Plasma-Assisted Hydrogen Production: Technologies, Challenges, and Future Prospects
Abstract
1. Introduction
1.1. The Potential of Plasma Technology in Hydrogen Production
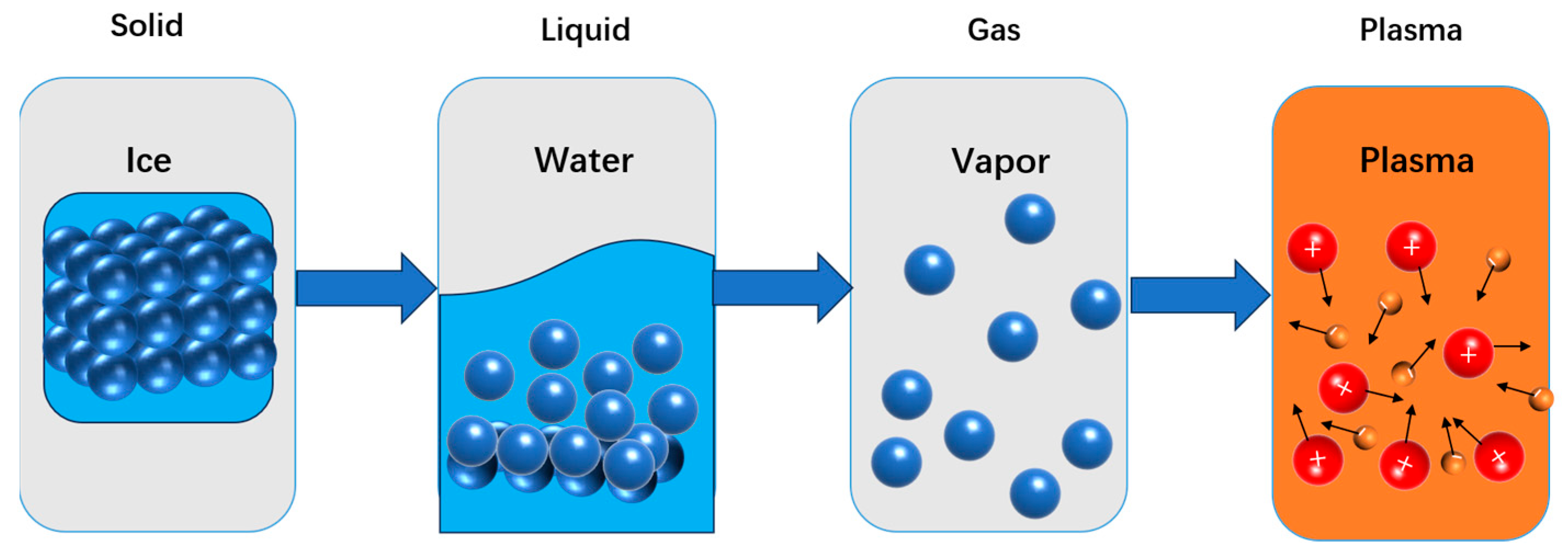
1.2. The Definition and Fundamental Properties of Plasma
1.3. Comparison of Traditional Hydrogen Production and Plasma-Assisted Hydrogen Production
1.3.1. Fundamental Principles of Traditional Hydrogen Production
1.3.2. Fundamental Principles of Plasma-Assisted Hydrogen Production
1.3.3. Additional Comparisons
1.3.4. Innovation and Research Contributions
2. Plasma-Assisted Hydrogen Production Methods
2.1. Plasma-Assisted Steam Cracking

2.2. Plasma-Assisted Hydrocarbon Cracking
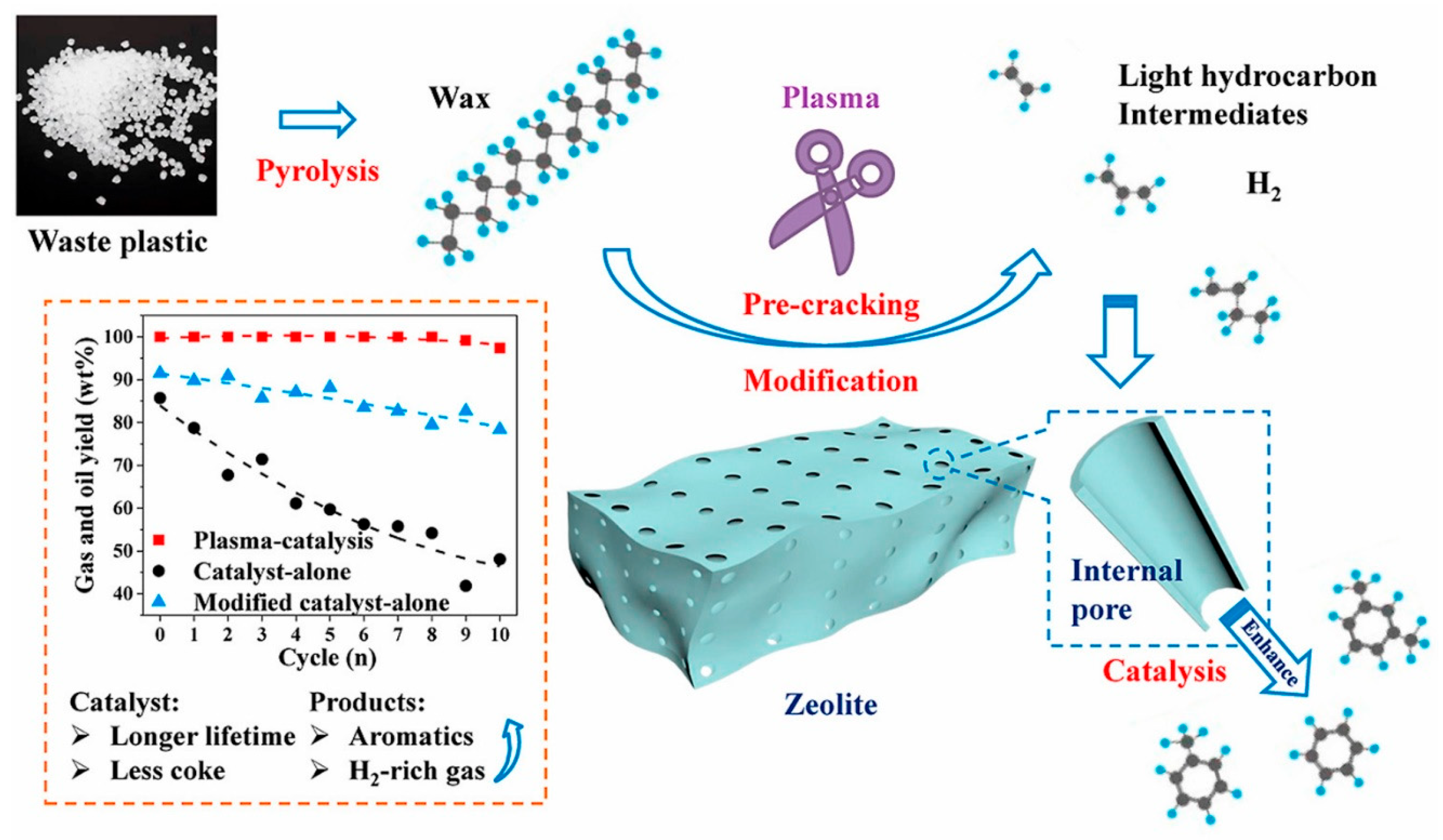
3. Plasma Generation Techniques for Hydrogen Production
3.1. Arc Discharge

3.2. Microwave Discharge

3.3. Dielectric Barrier Discharge (DBD)

3.4. Radio Frequency Discharge

3.5. Comparison of Hydrogen Production and Energy Efficiency
| Hydrogen Technology | Items | Feedstock | H2 Yield (%) | Energy Yield (L·(kW·h)−1) | References |
|---|---|---|---|---|---|
| Plasma generation techniques | Arc discharge | C7H8 | 48.6 | 60.2 | [39] |
| Arc discharge | NH3 | 34.8 | 1080.0 | [42] | |
| Arc discharge | C2H6O | 40.9 | - | [38] | |
| Arc discharge | C7H16 | 34–53 | 77–89 | [60] | |
| Microwave discharge | C2H6O | 58.1 | 23.97 | [50] | |
| Microwave discharge | CH4 | 74.0 | 136.6 | [45] | |
| Microwave discharge | CH4 | 9.5 | 21.28 | [44] | |
| Microwave discharge | NH3 | 54.4 | 274 | [51] | |
| Dielectric barrier discharge | NH3 | 15.1 | 48.6 | [61] | |
| Dielectric barrier discharge | NH3 | 99.9 | 430 | [59] | |
| Dielectric barrier discharge | CH4 and H2O | 80 | - | [62] | |
| Radio frequency discharge | CH4 and H2O | 64.2 | 6.72 | [36] |
4. Applications for Plasma-Based Hydrogen Production
4.1. Water Splitting for Hydrogen Production
| Hydrogen Technology | Items | Energy Consumption ($/GJ) | References |
|---|---|---|---|
| Plasma-assisted hydrogen production methods | Plasma-assisted steam cracking | 30.75 | [15] |
| Plasma-assisted hydrocarbon cracking | 0.83 | [72] | |
| Plasma generation techniques | Arc discharge | 12.81 | [73] |
| Microwave discharge | 2.8–5.1 | [57] | |
| Dielectric barrier discharge | 7.53–11.39 | [21] | |
| Radio frequency discharge | 0.88–1.38 | [57] |
4.2. Plasma Cracking of Natural Gas

4.3. Waste Gas Resource Utilization

5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, W.M.; Chan, V.S. Advances and challenges in computational plasma science. Plasma Phys. Control. Fusion 2005, 47, R1–R34. [Google Scholar] [CrossRef]
- Hirami, Y.; Hunge, Y.M.; Suzuki, N.; Rodríguez-González, V.; Kondo, T.; Yuasa, M.; Fujishima, A.; Teshima, K.; Terashima, C. Enhanced degradation of ibuprofen using a combined treatment of plasma and Fenton reactions. J. Colloid Interface Sci. 2023, 642, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Suzuki, N.; Hunge, Y.M.; Kuriyama, H.; Hayakawa, T.; Serizawa, I.; Terashima, C. Synergistic effect of Ag decorated in-liquid plasma treated titanium dioxide catalyst for efficient electrocatalytic CO2 reduction application. Sci. Total. Environ. 2023, 902, 166018. [Google Scholar] [CrossRef]
- Francis, F.C. Introduction to Plasma Physics and Controlled Fusion; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Thompson, W.B. An Introduction to Plasma Physics; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Nicholson, D.R. Introduction to Plasma Theory; Wiley: New York, NY, USA, 1983; Volume 1. [Google Scholar]
- Schram, D.C. Plasma processing and chemistry. Pure Appl. Chem. 2002, 74, 369–380. [Google Scholar] [CrossRef][Green Version]
- Miyamoto, K. Plasma Physics for Controlled Fusion; Springer: Berlin/Heidelberg, Germany, 2016; Volume 92. [Google Scholar]
- Lippens, P. Low-Pressure Cold Plasma Processing Technology; Woodhead Publishing: Cambridge, UK, 2007. [Google Scholar]
- Boretti, A.; Banik, B.K. Advances in hydrogen production from natural gas reforming. Adv. Energy Sustain. Res. 2021, 2, 2100097. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A comprehensive review on hydrogen production from coal gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Cook, M.; Nott, T.; Trompetter, W.J.; Futter, J.; Bumby, C.W.; Kennedy, J. Plasma mediated water splitting for hydrogen production. J. Phys. Energy 2025, 7, 022002. [Google Scholar] [CrossRef]
- Bromberg, L.; Cohn, D.; Rabinovich, A.; Alexeev, N. Plasma catalytic reforming of methane. Int. J. Hydrogen Energy 1999, 24, 1131–1137. [Google Scholar] [CrossRef]
- Petitpas, G.; Rollier, J.-D.; Darmon, A.; Gonzalez-Aguilar, J.; Metkemeijer, R.; Fulcheri, L. A comparative study of non-thermal plasma assisted reforming technologies. Int. J. Hydrogen Energy 2007, 32, 2848–2867. [Google Scholar] [CrossRef]
- Rehman, F.; Majeed, W.S.A.; Zimmerman, W.B. Hydrogen production from water vapor plasmolysis using DBD-Corona hybrid reactor. Energy Fuels 2013, 27, 2748–2761. [Google Scholar] [CrossRef]
- Bespalko, S.; Mizeraczyk, J. Overview of the hydrogen production by plasma-driven solution electrolysis. Energies 2022, 15, 7508. [Google Scholar] [CrossRef]
- Varne, M.; Dey, G.; Das, T.N. Evaluation of optimum conditions for hydrogen generation in argon-water vapor dielectric barrier discharge. Int. J. Hydrogen Energy 2016, 41, 22769–22774. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S. Comprehensive analysis of hydrogen production from various water types using plasma: Water vapour decomposition in the presence of ammonia and novel reaction kinetics analysis. Int. J. Hydrogen Energy 2022, 52, 14–30. [Google Scholar] [CrossRef]
- Jasiński, M.; Czylkowski, D.; Hrycak, B.; Dors, M.; Mizeraczyk, J. Atmospheric pressure microwave plasma source for hydrogen production. Int. J. Hydrogen Energy 2013, 38, 11473–11483. [Google Scholar] [CrossRef]
- Chehade, G.; Lytle, S.; Ishaq, H.; Dincer, I. Hydrogen production by microwave based plasma dissociation of water. Fuel 2020, 264, 116831. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Faisal, A.; Hafeez, A.; Javed, F.; Mustafa, M.; Rehman, F. Hydrogen production through water vapors using optimized corona-DBD hybrid plasma micro-reactor. Fuel 2022, 331, 125838. [Google Scholar] [CrossRef]
- Bilbao, D.C.; Machin, E.B.; Pedroso, D.T. Operating parameters’ influence on hydrogen production performance in microwave-induced plasma. Int. J. Hydrogen Energy 2024, 80, 956–979. [Google Scholar] [CrossRef]
- Budhraja, N.; Pal, A.; Mishra, R. Plasma reforming for hydrogen production: Pathways, reactors and storage. Int. J. Hydrogen Energy 2022, 48, 2467–2482. [Google Scholar] [CrossRef]
- Greensfelder, B.; Voge, H.; Good, G. Catalytic and thermal cracking of pure hydrocarbons: Mechanisms of Reaction. Ind. Eng. Chem. 1949, 41, 2573–2584. [Google Scholar] [CrossRef]
- Sadrameli, S. Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: A state-of-the-art review II: Catalytic cracking review. Fuel 2016, 173, 285–297. [Google Scholar] [CrossRef]
- Heijkers, S.; Aghaei, M.; Bogaerts, A. Plasma-based CH4 conversion into higher hydrocarbons and H2: Modeling to reveal the reaction mechanisms of different plasma sources. J. Phys. Chem. C 2020, 124, 7016–7030. [Google Scholar] [CrossRef]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A Gen. 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Gong, J. Study on preparation of fuel oil from three kinds of molecular sieve catalytic cracking waste lubricating oil. In Proceedings of the 2021 3rd International Conference on Advanced Materials and Ecological Environment (AMEE2021), Shanghai, China, 18–19 December 2021; IOP Publishing: Bristol, UK, 2022. [Google Scholar]
- Bao, S.; Liu, G.; Zhang, X.; Wang, L.; Mi, Z. New method of catalytic cracking of hydrocarbon fuels using a highly dispersed nano-HZSM-5 catalyst. Ind. Eng. Chem. Res. 2010, 49, 3972–3975. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, H.; Zhang, S.; Han, W.; Zhang, C.; Yang, Q.; Shao, T. COx-free co-cracking of n-decane and CH4 to hydrogen and acetylene using pulsed spark plasma. Chem. Eng. J. 2022, 436, 135190. [Google Scholar] [CrossRef]
- Akande, O.; Lee, B. Plasma steam methane reforming (PSMR) using a microwave torch for commercial-scale distributed hydrogen production. Int. J. Hydrogen Energy 2022, 47, 2874–2884. [Google Scholar] [CrossRef]
- Aminu, I.; Nahil, M.A.; Williams, P.T. Hydrogen production by pyrolysis–nonthermal plasma/catalytic reforming of waste plastic over different catalyst support materials. Energy Fuels 2022, 36, 3788–3801. [Google Scholar] [CrossRef]
- Kheirollahivash, M.; Rashidi, F.; Moshrefi, M. Hydrogen production from methane decomposition using a mobile and elongating arc plasma reactor. Plasma Chem. Plasma Process. 2019, 39, 445–459. [Google Scholar] [CrossRef]
- Kreuznacht, S.; Purcel, M.; Böddeker, S.; Awakowicz, P.; Xia, W.; Muhler, M.; Böke, M.; Keudell, A. Comparison of the performance of a microwave plasma torch and a gliding arc plasma for hydrogen production via methane pyrolysis. Plasma Process. Polym. 2023, 20, 2200132. [Google Scholar] [CrossRef]
- Nguyen, S.-C.V.T. Hydrogen Production in a Radio-Frequency Plasma Source Operating on Water Vapor. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2009. [Google Scholar]
- Rahim, I.; Nomura, S.; Mukasa, S.; Toyota, H. Decomposition of methane hydrate for hydrogen production using microwave and radio frequency in-liquid plasma methods. Appl. Therm. Eng. 2015, 90, 120–126. [Google Scholar] [CrossRef]
- Xiao, H.; Harding, J.; Lei, S.; Chen, W.; Xia, S.; Cai, N.; Chen, X.; Hu, J.; Chen, Y.; Wang, X. Hydrogen and aromatics recovery through plasma-catalytic pyrolysis of waste polypropylene. J. Clean. Prod. 2022, 350, 131467. [Google Scholar] [CrossRef]
- Du, C.; Mo, J.; Tang, J.; Huang, D.; Mo, Z.; Wang, Q.; Ma, S.; Chen, Z. Plasma reforming of bio-ethanol for hydrogen rich gas production. Appl. Energy 2014, 133, 70–79. [Google Scholar] [CrossRef]
- Wang, B.; Shize, L.; Yeping, P.; Chengyu, W. Gliding arc plasma reforming of toluene for onboard hydrogen production. Int. J. Hydrogen Energy 2020, 45, 6138–6147. [Google Scholar]
- Zhao, Y.; Wang, L.; Zhang, J.-L.; Guo, H.-C. Influence of Non-Thermal Plasma Discharge Mode and Reactor Structure on Ammonia Decomposition to Hydrogen. Acta Phys. Chim. Sin. 2014, 30, 738–744. [Google Scholar]
- Yan, S.; Jiang, Y.M.; Liu, C.Z.; Chen, L.W.; Zhang, W.J.; Ding, J.; Li, J.G. Gliding Arc Discharge Plasma Assisted Decomposition of Ammonia into Hydrogen. J. Combust. Sci. Technol. 2011, 17, 186–190. [Google Scholar]
- Lin, Q.F.; Mo, J.; Tang, J.; Huang, D.; Mo, Z.; Wang, Q.; Ma, S.; Chen, Z. Instantaneous hydrogen production from ammonia by non-thermal arc plasma combining with catalyst. Energy Rep. 2021, 7, 4064–4070. [Google Scholar] [CrossRef]
- Tatarova, E.; Bundaleska, N.; Dias, F.M.; Tsyganov, D.; Saavedra, R.; Ferreira, C.M. Hydrogen production from alcohol reforming in a microwave “tornado”-type plasma. Plasma Sources Sci. Technol. 2013, 22, 065001. [Google Scholar] [CrossRef]
- Czylkowski, D.; Hrycak, B.; Jasinski, M.; Dors, M.; Mizeraczyk, J. Microwave plasma-based method of hydrogen production via combined steam reforming of methane. Energy 2016, 113, 653–661. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Zhu, T.; Zhu, X.; Sun, B. Characteristics of methane wet reforming driven by microwave plasma in liquid phase for hydrogen production. Int. J. Hydrogen Energy 2021, 46, 34105–34115. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, X.; Xin, Y.; Zhu, X. Large capacity hydrogen production by microwave discharge plasma in liquid fuels ethanol. Int. J. Hydrogen Energy 2017, 42, 24047–24054. [Google Scholar] [CrossRef]
- Ikeda, A.; Hunge, Y.M.; Teshima, K.; Uetsuka, H.; Terashima, C. Enhancing CO2 reduction: Insights from In-liquid microwave plasma chemical vapor deposition. Energy Fuels 2024, 38, 11918–11926. [Google Scholar] [CrossRef]
- Tominaga, Y.; Hunge, Y.M.; Kubota, N.; Ishida, N.; Sato, S.; Kondo, T.; Yuasa, M.; Uetsuka, H.; Terashima, C. Ultra-high growth rate of boron-doped diamond films with optimized growth parameters using in-liquid microwave plasma CVD. Diam. Relat. Mater. 2023, 140, 110543. [Google Scholar] [CrossRef]
- Czylkowski, D.; Hrycak, B.; Miotk, R.; Jasinski, M.; Dors, M.; Mizeraczyk, J. Hydrogen production by conversion of ethanol using atmospheric pressure microwave plasmas. Int. J. Hydrogen Energy 2015, 40, 14039–14044. [Google Scholar] [CrossRef]
- Zhu, T.; Sun, B.; Zhu, X.; Wang, L.; Xin, Y.; Liu, J. Mechanism analysis of hydrogen production by microwave discharge in ethanol liquid. J. Anal. Appl. Pyrolysis 2021, 156, 105111. [Google Scholar] [CrossRef]
- Niu, Y.-L.; Li, S.-Z.; Wang, X.-C.; Yu, Q.-K.; Yang, D.; Wen, X.; Zhang, J. Characteristic study of nitrogen microwave plasma decomposition of ammonia at atmospheric pressure for hydrogen production. Plasma Sources Sci. Technol. 2024, 33, 105018. [Google Scholar] [CrossRef]
- Wang, B.; Sun, B.; Zhu, X.; Yan, Z.; Liu, Y.; Liu, H.; Liu, Q. Hydrogen production from alcohol solution by microwave discharge in liquid. Int. J. Hydrogen Energy 2016, 41, 7280–7291. [Google Scholar] [CrossRef]
- Snoeckx, R.; Bogaerts, A. Plasma technology–a novel solution for CO2 conversion? Chem. Soc. Rev. 2017, 46, 5805–5863. [Google Scholar] [CrossRef]
- Hu, Y.; Li, G.; Yang, Y.; Gao, X.; Lu, Z. Hydrogen generation from hydro-ethanol reforming by DBD-plasma. Int. J. Hydrogen Energy 2012, 37, 1044–1047. [Google Scholar] [CrossRef]
- Wang, B.; Lü, Y.; Zhang, X.; Hu, S. Hydrogen generation from steam reforming of ethanol in dielectric barrier discharge. J. Nat. Gas Chem. 2011, 20, 151–154. [Google Scholar] [CrossRef]
- Sato, T.; Kambe, M.; Nishiyama, H. Analysis of a methanol decomposition process by a nonthermal plasma flow. JSME Int. J. Ser. B 2005, 48, 432–439. [Google Scholar] [CrossRef]
- Longmier, B.W.; Gallimore, A.D.; Hershkowitz, N. Hydrogen production from methane using an RF plasma source in total nonambipolar flow. Plasma Sources Sci. Technol. 2012, 21, 015007. [Google Scholar] [CrossRef]
- Nguyen, S.; Lemmer, K.; Gallimore, A.; Foster, J. An experimental study of hydrogen production by dissociation of water vapor in a radio frequency plasma source. In Proceedings of the 2007 16th IEEE International Pulsed Power Conference, Albuquerque, NM, USA, 17–22 June 2007; IEEE: Piscataway, NJ, USA, 2008. [Google Scholar]
- Yi, Y.; Wang, L.; Guo, Y.; Sun, S.; Guo, H. Plasma-assisted ammonia decomposition over Fe-Ni alloy catalysts for COx-Free hydrogen. Aiche J. 2019, 65, 691–701. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y.; Yao, S. Oxidative reforming of n-heptane in gliding arc plasma reformer for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 22831–22840. [Google Scholar] [CrossRef]
- Andersen, J.A.; Christensen, J.M.; Ostberg, M.; Bogaerts, A.; Jensen, A.D. Plasma-catalytic ammonia decomposition using a packed-bed dielectric barrier discharge reactor. Int. J. Hydrogen Energy 2022, 47, 32081–32091. [Google Scholar] [CrossRef]
- Garcia-Villalva, R.; Biset-Peiró, M.; Alarcón, A.; Bacariza, C.; Murcia-López, S.; Guilera, J. Comparison of methane reforming routes for hydrogen production using dielectric barrier discharge plasma-catalysis. Int. J. Hydrogen Energy 2024, 59, 1367–1375. [Google Scholar] [CrossRef]
- Muellerlanger, F.; Tzimas, E.; Kaltschmitt, M.; Peteves, S. Techno-economic assessment of hydrogen production processes for the hydrogen economy for the short and medium term. Int. J. Hydrogen Energy 2007, 32, 3797–3810. [Google Scholar] [CrossRef]
- Hu, S.; Guo, B.; Ding, S.; Yang, F.; Dang, J.; Liu, B.; Gu, J.; Ma, J.; Ouyang, M. A comprehensive review of alkaline water electrolysis mathematical modeling. Appl. Energy 2022, 327, 120099. [Google Scholar] [CrossRef]
- Sebbahi, S.; Assila, A.; Alaoui Belghiti, A.; Laasri, S.; Kaya, S.; Hlil, E.K.; Rachidi, S.; Hajjaji, A. A comprehensive review of recent advances in alkaline water electrolysis for hydrogen production. Int. J. Hydrogen Energy 2024, 82, 583–599. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, H.; Wu, Y.; Wang, J.; Yamauchi, Y.; Kim, J.; Osman, S.M.; Xu, X.; Wang, L.; Wang, C.; et al. Construction of a 2D lamellar membrane for a combination of photocatalytic hydrogen evolution and photothermal water evaporation. Chem. Eng. J. 2023, 471, 144395. [Google Scholar] [CrossRef]
- Lee, B.; Chae, H.; Choi, N.H.; Moon, C.; Moon, S.; Lim, H. Economic evaluation with sensitivity and profitability analysis for hydrogen production from water electrolysis in Korea. Int. J. Hydrogen Energy 2017, 42, 6462–6471. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Jiao, L. PEM water electrolysis for hydrogen production: Fundamentals, advances, and prospects. Carbon Neutrality 2022, 1, 21. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.-B. The promise of hydrogen production from alkaline anion exchange membrane electrolyzers. Nano Energy 2021, 87, 106162. [Google Scholar] [CrossRef]
- Li, X.; Yao, Y.; Tian, Y.; Jia, J.; Ma, W.; Yan, X.; Liang, J. Recent advances in key components of proton exchange membrane water electrolysers. Mater. Chem. Front. 2024, 8, 2493–2510. [Google Scholar] [CrossRef]
- Taghvaei, H.; Jahanmiri, A.; Rahimpour, M.R.; Shirazi, M.M.; Hooshmand, N. Hydrogen production through plasma cracking of hydrocarbons: Effect of carrier gas and hydrocarbon type. Chem. Eng. J. 2013, 226, 384–392. [Google Scholar] [CrossRef]
- Blom, P.; Basson, G. Non-catalytic plasma-arc reforming of natural gas with carbon dioxide as the oxidizing agent for the production of synthesis gas or hydrogen. Int. J. Hydrogen Energy 2013, 38, 5684–5692. [Google Scholar] [CrossRef]
- Chaffin, J.H.; Bobbio, S.M.; Inyang, H.I.; Kaanagbara, L. Hydrogen Production by Plasma Electrolysis. J. Energy Eng. 2006, 132, 104–108. [Google Scholar] [CrossRef]
- Mizuno, T.; Akimoto, T.; Azumi, K.; Ohmori, T.; Aoki, Y.; Takahashi, A. Hydrogen Evolution by Plasma Electrolysis in Aqueous Solution. Jpn. J. Appl. Phys. 2005, 44, 396. [Google Scholar] [CrossRef]
- Yulianto, E.; Wardaya, A.Y.; Nur, M. Study of Plasma Electrolysis in Water: Alternative Technologies for Hydrogen Production. Trends Sci. 2022, 19, 1033. [Google Scholar] [CrossRef]
- Lee, D.H.; Kang, H.; Kim, Y.; Song, H.; Lee, H.; Choi, J.; Kim, K.-T.; Song, Y.-H. Plasma-assisted hydrogen generation: A mechanistic review. Fuel Process. Technol. 2023, 247, 107761. [Google Scholar] [CrossRef]
- Wang, G.; Chen, W.; Chen, G.; Huang, J.; Song, C.; Chen, D.; Du, Y.; Li, C.; Ostrikov, K.K. Trimetallic Mo–Ni–Co selenides nanorod electrocatalysts for highly-efficient and ultra-stable hydrogen evolution. Nano Energy 2020, 71, 104637. [Google Scholar] [CrossRef]
- Park, Y.K.; Kim, B.J.; Jeong, S.; Jeon, K.J.; Chung, K.H.; Jung, S.C. Characteristics of hydrogen production by photocatalytic water splitting using liquid phase plasma over Ag-doped TiO2 photocatalysts. Environ. Res. 2020, 188, 109630. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, X.; Wang, Q.; Li, D.-s.; Li, X.; Li, X.; Chen, L.; Zhang, X.; Tian, X.; Ostrikov, K. Plasma synthesis of Pt/g-C3N4 photocatalysts with enhanced photocatalytic hydrogen generation. J. Alloy. Compd. 2021, 873, 159871. [Google Scholar] [CrossRef]
- Gherardi, N.; Mizeraczyk, J.; Jasiński, M.; Marotta, E.; Paradisi, C. Plasma processing methods for hydrogen production. Eur. Phys. J. Appl. Phys. 2016, 75, 24702. [Google Scholar]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Isherwood, W.; Smith, J.R.; Aceves, S.M.; Berry, G.; Clark, W.; Johnson, R.; Das, D.; Goering, D.; Seifert, R. Remote power systems with advanced storage technologies for Alaskan villages. Energy 2000, 25, 1005–1020. [Google Scholar] [CrossRef]
- Abánades, A.; Rubbia, C.; Salmieri, D. Technological challenges for industrial development of hydrogen production based on methane cracking. Energy 2012, 46, 359–363. [Google Scholar] [CrossRef]
- Barni, R.; Benocci, R.; Spinicchia, N.; Roman, H.E.; Riccardi, C. An Experimental Study of Plasma Cracking of Methane Using DBDs Aimed at Hydrogen Production. Plasma Chem. Plasma Process. 2018, 39, 241–258. [Google Scholar] [CrossRef]
- Sehested, J.; Gelten, J.A.; Remediakis, I.N.; Bengaard, H.; Nørskov, J. Sintering of nickel steam-reforming catalysts: Effects of temperature and steam and hydrogen pressures. J. Catal. 2004, 223, 432–443. [Google Scholar] [CrossRef]
- Li, S.; Gong, J. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef]
- Sehested, J. Four challenges for nickel steam-reforming catalysts. Catal. Today 2006, 111, 103–110. [Google Scholar] [CrossRef]
- Putra, A.E.E.; Nomura, S.; Mukasa, S.; Toyota, H. Hydrogen production by radio frequency plasma stimulation in methane hydrate at atmospheric pressure. Int. J. Hydrogen Energy 2012, 37, 16000–16005. [Google Scholar] [CrossRef]
- Khalifeh, O.; Taghvaei, H.; Mosallanejad, A.; Rahimpour, M.R.; Shariati, A. Extra pure hydrogen production through methane decomposition using nanosecond pulsed plasma and Pt–Re catalyst. Chem. Eng. J. 2016, 294, 132–145. [Google Scholar] [CrossRef]
- Khoja, A.H.; Azad, A.K.; Saleem, F.; Khan, B.A.; Naqvi, S.R.; Mehran, M.T.; Amin, N.A.S. Hydrogen Production from Methane Cracking in Dielectric Barrier Discharge Catalytic Plasma Reactor Using a Nanocatalyst. Energies 2020, 13, 5921. [Google Scholar] [CrossRef]
- Gao, N.; Cheng, M.; Quan, C.; Zheng, Y. Syngas production via combined dry and steam reforming of methane over Ni-Ce/ZSM-5 catalyst. Fuel 2020, 273, 117702. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Zhang, S.; Sun, H.; Li, J.; Shao, T. Nanosecond pulsed plasma assisted dry reforming of CH4: The effect of plasma operating parameters. Appl. Energy 2019, 243, 132–144. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Q.; Qin, W.; Wu, H.; Liu, B.; Li, S.; Bogaerts, A. Plasma-catalytic dry reforming of CH4: Effects of plasma-generated species on the surface chemistry. Chem. Eng. J. 2024, 498, 155847. [Google Scholar] [CrossRef]
- Mountouris, A.; Voutsas, E.; Tassios, D. Plasma gasification of sewage sludge: Process development and energy optimization. Energy Convers. Manag. 2008, 49, 2264–2271. [Google Scholar] [CrossRef]
- Vij, D. Urbanization and Solid Waste Management in India: Present Practices and Future Challenges. Procedia Soc. Behav. Sci. 2012, 37, 437–447. [Google Scholar] [CrossRef]
- Leal-Quiros, E. Plasma Processing of Municipal Solid Waste. Braz. J. Phys. 2004, 34, 1587–1593. [Google Scholar] [CrossRef]
- Galeno, G.; Minutillo, M.; Perna, A. From waste to electricity through integrated plasma gasification/fuel cell (IPGFC) system. Int. J. Hydrogen Energy 2010, 36, 1692–1701. [Google Scholar] [CrossRef]
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef]
- Green, D.W. Perry’s Chemical Engineer Handbook; McGraw Hill: New York, NY, USA, 2007; p. 2400. [Google Scholar]
- Pourali, M. Application of Plasma Gasification Technology in Waste to Energy—Challenges and Opportunities. IEEE Trans. Sustain. Energy 2010, 1, 125–130. [Google Scholar] [CrossRef]

| Hydrogen Technology | Working Temperature/(°C) | Advantages | Disadvantages | Industrialization |
|---|---|---|---|---|
| AWE | 30~80 | low cost | low current density | industrialize |
| quick response; | ||||
| PEMWE | 55~65 | low power consumption; | high water quality requirements | special applications |
| AEMWE | 40~60 | low cost | poor thermal stability | laboratory phase |
| SOEC | 700~900 | high electrolysis efficiency | rapid material degradation; | laboratory phase |
| short operating life; high manufacturing costs | ||||
| PWE | energy consumption | high requirements for equipment | early stage of commercialization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Guo, X.; Liu, J.; Wang, C.; Wang, Y.; Qiu, Y.; Ling, Z.; Zeng, X.; Yuan, D. Plasma-Assisted Hydrogen Production: Technologies, Challenges, and Future Prospects. Processes 2025, 13, 1157. https://doi.org/10.3390/pr13041157
Wang L, Guo X, Liu J, Wang C, Wang Y, Qiu Y, Ling Z, Zeng X, Yuan D. Plasma-Assisted Hydrogen Production: Technologies, Challenges, and Future Prospects. Processes. 2025; 13(4):1157. https://doi.org/10.3390/pr13041157
Chicago/Turabian StyleWang, Lijian, Xiaowei Guo, Jianzheng Liu, Chao Wang, Yi Wang, Yi Qiu, Zhongqian Ling, Xianyang Zeng, and Dingkun Yuan. 2025. "Plasma-Assisted Hydrogen Production: Technologies, Challenges, and Future Prospects" Processes 13, no. 4: 1157. https://doi.org/10.3390/pr13041157
APA StyleWang, L., Guo, X., Liu, J., Wang, C., Wang, Y., Qiu, Y., Ling, Z., Zeng, X., & Yuan, D. (2025). Plasma-Assisted Hydrogen Production: Technologies, Challenges, and Future Prospects. Processes, 13(4), 1157. https://doi.org/10.3390/pr13041157








