Analysis of Carbon Dioxide Mineralization in Carbonates from Tampico-Misantla Basin, Mexico: Effect of Organic Matter Content
Abstract
1. Introduction
2. Materials and Methods
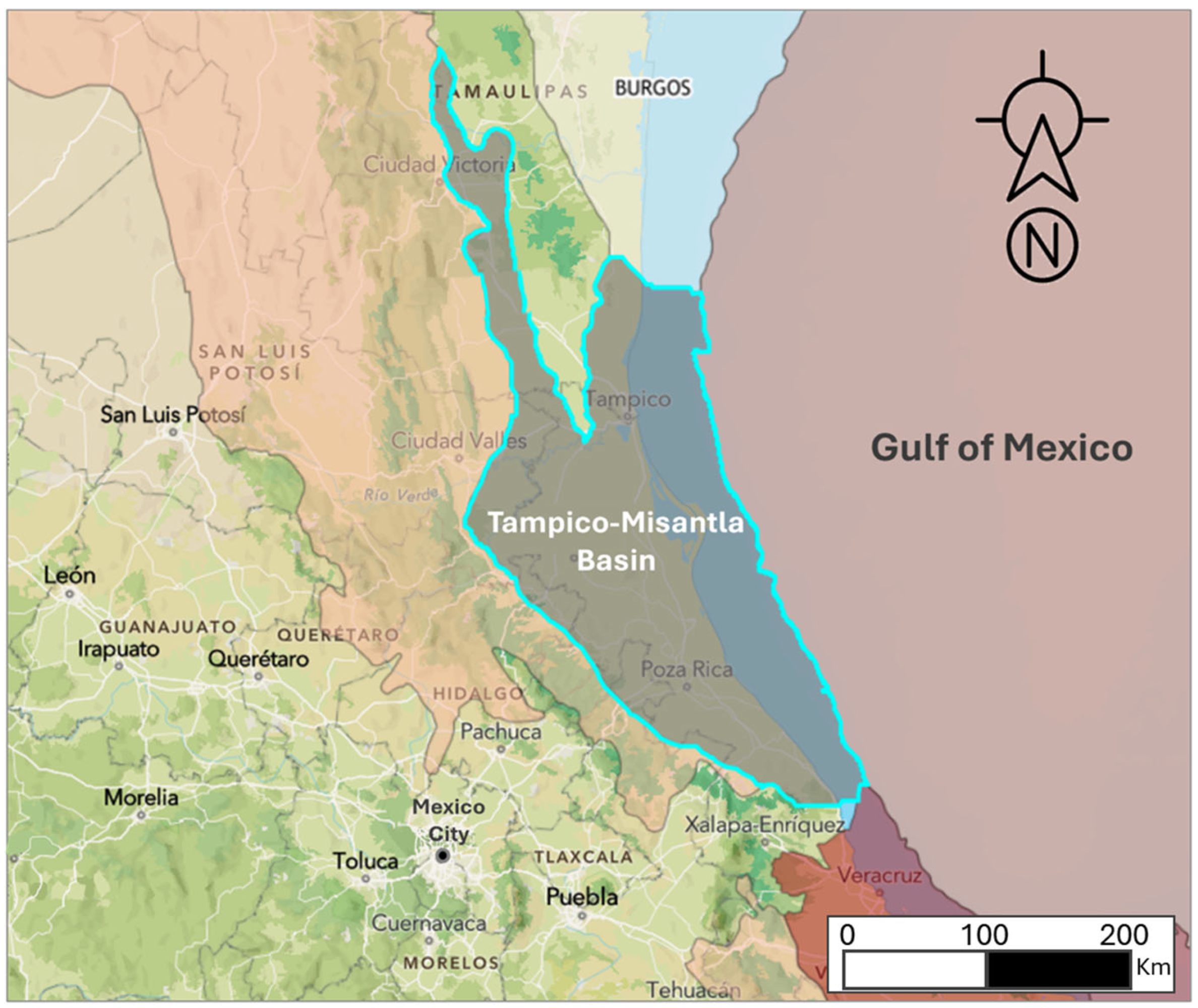
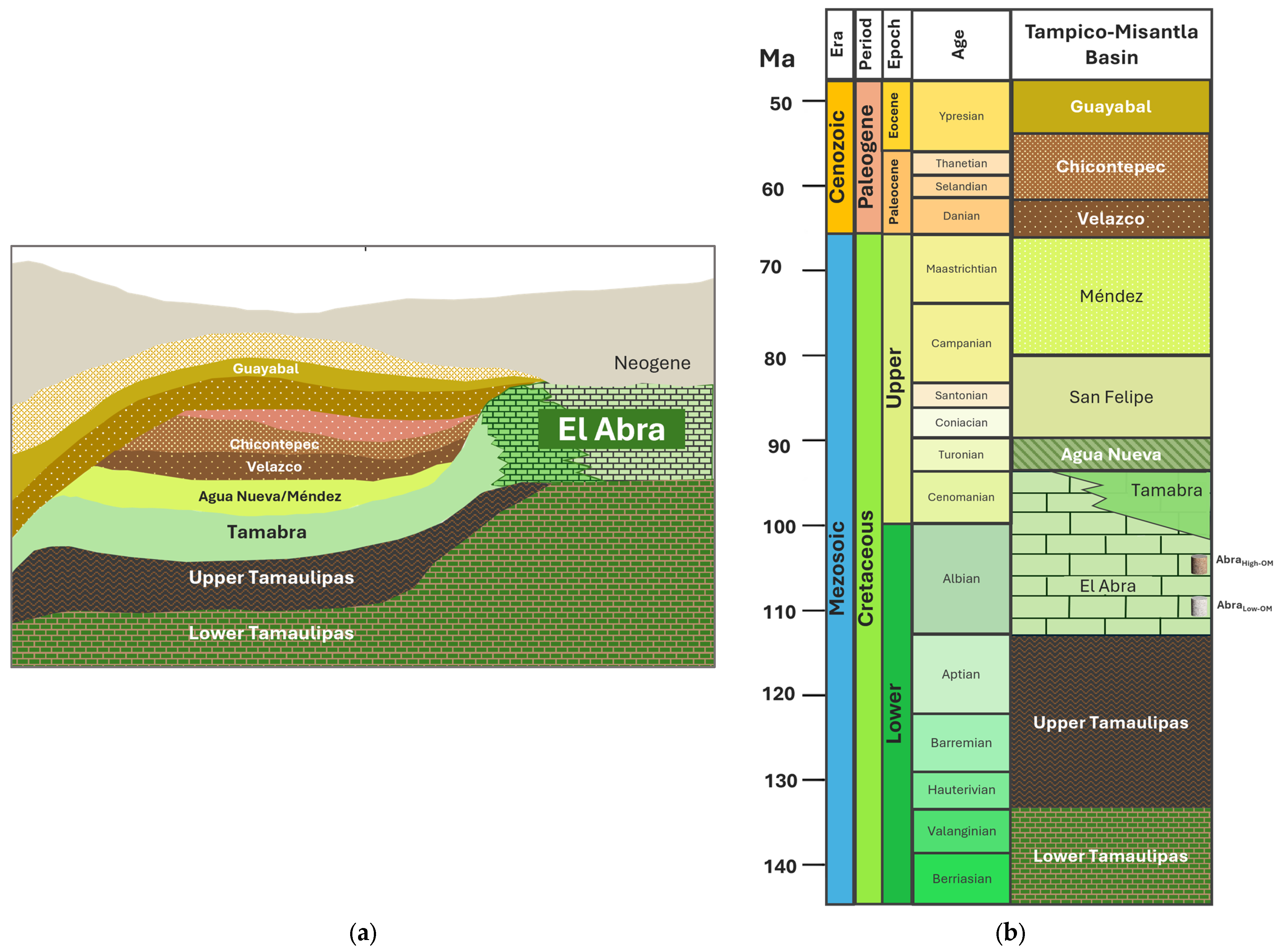
2.1. Selection Sample
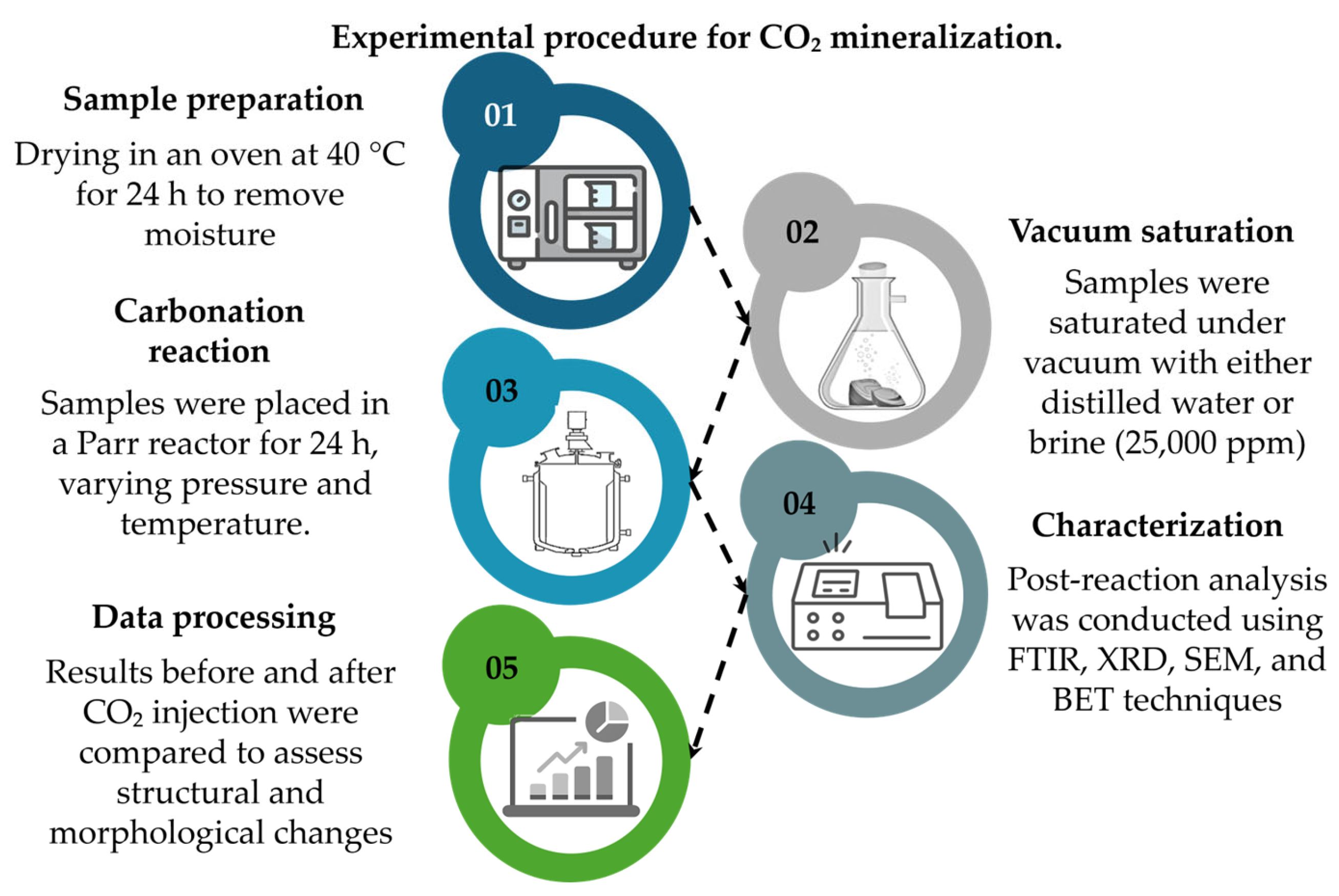
2.2. Sample Preparation
2.3. CO2 Injection in High Pressure and Temperature Reactor
2.4. Characterization Before and After CO2 Injection
- Fourier-transform infrared spectroscopy (FT-IR) using attenuated total reflectance (ATR). Infrared spectra were acquired in a PerkinElmer Spectrum 100 ONE spectrophotometer (PerkinElmer, Waltham, MA, USA)equipped with an ATR accessory of diamond crystal. This technique facilitated the identification of functional groups and structural modifications in the samples before and after CO2 injection. Measurements were conducted within the spectral range of 4000–400 cm−1, with a resolution of 4 cm−1 and 32 scans per spectrum;
- SEM with energy-dispersive X-ray spectroscopy (EDS). A JEOL JSM 7401-F field emission microscope (JEOL Ltd., Akishima, Tokyo, Japan) coupled with an EDAX X1 system was used to obtain high-resolution images of the rock surface. To ensure conductivity, the samples were coated with a thin carbon layer before analysis. Imaging was performed at an accelerating voltage of 15 kV, with magnifications ranging from 10× to 1000×, allowing for a detailed examination of microstructural changes;
- XRD analysis: The crystalline phases present in the samples were identified using a Bruker D2 PHASER diffractometer (Bruker Corporation, Billerica, MA, USA) equipped with a Cu-Kα radiation source (λ = 0.154 nm). Diffraction patterns were recorded over the 2θ range of 10–90°, with a step size of 0.02° and a counting time of 1 s per step, ensuring precise mineralogical characterization;
- Surface area and porosity analysis: The specific surface area of the samples was determined using the Brunauer–Emmett–Teller (BET) method on a Quantachrome Nova 2200e analyzer (Quantachrome Instruments, Boynton Beach, FL, USA). Before measurement, nitrogen physisorption was conducted at 77 K to generate adsorption isotherms. The resulting data were processed to calculate the specific surface area and assess the porous structure of the rock samples.
3. Results and Discussion
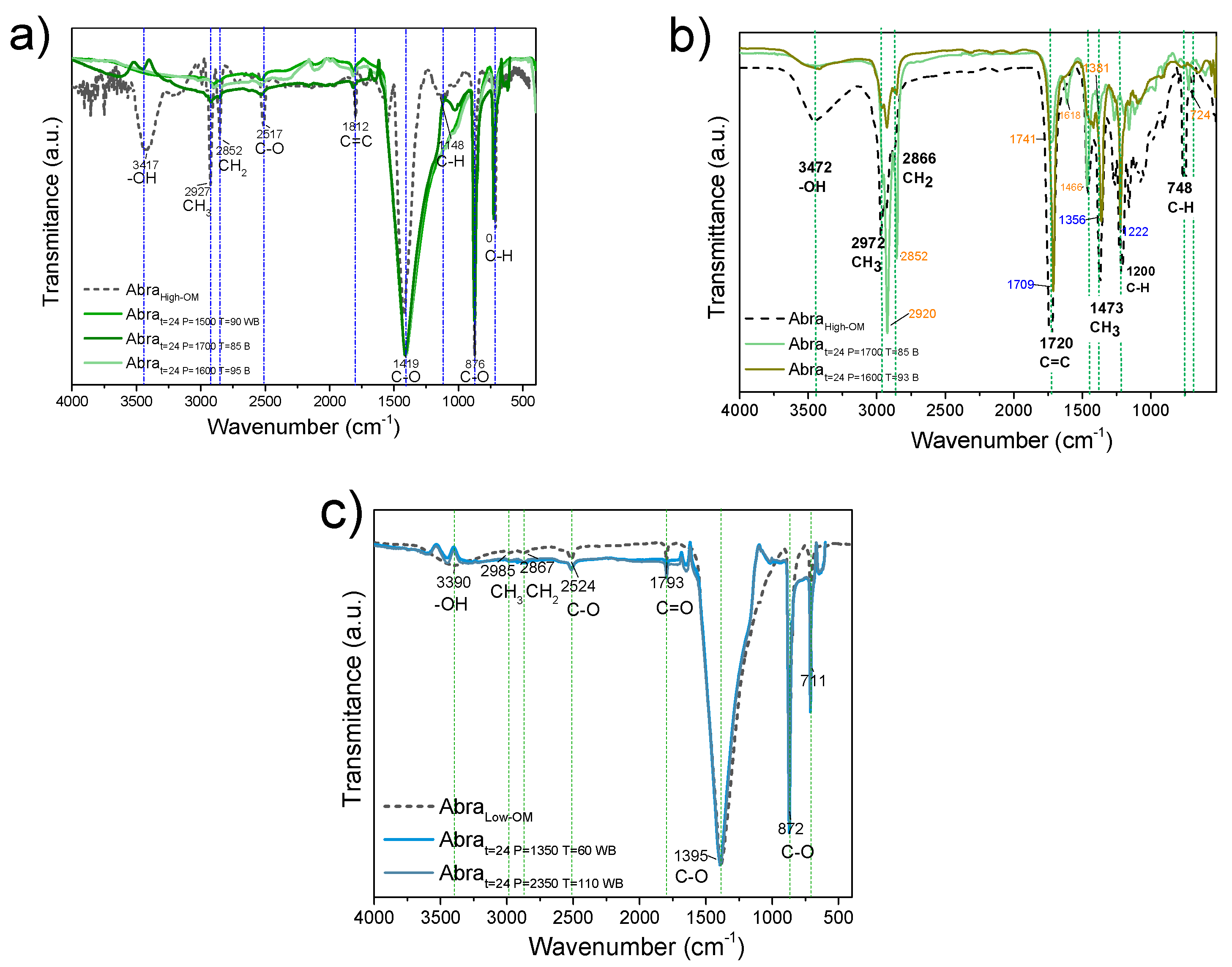
3.1. Fourier Transform Infrared (FT-IR) Spectroscopy to Monitor Functional Groups
3.2. Structural Analysis Through X-Ray Diffraction Method
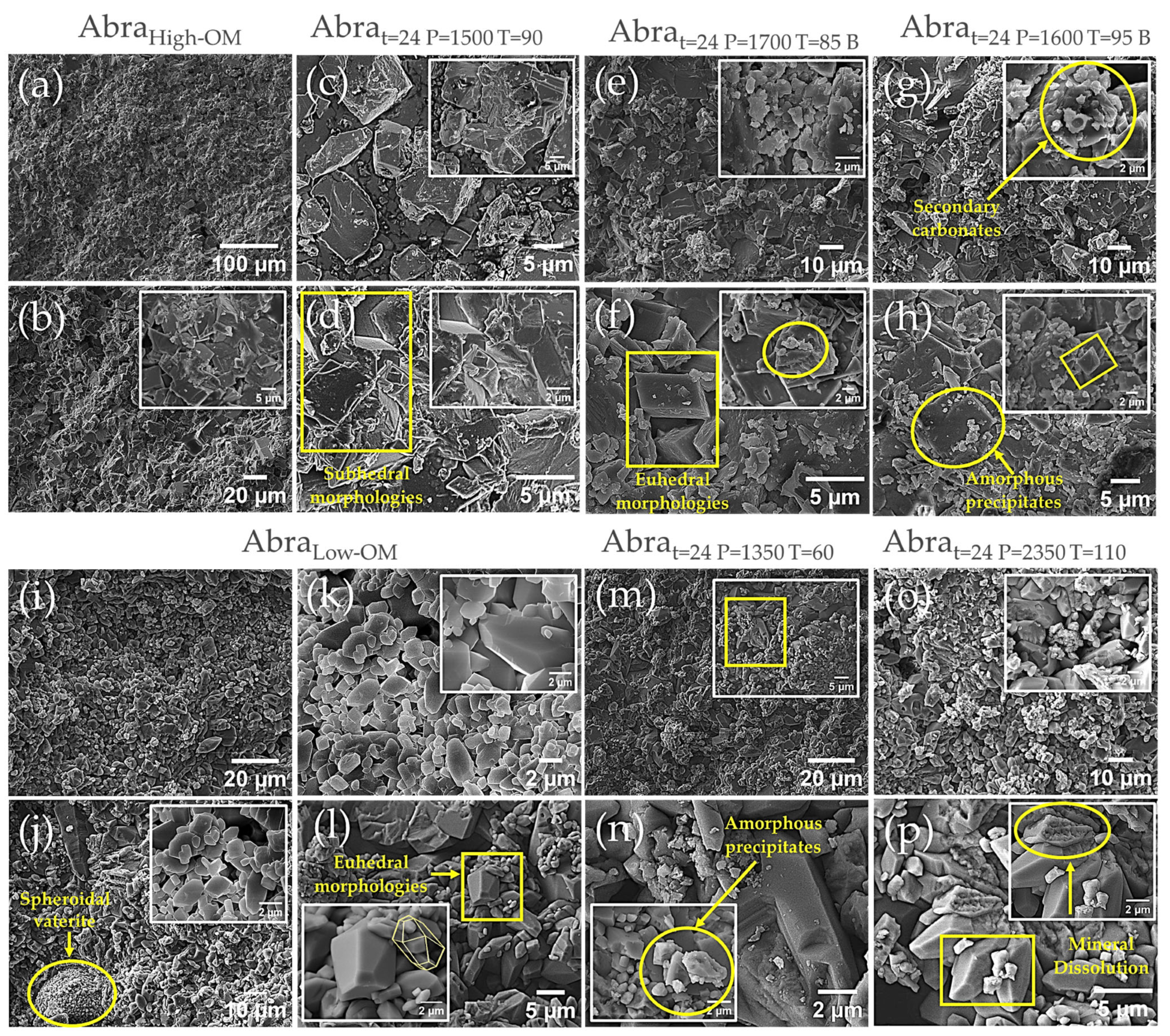
3.3. Morphology Evaluation Through Scanning Electron Microscopy
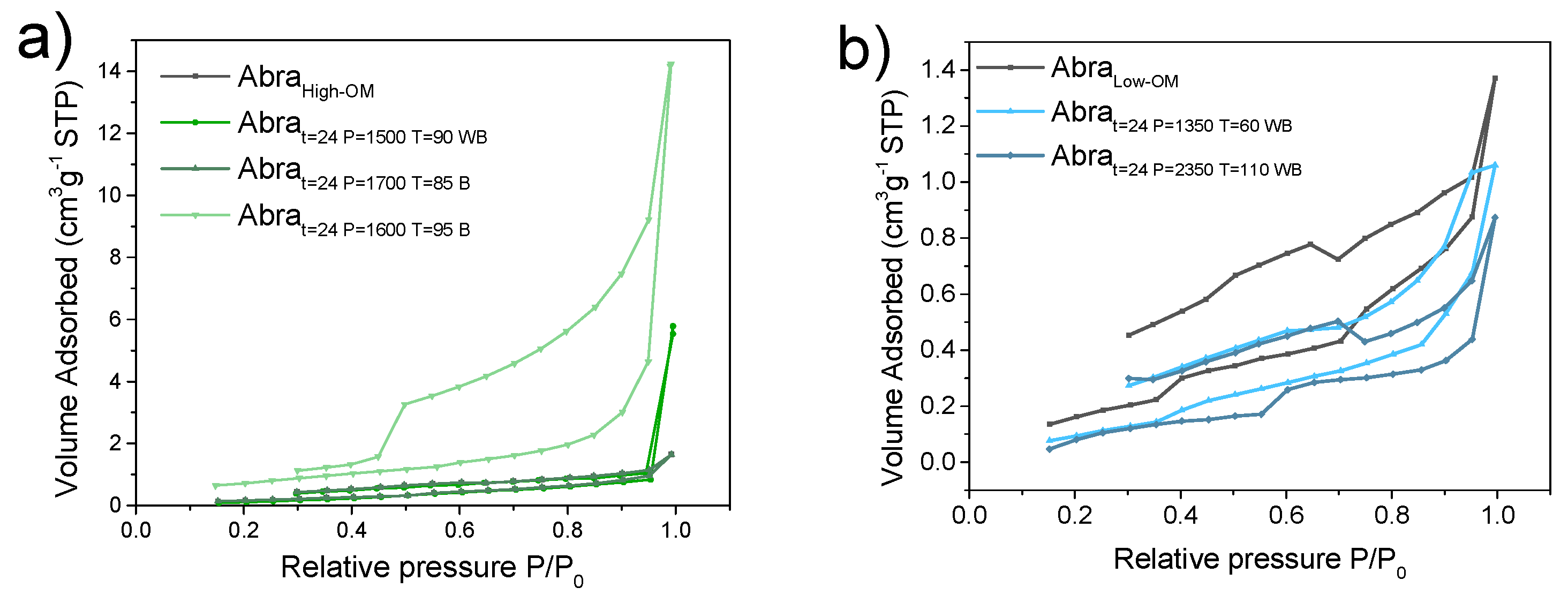
3.4. Specific Surface Analysis by Brunauer–Emmett–Teller Analysis
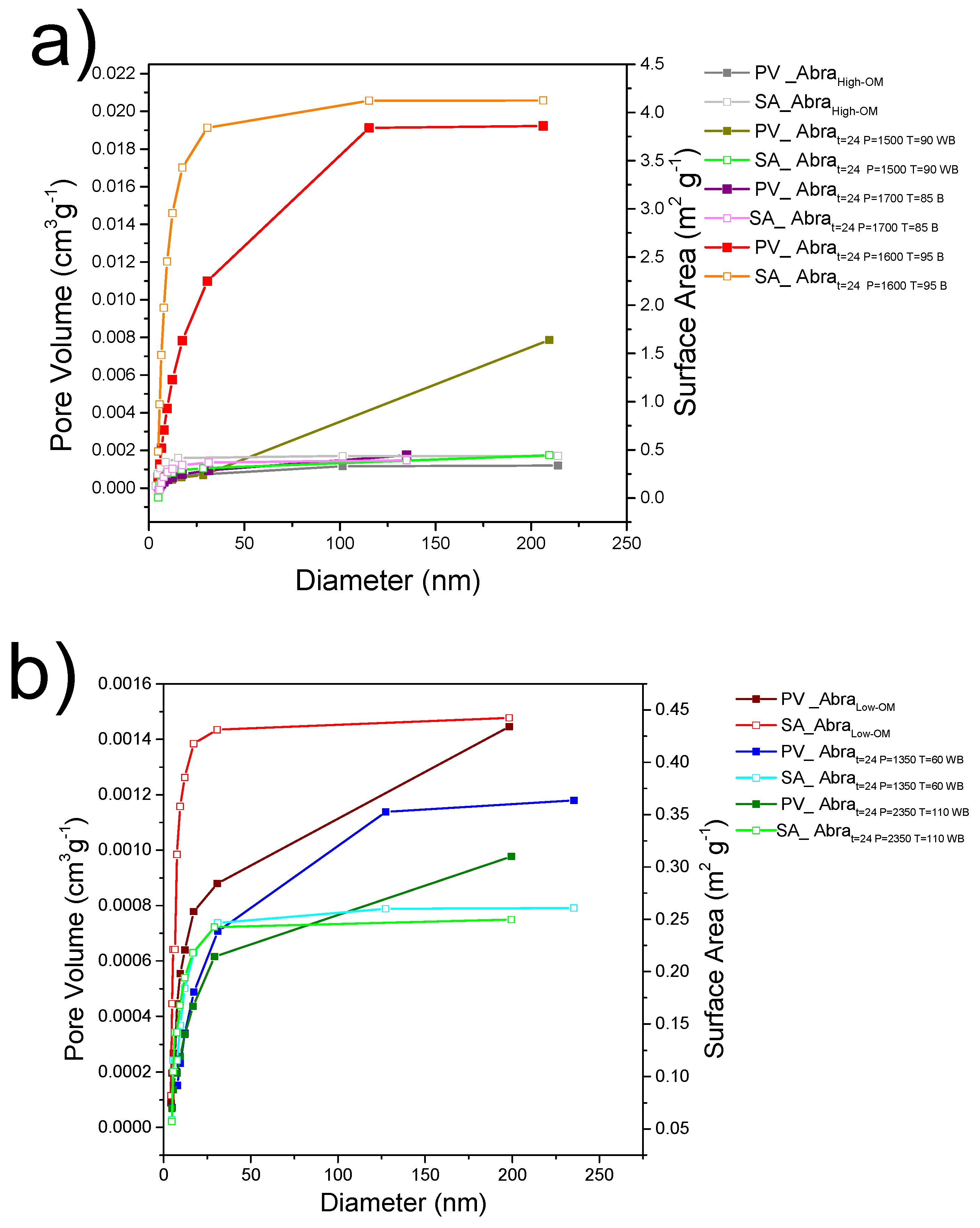
3.5. Analysis of Surface Area and Pore Size Distribution by the BJH Method
3.5.1. Pore Diameter and Pore Network Characteristics
3.5.2. Influence of Organic Matter on Pore Structure
- Dissolution/Precipitation of minerals: The injection of CO2 into the reactor, together with the high-pressure and -temperature conditions, may have induced the dissolution of minerals susceptible to acidification, such as carbonates. Dissolving these minerals creates new pore spaces, increasing the surface area and pore volume. At the same time, the alteration of the physicochemical conditions may have favored the precipitation of new mineral phases, such as silica or clays, which may also contribute to the modification of the pore structure [60]. SEM, XRD, and FTIR analyses provide evidence of these dissolution and precipitation processes;
- Organic matter maturation and hydrocarbon release: The Abra formation is hydrocarbon-producing and located in the Tampico-Misantla basin. The temperatures and pressures used in the treatments (90–110 °C and 1350–2350 PSI) are within the hydrocarbon generation window for source rocks. The oil expulsion observed in the organic matter-rich samples during the treatment suggests that an OM maturation process is being simulated, where kerogen is converted into oil and gas. The release of these hydrocarbons from the rock matrix could generate new pore spaces, improving permeability and the accessible surface area [61]. This process is particularly relevant in hydrocarbon source rocks, such as the Abra formation, where porosity and permeability are fundamental [62].
3.5.3. Effect on CO2 Storage Potential and EOR Implications
4. Conclusions
5. Recommendations for Future Research
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, A.; Boon, M.M.; Benson, S.M.; Watson, M.N.; Haese, R.R. Reconciling predicted and observed carbon mineralization in siliciclastic formations. Chem. Geol. 2023, 619, 121324. [Google Scholar] [CrossRef]
- Eyitayo, S.; Arbad, N.; Okere, C.; Gamadi, T.; Watson, M. Advancing geological storage of carbon dioxide (CO2) with emerging technologies for climate change mitigation. Int. J. Environ. Sci. Technol. 2025, 22, 5023–5056. [Google Scholar] [CrossRef]
- Rochelle, C.A.; Czernichowski-Lauriol, I.; Milodowski, A.E. The impact of chemical reactions on CO2 storage in geological formations: A brief review. In Geological Storage of Carbon Dioxide; Baines, S.J., Worden, R.H., Eds.; Geological Society of London: London, UK, 2004; Volume 233, pp. 87–106. [Google Scholar]
- Kim, K.; Kim, D.; Na, Y.; Song, Y.; Wang, J. A review of carbon mineralization mechanism during geological CO2 storage. Heliyon 2023, 9, e23135. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Levresse, G.; Carrera-Hernández, J.J.; Cerca, M. A basin scale assessment framework of onshore aquifer-based CO2 suitability storage in Tampico Misantla basin, Mexico. Int. J. Greenh. Gas Control 2023, 125, 103874. [Google Scholar] [CrossRef]
- Vega-Ortiz, C.; Beti, D.R.; Setoyama, E.; McLennan, J.D.; Ring, T.A.; Levey, R.; Martínez-Romero, N. Source rock evaluation in the central-western flank of the Tampico Misantla Basin, Mexico. J. South Am. Earth Sci. 2020, 100, 102552. [Google Scholar] [CrossRef]
- Comision Nacional de Hidrocarburos. Mapa de Hidrocarburos. 2025. Available online: https://mapa.hidrocarburos.gob.mx (accessed on 28 February 2025).
- Guzmán, A.E. Tampico-Misantla: A premier super basin in waiting. AAPG Bull. 2022, 106, 495–516. [Google Scholar] [CrossRef]
- Wendt, A.; Sheriff, A.; Shih, C.Y.; Vikara, D.; Grant, T. A multi-criteria CCUS screening evaluation of the Gulf of Mexico, USA. Int. J. Greenh. Gas Control 2022, 118, 103688. [Google Scholar] [CrossRef]
- Saleh, M.A.; Ryan, M.P.; Trusler, J.P.M.; Krevor, S. The interfacial processes controlling carbon dioxide mineralisation in magnesium and calcium silicates. Fuel 2025, 380, 132969. [Google Scholar] [CrossRef]
- Rahim, H.u.; Shah, M.M.; Khalil, R.; Jamil, M. Evaluation of fault and fracture controlled hydrothermal dolomitization in the Jurassic carbonate succession of the lesser Himalayas, NW Pakistan. Carbonates Evaporites 2025, 40, 32. [Google Scholar] [CrossRef]
- Jamil, M.; Ullah, I.; Rahim, H.U.; Khan, I.; Abbas, W.; Rehman, M.U.; Rashid, A.; Umar, M.; Ali, A.; Siddiqui, N.A. Tracking Depositional Architecture and Diagenetic Evolution in the Jurassic Carbonates, Trans Indus Ranges, NW Himalayas. Minerals 2024, 14, 1170. [Google Scholar] [CrossRef]
- Raza, A.; Glatz, G.; Gholami, R.; Mahmoud, M.; Alafnan, S. Carbon mineralization and geological storage of CO2 in basalt: Mechanisms and technical challenges. Earth-Sci. Rev. 2022, 229, 104036. [Google Scholar] [CrossRef]
- Pusparizkita, Y.M.; Schmahl, W.W.; Ambarita, M.; Kholid, H.N.; Sadewa, A.Y.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Mineralizing CO2 and producing polymorphic calcium carbonates from bitumen-rock asphalt manufacturing solid residues. Clean. Eng. Technol. 2023, 12, 100602. [Google Scholar] [CrossRef]
- Mexico Gobierno de la Republica. Atlas Geológico Cuenca Tampico—Misantla; National Hydrocarbons Commission: Mexico City, Mexico, 2023; Available online: https://hidrocarburos.gob.mx/media/3091/atlas_geologico_cuenca_tampico-misantla_v3.pdf (accessed on 28 February 2025).
- Isah, A.; Mahmoud, M.; Aljawad, M.S.; Arif, M.; Hussaini, S.R.; Amao, A.; Raza, A.; Kamal, M.S. Enforced CO2 mineralization in anhydrite-rich rocks. Energy 2024, 305, 132323. [Google Scholar] [CrossRef]
- Sanguinito, S.; Goodman, A.; Tkach, M.; Kutchko, B.; Culp, J.; Natesakhawat, S.; Fazio, J.; Fukai, I.; Crandall, D. Quantifying dry supercritical CO2-induced changes of the Utica Shale. Fuel 2018, 226, 54–64. [Google Scholar] [CrossRef]
- Moita, P.; Berrezueta, E.; Abdoulghafour, H.; Beltrame, M.; Pedro, J.; Mirão, J.; Miguel, C.; Galacho, C.; Sitzia, F.; Barrulas, P.; et al. Mineral Carbonation of CO2 in Mafic Plutonic Rocks, II—Laboratory Experiments on Early-Phase Supercritical CO2—Brine—Rock Interactions. Appl. Sci. 2020, 10, 5083. [Google Scholar] [CrossRef]
- Morais, M.M.; Lucas-Oliveira, E.; Bonagamba, T.J.; Aum, P.T.P.; Lucas, C.R.d.S.; da Silva, D.N.N.; Fortulan, C.A. Fabrication and petrophysical characterization of artificial carbonate rocks with multiscale porosity sintered in a CO2 atmosphere. Geoenergy Sci. Eng. 2023, 229, 212096. [Google Scholar] [CrossRef]
- Jayasekara, D.W.; Ranjith, P.G.; Wanniarachchi, W.A.M.; Rathnaweera, T.D.; Van Gent, D. CO2-brine-caprock interaction: Reactivity experiments on mudstone caprock of South-west Hub geo-sequestration project. J. Pet. Sci. Eng. 2020, 189, 107011. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, L.; Li, Y.; Zhang, J. Controllable crystallization of pure vaterite using CO2-storage material and different Ca2+ sources. J. CO2 Util. 2021, 48, 101520. [Google Scholar] [CrossRef]
- Durucan, S.; Korre, A.; Parlaktuna, M.; Senturk, E.; Wolf, K.-H.; Chalari, A.; Stork, A.; Nikolov, S.; Kunder, R.; Sigfusson, B.; et al. SUCCEED: A CO2 storage and utilisation project aimed at mitigating against greenhouse gas emissions from geothermal power production. SSRN Electron. J. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Cheng, Z.; Jiang, L.; Lv, P.; Song, Y. Review on Multiscale CO2 Mineralization and Geological Storage: Mechanisms, Characterization, Modeling, Applications and Perspectives. Energy Fuels 2023, 37, 14512–14537. [Google Scholar] [CrossRef]
- Meckel, T.A.; Bump, A.P.; Hovorka, S.D.; Trevino, R.H. Carbon capture, utilization, and storage hub development on the Gulf Coast. Greenh. Gases Sci. Technol. 2021, 11, 619–632. [Google Scholar] [CrossRef]
- Lu, H.; Wang, W.; Zhang, J.; Wu, M.; Deng, Y.; Li, G.; Tian, M. Accelerated Direct Mineralization of CO2 by Carbide Slag under Ambient Temperature and Pressure Reaction Conditions. Energy Fuels 2024, 38, 14483–14492. [Google Scholar] [CrossRef]
- Li, N.; Feng, W.; Yu, J.; Chen, F.; Zhang, Q.; Zhu, S.; Hu, Y.; Li, Y. Recent Advances in Geological Storage: Trapping Mechanisms, Storage Sites, Projects, and Application of Machine Learning. Energy Fuels 2023, 37, 10087–10111. [Google Scholar] [CrossRef]
- Jamaluddin, N.; Hsu, Y.-I.; Asoh, T.-A.; Uyama, H. Optically Transparent and Toughened Poly(methyl methacrylate) Composite Films with Acylated Cellulose Nanofibers. ACS Omega 2021, 6, 10752–10758. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.S.; Mahmud, M.B.; Omranpour, H.; Salehi, A.; Monfared, A.R.; Park, C.B. Highly Tough Yet Stiff, Transparent, and Recyclable PMMA Nanocomposites Incorporating TPU Nanofibril Networks with High Thermal Stability and Strong Interfacial Adhesion. ACS Appl. Mater. Interfaces 2024, 16, 42687–42703. [Google Scholar] [CrossRef]
- Ye, H.; Liu, Q.; Bao, Q.; Wang, Z.; Xie, Y.; Michelle, T.; Zhao, W.; Xian, C. Review on in-situ CO2 mineralization sequestration: Mechanistic understanding and research frontiers. Int. J. Coal Sci. Technol. 2025, 12, 15. [Google Scholar] [CrossRef]
- López-Dinorín, R.; Mendoza-Martínez, A.M.; Palma-Ramírez, D.; Luna-Dominguez, J.H.; Dorantes-Rosales, H. Structural and morphological evaluation of three types of carbonate hydrocarbon-bearing rocks from Tampico-Misantla basin: Structural effects after CO2 injection. MRS Adv. 2024, 9, 1023–1030. [Google Scholar] [CrossRef]
- Juárez-Arriaga, E.; Lawton, T.F.; Stockli, D.F.; Solari, L.; Martens, U. Late Cretaceous-Paleocene stratigraphic and structural evolution of the central Mexican fold and thrust belt, from detrital zircon (U-Th)/(He-Pb) ages. J. South Am. Earth Sci. 2019, 95, 102264. [Google Scholar] [CrossRef]
- Peter, A.; Jin, X.; Fan, X.; Eshiet, K.I.-I.; Sheng, Y.; Yang, D. Effect of CO2 Phase on Pore Geometry of Saline Reservoir Rock. Rock Mech. Rock Eng. 2022, 55, 1907–1930. [Google Scholar] [CrossRef]
- Xue, J.; Gao, H.; Ma, Z.; Shi, H.; Li, X.; Li, T.; Cheng, Z.; Wang, C.; Li, P.; Zhang, N.C. Analyzing the Microscopic Production Characteristics of CO2 Flooding after Water Flooding in Tight Oil Sandstone Reservoirs Utilizing NMR and Microscopic Visualization Apparatus. Atmosphere 2024, 15, 487. [Google Scholar] [CrossRef]
- González-Partida, E.; Camprubí, A.; Canet, C.; Sánchez, F. Fisicoquímica de salmueras e hidrocarburos en cuencas petroleras y en depósitos minerales tipo Mississippi Valley y asociados. Parte II: Ejemplos de la Cuenca de Sabinas y la Cuenca del Sureste, México. Boletín Soc. Geológica Mex. 2007, 60, 23–42. [Google Scholar] [CrossRef]
- Salem, A.M.; Shedid, S.A. Variation of petrophysical properties due to carbon dioxide (CO2) storage in carbonate reservoirs. J. Pet. Gas Eng. 2013, 4, 91–102. [Google Scholar]
- National Hydrocarbons Commission. Reporte de Reservas de Hidrocarburos; National Hydrocarbons Commission: Mexico City, Mexico, 2023; Available online: https://datos.gob.mx/busca/dataset/reporte-de-reservas-de-hidrocarburos (accessed on 28 February 2025).
- Kim, K.; Vilarrasa, V.; Makhnenko, R.Y. CO2 Injection Effect on Geomechanical and Flow Properties of Calcite-Rich Reservoirs. Fluids 2018, 3, 66. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of Micro-Fourier Transform Infrared Spectroscopy (FTIR) in the Geological Sciences—A Review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef]
- Pejcic, B.; Heath, C.; Pagès, A.; Normore, L. Analysis of carbonaceous materials in shales using mid-infrared spectroscopy. Vib. Spectrosc. 2021, 112, 103186. [Google Scholar] [CrossRef]
- Toffolo, M.B.; Regev, L.; Dubernet, S.; Lefrais, Y.; Boaretto, E. FTIR-Based Crystallinity Assessment of Aragonite–Calcite Mixtures in Archaeological Lime Binders Altered by Diagenesis. Minerals 2019, 9, 121. [Google Scholar] [CrossRef]
- Xu, B.; Poduska, K.M. Linking crystal structure with temperature-sensitive vibrational modes in calcium carbonate minerals. Phys. Chem. Chem. Phys. 2014, 16, 17634–17639. [Google Scholar] [CrossRef]
- Florek, M.; Fornal, E.; Gómez-Romero, P.; Zieba, E.; Paszkowicz, W.; Lekki, J.; Nowak, J.; Kuczumow, A. Complementary microstructural and chemical analyses of Sepia officinalis endoskeleton. Mater. Sci. Eng. C 2009, 29, 1220–1226. [Google Scholar] [CrossRef]
- Prasad, P.S.R.; Srinivasa Sarma, D.; Nirmal Charan, S. Mineral trapping and sequestration of carbon-dioxide in deccan basalts: SEM, FTIR and raman spectroscopic studies on secondary carbonates. J. Geol. Soc. India 2012, 80, 546–552. [Google Scholar] [CrossRef]
- Ortega-Villamagua, E.; Arcos, M.; Romero, M.; Vasquez, C.; Palma-Cando, A. Precipitación de carbonatos inducida microbiológicamente como potencial estrategia en la restauración de estructuras patrimoniales. Ge-Conservacion 2022, 21, 224–234. [Google Scholar] [CrossRef]
- Matalkah, F.; Soroushian, P. Role of CO2 in enhancing geopolymer properties formulated with fluidized bed combustion ash. J. CO2 Util. 2023, 71, 102462. [Google Scholar] [CrossRef]
- Krukowski, E.G.; Goodman, A.; Rother, G.; Ilton, E.S.; Guthrie, G.; Bodnar, R.J. FT-IR study of CO2 interaction with Na+ exchanged montmorillonite. Appl. Clay Sci. 2015, 114, 61–68. [Google Scholar] [CrossRef]
- Ali, M.; Jha, N.K.; Pal, N.; Keshavarz, A.; Hoteit, H.; Sarmadivaleh, M. Recent advances in carbon dioxide geological storage, experimental procedures, influencing parameters, and future outlook. Earth-Sci. Rev. 2022, 225, 103895. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Q.; Li, Z.; Hu, H.; Wang, C. Insights into the mechanochemical interfacial interaction between calcite and serpentine: Implications for ambient CO2 capture. J. Clean. Prod. 2023, 401, 136715. [Google Scholar] [CrossRef]
- Zoveidavianpoor, M.; Jalilavi, M.; Mohsin, R. Investigating mineral carbonation dynamics by variable CO2 injection in Bera sandstone and Tapah limestone under controlled weathering. Int. J. Pet. Eng. 2024, 4, 106–132. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Elhamdi, I.; Souissi, H.; Taktak, O.; Elghoul, J.; Kammoun, S.; Dhahri, E.; Costa, B.F.O. Experimental and modeling study of ZnO:Ni nanoparticles for near-infrared light emitting diodes. RSC Adv. 2022, 12, 13074–13086. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Alba-Rubio, A.C.; Cassidy, A.; Moreno-Tost, R.; García-Sancho, C.; Maireles-Torres, P. Tailoring the selectivity of Cu-based catalysts in the furfural hydrogenation reaction: Influence of the morphology of the silica support. Fuel 2022, 319, 123827. [Google Scholar] [CrossRef]
- Chen, F.; Xu, J.; Tang, L.; Yu, Q.; Gu, J. Microscopic investigation of chemical and physical alteration behaviors in sandstone minerals induced by CO2-H2O-rock interactions during CO2 saline aquifer storage. Sci. Total Environ. 2025, 958, 177897. [Google Scholar] [CrossRef]
- Md Yusof, M.A.; Ibrahim, M.A.; Idress, M.; Idris, A.K.; Saaid, I.M.; Mohd Rodzi, N.; Mohsin, S.; Azhari Awangku Matali, A.A. Effects of CO2/Rock/Formation Brine Parameters on CO2 Injectivity for Sequestration. In Proceedings of the Offshore Technology Conference Asia, Online, 2–6 November 2020. [Google Scholar]
- Duan, R.; Xu, Z.; Dong, Y.; Liu, W. Characterization and classification of pore structures in deeply buried carbonate rocks based on mono- and multifractal methods. J. Pet. Sci. Eng. 2021, 203, 108606. [Google Scholar] [CrossRef]
- Cheng, Y.; Zeng, M.; Lu, Z.; Du, X.; Yin, H.; Yang, L. Effects of Supercritical CO2 Treatment Temperatures on Mineral Composition, Pore Structure and Functional Groups of Shale: Implications for CO2 Sequestration. Sustainability 2020, 12, 3927. [Google Scholar] [CrossRef]
- Fu, S.; Fang, Q.; Li, A.; Li, Z.; Han, J.; Dang, X.; Han, W. Accurate characterization of full pore size distribution of tight sandstones by low-temperature nitrogen gas adsorption and high-pressure mercury intrusion combination method. Energy Sci. Eng. 2021, 9, 80–100. [Google Scholar] [CrossRef]
- Li, T.; Huang, Z.; Zhao, J.; Xu, X.; Guo, X. Pore structure characteristics and their influencing factors: A case study from the middle jurassic mixed siliciclastic carbonate rocks, Turpan-Hami basin, Northwest China. J. Pet. Sci. Eng. 2021, 203, 108611. [Google Scholar] [CrossRef]
- Kuila, U.; Prasad, M. Specific surface area and pore-size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar] [CrossRef]
- Zhao, H.; Luo, X.; Zhang, H.; Sun, N.; Wei, W.; Sun, Y. Carbon-based adsorbents for post-combustion capture: A review. Greenh. Gases Sci. Technol. 2018, 8, 11–36. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Masoudi Soltani, S. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Rashid, A.; Siddiqui, N.A.; Ahmed, N.; Jamil, M.; El-Ghali, M.A.K.; Ali, S.H.; Zaidi, F.K.; Wahid, A. Field attributes and organic geochemical analysis of shales from early to middle Permian Dohol Formation, Peninsular Malaysia: Implications for hydrocarbon generation potential. J. King Saud Univ.-Sci. 2022, 34, 102287. [Google Scholar] [CrossRef]


| Sample | Depth (m) | OM Content | Hydrocarbon Generating Potential | Additional Details |
|---|---|---|---|---|
| AbraHigh-OM | ~1010 | High | High hydrocarbon generating potential (CNH) | Representative high-OM sample; evaluated for CO2 storage potential and diagenetic alteration. |
| AbraLow-OM | ~1450 | Low | Low hydrocarbon generating potential (CNH) | Representative low-OM sample; compared against high-OM sample to assess differences in secondary carbonate precipitation. |
| Sample | Time (h) | Pressure (PSI) | Temperature (°C) | B or WB |
|---|---|---|---|---|
| Abrat=24 P=1500 T=90 WB | 24 | 1500 | 90 | WB |
| Abrat=24 P=1700 T=85 B | 24 | 1700 | 85 | B |
| Abrat=24 P=1600 T=95 B | 24 | 1600 | 95 | B |
| Abrat=24 P=1350 T=60 WB | 24 | 1350 | 60 | WB |
| Abrat=24 P=2350 T=110 WB | 24 | 2350 | 110 | WB |
| Sample | Particle Size (μm) |
|---|---|
| AbraHigh-OM | 13 |
| Abrat=24 P=1500 T=90 WB | 2.11 |
| Abrat=24 P=1700 T=85 B | 1.88 |
| Abrat=24 P=1600 T=95 B | 2.12 |
| AbraLow-OM | 1.6 |
| Abrat=24 P=1350 T=60 WB | 3.26 |
| Abrat=24 P=2350 T=110 WB | 4.12 |
| Sample | BET Surface Area (m2 g−1) | Pore Surface Area (m2 g−1) | Pore Diameter (nm) |
|---|---|---|---|
| AbraHigh-OM | 0.611 | 0.436 | 3.587 |
| Abrat=24 P=1500 T=90 WB | 0.977 | 0.444 | 5.5564 |
| Abrat=24 P=1700 T=85 B | 0.831 | 0.391 | 4.917 |
| Abrat=24 P=1600 T=95 B | 2.918 | 4.125 | 4.884 |
| AbraLow-OM | 0.744 | 0.442 | 4.327 |
| Abrat=24 P=1350 T=60 WB | 0.539 | 0.261 | 4.896 |
| Abrat=24 P=2350 T=110 WB | 1.893 | 0.25 | 4.847 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Dinorín, R.; Mendoza-Martínez, A.M.; Palma-Ramírez, D.; Dorantes-Rosales, H.; García-Alamilla, R.; Romero-Ibarra, I.C.; García-Zaleta, D.S. Analysis of Carbon Dioxide Mineralization in Carbonates from Tampico-Misantla Basin, Mexico: Effect of Organic Matter Content. Processes 2025, 13, 1087. https://doi.org/10.3390/pr13041087
López-Dinorín R, Mendoza-Martínez AM, Palma-Ramírez D, Dorantes-Rosales H, García-Alamilla R, Romero-Ibarra IC, García-Zaleta DS. Analysis of Carbon Dioxide Mineralization in Carbonates from Tampico-Misantla Basin, Mexico: Effect of Organic Matter Content. Processes. 2025; 13(4):1087. https://doi.org/10.3390/pr13041087
Chicago/Turabian StyleLópez-Dinorín, Roxana, Ana María Mendoza-Martínez, Diana Palma-Ramírez, Héctor Dorantes-Rosales, Ricardo García-Alamilla, Issis Claudette Romero-Ibarra, and David Salvador García-Zaleta. 2025. "Analysis of Carbon Dioxide Mineralization in Carbonates from Tampico-Misantla Basin, Mexico: Effect of Organic Matter Content" Processes 13, no. 4: 1087. https://doi.org/10.3390/pr13041087
APA StyleLópez-Dinorín, R., Mendoza-Martínez, A. M., Palma-Ramírez, D., Dorantes-Rosales, H., García-Alamilla, R., Romero-Ibarra, I. C., & García-Zaleta, D. S. (2025). Analysis of Carbon Dioxide Mineralization in Carbonates from Tampico-Misantla Basin, Mexico: Effect of Organic Matter Content. Processes, 13(4), 1087. https://doi.org/10.3390/pr13041087






