benign Process for Cobalt and Lithium Recovery from Spent Lithium-Ion Batteries by Mechanochemical Approach. Waste Manag. 2016, 51, 239–244. [Google Scholar] [CrossRef]
- Xie, J.; Huang, K.; Nie, Z.; Yuan, W.; Wang, X.; Song, Q.; Zhang, X.; Zhang, C.; Wang, J.; Crittenden, J.C. An Effective Process for the Recovery of Valuable Metals from Cathode Material of Lithium-Ion Batteries by Mechanochemical Reduction. Resour. Conserv. Recycl. 2021, 168, 105261. [Google Scholar] [CrossRef]
- Guan, J.; Li, Y.; Guo, Y.; Su, R.; Gao, G.; Song, H.; Yuan, H.; Liang, B.; Guo, Z. Mechanochemical Process Enhanced Cobalt and Lithium Recycling from Wasted Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2017, 5, 1026–1032. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, X.; Yan, S.; Ou, Y.; Zhou, T. Mechanochemistry-Induced Recycling of Spent Lithium-Ion Batteries for Synergistic Treatment of Mixed Cathode Powders. Green Chem. 2022, 24, 5987–5997. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Fei, Z.; Fan, C.; Meng, Q.; Peng, X.; Dong, P. Review and Perspectives on Direct Regeneration of Spent Ternary Cathode Materials Based on Failure Mechanisms. Energy Fuels 2025, 39, 104–131. [Google Scholar] [CrossRef]
- Gupta, V.; Appleberry, M.; Li, W.; Chen, Z. Direct Recycling Industrialization of Li-Ion Batteries: The Pre-Processing Barricade. Next Energy 2024, 2, 100091. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Zhuang, Z.; Liang, Z.; Jia, K.; Ji, G.; Zhou, G.; Cheng, H.-M. Toward Direct Regeneration of Spent Lithium-Ion Batteries: A Next-Generation Recycling Method. Chem. Rev. 2024, 124, 2839–2887. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.J.; Phuong, D.M.; Verma, V.; Chaudhary, R.; Carboni, M.; Meyer, D.; Cao, B.; Srinivasan, M. Direct Recycling of Li-ion Batteries from Cell to Pack Level: Challenges and Prospects on Technology, Scalability, Sustainability, and Economics. Carbon Energy 2024, 6, e492. [Google Scholar] [CrossRef]
- Ji, Y.; Kpodzro, E.E.; Jafvert, C.T.; Zhao, F. Direct Recycling Technologies of Cathode in Spent Lithium-Ion Batteries. Clean Technol. Recycl. 2021, 1, 124–151. [Google Scholar] [CrossRef]
- Sloop, S.; Crandon, L.; Allen, M.; Koetje, K.; Reed, L.; Gaines, L.; Sirisaksoontorn, W.; Lerner, M. A Direct Recycling Case Study from a Lithium-Ion Battery Recall. Sustain. Mater. Techno. 2020, 25, e00152. [Google Scholar] [CrossRef]
- Xu, P.; Tan, D.H.S.; Jiao, B.; Gao, H.; Yu, X.; Chen, Z. A Materials Perspective on Direct Recycling of Lithium-Ion Batteries: Principles, Challenges and Opportunities. Adv. Funct. Mater. 2023, 33, 2213168. [Google Scholar] [CrossRef]
- Mancini, M.; Hoffmann, M.F.; Martin, J.; Weirather-Köstner, D.; Axmann, P.; Wohlfahrt-Mehrens, M. A Proof-of-Concept of Direct Recycling of Anode and Cathode Active Materials: From Spent Batteries to Performance in New Li-Ion Cells. J. Power Sources 2024, 595, 233997. [Google Scholar] [CrossRef]
- Zhou, B.; Su, H.; Liu, W.; Zhu, Z.; Wang, L.; Qi, T. Solvent Extraction of Metal Ions from the Leaching Solutions of Waste Lithium-Ion Battery Materials: A Review. Sep. Purif. Technol. 2025, 354, 129173. [Google Scholar] [CrossRef]
- Lei, S.; Sun, W.; Yang, Y. Solvent Extraction for Recycling of Spent Lithium-Ion Batteries. J. Hazard. Mater. 2022, 424, 127654. [Google Scholar] [CrossRef]
- Meshram, P.; Virolainen, S.; Abhilash, A.; Sainio, T. Solvent Extraction for Separation of 99.9% Pure Cobalt and Recovery of Li, Ni, Fe, Cu, Al from Spent LIBs. Metals 2022, 12, 1056. [Google Scholar] [CrossRef]
- Kang, J.; Senanayake, G.; Sohn, J.; Shin, S.M. Recovery of Cobalt Sulfate from Spent Lithium Ion Batteries by Reductive Leaching and Solvent Extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef]
- Zhao, J.M.; Shen, X.Y.; Deng, F.L.; Wang, F.C.; Wu, Y.; Liu, H.Z. Synergistic Extraction and Separation of Valuable Metals from Waste Cathodic Material of Lithium Ion Batteries Using Cyanex272 and PC-88A. Sep. Purif. Technol. 2011, 78, 345–351. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Rui, H.; Shi, D.; Peng, X.; Ji, L.; Song, X. Lithium Recovery from Effluent of Spent Lithium Battery Recycling Process Using Solvent Extraction. J. Hazard. Mater. 2020, 398, 122840. [Google Scholar] [CrossRef]
- Wesselborg, T.; Virolainen, S.; Sainio, T. Recovery of Lithium from Leach Solutions of Battery Waste Using Direct Solvent Extraction with TBP and FeCl3. Hydrometallurgy 2021, 202, 105593. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, S.; Song, S.; Sun, W.; Wang, L. Stepwise Recycling of Valuable Metals from Ni-Rich Cathode Material of Spent Lithium-Ion Batteries. Waste Manag. 2020, 102, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Sahu, S.; Sukla, L.B.; Devi, N. Application of Various Processes to Recycle Lithium-Ion Batteries (LIBs): A Brief Review. Mater. Today Proc. 2021, 47, 1203–1212. [Google Scholar] [CrossRef]
- Chang, H.-F.; Lin, J.-Y.; Cheng, T.-M.; Lai, C.-H. Advanced Absolute Chemical Precipitation for High-Purity Metal Recovery in All-Types of Lithium-Ion Battery Recycling. Sep. Purif. Technol. 2025, 361, 131454. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Meng, Q.; Dong, P.; Ning, P.; Li, Q. Recovery of Valuable Metals from Mixed Spent Lithium-Ion Batteries by Multi-Step Directional Precipitation. RSC Adv. 2021, 11, 268–277. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.; Villen-Guzman, M.; Vereda-Alonso, C.; Rodriguez-Maroto, J.; Paz-Garcia, J. Towards Sustainable Lithium-Ion Battery Recycling: Advancements in Circular Hydrometallurgy. Processes 2024, 12, 1485. [Google Scholar] [CrossRef]
- Arnold, S.; Ruthes, J.G.A.; Kim, C.; Presser, V. Electrochemical Recycling of Lithium-ion Batteries Advancements and Future Directions. EcoMat 2024, 6, e12494. [Google Scholar] [CrossRef]
- Chan, K.H.; Malik, M.; Azimi, G. Separation of Lithium, Nickel, Manganese, and Cobalt from Waste Lithium-Ion Batteries Using Electrodialysis. Resour. Conserv. Recycl. 2022, 178, 106076. [Google Scholar] [CrossRef]
- Wang, Q.; Fox, R.V.; Shi, M.; Snyder, S.W.; Ilevbare, G.O.; Ginosar, D.M. Electrodialysis: An Effective Methodology to Purify the Leachate of Spent Li-Ion Batteries. Sep. Purif. Technol. 2025, 359, 130430. [Google Scholar] [CrossRef]
- Yu, L.; Bai, Y.; Belharouak, I. Recycling of Lithium-Ion Batteries via Electrochemical Recovery: A Mini-Review. Batteries 2024, 10, 337. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Jiang, Y.; Zhou, T.; Zhao, Y.; Chen, X. Novel Electrochemically Driven and Internal Circulation Process for Valuable Metals Recycling from Spent Lithium-Ion Batteries. Waste Manag. 2021, 136, 18–27. [Google Scholar] [CrossRef]
- Kim, K.; Raymond, D.; Candeago, R.; Su, X. Selective Cobalt and Nickel Electrodeposition for Lithium-Ion Battery Recycling through Integrated Electrolyte and Interface Control. Nat. Commun. 2021, 12, 6554. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Yang, J.; He, Z.; Zheng, J.; Li, Y. Electrochemical Methods Contribute to the Recycling and Regeneration Path of Lithium-Ion Batteries. Energy Storage Mater. 2023, 55, 606–630. [Google Scholar] [CrossRef]
- Lv, L.; Zhou, S.; Liu, C.; Sun, Y.; Zhang, J.; Bu, C.; Meng, J.; Huang, Y. Recycling and Reuse of Spent LIBs: Technological Advances and Future Directions. Molecules 2024, 29, 3161. [Google Scholar] [CrossRef]
- Tran, T.T.; Lee, M.S. Ion Exchange for the Purification of Co(II) or Ni(II) from Acidic and Ammonia Solutions in the Recycling of Spent Lithium-Ion Batteries. Korean J. Met. Mater. 2022, 60, 902–911. [Google Scholar] [CrossRef]
- Virolainen, S.; Wesselborg, T.; Kaukinen, A.; Sainio, T. Removal of Iron, Aluminium, Manganese and Copper from Leach Solutions of Lithium-Ion Battery Waste Using Ion Exchange. Hydrometallurgy 2021, 202, 105602. [Google Scholar] [CrossRef]
- Strauss, M.L.; Diaz, L.A.; McNally, J.; Klaehn, J.; Lister, T.E. Separation of Cobalt, Nickel, and Manganese in Leach Solutions of Waste Lithium-Ion Batteries Using Dowex M4195 Ion Exchange Resin. Hydrometallurgy 2021, 206, 105757. [Google Scholar] [CrossRef]
- Du, K.; Ang, E.H.; Wu, X.; Yichun Liu, Y. Progresses in Sustainable Recycling Technology of Spent Lithium-Ion Batteries. Energy Environ. Mater. 2022, 5, 1012–1036. [Google Scholar] [CrossRef]
- Lima, M.C.C.; Pontes, L.P.; Vasconcelos, A.S.M.; De Araujo Silva Junior, W.; Wu, K. Economic Aspects for Recycling of Used Lithium-Ion Batteries from Electric Vehicles. Energies 2022, 15, 2203. [Google Scholar] [CrossRef]
- Latini, D.; Vaccari, M.; Lagnoni, M.; Orefice, M.; Mathieux, F.; Huisman, J.; Tognotti, L.; Bertei, A. A Comprehensive Review and Classification of Unit Operations with Assessment of Outputs Quality in Lithium-Ion Battery Recycling. J. Power Sources 2022, 546, 231979. [Google Scholar] [CrossRef]
- Vaccari, M.; Parlanti, F.; Manni, F.M.; Orefice, M.; Mathieux, F.; Pannocchia, G.; Tognotti, L.; Bertei, A. Assessing Performance in Lithium-Ion Batteries Recycling Processes: A Quantitative Modeling Perspective. Resour. Conserv. Recycl. 2024, 206, 107643. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; Sun, W.; He, Y. On the Sustainability of Lithium Ion Battery Industry—A Review and Perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar] [CrossRef]
- Tian, X.; Ma, Q.; Xie, J.; Xia, Z.; Liu, Y. Environmental Impact and Economic Assessment of Recycling Lithium Iron Phosphate Battery Cathodes: Comparison of Major Processes in China. Resour. Conserv. Recycl. 2024, 203, 107449. [Google Scholar] [CrossRef]
- Alipanah, M.; Jin, H.; Zhou, Q.; Barboza, C.; Gazzo, D.; Thompson, V.; Fujita, Y.; Liu, J.; Anderko, A.; Reed, D. Sustainable Bioleaching of Lithium-Ion Batteries for Critical Metal Recovery: Process Optimization through Design of Experiments and Thermodynamic Modeling. Resour. Conserv. Recycl. 2023, 199, 107293. [Google Scholar] [CrossRef]
- Reinhart, L.; Vrucak, D.; Woeste, R.; Lucas, H.; Rombach, E.; Friedrich, B.; Letmathe, P. Pyrometallurgical Recycling of Different Lithium-Ion Battery Cell Systems: Economic and Technical Analysis. J. Clean. Prod. 2023, 416, 137834. [Google Scholar] [CrossRef]
- Wang, M.; Liu, K.; Dutta, S.; Alessi, D.S.; Rinklebe, J.; Ok, Y.S.; Tsang, D.C.W. Recycling of Lithium Iron Phosphate Batteries: Status, Technologies, Challenges, and Prospects. Renew. Sust. Energ. Rev. 2022, 163, 112515. [Google Scholar] [CrossRef]
- Mayyas, A.; Steward, D.; Mann, M. The Case for Recycling: Overview and Challenges in the Material Supply Chain for Automotive Li-Ion Batteries. Sustain. Mater. Techno. 2019, 19, e00087. [Google Scholar] [CrossRef]
- Arshad, F.; Lin, J.; Manurkar, N.; Fan, E.; Ahmad, A.; Tariq, M.-N.; Wu, F.; Chen, R.; Li, L. Life Cycle Assessment of Lithium-Ion Batteries: A Critical Review. Resour. Conserv. Recycl. 2022, 180, 106164. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, X.-D.; Lin, A.-J.; Dong, Y.; Duan, G.-L. Environmental Life Cycle Assessment on the Recycling Processes of Power Batteries for New Energy Vehicles. J. Clean. Prod. 2025, 488, 144641. [Google Scholar] [CrossRef]
- Yu, M.; Bai, B.; Xiong, S.; Liao, X. Evaluating Environmental Impacts and Economic Performance of Remanufacturing Electric Vehicle Lithium-Ion Batteries. J. Clean. Prod. 2021, 321, 128935. [Google Scholar] [CrossRef]
- Wu, F.; Li, L.; Crandon, L.; Cao, Y.; Cheng, F.; Hicks, A.; Zeng, E.Y.; You, J. Environmental Hotspots and Greenhouse Gas Reduction Potential for Different Lithium-Ion Battery Recovery Strategies. J. Clean. Prod. 2022, 339, 130697. [Google Scholar] [CrossRef]
- Alipanah, M.; Reed, D.; Thompson, V.; Fujita, Y.; Jin, H. Sustainable Bioleaching of Lithium-Ion Batteries for Critical Materials Recovery. J. Clean. Prod. 2023, 382, 135274. [Google Scholar] [CrossRef]

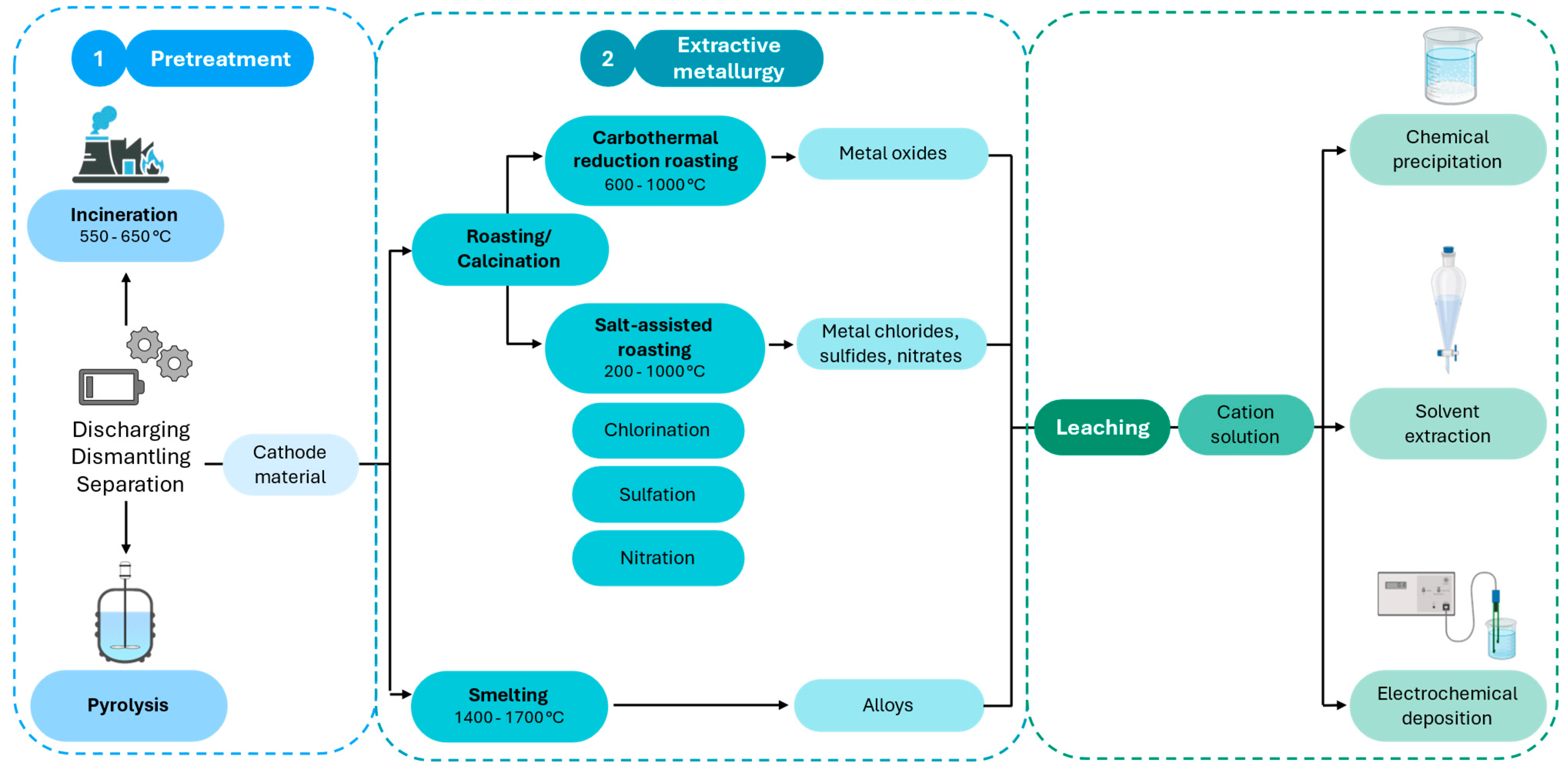


| Sample | Applied Technique | Operational Conditions (Temp. and Time) | Efficiency | Reference |
|---|---|---|---|---|
| Li(NixMnyCo1−x−y)O2 cathode material | Carbothermal reduction; water leaching | 700–1200 °C 1 h | Li: 93% | [77] |
| LiNiMnCoO2 cathode material | Microwave carbothermal reduction; acid leaching | 900 °C (500 W) 30 min | Li: 99.68% Co: 97.85% Ni: 97.65% Mn: 96.73% | [80] |
| LiCoNiO2 cathode material | Smelting | 1450 °C 30 min | Co: 98.83% Ni: 98.39% Cu: 93.57% | [85] |
| Mixed cathode material | Calcination; organic acid leaching | 700 °C 2 h | Li: 91.5% Co: 95.02% | [86] |
| LiFePO4 cathode material | Salt-assisted roasting (1. Na2CO3; 2. NaOH) | 1. 900 °C; 4 h 2. 600 °C; 2 h | 1. Li: 99.2% 2. Li: 92.7% | [87] |
| LiFePO4 cathode material | Salt-assisted roasting (Na2CO3); inorganic acid leaching | 600 °C 2 h | Li: 99.2% | [88] |
| LiCoO2 cathode material | Chlorination roasting (NH4Cl); water leaching | 400 °C 20 min | Li: 99.43% Co: 99.05% | [89] |
| Sample | Leaching Reagents | Operational Conditions (Temp. and Time) | S/L Ratio | Efficiency | Reference |
|---|---|---|---|---|---|
| LiCoO2 cathode material | HCl (5 M) | 95 °C 70 min | 10 g/L | Li: 98% Co: 99% | [104] |
| LiNixCoyMnzO2 cathode material | H2SO4 (3 M) FeS2 | 80 °C 2 h | 40 g/L | Li: 99.9% Co: 99.5% Mn: 98% Ni: 98.9% | [105] |
| LiCoO2 cathode material | HNO3 (1 M) H2O2 (1.7 vol%) | 75 °C 30 min | 10-20 g/L | Li: 99% Co: 99% | [106] |
| LiNixCoyMnzO2 cathode material | Ethylene glycol | 200 °C 20 h | 50 g/L | Li: 99.2% | [107] |
| Cathode material | Citric acid (2 M) H2O2 (0.25 M) | 80 °C 2 h | 20 g/L | Li: 99% Co: 99% Mn: 92% Ni: 90% | [108] |
| Cathode material | Propionic acid (2 M) H2O2 (2 v/v%) | 80 °C 2 h | 30 g/L | Li: 87.4% Co: 92.9% Mn: 92.7% Ni: 94.0% | [109] |
| LiNixCoyMnzO2 cathode material | NH3·H2O (6 M) (NH4)2CO3 (0.5 M) Na2SO3 (0.5 M) | 150 °C 30 min | 10 g/L | Li: 87.0% Co: 99.5% Ni: 91.1% | [102] |
| LiNixCoyMnzO2 cathode material | NH3·H2O (6 M) (NH4)2SO3 (0.5 M) | 150 °C 30 min | 10 g/L | Li: 97.8% Co: 100% Ni: 73.7% | [102] |
| Sample | Leaching Reagents | Operational Conditions (Temp. and Time) | S/L Ratio | Efficiency | Reference |
|---|---|---|---|---|---|
| LiMnO2 cathode material | Acidithiobacillus thiooxidans | 30 °C 8 days | 60 g/L | Li: 93% Mn: 53% | [118] |
| LiNixCoyMnzO2 cathode material | Acidithiobacillus ferrooxidans | 30 °C 72 h | 100 g/L | Li: 89% Co: 82% Mn: 92% Ni: 90% | [125] |
| LiCoO2 cathode material | Acidithiobacillus caldus and Sulfobacillus thermosulfidooxidans | 30 °C 2 days | 20 g/L | Li: 94% Co: 95% | [126] |
| LiCoO2 cathode material | Penicillium | 25 °C 30 days | 0.1% pulp density | Li: 99.88% Co: 77.87% | [121] |
| Mixed cathode materials | Aspergillus niger | 30 °C 30 days | 1% pulp density | Li: 100% Cu: 94% Mn: 72% Al: 62% Ni: 45% Co: 38% | [127] |
| Traditional Approaches | ||
| Advantages | Disadvantages | |
| Pyrometallurgy |
|
|
| Hydrometallurgy |
|
|
| Innovative Approaches | ||
| Advantages | Disadvantages | |
| Bioleaching |
|
|
| Mechanochemistry |
|
|
| Direct recycling |
|
|
| Extractant | Operational Conditions (Conc. and pH) | Selectivity | Efficiency | References |
|---|---|---|---|---|
| D2EHPA | 1 M pH 3 | Mn > Co > Ni | Mn: 90% | [143] |
| Cyanex 272 | 0.4–1 M pH 5–6 | Co > Ni > Li | Co: 95–98% | [145,146] |
| PC88A | 30 vol% pH 5 | Mn > Co > Li | Mn: 98% Co: 90% | [147,150] |
| HBTA + TOPO | 0.4 M pH 8.5 | Li | Li: 97% | [148] |
| Advantages | Disadvantages | |
|---|---|---|
| Solvent extraction |
|
|
| Chemical precipitation |
|
|
| Electrodialysis |
|
|
| Electrochemical deposition |
|
|
| Ion exchange |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Sardo, C.; Cacciatore, G.; Cappuccino, G.; Aiello, D.; Napoli, A. Spent Lithium Battery Recycling: Traditional and Innovative Approaches. Processes 2025, 13, 950. https://doi.org/10.3390/pr13040950
Lo Sardo C, Cacciatore G, Cappuccino G, Aiello D, Napoli A. Spent Lithium Battery Recycling: Traditional and Innovative Approaches. Processes. 2025; 13(4):950. https://doi.org/10.3390/pr13040950
Chicago/Turabian StyleLo Sardo, Carmen, Giuseppina Cacciatore, Gregorio Cappuccino, Donatella Aiello, and Anna Napoli. 2025. "Spent Lithium Battery Recycling: Traditional and Innovative Approaches" Processes 13, no. 4: 950. https://doi.org/10.3390/pr13040950
APA StyleLo Sardo, C., Cacciatore, G., Cappuccino, G., Aiello, D., & Napoli, A. (2025). Spent Lithium Battery Recycling: Traditional and Innovative Approaches. Processes, 13(4), 950. https://doi.org/10.3390/pr13040950







