Comparison of Thermochemical Conversion Processes for Antibiotic Residues: Insights from Life Cycle Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Goal and Scope Definition
2.2. Life Cycle Inventory
2.2.1. Composition of Antibiotic Residue
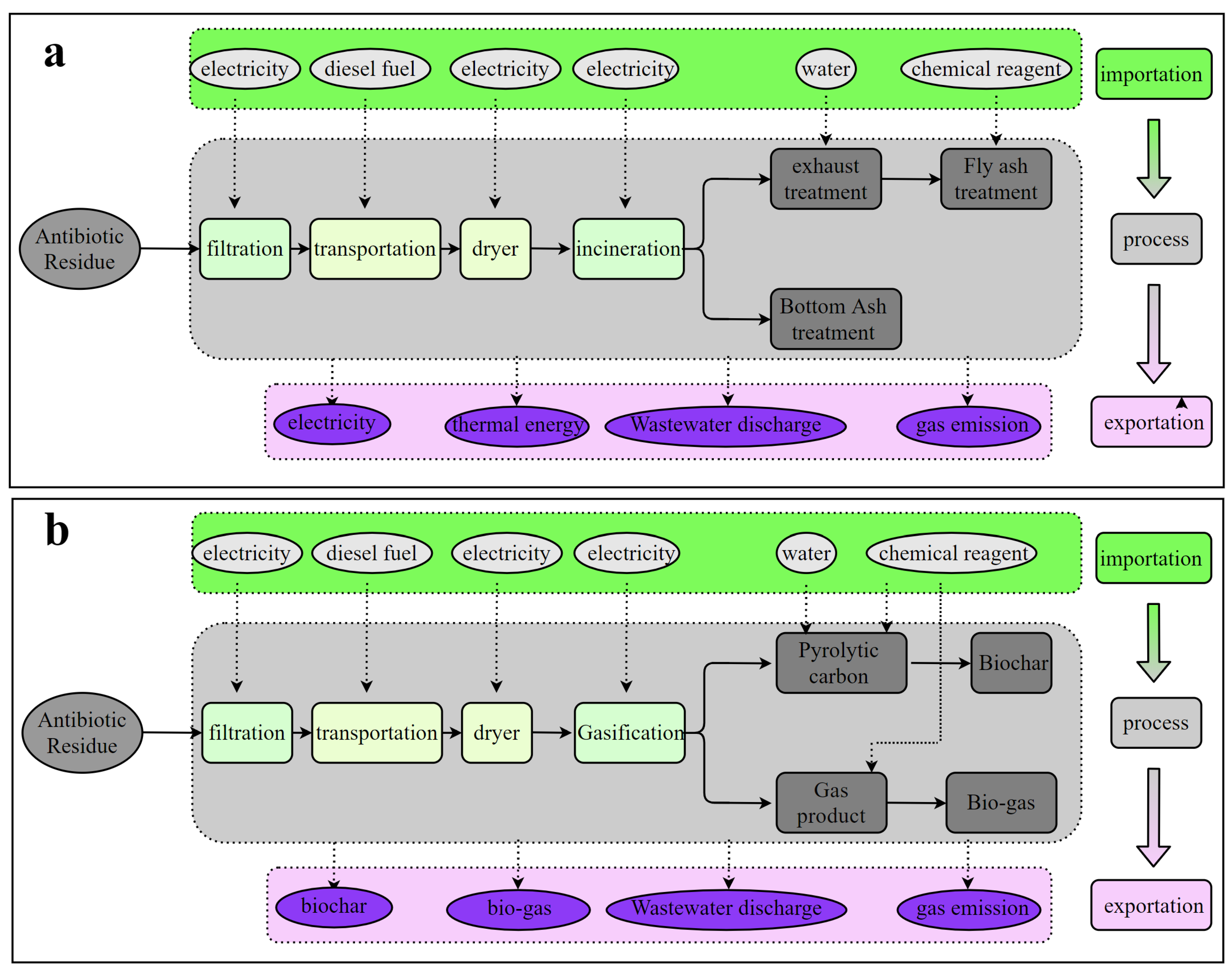
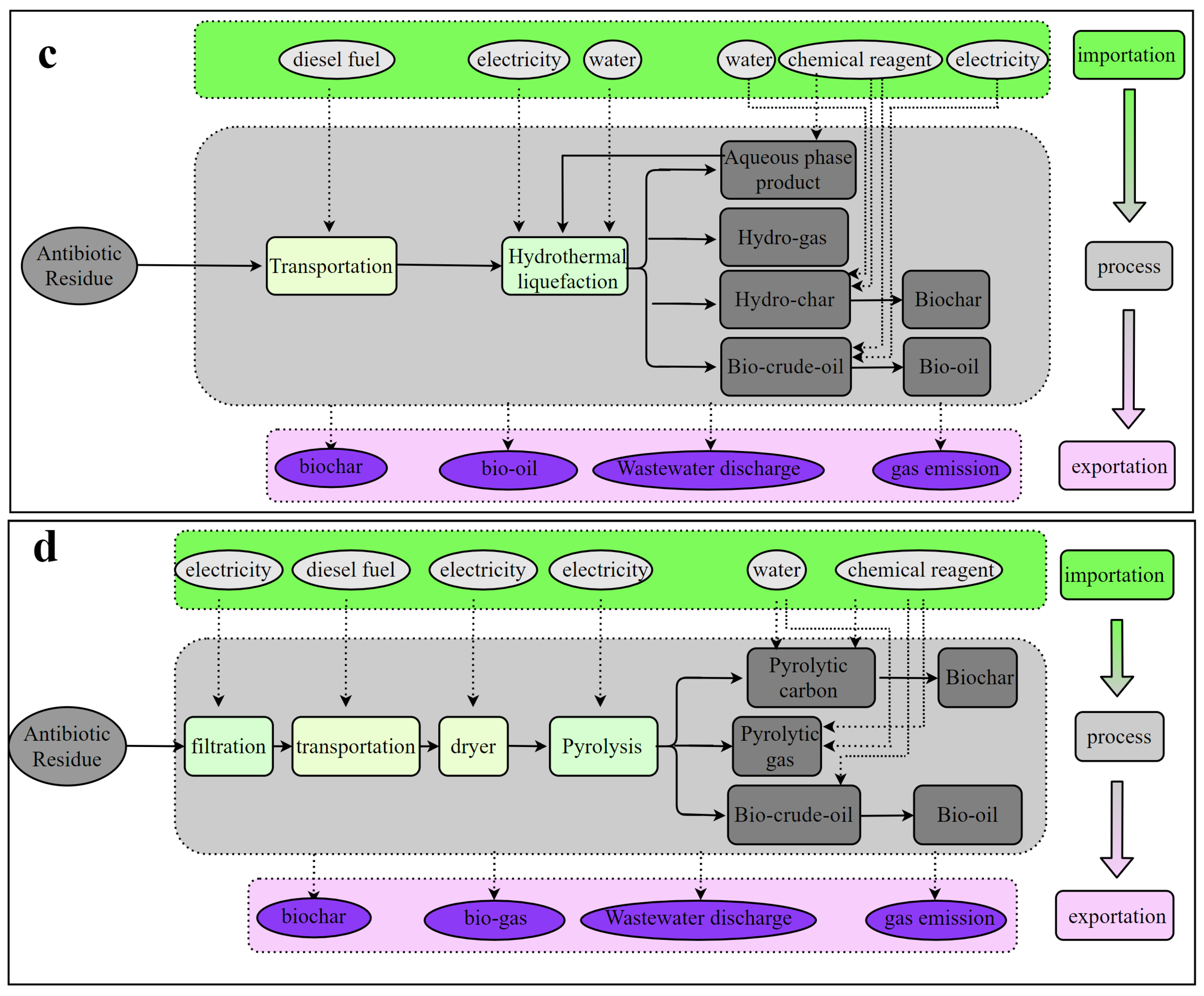
2.2.2. Technology Configuration
2.3. Sensitivity Ratios of LCA
2.4. Impact Assessment and Interpretation of Results
3. Results and Discussion
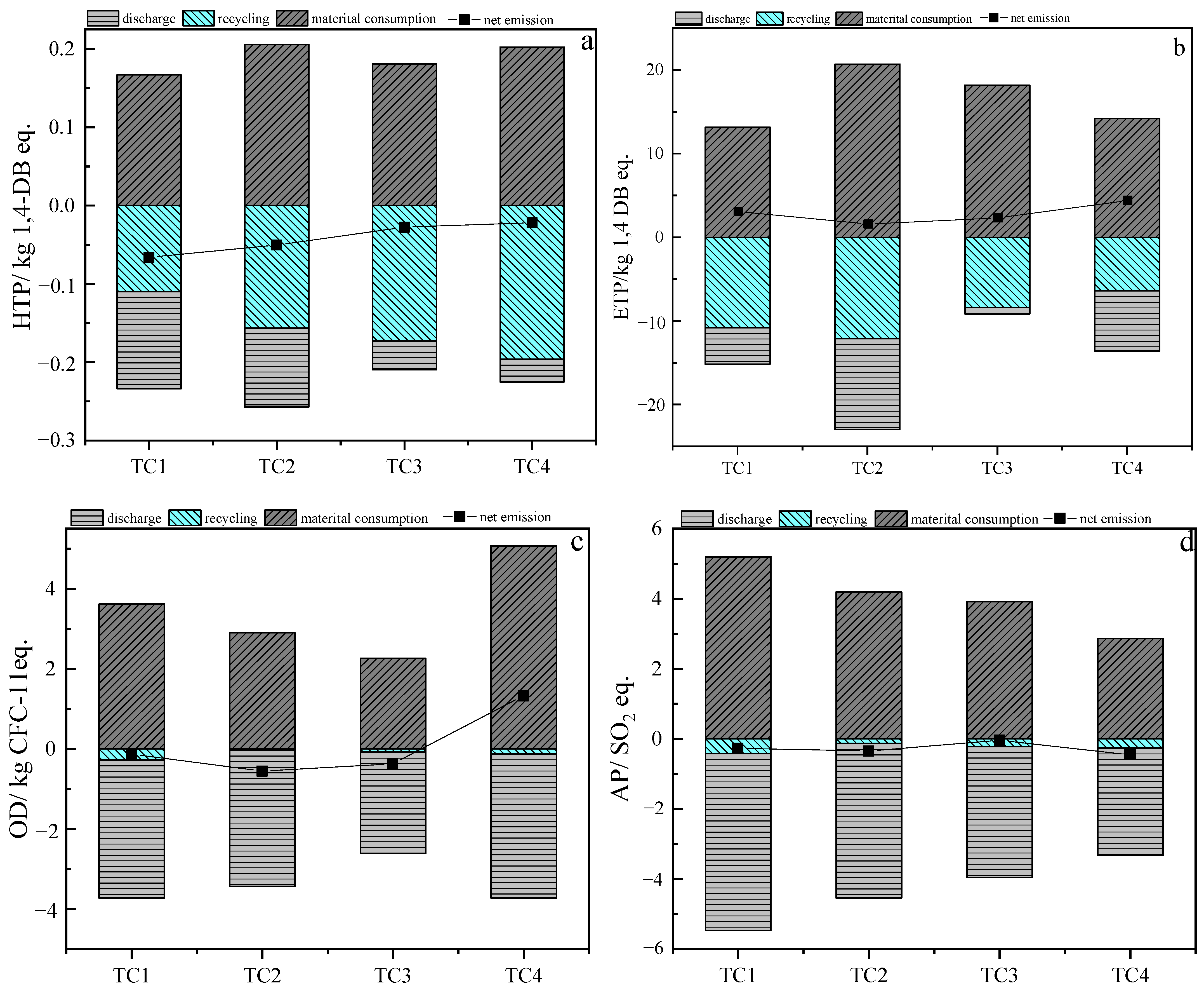
3.1. Analysis of All Midpoint Indicators for the Life Cycle Assessment of Four Processes
3.1.1. Effect of Low Differences Middle-Points
3.1.2. Analysis of Resource Consumption and Environmental Effects of Thermochemical Transformation of Antibiotic Residues
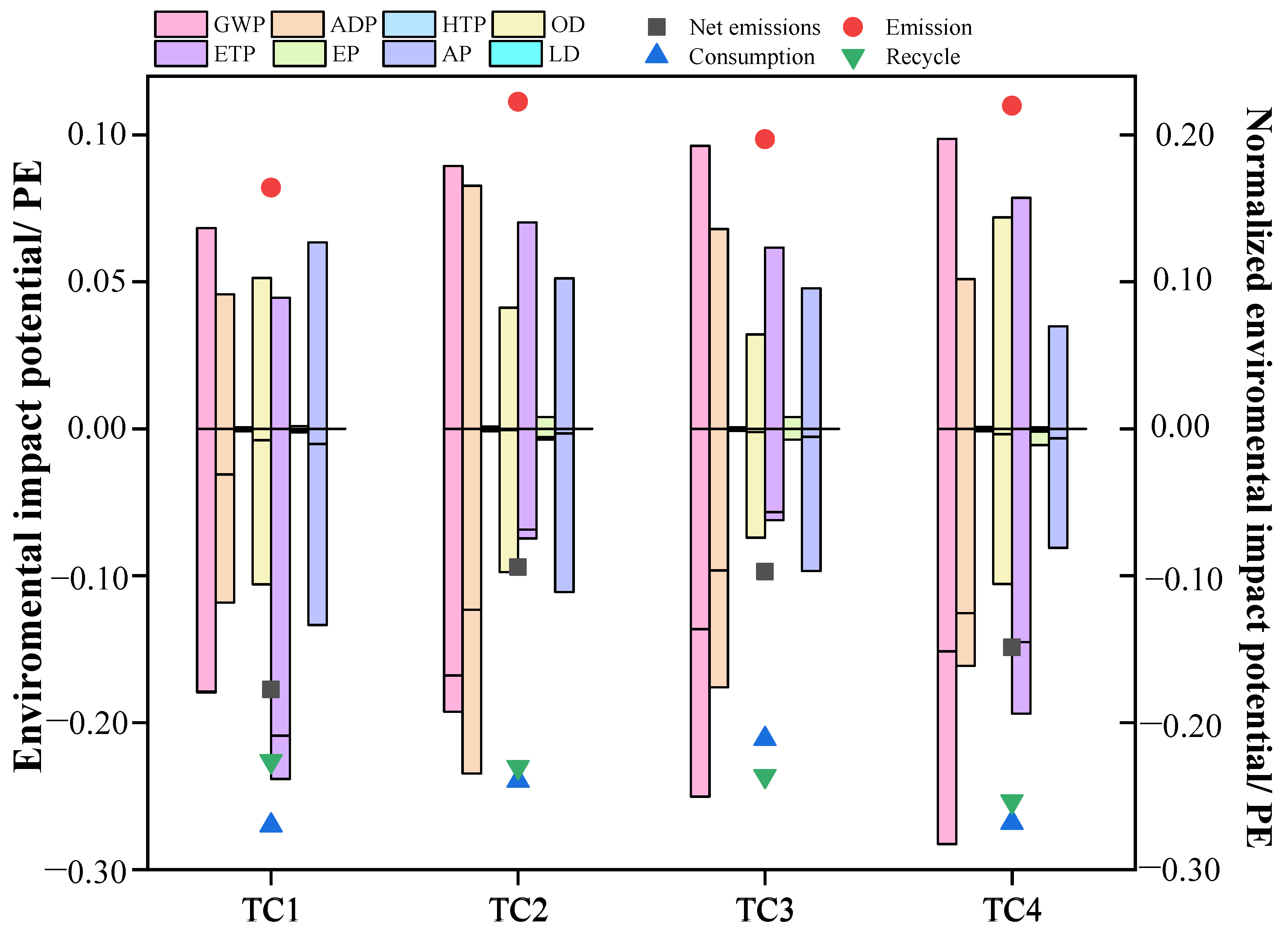
3.1.3. Integrated Assessment of Human Health and Ecological Impact Potentials
3.2. Sensitivity Analysis
3.3. Normalized Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LCA | Life cycle assessment |
| GWP | Global warming potential |
| ODP | Ozone depletion potential |
| AP | Acidification potential |
| EP | Eutrophication potential |
| HTP | Human toxicity potential |
| ETP | Ecotoxicity potential |
| ADP | Abiotic depletion potential |
| FC | Freshwater consumption |
| FE | Freshwater ecotoxicity |
| TE | Terrestrial ecotoxicity |
References
- Yang, J.; Ma, R.; Xing, Y.; Ma, H.Z.; Dong, B.Q.; Li, H.X.; Hong, C.; Xia, C. Study on the prepared bio-oil by catalytic hydrothermal liquefaction of antibiotic residues in ethanol-water reaction system with transition metal modified hzsm-5. Fuel 2023, 331, 125413. [Google Scholar] [CrossRef]
- Yang, W.Q.; Li, J.; Yao, Z.L.; Li, M. A review on the alternatives to antibiotics and the treatment of antibiotic pollution: Current development and future prospects. Sci. Total Environ. 2024, 926, 171757. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hong, C.; Li, Z.X.; Xing, Y.; Zhao, X.M. Study on hydrothermal liquefaction of antibiotic residues for bio-oil in ethanol-water system. Waste Manag. 2021, 120, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ying, G.G.; Deng, W.J. Antibiotic residues in food: Extraction, analysis, and human health concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Ben, Y.J.; Fu, C.X.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C.M. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Robles-Jimenez, L.E.; Aranda-Aguirre, E.; Castelan-Ortega, O.A.; Shettino-Bermudez, B.S.; Ortiz-Salinas, R.; Miranda, M.; Li, X.; Angeles-Hernandez, J.C.; Vargas-Bello-Pérez, E.; Gonzalez-Ronquillo, M. Worldwide traceability of antibiotic residues from livestock in wastewater and soil: A systematic review. Animals 2022, 12, 60. [Google Scholar] [CrossRef]
- Ohno, A.; Ano, T.; Shoda, M. Use of soybean curd residue, okara, for the solid state substrate in the production of a lipopeptide antibiotic, iturin A, by bacillus subtilis nb22. Process. Biochem. 1996, 31, 801–806. [Google Scholar] [CrossRef]
- Wei, W.W.; Liu, S.; Li, X.Y.; Li, L.H.; Cao, W. Thermodynamic and environmental analysis of self-thermal antibiotic residues supercritical water gasification system for hydrogen and power production. Process. Saf. Environ. Prot. 2023, 179, 168–179. [Google Scholar] [CrossRef]
- Li, Y.F.; Hong, C.; Wang, Y.J.; Xing, Y.; Chang, X.N.; Zheng, Z.X.; Li, Z.; Zhao, X. Nitrogen migration mechanism during pyrolysis of penicillin fermentation residue based on product characteristics and quantum chemical analysis. ACS Sustain. Chem. Eng. 2020, 8, 7721–7740. [Google Scholar] [CrossRef]
- Zheng, Q.Y.; Zhu, Z.H.; Guo, Y.Y.; Jiang, X.P.; Xing, X.; Chen, W.B.; Zhao, Y.C.; Zhu, Y.; Zhou, T.; Xu, B. Highly collaborative of wasted activated sludge deconstruction and incineration gas utilization in the presence of nitrogen oxides. J. Clean. Prod. 2025, 493, 144927. [Google Scholar] [CrossRef]
- Umemoto, S.; Kajitani, S.; Hara, S. Modeling of coal char gasification in coexistence of CO2 and H2O considering sharing of active sites. Fuel 2013, 103, 14–21. [Google Scholar] [CrossRef]
- Rahman, M.M.; Oni, A.O.; Gemechu, E.; Kumar, A. Assessment of energy storage technologies: A review. Energy Convers. Manag. 2020, 223, 113295. [Google Scholar] [CrossRef]
- Riek, R.F. Studies on reactions of animals to infestation with ticks. 6. Resistance of cattle to infestation with tick boophilus microplus (canestrini). Aust. J. Agric. Res. 1962, 13, 532. [Google Scholar] [CrossRef]
- Hoogmartens, R.; Van Passel, S.; Van Acker, K.; Dubois, M. Bridging the gap between LCA, LCC and CBA as sustainability assessment tools. Environ. Impact Assess. Rev. 2014, 48, 27–33. [Google Scholar] [CrossRef]
- Han, Y.L.; Yang, Z.H.; Ding, T.; Xiao, J.Z. Environmental and economic assessment on 3d printed buildings with recycled concrete. J. Clean. Prod. 2021, 278, 123884. [Google Scholar] [CrossRef]
- Ludin, N.A.; Mustafa, N.I.; Hanafiah, M.M.; Ibrahim, M.A.; Teridi, M.; Sepeai, S.; Zaharim, A.; Sopian, K. Prospects of life cycle assessment of renewable energy from solar photovoltaic technologies: A review. Renew. Sustain. Energy Rev. 2018, 96, 11–28. [Google Scholar] [CrossRef]
- Spierling, S.; Knüpffer, E.; Behnsen, H.; Mudersbach, M.; Krieg, H.; Springer, S.; Albrecht, S.; Herrmann, C.; Endres, H.-J. Bio-based plastics—A review of environmental, social and economic impact assessments. J. Clean. Prod. 2018, 185, 476–491. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sivakumar, N.; Lukk, T.; Pecoraro, L.; Thakur, V.K.; Roberts, D.; Newbold, J.; Gupta, V.K. Bioprocessing of waste biomass for sustainable product development and minimizing environmental impact. Bioresour. Technol. 2021, 322, 124548. [Google Scholar] [CrossRef]
- Huang, J.S.; Wang, J.Q.; Huang, Z.C.; Liu, T.Y.; Li, H. Photothermal technique-enabled ambient production of microalgae biodiesel: Mechanism and life cycle assessment. Bioresour. Technol. 2023, 369, 128390. [Google Scholar] [CrossRef]
- Arias, A.; Nika, C.E.; Feijoo, G.; Moreira, M.T.; Katsou, E. Sustainability and circularity assessment of the potential of a biofuel produced from black liquor as a substitute for conventional fuels. Chem. Eng. J. 2024, 498, 155335. [Google Scholar] [CrossRef]
- Ma, H.Z.; Wei, Y.L.; Fei, F.; Gao, M.; Wang, Q.H. Whether biorefinery is a promising way to support waste source separation? From the life cycle perspective. Sci. Total Environ. 2024, 912, 168731. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Koelewijn, S.F.; Van den Bossche, G.; Van Aelst, J.; Van den Bosch, S.; Renders, T.; Navare, K.; Nicolaï, T.; Van Aelst, K.; Maesen, M.; et al. A sustainable wood biorefinery for low-carbon footprint chemicals production. Science 2020, 367, 1385. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Burn, S.; Crossin, E.; Othman, M. Life cycle costing of municipal food waste management systems: The effect of environmental externalities and transfer costs using local government case studies. Resour. Conserv. Recycl. 2018, 138, 118–129. [Google Scholar] [CrossRef]
- Astrup, T.F.; Tonini, D.; Turconi, R.; Boldrin, A. Life cycle assessment of thermal waste-to-energy technologies: Review and recommendations. Waste Manag. 2015, 37, 104–115. [Google Scholar] [CrossRef]
- Chan, F.L.; Tanksale, A. Review of recent developments in ni-based catalysts for biomass gasification. Renew. Sustain. Energy Rev. 2014, 38, 428–438. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, S.; Brown, R.C.; Kelkar, A.; Bai, X.L. Fast pyrolysis of biomass and waste plastic in a fluidized bed reactor. Fuel 2015, 156, 40–46. [Google Scholar] [CrossRef]
- Tian, C.Y.; Li, B.M.; Liu, Z.D.; Zhang, Y.H.; Lu, H.F. Hydrothermal liquefaction for algal biorefinery: A critical review. Renew. Sustain. Energy Rev. 2014, 38, 933–950. [Google Scholar] [CrossRef]
- Heidenreich, S.; Foscolo, P.U. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Gai, C.; Zhang, Y.H.; Chen, W.T.; Zhang, P.; Dong, Y.P. An investigation of reaction pathways of hydrothermal liquefaction using chlorella pyrenoidosa and spirulina platensis. Energy Convers. Manag. 2015, 96, 330–339. [Google Scholar] [CrossRef]
- Hu, Q.; Shen, Y.; Chew, J.W.; Ge, T.S.; Wang, C.H. Chemical looping gasification of biomass with Fe2O3/Cao as the oxygen carrier for hydrogen-enriched syngas production. Chem. Eng. J. 2020, 379, 122346. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Rincón, L.; Vilariño, V.; Pérez, G.; Castell, A. Life cycle assessment (LCA) and life cycle energy analysis (LCEA) of buildings and the building sector: A review. Renew. Sustain. Energy Rev. 2014, 29, 394–416. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Guo, Y.; Yeh, T.; Song, W.H.; Xu, D.H.; Wang, S.Z. A review of bio-oil production from hydrothermal liquefaction of algae. Renew. Sustain. Energy Rev. 2015, 48, 776–790. [Google Scholar] [CrossRef]
- Ma, W.C.; Wenga, T.; Frandsen, F.J.; Yan, B.B.; Chen, G.Y. The fate of chlorine during MSW incineration: Vaporization, transformation, deposition, corrosion and remedies. Prog. Energy Combust. Sci. 2020, 76, 100789. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.Y.; Tahir, N.; Awasthi, M.K. Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: A review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef]
- Ghimpeteanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Dragotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic use in livestock and residues in food-a public health threat: A review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, X.M. Detoxification, solidification and recycling of municipal solid waste incineration fly ash: A review. Chem. Eng. J. 2021, 420, 130349. [Google Scholar] [CrossRef]
- Li, J.; Xiao, F.P.; Zhang, L.F.; Amirkhanian, S.N. Life cycle assessment and life cycle cost analysis of recycled solid waste materials in highway pavement: A review. J. Clean. Prod. 2019, 233, 1182–1206. [Google Scholar] [CrossRef]
- Quinn, J.C.; Davis, R. The potentials and challenges of algae based biofuels: A review of the techno-economic, life cycle, and resource assessment modeling. Bioresour. Technol. 2015, 184, 444–452. [Google Scholar] [CrossRef]
- Chang, H.; Yuan, J.; Zhao, Y.; Bisinella, V.; Damgaard, A.; Christensen, T.H. Carbon footprints of incineration, pyrolysis, and gasification for sewage sludge treatment. Resour. Conserv. Recycl. 2025, 212, 107939. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Zhu, X.; Zhou, S.; Zhang, X.; Li, J.; Yan, B.; Chen, G. Comparative analysis of microwave wet torrefaction and anaerobic digestion for energy recovery from antibiotic mycelial residue. Fuel 2024, 357, 129776. [Google Scholar] [CrossRef]
- Mayer, F.; Bhandari, R.; Gäth, S.A. Life cycle assessment on the treatment of organic waste streams by anaerobic digestion, hydrothermal carbonization and incineration. Waste Manag. 2021, 130, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Geng, Y.; Hong, J.; Kua, H.W.; Xu, C.; Yu, N. Life cycle assessment of antibiotic mycelial residues management in China. Renew. Sustain. Energy Rev. 2017, 79, 830–838. [Google Scholar] [CrossRef]
- Haque, A.; Rogerson, L.; Nath, N.D.; Haruna, S.; Ahn, J.; Johnston, T.V.; Lin, C.S.K.; Chong, L.; Na, L.; Jang, M.J.; et al. Sustainable management and valorization of antibiotic waste. Chem. Eng. J. 2024, 498, 155372. [Google Scholar] [CrossRef]
- Paudel, P.P.; Kafle, S.; Park, S.; Kim, S.J.; Cho, L.; Kim, D.H. Advancements in sustainable thermochemical conversion of agricultural crop residues: A systematic review of technical progress, applications, perspectives, and challenges. Renew. Sustain. Energy Rev. 2024, 202, 114723. [Google Scholar] [CrossRef]
- Cormos, C.-C.; Dragan, M.; Petrescu, L.; Cormos, A.-M.; Dragan, S.; Bathori, A.-M.; Galusnyak, S.-C. Synthetic natural gas (SNG) production by biomass gasification with CO2 capture: Techno-economic and life cycle analysis (LCA). Energy 2024, 312, 133507. [Google Scholar] [CrossRef]
- Nkuna, S.G.; Olwal, T.O.; Chowdhury, S.P.D.; Ndambuki, J.M. A review of wastewater sludge-to-energy generation focused on thermochemical technologies: An improved technological, economical and socio-environmental aspect. Clean. Waste Syst. 2024, 7, 100130. [Google Scholar] [CrossRef]
- Poornima, S.; Manikandan, S.; Prakash, R.; Deena, S.R.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Biofuel and biochemical production through biomass transformation using advanced thermochemical and biochemical processes—A review. Fuel 2024, 372, 132204. [Google Scholar] [CrossRef]
- Sapkota, B.; Pariatamby, A. Pharmaceutical waste management system–Are the current techniques sustainable, eco-friendly and circular? A review. Waste Manag. 2023, 168, 83–97. [Google Scholar] [CrossRef]
- Andooz, A.; Eqbalpour, M.; Kowsari, E.; Ramakrishna, S.; Cheshmeh, Z.A. A comprehensive review on pyrolysis from the circular economy point of view and its environmental and social effects. J. Clean. Prod. 2023, 388, 136021. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, C.; Xing, Y.; Li, Z.; Li, Y.; Yang, J.; Feng, L.; Hu, J.; Sun, H. Thermal characteristics and product formation mechanism during pyrolysis of penicillin fermentation residue. Bioresour. Technol. 2019, 277, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Demichelis, F.; Fino, D.; Tommasi, T. A closed-loop valorization of the waste biomass through two-stage anaerobic digestion and digestate exploitation. Renew. Sustain. Energy Rev. 2025, 207, 114938. [Google Scholar] [CrossRef]
- Dutta, S.; Kataki, S.; Banerjee, I.; Pohrmen, C.B.; Jaiswal, K.K.; Jaiswal, A.K. Microalgal biorefineries in sustainable biofuel production and other high-value products. New Biotechnol. 2025, 87, 39–59. [Google Scholar] [CrossRef]
- Mazhkoo, S.; Soltanian, S.; Odebiyi, H.O.; Norouzi, O.; Ubene, M.; Hayder, A.; Pourali, O.; Santos, R.M.; Brown, R.C.; Dutta, A. Process intensification in hydrothermal liquefaction of biomass: A review. J. Environ. Chem. Eng. 2025, 13, 115722. [Google Scholar] [CrossRef]
- Alsaeedi, S.; Yan, B.; Wang, Z.; Chen, G.; Song, Y.; Al-Hakeem, B. Current utilization technologies and products of antibiotic fermentation residues: A review. J. Environ. Chem. Eng. 2025, 13, 116182. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wei, Y.; Ma, R.; Ma, H.; Dong, B.; Wang, Y. Comparison of Thermochemical Conversion Processes for Antibiotic Residues: Insights from Life Cycle Assessment. Processes 2025, 13, 1139. https://doi.org/10.3390/pr13041139
Yang J, Wei Y, Ma R, Ma H, Dong B, Wang Y. Comparison of Thermochemical Conversion Processes for Antibiotic Residues: Insights from Life Cycle Assessment. Processes. 2025; 13(4):1139. https://doi.org/10.3390/pr13041139
Chicago/Turabian StyleYang, Jian, Yulian Wei, Rui Ma, Hongzhi Ma, Biqin Dong, and Ying Wang. 2025. "Comparison of Thermochemical Conversion Processes for Antibiotic Residues: Insights from Life Cycle Assessment" Processes 13, no. 4: 1139. https://doi.org/10.3390/pr13041139
APA StyleYang, J., Wei, Y., Ma, R., Ma, H., Dong, B., & Wang, Y. (2025). Comparison of Thermochemical Conversion Processes for Antibiotic Residues: Insights from Life Cycle Assessment. Processes, 13(4), 1139. https://doi.org/10.3390/pr13041139







