Abstract
Shale oil is abundant in geological reserves, but its recovery rate is low due to its unique characteristics of ultra-low porosity, ultra-low permeability, and high clay content. This study investigated the effect of mixed-charge surfactants (PSG) on enhanced oil recovery (EOR) in high-temperature shale reservoirs, building on our previous research. The results indicate that PSG not only has outstanding interfacial activity, anti-adsorption, and high-temperature resistance but can also alter the wettability of shale. After aging at 150 °C for one month, a 0.2% PSG solution exhibited minimal influence on the viscosity reduction and oil-washing properties but significantly altered the oil/water interfacial tension (IFT). Compared to field water, the 0.2% PSG solution enhances the static oil-washing efficiency by over 25.85% at 80 °C. Moreover, its imbibition recovery rate stands at 29.03%, in contrast to the mere 9.84% of field water. Because of the small adhesion work factor of the PSG solution system, it has a strong ability to improve shale wettability and reduce oil/water IFT, thereby improving shale oil recovery. This study provides the results of a laboratory experiment evaluation for enhancing shale oil recovery with surfactants. Furthermore, it holds significant potential for application in the single-well surfactant huff-n-puff process within shale reservoirs.
1. Introduction
With the rapid development of society and the economy, the disparity between the supply and demand of oil and gas has become increasingly prominent [1]. At present, the significance of unconventional oil and gas sources, such as shale oil, heavy oil, and oil sands, is increasingly underscored [2,3]. Notably, China possesses abundant reserves of shale oil. According to statistics from Sinopec in 2019, China’s shale oil reserves are estimated at approximately 37.2 billion tons, with recoverable reserves of 4.39 billion tons after the United States and Russia, ranking third in the world [4,5]. However, it is noteworthy that the oil recovery rate is generally low (<10%) [6]. In contrast to the marine shale oil found in North America, China’s terrestrial shale oil is characterized by strong heterogeneity and poor compressibility, thus resulting in significant challenges to its exploration and development [7]. Over the past decade, shale oil has been discovered in multiple oil-bearing basins in China, and breakthroughs have been achieved successively in large-scale horizontal-well volume-fracturing technology in places such as Gulong, Jimusar, Longdong, Cangdong, Jiyang, etc. [8,9,10]. Nevertheless, the production of shale oil exhibits significant variability among different wells. Additionally, the energy within these wells diminishes rapidly following the initial fracturing development, leading to a corresponding decline in shale oil production [11,12]. Therefore, there is an urgent need to seek technologies for energy replenishment, production increase, and enhanced oil recovery.
In contrast to conventional reservoirs, the lithology of shale reservoirs is compact, and the nano-network is well-developed [13]. The application of waterflooding development, a common practice in conventional reservoirs, is particularly challenging in shale oil reservoirs because of nanoconfinement [14]. Hao et al. discovered that CO2 injections for the development of shale oil reservoirs are more effective than water injection development due to their operational simplicity and minimal formation damage [15]. However, gas channeling frequently occurs during gas injections [16]. Additionally, injecting CO2 can result in pipeline corrosion [17]. By contrast, numerous studies have revealed that surfactants can reduce the oil–water interfacial tension (IFT), thereby reducing capillary resistance [11,18,19]. Moreover, surfactants can also modify the wettability of the reservoir, thereby enhancing oil recovery [20]. Tu and Sheng et al. confirmed that surfactants with wettability alteration function while maintaining relatively high interfacial tension (IFT) are the best candidates to stimulate spontaneous imbibition by their experimental and simulation results [21]. Further, Xu et al. demonstrated that zwitterionic surfactant (i.e., cocoamidopropyl betaine, CAB) solutions significantly enhance the imbibition recovery of shale oil reservoirs, and the 1.167 mM CAB system exhibits the highest imbibition recovery rate at 25.6%, marking an increase of 18.69% compared to formation water [22].
Both laboratory and field tests at Bakken have confirmed that surfactant solutions do not present injectivity problems in shale reservoirs, in contrast to water injection [11,23]. Nevertheless, the temperature of shale oil reservoirs is typically higher than that of conventional oil reservoirs. In some cases, these temperatures can soar to a range of 150–160 °C [24]. This elevated temperature complicates the application of the surfactants or oil displacement agents traditionally used in conventional oil reservoirs, rendering them less effective in shale oil reservoirs. We hypothesize that a high-temperature-resistant surfactant system is developed for the huff-n-puff stimulation of shale oil wells. This could potentially improve the imbibition displacement efficiency of shale oil by reducing IFT and changing the wettability, thereby improving shale oil recovery. In our previous work, we prepared a catanionic surfactant (i.e., PSG) that can be used for the emulsification and reduction of viscosity for shale oil [25]. However, this applies only to the well lift and surface gathering of shale oil, and the effect of EOR in shale reservoirs is not clear. In this work, we conducted a comprehensive evaluation of this surfactant system for EOR, examining its interfacial properties, anti-adsorption performance, temperature resistance, emulsification performance, and oil-washing effect. Meanwhile, the mechanism of improving shale oil recovery by this system is analyzed in detail. This study is expected to provide theoretical and technical support for the efficient development of shale oil in the Bohai Bay Basin, located in East China.
2. Materials and Methods
2.1. Reagents and Materials
Petroleum ether (boiling range 90–120 °C, AR) was purchased from Tianjin Kemiou Chemical Reagent Co., Ltd, Tianjin, China. Three types of shale oil (GY152H and GY734H) were taken from the second member of the Kongdian Formation (EK2) of Cangdong Sag in PetroChina Dagang Oilfield, Tianjin, China and their physicochemical properties are shown in Table 1. The mixed-charge surfactant (i.e., PSG) was prepared according to the previously reported method [25]. Specifically, PSG is a pseudogemini surfactant assembled by the electrostatic interaction of cetyltrimethyl ammonium bromide (CTAB) and α-olefin sulfonate (AOS) at the molar ratio of 6:4 in the water phase; its critical micelle concentration (CMC) and hydrophilic–lipophilic balance (HLB) values are 0.4271 mmol/L and 13.8, respectively. The water used in the experiment was field water from the Dagang oilfield, Tianjin, Chian and its ion composition and content are shown in Table 2. Quartz sand (70–110 mesh) was purchased from Henan Minghai Environmental Protection Technology Co., Ltd., Zhengzhou, China.

Table 1.
Physicochemical properties of shale oil.

Table 2.
Composition and content of ions in field water from Dagang oilfield.
2.2. Interfacial Tension Measurement
The IFT between PSG surfactant solutions and shale oil was determined using a rotating drop interfacial tensiometer (701, CNG, Chicago, IL, USA). Initially, a 0.2% PSG surfactant solution was injected into the test tube by an injection syringe with a long needle, and then a small amount of shale oil was attached to the inner wall of the test tube using a needle tip. Following the installation of the test tube, the IFT was measured with a rotation speed of 5000 rpm at 90 °C for 30 min, and a picture was taken every minute. Finally, dynamic interfacial tension and equilibrium interfacial tension were obtained.
2.3. Anti-Adsorption Performance Measurement
The average content of clay minerals in the shale reservoir of the EK2 of Cangdong Sag in the Bohai Bay Basin is higher than 15%. The presence of clay minerals leads to the adsorption loss of surfactants, thereby reducing their application performance. For this purpose, the anti-adsorption performance of surfactants was evaluated by measuring the interfacial tension after adsorption according to the enterprise standard of China National Petroleum Group Co., Ltd., Beijing, China (Q/SY 17583-2018) [26] and the method of literature [27,28].
2.4. High-Temperature Resistance Evaluation Experiment
A 0.2% PSG surfactant solution was first prepared with field water and then sealed in a high-temperature and high-pressure reactor, whereafter it was placed in a 150 °C oven for one month. The oil–water IFT between the 0.2% PSG surfactant solution and shale oil was monitored for 1 day, 3 days, 5 days, 7 days, 10 days, 15 days, 20 days, and 30 days. Additionally, the emulsification and oil-washing performances of the surfactant solution after 30 days of aging were used to evaluate its temperature resistance.
2.5. Static Oil-Washing Experiment
According to the standard Q/SH 1020 2191-2021 [29], quartz sand and shale oil in the target block were first mixed at a mass ratio of 4:1 and then aged at a constant temperature in an oven at 80 °C for 7 days, stirring once a day to prepare the experimental oil sand. Next, 10 g of oil sand was weighed into a 100 mL jar, and the total mass of the jar and oil sand was accurately weighed (accurate to 0.001 g) and recorded as m1. Whereafter, 50 g of 0.2% surfactant solution was added to the jar, the glass plug was covered and stood in the oven at 80 °C for 48 h so that the shale oil in the oil sands could be fully washed out. Subsequently, the washed shale oil and surfactant solution were slowly removed, and the jar was dried in a 110 °C oven for 12 h. The jar was weighed and recorded as m2 after the fully dried moisture. Finally, the remaining shale oil in the oil sands was washed with petroleum ether, and the jar and cleaned sand were dried in a 110 °C oven for 12 h, weighed, and recorded as m3. Following the same procedure, field water was used as a blank control group. The oil-washing efficiency is calculated according to Equation (1).
where is the static washing efficiency, %; m1 and m2 are the total mass of the jar and oil sand before and after washing, respectively; and g. m3 is the total mass of the jar and cleaned sand after washing by petroleum ether, g.
2.6. Dynamic Imbibition Experiment
Initially, the shale core was cleaned and dried in an oven at 110 °C for 6 h, and then the core was weighed and recorded as m0. Subsequently, the core was placed into a high-temperature and high-pressure device and pressurized to 30 MPa at 80 °C to saturate the shale oil. After the core was aged in an oven at 80 °C for 7 days, the core with saturated shale oil was weighed and recorded as m1. The saturated shale oil core was then immersed in an Amott cell filled with 0.2% PSG surfactant solution, and the volume of shale oil after 0.5 h, 1 h, 2 h, 6 h, 9 h, 12 h, 24 h, 36 h, 48 h, 60 h, and 72 h were recorded at 80 °C, respectively. Furthermore, field water was used as a blank control group. The imbibition efficiency is calculated according to Equation (2).
where is the dynamic imbibition efficiency, %; ρ is the density of shale oil, g/cm3; Vt is the volume of shale oil imbibed, mL; and m0 and m1 are the core masses before and after saturated shale oil, g.
2.7. Wettability Measurement
The first step is sample treatment. First, the natural core was cut into an initial core with a diameter of 2.5 cm and a height of 1 cm, and the surface was polished and smooth. Subsequently, the initial core was dried in an oven at 110 °C with constant weight and marked as the 0# core. Then, the three initial cores were immersed in shale oil, field water, and 0.2% PSG surfactant solution at 80 °C for 48 h to make them fully imbibition. Their surfaces were wiped dry after removal and labeled as the 1#, 2#, and 3# cores, respectively. The second step is wettability measurement. The contact angles between the field water and four cores were measured by the automatic contact angle measuring instrument (OCA50, Dataphysics, Stuttgart, Germany). First, 10 μL of water was dropped onto the core surface at room temperature through a micro-syringe, and then photos were taken after contact between the water droplet and the core for 5 s. Finally, the contact angle was calculated by using the software of the instrument.
3. Results and Discussion
3.1. Interfacial Tension Evaluation
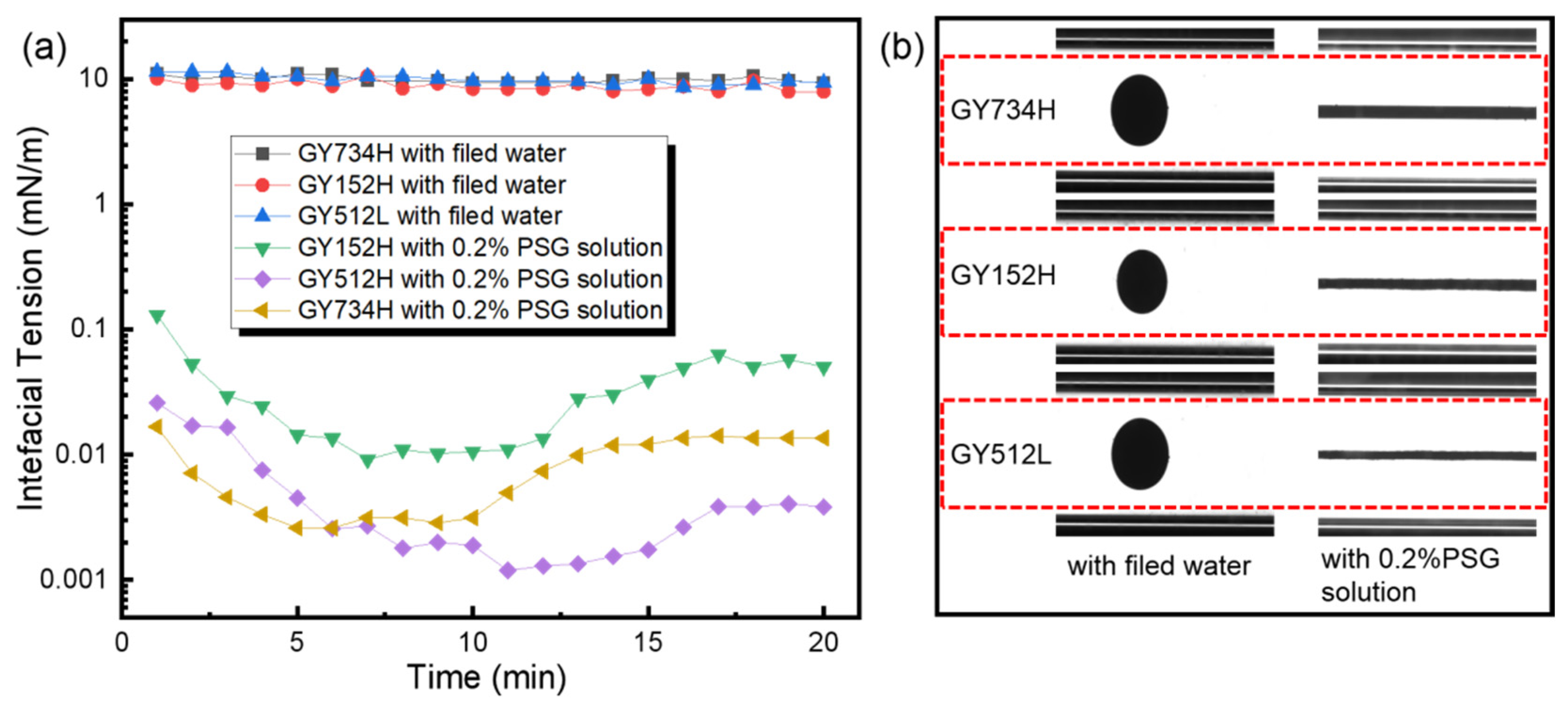
The capacity of surfactants to reduce the IFT significantly impacts emulsification and the reduction in capillary force [30]. Here, the IFT between shale oil and water in the presence of PSG is observed. As shown in Figure 1a, the IFT between field water and the three different shale oils does not change significantly with the increase in test time, and their IFTs are close to 10 mN/m. However, after adding 0.2% PSG, the IFT decreased significantly and showed a trend of decreasing first and then increasing steadily with the increase in test time. The trend of the IFT can be attributed to the dynamic adsorption and rearrangement of surfactant molecules at the oil/water interface. Initially, PSG molecules rapidly migrate to the interface, reducing the IFT by lowering the interfacial energy through adsorption. However, the adsorption of PSG at the oil/water interface reaches saturation over time. Whereafter, excess PSG molecules form micelles in the bulk phase rather than further populating the interface, leading to a depletion of free surfactants available for interfacial activity. Eventually, the interfacial tension increases slightly. Moreover, the IFTs of the three shale oils were below 0.01 mN/m at 7 min. These results indicate that the PSG has strong interfacial activity and is beneficial in reducing capillary resistance. Furthermore, the extent to which 0.2%PSG reduces oil–water interfacial tension is different for these three shale oils. After 10 min, the IFT between 0.2%PSG solution and shale oil is successively GY152H > GY734H > GY512L. This difference is related to the composition of shale oil and its interaction with surfactants. As shown in Table 1, the wax contents of GY152H, GY734H, and GY512L are 30.49%, 23.54%, and 20.24%, respectively. That is to say, the content of non-polar components (e.g., wax) in GY512L was lower than that in GY152H. Conversely, the content of polar components (e.g., aromatic hydrocarbons, asphaltenes, etc.) in GY512L was higher than that in GY152H. These polar components enhance surfactant adsorption at the oil/water interface, facilitating micelle formation or monolayer packing, thereby lowering interfacial energy [31]. Therefore, the IFT between GY512H and the 0.2% PSG solution becomes the lowest. Additionally, as shown in Figure 1b, in the field water without 0.2% PSG, the oil droplets are ellipsoid during the process of interfacial tension test due to the high IFT, which is not conducive to passing through the shale pore throats and micro-fractures. However, in the field water with 0.2% PSG, the oil droplets are easily deformed and elongated in the test process, which is conducive to the oil droplets passing through the shale pore throat and micro-fractures and can effectively peel the crude oil on the shale surface, thereby improving oil recovery.
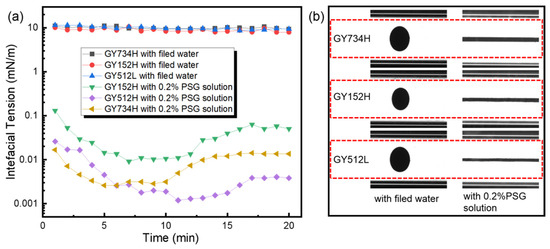
Figure 1.
(a) Dynamic interfacial tension between 0.2% PSG solution and shale oil, and (b) images of oil droplets after 10 min for interfacial tension measurement.
3.2. Anti-Adsorption Performance
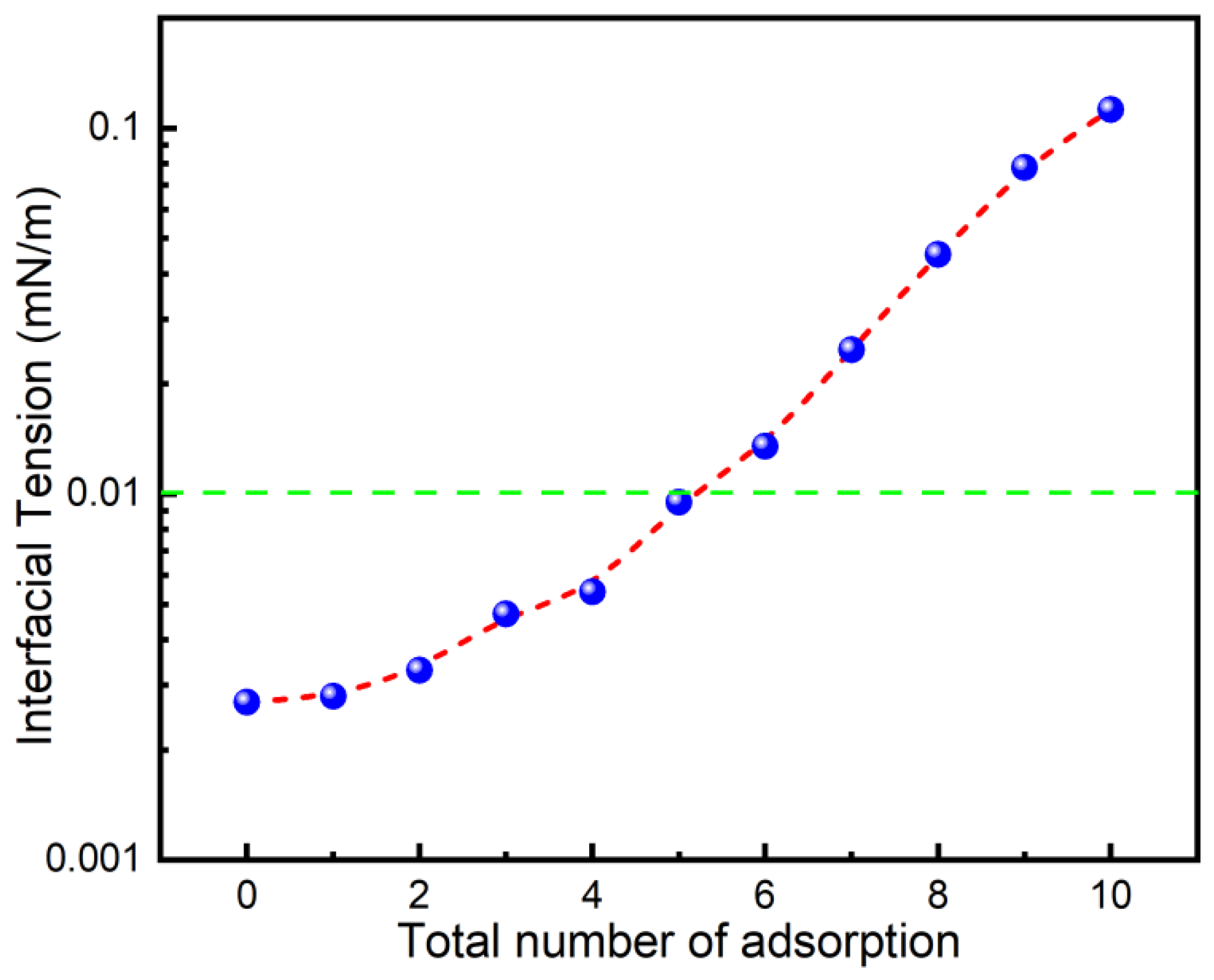
In the injection of surfactant huff-n-puff or the flooding process, the adsorption loss of surfactant on rock is one of the main reasons for the low oil recovery [32,33]. This is particularly significant in shale reservoirs with high mudstone content [5]. To understand whether the IFT of the 0.2% PSG solution and shale oil after adsorption onto oil sands can still reach the desired levels, the IFT was used to evaluate the anti-adsorption capability of PSG surfactant. As shown in Figure 2, the IFT between the PSG solution and shale oil gradually increases with increasing adsorption times, indicating that the adsorption amount of PSG molecules on the surface of the oil sand gradually increases, resulting in a decrease in the effective concentration of PSG in the solution. Interestingly, the IFT was still lower than 0.01 mN/m after five adsorptions, revealing that PSG surfactants had strong anti-adsorption properties. Additionally, it should be noted that a small amount of PSG adsorption on the mineral surface is conducive to changing the shale wettability.
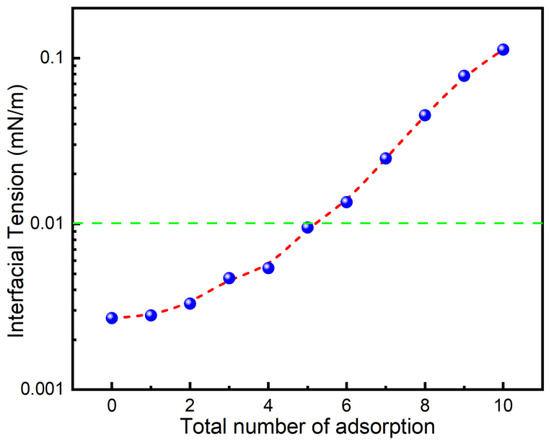
Figure 2.
Interfacial tension between 0.2% PSG solution and shale oil after multiple adsorptions on oil sand at 80 °C.
3.3. Static Oil-Washing Efficiency
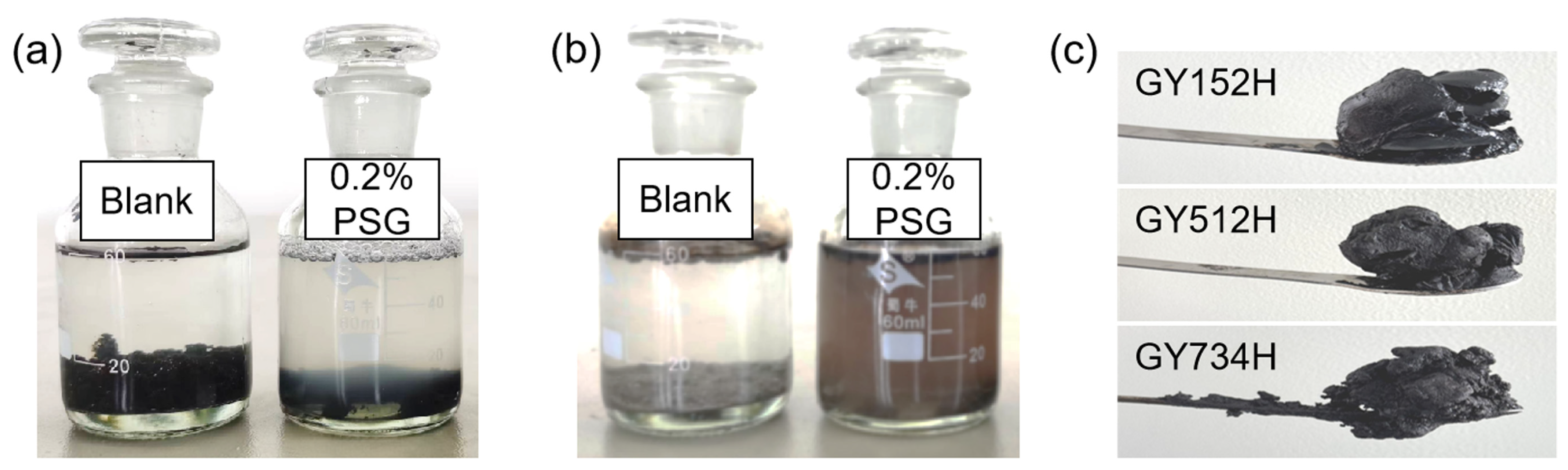
Static oil-washing efficiency is one of the important ways to evaluate the application of surfactants in EOR. The results of static washing efficiency before and after adding 0.2% PSG surfactants are listed in Table 3. Generally, field water contains a substantial quantity of inorganic salts. The total salinity of the field water used in this experiment is 27,119 mg/L, with a total calcium and magnesium ion content of 333 mg/L (Table 2). In the absence of PSG, the static washing efficiency of the GY152H, GY512L, and GY734H oil sands is 31.15%, 26.34%, and 21.63% by using the field water, respectively. In the presence of 0.2% PSG, the static oil-washing efficiency increased to 66.24%, 55.67%, and 47.48%, with an increase of more than 25%. In addition, as shown in Figure 3a, the solution in the jar was colorless and transparent with no floating oil phase at the beginning, while the black shale oil floated on the upper layer of the solution, and the system solution was brown after adding 0.2% PSG and standing at 80 °C for 48 h (Figure 3b), indicating that a small amount of shale oil was solubilized into the solution. These results indicate that PSG can significantly improve the oil-washing efficiency of shale oil, regardless of whether PSG surfactant is added to field water or not. Additionally, the viscosities of GY152H, GY512L, and GY734H shale oil at 50 °C are 143 mPa·s, 726 mPa·s, and 2220 mPa·s, respectively (Table 1), and their appearance images are shown in Figure 3c. The higher the viscosity of shale oil, the lower the washing efficiency of the corresponding oil sand because the high viscosity reduces the mass transfer efficiency of the system. A similar phenomenon was observed in our study on the surfactant-enhanced sludge-washing process [34]. Our research has determined that heavy oils with a high viscosity result in the production of oily sludge with a high viscosity, and the corresponding efficiency of chemical washing using surfactant is also reduced.

Table 3.
Comparative results of static oil-washing efficiency.

Figure 3.
Images of the systems before and after washing oil for oil sand (GY734): (a) at the beginning and (b) after 48 h. (c) Appearance images of shale oils.
3.4. High-Temperature Resistance
In the EK2 of Cangdong Sag in Bohai Bay Basin, the temperature of the shale reservoir is between 120 °C and 150 °C [24,35]. Such elevated temperatures may cause the surfactant to be inactivated or even decomposed [36]. Therefore, it is very vital to evaluate the temperature resistance of the surfactants used in enhanced shale oil recovery. Here, we used GY734H shale oil as a representative example to study the temperature resistance of PSG surfactants. The main performance indexes of the 0.2% PSG solution after aging at 150 °C for 1 month are listed in Table 4. The results show that the IFT increases from 0.0027 mN/m to 0.0316 mN/m after aging in a high-temperature environment, but the emulsification performance and oil-washing efficiency are not affected. In addition to 98.60% before aging, the emulsification and viscosity reduction rate after aging is still 95.41%. In addition, at 80 °C for 2 h, the demulsification rate before aging was 88.75%, while the demulsification rate after aging was 92.25%. Compared to 47.48% before aging, the static oil-washing efficiency after aging increased slightly to 48.54%, which may be caused by errors. In brief, these results indicate that the surfactant PSG has excellent temperature resistance and can be applied to the high-temperature environment of shale reservoirs.

Table 4.
Comparison of performance indexes of 0.2% PSG solution before and after aging at 150 °C for 1 month.
3.5. Wettability Analysis
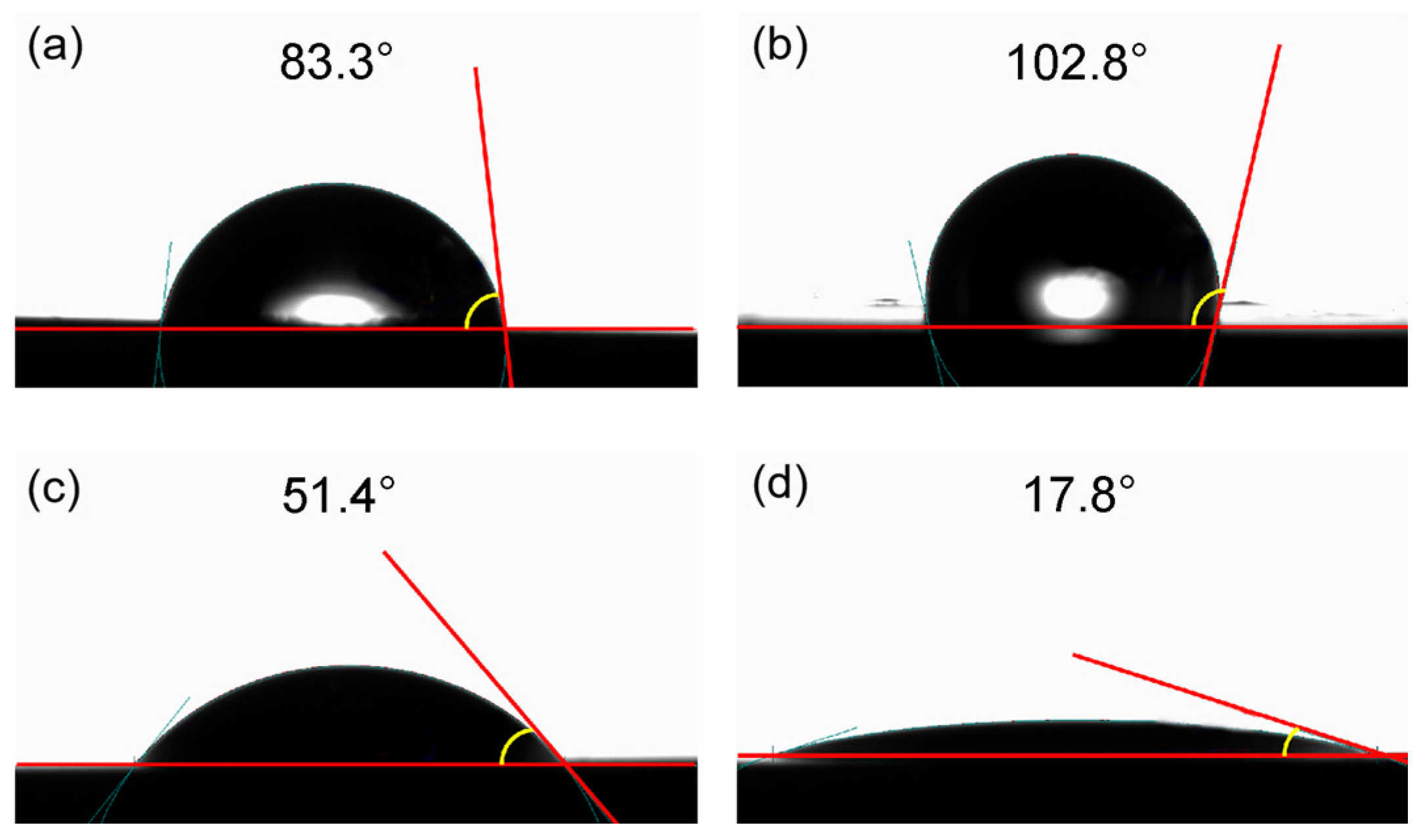
The wetting inversion of the shale surface is a significant factor that influences EOR efficiency. Shale reservoirs are usually neutral or oil-wet due to the strong boundary effect from the pore wall to the pore throat [37]. To solve this problem, several researchers have found that surfactants can alter shale wettability from oil-wet to water-wet [22,32,33,38,39]. The water contact angle can often be used to evaluate wettability. Specifically, the contact angles indicated water-wet conditions at 0°–75°, neutral wetting at 75°–105°, and oil wetting at 105°–180° [40]. As shown in Figure 4a, we observed that the contact angle between the initial shale core and water is 83.3°, showing a neutral wetting state. After immersing the core in shale oil at 80 °C for 24 h, the contact angle increases to 102.8° (Figure 4b), indicating a significant increase in hydrophobicity and showing an oil-wet state. After soaking the core in field water at 80 °C for 24 h, the contact angle decreases to 51.4° (Figure 4c), suggesting a slight increase in hydrophilicity and a weak water-wet state. After immersing the core in 0.2% PSG solution at 80 °C for 24 h, the contact angle decreased to 17.8° (Figure 4d), indicating a significant enhancement in hydrophilicity, thereby showing a strong water-wet state. These results show that PSG can significantly alter the wettability of shale, transforming it from neutral wet to strong water-wet, which is beneficial for improving shale oil recovery.
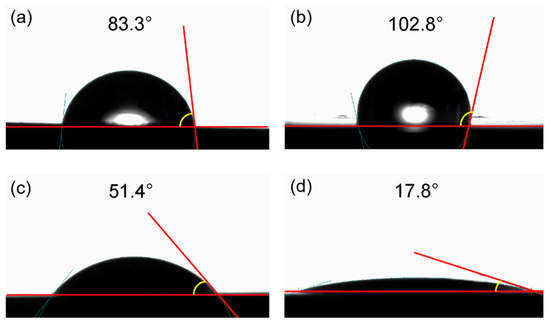
Figure 4.
Effect of PSG on shale surface wettability: (a) initial shale core and (b–d) cores after soaking in shale oil, field water, and 0.2% PSG solution.
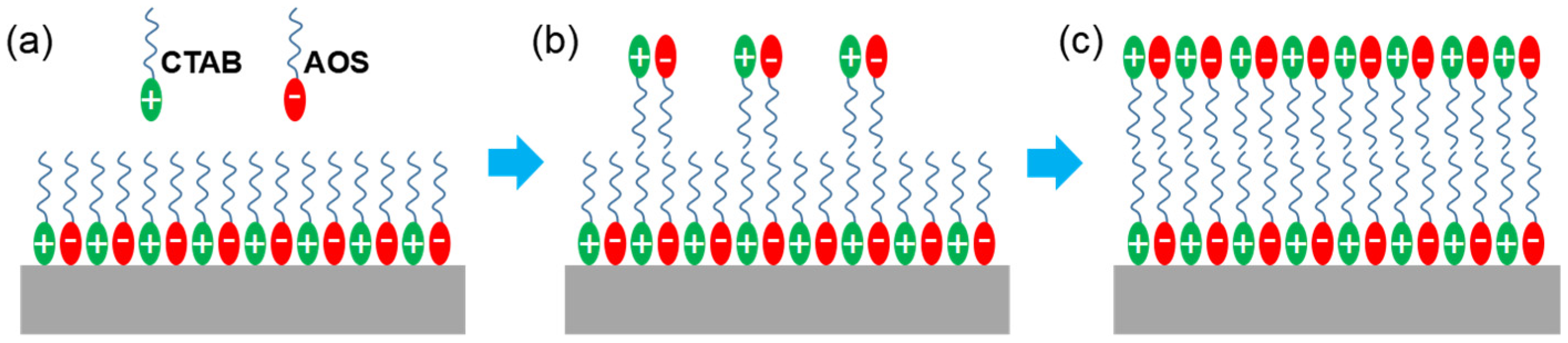
The shale reservoir of the EK2 in Cangdong Sag contains many clay minerals, which are typically laminated shale [41], which results in a negatively charged surface. The PSG surfactant is assembled by CTAB and AOS in the aqueous phase at a molar ratio of 6:4 [25]. The head group of PSG can generate a weak electrostatic interaction with the shale surface, which, over time, results in its adsorption to form the first adsorption layer (Figure 5a). With an increase in soaking time, a secondary adsorption layer can be formed through a hydrophobic interaction between the tail chains (Figure 5b). Eventually, the hydrophilic head group is exposed to the outermost layer (Figure 5c), resulting in a strong water-wet state on the shale surface.
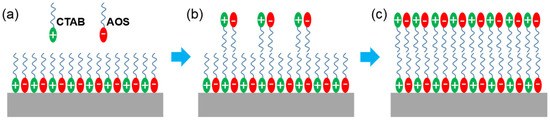
Figure 5.
Mechanism of PSG changing wettability. (a) The first adsorption layer formed by weak electrostatic interaction with the shale surface, (b) the secondary adsorption layer over time, and (c) the secondary adsorption layer formed by hydrophobic interaction between the tail chains.
3.6. Imbibition Efficiency
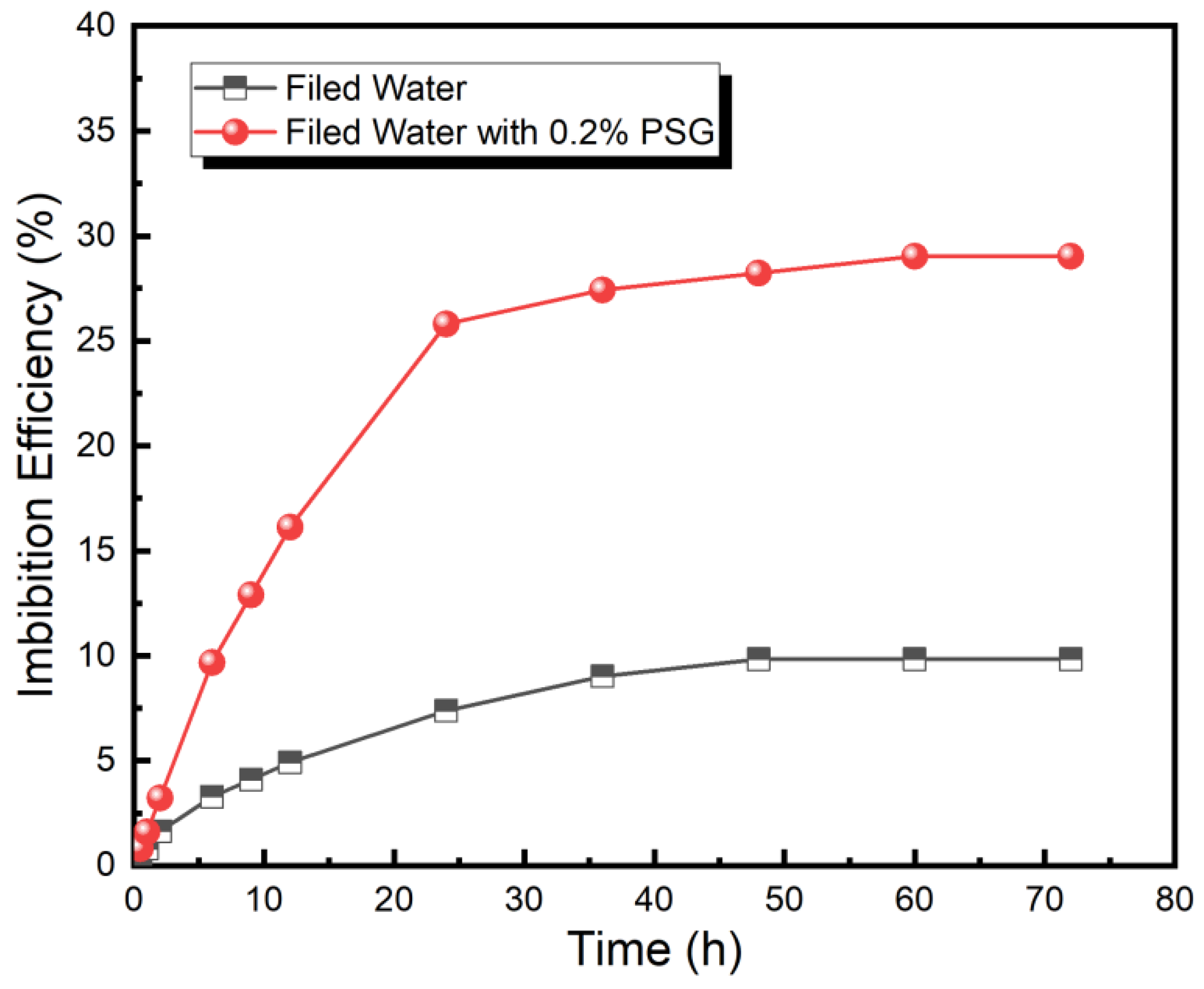
For shale oil reservoirs, the PSG surfactant has demonstrated an ability to reduce interfacial tension, strong anti-adsorption, excellent high-temperature resistance, improve wettability, and enhance static oil-washing efficiency, thus laying a foundation for its application in enhancing shale oil recovery. Thereafter, the EOR efficiency of the PSG surfactant was investigated using the spontaneous imbibition tests. As shown in Figure 6, the imbibition process is divided into three stages. The first stage (0–24 h) is a rapid imbibition stage, and the imbibition efficiency increases rapidly with the increasing imbibition time. The second stage (24–48 h) is the slow imbibition stage, and the imbibition efficiency increases slowly with increasing imbibition time. The third stage (48–72 h) is the equilibrium imbibition stage, and the imbibition efficiency reaches equilibrium with an increase in imbibition time. After imbibition at 80 °C for 72 h, the imbibition efficiency of the 0.2% PSG solution reached 29.03%, compared to only 9.84% for field water. Furthermore, during the imbibition whole process, the imbibition efficiency of the 0.2% PSG solution is always higher than that of field water, indicating that the PSG surfactant can effectively enhance imbibition efficiency and thus improve the recovery of shale oil.
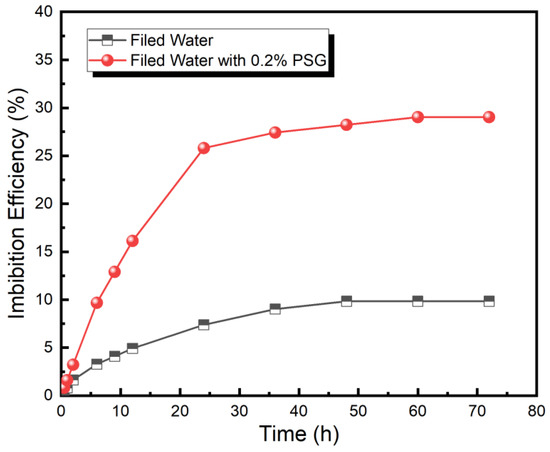
Figure 6.
Comparison of dynamic imbibition efficiency of field water and 0.2% PSG solution.
3.7. Mechanism Analysis of Enhanced Oil Recovery
To evaluate the effect of surfactants on the enhanced oil recovery of shale oil, two of the direct performance indexes are the oil-washing efficiency of the shale surface and the imbibition efficiency of the shale matrix. Generally, both the wetted and non-wetted phases in the core are predominantly affected by capillary forces and gravity during the imbibition process [22]. The shale reservoir of the EK2 in Cangdong Sag has the characteristics of high temperature, ultra-low porosity, and ultra-low permeability [35]. The effect of capillary force is significantly greater than that of gravity because of the nanoconfinement effect for this reservoir [14]. Therefore, capillary force is the primary driving force in the process of enhanced shale oil recovery via imbibition. For shale reservoirs, a surfactant system with excellent performance should have the advantages of reducing the interfacial tension between oil and water and improving the wettability of the rock surface. The combined effects of the surfactant system on these two aspects can be evaluated by the adhesion work factor (Equation (3)) [42].
where Ws and Ww are the adhesion work of shale oil on a shale surface in a surfactant solution/shale oil/shale system and field water/shale oil/shale system, respectively, mJ. Eσ is the interfacial tension factor, and Eθ is the wettability factor. σs is the interfacial tension between the surfactant solution and shale oil, and σw is the interfacial tension between field water and shale oil, mN/m. θs and θw are the contact angles of the surfactant solution and field water on the shale surface, °.
The adhesion work factor (E) of the surfactant solution reveals its capability for imbibition oil displacement [43]. As shown in Table 5, the adhesion work of the 0.2% PSG solution system is significantly lower than that of field water. Compared to field water, the E of the 0.2% PSG solution is only 3.44 × 10−5. The low adhesion work suggests that the PSG solution system facilitates the easy detachment of shale oil from the shale surface, thereby enhancing oil-washing efficiency. A small E value indicates the strong capacity of the PSG solution system to improve wettability and reduce interfacial tension between the shale oil and water, resulting in a high ultimate imbibition recovery.

Table 5.
Comparison of imbibition effect factors of 0.2% PSG solution and field water on GY734H shale oil.
4. Conclusions
In the present study, we built on previous work to investigate the effects of mixed-charge surfactants on enhanced oil recovery in high-temperature shale reservoirs. The results show that PSG surfactants exhibit excellent interfacial activity, significantly reducing the oil/water IFT of three shale oils in the Dagang oilfield from 10 mN/m to less than 0.01 mN/m. Additionally, this surfactant has strong anti-adsorption and excellent high-temperature resistance. After five times of adsorption onto oil sand, the IFT between the PSG solution and shale oil is lower than 0.01 mN/m, while the IFT exceeds 0.1 mN/m after 10 times of adsorption. Moreover, after aging at 150 °C for one month, the 0.2% PSG solution has minimal influence on the viscosity reduction, demulsification, and static oil-washing properties, but the oil/water IFT increases by an order of magnitude. Furthermore, PSG surfactants can alter shale wettability, reducing the contact angle between shale and water from 83.3° to 17.8°, thereby achieving strong water-wetting. Compared to field water, using a 0.2% PSG solution can improve the static oil-washing efficiency by more than 25.85% at 80 °C. The static imbibition experiment shows that the imbibition recovery rate of a 0.2% PSG solution is 29.03%, while the field water is only 9.84%. Finally, the EOR mechanism is explained by the adhesion work factor. This work lays a theoretical and experimental foundation for PSG surfactants in enhancing oil recovery, and it is expected to play an important role in the single-well surfactant huff-n-puff in shale reservoirs.
Author Contributions
Conceptualization, X.W.; methodology, Q.L.; software, Y.T.; formal analysis, X.Z.; investigation, Q.L., Y.T. and N.Z.; resources, H.G., X.W., X.Z. and D.L.; data curation, Q.L.; writing—original draft preparation, Q.L.; writing—review and editing, Q.L.; visualization, Y.Z.; supervision, X.W., H.G. and D.L.; project administration, Q.L., Y.Z. and X.W.; funding acquisition, H.W. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the CNPC Major Science and Technology Project (No.2023ZZ15YJ04), the Post-doctoral Project of PetroChina Dagang Oilfield Company (No.2023BO59), and the Funding Project of Tianjin Post-doctoral Innovation Post (No.2024072061).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the Unconventional Research Institute of China University of Petroleum (Beijing) for their testing support of some of the experiments.
Conflicts of Interest
Authors Qi Li, Xiaoyan Wang, Hongjiang Ge, Xiaoyu Zhou, Dongping Li, Haifeng Wang, Nan Zhang, Yang Zhang and Wei Wang were employed by the PetroChina Dagang Oilfield Company. The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PSG | Mixed-charge surfactants, i.e., a pseudogemini surfactant |
| IFT | Interfacial tension |
| EOR | Enhancing oil recovery |
| CAB | Cocoamidopropyl betaine |
| EK2 | The second member of Kongdian Formation of Cangdong Sag |
| CTAB | Cetyltrimethyl ammonium bromide |
| AOS | α-olefin sulfonate |
| CNPC | China National Petroleum Corporation |
References
- Wang, M.; Guo, Z.; Jiao, C.; Lu, S.; Li, J.; Xue, H.; Li, J.; Li, J.; Chen, G. Exploration progress and geochemical features of lacustrine shale oils in China. J. Pet. Sci. Eng. 2019, 178, 975–986. [Google Scholar] [CrossRef]
- Zou, C.; Pan, S.; Jing, Z.; Gao, J.; Yang, Z.; Wu, S.; Zhao, Q. Shale oil and gas revolution and its impact. Acta Pet. Sin. 2020, 41, 1–12. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Blackbourn, G.; Ma, F.; He, Z.; Wen, Z.; Wang, Z.; Yang, Z.; Luan, T.; Wu, Z. Heavy oils and oil sands: Global distribution and resource assessment. Acta Geol. Sin.-Engl. Ed. 2019, 93, 199–212. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, M.; Xu, L.; Du, S.; Liu, Y.; Yang, E.; Shan, J. Study on oil extraction characteristics in micropores of a typical terrestrial shale reservoir in China by CO2 injection and surfactant imbibition. Energy Fuels 2024, 38, 6927–6937. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S.; Liu, Y.; Liu, D.; Gao, M.; Tian, Y.; Wang, J. A review on shale oil and gas characteristics and molecular dynamics simulation for the fluid behavior in shale pore. J. Mol. Liq. 2023, 376, 121507–121541. [Google Scholar] [CrossRef]
- Xia, Z.; Yin, H.; Wang, X.; Xu, G. Surfactant slug assisting CO2 huff and puff in enhancing shale oil reservoir recovery. Phys. Fluids 2024, 36, 16601–16610. [Google Scholar] [CrossRef]
- Lei, Q.; Weng, D.; Guan, B.; Shi, J.; Cai, B.; He, C.; Sun, Q.; Huang, R. Shale oil and gas exploitation in China: Technical comparison with US anddevelopment suggestions. Pet. Explor. Dev. 2023, 50, 824–831. [Google Scholar] [CrossRef]
- Zhao, Z.; Bai, B.; Liu, C.; Wang, L.; Zhou, H.; Liu, Y. Current status, advances, and prospects of CNPC’s exploration of onshoremoderately to highly mature shale oil reservoirs. Oil Gas Geol. 2024, 45, 327–340. [Google Scholar] [CrossRef]
- Jia, C.; Wang, Z.; Jiang, L.; Zhao, W. Progress and key scientific and technological problems of shale oil exploration and development in China. World Pet. Ind. 2024, 31, 1–11. [Google Scholar] [CrossRef]
- Zou, C.; Ma, F.; Pan, S.; Zhang, X.; Wu, S.; Fu, G.; Wang, H.; Yang, Z. Formation and distribution potential of global shale oil and the developments of continental shale oil theory and technology in China. Earth Sci. Front. 2023, 30, 128–142. [Google Scholar] [CrossRef]
- Saputra, I.W.R.; Park, K.H.; Zhang, F.; Adel, I.A.; Schechter, D.S. Surfactant-assisted spontaneous imbibition to improve oil recovery on the Eagle Ford and Wolfcamp shale oil reservoir: Laboratory to field analysis. Energy Fuels 2019, 33, 6904–6920. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Cao, H.; Wu, J.; Wang, F.; Wang, Y. The Crack Propagation Behaviour of CO2 Fracturing Fluid in Unconventional Low Permeability Reservoirs: Factor Analysis and Mechanism Revelation. Processes 2025, 13, 159. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Y.; Yu, X.; Wang, C.; Yao, B.; Wang, S.; Winterfeld, P.H.; Wang, X.; Yang, Z.; Wang, Y.; et al. Advances in improved/enhanced oil recovery technologies for tight and shale reservoirs. Fuel 2017, 210, 425–445. [Google Scholar] [CrossRef]
- Liu, B.; Lei, X.; Feng, D.; Ahmadi, M.; Wei, Z.; Chen, Z.; Jiang, L. Nanoconfinement effect on the miscible behaviors of CO2/shale oil/surfactant systems in nanopores: Implications for CO2 sequestration and enhanced oil recovery. Sep. Purif. Technol. 2025, 356, 129826–129838. [Google Scholar] [CrossRef]
- Hao, Y.; Wu, Z.; Chen, Z.; Li, L.; Sun, Y.; Liu, R.; Guo, F. The characteristics and effects of Huff-n-Puff in shale with brine, aqueous surfactant solutions and CO2. J. CO2 Util. 2024, 79, 102655–102667. [Google Scholar] [CrossRef]
- Yao, C.; Zhao, J.; Ji, Z.; Cao, M.; Xu, L.; Ma, Y. Effect of CO2 Huff-n-Puff mode with a horizontal well on shale oil recovery: A three-dimensional experimental study. Energy Fuels 2023, 37, 9318–9328. [Google Scholar] [CrossRef]
- Cosultchi, A.; Rossbach, P.; Hernandez-Calderon, I. XPS analysis of petroleum well tubing adherence. Surf. Interface Anal. 2003, 35, 239–245. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, J.J. Experimental investigation of surfactant enhanced spontaneous imbibition in Chinese shale oil reservoirs using NMR tests. J. Ind. Eng. Chem. 2019, 72, 414–422. [Google Scholar] [CrossRef]
- Alizadehmojarad, A.A.; Fazelabdolabadi, B.; Vuković, L. Surfactant-controlled mobility of oil droplets in mineral nanopores. Langmuir 2020, 36, 12061–12067. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, J.J.; Wang, X.; Ge, H.; Yao, E. Experimental study of wettability alteration and spontaneous imbibition in Chinese shale oil reservoirs using anionic and nonionic surfactants. J. Pet. Sci. Eng. 2019, 175, 624–633. [Google Scholar] [CrossRef]
- Tu, J.; Sheng, J.J. Experimental and numerical study of surfactant solution spontaneous imbibition in shale oil reservoirs. J. Taiwan Inst. Chem. Eng. 2020, 106, 169–182. [Google Scholar] [CrossRef]
- Xu, N.; Wang, Y.; Zhang, C.; Bai, B.; Li, D.; Zhang, Y.; Shi, W.; Ding, W. Effect of surfactants on the interface characteristics and imbibition processes in shale oil reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2025, 706, 135818–135828. [Google Scholar] [CrossRef]
- Pospisil, G.; Griffin, L.; Souther, T.; Strickland, S.; McChesney, J.; Pearson, C.M.; Dalkhaa, C.; Sorensen, J.; Hamling, J.; Kurz, B.; et al. East Nesson Bakken enhanced oil recovery pilot: Coinjection of produced gas and a water-surfactant mixture. In Proceedings of the 10th Unconventional Resources Technology Conference, URTEC-3722974-MS, Houston, TX, USA, 20–22 June 2022. [Google Scholar] [CrossRef]
- Zhao, W.; Bian, C.; Li, Y.; Liu, W.; Qin, B.; Pu, X.; Jiang, J.; Liu, S.; Guan, M.; Dong, J.; et al. “Component flow” conditions and its effects on enhancing production of continental medium-to-high maturity shale oil. Pet. Explor. Dev. 2024, 51, 720–730. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Li, D.; Ge, H.; Han, X.; Xue, E. Catanionic surfactant systems for emulsifying and viscosity reduction of shale oil. Energies 2024, 17, 5780. [Google Scholar] [CrossRef]
- Q/SY 17583-2018; Specifications of Surfactants Used in SP Binary Combination Flooding. China National Petroleum Group Co., Ltd.: Beijing, China, 2018.
- Cheng, J.; Wu, J.; Wu, H.; Liu, X.; He, J.; Jia, S. Alkali-free three-component emulsification flooding system. Acta Pet. Sin. 2023, 44, 636–646. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, X.; Zhao, O.; Huang, J.; Liu, C.; Zhao, B. Mixed cationic and anionic surfactant systems achieve ultra-low interfacial tension in the Karamay oil field. Acta Phys.-Chim. Sin. 2014, 30, 693–698. [Google Scholar] [CrossRef]
- Q/SH 1020 2191-2021; Technical Requirements of Surfactants for Oil Displacement. Sinopec Shengli Petroleum Administration Bureau: Dongying, China, 2021.
- Sheng, J.J. What type of surfactants should be used to enhance spontaneous imbibition in shale and tight reservoirs? J. Pet. Sci. Eng. 2017, 159, 635–643. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Lu, G.; Mu, M.; Liu, H.; Guo, S.; Tang, X.; Zhang, Y. Sodium fatty alcohol polyoxyethylene ether carboxylate/cationic surfactant binary system for high-salt oil reservoir. J. Surfactants Deterg. 2023, 26, 843–852. [Google Scholar] [CrossRef]
- Duan, Y.; Li, Y.; Chen, B.; Ai, C.; Wu, J. Preparation and performance evaluation of a novel temperature-resistant anionic/nonionic surfactant. Sci. Rep. 2024, 14, 5710–5720. [Google Scholar] [CrossRef]
- Li, Q.; Sun, D.; Hua, J.; Jiang, K.; Xu, Z.; Tong, K. Enhancing low-temperature thermal remediation of petroleum sludge by solvent deasphalting. Chemosphere 2022, 304, 135278–135287. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, C.; Yang, F.; Cui, Y.; Song, S.; Guan, Q.; Zhou, F. Research and breakthrough of benefit shale oil development in Cangdong Sag, Bohai Bay Basin. China Pet. Explor. 2023, 28, 24–33. [Google Scholar] [CrossRef]
- Hou, B.; Jia, R.; Fu, M.; Wang, Y.; Ma, C.; Jiang, C.; Yang, B. A novel high temperature tolerant and high salinity resistant gemini surfactant for enhanced oil recovery. J. Mol. Liq. 2019, 296, 112114–112122. [Google Scholar] [CrossRef]
- Saputra, I.W.R.; Adebisi, O.; Ladan, E.B.; Bagareddy, A.; Sarmah, A.; Schechter, D.S. The influence of oil composition, rock mineralogy, aging time, and brine pre-soak on shale wettability. ACS Omega 2022, 7, 85–100. [Google Scholar] [CrossRef]
- Torabi, F.; Gandomkar, A. Experimental evaluation of CO2-soluble nonionic surfactants for wettability alteration to intermediate CO2-oil wet during immiscible gas injection. SPE J. 2024, 29, 5071–5086. [Google Scholar] [CrossRef]
- Liu, R.; Liu, R.; Shi, J.; Zhao, K.; Chu, Y.; Du, D. Interfacial properties and efficient imbibition mechanism of anionic–nonionic surfactants in shale porous media. Energy Fuels 2023, 37, 11955–11968. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, J.J.; Tu, J. Effect of spontaneous emulsification on oil recovery in tight oil-wet reservoirs. Fuel 2020, 279, 118456–118466. [Google Scholar] [CrossRef]
- Zhao, X.; Pu, X.; Jin, F.; Chen, C.; Shi, Z.; Chai, G.; Han, W.; Jiang, W.; Guan, Q.; Zhang, W.; et al. Enrichment law and favorable exploration area of shale-type shale oil in Huanghua depression. Acta Pet. Sin. 2023, 44, 158–175. [Google Scholar] [CrossRef]
- Li, J. The Effect of Surfactant System on Imbibition Bahavior. Ph.D. Thesis, China University of Petroleum (Beijing), Beijing, China, 2006. [Google Scholar]
- Yao, T.; Li, J.; Zhou, G. Analysis of parameters influencing oil displacement efficiency of oil displacement agent. J. China Univ. Pet. (Ed. Nat. Sci.) 2008, 32, 99–102. Available online: http://zgsydxxb.ijournals.cn/zgsydxxben/ch/reader/view_abstract.aspx?file_no=20080321&flag=1 (accessed on 15 January 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).