Abstract
Wastewater treatment plants (WWTPs) play a crucial role in treating sewage, which undergoes multiple treatment stages to ensure a safe treated effluent. However, any interference during these stages can compromise the final effluent quality. Such is the case of the overgrowth of the microcrustacean Daphnia spp., known to inhabit WWTPs, but with its presence in the decantation stage negatively impacting effluent clarification and further disinfection. This study aimed to evaluate how the effluent quality parameters influence the occurrence of Daphnia spp. in the secondary decanter of a WWTP. Wastewater monitoring data collected from 2017 to 2022 were analyzed. Firstly, as the COVID-19 pandemic occurred during the studied period, it was assessed whether the quality and load of the raw wastewater changed. Subsequently, an analysis was carried out using multivariate statistical methods for all the steps of WWTP. Comparing the periods before and during the pandemic, the raw wastewater volume decreased by 19.58%, and the BOD, COD, and TSS decreased by 37.78%, 16.86%, and 35.75%, respectively. These were the parameters affected the most. The statistical analysis revealed correlations between the presence of Daphnia spp. and specific effluent quality parameters, including raw wastewater BOD values below 500 mg L−1, treated effluent BOD values below 13 mg L−1, and pH levels exceeding 7.3. Additionally, BOD and pH were highlighted as critical parameters influencing their presence or absence.
1. Introduction
Wastewater treatment plants (WWTPs) are facilities with the purpose of reducing the pollutants present in wastewater so that it can be returned to the receiving environment without negatively affecting it [1,2,3]. For this to happen, wastewater undergoes treatment processes in which the pollutants present are eliminated through physical, biological, and, if necessary, chemical treatments [3,4]. In a general context, wastewater treatment generally comprises four key stages: pre-treatment, primary treatment, secondary treatment, and tertiary treatment [3,4,5,6]. During pre-treatment, mechanical methods are employed to remove up to 80% of solid material, including sand, plastics, and food waste [7]. Following this, primary treatment involves sedimentation, where suspended solids are separated, allowing the clarified wastewater to proceed to the secondary treatment phase. In the secondary treatment phase occurs the biological treatment that relies on aerobic microorganisms, commonly known as activated sludge, to degrade the organic matter present in the wastewater [1,8]. Finally, the tertiary treatment, conducted after the secondary sedimentation phase, incorporates advanced processes, such as chlorination, ultraviolet irradiation, and membrane filtration, that are designed to eliminate residual contaminants, such as pathogens and other harmful microorganisms [9]. However, the presence of Daphnia spp., commonly known as water fleas, is often significant in secondary decanters, before the treated effluent proceeds to the tertiary treatment, due to the already increased water quality guaranteed by the secondary treatment, and its presence is easily detected by their large size and rapid parthenogenetic reproduction [10].
Daphnia spp. presence comes with both benefits and disadvantages. On one hand, they contribute to controlling algae growth and to removing organic matter and nutrients from wastewater [11,12]. On the other hand, their overgrowth in secondary decanters can pose substantial challenges for the final stages of treatment, mainly when disinfection is needed. Their movement and feeding behavior resuspend solids, leading to increased turbidity and higher solids load, compromising the applied ultraviolet-based tertiary treatment by blocking the radiation from effectively passing through the system to disinfect the final effluent, as reported in this case study. This not only challenges the system’s ability to meet legal discharge limits but also disrupts disinfection processes, as the increased turbidity and particulate matter reduce the effectiveness of disinfection treatments following the decantation step. Understanding why Daphnia spp. occurs in this specific treatment step is crucial for managing their presence and preventing their uncontrolled proliferation, assuring the quality of the final effluent.
Daphnia spp. exhibits remarkable adaptability to various environmental conditions, including pollution, temperature changes, photoperiod, salinity, and nutrient levels [13,14]. When under highly stressful conditions, Daphnia spp. populations switch to sexual reproduction, producing dormant eggs that facilitate colonization of new habitats and the re-establishment of their population [13,15]. These characteristics are the primary reason why Daphnia spp. has successfully colonized WWTPs and continues to thrive by repeatedly re-establishing their populations. Indeed, the presence of Daphnia spp. in WWTPs is well-documented. For example, Kumar and Kiran identified Daphnia spp. and other zooplankton in water collected from a sewage-fed tank, demonstrating that nutrient-rich waters coupled with aquatic weeds favored the abundance of zooplankton [16]. In their study, water quality was assessed from samples taken during the occurrence of Daphnia spp., revealing a water temperature range of 22.5 to 32 °C, an alkaline pH, high dissolved oxygen levels (2.4–4.8 mg L−1), low biochemical oxygen demand (BOD) levels (4.8–16.8 mg L−1), and nutrient concentrations such as nitrates (14.6–54.4 mg L−1) and phosphates (0.28–1.08 mg L−1) [16]. Other studies have sought to explore their potential to enhance wastewater treatment efficiency, as they have shown the capacity to remove particulate matter and reduce bacteria and coliform contents [17,18,19,20,21,22]. Some studies, such as Pau et al., reported the Daphnia magna ability to filter sludge particles from the treated wastewater on secondary decanters, with laboratory tests reporting a 29.4% reduction in the suspended particles and in situ tests reporting a 30.4% reduction [19]. Tertiary treatment based on Daphnia magna filtration has even been considered by Serra et al., showing a 35% reduction in the concentration of particles with diameters below 30 µm and a significant inactivation of E. coli by 1.2 log units, although lower than conventional ultraviolet irradiation (inactivation between 1.5 and 4.0 log units) depending on temperature and hydraulic retention time [22]. However, no research is found on their occurrence when they become problematic. In cases such as the overgrowth reported herein, there is a lack of knowledge of the factors driving their proliferation or of strategies to predict and effectively control their presence.
Considering the above, the main objective of this work was to study the occurrence of Daphnia spp. in an urban WWTP secondary decanter. The specific objectives consisted of processing the monitoring data of the WWTP from 2017 to 2022 and identifying the most relevant physicochemical conditions for their presence. To this end, statistical tools were used to identify patterns in the quality of the effluent when Daphnia spp. appeared. It was also sought to identify possible ways of controlling this organism in WWTP without loss of quality in the final treated effluent.
2. Materials and Methods
2.1. Case Study
The analyzed urban WWTP is located in Fátima, Portugal, and comprises a primary inlet channel, which receives raw wastewater that is subsequently distributed across two independent lines (Figure 1). Each line includes an oxidation ditch followed by a secondary decanter. Post-decantation, the treated effluents from both lines converge and undergo ultraviolet (UV) disinfection treatment prior to discharge into the receiving water body. Upon analyzing the WWTP’s treatment stages, the following six data collection points were identified: (1) influent or raw wastewater, (2) secondary sludge line 1, (3) secondary sludge line 2, (4) aerobic zone 1, (5) aerobic zone 2, and (6) final effluent.

Figure 1.
Satellite overview of the studied WWTP (Fátima, Portugal) and collection points: (1) raw wastewater, (2) secondary sludge line (1, 3) secondary sludge line (2, 4) aerobic zone (1, 5) aerobic zone 2, and (6) final effluent.
These points were chosen to provide a comprehensive assessment of the WWTP performance, covering upstream, within, and downstream locations of the secondary treatment system. Raw wastewater (1) reflects the characteristics of wastewater entering the plant. As the effluent subsequently flows through two independent lines, it is necessary to identify whether the treatment is equally efficient in both lines. For this reason, points (2) to (5) were identified as important for this study. After the secondary decantation, the treated effluent joins up again in just one line, with this monitoring point considered the final effluent (6). From these 6 points it was possible to characterize the raw wastewater, the WWTP treatment influence on the improvement of water quality, and, after all the treatment stages, characterize the treated effluent.
The urban WWTP provided an extensive dataset of analytical parameters obtained through monthly monitoring from 2017 to 2022. From this dataset, 12 parameters were selected for analysis due to their availability through weekly monitoring, resulting in a robust and comprehensive database. The selected parameters include volumetric flow, biochemical oxygen demand (BOD), chemical oxygen demand (COD), ammonium ion (NH4+), nitrate (NO3−), total nitrogen (TN), pH, total phosphorus (TP), total and volatile suspended solids (TSS and VSS), and total and volatile solids (TS and VS). In addition, records documenting the occurrence of Daphnia spp. within WWTP were also incorporated, enabling a detailed assessment of the factors influencing their presence and potential impacts on the treatment process.
As the problem with Daphnia spp. was identified at an unusual time, during a pandemic period, a study was carried out to identify whether this event influenced the load and quality of the raw wastewater. For this analysis, the following parameters were compared: volumetric flow, BOD, COD, pH, TN, TP, and TSS. All monthly values monitored at the WWTP were averaged between June 2017 and February 2020, a period prior to COVID-19. These values were next compared with the period during the COVID-19 pandemic, between March 2020 and February 2022.
Statistical tools were then used to enlighten the occurrence patterns of Daphnia spp. in the WWTP, relating to the selected parameters (monitored at 6 different points in the WWTP). A total of 23 sample points were chosen due to their representativeness of the WWTP treatment stages and data availability from 2017 to 2022, namely: Flow, Biochemical Oxygen Demand-Raw Wastewater (BOD-RW), Biochemical Oxygen Demand-Effluent (BOD-E), Chemical Oxygen Demand-Raw Wastewater (COD-RW), Chemical Oxygen Demand-Effluent (COD-E), Ammonium Ion-Effluent (NH4-E), Nitrate-Effluent (NO3-E), Nitrogen-Raw Wastewater (TN-RW), Nitrogen-Effluent (TN-E), pH-A, pH-E, Phosphorus-Raw Wastewater (TP-RW), Phosphorus-Effluent (TP-E), Total Suspended Solids-Raw Wastewater (TSS-RW), Total Suspended Solids-Effluent (TSS-E), Total Suspended Solids-Aerobic Zone 1 (TSS-AZ1), Volatile Suspended Solids-Aerobic Zone 1 (VSS-AZ1), Total Solids-Secondary Sludge 1 (TS-SS1), Volatile Solids-Secondary Sludge 1 (VS-SS1), Total Suspended Solids-Aerobic Zone 2 (TSS-AZ2), Volatile Suspended Solids-Aerobic Zone 2 (VSS-AZ2), Total Solids-Secondary Sludge 2 (TS-SS2), and Volatile Solids-Secondary Sludge 2 (VS-SS2). The selected WWTP points include essential parameters commonly monitored in WWTPs, allowing us to track how the raw wastewater content in organic matter (BOD, COD, and TSS), nutrients (TN and TP), and pH fluctuate as they enter the biological treatment and ultimately impact the final effluent. By including measurements at different treatment stages, the assessment of the treatment performance and operational stability can be carried out. The content on TSS and vs. on both the anaerobic zones and decanted sludge, 1 and 2, are of extreme importance to evaluate if both streams are operating at similar levels.
2.2. Statistical Analysis
Pearson’s correlation matrix, logistic regression, and Principal Component Analysis (PCA) were computed using the R program (R Core Team 4.3.1, 2023), adopting a significance level of <0.05 for statistical differences [23].
2.2.1. Pearson’s Correlation Matrix
The first approach consisted of submitting the selected parameters to a Pearson’s correlation matrix in order to investigate how they are correlated and to identify and remove collinear parameters. A correlation coefficient above 0.7 was adopted as the cut-off value for removing colinear parameters [24].
2.2.2. Principal Component Analysis (PCA)
The subset of the retained parameters was next subjected to a statistical procedure, PCA [25,26,27], to reduce the dimensionality of the dataset and identify possible clusters regarding the absence and presence of Daphnia spp. This second approach assumes that the occurrence of Daphnia spp. can be related to various analytical parameters, so the scenarios of ‘absence’ and ‘presence’ of the microcrustacean would form two distinct clusters within a multivariate space. The analytical parameters were first log-normalized for standardization purposes and to avoid possible data non-normality effects. In addition, Student’s t-tests were carried out to check for statistical differences between the absence and presence of Daphnia spp. (categorical parameters) using the first three axes of the PCA (response parameters) of the analytical parameters.
2.2.3. Logistic Regression
The third approach aimed to understand the relative contribution of the analytical parameters that best explain the occurrence of Daphnia spp. in the urban WWTP. In this case, a logistic regression was applied, with the response parameter being binary (0 = absence and 1 = presence) and the categorical parameters being the analytical parameters [28,29]. Once again, the analytical parameters were log-normalized to standardize the parameters in terms of units. The explanatory performance of the logistic model was assessed using the Wald test (chi-squared) and the Akaike criterion (AIC) [30]. For this set of analyses, a significance level of <0.05 was adopted to assess significant statistical differences.
2.2.4. Classification Tree
Finally, a classification tree analysis was performed using the parameters identified by the logistic regression as most influential to Daphnia spp. presence. The most predictive parameter determines the first split (branches), with subsequent branches added until meeting the stopping criteria. Unlike regression models, which fit a single relationship across the entire domain, classification trees adapt to changing relationships within different input regions. Model performance is evaluated using confusion matrices and receiver operating characteristic (ROC) curves that quantify true/false positives and negatives, enabling the calculation of sensitivity (true positive rate), specificity (true negative rate), and accuracy (correct classification rate). A well-balanced model exhibits high and similar sensitivity and specificity, minimizing false classifications [31].
3. Results
3.1. Effect of the COVID-19 Pandemic
This first assessment of the quality of the raw wastewater is essential because the first case of the presence of Daphnia spp. was identified in December 2020, 10 months after the declaration of the COVID-19 global pandemic. Between the date of this first appearance and June 2022, approximately 30 interferences in the treatment were identified caused by the presence of the microcrustacean. The first sign of the appearance of microcrustaceans was an increase in turbidity in the final treated effluent. After that, WWTP technicians carried out a visual inspection, observing red clouds in the secondary decanter and registering their occurrence.
Table 1 shows the variations in the raw inflow between the period before (June 2017–February 2020) and during (March 2020–February 2022) the pandemic.

Table 1.
Variation in raw wastewater quality caused by COVID-19 (mean ± standard deviation).
3.2. Pearson’s Correlation Matrix
The Pearson’s correlation matrix shows a series of highly correlated parameters (Figure 2). In fact, 10 of the parameters analyzed presented correlation coefficients (r) greater than 0.7, leading to the following being removed: COD_RW, COD_E, TP_RW, TP_E, TSS_A, TSS_E, VSS_AZ1, TS_SS1, VSS_AZ2, and TS_SS2. Such high correlations are due to the WWTP operating structure, with the raw wastewater following a single treatment line through the initial stages (grading/sampling and desanding/degreasing) before being split into two separate biological reactor lines and secondary decantation stages, as seen in Figure 1. This division results in non-correlated parameters between both streams (VSS_AZ1, TS_SS1, VSS_AZ2, and TS_SS2) and could further be affecting the correlation of the COD and TP parameters between the raw wastewater and treated effluent.
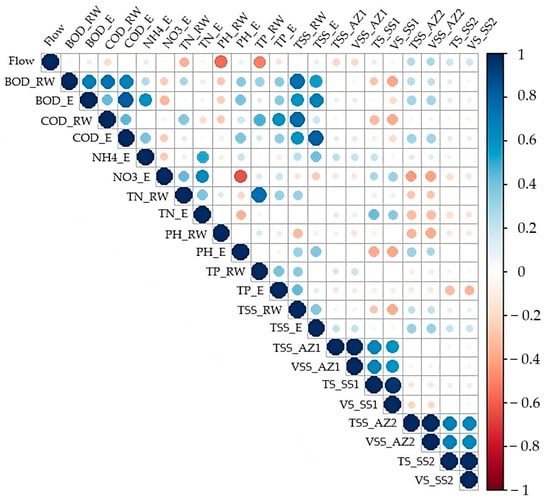
Figure 2.
Pearson’s correlation matrix with the measured analytical parameters. The circle’s size corresponds to the correlation’s magnitude, varying from −1 to 1 (bar on the right side). Red indicates negative correlations, while blue indicates positive correlations.
3.3. Principal Component Analysis
The first three principal components of the PCA explained around 60% of the total variability of the data: PC 1 (27.9%), PC 2 (16.4%), and PC 3 (14.7%). A high degree of overlap between the two groups (absence and presence of Daphnia spp.) is apparent, with only a slight tendency towards segregation, as observed by the distance of the centroids (Figure 3a–c).
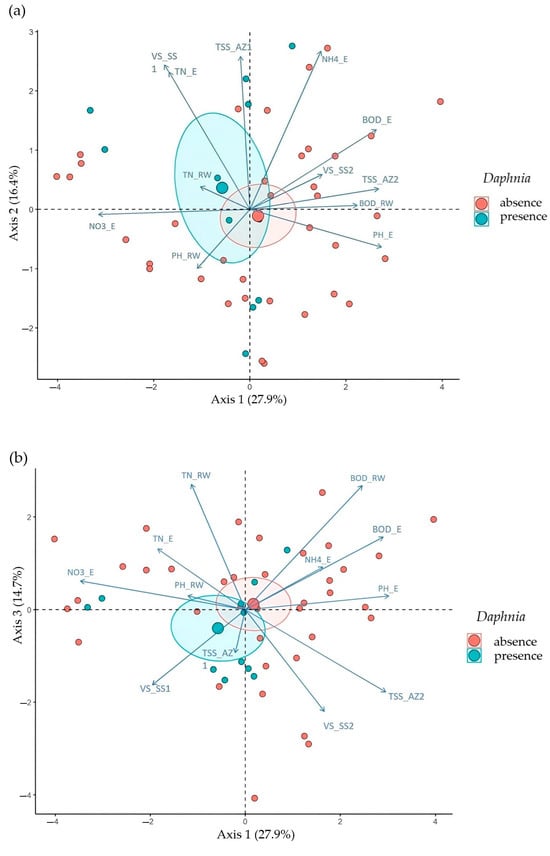
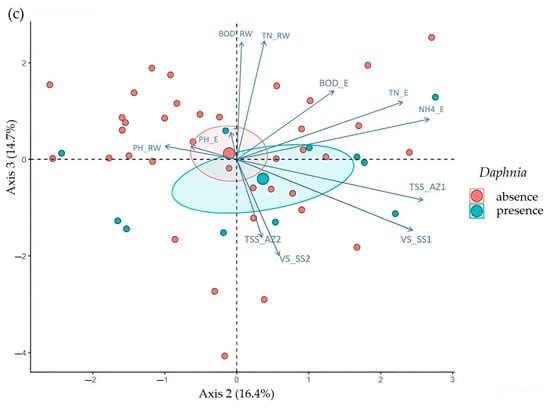
Figure 3.
PCA biplots demonstrate a pattern of high overlap between the absence (red) and presence (blue) groups of Daphnia spp., with a slight tendency towards segregation (distance between centroids). (a) PC 1 × PC 2; (b) PC 1 × PC 3; (c) PC 2 × PC 3.
The Daphnia spp. presence pattern, though not statistically significant as next determined, was more related to lower PC1 and PC3 values. Analyzing Figure 3b, it is also clear that the higher values of PC1 and PC3 (and hence of lower Daphnia spp. occurrence) correspond to the direction of the BOD_RW and BOD_E (in both cases corroborated by the logistic regression) and NH4_E and are opposite to the direction of VS_SS1 (in these two later cases just shortly failing to be considered as significant drivers by the logistic regression). It is also possible to verify in Figure 3a that larger values of VS_SS1, TN_E, and TN_RW correspond to a greater distinction between the presence and absence of Daphnia spp. Finally, Figure 3c suggests that higher values of VS_SS1, VS_SS2, TSS_AZ1, and TSS_AZ2 are associated with a more pronounced distinction between the presence and absence of Daphnia spp. However, in these two later figures (Figure 3b,c), the fact that PC2 does not provide for any distinction between Daphnia presence or absence limits their use in this analysis.
3.4. T Test
Regarding Student’s t-test, it did not register any significant difference in the three principal components (axes) of the PCA (environmental parameters) between the “absence” and “presence” groups of Daphnia (Axis 1 (PC 1): t = 1.439, p = 0.162; Axis 2 (PC 2): t = −0.840, p = 0.415; Axis 3 (PC 3): t = 1.401, p = 0.173). These results corroborate the pattern of high overlap between the absence and presence of Daphnia spp. found by the PCA (Figure 4a–c).
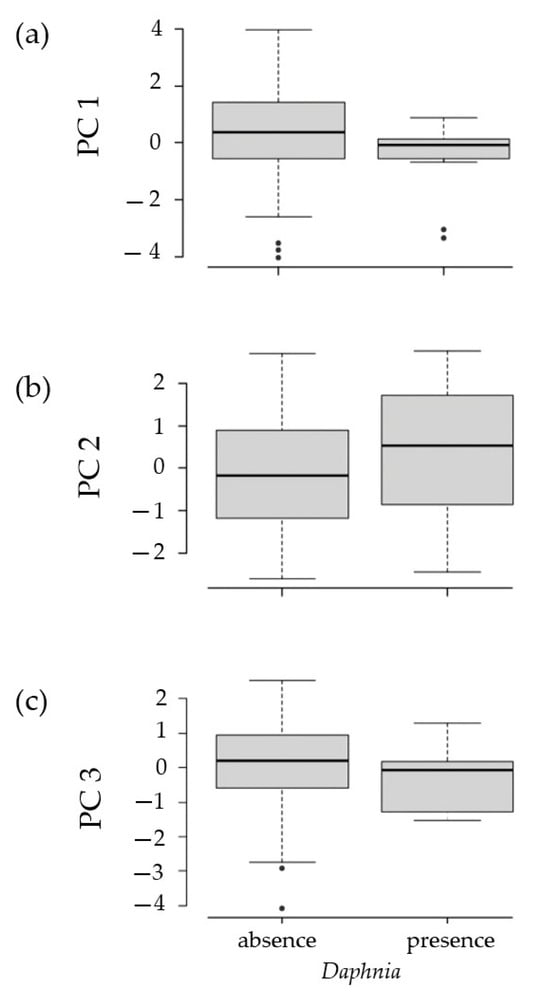
Figure 4.
Boxplot graphs of the T-test results show no significant differences between the “absence” and “presence” groups of Daphnia for PC 1 (a), PC 2 (b), and PC 3 (c), used as proxies for analytical parameters.
3.5. Logistic Regression
Logistic regression identified three parameters with different levels of influence on the occurrence of Daphnia spp. The biochemical oxygen demand of both the raw wastewater (BOD_RW) and the effluent (BOD_E) had a moderate negative effect (i.e., marginally significant p-value) on the response parameter, i.e., the presence of Daphnia spp. was related to low levels of BOD_RW (<500 mg/L) and BOD_E (<13 mg/L) (Figure 5a,b).
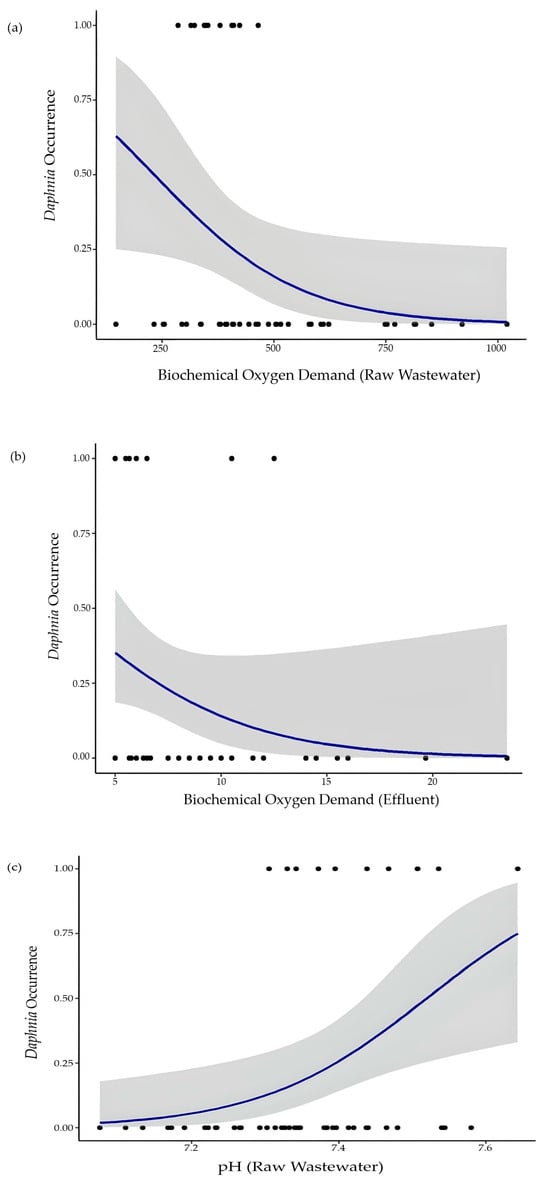
Figure 5.
Graphs of the logistic regression model showing the influence of biochemical oxygen deficiency (a,b) and pH (c) on the occurrence of Daphnia (0 = absence/1 = presence). (Dots represent the observational data; blue line depicts the predict mean; shadow indicates 95% confidence intervals).
These relationships produced close AIC and Wald (chi-squared) values (Table 2), which shows the similar effects of these variables.

Table 2.
Summary of logistic regression results testing the influences of analytical parameters on Daphnia’s occurrence (absence/presence).
By far the parameter with the greatest influence on the absence/presence of Daphnia spp. was the pH of the raw wastewater (pH_RW), with the occurrence of the crustacean found at higher pH levels (>7.3) (Figure 5c). This result was reinforced by the model’s explanatory power, with the highest Wald test value and the lowest AIC (Table 2). The (low) raw wastewater and effluent BOD were also identified as drivers for Daphnia occurrence by the logistic regression. Further, it is considered that the raw effluent characteristics influence the operating conditions within the WWTP aerated tanks (and treated effluent to some degree). This assumption also holds true for the raw effluent BOD, presenting a moderate to strong positive correlation with the treated effluent BOD (between 5 mg L−1 and 33 mg L−1 and averaging 8.6 mg L−1), with both being found to drive Daphnia outbreaks. Hence, it seems that the combined effect of BOD reduction and pH increase (towards more alkaline values) may have potentiated Daphnia outgrowth. It should also be kept in mind that, although the determination of the Daphnia occurrences was performed in the secondary clarifier, it seems licit to infer that also within the aerated tanks (influenced by the raw effluent wastewater characteristics), this microorganism would also be present (at least via dormant eggs present in sludge recirculation).
3.6. Classification Tree
A classification tree was also performed with the parameters found by the logistic regression to influence Daphnia presence the most (BOD_RW, BOD_E, and pH_RW). The raw wastewater BOD was the parameter that governed the most the presence of Daphnia in this case, namely for values below 476.5 mg L−1 (all 18 samples above this value did not present Daphnia), alongside a pH value above 7.35 (11 out of the 14 samples below this value in the classification tree did not present Daphnia). Indeed, 8 out of the 11 (72.7%) samples presenting Daphnia fell within the above range (BOD_RW < 476.5 mg L−1 and pH_E > 7.35), whereas 29 out of the 37 (78.4%) samples lacking Daphnia fell outside. These results are comparable to the ones found by the logistic regression (BOD_RW < 500 mg L−1 and pH_E > 7.3) (Figure 6).
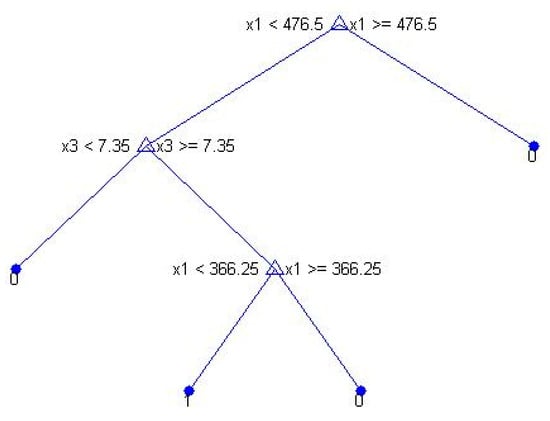
Figure 6.
Results of the classification tree on the occurrence of Daphnia (0 = absence/1 = presence). x1—BOD_RW and x3—pH_E.
4. Discussion
The appearance of Daphnia spp. began in a year marked by the start of the global COVID-19 pandemic. Pandemic control measures began in Portugal in March 2020. This event led to some changes in the load and quality of the raw wastewater arriving at WWTPs.
This study revealed a significant reduction in the volume of raw wastewater entering the studied urban WWTP during the period from 2020 to 2022, coinciding with the restrictive measures implemented during the COVID-19 pandemic. Over this period, the WWTP experienced a significant reduction (p = 0.00016) in the average flow (20% less). This decrease was accompanied by changes in several wastewater parameters. The most significant was the BOD (p = 0.00002), which dropped by 38%, followed by the TSS (p = 0.000428), which dropped 36%, with the COD exhibiting a marginally significant (p = 0.04769) reduction of 17%. Conversely, nutrient levels, such as nitrogen and phosphorus, showed a slight increase during this time; however, these changes were not statistically significant (p = 0.46737 for N and p = 0.21921 for P). Additionally, the pH demonstrated a significant change during the period (p = 0.00019), though only increasing 2%.
The observed changes in the wastewater composition and flow rate within the COVID-19 period possibly created more favorable conditions for the emergence of Daphnia spp. in the studied WWTP. The observed pH increase and BOD reduction in the raw effluent (translated also in the reduction in the final effluent BOD), as seen by the logistic regression results, can be considered as one of the major drivers behind Daphnia spp. overgrowth within the COVID-19 period. Indeed, reductions in organic loads, particularly the significant decreases in BOD (37.8%), TSS (35.8%), and COD (16.9%), suggest a decline in the content of organic pollutants and suspended solids, potentially leading to improved water clarity and quality. Such changes could improve oxygen availability and light penetration, which could help the proliferation of Daphnia spp. [32]. Additionally, slight increases in nutrient levels, 8.4% for TN and 13.8% for TP, support the growth of phytoplankton, the primary food source for Daphnia spp. and responsible for their productivity [33]. The observed changes in pH (2.0%, 0.145 in value), though minor, could have influenced the suitability of the environment for Daphnia spp. colonization.
These changes were also noticed in other studies, as in Çolak and Öztekin, who reported a decrease in COD (8.8%) and BOD (12.4%) from 2020 to 2021 [34]. In another study, it was reported that the amount of BOD and COD decreased 41% and 39.4%, respectively [35]. Yazdian and Jamshidi (2021) also stated changes in the wastewater quality and load flowing to 23 analyzed WWTPs in Iran during COVID-19 prevention measures, referring to the fact that the closing of industrial and business activities may have contributed to their reduction [36].
The correlation between the parameters monitored across the two treatment lines identified positive and negative correlations as reported by Tanyol and Demir [37]. Regarding the statistical analysis, the parameters identified as most influencing the presence of Daphnia spp. were BOD and pH. Indeed, the logistic regression showed that a pH larger than 7.3 and BOD in the incoming raw wastewater below 500 mg L−1 and in the outgoing effluent below 13 mg L−1 statistically influenced the presence and absence of Daphnia spp. in the WWTP. Further, BOD was the monitored parameter that underwent the most changes because of the COVID-19 control measures, with a decrease of approximately 38%, reflecting a less polluted and more favorable environment for Daphnia spp. to grow. Additionally, low BOD values (4.8–16.8 mg L−1) were also reported by Kumar and Kiran when Daphnia spp. appeared [15].
In relation to the influence of pH on Daphnia spp., several studies report its impacts, including threshold values for mortality between pH 10.5 and 11.5, with a population mortality increase already documented for pH above these values [38]. Additionally, a reduction in the number of newborns (50–80%) due to egg degeneration and stillbirths was observed at a pH of 10.0 and 10.5, given that alkaline pH tends to substantially reduce the viability of eggs and the resilience of Daphnia spp. [39]. Another study, focusing on Daphnia magna, one of the most studied species, reported a suitable pH between 4.6 and 10.1, with the optimal pH situated between 7.9 and 8.3, slightly higher than the ones observed in the monitored WWTP [40].
These findings suggest that the reduced pollutant and effluent load generated by the COVID-19 prevention measures, coupled with increased nutrient density, may have created more favorable conditions for the proliferation of Daphnia spp. in the studied urban WWTP.
5. Conclusions
The COVID-19 pandemic restrictions significantly altered the flow and composition of raw wastewater. Reduced human activity, industrial shutdowns, and changes in water consumption patterns led to a notable decrease in overall raw wastewater loads entering WWTPs, influencing the operating conditions within the WWTP aerated tanks and, consequently, the treated effluent. However, this reduction often resulted in increased nutrient concentration, such as nitrogen and phosphorus, within the wastewater, which may have influenced the dynamics of the biota present within WWTPs. Indeed, the higher availability of nutrients, paired with the reduction in organic pollutants, seems to have created an environment conducive to the proliferation of species such as Daphnia spp., which thrive in nutrient-rich and low-pollution conditions. Understanding these changes is essential for optimizing treatment processes and managing ecological impacts in wastewater treatment systems during periods of altered effluent flow and composition.
The statistical analysis of the available data, combined with the periods of Daphnia spp. occurrence, allowed for the estimation of the parameters most influencing their presence in the studied WWTP. Among these, BOD and pH were identified as having the greatest impact. Considering that the goal of WWTPs is to originate high-quality treated wastewater in which these microcrustaceans thrive, care should be taken to restrain them from entering these systems. Hence, as recommendations, a comprehensive investigation should be conducted to determine the main entrance gates of Daphnia spp. in WWTPs. The presence of Daphnia spp. in WWTPs is likely due to their dormant eggs, which can withstand harsh environmental conditions for extended periods of time [41]. Potential entry points can include their use as fish food, leading to their introduction from urban households or industrial sources that discharge them into sewers and ultimately reaching WWTPs. Additionally, dormant eggs are known to survive the digestive tract of water birds, making it another possible source of contamination [42].
Sampling the raw wastewater at a plant’s inlet would be valuable in identifying whether these species mostly (re)enter the system periodically or are a result of a one-time introduction, with subsequent survival and reproduction facilitated by its defense mechanisms. If the organism is confirmed to enter through the sewage system, further studies outside the WWTP will be necessary to trace their origin.
Additionally, strategies for controlling Daphnia spp. within wastewater treatment systems should be explored through chemical, biological, or physical methods. Chemical control could be an effective option, as the pH tolerance of Daphnia spp. is well-established, and numerous studies have examined the toxic effects of various chemicals on this species. However, a few chemicals can be applied in WWTPs without compromising treatment efficiency and effluent quality. A promising alternative is the use of commonly administered disinfection agents, such as sodium hypochlorite. Also, biological methods could leverage their central role in food webs, as Daphnia spp. have many natural predators, including fish, that could be introduced to control their proliferation. Physical control, on the other hand, could involve the implementation of filtration systems to prevent the circulation of Daphnia spp. between different treatment steps, effectively limiting their impact on the process. Further research into alternative and sustainable solutions for managing Daphnia spp. in urban WWTPs is needed.
Author Contributions
Conceptualization, P.E. and R.E.; methodology, R.E., A.L.A. and C.R.; software, R.E. and A.L.A.; validation, A.L.A., C.R. and V.O.; formal analysis, R.E.; investigation, R.E.; writing—original draft preparation, P.E. and R.E.; writing—review and editing, V.O., A.L.A. and C.R.; visualization, P.E., V.O., A.L.A. and C.R.; supervision, A.L.A. and C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy issues.
Acknowledgments
The researchers thank AdCL (Águas do Centro Litoral) for providing the data used for the development of this work. V. Oliveira thanks the national funding by FCT—Foundation for Science and Technology, P.I., through the institutional scientific employment program contract (https://doi.org/10.54499/CEECINST/00077/2021/CP2798/CT0002).
Conflicts of Interest
Author Rômulo Egito was employed by the Public Waters of Alentejo Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Várhelyi, M.; Vasile, M.; Marius, A. Improving wastewater treatment plant operation by ammonia-based aeration and return activated sludge control. Comput. Aid. Chem. Eng. 2019, 46, 1165–1170. [Google Scholar] [CrossRef]
- Muga, H.E.; Mihelcic, J.R. Sustainability of wastewater treatment technologies. J. Environ. Manag. 2008, 88, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A. Wastewater Treatment and Reuse for Sustainable Water Resources Management: A Systematic Literature Review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Englande, A.J., Jr.; Krenkel, P.A. Waste Water Treatment and Water Reclamation. Encycl. Phys. Sci. Technol. 2003, 13, 661–677. [Google Scholar]
- Riffat, R.; Husnain, T. Fundamentals of Wastewater Treatment and Engineering, 2nd ed.; CRC Press: London, UK, 2012; p. 430. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Chapter 1: Wastewater Treatment: An Overview. In Green Adsorbents for Pollutant Removal, Environmental Chemistry for a Sustainable World; Springer Nature: Cham, Switzerland, 2018; Volume 18, pp. 1–21. [Google Scholar] [CrossRef]
- Suzenet, G.; Tal, A.; Boymanns, D. Sustainable water management for the city: Technologies for improving domestic water supply. Built Environ. 2002, 28, 138–151. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I. Wastewater treatment by biological methods. In Environmental Water; Elsevier: Amsterdam, The Netherlands, 2013; pp. 179–204. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Bampos, G. Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects. Processes 2022, 10, 2304. [Google Scholar] [CrossRef]
- Bethke, K.; Kwidzińska, K.; Caban, M. Investigation of pharmaceutical bioaccumulation in Daphnia sp. living in a wastewater treatment plant. Sci. Total. Environ. 2024, 950, 174915. [Google Scholar] [CrossRef]
- Ebert, D. Introduction to Daphnia biology. In Ecology, Epidemiology, and Evolution of Parasitism in Daphnia; National Library of Medicine (US), National Center for Biotechnology Information: Bethesda, MD, USA, 2005; pp. 5–18, ISBN-10: 1-932811-06-0. [Google Scholar]
- Roche, K.F. Growth potential of Daphnia magna Straus in the water of dairy waste stabilization ponds. Water Res. 1998, 32, 1325–1328. [Google Scholar] [CrossRef]
- Ebert, D. Daphnia as a versatile model system in ecology and evolution. EvoDevo 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Santos-Medrano, G.E.; Rico-Martínez, R. Acute sensitivity comparison among Daphnia magna Strauss, 1820 daphnia pulex leydig, 1860 and simocephalus vetulus muller, 1776, exposed to nine toxicants. Turk. J. Fish. Aquat. Sci. 2019, 19, 615–623. [Google Scholar] [CrossRef]
- La, G.-H.; Choi, J.-Y.; Chang, K.-H.; Jang, M.-H.; Joo, G.-J.; Kim, H.-W. Mating Behavior of Daphnia: Impacts of Predation Risk, Food Quantity, and Reproductive Phase of Females. PLoS ONE 2014, 9, e104545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, K.; Kiran, B. A report on diversity of Cladocera in sewage fed tank of Bhadravathi taluk, Karnataka. Int. J. Fauna Biol. Stud. 2016, 3, 18–20. [Google Scholar]
- Miner, B.E.; De Meester, L.; Pfrender, M.E.; Lampert, W.; Hairston, N.G. Linking genes to communities and ecosystems: Daphnia as an ecogenomic model. Proc. R. Soc. B Biol. Sci. 2012, 279, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Serra, T.; Barcelona, A.; Pous, N.; Salvadó, V.; Colomer, J. Disinfection and particle removal by a nature-based Daphnia filtration system for wastewater treatment. J. Water Process. Eng. 2022, 50, 103238. [Google Scholar] [CrossRef]
- Pau, C.; Serra, T.; Colomer, J.; Casamitjana, X.; Sala, L.; Kampf, R. Filtering capacity of Daphnia magna on sludge particles in treated wastewater. Water Res. 2013, 47, 181–186. [Google Scholar] [CrossRef]
- Shiny, K.J.; Remani, K.N.; Nirmala, E.; Jalaja, T.K.; Sasidharan, V.K. Biotreatment of wastewater using aquatic invertebrates, Daphnia magna and Paramecium caudatum. Bioresour. Technol. 2005, 96, 55–58. [Google Scholar] [CrossRef]
- Burnet, J.-B.; Faraj, T.; Cauchie, H.-M.; Joaquim-Justo, C.; Servais, P.; Prévost, M.; Dorner, S.M. How does the cladoceran Daphnia pulex affect the fate of Escherichia coli in water? PLoS ONE 2017, 12, e0171705. [Google Scholar] [CrossRef]
- Serra, T.; Colomer, J.; Pau, C.; Marín, M.; Sala, L. Tertiary treatment for wastewater reuse based on the Daphnia magna filtration—Comparison with conventional tertiary treatments. Water Sci. Technol. 2014, 70, 705–711. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statical Computing: Vienna, Austria, 2023. Available online: https://www.R-project.org/ (accessed on 2 March 2024).
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2009; ISBN 978-0-13-100lW6·5. [Google Scholar]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Hongyu, K.; Sandanielo, V.L.; Junior, G.J. Principal Component Analysis: Theoretical Summary, Application, and Interpretation. E&S–Eng. Sci. 2015, 5, 1. [Google Scholar] [CrossRef]
- Mishra, S.P.; Sarkar, U.; Taraphder, S.; Datta, S.; Swain, D.P.; Saikhom, R.; Panda, S.; Laishram, M. Multivariate Statistical Data Analysis—Principal Component Analysis (PCA). Int. J. Livest. Res. 2017, 7, 60. [Google Scholar]
- Gonzalez, L. Logistic regression and its application. Monograph (Specialisation)—Computer Science Course; Universidade Federal do Maranhão: São Luís, 2018. Available online: https://monografias.ufma.br/jspui/bitstream/123456789/3572/1/LEANDRO-GONZALEZ.pdf (accessed on 8 March 2025).
- Fernandes, A.A.T.; Filho, D.B.F.; da Rocha, E.C.; Nascimento, W.d.S. Read this paper if you want to learn logistic regression. Rev. Sociol. Polit. 2020, 28, 1–20. [Google Scholar] [CrossRef]
- Freitas, L. WALD Test for Evaluating Regression and Dispersion Parameters in Multivariate Generalized Linear Covariance Models. Master’s Dissertation, University Federal do Paraná, Curitiba, Brazil, 2022. Available online: https://hdl.handle.net/1884/78069 (accessed on 11 November 2024).
- Bergman, L.E.; Wilson, J.M.; Small, M.J.; VanBriesen, J.M. Application of Classification Trees for Predicting Disinfection By-Product Formation Targets from Source Water Characteristics. Environ. Eng. Sci. 2016, 33, 455–470. [Google Scholar] [CrossRef]
- Serra, T.; Müller, M.F.; Barcelona, A.; Salvadó, V.; Pous, N.; Colomer, J. Optimal light conditions for Daphnia filtration. Sci. Total. Environ. 2019, 686, 151–157. [Google Scholar] [CrossRef]
- Striebel, M.; Singer, G.; Herwig, S.; Andersen, T. “Trophic overyielding”: Phytoplankton diversity promotes zooplankton productivity. Ecology 2012, 93, 2719–2727. [Google Scholar] [CrossRef]
- Çolak, S.; Öztekin, E. Effect of COVID-19 Pandemic on Chemical Parameters of Wastewater Treatment Plant: A Case Study in Zonguldak City, Turkey. Environ. Eng. Manag. J. 2022, 21, 805–815. [Google Scholar] [CrossRef]
- Patel, P.P.; Mondal, S.; Ghosh, K.G. Some respite for India’s dirtiest river? Examining the Yamuna’s water quality at Delhi during the COVID-19 lockdown period. Sci. Total. Environ. 2020, 744, 140851. [Google Scholar] [CrossRef]
- Yazdian, H.; Jamshidi, S. Performance evaluation of wastewater treatment plants under the sewage variations imposed by COVID-19 spread prevention actions. J. Environ. Health Sci. Eng. 2021, 19, 1613–1621. [Google Scholar] [CrossRef]
- Tanyol, M.; Demir, V. Correlations between some operation parameters and efficiency evaluation of domestic wastewater treatment plant in Tunceli (Turkey). Desalination Water Treat. 2016, 57, 28115–28121. [Google Scholar] [CrossRef]
- Oberholster, P.; Dabrowski, J.; Botha, A. Assessing daphnia population dynamics and recovery patterns after exposure to multiple environmental stressors in a eutrophic lake. In Eutrophication: Causes, Economic Implications and Future Challenges, 1st ed.; Lambert, A., Roux, C., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 127–154. ISBN 978-1-62808-499-3. [Google Scholar]
- Vijverberg, J.; Kalf, D.F.; Boersma, M. Decrease in Daphnia egg viability at elevated pH. Limnol. Oceanogr. 1996, 41, 789–794. [Google Scholar] [CrossRef]
- El-Deed, M.; Habashy, M.; Mohammady, E. Effects of pH on Survival, Growth and Reproduction Rates of The Daphnia Magna. Aust. J. Basic Appl. Sci. 2011, 5, 1–10. [Google Scholar]
- Issa, S.; Simonsen, A.; Jaspers, V.L.; Einum, S. Population dynamics and resting egg production in Daphnia: Interactive effects of mercury, population density and temperature. Sci. Total. Environ. 2021, 755, 143625. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.; Miranda, N.A.F.; Cumming, G.S. The role of waterbirds in the dispersal of aquatic alien and invasive species. Divers. Distrib. 2015, 21, 744–754. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).