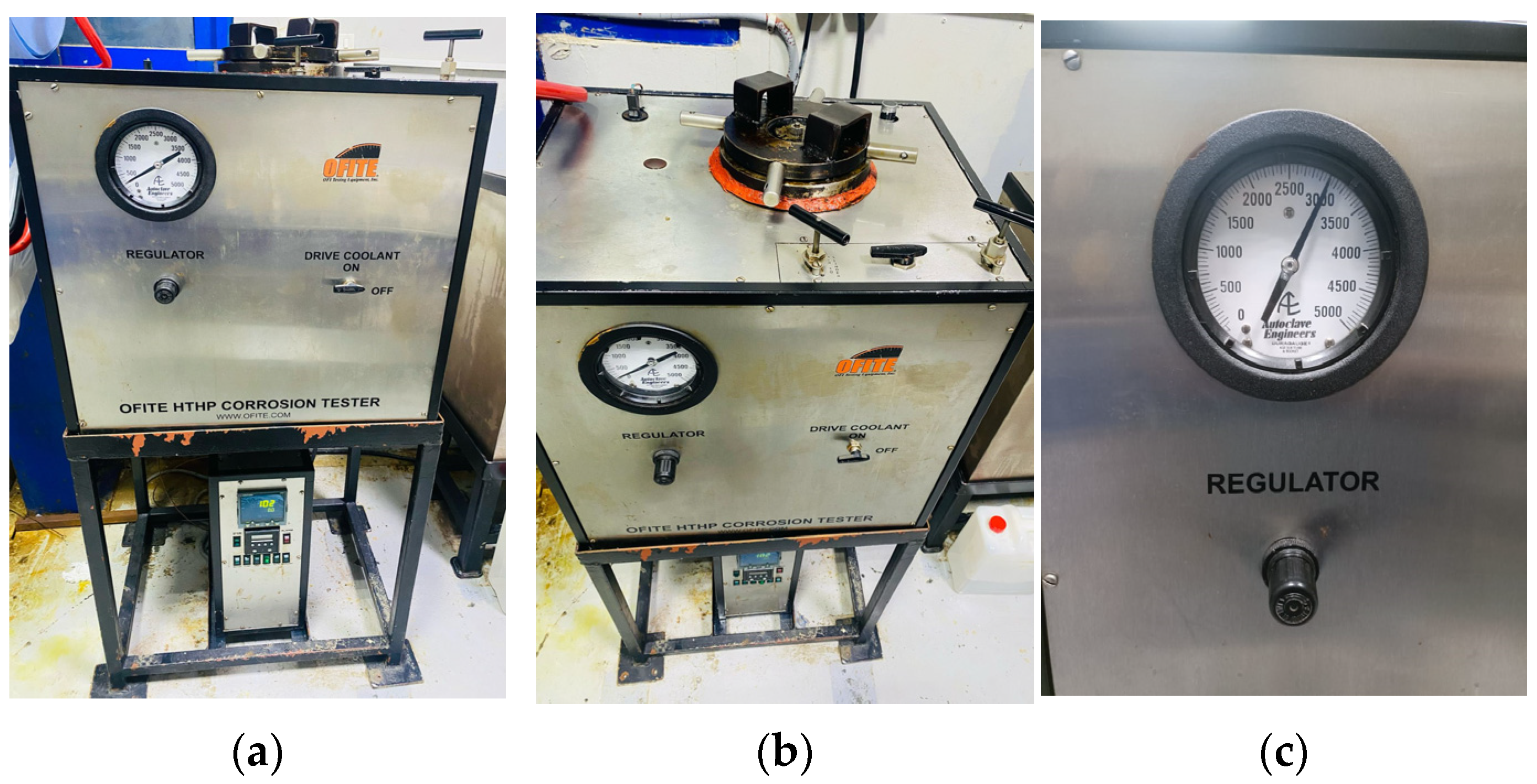
2.2. Laboratory Steps and Mixture Formulation and Experiments
Prior to deploying the emulsified acid treatment in field applications, substantial laboratory testing was performed to guarantee the formulation’s stability, homogeneity, and effectiveness under simulated reservoir conditions. The primary goal of these tests was to ensure that the emulsified acid retains its two-phase stability, efficiently mixes the organic diesel phase (external) and inorganic acid phase (interior), and operates consistently under different temperature, pressure, and salt conditions.
Laboratory studies to create and sustain a consistent emulsion over 24 h at room temperature was a challenge. Trials were performed in the UAE in Superior Abu Dhabi company laboratory with the help of engineers that put effort to test many emulsifiers and be able to create the required one phase product, having organic external phase and inorganic internal phase.
The laboratory was equipped with the following equipment:
- -
Corrosion tester machine;
- -
Emulsion stabilizer;
- -
High performance digital oven;
- -
Testing bottles and tubes.
Step 1: Selecting the Right Emulsifying Additive:
- 1.
The first step is to select a specific emulsifying agent that ensures steady and homogeneous mixing of the two immiscible phases: organic diesel (external phase) and inorganic acid (interior phase).
A thorough series of tests was carried out to assure the creation of a stable emulsion between diesel and acid. These tests were necessary because emulsions including hydrocarbons and acids are intrinsically unstable, resulting in phase separation due to variations in polarity, density, and solubility. Achieving a steady emulsion necessitated the careful optimization of elements such as the diesel to acid ratio, the type and quantity of emulsifying agents, and the mechanical conditions under which emulsification occurred, including stirring speed and temperature. Various surfactants and stabilizing agents were tried to see how well they could induce a consistent and long-lasting dispersion of acid droplets in the diesel phase.
The stability of the generated emulsions was assessed over time using factors such as droplet size distribution, coalescence tendencies, and the possibility of phase inversion. Ensuring the emulsion’s stability was critical, since any phase separation may cause inefficiencies in following corrosion protection testing and jeopardize the formulation’s overall efficacy [
7].
- 2.
This addition is essential for ensuring long-term stability and preventing phase separation, both of which are required for effective acid transport and penetration.
Once a stable and reproducible emulsion had been established, several attempts were undertaken to improve its corrosion resistance by introducing other families of corrosion inhibitors. These inhibitors were chosen due to their known chemical characteristics, methods of action, and compatibility with the diesel–acid system.
Step 2: Compatibility Testing:
The prepared emulsified acid is subjected to comprehensive compatibility testing to determine its effectiveness and stability under a variety of temperature and pressure conditions.
Tests are run at room temperature and elevated reservoir temperatures to confirm that the mixture is stable and does not separate prematurely.
Compatibility testing also evaluates how the emulsified acid interacts with formation minerals, wellbore fluids, and stimulation additives.
Step 3: Optimization of Chemical Dosage:
- 1.
The ideal acid-to-diesel ratios, emulsifier concentrations, and other additions are found via laboratory tests and field simulations.
Emulsified acid treatment is optimized using a systematic approach that includes laboratory testing and field simulations to establish the optimal acid-to-diesel ratios, emulsifier concentrations, and extra chemical additions. Extensive laboratory testing determined that a 70:30 acid-to-diesel ratio achieves the optimal blend of emulsion stability, controlled acid release, and deep formation penetration, ensuring excellent mineral dissolving while avoiding premature acid spending. The choice and concentration of emulsifiers were also important, with 3–5 wt% of nonionic and anionic surfactants being ideal for maintaining phase stability at high reservoir temperatures and pressures.
- 2.
This phase assures that the treatment increases reservoir stimulation efficiency while lowering operational costs and dangers.
- 3.
The proper dosage is required to achieve a regulated acid reaction rate, which reduces excessive acid spending and extends wormhole growth.
Step 4: Corrosion Testing for Different Well Conditions:
- 1.
To measure its impact on downhole metallurgy, the emulsified acid is tested under various well conditions with a variety of corrosion coupons.
Extensive studies were conducted to examine the stability of the emulsion and the performance of various corrosion inhibitors under both low and extremely high temperature circumstances in order to successfully stop the acidic emulsion treatment over a wide range of temperatures. Temperature is an important factor in determining emulsion stability and the efficiency of corrosion inhibitors since temperature changes can affect the system’s solubility, reaction kinetics, and phase behavior. At low temperatures, the fundamental problem was preventing phase separation and ensuring that the acid was evenly distributed throughout the fuel. This required the selection of surfactants and emulsifying agents that could perform efficiently at low kinetic energy levels, ensuring that the acid droplets remained finely dispersed without coagulating. Furthermore, corrosion inhibitors at lower temperatures had to be tested for adsorption efficacy on metal surfaces, as decreased molecular mobility could affect the creation of a protective inhibitor layer. In contrast, at extremely high temperatures, the problem switched to preventing thermal deterioration of the emulsion and inhibitors while maintaining corrosion protection. At high temperatures, increased molecular activity accelerates phase separation, weakening the emulsion and potentially exposing metal surfaces to acid attack. To address this, high-temperature-resistant emulsifiers were carefully chosen, and thermally stable corrosion inhibitors were added to ensure that the protective barrier remained effective even in harsh conditions.
- 2.
To verify that the treatment does not severely erode tubulars and well equipment, tests are carried out at various temperatures, pressures, and acid strengths.
The inhibitor families included film-forming inhibitors, passivating compounds, and adsorption-type inhibitors, each with a unique protective mechanism. Film-forming inhibitors were evaluated for their capacity to build a physical barrier on metal surfaces, preventing direct contact between corrosive acid and metal substrate. Passivating inhibitors were examined for their ability to modify the electrochemical behavior of the metal, hence reducing its susceptibility to oxidation. Adsorption inhibitors, on the other hand, were evaluated for their ability to bind to the metal surface and form a protective molecular layer, thereby reducing acid assault. The performance of each inhibitor family was rigorously assessed, taking into account variables such as inhibitor concentration, exposure time, temperature fluctuations, and the emulsion’s general chemical stability in the presence of these additives.
- 2.1.
Standard Corrosion Testing Procedure
Gravimetry, the most commonly used method for corrosion measurement, was employed to quantify inhibitor performance by tracking material weight loss over time. Metal samples were subjected to a diesel–acid emulsion containing various inhibitors [
7], then cleaned and weighed before and after the exposure. Unlike electrochemical approaches, which can add uncertainty owing to current variations, gravimetric analysis provides a precise, repeatable assessment of metal degradation, making it suitable for evaluating inhibitor efficiency and determining the most effective formulations.
Standardized gravimetric corrosion tests were carried out on L-80 (production tubing material), QT-800 (coiled tubing material), and Alloy-28 (offshore wells). Coupons were immersed in emulsions containing varied inhibitor concentrations, treated to regulated temperatures for 6 or 12 h, then cleaned, dried, and weighed to assess mass loss and corrosion rate.
Analyzing these results revealed the most effective inhibitor formulations—such as quaternary ammonium and quinoline chloride—for both low- and high-temperature applications, ensuring optimal corrosion protection while retaining emulsion stability.
- 3.
Results show that emulsified acid has much lower corrosion rates than standard HCl, making it a safer option for long-term use in high-temperature and deep reservoirs.
Steps 5: Validation of Unique Advantages.
The emulsified acid has a significant benefit over typical acid treatments: it is immiscible with water, limiting uncontrolled acid loss into the formation.
Unlike conventional acids, emulsified acid only reacts when in contact with oil, allowing for deep formation cleaning, extended wormhole propagation, and increased permeability.
This selective reaction improves reservoir fluid dynamics by increasing oil flow efficiency and reducing water breakthrough and formation damage.
Its gradual investment promotes deeper penetration into the formation, hence increasing stimulation efficiency in tight and ultra-low permeability reservoirs.
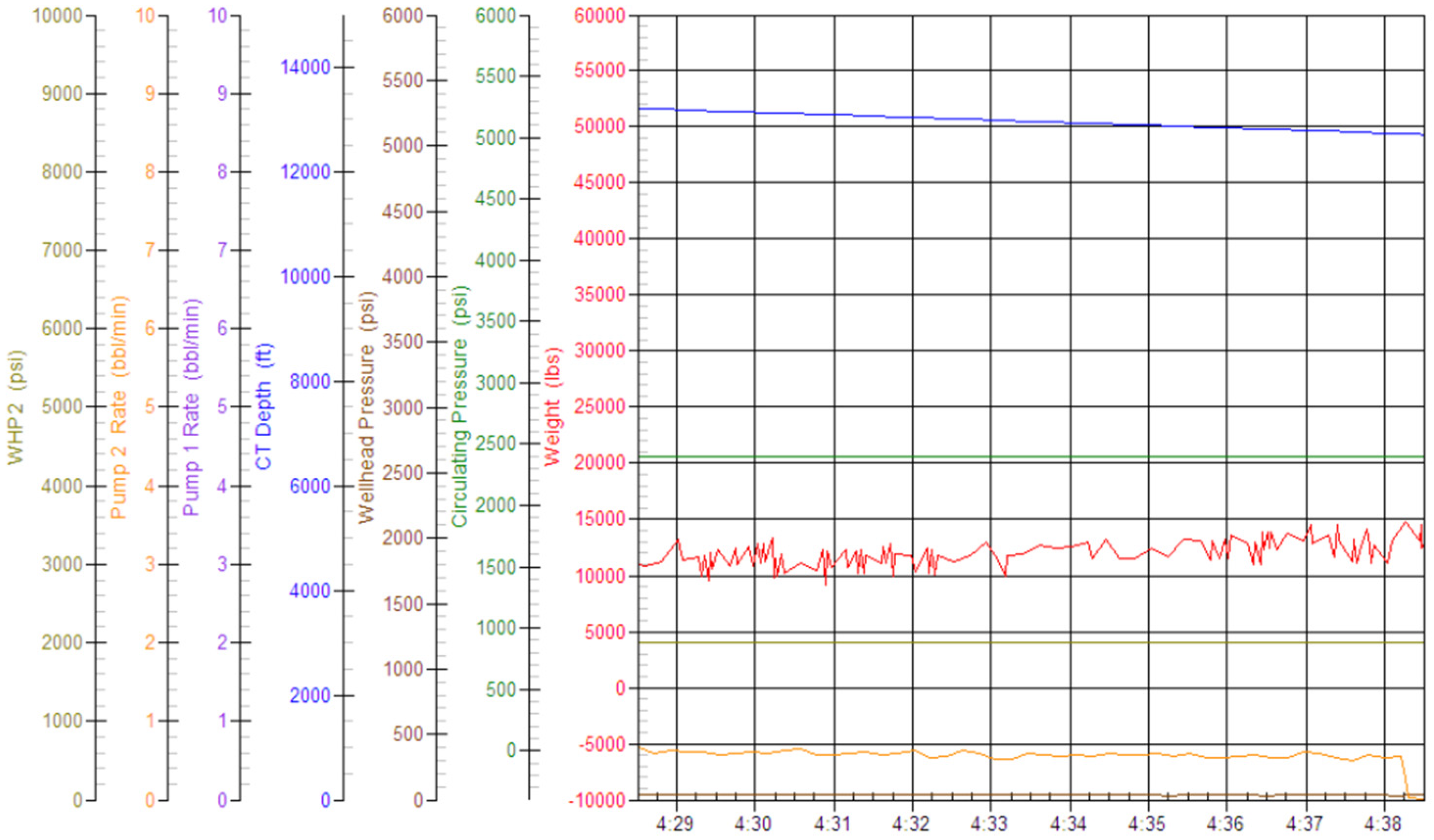
Step 6: Field trials and Performance Validation:
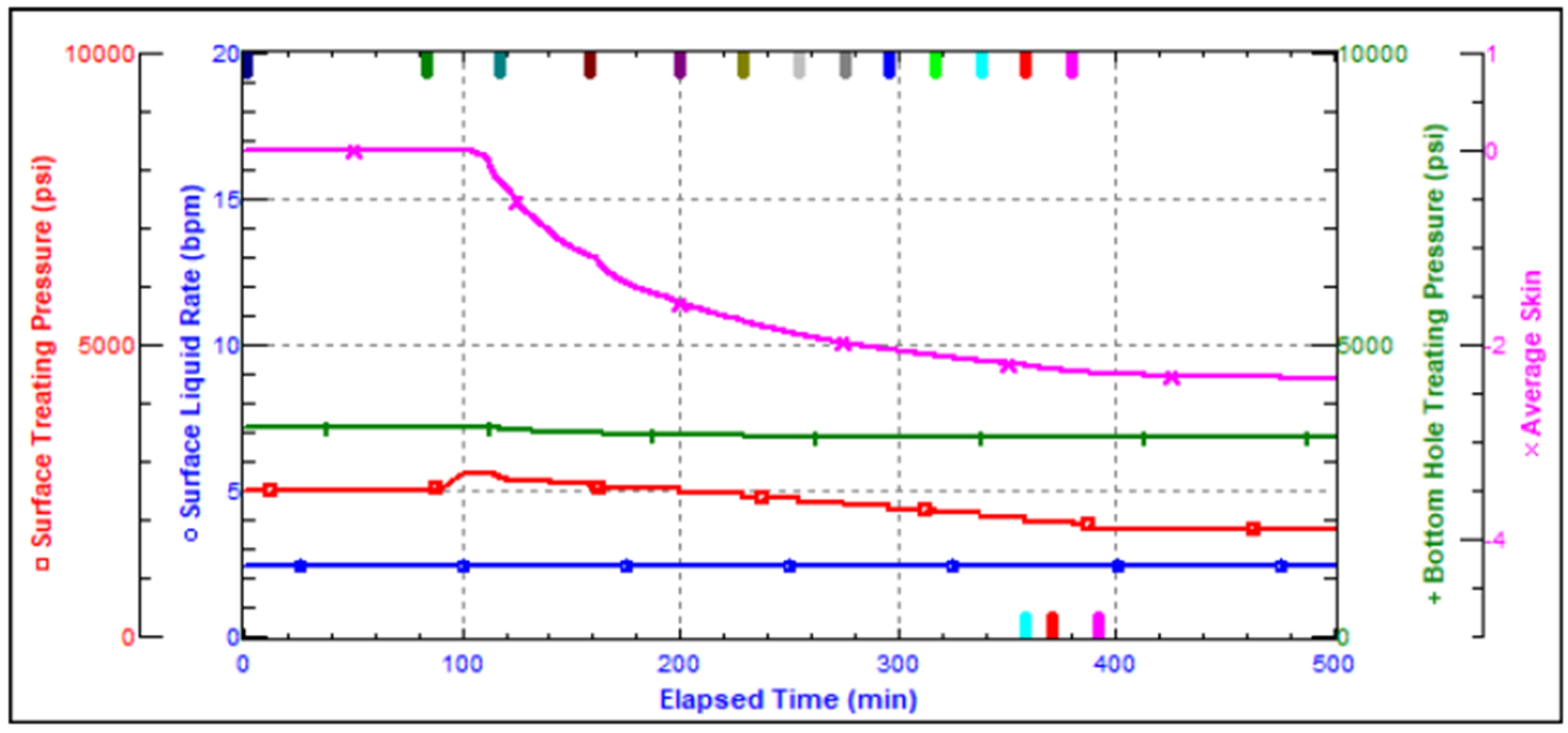
Advanced stimulation techniques are required to address the difficulty of low injection rates and reduced oil production in carbonate reservoirs, which is frequently caused by precipitates and deposits that generate detrimental skin and hinder fluid flow. Darcy’s Law describes the relationship between flow rate (Q), permeability (K), and pressure gradient (ΔP) in reservoirs. However, the presence of skin, a measure of near-wellbore damage, considerably modifies this relationship, resulting in decreased permeability (Ks) and a positive skin effect (s), which drastically affects injectivity and productivity.
- 1.
To test the efficacy of emulsified acid, field studies were undertaken versus traditional 15% HCl acid treatments in a variety of well types, including oil producers, gas producers, and water injectors.
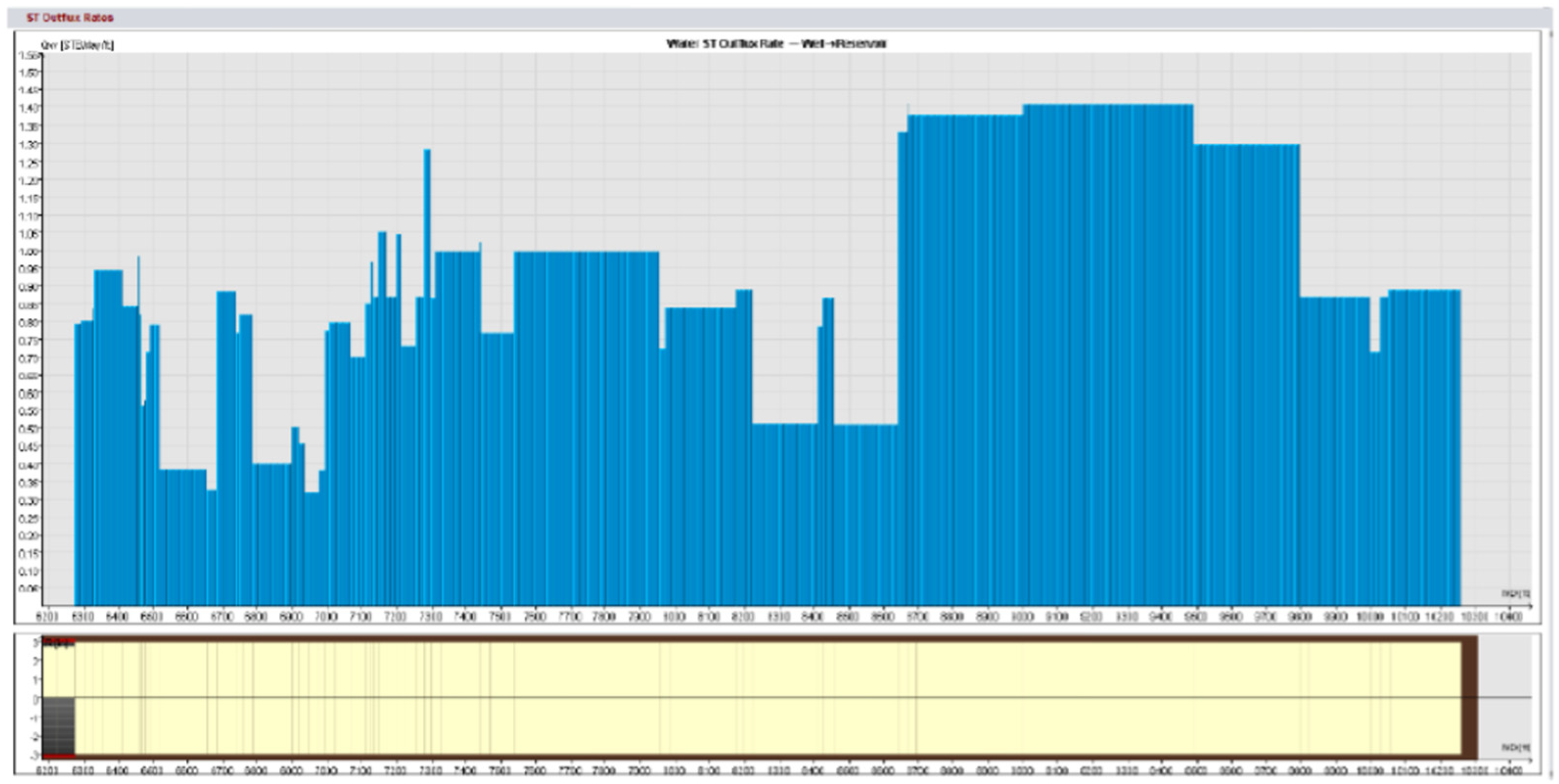
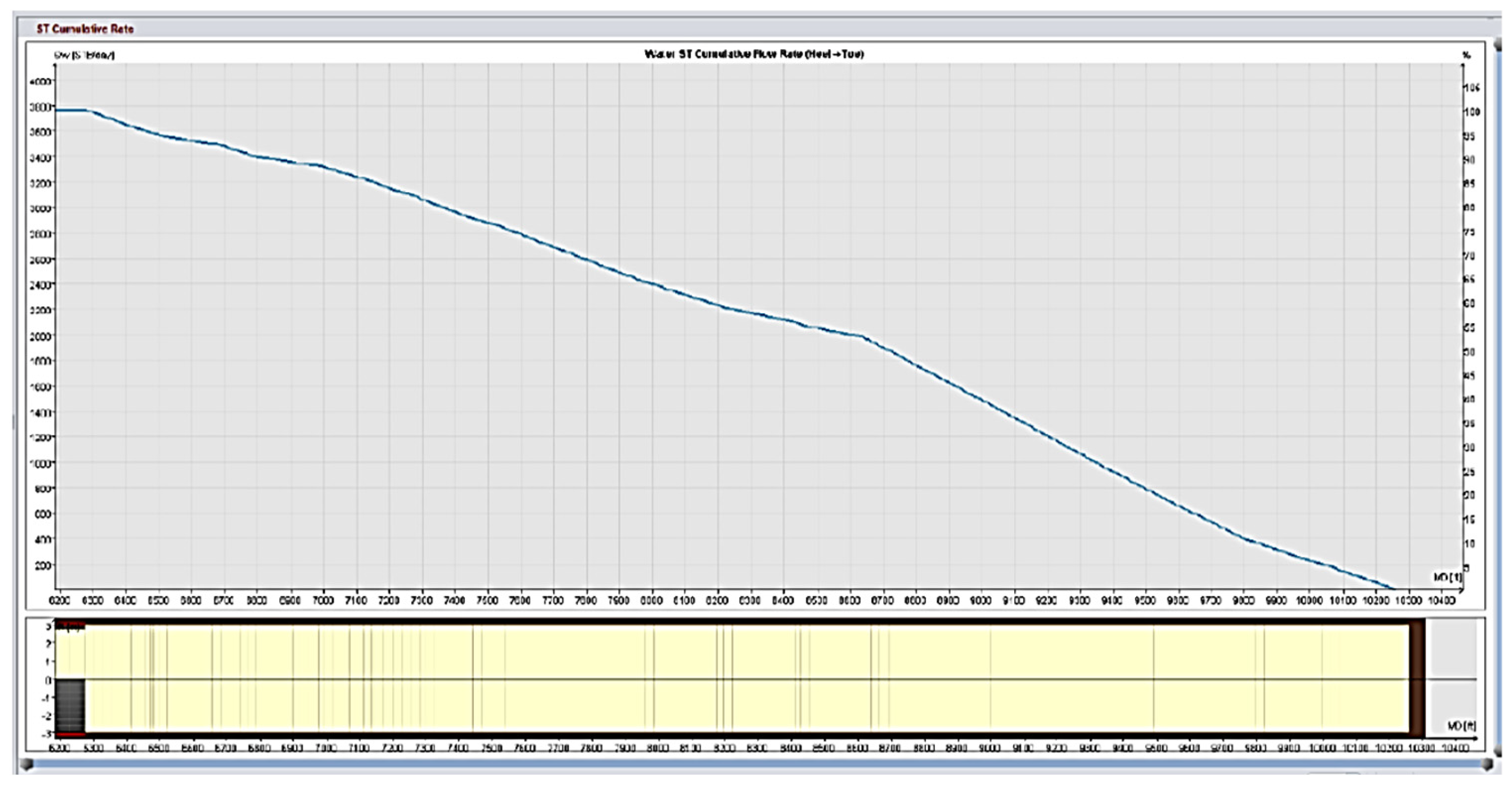
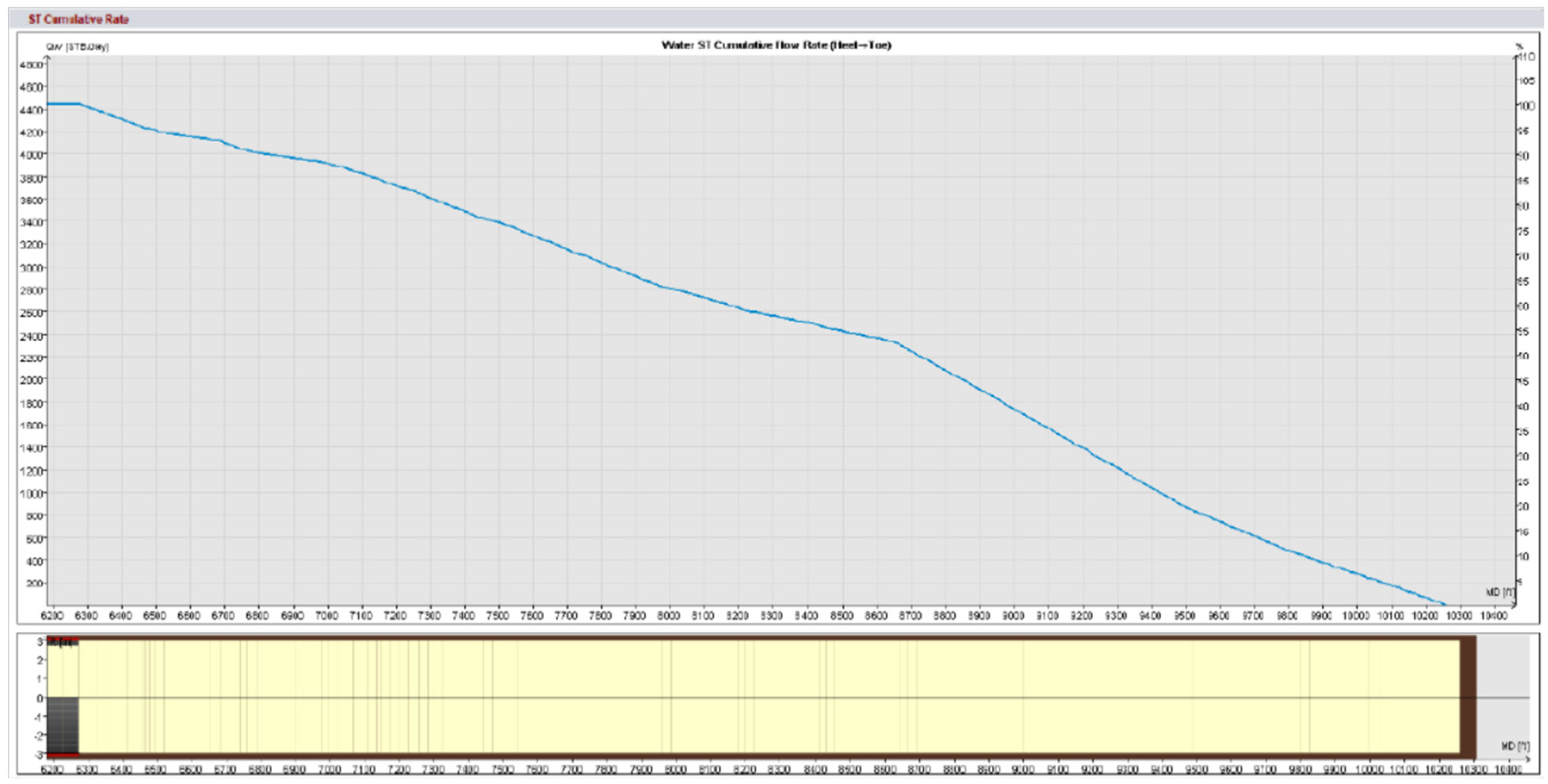
Field trials using standard matrix acidification with conventional HCl was ineffective in addressing these concerns. HCl’s high reactivity resulted in limited penetration because the acid preferentially reacted with near-wellbore damage while leaving deeper formation pores untouched. This resulted in long-lasting favorable skin effects, which hampered injection and manufacturing. To address these constraints, a unique emulsified acid treatment was developed to function as a delayed acid system. The designed emulsified acid, a two-phase solution with HCl droplets dispersed in a continuous diesel phase, provided a regulated reaction rate, allowing for deeper acid penetration. This retardation effect was achieved by strategically using patented emulsifiers (SUP-AE-03), which retarded the interaction between HCl and carbonate production, allowing the acid to permeate deeper into the reservoir and stimulate low-permeability pores. This is in stark contrast to conventional HCl, which quickly depletes at the wellbore face.
The retardation mechanism is directly proportional to the volume percentage of the acid internal phase. A 70:30 acid-to-diesel ratio was chosen to balance viscosity and penetration depth. This formulation, which had the same raw acid dosage as a traditional 15% HCl treatment, provided optimum retardation and deep live acid penetration even at high bottomhole temperatures (275 °F).
- 2.
The results of the field trials demonstrated:
The effectiveness of stimulation techniques in oil and gas reservoirs is usually evaluated according to three main categories of criteria where geological and physical factors significantly influence their success. These elements include various reservoir attributes, such as the characteristics of reservoir fluids including:
- -
viscosity;
- -
density;
- -
formation composition;
- -
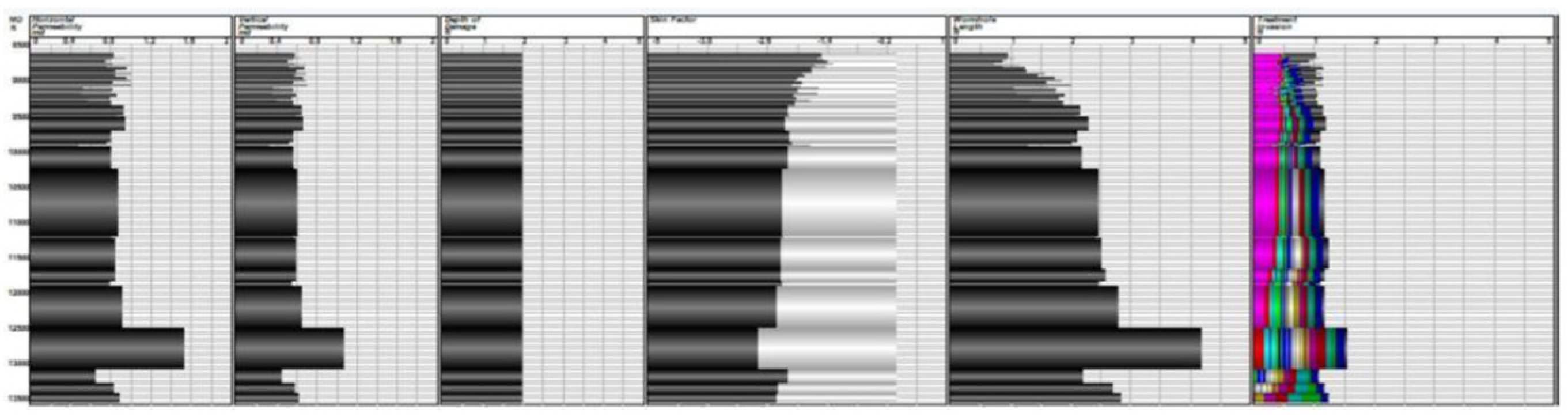
petrophysical data, such as permeability, porosity, thickness, and lithology;
- -
bottom hole pressure;
- -
bottom hole temperature.
Where these factors affect fluid mobility and the success of stimulation procedures. The depth and circumstances surrounding an oil-saturated reservoir also influence the choice of treatment fluids and methods, as deeper formations typically necessitate more resilient stimulation approaches due to increased pressures and temperatures. To address this challenge, emulsified acid, by its formulation, overcomes water’s diluting effects due to its combinations of the diesel phase’s outer shell, allowing the oily emulsion to have a deeper penetration towards the reservoir, seeking extensive wormholing to reach out to oil pores and clean damaged skin.
- 2.1.
Injectivity increased by 2.5 to 5 times compared to the initial well performance.
Additionally, the petrophysical characteristics of the reservoir, including porosity and permeability, are vital for comprehending how fluids will move through the formation and how efficiently stimulation can improve injectivity and productivity. The traits of an oil-bearing reservoir, such as its lithology, level of heterogeneity, and natural fracture systems, influence how stimulation fluids can penetrate and interact with the formation. Furthermore, the filling of the pore space with reservoir fluids—be it oil, water, or gas—determines the effectiveness of displacement and possible enhancements after stimulation.
- 2.2.
Superior wormhole propagation and acid penetration compared to standard HCl.
For gas-saturated reservoirs, utilizing emulsified acid treatment has demonstrated a marked increase in injectivity and productivity, with enhancements between 2.5 and 5 times the initial performance. This improvement is mainly due to the acid’s capacity to uniformly infiltrate the formation, dissolve blocking minerals, and establish highly conductive flow pathways, thus enhancing gas movement and reservoir connectivity. Consequently, the productivity index, reflecting the reservoir’s capacity to supply hydrocarbons to the wellbore, shows significant enhancements, guaranteeing a more effective and continuous production rate.
The heightened injectivity facilitates improved reservoir pressure upkeep and greater hydrocarbon extraction, resulting in optimized well efficiency and a more cost-effective operation. Through the meticulous assessment of these geological and physical factors, Superior Abu Dhabi engineers can customize stimulation treatments to suit particular reservoir conditions, guaranteeing optimal efficiency and sustainable production over long term.
The emulsified acid therapy effectively addressed the difficulties of limited injectivity while also providing favorable skin effects. By disintegrating damaged skin and forming conductive wormholes, it greatly enhanced permeability and reduced the skin effect, resulting in a dramatic rise in injectivity of five times, depending on well conditions. This large improvement in injectivity directly translated into increased oil production rates, as indicated by the significant pressure drop recorded during emulsified acid injection. The high-velocity jetting instrument, when combined with the emulsified acid, improved acid penetration and distribution, ensuring that the acid reached and stimulated all zones of interest. This combination efficiently creates conductives channels known as wormholes, which improved rock qualities like wettability, permeability, and differential pressure.
- 2.3
Significant reduction in post-stimulation skin factor, resulting in sustained high productivity gains.
- 3
Reduced corrosion influence on wellbore materials, resulting in longer operating life.
The comparison testing indicate that emulsified acid is not only a feasible alternative to HCl, but also a long-term option for improving well performance while decreasing environmental effect. Furthermore, the emulsified acid system provided considerable benefits in terms of decreased acid leakage and spent acid amounts. The regulated reaction rate reduced the quantity of acid necessary for effective stimulation, resulting in lower chemical consumption and environmental impact. The stability of the emulsified acid system was carefully examined, including corrosion tests and viscosity studies under reservoir conditions. The adoption of a tuned corrosion inhibitor guaranteed that the system remained stable and effective at high temperatures, which contributed to its overall success.
In summary, the developed emulsified acid treatment proved to be a highly successful and long-term solution for increasing oil production in oil-saturated reservoirs, gas-saturated and reservoir injectivity in the Fields. Its capacity to operate as a retarded acid, along with the use of high-velocity jetting instruments, resulted in a huge improvement in well performance, highlighting the significant benefits of this novel strategy over traditional acidizing approaches to improve deep clean into formation leading to better reservoir performance.
The experimental data in
Table 1 offer a detailed overview of the important parameters controlling the performance of the emulsified acid system. The ideal acid-to-diesel ratio was determined to be 70:30, which ensures consistent emulsion formation while successfully limiting acid discharge for deep reservoir penetration. The emulsifier concentration, ranging from 3 to 5 wt%, was critical in maintaining the stability of the two-phase emulsion, especially under high-temperature conditions of up to 275 °F, which are typical of deep carbonate reservoirs. Reaction retardation was well controlled, allowing for longer acid–rock interaction, resulting in deeper wormhole propagation and increased acid penetration efficiency. The formulated system had a major advantage in that it greatly reduced corrosion rates when compared to standard hydrochloric acid (HCl) treatments, improving wellbore integrity and minimizing equipment degradation. Furthermore, the injectivity of treated wells increased by 2.5 to 5 times, demonstrating the better stimulating effectiveness of the emulsified acid treatment. These findings show that the improved formulation has the potential to increase well productivity while reducing operational constraints associated with conventional acidizing procedures.