Study of Nickel–Chromium-Containing Ferroalloy Production
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
- −
- Nickel recovery: The second option ensures a high nickel recovery rate of 91%, which is only 1% lower than the maximum (with 100% coke), but significantly higher than the equal ratio of coke and coal (88%).
- −
- Coke savings: The 75:25 option reduces coke consumption from 7.193 g to 5.93 g, improving the economic efficiency of the process.
- −
- Slag ratio: The value of 3.07 indicates a more stable slag process compared to the 50:50 option (3.02), contributing to better metal quality and lower nickel losses.
- Iron is present in the form of Fe2O3, Fe3O4, and FeO(OH). These oxides are reduced sequentially as the temperature increases:
- 2.
- Chromium in the ore is present in the form of CrO(OH) and FeCr2O4.
- 3.
- Nickel in the ore is present in the form of silicates (Ni2SiO4). According to the authors of [25], nickel silicate is reduced by carbon as follows:
- 4.
- Silicon in the ore is present in the form of quartz. Up to 80% of SiO2 binds with CaO and transitions into the slag, while 20% is reduced according to the reaction at temperatures above 1500 °C [29].
4. Conclusions
- −
- Enables the processing of low-grade and substandard nickel ores from Kazakhstan without the need for prior agglomeration;
- −
- Demonstrates flexibility with respect to raw material composition;
- −
- Features lower energy consumption compared to electric smelting;
- −
- Has a reduced environmental impact due to the absence of slag foaming;
- −
- Ensures the production of an industrially applicable semi-product that meets the needs of the domestic market.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shofi, A.; Supriyatna, Y.I.; Prasetyo, A.B. Selective reduction of southeast sulawesi nickel laterite using palm kernel shell charcoal: Kinetic studies with addition of Na2SO4 and NaCl as additives. Bull. Chem. React. Eng. Catal. 2020, 15, 501–513. [Google Scholar] [CrossRef]
- Sari, Y.; Manaf, A.; Astuti, W.; Nurjaman, F.; Susanti, D.; Sipahutar, W.S.; Bahfie, F. Nickel Recovery in Ferronickel Concentrate by Green Selective Reduction of Nickel Laterite. Eng. Sci. Technol. Int. J. 2024, 57, 101798. [Google Scholar] [CrossRef]
- Sari, Y.; Manaf, A.; Astuti, W.; Haryono, T.; Nurjaman, F.; Bahfie, F. Recovery of Ferronickel by Green Selective Reduction of Nickel Laterite. IOP Conf. Ser. Earth Environ. Sci. 2024, 1388, 012026. [Google Scholar] [CrossRef]
- Tolymbekov, M.Z.; Kelamanov, B.S.; Baisanov, A.S.; Kaskin, K.K. Processing Kazakhstan’s Chromonickel Ore. Steel Transl. 2008, 38, 660–663. [Google Scholar] [CrossRef]
- Kelamanov, B.; Yessengaliyev, D.; Sariev, O.; Akuov, A.; Samuratov, Y.; Zhuniskaliyev, T.; Kuatbay, Y.; Mukhambetgaliyev, Y.; Kolesnikova, O.; Zhumatova, A.; et al. Technological Analysis of the Production of Nickel-Containing Composite Materials. J. Compos. Sci. 2024, 8, 179. [Google Scholar] [CrossRef]
- Kelamanov, B.; Samuratov, Y.; Zhumagaliyev, Y.; Akuov, A.; Sariev, O. Titanium and Chrome Oxides System Thermodynamic Diagram Analysis. Metallurgija 2020, 59, 101–104. [Google Scholar]
- Kelamanov, B.; Sariev, O.; Akuov, A.; Samuratov, Y.; Sultamuratova, Z.; Orynbassar, R. Study of Nickel Briquettes by Thermographic Method. Metallurgija 2022, 61, 217–220. [Google Scholar]
- Agatzini-Leonardou, S.; Tsakiridis, P.E.; Oustadakis, P.; Karidakis, T.; Katsiapi, A. Hydrometallurgical process for the separation and recovery of nickel from sulphate heap leach liquor of nickeliferrous laterite ores. Miner. Eng. 2009, 22, 1181–1192. [Google Scholar] [CrossRef]
- Chen, J.; Hayes, P.C. Mechanisms and Kinetics of Reduction of Solid NiO in CO/CO2 and CO/Ar Gas Mixtures. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2019, 50, 2623–2635. [Google Scholar] [CrossRef]
- Qu, G.; Zhou, S.; Wang, H.; Li, B.; Wei, Y. Production of ferronickel concentrate from low-grade nickel laterite ore by non-melting reduction magnetic separation process. Metals 2019, 9, 1340. [Google Scholar] [CrossRef]
- Sadykhov, G.B.; Kop’Ev, D.Y.; Olyunina, T.V.; Anisonyan, K.G. Bloomery Processing of Oxidized Magnesia Nickel Ores. Russian Metall. (Metally) 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Khasanov, M.S.; Sadykhov, G.B.; Anisonyan, K.G.; Zablotskaya, Y.V.; Olyunina, T.V. Effect of the Temperature of Reduction Roasting of Ferriferrous Oxidized Nickel Ores on the Nickel and Cobalt Recovery during Hydrometallurgical Processing of a Cinder. Russ. Metall. 2022, 714–718. [Google Scholar] [CrossRef]
- Zayakin, O.V. Development of a Rational Composition and Technology for the Production of Nickel-Containing Ferroalloysfrom Low-Grade Oxidized Nickel Ores. Ph.D. Thesis, Institute of Metallurgy, Ural Branch of the Russian Academy of Sciences, Yekaterinburg, Russia, 2002. [Google Scholar]
- Sadykhov, G.B.O.; Kisilev, A.I.; Lainer, Y.A. Method for Processing Nickel Laterite Ores Resulting in the Direct of Ferronickel. Patent WO 2014133421, 4 September 2014. [Google Scholar]
- Zhang, Z.F.; Zhang, W.B.; Zhang, Z.G.; Chen, X.F. Nickel extraction from nickel laterites: Processes, resources, environment and cost. China Geol. 2025, 1, 187–213. [Google Scholar] [CrossRef]
- Zevgolis, E.N.; Daskalakis, K.A. The Nickel Production Methods from Laterites and the Greek Ferronickel Production among Them. Mater. Proc. 2021, 5, 104. [Google Scholar] [CrossRef]
- Lin, B. Rotary Kiln for Producing Ferronickel from Indonesia Sulawesi Nickel Laterite Ore Through RKEF (Rotary Kiln-Electric Furnace) Technical Processing. Patent CN 202973836, 5 June 2013. [Google Scholar]
- Quintero-Coronel, D.A.; Guillin-Estrada, W.D.; Echeverri-Roman, J.L.; Maury, H.; Corredor, L.; Ruiz, J.A.; Rueda, B.S.; Gonzalez-Quiroga, A. Large- and Particle-Scale energy assessment of reduction roasting of nickel laterite ore for Ferronickel production via the rotary Kiln-Electric furnace process. Therm. Sci. Eng. Prog. 2022, 32, 101331. [Google Scholar] [CrossRef]
- Romero, J.M.; Pardo, Y.S.; Parra, M.; Castillo, A.D.J.; Maury, H.; Corredor, L.; Sánchez, I.; Rueda, B.; Gonzalez-Quiroga, A. Improving the rotary kiln-electric furnace process for ferronickel production: Data analytics-based assessment of dust insufflation into the rotary kiln flame. Alex. Eng. J. 2022, 61, 3215–3228. [Google Scholar] [CrossRef]
- Voskoboynikov, V.G.; Kudrin, V.A.; Yakushev, A.M. General Metallurgy: University Textbook, 6th ed.; Revised and Updated; RJSC “Akademkniga”: Moscow, Russia, 2002; 768p. [Google Scholar]
- GOST 22772.4-77; Manganese Ores, Concentrates and Agglomerates. Methods for Determination of Iron Content (Total). State Committee for Standards of the Council of Ministers of the USSR: Moscow, Russia, 1979.
- GOST 22772.6-77; Manganese Ores, Concentrates and Agglomerates. Methods for the Determination of Phosphorus. Committee for Standards of the Council of Ministers of the USSR: Moscow, Russia, 1979.
- GOST 22772.7-96; Manganese Ores, Concentrates and Agglomerates. Methods for Determination of Sulfur. Committee for Standards of the Council of Ministers of the USSR: Moscow, Russia, 1999.
- Fazlutdinov, K.K. Physicochemical Features of Cr(VI) Solution Utilization Using Steel Shavings: Reduction Kinetics, Phase Formation, Structure, and Morphology of Precipitates. Ph.D. Dissertation, Ural Federal University named after the first President of Russia B. N. Yeltsin (UrFU), Yekaterinburg, Russia, 2017. 154p. [Google Scholar]
- Demidov, A.I.; Markelov, I.A. Thermodynamics of Iron Reduction from Oxides by Carbon Monoxide in the Presence of Carbon. Mater. Sci. Power Eng. 2021, 27, 162. [Google Scholar] [CrossRef]
- Senin, A.V.; Mikhailov, G.G.; Pashkeev, I.Y.; Kuznetsova, O.V. Thermodynamic Analysis of Carbothermic Reduction of Iron Chromite. Bull. South Ural State Univ. Ser. Math. Mech. Phys. 2003. Available online: https://cyberleninka.ru/article/n/termodinamicheskiy-analiz-karbotermicheskogo-vosstanovleniya-hromita-zheleza (accessed on 18 April 2025).
- Kryukov, R.E.; Goryushkin, V.F.; Bendre, Y.V.; Bashchenko, L.P.; Kozyrev, N.A. Some Thermodynamic Aspects of Cr2O3 Reduction by Carbon. Izv. Vuzov. Black Metall. 2019, 62, 950–956. [Google Scholar]
- Bajraktari-Gashi, Z.; Halilaj, B. Material Balance of the Technological Process in the New Foundry of New Ferronickel in Drenas 2017. J. Technol. Exploit. Mech. Eng. 2018, 4, 29–35. [Google Scholar] [CrossRef]
- Janelidze, I.; Jandieri, G.; Tsertsvadze, T. Thermodynamic Analysis of Interaction of Components in the SiO2-C System: Improvement of Technical Silicon Production Technological Process. Phys. Chem. Solid State 2021, 22, 345–352. [Google Scholar] [CrossRef]

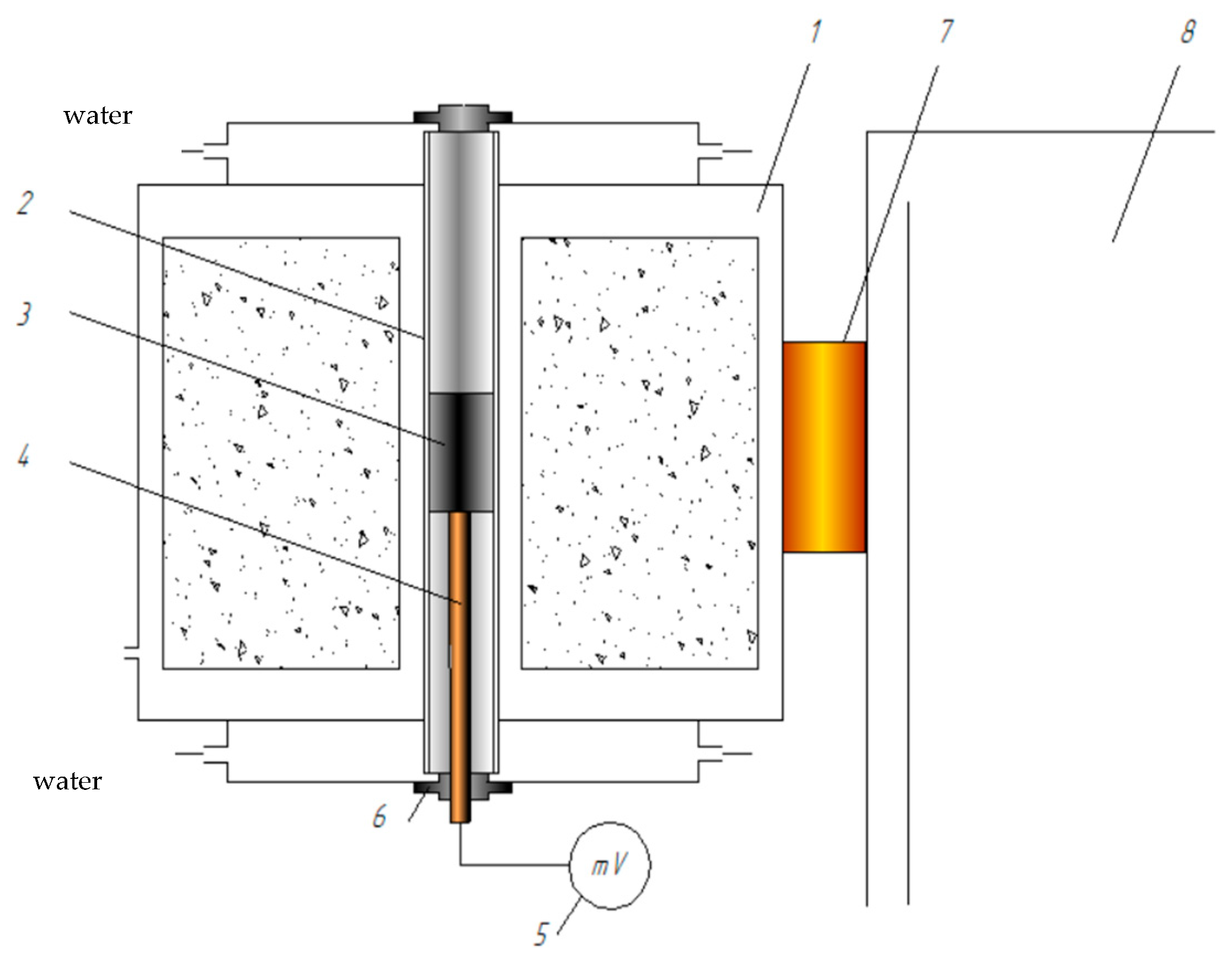

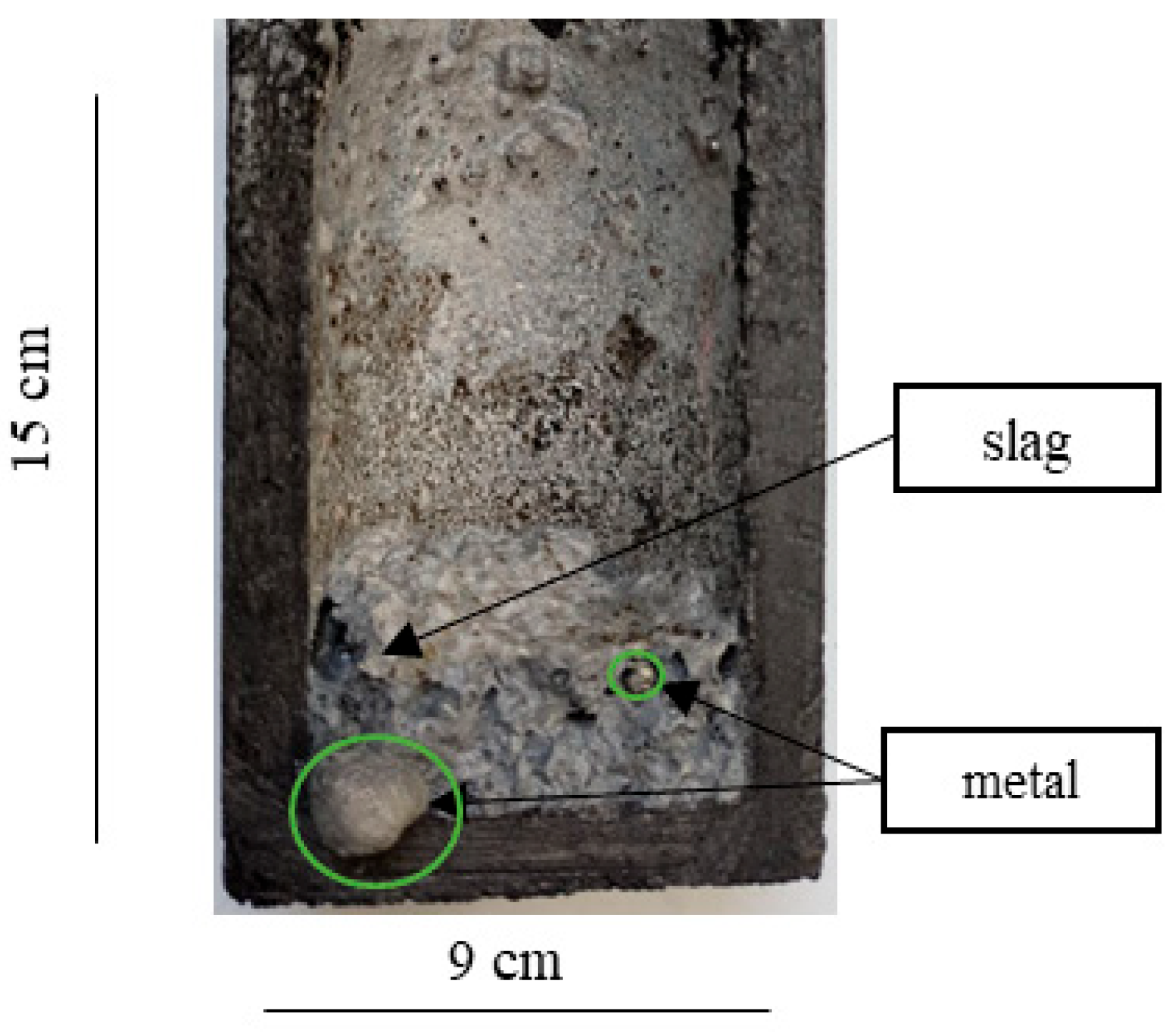
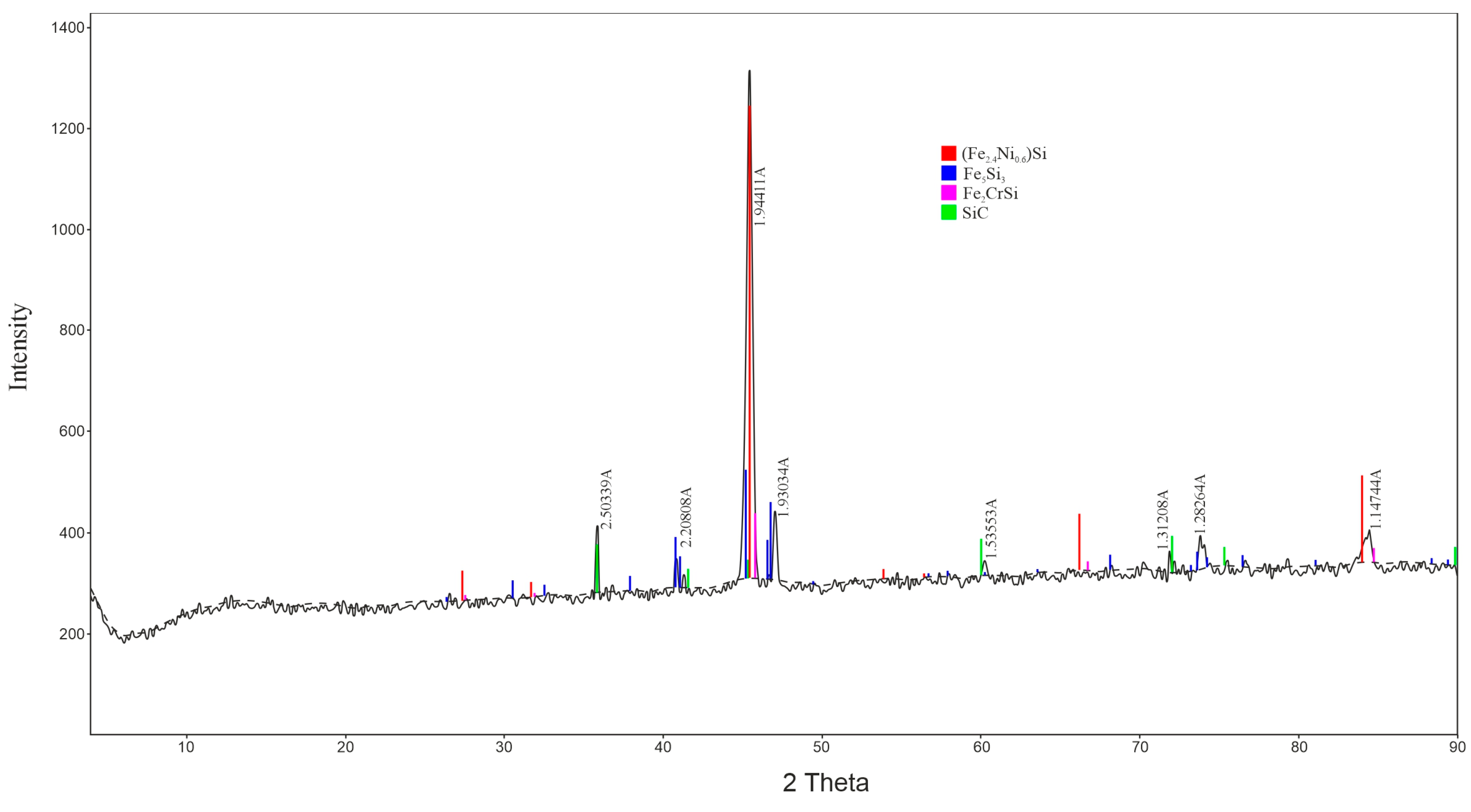

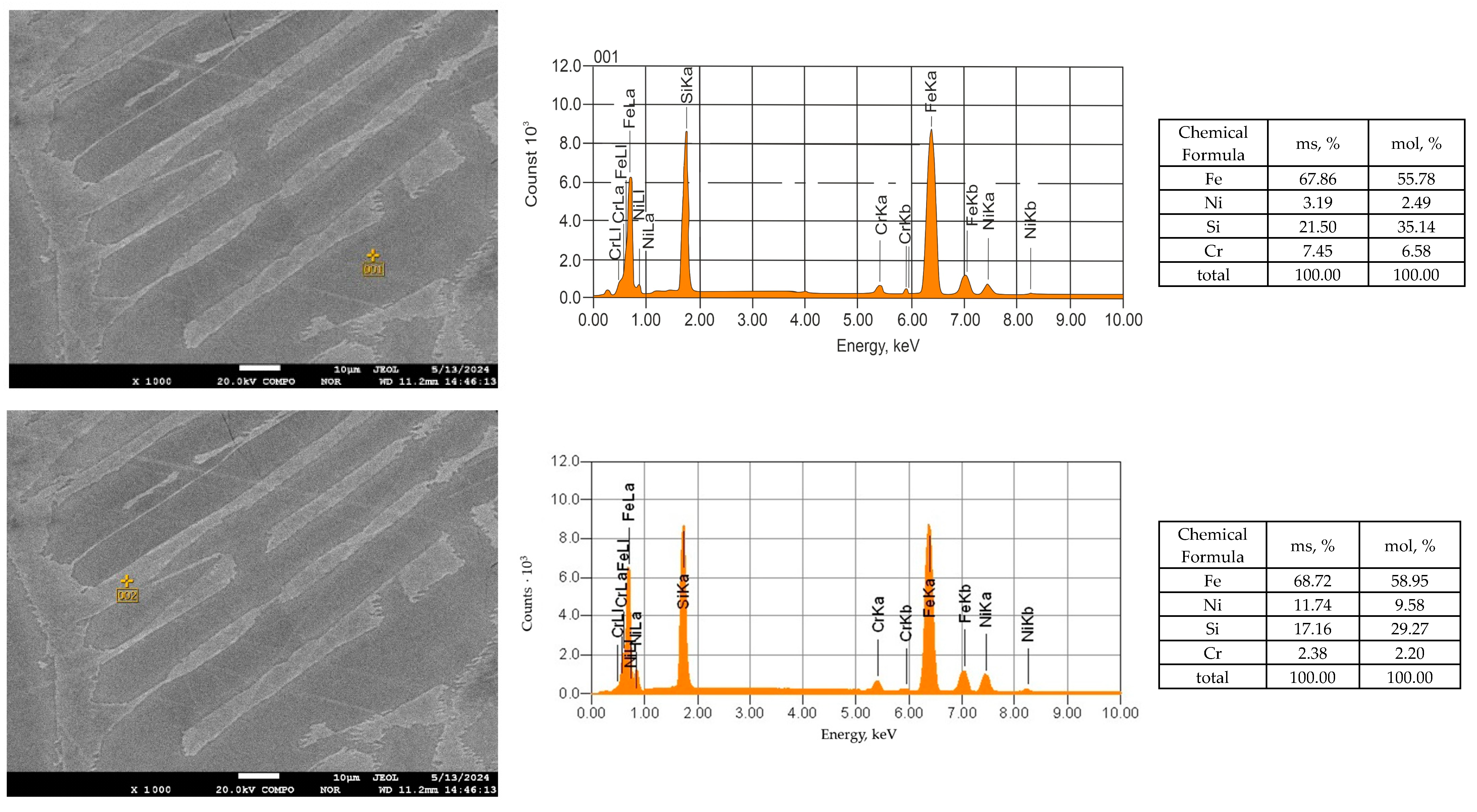
| Material | Content, % | ||||||||
| FC | Ash | Wp | V | SiO2 | Al2O3 | MgO | Fetot | Ptot | |
| Coke | 75.73 | 20.4 | 1.07 | 3.83 | |||||
| Coke Ash | 48.50 | 14.57 | 4.34 | 11.84 | 0.04 | ||||
| Coal | 55.28 | 9.24 | 5.44 | 37.71 | |||||
| Coal Ash | 51.80 | 32.54 | 3.12 | 3.99 | 0.14 | ||||
| Material | Content, % | ||||||||
| Crtot | Nitot | Fetot | CaO | SiO2 | Al2O3 | MgO | |||
| Nickel Ore | 0.30 | 1.05 | 19.62 | 0.46 | 42.83 | 4.39 | 7.07 | ||
| Lime | 1.15 | 90 | 1.96 | 3.10 | 3.27 | ||||
| Parameters | Units of Measurement | Indicators |
| Power Consumption | kW | 80 |
| Mains Voltage | V | 380 |
| Maximum Voltage on Furnace Busbars | V | 15 |
| Heating Time to Maximum Temperature | data | 0.5 |
| Overall Dimensions | ||
| Length | mm | 930 |
| Width | mm | 630 |
| Height | mm | 1000 |
| Oxides | NiO | Cr2O3 | SiO2 | FeO | Al2O3 | CaO | MgO |
|---|---|---|---|---|---|---|---|
| Reduced, % | 98 | 99 | 20 | 90 | 0 | 0 | 0 |
| Transition into slag, % | 2 | 1 | 80 | 10 | 100 | 100 | 100 |
| Element | Ni | Cr | Fe | Si | P | S |
|---|---|---|---|---|---|---|
| Transitions into Metal, % | 95 | 90 | 98 | 85 | 0 | 0 |
| Evaporates, % | 5 | 10 | 2 | 15 | 100 | 100 |
| Indicators | Options | ||
|---|---|---|---|
| I | II | III | |
| Reducer Ratio (Coke to Coal) | 100:0 | 75:25 | 50:50 |
| Material Consumption, g: | |||
| Nickel Ore | 100 | 100 | 100 |
| RK Coke | 7.193 | 5.93 | 4.39 |
| Shubarkol Coal | - | 1.98 | 4.39 |
| Lime | 24.22 | 24.22 | 24.22 |
| Basicity (CaO/SiO2) | 0.4 | 0.4 | 0.4 |
| Slag Ratio | 3.44 | 3.07 | 3.02 |
| Average Nickel Recovery, % | 85–92 | 88–91 | 85–88 |
| Material | Content, % | ||||||
|---|---|---|---|---|---|---|---|
| Alloy | Ni | Cr | Fe | Si | C | S | P |
| Sample 1 | 3.68 | 4.50 | 73.8 | 7.21 | 4.38 | 0.022 | 0.08 |
| Sample 2 | 3.2 | 4.4 | 62.50 | 21.58 | 2.51 | − | 0.021 |
| Sample 3 | 6.54 | 2.65 | 73.2 | 2.22 | 4.1 | 0.013 | 0.033 |
| Sample 4 | 3.21 | 3.40 | 70.57 | 24.90 | 0.60 | 0.005 | 0.021 |
| Sample 5 | 2.96 | 2.60 | 73.69 | 19.19 | 1.01 | 0.004 | 0.003 |
| Slag | NiO | Cr2O3 | FeO | SiO2 | Al2O3 | CaO | MgO |
| Sample 1 | 0.2 | 0.16 | 3.22 | 43.37 | 4.24 | 41.12 | 8.68 |
| Sample 2 | 0.069 | 0.54 | 4.62 | 42.25 | 3.61 | 5.27 | 14.88 |
| Sample 3 | 0.15 | 1.05 | 5.26 | 69.12 | 3.94 | 2.38 | 10.34 |
| Sample 4 | 0.102 | 0.58 | 7.72 | 77.00 | 2.48 | 1.76 | 10.09 |
| Sample 5 | 0.069 | 0.36 | 5.94 | 66.48 | 16.42 | 1.76 | 3.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdirashit, A.; Kelamanov, B.; Sariyev, O.; Yessengaliyev, D.; Abilberikova, A.; Zhuniskaliyev, T.; Kuatbay, Y.; Naurazbayev, M.; Nazargali, A. Study of Nickel–Chromium-Containing Ferroalloy Production. Processes 2025, 13, 1258. https://doi.org/10.3390/pr13041258
Abdirashit A, Kelamanov B, Sariyev O, Yessengaliyev D, Abilberikova A, Zhuniskaliyev T, Kuatbay Y, Naurazbayev M, Nazargali A. Study of Nickel–Chromium-Containing Ferroalloy Production. Processes. 2025; 13(4):1258. https://doi.org/10.3390/pr13041258
Chicago/Turabian StyleAbdirashit, Assylbek, Bauyrzhan Kelamanov, Otegen Sariyev, Dauren Yessengaliyev, Aigerim Abilberikova, Talgat Zhuniskaliyev, Yerbol Kuatbay, Magauiya Naurazbayev, and Alibek Nazargali. 2025. "Study of Nickel–Chromium-Containing Ferroalloy Production" Processes 13, no. 4: 1258. https://doi.org/10.3390/pr13041258
APA StyleAbdirashit, A., Kelamanov, B., Sariyev, O., Yessengaliyev, D., Abilberikova, A., Zhuniskaliyev, T., Kuatbay, Y., Naurazbayev, M., & Nazargali, A. (2025). Study of Nickel–Chromium-Containing Ferroalloy Production. Processes, 13(4), 1258. https://doi.org/10.3390/pr13041258






