1. Introduction
Food packaging preserves food quality and microbiological integrity while also serving as a means of conveying product information. Various materials, including paper, glass, metal, and plastic, have long been utilized in food packaging [
1]. At present, around half of all food packaging materials originate from fossil fuels [
2]. According to the data from the Center for Environmental Improvement (2022), as much as 79% of plastic originating from fossil fuels ends up in landfills. As a consequence of the degradation of plastic, microplastic particles are created and can easily accumulate and enter the food chain [
3]. Hence, it is imperative to prevent and reduce the disposal of plastic and preserve resources. One of the solutions is the replacement of plastic and the introduction of packaging materials made of natural biopolymers [
4,
5]. Edible films can enhance the shelf life of packaged food by offering protection against microorganisms and oxidation, while also boosting its nutritional content and sensory attributes [
6]. Certain types of food packed in edible films can be consumed without removing the packaging, pointing out one more advantage of this packaging [
7,
8].
Starch is a widely available biopolymer in nature, making it a highly economical choice for producing edible films [
1,
9]. Thanks to characteristics such as transparency, the absence of smell and taste, and gelling ability, starch can compete with most polymers obtained from fossil fuels [
10]. For the production of films, it can often be combined with other biopolymers, such as gelatin [
11,
12], chitosan [
13], and carrageenan [
14], to enhance mechanical and barrier characteristics. To improve the antioxidant and antimicrobial properties of edible films, different fortifying agents can be added, such as essential oils [
15,
16], probiotics and prebiotics [
17], or plant extracts [
18,
19]. Intelligent packaging has the ability to monitor the integrity of food, detect changes and irregularities, indicate the condition of packaged food and thus warn of potential problems [
20]. The advancement of intelligent starch-based packaging films is attracting considerable attention, especially in the design of pH-responsive films. These films can detect and signal pH changes in packaged food, which often result from spoilage [
13,
18,
21,
22].
Wild blackberry (
Rubus sp.), a perennial species in the
Rosaceae family, contains a diverse range of bioactive compounds, including essential vitamins, minerals, flavonoids, terpenes, tannins, a variety of acids, and lipids, all of which contribute to its nutritional and therapeutic value [
23]. Among the phenolic compounds, anthocyanins are the most common, while ellagitannins, flavonols, and hydroxybenzoic acids are also present [
24,
25]. Anthocyanins are pigments responsible for the color of the wild blackberries, but can also be hydrogen donors and have the ability to neutralize monovalent oxygen. These properties contribute to the pronounced antioxidant activity of blackberries [
26]. Apart from polyphenols, the antioxidant activity of blackberries mostly relies on the present ascorbic acid [
27]. Additionally, polyphenols present in blackberry extract can have antimicrobial activity [
27,
28]. In addition to their antimicrobial and antioxidant potential, anthocyanins can to change color at different pH values, so they can be used as indicators [
29]. Blackberry extract can be utilize in the pH-sensitive packaging production due to its high anthocyanin content, which is sensitive to pH fluctuations in the surrounding environment [
30].
In response to the increasing demand for alternative food packaging solutions, this study focuses on developing and characterizing starch-based pH-sensitive packaging films that are enriched with different concentrations of wild blackberry extract (WBE) to improve the properties of the films.
2. Materials and Methods
2.1. Preparation of Wild Blackberry Extract
A water-based extract of wild blackberry (
Rubus sp.) was obtained using a modified protocol adapted from Norajit et al. (2010) [
31]. Fresh wild blackberry fruits were initially finely chopped and then subjected to extraction with distilled water at a 1:10 ratio. The extraction process was carried out using a SCILOGEX SCI280-Pro stirrer (Rocky Hill, CT, USA) at 50 °C for a duration of two hours. Following extraction, the mixture was passed through Whatman No. 3 filter paper to eliminate any remaining fruit residues. The purified extract was then stored in a refrigerator until it was needed for further analysis.
2.2. Determination of Dry Matter Content in the Wild Blackberry Extract
To determine the dry matter content, the wild blackberry extract (WBE) sample was dehydrated at 105 °C until a constant mass was reached. A 10 mL portion of WBE was transferred into a pre-weighed flask and subjected to drying. After the drying process, the sample was placed in a desiccator to cool, and its weight was subsequently recorded using an analytical balance (Joanlab, Ningbo, China). The dry matter content was determined based on dry residue content and expressed as mg/mL of extract.
2.3. Preparation of Starch-Based Films
Edible packaging films were produced following the methodology described by Al-Hashimi et al. (2020) [
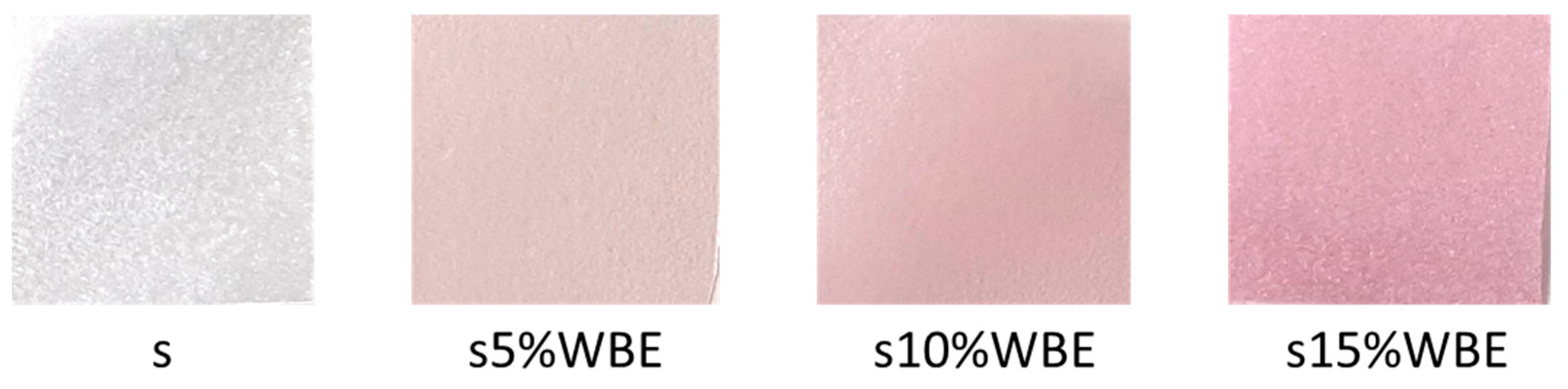
16]. The films were fabricated using the casting technique, where 4.5 g of starch (Centrohem doo, Stara Pazova, Serbia) was dissolved in 130 mL of distilled water. The solution was continuously stirred on a magnetic stirrer (SCILOGEX SCI280-Pro, Rocky Hill, CT, USA) at 75 °C and 400 rpm until it formed a gelatinous consistency. Subsequently, 1.1 mL of glycerol was introduced, and stirring continued for an additional 10 min. Wild blackberry extract (WBE) was incorporated into the starch matrix at concentrations of 0, 5, 10, and 15%
w/w (expressed as grams of dry extract per gram of prepared film). The mixture was then homogenized using an ultrasonic homogenizer (Witeg, Wertheim, Germany) at 13,500 rpm for 3 min. Finally, the prepared solution was poured into Petri dishes (ϕ16 cm) and allowed to dry at room temperature for 48 h.
2.4. Determination pH Sensitive Properties of Starch-Based Films
The color-responsive behavior of starch-based films in different pH environments was analyzed using a modified approach based on Prietto et al. (2017) [
32]. To evaluate pH sensitivity, a 2 cm × 2 cm film sample was exposed to a drop of either 0.1 M HCl solution, distilled water, or 0.1 M NaOH. The resulting color change was visually assessed by comparing the film’s appearance to its original color in contact with water. Each experiment was performed in triplicate to ensure reliability and accuracy.
2.5. Determination of Mechanical Properties of Starch-Based Films
The thickness of the films was measured using a thickness gauge (INSIZE 2364-10, Precision Measurement, Suzhou, China) following the ISO 4593:1993 standard [
33]. Measurements were taken at five different locations to ensure accuracy [
34].
Tensile strength (MPa) and elongation at break (%) were determined using a texture analyzer (TA.XT plus, Stable Micro Systems, Godalming, UK) according to the method described by Dordevic et al. (2023) [
35]. Film strips measuring 15 cm × 1 cm were cut and tested following the ASTM D882-02 standard [
36].
2.6. Assessment of Water Content, Swelling Behavior, and Solubility of Starch-Based Films
The films were analyzed for their water content, swelling behavior, and solubility using a modified procedure based on Souza et al. (2017) [
37]. Film samples were cut into 2 cm × 2 cm squares and initially weighed on an analytical scale, with the recorded mass designated as W
1. The samples were then dried in a laboratory dryer (Sutjeska, Belgrade, Serbia) at 105 °C for 2 h and reweighed (W
2). Following this, they were submerged in 25 mL of water and allowed to soak at room temperature for 24 h. After the immersion period, the samples were carefully blotted dry using filter paper and weighed again (W
3). Finally, the films underwent a second drying process at 105 °C for 24 h, after which their final weight (W
4) was recorded. The analyses were performed in triplicate. The values were calculated according to Equations (1)–(3):
2.7. Measurement of Water Vapor Permeability in Starch-Based Films
A modified gravimetric method was employed to assess the water vapor permeability of the films, following the procedure described by [
38]. A vial was filled with silica gel, and its opening was sealed with the film sample under evaluation. The vial was then weighed using an analytical scale and subsequently placed in a desiccator containing distilled water at room temperature. During three days, the samples were weighted and the water vapor permeability was calculated as (Equation (4)):
where W—increase in the sample weight (g), x—film thickness (m), t—elapsed time (s), A—surface of leakage area (m
2), and P—partial pressure difference between pure water vapor and dry atmosphere (2339 Pa at 20 °C).
The experiment was performed in three replications and expressed as µg × m−1 × s−1 × Pa−1.
2.8. Determination of Antioxidant Activity of Extract and Starch-Based Films
The antioxidant activity was evaluated using the DPPH method [
34]. To prepare the samples, starch-based films were crushed, and 0.1 g of each was mixed with 20 mL of ethanol. The same process was used to evaluate the antioxidant properties of WBE. The samples were subjected to sonication for 30 min and then filtered. A 3 mL aliquot of the resulting filtrate was combined with 1 mL of a 0.1 mM DPPH radical solution (Sigma Aldrich, St. Louis, MO, USA) in ethanol and left to incubate for 30 min in the dark. The absorbance at 517 nm was measured using a UV-VIS spectrophotometer (model UV-M51, Bel Engineering, Monza, Italy), with ethanol serving as the blank. The DPPH radical neutralization capacity was determined according to Equation (5):
where AbsDPPH—DPPH solution absorbance, Abs sample—sample absorbance.
2.9. Determination of Total Polyphenols of Starch-Based Films
The total polyphenol content was measured using the method described by Dou et al. (2018) [
39]. The samples were dissolved in distilled water at a ratio of 1:40. After 10 min, 5 mL of Folin–Ciocalteu reagent was added to 1 mL of the solution, followed by the addition of 4 mL of 7.5% Na
2CO
3. The mixture was then left in the dark for 30 min. Absorbance was recorded at 765 nm, with distilled water serving as the blank and gallic acid being used as the standard for quantification.
2.10. Measurement of Bioactive Component Migration from Starch-Based Films
The migration of bioactive components was assessed using the method outlined by Dordevic et al. (2021) [
34]. Film samples measuring 1 cm × 1 cm were placed in pre-labeled tubes containing 2.5 mL of a 10% ethanol solution and incubated at 30 °C for 10 days. After this period, the solution was filtered to remove any film remnants. The analysis involved measuring both the total polyphenol content and the DPPH radical scavenging ability of the filtrate.
2.11. Assessment of Antimicrobial Properties of Extract and Starch-Based Films
The disk diffusion method, described in the guidelines of the European Committee for Antimicrobial Susceptibility Testing (EUCAST) was used for determination of antimicrobial activity. Disks, each 5 mm in diameter, were cut from the films and disinfected by exposure to UV light (260 nm) for 60 s. The effectiveness was tested against Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, Proteus vulgaris ATCC 8427, Pseudomonas aeruginosa ATCC 27853, Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 25923, Candida albicans ATCC 2091, and Bacillus cereus. The inoculum concentration was approximately 1–2 × 108 CFU/mL, in line with the McFarland 0.5 turbidity standard. Using sterile swabs, the inoculum was spread across the surface of the substrates. For bacterial cultures, nutrient agar (Torlak, Belgrade, Serbia) was used, and for yeast, Sabouraud maltose agar (Torlak, Belgrade, Serbia). For the determination of the antimicrobial activity of the extract, 50 µL of WBE was placed on paper disks 5 mm in diameter, with water used as a control sample. The disks of films and paper disks with WBE were placed on the inoculated surfaces, and the plates were incubated at 35–37 °C for 24 h. Antimicrobial activity was assessed visually, and inhibition zones were expressed in mm.
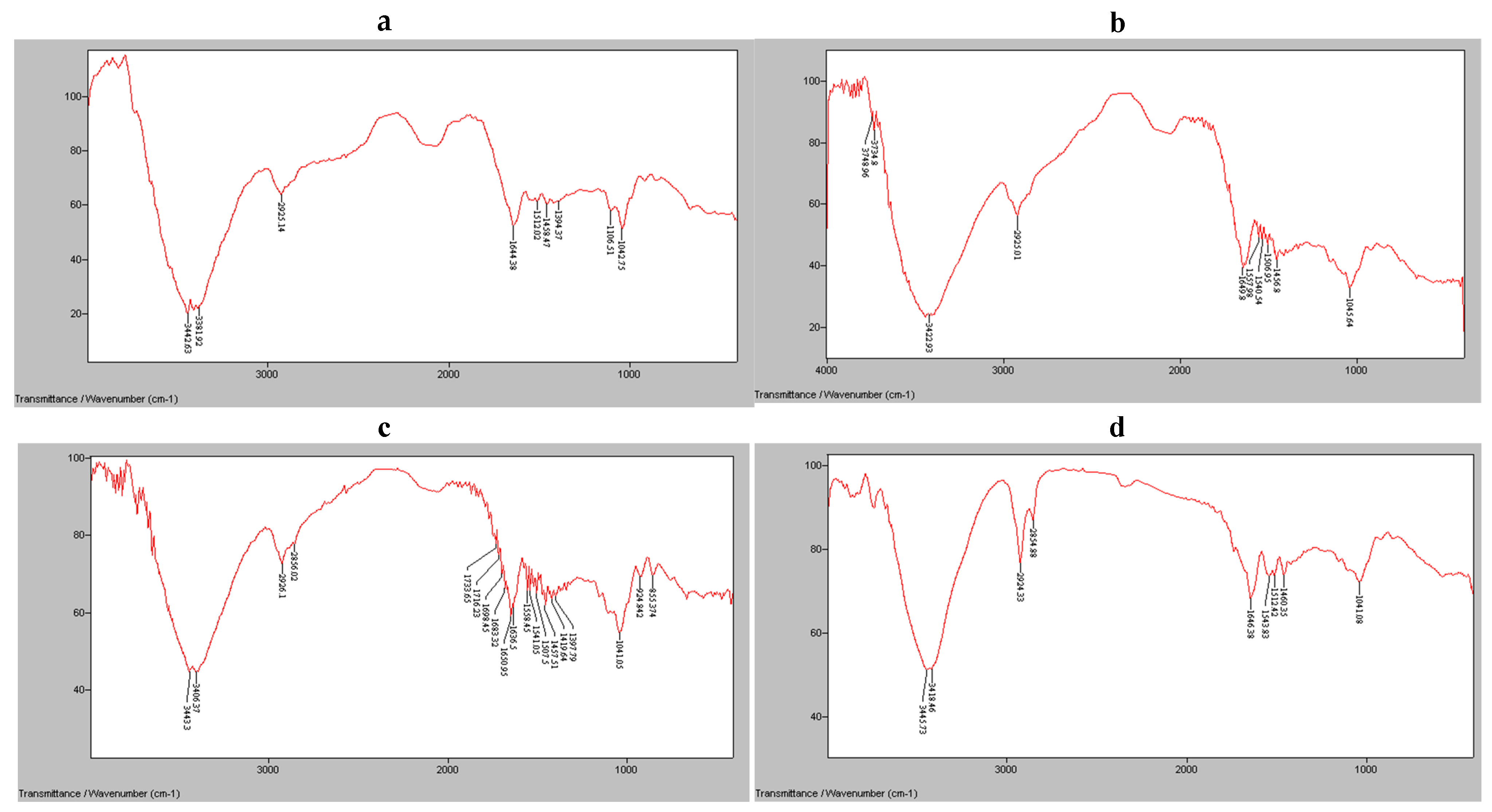
2.12. FTIR Analysis of Starch-Based Films
Fourier transform infrared (FTIR) spectroscopy was used to analyze starch-based edible films containing blackberry extract. The FTIR spectra were obtained in the range of 400–4000 cm
−1 [
40]. To prepare the KBr pellet, 0.1 g of the film was finely ground and blended with 1.5 g of KBr. The measurements were performed using a BOMEM MB-100 spectrometer (Hartmann & Braun, Brampton, ON, Canada), which was equipped with a KBr detector and had a resolution of 4 cm
−1.
2.13. Statistical Analysis
The data are expressed as mean values ± standard deviation. To determine statistically significant differences among the samples, a one-way ANOVA was conducted, followed by Tukey’s post hoc test. Statistical analysis was carried out using SPSS 21.0 software (IBM, New York, NY, USA), with a significance threshold set at p < 0.05.
4. Conclusions
The incorporation of WBE into starch-based films significantly altered their mechanical, physical, barrier, and functional properties, making them suitable for use in packaging perishable products. The films exhibited pH-sensitive behavior, changing color in response to environmental pH variations, and demonstrated strong antimicrobial activity against a range of bacterial strains. Although the addition of WBE led to a decrease in tensile strength, it improved the films’ flexibility and toughness. Additionally, the increased water content and solubility suggest that the hydrophilic nature of the extract influenced the films’ water absorption properties. The films also displayed notable antioxidant activity, further enhancing their potential for packaging sensitive ingredients. In conclusion, these findings emphasize the potential of using WBE to create biodegradable and intelligent packaging materials with antimicrobial, pH-sensitive, and antioxidant properties.