Cortisone Analysis by FTIR Spectroscopy: In Vitro Study
Abstract
1. Introduction
2. Material and Methods
2.1. Spectrum of Cortisone and Reliability of Spectra Obtained by FTIR
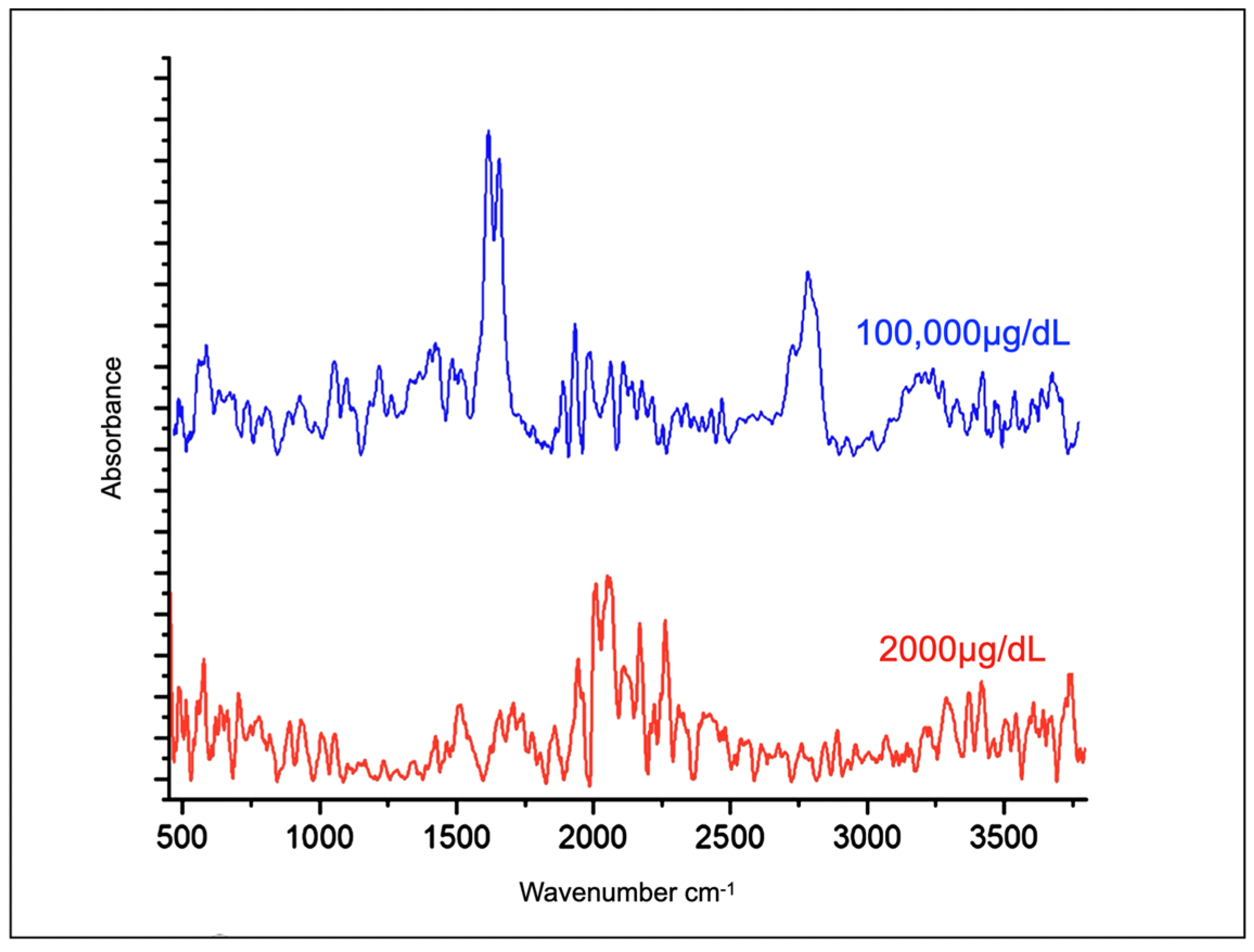
2.2. Evaluation of Cortisone Spectra in Water
2.3. Analysis of Surfactant Increment in Obtaining Cortisone Spectra
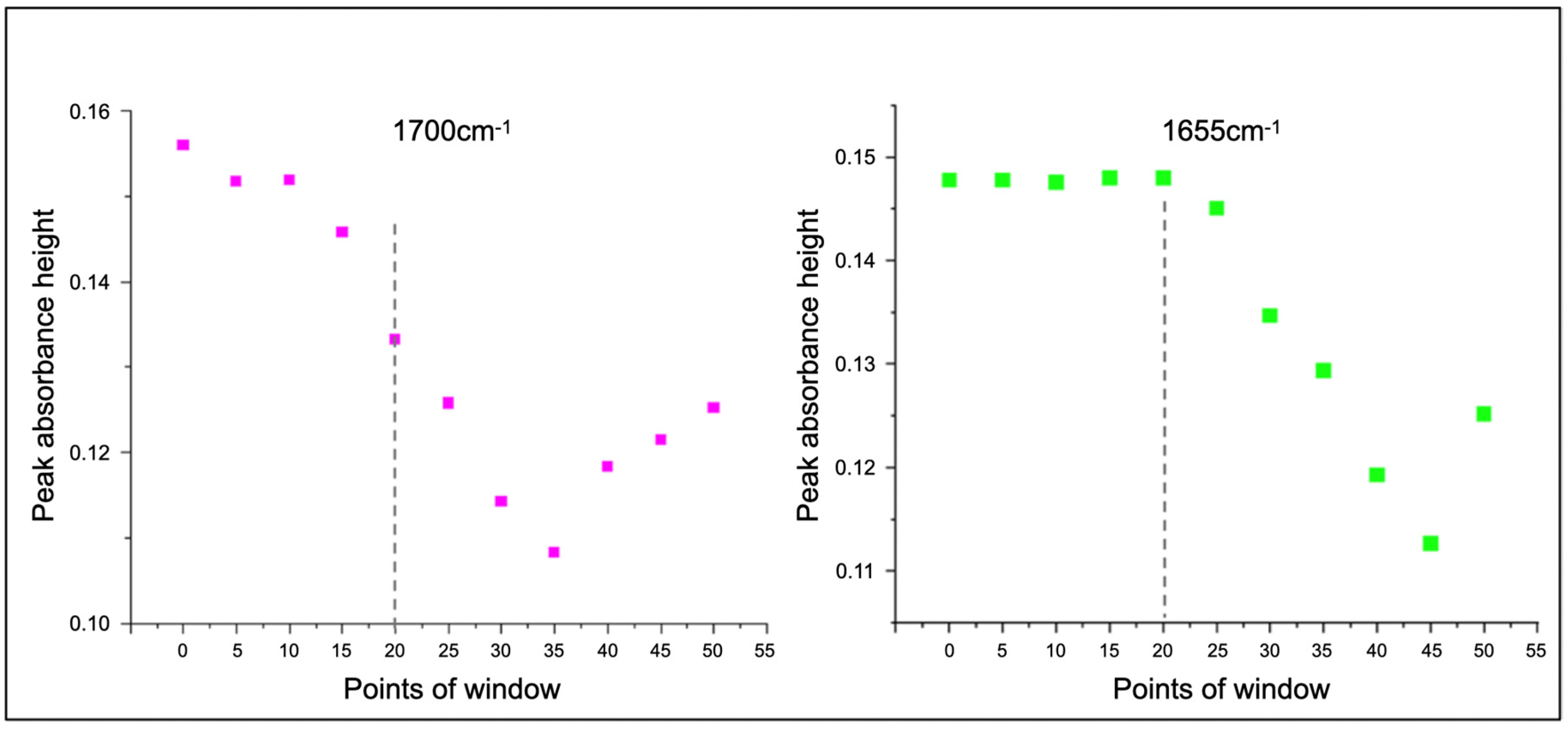
2.4. Signal Processing and Data Treatment
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Festa, F.; Rotelli, C.; Scarano, A.; Navarra, R.; Caulo, M.; Macrì, M. Functional magnetic resonance connectivity in patients with temporomadibular joint disorders. Front. Neurol. 2021, 12, 629211. [Google Scholar] [CrossRef] [PubMed]
- Villa, J.E.L.; Garcia, I.; Jimenez de Aberasturi, D.; Pavlov, V.; Sotomayor, M.D.P.T.; Liz-Marzán, L.M. SERS-based immunoassay for monitoring cortisol-related disorders. Biosens. Bioelectron. 2020, 165, 112418. [Google Scholar] [CrossRef] [PubMed]
- Hellhammer, D.H.; Wüst, S.; Kudielka, B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Kirschbaum, C.; Kudielka, B.M.; Adam, E.K.; Pruessner, J.C.; Wüst, S.; Dockray, S.; Smyth, N.; Evans, P.; Hellhammer, D.H.; et al. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 2016, 63, 414–432. [Google Scholar] [CrossRef]
- de Jesus Pinto, W.; Muricy, V.C.; Treto, R.R.R. Ritmos Biológicos No Sistema Endócrino. Braz. J. Dev. 2020, 6, 53677–53696. [Google Scholar] [CrossRef]
- Titman, A.; Price, V.; Hawcutt, D.; Chesters, C.; Ali, M.; Cacace, G.; Lancaster, G.A.; Peak, M.; Blair, J.C. Salivary cortisol, cortisone and serum cortisol concentrations are related to age and body mass index in healthy children and young people. Clin. Endocrinol. 2020, 93, 572–578. [Google Scholar] [CrossRef]
- Blair, J.; Adaway, J.; Keevil, B.; Ross, R. Salivary cortisol and cortisone in the clinical setting. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 161–168. [Google Scholar] [CrossRef]
- Harrison, R.F.; Debono, M.; Whitaker, M.J.; Keevil, B.G.; Newell-Price, J.; Ross, R.J. Salivary Cortisone to Estimate Cortisol Exposure and Sampling Frequency Required Based on Serum Cortisol Measurements. J. Clin. Endocrinol. Metab. 2018, 104, 765–772. [Google Scholar] [CrossRef]
- Debono, M.; Harrison, R.F.; Whitaker, M.J.; Eckland, D.; Arlt, W.; Keevil, B.G.; Ross, R.J. Salivary cortisone reflects cortisol exposure under physiological conditions and after hydrocortisone. J. Clin. Endocrinol. Metab. 2016, 101, 1469–1477. [Google Scholar] [CrossRef]
- Bäcklund, N.; Brattsand, G.; Israelsson, M.; Ragnarsson, O.; Burman, P.; Engström, B.E.; Høybye, C.; Berinder, K.; Wahlberg, J.; Olsson, T.; et al. Reference intervals of salivary cortisol and cortisone and their diagnostic accuracy in Cushing’s syndrome. Eur. J. Endocrinol. 2020, 182, 569–582. [Google Scholar] [CrossRef]
- Shin, S.; Oh, H.; Park, H.R.; Joo, E.Y.; Lee, S.Y. A Sensitive and Specific Liquid Chromatography-Tandem Mass Spectrometry Assay for Simultaneous Quantification of Salivary Melatonin and Cortisol: Development and Comparison with Immunoassays. Ann. Lab. Med. 2020, 41, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Holčapek, M.; Kolářová, L.; Nobilis, M. High-performance liquid chromatography-tandem mass spectrometry in the identification and determination of phase I and phase II drug metabolites. Anal. Bioanal. Chem. 2008, 391, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, H.; Li, M.; Zeng, J.; Wang, J.; Long, Q.; Wang, Y.; Zhang, C.; Chen, W. Development and validation of a candidate reference method for serum cortisol by isotope dilution liquid chromatography-tandem mass spectrometry combined with dextran sulfate-Mg2+ precipitation. Anal. Bioanal. Chem. 2020, 412, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Nugrahani, I.; Fauzia, R. Quantitative vibrational methods development and its performance comparison to colorimetry on the assay of kanamycin sulfate. Int. J. Appl. Pharm. 2019, 11, 426–435. [Google Scholar] [CrossRef]
- Perogamvros, I.; Owen, L.J.; Newell-Price, J.; Ray, D.W.; Trainer, P.J.; Keevil, B.G. Simultaneous measurement of cortisol and cortisone in human saliva using liquid chromatography-tandem mass spectrometry: Application in basal and stimulated conditions. J. Chromatogr. B 2009, 877, 3771–3775. [Google Scholar] [CrossRef]
- Fahelelbom, K.M.; Saleh, A.; Al-Tabakha, M.M.A.; Ashames, A.A. Recent applications of quantitative analytical FTIR spectroscopy in pharmaceutical, biomedical, and clinical fields: A brief review. Rev. Anal. Chem. 2022, 41, 21–33. [Google Scholar] [CrossRef]
- Fadlelmoula, A.; Pinho, D.; Carvalho, V.H.; Catarino, S.O.; Minas, G. Spectroscopy to Analyse Human Blood over the Last 20 Years: A Review towards Lab-on-a-Chip Devices. Micromachines 2022, 13, 187. [Google Scholar] [CrossRef]
- Mayerhöfer, T.G.; Ivanovski, V.; Popp, J. Infrared Refraction Spectroscopy. Appl. Spectrosc. 2021, 75, 1526–1531. [Google Scholar] [CrossRef]
- Qu, S.; Wu, G.; Fang, J.; Zang, D.; Xing, H.; Wang, L.; Wu, H. Dielectric and Magnetic Loss Behavior of Nanooxides. In Dielectric and Magnetic Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 323–461. [Google Scholar] [CrossRef]
- Nerdy, N.; Margata, L.; Sembiring, B.M.; Ginting, S.; Putra, E.d.L.; Bakri, T.K. Validation of the developed zero-order infrared spectrophotometry method for qualitative and quantitative analyses of tranexamic acid in marketed tablets. Molecules 2021, 26, 6985. [Google Scholar] [CrossRef]
- Amais, R.S.; Rocha, F.R.P.; Nóbrega, J.A. Sinais de fundo em análise instrumental: Uma discussão essencial em cursos de graduação. Quim. Nova 2017, 40, 228–237. [Google Scholar] [CrossRef]
- Luo, J.; Ying, K.; Bai, J. Savitzky-Golay smoothing and differentiation filter for even number data. Signal Process. 2005, 85, 1429–1434. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Leal, L.B.; Macarini, W.; Pimentel, R.L.; Muller, M.; Vassallo, P.F.; Campos, L.C.G.; dos Santos, L.; Luiz, W.B.; Mill, J.G.; et al. Rapid diagnosis of COVID-19 using FT-IR ATR spectroscopy and machine learning. Sci. Rep. 2021, 11, 15409. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.S.; Barreto, A.L.; Furukawa, M.; Rovai, E.S.; Bastos, A.; Bertoncello, G.; Carvalho, L.F.D.C.E.S.D. FTIR spectroscopy as a point of care diagnostic tool for diabetes and periodontitis: A saliva analysis approach. Photodiagnosis Photodyn. Ther. 2022, 40, 103036. [Google Scholar] [CrossRef] [PubMed]
- Manna, L.; Banchero, M. Solubility of Cortisone and Hydrocortisone in Supercritical Carbon Dioxide and Ethanol. J. Chem. Eng. Data 2023, 68, 601–611. [Google Scholar] [CrossRef]
- Schmidt, A.; Koulov, A.; Huwyler, J.; Mahler, H.C.; Jahn, M. Stabilizing Polysorbate 20 and 80 Against Oxidative Degradation. J. Pharm. Sci. 2020, 109, 1924–1932. [Google Scholar] [CrossRef]
- Chemistry Database. Infrared Spectroscopy Absorption Table—OChemOnline. LibreTexts 2020, 1–6. Available online: https://chem.libretexts.org/@go/page/22645 (accessed on 31 March 2025).
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Schmid, M.; Rath, D.; Diebold, U. Why and How Savitzky-Golay Filters Should Be Replaced. ACS Meas. Sci. Au 2022, 2, 185–196. [Google Scholar] [CrossRef]
- Fernandes, J.; Silva, R.; Oliveira, L.; Santos, A. Line Shape Analysis of Cortisol Infrared Spectra for Salivary Sensors: Theoretical and Experimental Observations. In XXVII Brazilian Congress on Biomedical Engineering; Springer: Cham, Switzerland, 2020; pp. 1345–1352. [Google Scholar] [CrossRef]
- Kartsova, L.A.; Solov’eva, S.A.; Bessonova, E.A. Microemulsion Preconcentration of Steroid Hormones from Aqueous Solutions and Urine Samples. J. Anal. Chem. 2021, 76, 1058–1064. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Cui, X.; Ding, S.; Chen, Z. Three different types of solubilization of thymol in Tween 80: Micelles, solutions, and emulsions—A mechanism study of micellar solubilization. J. Mol. Liq. 2020, 306, 112901. [Google Scholar] [CrossRef]
- Szymczyk, K.; Taraba, A. Properties of aqueous solutions of nonionic surfactants, Triton X-114 and Tween 80, at temperatures from 293 to 318 K: Spectroscopic and ultrasonic studies. Chem. Phys. 2017, 483–484, 96–102. [Google Scholar] [CrossRef]
- Lemes, L.C.; Cesar, P.; Júnior, C.; Strixino, J.F.; Aguiar, J. Analysis of serum cortisol levels by Fourier Transform Infrared Spectroscopy for diagnosis of stress in athletes. Res. Biomed. Eng. 2016, 32, 293–300. [Google Scholar] [CrossRef][Green Version]
- Chechekina, O.G.; Tropina, E.V.; Fatkhutdinova, L.I.; Zyuzin, M.V.; Bogdanov, A.A.; Ju, Y.; Boldyrev, K.N. Machine learning assisted rapid approach for quantitative prediction of biochemical parameters of blood serum with FTIR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 326, 125283. [Google Scholar] [CrossRef] [PubMed]
- AlKadi, H.; Alzier, A.; Mando, H.; Mando, Z.; Darwicha, J.A.N.; Allaf, A.W. Quantitative Determination of Pitavastatin in Tablets Using FTIR and RP-HPLC Analysis: A Comparative Study. Infect. Disord. Drug Targets 2022, 22, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Vigo, F.; Tozzi, A.; Disler, M.; Gisi, A.; Kavvadias, V.; Kavvadias, T. Vibrational Spectroscopy in Urine Samples as a Medical Tool: Review and Overview on the Current State-of-the-Art. Diagnostics 2023, 13, 27. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Ji, R.; Wang, M.; Zhang, Y.; Zheng, L. Mark-Spectra: A convolutional neural network for quantitative spectral analysis overcoming spatial relationships. Comput. Electron. Agric. 2022, 192, 106624. [Google Scholar] [CrossRef]
- Brum, M.C.B.; Senger, M.B.; Schnorr, C.C.; Ehlert, L.R.; da Costa Rodrigues, T. Effect of night-shift work on cortisol circadian rhythm and melatonin levels. Sleep Sci. 2022, 15, 143–148. [Google Scholar] [CrossRef]

| Serial Dilution (1:50—1 mL Solution + 49 mL Ultrapure Water Type I) | ||
|---|---|---|
| Stage | Initial Concentration (µg/dL) | Concentration After Dilution (µg/dL) |
| 1st | 5,000,000 | 100,000 |
| 2nd | 100,000 | 2000 |
| 3rd | 2000 | 40 |
| 4th | 40 | 0.8 |
| 5th | 0.8 | 0.016 |
| 1 mL Solution + 9 mL Ultrapure Water Type 1 | ||
|---|---|---|
| Stage | Cortisone (µg/dL) | Tween 80 (%) |
| 1st | 416,666.16 | 1.66 |
| 2nd | 41,666.66 | 0.16 |
| 3rd | 4166.66 | 0.016 |
| 4th | 416.66 | 0.0016 |
| 5th | 41.66 | 0.00016 |
| 1 mL Solution + 9 mL Ultrapure Water Type 1 | ||
|---|---|---|
| Stage | Cortisone (µg/dL) | Tween 80 (%) |
| 1st | 333,333.33 | 3.33 |
| 2nd | 33,333.33 | 0.33 |
| 3rd | 3333.33 | 0.033 |
| 4th | 333.33 | 0.0033 |
| 5th | 33.33 | 0.00033 |
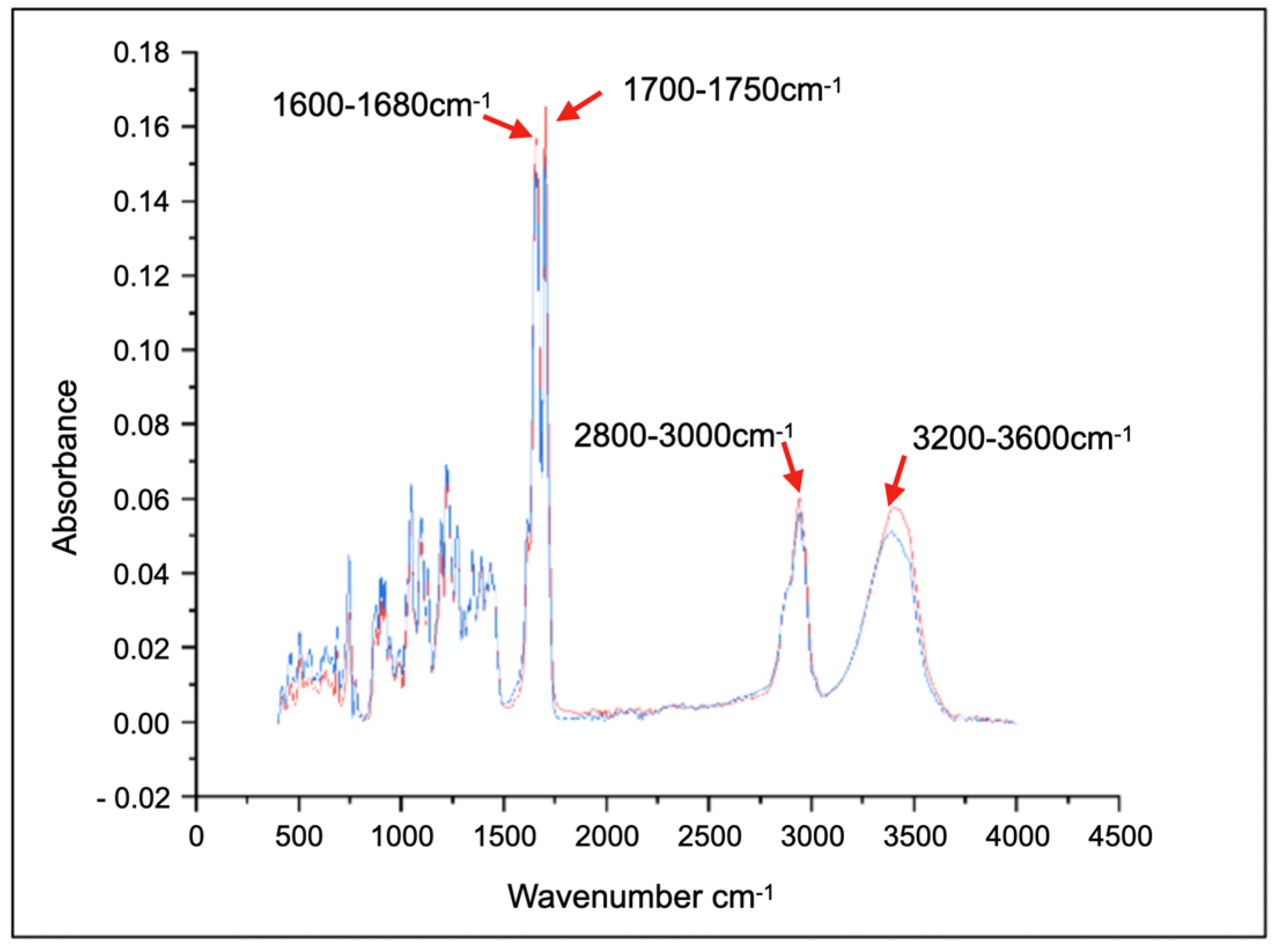
| Wavenumber Range (cm−1) | Intensity | Shape | Bond Type | Functional Group |
|---|---|---|---|---|
| 1600–1680 | Strong | Sharp | C=C | Alkene |
| 1700–1750 | Strong | Sharp | C-O | Ketone |
| 2800–3000 | Medium | Sharp | C-H | Alkyl and Methyl |
| 3200–3600 | Medium | Broad | OH | Hydroxyl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcas, L.P.B.; da Silva, S.M.S.D.; Arcas, F.C.D.; Alves, F.H.; de Carvalho, L.F.d.C.e.S.; Amaral, M. Cortisone Analysis by FTIR Spectroscopy: In Vitro Study. Processes 2025, 13, 1112. https://doi.org/10.3390/pr13041112
Arcas LPB, da Silva SMSD, Arcas FCD, Alves FH, de Carvalho LFdCeS, Amaral M. Cortisone Analysis by FTIR Spectroscopy: In Vitro Study. Processes. 2025; 13(4):1112. https://doi.org/10.3390/pr13041112
Chicago/Turabian StyleArcas, Luciana Paula Benício, Sara Maria Santos Dias da Silva, Felipe Carlos Dias Arcas, Flávio Henrique Alves, Luís Felipe das Chagas e Silva de Carvalho, and Marina Amaral. 2025. "Cortisone Analysis by FTIR Spectroscopy: In Vitro Study" Processes 13, no. 4: 1112. https://doi.org/10.3390/pr13041112
APA StyleArcas, L. P. B., da Silva, S. M. S. D., Arcas, F. C. D., Alves, F. H., de Carvalho, L. F. d. C. e. S., & Amaral, M. (2025). Cortisone Analysis by FTIR Spectroscopy: In Vitro Study. Processes, 13(4), 1112. https://doi.org/10.3390/pr13041112











