Malodorous Gases in Aquatic Environments: A Comprehensive Review from Microbial Origin to Detection and Removal Techniques
Abstract
1. Introduction
2. Emission Sources of Malodorous Gases
2.1. Total Reduced Sulfur (TRS) and Ammonia (NH3) as Major Malodorants
2.2. Emission Sources
2.2.1. Anthropogenic Emissions
2.2.2. Natural Emissions
2.2.3. Hybrid Emissions
3. Regulatory Framework and Odor Thresholds
3.1. International Standards and Legislation
3.2. Regulation and Air Quality Standards in Colombia
4. Thresholds, Exposure Times, and Concentrations of H2S
5. Health Effects of Odorous Gases
5.1. Health Effects of TRS
5.2. Health Effects of NH3
6. Biogeochemical and Microbial Processes Leading to Gas Formation
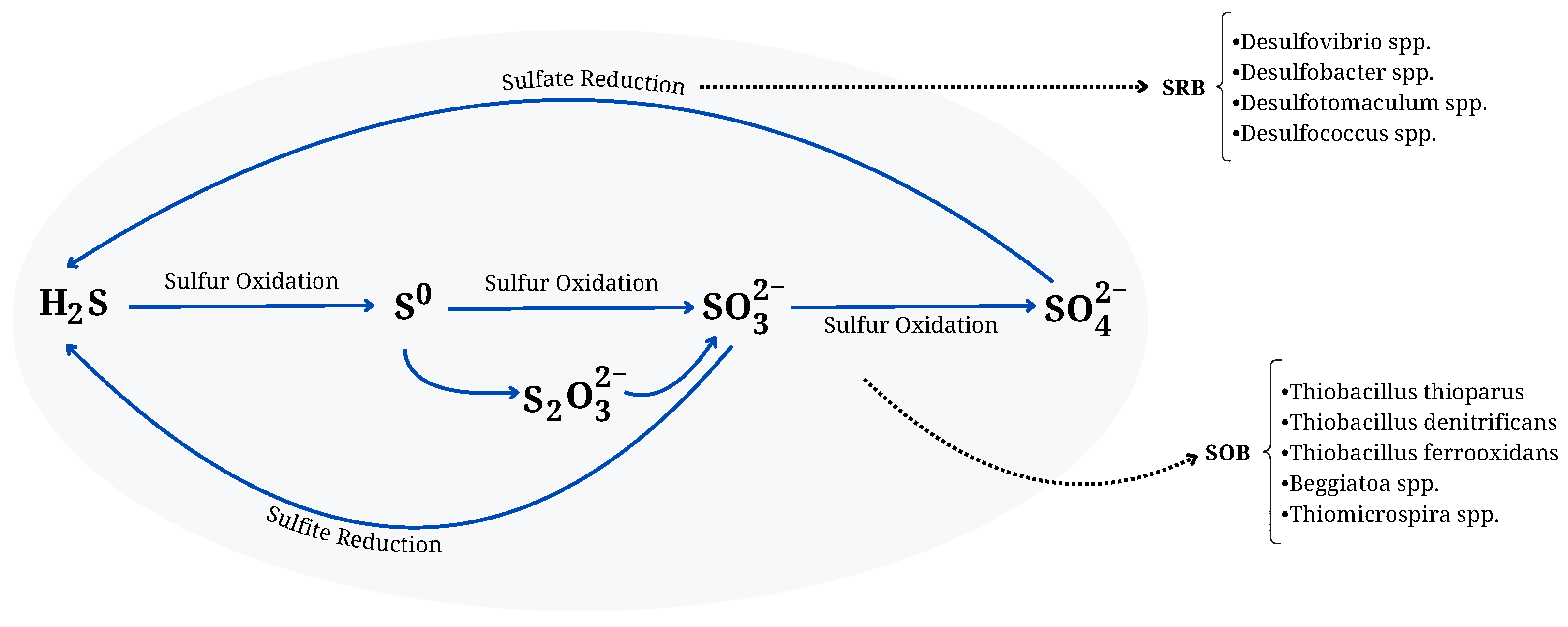
6.1. Biological Sulfur Cycle and Microbial Processes Leading to H2S Production
6.2. Formation Mechanisms of TRS Compounds
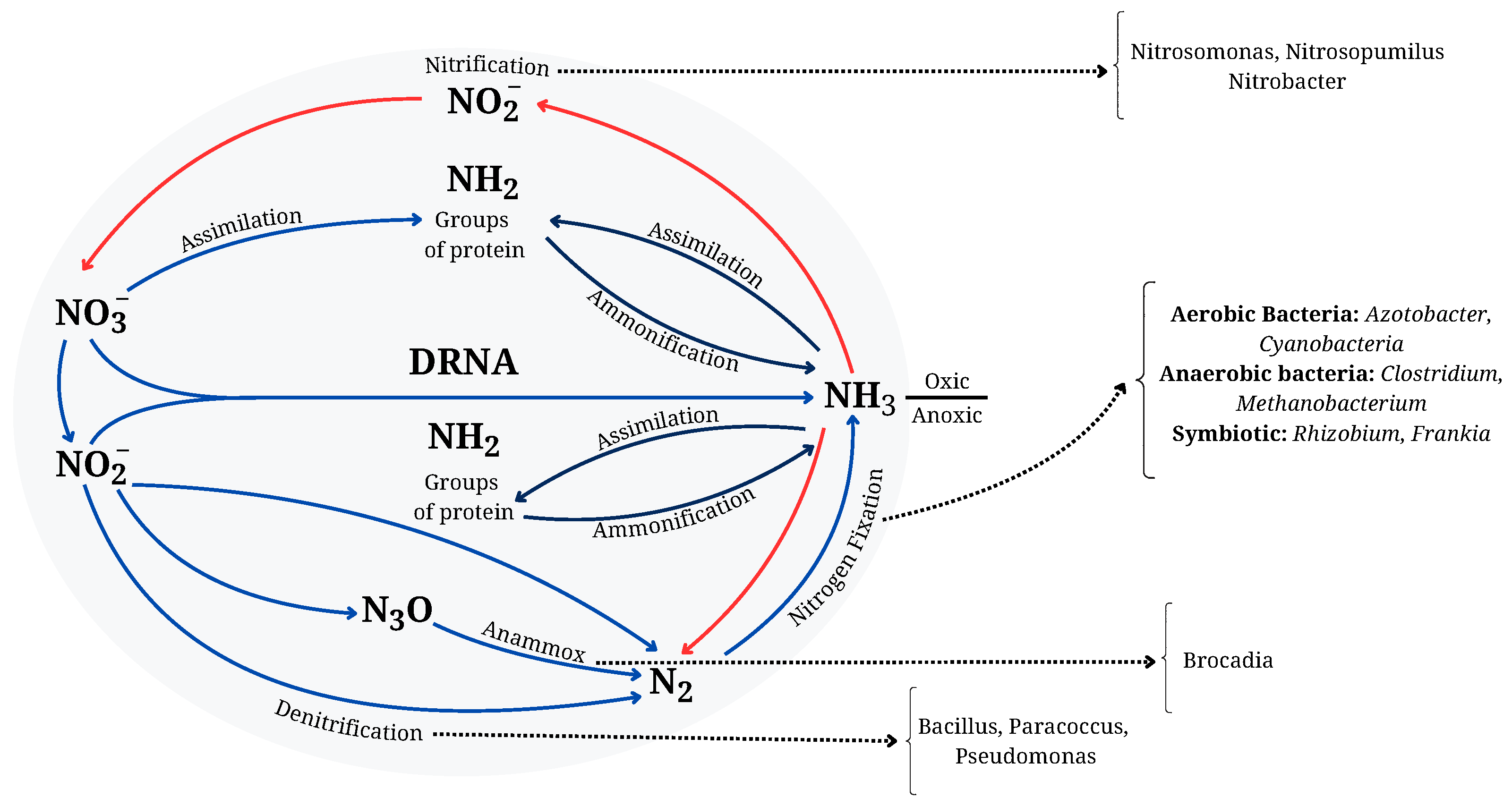
6.3. Biological Nitrogen Cycle and Microbial Processes Leading to NH3 Production
7. Factors Affecting Biological Gas Formation
8. Linking Water Quality and Malodorous Gas Emissions
8.1. Effects of Contaminants and Dissolved Oxygen on Odor Formation
8.2. Real-World Data from Different Contaminated Water Sources: Case Studies from Tianziling Landfill and the Wuxi Crisis
8.3. Case Study: The Bogotá River
9. Detection Technologies for Odorous Gases
9.1. Traditional Analytical Techniques
9.2. Advanced Optical and Spectroscopic Methods
9.3. Electrochemical Sensors and Colorimetric Methods
9.4. Emerging Technologies
10. Advantages and Limitations of Different Measurement Methods
11. Mitigation and Control Strategies
11.1. Removal Challenges
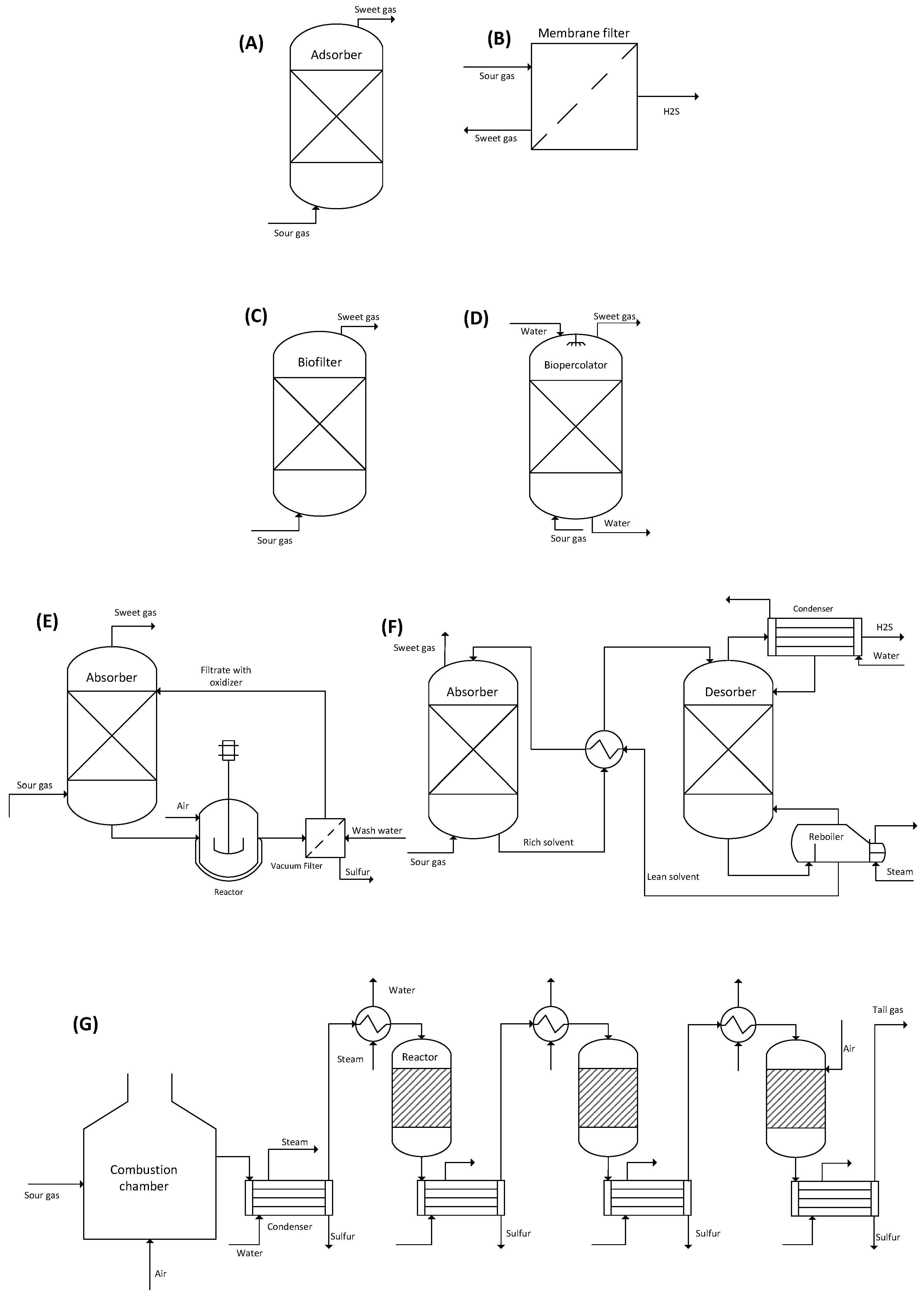
11.2. Methods for H2S Removal
11.2.1. Absorption
11.2.2. Adsorption
11.2.3. Membrane Separation
11.2.4. Chemical Conversion
Biofilter
Biopercolators
Comparison in Diverse Settings
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, E.R.; van Vliet, M.T.; Qadir, M.; Bierkens, M.F. Spatially-explicit estimates of global wastewater production, collection, treatment and re-use. Earth Syst. Sci. Data Discuss. 2020, 13, 237–254. [Google Scholar] [CrossRef]
- Sandoval, M.A.; Calzadilla, W.; Vidal, J.; Brillas, E.; Salazar-González, R. Contaminants of emerging concern: Occurrence, analytical techniques, and removal with electrochemical advanced oxidation processes with special emphasis in Latin America. Environ. Pollut. 2024, 345, 123397. [Google Scholar] [PubMed]
- Henao-Herreño, L.X.; López-Tamayo, A.M.; Ramos-Bonilla, J.P.; Haas, C.N.; Husserl, J. Risk of illness with Salmonella due to consumption of raw unwashed vegetables irrigated with water from the Bogotá River. Risk Anal. 2017, 37, 733–743. [Google Scholar]
- Zhang, L.; Qiu, Y.Y.; Sharma, K.R.; Shi, T.; Song, Y.; Sun, J.; Liang, Z.; Yuan, Z.; Jiang, F. Hydrogen sulfide control in sewer systems: A critical review of recent progress. Water Res. 2023, 240, 120046. [Google Scholar]
- Sheikh, M.; Harami, H.R.; Rezakazemi, M.; Valderrama, C.; Cortina, J.L.; Aminabhavi, T.M. Efficient NH3-N recovery from municipal wastewaters via membrane hybrid systems: Nutrient-Energy-Water (NEW) nexus in circular economy. Chem. Eng. J. 2023, 465, 142876. [Google Scholar] [CrossRef]
- Namour, P. The biogeochemical origin of sewage gases and control of their generation. J. Hazard. Mater. Adv. 2022, 7, 100124. [Google Scholar]
- Lee, J.; Lee, S.; Park, Y.K. Reduction of odor-causing compounds in wastewater using biochar: A review. Bioresour. Technol. 2023, 385, 129419. [Google Scholar] [CrossRef]
- Watson, S.B.; Jüttner, F. Malodorous volatile organic sulfur compounds: Sources, sinks and significance in inland waters. Crit. Rev. Microbiol. 2017, 43, 210–237. [Google Scholar]
- Morral, E.; Gabriel, D.; Dorado, A.D.; Gamisans, X. A Review of Biotechnologies for the Abatement of Ammonia Emissions. Chemosphere 2021, 273, 128606. [Google Scholar] [CrossRef]
- Tran, H.T.; Binh, Q.A.; Van Tung, T.; Pham, D.T.; Hoang, H.G.; Hai Nguyen, N.S.; Xie, S.; Zhang, T.; Mukherjee, S.; Bolan, N.S. A Critical Review on Characterization, Human Health Risk Assessment and Mitigation of Malodorous Gaseous Emission during the Composting Process. Environ. Pollut. 2024, 351, 124115. [Google Scholar]
- Moreno, A.I.; Arnáiz, N.; Font, R.; Carratalá, A. Chemical Characterization of Emissions from a Municipal Solid Waste Treatment Plant. Waste Manag. 2014, 34, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Saral, A.; Demir, S.; Yıldız, Ş. Assessment of Odorous VOCs Released from a Main MSW Landfill Site in Istanbul-Turkey via a Modelling Approach. J. Hazard. Mater. 2009, 168, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, W.h.; Deng, Y.; Li, W.; Ma, X.; Chen, Y. Removal of Low Concentration CH3SH with Regenerable Cu-doped Mesoporous Silica. J. Colloid Interface Sci. 2018, 513, 903–910. [Google Scholar] [CrossRef]
- National Research Council; Division on Earth and Life Studies; Board on Environmental Studies and Toxicology; Committee on Toxicology; Committee on Acute Exposure Guideline Levels. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 12; Technical Report; National Research Council: Ottawa, ON, Canada, 2012. [Google Scholar]
- Damme, M.V.; Clarisse, L.; Whitburn, S.; Hadji-Lazaro, J.; Hurtmans, D.; Clerbaux, C.; Coheur, P.F. Industrial and Agricultural Ammonia Point Sources Exposed. Nature 2018, 564, 99–103. [Google Scholar] [CrossRef]
- Bekyarova, E.; Davis, M.M.; Burch, T.; Itkis, M.E.; Zhao, B.; Sunshine, A.S.; Haddon, R.C. Chemically Functionalized Single-Walled Carbon Nanotubes as Ammonia Sensors. J. Phys. Chem. B 2004, 108, 19717–19720. [Google Scholar] [CrossRef]
- Guo, H.; Otjes, R.P.; Schlag, P.; Kiendler-Scharr, A.; Nenes, A.; Weber, R.J. Effectiveness of Ammonia Reduction on Control of Fine Particle Nitrate. Atmos. Chem. Phys. 2018, 18, 12241–12256. [Google Scholar] [CrossRef]
- de Vries, W.; Cellier, P.; Erisman, J.W.; Sutton, M.A. Assessment of Nitrogen Fluxes to Air and Water from Site Scale to Continental Scale: An Overview. Environ. Pollut. 2011, 159, 3143–3148. [Google Scholar] [CrossRef]
- Chan, Y.H.; Lock, S.S.M.; Wong, M.K.; Yiin, C.L.; Loy, A.C.M.; Cheah, K.W.; Chai, S.Y.W.; Li, C.; How, B.S.; Chin, B.L.F.; et al. A State-of-the-Art Review on Capture and Separation of Hazardous Hydrogen Sulfide (H2S): Recent Advances, Challenges and Outlook. Environ. Pollut. 2022, 314, 120219. [Google Scholar] [CrossRef]
- Marriott, R.A.; Pirzadeh, P.; Marrugo-Hernandez, J.J.; Raval, S. Hydrogen Sulfide Formation in Oil and Gas. Can. J. Chem. 2016, 94, 406–413. [Google Scholar] [CrossRef]
- Cui, G.; Bhat, S.A.; Li, W.; Ishiguro, Y.; Wei, Y.; Li, F. H2S, MeSH, and NH3 Emissions from Activated Sludge: An Insight towards Sludge Characteristics and Microbial Mechanisms. Int. Biodeterior. Biodegrad. 2022, 166, 105331. [Google Scholar] [CrossRef]
- Omri, I.; Aouidi, F.; Bouallagui, H.; Godon, J.J.; Hamdi, M. Performance study of biofilter developed to treat H2S from wastewater odour. Saudi J. Biol. Sci. 2013, 20, 169–176. [Google Scholar] [PubMed]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia Emissions from Agriculture and Their Contribution to Fine Particulate Matter: A Review of Implications for Human Health. J. Environ. Manag. 2022, 323, 116285. [Google Scholar]
- Xu, R.; Tian, H.; Pan, S.; Prior, S.A.; Feng, Y.; Batchelor, W.D.; Chen, J.; Yang, J. Global Ammonia Emissions from Synthetic Nitrogen Fertilizer Applications in Agricultural Systems: Empirical and Process-based Estimates and Uncertainty. Glob. Change Biol. 2018, 25, 314–326. [Google Scholar]
- Misselbrook, T.H.; Sutton, M.A.; Scholefield, D. A Simple Process-based Model for Estimating Ammonia Emissions from Agricultural Land after Fertilizer Applications. Soil Use Manag. 2004, 20, 365–372. [Google Scholar]
- Zhang, C.; Geng, X.; Wang, H.; Zhou, L.; Wang, B. Emission Factor for Atmospheric Ammonia from a Typical Municipal Wastewater Treatment Plant in South China. Environ. Pollut. 2017, 220 Pt B, 963–970. [Google Scholar]
- Li, Q.; Jiang, J.; Cai, S.; Zhou, W.; Wang, S.; Duan, L.; Hao, J. Gaseous Ammonia Emissions from Coal and Biomass Combustion in Household Stoves with Different Combustion Efficiencies. Environ. Sci. Technol. Lett. 2016, 3, 98–103. [Google Scholar]
- ATSDR. Toxicological Profile for Ammonia; Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2004. [Google Scholar]
- Habeeb, O.A.; Kanthasamy, R.; Ali, G.A.M.; Sethupathi, S.; Yunus, R.B.M. Hydrogen Sulfide Emission Sources, Regulations, and Removal Techniques: A Review. Rev. Chem. Eng. 2018, 34, 837–854. [Google Scholar]
- Berresheim, H.; Jaeschke, W. The Contribution of Volcanoes to the Global Atmospheric Sulfur Budget. J. Geophys. Res. Ocean. 1983, 88, 3732–3740. [Google Scholar]
- Chou, C.H.; Ogden, J.M.; Pohl, H.R.; Scinicariello, F.; Ingerman, L.; Barber, L.; Citra, M.J. Toxicological Profile for Hydrogen Sulfide and Carbonyl Sulfide; Technical Report; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016. [Google Scholar]
- Ward, B.B.; Jensen, M.M. The microbial nitrogen cycle. Front. Microbiol. 2014, 5, 553. [Google Scholar]
- van de Leemput, I.A.; Veraart, A.J.; Dakos, V.; de Klein, J.J.M.; Strous, M.; Scheffer, M. Predicting Microbial Nitrogen Pathways from Basic Principles. Environ. Microbiol. 2011, 13, 1477–1487. [Google Scholar]
- Kowalchuk, G.A.; Stephen, J.R. Ammonia-oxidizing bacteria: A model for molecular microbial ecology. Annu. Rev. Microbiol. 2001, 55, 485–529. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Aschenbach, J.R. Ureases in the gastrointestinal tracts of ruminant and monogastric animals and their implication in urea-N/ammonia metabolism: A review. J. Adv. Res. 2018, 13, 39–50. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Hydrogen Sulfide and Carbonyl Sulfide; Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2016. [Google Scholar]
- Durand, M. Toxic gases and dead birds at Sulphur Bay, Rotorua, North Island, New Zealand. Notornis 2007, 54, 42. [Google Scholar]
- Gao, X.; Yang, F.; Cheng, J.; Xu, Z.; Zang, B.; Li, G.; Xie, X.; Luo, W. Emission of Volatile Sulphur Compounds during Swine Manure Composting: Source Identification, Odour Mitigation and Assessment. Waste Manag. 2022, 153, 129–137. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Fang, Y.; Huang, R. Analysis on the formation condition of the algae-induced odorous black water agglomerate. Saudi J. Biol. Sci. 2014, 21, 597–604. [Google Scholar] [CrossRef]
- Tian, L.; Han, C.; Zhang, J.; Ouyang, Y.; Xi, J. Development of an H2S Emission Model for Wastewater Treatment Plants. J. Air Waste Manag. Assoc. 2021, 71, 1303–1311. [Google Scholar] [CrossRef]
- Bazemo, U.; Gardner, E.; Romero, A.; Hauduc, H.; Al-Omari, A.; Takacs, I.; Murthy, S.; Torrents, A.; De Clippeleir, H. Investigating the Dynamics of Volatile Sulfur Compound Emission from Primary Systems at a Water Resource Recovery Facility. Water Environ. Res. 2021, 93, 316–327. [Google Scholar] [CrossRef]
- Peu, P.; Picard, S.; Diara, A.; Girault, R.; Béline, F.; Bridoux, G.; Dabert, P. Prediction of Hydrogen Sulphide Production during Anaerobic Digestion of Organic Substrates. Bioresour. Technol. 2012, 121, 419–424. [Google Scholar] [CrossRef]
- Langevin, B.; Génermont, S.; Basset-Mens, C.; Lardon, L. Simulation of field NH3 and N2O emissions from slurry spreading. Agron. Sustain. Dev. 2015, 35, 347–358. [Google Scholar] [CrossRef]
- Jeon, E.C.; Sa, J.H.; Park, J.H. Development of NH3 Emission Factors using a Dynamic Flux Chamber in a Sewage Treatment Plant. J. Environ. Impact Assess. 2005, 14, 263–273. [Google Scholar]
- Wang, S.; Zeng, Y. Ammonia emission mitigation in food waste composting: A review. Bioresour. Technol. 2018, 248, 13–19. [Google Scholar] [PubMed]
- UNE-EN 13725:2022; Determinación de la Concentración de olor por Olfatometría dináMica y cáLculo de la Emisión de olor. Asociación Española de Normalización: Madrid, Spain, 2022.
- UNE-EN 15259:2008; Calidad del Aire. Emisiones de Fuentes Estacionarias. Requisitos de las Secciones y Sitios de Medición y Para el Objetivo, Plan e Informe de Medición. Asociación Española de Normalización: Madrid, Spain, 2008.
- ISO 16911-1:2013; Stationary Source Emissions—Manual and Automatic Determination of Velocity and Volume Flow Rate in Ducts. International Organization for Standardization: Geneva, Switzerland, 2013.
- Corbett, S. NSW Public. NSW Public Health Bull. 2003, 14, 161. [Google Scholar] [CrossRef]
- Ontario Ministry of the Environment. Summary of Point of Impingement Guidelines, and Ambient Air Quality Criteria (AAQCs); Ontario Ministry of the Environment: Toronto, ON, Canada, 2011. [Google Scholar]
- Alberta Environment. Alberta’s Ambient Air Quality Objectives (AAAQOs) and Guidelines (AAAQGs); Alberta Environment: Edmonton, AB, Canada, 2013. [Google Scholar]
- Diaz, C.; Izquierdo, C.; Capelli, L.; Arias, R.; Salas Seoane, N. Analysis of Existing Regulations in Odour Pollution, Odour Impact Criteria 1; H2020SwafS-23-2017-789315; D-NOSES: Barcelona, Spain, 2019. [Google Scholar]
- Lopicich Catalán, B.A. Legislación en Materia de Contaminación de Olores: Evaluación Crítica de una Posible Dispersión Normativa; Consultado el 10 de Diciembre de 2024; Biblioteca del Congreso Naciona: Buenos Aires, Argentina, 2024. [Google Scholar]
- Congreso de la República de Colombia. Ley 9 de 1979: Por la Cual se Dictan Medidas Sanitarias; Consultado el 10 de diciembre de 2024; Congreso de la República de Colombia: Bogotá, Colombia, 1979. [Google Scholar]
- Presidencia de la República de Colombia. Decreto 948 de 1995. 1995. Available online: https://www.sgc.gov.co (accessed on 10 December 2024).
- Minambiente. Resolución 601 de 2006: Por la Cual se Establece la Norma de Calidad del Aire o Nivel de Inmisión, para Todo el Territorio Nacional en Condiciones de Referencia; Technical Report; Ministerio de Ambiente, Vivienda y Desarrollo Territorial: Bogotá, Colombia, 2006. [Google Scholar]
- Minambiente. Resolución 610 de 2010: Por la Cual se Modifica la Resolución 601 del 4 de Abril de 2006; Technical Report; Ministerio de Ambiente, Vivienda y Desarrollo Territorial: Bogotá, Colombia, 2010. [Google Scholar]
- Minambiente. Resolución 1541 de 2013: Por la Cual se Establecen los Niveles Permisibles de Calidad del Aire o de Inmisión, el Procedimiento para la Evaluación de Actividades que Generan Olores Ofensivos y se Dictan Otras Disposiciones; Technical Report; Ministerio de Ambiente, Vivienda y Desarrollo Territorial: Bogotá, Colombia, 2013. [Google Scholar]
- Minambiente. Resolución 2087 de 2014: Por la Cual se Adopta el Protocolo para el Monitoreo, Control y Vigilancia de Olores Ofensivos; Technical Report; Ministerio de Ambiente, Vivienda y Desarrollo Territorial: Bogotá, Colombia, 2014. [Google Scholar]
- NTC 6012-1:2013; Evaluación de los Efectos y Molestias Causadas por los Olores. Parte 1: Evaluación sicoméTrica de las Molestias por Olores. Instituto Colombiano de Normas Técnicas y Certificación: Bogotá, Colombia, 2013.
- CNCT. NTP 320: Umbrales Olfativos y Seguridad de Sustancias quíMicas Peligrosas; Technical Report; Centro Nacional de Condiciones de Trabajo: Madrid, Spain, 2011. [Google Scholar]
- Philipopoulos, G.; Tat, J.; Chan, A.; Jiang, J.; Mukai, D.S.; Burney, T.; Doosty, M.; Mahon, S.; Patel, H.H.; White, C.W.; et al. Methyl Mercaptan Gas: Mechanisms of Toxicity and Demonstration of the Effectiveness of Cobinamide as an Antidote in Mice and Rabbits. Clin. Toxicol. 2022, 60, 615–622. [Google Scholar]
- Setoguchi, T.; Machigashira, M.; Yamamoto, M.; Yotsumoto, Y.; Yoshimori, M.; Izumi, Y.; Yaegaki, K. The Effects of Methyl Mercaptan on Epithelial Cell Growth and Proliferation. Int. Dent. J. 2002, 52 (Suppl. S3), 241–246. [Google Scholar]
- Wang, B.; Sivret, E.C.; Parcsi, G.; Stuetz, R.M. Determination of VOSCs in sewer headspace air using TD-GC-SCD. Talanta 2015, 137, 71–79. [Google Scholar] [CrossRef]
- Encarnação, T.; Pais, A.A.C.C.; Campos, M.G.; Burrows, H.D. Endocrine Disrupting Chemicals: Impact on Human Health, Wildlife and the Environment. Sci. Prog. 2019, 102, 3–42. [Google Scholar]
- Rippey, J.C.; Charles, J.; Rippey, E.R. Nine Cases of Accidental Exposure to Dimethyl Sulphate—A Potential Chemical Weapon. Emerg. Med. J. 2005, 22, 878–879. [Google Scholar]
- Lewis, R.J.; Copley, G.B. Chronic Low-Level Hydrogen Sulfide Exposure and Potential Effects on Human Health: A Review of the Epidemiological Evidence. Crit. Rev. Toxicol. 2015, 45, 123–193. [Google Scholar] [CrossRef]
- Curren, J.; Hallis, S.A.; Snyder, C.L.C.; Suffet, I.H.M. Identification and quantification of nuisance odors at a trash transfer station. Waste Manag. 2016, 58, 52–61. [Google Scholar]
- Blanes-Vidal, V.; Bælum, J.; Schwartz, J.D.; Løfstrøm, P.; Christensen, L.P. Respiratory and Sensory Irritation Symptoms among Residents Exposed to Low-to-Moderate Air Pollution from Biodegradable Wastes. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 388–397. [Google Scholar] [CrossRef]
- Wu, Y.N.; Yan, F.F.; Hu, J.Y.; Chen, H.; Tucker, C.M.; Green, A.R.; Cheng, H.W. The Effect of Chronic Ammonia Exposure on Acute-phase Proteins, Immunoglobulin, and Cytokines in Laying Hens. Poult. Sci. 2017, 96, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Turner, M.C.; Burnett, R.T.; Jerrett, M.; Gapstur, S.M.; Diver, R.W.; Krewski, D.; Brook, R.D. Relationships between Fine Particulate Air Pollution, Cardiometabolic Disorders, and Cardiovascular Mortality. Circ. Res. 2015, 116, 108–115. [Google Scholar] [PubMed]
- Fike, D.A.; Bradley, A.S.; Rose, C.V. Rethinking the Ancient Sulfur Cycle. Annu. Rev. Earth Planet. Sci. 2015, 43, 593–622. [Google Scholar]
- Gupta, S.; Plugge, C.M.; Muyzer, G.; Sánchez-Andrea, I. Harnessing the Potential of the Microbial Sulfur Cycle for Environmental Biotechnology. Curr. Opin. Biotechnol. 2024, 88, 103164. [Google Scholar]
- Wang, R.; Lin, J.q.; Liu, X.m.; Pang, X.; Zhang, C.J.; Yang, C.L.; Gao, X.; Lin, C.; Li, Y.Q.; Li, Y.; et al. Sulfur Oxidation in the Acidophilic Autotrophic Acidithiobacillus spp. Front. Microbiol. 2019, 9, 3290. [Google Scholar] [CrossRef]
- Beller, H.R.; Chain, P.S.G.; Letain, T.E.; Chakicherla, A.; Larimer, F.W.; Richardson, P.M.; Coleman, M.A.; Wood, A.P.; Kelly, D.P. The Genome Sequence of the Obligately Chemolithoautotrophic, Facultatively Anaerobic Bacterium Thiobacillus Denitrificans. J. Bacteriol. 2006, 188, 1473–1488. [Google Scholar]
- Lin, S.; Mackey, H.R.; Hao, T.; Guo, G.; van Loosdrecht, M.C.M.; Chen, G. Biological Sulfur Oxidation in Wastewater Treatment: A Review of Emerging Opportunities. Water Res. 2018, 143, 399–415. [Google Scholar]
- Hao, T.w.; Xiang, P.y.; Mackey, H.R.; Chi, K.; Lu, H.; Chui, H.k.; van Loosdrecht, M.C.M.; Chen, G.H. A Review of Biological Sulfate Conversions in Wastewater Treatment. Water Res. 2014, 65, 1–21. [Google Scholar]
- Tran, P.Q.; Bachand, S.C.; Hotvedt, J.C.; Kieft, K.; McDaniel, E.A.; McMahon, K.D.; Anantharaman, K. Physiological and genomic evidence of cysteine degradation and aerobic hydrogen sulfide production in freshwater bacteria. mSystems 2023, 8, e00201-23. [Google Scholar]
- Mutegoa, E.; Sahini, M.G. Approaches to Mitigation of Hydrogen Sulfide during Anaerobic Digestion Process—A Review. Heliyon 2023, 9, e19768. [Google Scholar]
- Lens, P. Sulfur Cycle. In Encyclopedia of Microbiology, 3rd ed.; Academic Press: Oxford, UK, 2009; pp. 361–369. [Google Scholar]
- Leavitt, W.D.; Cummins, R.C.; Schmidt, M.L.; Sim, M.S.; Ono, S.; Bradley, A.S.; Johnston, D.T. Multiple Sulfur Isotope Signatures of Sulfite and Thiosulfate Reduction by the Model Dissimilatory Sulfate-Reducer, Desulfovibrio Alaskensis Str. G20. Front. Microbiol. 2014, 5, 591. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sugita, K.; Okabe, S. Isolation, Characterization, and in Situ Detection of a Novel Chemolithoautotrophic Sulfur-Oxidizing Bacterium in Wastewater Biofilms Growing under Microaerophilic Conditions. Appl. Environ. Microbiol. 2004, 70, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- Luther, G.W.; Findlay, A.J.; MacDonald, D.J.; Owings, S.M.; Hanson, T.E.; Beinart, R.A.; Girguis, P.R. Thermodynamics and Kinetics of Sulfide Oxidation by Oxygen: A Look at Inorganically Controlled Reactions and Biologically Mediated Processes in the Environment. Front. Microbiol. 2011, 2, 62. [Google Scholar] [CrossRef]
- Camacho, D.; Frazao, R.; Fouillen, A.; Nanci, A.; Lang, B.F.; Apte, S.C.; Baron, C.; Warren, L.A. New Insights into Acidithiobacillus Thiooxidans Sulfur Metabolism through Coupled Gene Expression, Solution Chemistry, Microscopy, and Spectroscopy Analyses. Front. Microbiol. 2020, 11, 411. [Google Scholar] [CrossRef]
- Sander, J.; Engels-Schwarzlose, S.; Dahl, C. Importance of the DsrMKJOP Complex for Sulfur Oxidation in Allochromatium Vinosum and Phylogenetic Analysis of Related Complexes in Other Prokaryotes. Arch. Microbiol. 2006, 186, 357–366. [Google Scholar] [CrossRef]
- Tang, K. Chemical Diversity and Biochemical Transformation of Biogenic Organic Sulfur in the Ocean. Front. Mar. Sci. 2020, 7, 68. [Google Scholar]
- Li, K.; Xin, Y.; Xuan, G.; Zhao, R.; Liu, H.; Xia, Y.; Xun, L. Escherichia coli Uses Separate Enzymes to Produce H2S and Reactive Sulfane Sulfur from L-Cysteine. Front. Microbiol. 2019, 10, 298. [Google Scholar] [CrossRef]
- Lehnert, A.S.; Cooper, R.E.; Ignatz, R.M.; Ruecker, A.; Gomes-Alves, E.; Küsel, K.; Pohnert, G.; Trumbore, S.E. Dimethyl Sulfide Emissions from a Peatland Result More from Organic Matter Degradation than Sulfate Reduction. J. Geophys. Res. Biogeosci. 2024, 129, e2023JG007449. [Google Scholar] [CrossRef]
- Tchong, S.I.; Xu, H.; White, R.H. L-Cysteine Desulfidase: An [4Fe-4S] Enzyme Isolated from Methanocaldococcus Jannaschii That Catalyzes the Breakdown of L-cysteine into Pyruvate, Ammonia, and Sulfide. Biochemistry 2005, 44, 1659–1670. [Google Scholar] [CrossRef]
- Loddeke, M.; Schneider, B.L.; Oguri, T.; Mehta, I.; Xuan, Z.; Reitzer, L. Anaerobic Cysteine Degradation and Potential Metabolic Coordination in Salmonella enterica and Escherichia coli. J. Bacteriol. 2017, 199. [Google Scholar] [CrossRef]
- Mishanina, T.V.; Libiad, M.; Banerjee, R. Biogenesis of Reactive Sulfur Species for Signaling by Hydrogen Sulfide Oxidation Pathways. Nat. Chem. Biol. 2015, 11, 457–464. [Google Scholar] [PubMed]
- Lei, Q.; Boatright, W.L. Methionine Is the Methyl Group Donor for Sulfite-Associated Methanethiol Formation in Isolated Soy Proteins. J. Food Sci. 2006, 71, C527–C531. [Google Scholar] [CrossRef]
- He, R.; Yao, X.Z.; Chen, M.; Ma, R.; Li, H.J.; Wang, C.; Ding, S. Conversion of Sulfur Compounds and Microbial Community in Anaerobic Treatment of Fish and Pork Waste. Waste Manag. 2018, 76, 383–393. [Google Scholar] [CrossRef]
- Gallardo-Benavente, C.; Carrión, O.; Todd, J.D.; Pieretti, J.C.; Seabra, A.B.; Durán, N.; Rubilar, O.; Pérez-Donoso, J.M.; Quiroz, A. Biosynthesis of CdS Quantum Dots Mediated by Volatile Sulfur Compounds Released by Antarctic Pseudomonas fragi. Front. Microbiol. 2019, 10, 1866. [Google Scholar]
- Visscher, P.T.; Baumgartner, L.K.; Buckley, D.H.; Rogers, D.R.; Hogan, M.E.; Raleigh, C.D.; Turk, K.A.; Marais, D.J.D. Dimethyl Sulphide and Methanethiol Formation in Microbial Mats: Potential Pathways for Biogenic Signatures. Environ. Microbiol. 2003, 5, 296–308. [Google Scholar]
- Findlay, A.J. Microbial Impact on Polysulfide Dynamics in the Environment. FEMS Microbiol. Lett. 2016, 363, fnw103. [Google Scholar]
- Avetisyan, K.; Zweig, I.; Luther, G.W.; Kamyshny, A. Kinetics and Mechanism of Polysulfides and Elemental Sulfur Formation by a Reaction between Hydrogen Sulfide and δ-MnO2. Geochim. Cosmochim. Acta 2021, 313, 21–37. [Google Scholar] [CrossRef]
- Mashkina, A.V. Catalytic Reactions of Dialkyl Disulfides. Russ. Chem. Rev. 2014, 83, 733–757. [Google Scholar] [CrossRef]
- Yücel, M.; Sommer, S.; Dale, A.W.; Pfannkuche, O. Microbial Sulfide Filter along a Benthic Redox Gradient in the Eastern Gotland Basin, Baltic Sea. Front. Microbiol. 2017, 8, 169. [Google Scholar]
- Wilkening, J.V.; Turchyn, A.V.; Redeker, K.R.; Mills, J.V.; Antler, G.; Carrión, O.; Todd, J.D. The Production and Fate of Volatile Organosulfur Compounds in Sulfidic and Ferruginous Sediment. J. Geophys. Res. Biogeosci. 2019, 124, 3390–3402. [Google Scholar] [CrossRef]
- Yoch, D.C. Dimethylsulfoniopropionate: Its Sources, Role in the Marine Food Web, and Biological Degradation to Dimethylsulfide. Appl. Environ. Microbiol. 2002, 68, 5804–5815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Liu, J.; Yang, G.; Xue, C.X.; Curson, A.R.J.; Todd, J.D. Biogenic Production of DMSP and Its Degradation to DMS—Their Roles in the Global Sulfur Cycle. Sci. China Life Sci. 2019, 62, 1296–1319. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.K.; Sekar, J.; Ramalingam, P.V. Recent Insights into Oceanic Dimethylsulfoniopropionate Biosynthesis and Catabolism. Environ. Microbiol. 2022, 24, 2669–2700. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tan, Q.; Zhao, H.; Xia, F.; Li, C.; Ma, X.; Li, W. Eutrophication-Driven Infochemical Dimethylsulfide Accelerates Carbon Transfer in Freshwater Food Chain. J. Environ. Manag. 2024, 353, 120155. [Google Scholar] [CrossRef]
- Zanello, P. Structure and Electrochemistry of Proteins Harboring Iron-Sulfur Clusters of Different Nuclearities. Part V. Nitrogenases. Coord. Chem. Rev. 2019, 398, 113004. [Google Scholar] [CrossRef]
- Zehr, J.P.; Jenkins, B.D.; Short, S.M.; Steward, G.F. Nitrogenase Gene Diversity and Microbial Community Structure: A Cross-System Comparison. Environ. Microbiol. 2003, 5, 539–554. [Google Scholar] [CrossRef]
- Rana, A.; Pandey, R.K.; Ramakrishnan, B. Chapter 3—Enzymology of the Nitrogen Cycle and Bioremediation of Toxic Nitrogenous Compounds. In Smart Bioremediation Technologies; Academic Press: Cambridge, MA, USA, 2019; pp. 45–61. [Google Scholar]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Xu, P.; Wang, E. Diversity and Regulation of Symbiotic Nitrogen Fixation in Plants. Curr. Biol. 2023, 33, R543–R559. [Google Scholar] [CrossRef]
- Santi, C.; Bogusz, D.; Franche, C. Biological Nitrogen Fixation in Non-Legume Plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef]
- Berman-Frank, I.; Lundgren, P.; Chen, Y.B.; Küpper, H.; Kolber, Z.S.; Bergman, B.; Falkowski, P.G. Segregation of Nitrogen Fixation and Oxygenic Photosynthesis in the Marine Cyanobacterium Trichodesmium. Science 2001, 294, 1534–1537. [Google Scholar] [CrossRef]
- Romillac, N. Ammonification. Encycl. Ecol. 2019, 2, 256–263. [Google Scholar]
- Ng, C.W.; Yan, W.H.; Tsim, K.W.; San So, P.; Xia, Y.T.; To, C.T. Effects of Bacillus Subtilis and Pseudomonas Fluorescens as the Soil Amendment. Heliyon 2022, 8, e11674. [Google Scholar] [PubMed]
- Ward, B.B. Nitrification and Ammonification in Aquatic Systems. Life Support Biosph. Sci. 1996, 3, 25–29. [Google Scholar]
- Lea, P.J.; Miflin, B.J. Nitrogen Assimilation and Its Relevance to Crop Improvement. In Annual Plant Reviews; Blackwell Publishing Ltd.: Oxford, UK, 2010. [Google Scholar]
- Heldt, H.W.; Piechulla, B. Nitrate Assimilation Is Essential for the Biosynthesis of Organic Matter. In Plant Biochemistry; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Morot-Gaudry, J.F. Nitrogen Assimilation by Plants: Physiological, Biochemical, and Molecular Aspects. In Nitrogen Assimilation by Plants; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Pierzynowski, S.G.; Pierzynowska, K.G. Alpha-Ketoglutarate, a Key Molecule Involved in Nitrogen Circulation in Both Animals and Plants, in the Context of Human Gut Microbiota and Protein Metabolism. Adv. Med. Sci. 2022, 67, 142–147. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and Signaling Aspects Underpinning the Regulation of Plant Carbon Nitrogen Interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar]
- Wallace, W.; Nicholas, D.J.D. The Biochemistry of Nitrifying Microorganisms. Biol. Rev. 1969, 44, 359–389. [Google Scholar]
- Divya, M.; Aanand, S.; Srinivasan, A.; Ahilan, B. Bioremediation–an Eco-Friendly Tool for Effluent Treatment: A Review. Int. J. Appl. Res. 2015, 1, 530–537. [Google Scholar]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.W.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete Nitrification by Nitrospira Bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ito, O.; Sahrawat, K.L.; Berry, W.L.; Nakahara, K.; Ishikawa, T.; Watanabe, T.; Suenaga, K.; Rondón, M.A.; Rao, I.M. Scope and Strategies for Regulation of Nitrification in Agricultural Systems—Challenges and Opportunities. Crit. Rev. Plant Sci. 2006, 25, 303–335. [Google Scholar]
- Beeckman, F.; Motte, H.; Beeckman, T. Nitrification in Agricultural Soils: Impact, Actors and Mitigation. Curr. Opin. Biotechnol. 2018, 50, 166–173. [Google Scholar]
- Olaya-Abril, A.; Hidalgo-Carrillo, J.; Luque-Almagro, V.M.; Fuentes-Almagro, C.A.; Urbano, F.J.; Moreno-Vivián, C.; Richardson, D.J.; Roldán, M.D. Exploring the Denitrification Proteome of Paracoccus Denitrificans PD1222. Front. Microbiol. 2018, 9, 1137. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ni, B.J.; Bond, P.L.; Ye, L.; Yuan, Z. Electron Competition among Nitrogen Oxides Reduction during Methanol-Utilizing Denitrification in Wastewater Treatment. Water Res. 2013, 47, 3273–3281. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, L. Biological Nitrate Removal from Water and Wastewater by Solid-Phase Denitrification Process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Rooke, J.A.; Cabeza, I.; Wallace, R.J. Nitrate and Inhibition of Ruminal Methanogenesis: Microbial Ecology, Obstacles, and Opportunities for Lowering Methane Emissions from Ruminant Livestock. Front. Microbiol. 2016, 7, 132. [Google Scholar] [CrossRef]
- Richardson, D.J.; Felgate, H.; Watmough, N.J.; Thomson, A.; Baggs, E.M. Mitigating Release of the Potent Greenhouse Gas N2O from the Nitrogen Cycle—Could Enzymic Regulation Hold the Key? Trends Biotechnol. 2009, 27, 388–397. [Google Scholar] [CrossRef]
- Oshiki, M.; Satoh, H.; Okabe, S. Ecology and Physiology of Anaerobic Ammonium Oxidizing Bacteria. Environ. Microbiol. 2016, 18, 2784–2796. [Google Scholar] [CrossRef]
- Kartal, B.; Keltjens, J.T. Anammox Biochemistry: A Tale of Heme c Proteins. Trends Biochem. Sci. 2016, 41, 998–1011. [Google Scholar] [CrossRef]
- op den Camp, H.J.M.; Kartal, B.; Guven, D.; van Niftrik, L.; Haaijer, S.C.M.; van der Star, W.R.L.; van de Pas-Schoonen, K.T.; Cabezas, A.; Ying, Z.; Schmid, M.; et al. Global Impact and Application of the Anaerobic Ammonium-Oxidizing (Anammox) Bacteria. Biochem. Soc. Trans. 2006, 34 Pt 1, 174–178. [Google Scholar] [CrossRef]
- Suneethi, S.; Joseph, K. ANAMMOX Process Start up and Stabilization with an Anaerobic Seed in Anaerobic Membrane Bioreactor (AnMBR). Bioresour. Technol. 2011, 102, 8860–8867. [Google Scholar] [CrossRef]
- Yan, L.; Ye, J.; Zhang, P.; Xu, D.y.; Wu, Y.; Liu, J.; Zhang, H.; Fang, W.; Wang, B.; Zeng, G. Hydrogen Sulfide Formation Control and Microbial Competition in Batch Anaerobic Digestion of Slaughterhouse Wastewater Sludge: Effect of Initial Sludge pH. Bioresour. Technol. 2018, 259, 67–74. [Google Scholar] [CrossRef]
- Weng, H.x.; Dai, Z.; Ji, Z.q.; Gao, C.; Liu, C. Release and Control of Hydrogen Sulfide during Sludge Thermal Drying. J. Hazard. Mater. 2015, 296, 61–67. [Google Scholar] [PubMed]
- Matos, R.V.; Matias, N.; Ferreira, F.; Silva, J.S.; Matos, J.S. Assessment of Sulfide Production in a Full Scale Wastewater Sludge Rising Main. J. Environ. Manag. 2018, 209, 505–514. [Google Scholar]
- Vásquez, Y.; Escobar, M.C.; Neculita, C.M.; Arbeli, Z.; Roldán, F. Biochemical Passive Reactors for Treatment of Acid Mine Drainage: Effect of Hydraulic Retention Time on Changes in Efficiency, Composition of Reactive Mixture, and Microbial Activity. Chemosphere 2016, 153, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Hülse, D.; Arndt, S.; Ridgwell, A. Mitigation of Extreme Ocean Anoxic Event Conditions by Organic Matter Sulfurization. Paleoceanogr. Paleoclimatol. 2019, 34, 476–489. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.r.; Zhao, W.L.; Yang, S.w.; Hu, B.; Yang, S.g.; Lu, Q. Mechanical Insight into the Formation of H2S from Thiophene Pyrolysis: The Influence of H2O. Chemosphere 2021, 279, 130628. [Google Scholar]
- Madigan, M.T.; Martinko, J.M.; Parker, J. Brock Biology of Microorganisms; Prentice Hall Upper: Saddle River, NJ, USA, 1997; Volume 11. [Google Scholar]
- Yang, F.; Han, Y.; Bi, H.; Wei, X.m.; Luo, W.; Li, G. Ammonia Emissions and Their Key Influencing Factors from Naturally Ventilated Dairy Farms. Chemosphere 2022, 307, 135747. [Google Scholar]
- Sha, Z.; Ma, X.; Liu, H.; Wang, J.; Lv, T.; Goulding, K.W.T.; Liu, X. Crop-Specific Ammonia Volatilization Rates and Key Influencing Factors in the Upland of China—A Data Synthesis. J. Environ. Manag. 2023, 336, 117676. [Google Scholar]
- Kemmou, L.; Amanatidou, E. Factors Affecting Nitrous Oxide Emissions from Activated Sludge Wastewater Treatment Plants—A Review. Resources 2023, 12, 114. [Google Scholar] [CrossRef]
- Zhang, H.; Schuchardt, F.; Li, G.; Yang, J.b.; Yang, Q.Y. Emission of Volatile Sulfur Compounds during Composting of Municipal Solid Waste (MSW). Waste Manag. 2013, 33, 957–963. [Google Scholar]
- Xu, Z.; Yang, Q.; Jiang, X.; Dong, Y.; Hu, Z.; Shi, L.; Zhao, R. Multi-Dimension Analysis of Volatile Sulfur Compound Emissions from an Urban Wastewater Treatment Plant. J. Environ. Manag. 2023, 345, 118632. [Google Scholar] [CrossRef]
- Barczak, R.J.; Fisher, R.M.; Le-Minh, N.; Stuetz, R.M. Identification of Volatile Sulfur Odorants Emitted from Ageing Wastewater Biosolids. Chemosphere 2022, 287 Pt 2, 132210. [Google Scholar] [CrossRef]
- Xin, L.; Nan’an, X.; Wang, X.; Dai, X.; Guo, Y.; Dong, B. New Insight into Volatile Sulfur Compounds Conversion in Anaerobic Digestion of Excess Sludge: Influence of Free Ammonia Nitrogen and Thermal Hydrolysis Pretreatment. J. Clean. Prod. 2020, 277, 123366. [Google Scholar]
- Han, Z.; Qi, F.; Wang, H.; Liu, B.; Shen, X.; Song, C.; Bao, Z.; Zhao, X.; Xu, Y.; Sun, D. Emission characteristics of volatile sulfur compounds (VSCs) from a municipal sewage sludge aerobic composting plant. Waste Manag. 2018, 77, 593–602. [Google Scholar] [CrossRef]
- Li, R.; Han, Z.; Shen, H.; Qi, F.; Ding, M.; Song, C.; Sun, D. Emission characteristics of odorous volatile sulfur compound from a full-scale sequencing batch reactor wastewater treatment plant. Sci. Total Environ. 2021, 776, 145991. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Kulig, A.; Lelicińska-Serafin, K. The importance of the microclimatic conditions inside and outside of plant buildings in odorants emission at municipal waste biogas installations. Energies 2020, 13, 6463. [Google Scholar] [CrossRef]
- Ding, Y.; Cai, C.; Hu, B.; Xu, Y.; Zheng, X.; Chen, Y.; Wu, W. Characterization and control of odorous gases at a landfill site: A case study in Hangzhou, China. Waste Manag. 2012, 32, 317–326. [Google Scholar]
- Hernández, D.; Zambra, C.; Astudillo, C.; Gabriel, D.; Díaz, J. Evolution of physico-chemical parameters, microorganism diversity and volatile organic compound of apple pomace exposed to ambient conditions. Heliyon 2023, 9, e19770. [Google Scholar] [CrossRef]
- Vela Aparicio, D.; Muñoz Lasso, C.; Cabeza Rojas, I.; Brandao, P. Evaluación de las Emisiones de H2S, NH3 y Compuestos orgáNicos Volátiles en la PTAR el Salitre a Través del réGimen climáTico de Bogotá; Universidad Santo Tomás: Santiago, Chile, 2020. [Google Scholar]
- Fisher, R.; Le-Minh, N.; Alvarez-Gaitan, J.; Moore, S.; Stuetz, R. Emissions of volatile sulfur compounds (VSCs) throughout wastewater biosolids processing. Sci. Total Environ. 2018, 616, 622–631. [Google Scholar] [CrossRef]
- Lomans, B.P.; Smolders, A.; Intven, L.M.; Pol, A.; Op, D.; Van Der Drift, C. Formation of dimethyl sulfide and methanethiol in anoxic freshwater sediments. Appl. Environ. Microbiol. 1997, 63, 4741–4747. [Google Scholar] [CrossRef]
- Paternina, M.F.; Fern, A.A.; Cabeza, R.I.; Forero, D.F. Evaluación de Diferentes Variables Operacionales en un Sistema de Biofiltración con Tres Tipos de Lechos Orgánicos para la Remoción Simultánea de Sulfuro de Hidrógeno (H2S) y Amoniaco (NH3); FAO: Roma, Italy, 2020. [Google Scholar]
- Ras, M.R.; Borrull, F.; Marcé, R.M. Determination of volatile organic sulfur compounds in the air at sewage management areas by thermal desorption and gas chromatography–mass spectrometry. Talanta 2008, 74, 562–569. [Google Scholar] [CrossRef]
- Wenzhu, Y.; Hu, Y.; Song, C.; Yu, Y.; Jiao, Y. Interactive effects of soil moisture, nitrogen fertilizer, and temperature on the kinetic and thermodynamic properties of ammonia emissions from alkaline soil. Atmos. Pollut. Res. 2023, 14, 101805. [Google Scholar] [CrossRef]
- Drame, M.; Carswell, A.; Roberts, W.; Hood, J.; Jemo, M.; Heuer, S.; Kirk, G.; Pawlett, M.; Misselbrook, T. Effects of hotter, drier conditions on gaseous losses from nitrogen fertilisers. J. Environ. Manag. 2023, 345, 118671. [Google Scholar] [CrossRef]
- Vangeli, S.; Posse, G.; Beget, M.E.; Otero Estrada, E.; Valdettaro, R.A.; Oricchio, P.; Kandus, M.; Di Bella, C.M. Effects of fertilizer type on nitrous oxide emission and ammonia volatilization in wheat and maize crops. Soil Use Manag. 2022, 38, 1519–1531. [Google Scholar] [CrossRef]
- Alvarado, M.d.R.T.; Muñoz, T.P.; Vela, N.B.M. Methanogenesis in sediments of a tropical coastal wetland: A culture-dependent method. Rev. Biol. Trop. 2024, 72, 28. [Google Scholar]
- Wang, M.; Hou, J.; Deng, R. Co-exposure of environmental contaminants with unfavorable temperature or humidity/moisture: Joint hazards and underlying mechanisms. Ecotoxicol. Environ. Saf. 2023, 264, 115432. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, Y.; Shi, Y.; Zhou, X.; Lin, J.; Ruan, A. The Response Mechanism of Microorganisms to the Organic Carbon-Driven Formation of Black and Odorous Water. Environ. Res. 2023, 231, 116255. [Google Scholar] [CrossRef]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining Oxygen in the Global Ocean and Coastal Waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef]
- Díaz, R.J.; Rosenberg, R. Spreading Dead Zones and Consequences for Marine Ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Jouanneau, S.; Recoules, L.; Durand, M.J.; Boukabache, A.; Picot, V.S.; Primault, Y.; Lakel, A.; Sengelin, M.; Barillon, B.C.G.; Thouand, G. Methods for Assessing Biochemical Oxygen Demand (BOD): A Review. Water Res. 2014, 49, 62–82. [Google Scholar] [CrossRef]
- Bonsdorff, E. Eutrophication: Early Warning Signals, Ecosystem-Level and Societal Responses, and Ways Forward. Ambio 2021, 50, 753–758. [Google Scholar] [CrossRef]
- Heisler, J.C.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.P.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.M.; et al. Eutrophication and Harmful Algal Blooms: A Scientific Consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar]
- Olivera, C.; Tondo, M.L.; Girardi, V.; Fattobene, L.; Herrero, M.S.; Pérez, L.M.; Salvatierra, L.M. Early-Stage Response in Anaerobic Bioreactors Due to High Sulfate Loads: Hydrogen Sulfide Yield and Other Organic Volatile Sulfur Compounds as a Sign of Microbial Community Modifications. Bioresour. Technol. 2022, 350, 126947. [Google Scholar]
- Yu, C.; Shi, C.; Tang, J.; Ji, Q.; Wang, X.; Xu, X.; Wang, G. Release of Taste and Odour Compounds during Zizania Latifolia Decay: A Microcosm System Study. Environ. Pollut. 2019, 254 Pt A, 112954. [Google Scholar] [CrossRef]
- Darnajoux, R.; Inomura, K.; Zhang, X. A Diazotrophy-Ammoniotrophy Dual Growth Model for the Sulfate Reducing Bacterium Desulfovibrio Vulgaris Var. Hildenborough. Comput. Struct. Biotechnol. J. 2023, 21, 3136–3148. [Google Scholar]
- Parshina, S.N.; Sipma, J.; Nakashimada, Y.; Henstra, A.M.; Smidt, H.; Lysenko, A.M.; Lens, P.N.; Lettinga, G.; Stams, A.J. Desulfotomaculum Carboxydivorans Sp. Nov., a Novel Sulfate-Reducing Bacterium Capable of Growth at 100% CO. Int. J. Syst. Evol. Microbiol. 2005, 55, 2159–2165. [Google Scholar] [CrossRef]
- Ghorbel, L.; Coudert, L.; Gilbert, Y.G.; Mercier, G.; Blais, J.F. Determination of Critical Operational Conditions Favoring Sulfide Production from Domestic Wastewater Treated by a Sulfur-Utilizing Denitrification Process. J. Environ. Manag. 2017, 198 Pt 1, 16–23. [Google Scholar]
- Guerrero, L.; Montalvo, S.; Huiliñir, C.; Campos, J.L.; Barahona, A.; Borja, R. Advances in the Biological Removal of Sulphides from Aqueous Phase in Anaerobic Processes: A Review. Environ. Rev. 2016, 24, 84–100. [Google Scholar]
- Murei, A.; Kamika, I.; Samie, A.; Momba, M.N.B. Assessment of the Water Sources for Potential Channels of Faecal Contamination within Vhembe District Municipality Using Sanitary Inspections and Hydrogen Sulphide Test. Sci. Rep. 2023, 13, 6250. [Google Scholar]
- Zuo, Z. Emission of Cyanobacterial Volatile Organic Compounds and Their Roles in Blooms. Front. Microbiol. 2023, 14, 1097712. [Google Scholar]
- Dong, Z.Y.; Lin, Y.L.; Zhang, T.Y.; Hu, C.y.; Pan, Y.; Zheng, Z.X.; Tang, Y.L.; Xu, B.; Gao, N.y. The Formation, Analysis, and Control of Chlor(Am)Ination-Derived Odor Problems: A Review. Water Res. 2021, 203, 117549. [Google Scholar]
- Zhang, X.j.; Chen, C.; Ding, J.q.; Hou, A.; Li, Y.; Niu, Z.b.; Su, X.y.; Xu, Y.j.; Laws, E.A. The 2007 water crisis in Wuxi, China: Analysis of the origin. J. Hazard. Mater. 2010, 182, 130–135. [Google Scholar] [CrossRef]
- Jefferson, G.M.; Ciro, P.M.; Andrea, M.S.M. Environmental Education and the Bogotá River: An Intervention to Be Carried out in Cundinamarca (Colombia). Int. Res. Geogr. Environ. Educ. 2017, 26, 281–296. [Google Scholar] [CrossRef]
- Díaz-Casallas, D.M.; Castro-Fernández, M.F.; Bocos, E.; Montenegro-Marín, C.E.; Crespo, R.G. 2008–2017 Bogota River Water Quality Assessment Based on the Water Quality Index. Sustainability 2019, 11, 1668. [Google Scholar] [CrossRef]
- Fernández, M.F.C.; Manosalva, I.R.C.; Quintero, R.F.C.; Marín, C.E.M.; Cuesta, Y.E.D.; Mahecha, D.E.; Vásquez, P.A.P. Multitemporal Total Coliforms and Escherichia coli Analysis in the Middle Bogotá River Basin, 2007–2019. Sustainability 2022, 14, 1769. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ndabambi, M.; Choi, S.; Oh, J.e. Legacy and Novel Perfluoroalkyl and Polyfluoroalkyl Substances in Industrial Wastewater and the Receiving River Water: Temporal Changes in Relative Abundances of Regulated Compounds and Alternatives. Water Res. 2021, 191, 116830. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.M.; Boobis, A.R.; DeWitt, J.C.; Lau, C.; Ng, C.A.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2020, 40, 606–630. [Google Scholar] [CrossRef]
- Ruíz-Ordóñez, D.M.; Solano-Correa, Y.T.; Maysels, R.; Figueroa-Casas, A. Land-Use Dynamics and Water Quality in Andean Basins. Sustainability 2023, 15, 15965. [Google Scholar] [CrossRef]
- Kennish, M.J.; Townsend, A.R. Nutrient Enrichment and Estuarine Eutrophication1. Ecol. Appl. 2007, 17, S1–S2. [Google Scholar] [CrossRef]
- Ma, R.; Chen, Z.; Wang, B.; Xu, C.; Jia, Z.; Li, L.; Hu, J. Spatiotemporal Variations and Controlling Mechanism of Low Dissolved Oxygen in a Highly Urbanized Complex River System. J. Hydrol. Reg. Stud. 2024, 52, 101691. [Google Scholar] [CrossRef]
- Rixen, T.; Baum, A.; Sepryani, H.; Pohlmann, T.; Jose, C.; Samiaji, J. Dissolved Oxygen and Its Response to Eutrophication in a Tropical Black Water River. J. Environ. Manag. 2010, 91, 1730–1737. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Díaz, R.J.; Levin, L.A.; Turner, R.E.; Gilbert, D.; Zhang, J. Dynamics and Distribution of Natural and Human-Caused Hypoxia. Biogeosciences 2010, 7, 585–619. [Google Scholar] [CrossRef]
- Cao, J.; Sun, Q.; Zhao, D.; Xu, M.; Shen, Q.; Wang, D.; Wang, Y.; Ding, S. A Critical Review of the Appearance of Black-Odorous Waterbodies in China and Treatment Methods. J. Hazard. Mater. 2019, 385, 121511. [Google Scholar] [CrossRef]
- Peña-Guzmán, C.A.; Soto, L.; Diaz, A. A proposal for redesigning the water quality network of the Tunjuelo River in Bogotá, Colombia through a spatio-temporal analysis. Resources 2019, 8, 64. [Google Scholar] [CrossRef]
- Martinez Castro, C.Y. Aplicabilidad de la Producción más Limpia (PML) y el Modelo de Economía Azul en las Cuencas del Río Bogotá; Repositorio UMNG; Universidad Militar Nueva Granada: Bogotá, Colombia, 2020. [Google Scholar]
- Giraldo, E.; Garzon, A. The potential for water hyacinth to improve the quality of Bogota River water in the Muña Reservoir: Comparison with the performance of waste stabilization ponds. Water Sci. Technol. 2002, 45, 103–110. [Google Scholar] [CrossRef]
- Peña-Guzmán, C.A.; Melgarejo, J.; Lopez-Ortiz, I.; Mesa, D.J. Simulation of infrastructure options for urban water management in two urban catchments in Bogotá, Colombia. Water 2017, 9, 858. [Google Scholar] [CrossRef]
- Sun, J.; Hu, S.; Sharma, K.R.; Keller-Lehmann, B.; Yuan, Z. An Efficient Method for Measuring Dissolved VOSCs in Wastewater Using GC-SCD with Static Headspace Technique. Water Res. 2014, 52, 208–217. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Whelan, M.E.; Rhew, R.C. Analysis of Low Concentration Reduced Sulfur Compounds (RSCs) in Air: Storage Issues and Measurement by Gas Chromatography with Sulfur Chemiluminescence Detection. Talanta 2012, 88, 581–586. [Google Scholar] [CrossRef]
- Rabaud, N.E.; Ebeler, S.E.; Ashbaugh, L.L.; Flocchini, R.G. The Application of Thermal Desorption GC/MS with Simultaneous Olfactory Evaluation for the Characterization and Quantification of Odor Compounds from a Dairy. J. Agric. Food Chem. 2002, 50, 5139–5145. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, K.H. A Novel Quantitation Approach for Maximizing Detectable Targets for Offensive/Volatile Odorants with Diverse Functional Groups by Thermal Desorption-Gas Chromatography-Mass Spectrometry. Sci. Rep. 2016, 6, 29248. [Google Scholar] [CrossRef]
- Conti, C.; Guarino, M.; Bacenetti, J. Measurements Techniques and Models to Assess Odor Annoyance: A Review. Environ. Int. 2019, 134, 105261. [Google Scholar] [CrossRef]
- Yu, W.; Lewis, N.S.; Gray, H.B.; Dalleska, N.F. Isotopically Selective Quantification by UPLC-MS of Aqueous Ammonia at Submicromolar Concentrations Using Dansyl Chloride Derivatization. Acs Energy Lett. 2020, 5, 1532–1536. [Google Scholar]
- Leifer, I.; Melton, C.; Tratt, D.M.; Buckland, K.N.; Chang, C.S.; Clarisse, L.; Franklin, M.; Hall, J.L.; Leen, J.B.; Lundquist, T.J.; et al. Estimating Exposure to Hydrogen Sulfide from Animal Husbandry Operations Using Satellite Ammonia as a Proxy: Methodology Demonstration. Sci. Total Environ. 2019, 709, 134508. [Google Scholar]
- Noppen, L.; Clarisse, L.; Tack, F.; Ruhtz, T.; Merlaud, A.; Damme, M.V.; Roozendael, M.V.; Schuettemeyer, D.; Coheur, P.F. Constraining Industrial Ammonia Emissions Using Hyperspectral Infrared Imaging. Remote. Sens. Environ. 2023, 291, 113559. [Google Scholar] [CrossRef]
- Zhu, X.; Roberts, W.L.; Guiberti, T.F. UV-visible Chemiluminescence Signature of Laminar Ammonia-Hydrogen-Air Flames. Proc. Combust. Inst. 2022, 39, 4227–4235. [Google Scholar] [CrossRef]
- Yang, W.; Que, H.; Wang, S.; Zhu, A.; Zhang, Y.; He, Y.; Xin, X.; Zhang, X.; Ding, S. High Temporal Resolution Measurements of Ammonia Emissions Following Different Nitrogen Application Rates from a Rice Field in the Taihu Lake Region of China. Environ. Pollut. 2019, 257, 113489. [Google Scholar] [CrossRef]
- Humbert, K.; Debret, M.; Morin, C.; Cosme, J.; Portet-Koltalo, F. Direct Thermal Desorption-Gas Chromatography-Tandem Mass Spectrometry versus Microwave Assisted Extraction and GC-MS for the Simultaneous Analysis of Polyaromatic Hydrocarbons (PAHs, PCBs) from Sediments. Talanta 2022, 250, 123735. [Google Scholar]
- Martin, N.J.; Smith, P.A.; Brown, C.W.; Achee, N.L.; DeLong, G.T. Dichlorodiphenyltrichloroethane Determination in Air by Thermal Desorption Gas Chromatography-Mass Spectrometry. Pest Manag. Sci. 2012, 68, 1360–1367. [Google Scholar]
- Saji, S.; Lu, H.; Lu, Z.; Carroll, A.J.; Yin, Z. An Experimentally Verified LC-MS Protocol toward an Economical, Reliable, and Quantitative Isotopic Analysis in Nitrogen Reduction Reactions. Small Methods 2021, 5, e2000694. [Google Scholar]
- Joo, K.M.; Shin, M.; Jung, J.h.; Kim, B.M.; Lee, J.W.; Jeong, H.J.; Lim, K.M. Determination of N-nitrosodiethanolamine, NDELA in Cosmetic Ingredients and Products by Mixed Mode Solid Phase Extraction and UPLC-tandem Mass Spectrometry with Porous Graphitic Carbon Column through Systemic Sample Pre-Cleanup Procedure. Talanta 2015, 137, 109–119. [Google Scholar]
- da Silveira Petruci, J.F.; Wilk, A.; Cardoso, A.A.; Mizaikoff, B. Online analysis of H2S and SO2 via advanced mid-infrared gas sensors. Anal. Chem. 2015, 87, 9605–9611. [Google Scholar]
- Dong, F.; Junaedi, C.; Roychoudhury, S.; Gupta, M.X. Rapid, Online Quantification of H2S in JP-8 Fuel Reformate Using near-Infrared Cavity-Enhanced Laser Absorption Spectroscopy. Anal. Chem. 2011, 83, 4132–4136. [Google Scholar] [CrossRef]
- de Cumis, M.S.; Viciani, S.; Borri, S.; Patimisco, P.; Sampaolo, A.; Scamarcio, G.; Natale, P.D.; D’Amato, F.; Spagnolo, V. Widely-Tunable Mid-Infrared Fiber-Coupled Quartz-Enhanced Photoacoustic Sensor for Environmental Monitoring. Opt. Express 2014, 22, 28222–28231. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Wang, Q.; Borri, S.; Galli, I.; Sampaolo, A.; Patimisco, P.; Spagnolo, V.; Natale, P.D.; Ren, W. Parts-per-Billion-Level Detection of Hydrogen Sulfide Based on Doubly Resonant Photoacoustic Spectroscopy with Line-Locking. Photoacoustics 2022, 29, 100436. [Google Scholar] [CrossRef]
- Kamp, J.N.; Chowdhury, A.; Adamsen, A.P.S.; Feilberg, A. Negligible Influence of Livestock Contaminants and Sampling System on Ammonia Measurements with Cavity Ring-down Spectroscopy. Atmos. Meas. Tech. 2018, 12, 2837–2850. [Google Scholar] [CrossRef]
- Ceballos, S.; Sala, A.; Gil, S.; Rodríguez-Nuévalos, S.; Costero, A.M.; Doménech, A. Chemical and Electrochemical Behaviour of 4,4′,4″,4‴-Tetrakis(Dimethylamino)-Tetraphenylethylene in an Oxidant Environment: Toward a New Sensor for NO2 and SO2 in Gas Phase. Sensors Actuators B Chem. 2020, 311, 127929. [Google Scholar] [CrossRef]
- Hall, J.R.; Schoenfisch, M.H. Direct Electrochemical Sensing of Hydrogen Sulfide without Sulfur Poisoning. Anal. Chem. 2018, 90, 5194–5200. [Google Scholar] [CrossRef]
- Balasubramani, V.; Chandraleka, S.; Rao, T.S.; Sasikumar, R.; Kuppusamy, M.R.; Sridhar, T.M. Review—Recent Advances in Electrochemical Impedance Spectroscopy Based Toxic Gas Sensors Using Semiconducting Metal Oxides. J. Electrochem. Soc. 2020, 167, 037572. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Jiang, L.; Jones, T.G.J.; Compton, R.G. A Thin-Layer Amperometric Sensor for Hydrogen Sulfide: The Use of Microelectrodes to Achieve a Membrane-Independent Response for Clark-type Sensors. Anal. Chem. 2003, 75, 2499–2503. [Google Scholar] [CrossRef]
- Hayward, T.; Thurbide, K.B. Quenching-Resistant Multiple Micro-Flame Photometric Detector for Gas Chromatography. Anal. Chem. 2009, 81, 8858–8867. [Google Scholar] [CrossRef]
- Ni, L.; Li, S.; Ding, K.; Geng, X.; Duan, C.f.; Guan, Y. Enhancement of Chemiluminescence Intensity of S2* in Non-Premixed Hydrogen Microjet Flame in the Photometric Detector for Sulfur Detection. Anal. Chem. 2021, 93, 1969–1975. [Google Scholar] [CrossRef]
- Kendler, S.; Reidy, S.M.; Lambertus, G.R.; Sacks, R.D. Ultrafast Gas Chromatographic Separation of Organophosphor and Organosulfur Compounds Utilizing a Microcountercurrent Flame Photometric Detector. Anal. Chem. 2006, 78, 6765–6773. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.H. The Fundamental Properties of the Direct Injection Method in the Analysis of Gaseous Reduced Sulfur by Gas Chromatography with a Pulsed Flame Photometric Detector. Anal. Chim. Acta 2008, 615, 165–173. [Google Scholar] [CrossRef]
- Vargas-Muñoz, M.; Morales, J.; Cerdà, V.; Ferrer, L.; Palacio, E. Paper Sensor-Based Method Using a Portable 3D-printed Platform and Smartphone-Assisted Colorimetric Detection for Ammonia and Sulfide Monitoring in Anaerobic Digesters and Wastewater. Microchem. J. 2023, 188, 108469. [Google Scholar] [CrossRef]
- Escobedo, P.; Ramos-Lorente, C.E.; Ejaz, A.; Erenas, M.M.; Martínez-Olmos, A.; Carvajal, M.A.; Garcia-Núñez, C.; de Orbe-Payá, I.; Capitán-Vallvey, L.F.; Palma, A.J. QRsens: Dual-purpose Quick Response Code with Built-in Colorimetric Sensors. Sensors Actuators B Chem. 2022, 376, 133001. [Google Scholar] [CrossRef]
- Gao, Z.; Tang, D.; Tang, D.; Niessner, R.; Knopp, D. Target-Induced Nanocatalyst Deactivation Facilitated by Core@shell Nanostructures for Signal-Amplified Headspace-Colorimetric Assay of Dissolved Hydrogen Sulfide. Anal. Chem. 2015, 87, 10153–10160. [Google Scholar] [CrossRef]
- Amirjani, A.; Fatmehsari, D.H. Colorimetric Detection of Ammonia Using Smartphones Based on Localized Surface Plasmon Resonance of Silver Nanoparticles. Talanta 2018, 176, 242–246. [Google Scholar] [CrossRef]
- Shaik, R.; Kampara, R.K.; Kumar, A.; Sharma, C.S.; Kumar, M. Metal oxide nanofibers based chemiresistive H2S gas sensors. Coord. Chem. Rev. 2022, 471, 214752. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Kwoka, M.; Gang, M.; Kumar, M. IoT-enabled surface-active Pd-anchored metal oxide chemiresistor for H2S gas detection. Sensors Actuators B Chem. 2024, 402, 135065. [Google Scholar] [CrossRef]
- Pourrahmani, H.; Javadi, A.; Monazzah, A.M.H.; Herle, J.V. Real-time H2S detection kit for hydrogen fuel cell. Heliyon 2024, 10, e33321. [Google Scholar] [CrossRef]
- Ali, F.I.; Awwad, F.; Greish, Y.E.; Mahmoud, S.T. Hydrogen sulfide (H2S) gas sensor: A review. IEEE Sensors J. 2018, 19, 2394–2407. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Horrillo, M.C. Advances in artificial olfaction: Sensors and applications. Talanta 2014, 124, 95–105. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Song, Z.; Ye, W.; Fan, Z. Smart Gas Sensor Arrays Powered by Artificial Intelligence. J. Semicond. 2019, 40, 111601. [Google Scholar]
- Chen, J.; Chen, Z.; Boussaid, F.; Zhang, D.; Pan, X.; Zhao, H.; Bermak, A.; Tsui, C.Y.; Wang, X.; Fan, Z. Ultra-Low-Power Smart Electronic Nose System Based on Three-Dimensional Tin Oxide Nanotube Arrays. ACS Nano 2018, 12, 6079–6088. [Google Scholar]
- Moshayedi, A.J.; Sohail Khan, A.; Hu, J.; Nawaz, A.; Zhu, J. E-Nose-Driven Advancements in Ammonia Gas Detection: A Comprehensive Review from Traditional to Cutting-Edge Systems in Indoor to Outdoor Agriculture. Sustainability 2023, 15, 11601. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, D.; Tang, M.; Wang, Z. Deep Learning-Assisted Colorimetric/Electrical Dual-Sensing System for Ultrafast Detection of Hydrogen Sulfide. ACS Sens. 2024, 9, 2000–2009. [Google Scholar] [CrossRef]
- Jornet-Martínez, N.; Hakobyan, L.; Argente-García, A.I.; Molins-Legua, C.; Campíns-Falcó, P. Nylon-Supported Plasmonic Assay Based on the Aggregation of Silver Nanoparticles: In Situ Determination of Hydrogen Sulfide-like Compounds in Breath Samples as a Proof of Concept. ACS Sens. 2019, 4, 2164–2172. [Google Scholar] [CrossRef]
- Pla-Tolós, J.; Moliner-Martínez, Y.; Verdú-Andrés, J.; Casanova-Chafer, J.; Molins-Legua, C.; Campíns-Falcó, P. New Optical Paper Sensor for in Situ Measurement of Hydrogen Sulphide in Waters and Atmospheres. Talanta 2016, 156–157, 79–86. [Google Scholar]
- Okamoto, J.; Matsubara, T.C.M.; Kitagawa, T.; Umeda, T. Determination of a Sulfur-Containing Drug in Human Plasma by an Improved Method for Sulfur Chemiluminescence Detection in Combination with Capillary Gas Chromatography. Anal. Chem. 2000, 72, 634–638. [Google Scholar]
- Siebert, T.E.; Solomon, M.; Pollnitz, A.P.; Jeffery, D.W. Selective Determination of Volatile Sulfur Compounds in Wine by Gas Chromatography with Sulfur Chemiluminescence Detection. J. Agric. Food Chem. 2010, 58, 9454–9462. [Google Scholar] [CrossRef]
- García, C.L.; Becchi, M.; Grenier-loustalot, M.F.; Païsse, O.; Szymanski, R. Analysis of Aromatic Sulfur Compounds in Gas Oils Using GC with Sulfur Chemiluminescence Detection and High-Resolution MS. Anal. Chem. 2002, 74, 3849–3857. [Google Scholar]
- Nolvachai, Y.; Kulsing, C.; Marriott, P.J. Pesticides Analysis: Advantages of Increased Dimensionality in Gas Chromatography and Mass Spectrometry. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2135–2173. [Google Scholar]
- Sapozhnikova, Y.; Lehotay, S.J. Review of Recent Developments and Applications in Low-Pressure (Vacuum Outlet) Gas Chromatography. Anal. Chim. Acta 2015, 899, 13–22. [Google Scholar]
- Dymerski, T. Two-Dimensional Gas Chromatography Coupled with Mass Spectrometry in Food Analysis. Crit. Rev. Anal. Chem. 2018, 48, 252–278. [Google Scholar]
- Kauna-Czaplińska, J. Current Applications of Gas Chromatography/Mass Spectrometry in the Study of Organic Acids in Urine. Crit. Rev. Anal. Chem. 2011, 41, 114–123. [Google Scholar]
- Wu, H.; Dong, L.; Zheng, H.; Yu, Y.; Ma, W.; Zhang, L.; Yin, W.; Xiao, L.; Jia, S.; Tittel, F.K. Beat Frequency Quartz-Enhanced Photoacoustic Spectroscopy for Fast and Calibration-Free Continuous Trace-Gas Monitoring. Nat. Commun. 2017, 8, 15331. [Google Scholar]
- Yang, Z.L.; Lin, H.; Montano, B.A.Z.; Zhu, W.; Zhong, Y.; Yuan, B.; Yu, J.; Kan, R.; Shao, M.; Zheng, H. High-Power near-Infrared QEPAS Sensor for Ppb-Level Acetylene Detection Using a 28 kHz Quartz Tuning Fork and 10 W EDFA. Opt. Express 2022, 30, 6320–6331. [Google Scholar]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2014, 87, 230–249. [Google Scholar]
- Bogomolova, A.; Komarova, E.S.; Reber, K.; Gerasimov, T.; Yavuz, Ö.; Bhatt, S.; Aldissi, M. Challenges of Electrochemical Impedance Spectroscopy in Protein Biosensing. Anal. Chem. 2009, 81, 3944–3949. [Google Scholar] [CrossRef]
- Thurbide, K.B.; Anderson, C.D. Flame Photometric Detection inside of a Capillary Gas Chromatography Column. Analyst 2003, 128, 616–622. [Google Scholar]
- Lackner, M. Tunable Diode Laser Absorption Spectroscopy (Tdlas) in the Process Industries—A Review. Rev. Chem. Eng. 2007, 23, 147–165. [Google Scholar] [CrossRef]
- Lin, S.; Chang, J.; Sun, J.; Xu, P.F. Improvement of the Detection Sensitivity for Tunable Diode Laser Absorption Spectroscopy: A Review. Front. Phys. 2022, 10, 853966. [Google Scholar] [CrossRef]
- Martínez-Aviñó, A.; Molins-Legua, C.; Pilar, C.F. Scaling the Analytical Information given by Several Types of Colorimetric and Spectroscopic Instruments Including Smartphones: Rules for Their Use and Establishing Figures of Merit of Solid Chemosensors. Anal. Chem. 2021, 93, 6043–6052. [Google Scholar] [CrossRef]
- Kleine, D.; Mürtz, M.; Lauterbach, J.; Dahnke, H.; Urban, W.; Hering, P.; Kleinermanns, K. Atmospheric Trace Gas Analysis with Cavity Ring-down Spectroscopy. Isr. J. Chem. 2001, 41, 111–116. [Google Scholar] [CrossRef]
- Sommer, S.G.; Misselbrook, T.H. A Review of Ammonia Emission Measured Using Wind Tunnels Compared with Micrometeorological Techniques. Soil Use Manag. 2016, 32, 101–108. [Google Scholar]
- Ellis, R.; Murphy, J.G.; Pattey, E.; van Haarlem, R.P.; O’brien, J.; Herndon, S.C. Characterizing a Quantum Cascade Tunable Infrared Laser Differential Absorption Spectrometer (QC-TILDAS) for Measurements of Atmospheric Ammonia. Atmos. Meas. Tech. 2009, 3, 397–406. [Google Scholar]
- Pan, Y.; Gu, M.; Song, L.; Tian, S.; Wu, D.; Walters, W.W.; Yu, X.; Lü, X.; Ni, X.; Wang, Y.J.; et al. Systematic Low Bias of Passive Samplers in Characterizing Nitrogen Isotopic Composition of Atmospheric Ammonia. Atmos. Res. 2020, 243, 105018. [Google Scholar]
- Almenglo, F.; González-Cortés, J.; Ramírez, M.; Cantero, D. Recent advances in biological technologies for anoxic biogas desulfurization. Chemosphere 2023, 321, 138084. [Google Scholar]
- Babamohammadi, S.; Shamiri, A.; Aroua, M. A Review of CO2 Capture by Absorption in Ionic Liquid-Based Solvents. Rev. Chem. Eng. 2015, 31, 383–412. [Google Scholar]
- Mugwili, M.E.; Waanders, F.B.; Masindi, V.; Fosso-Kankeu, E. An update on sustainabilities and challenges on the removal of ammonia from aqueous solutions: A state-of-the-art review. J. Environ. Manag. 2023, 347, 119172. [Google Scholar]
- Zulkifli, M.; Hasan, H.A.; Abdullah, S.R.S.; Muhamad, M.H. A review of ammonia removal using a biofilm-based reactor and its challenges. J. Environ. Manag. 2022, 315, 115162. [Google Scholar]
- Winkler, M.K.; Straka, L. New directions in biological nitrogen removal and recovery from wastewater. Curr. Opin. Biotechnol. 2019, 57, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Aminuddin, M.S.; Bustam, M.A.; Johari, K. Latest Technological Advances and Insights into Capture and Removal of Hydrogen Sulfide: A Critical Review. RSC Sustain. 2024, 2, 757–803. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-Combustion CO2 Capture with Chemical Absorption: A State-of-the-Art Review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Chapter 14—Physical Solvents for Acid Gas Removal. In Gas Purification, 5th ed.; Kohl, A.L., Nielsen, R.B., Eds.; Gulf Professional Publishing: Houston, TX, USA, 1997; pp. 1187–1237. [Google Scholar]
- Liu, X.; Wang, B.; Lv, X.; Meng, Q.; Li, M. Enhanced Removal of Hydrogen Sulfide Using Novel Nanofluid System Composed of Deep Eutectic Solvent and Cu Nanoparticles. J. Hazard. Mater. 2021, 405, 124271. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Chapter 2—Alkanolamines for Hydrogen Sulfide and Carbon Dioxide Removal. In Gas Purification, 5th ed.; Kohl, A.L., Nielsen, R.B., Eds.; Gulf Professional Publishing: Houston, TX, USA, 1997; pp. 40–186. [Google Scholar]
- Kouser, S.; Waghmare, U.V.; Tit, N. Adsorption and Splitting of H2S on 2D-ZnO1−xNy: First-Principles Analysis. Phys. Chem. Chem. Phys. PCCP 2014, 16, 10719–10726. [Google Scholar] [CrossRef]
- Ahmadi, R.; Alivand, M.S.; Haj Mohammad Hossein Tehrani, N.; Ardjmand, M.; Rashidi, A.; Rafizadeh, M.; Seif, A.; Mollakazemi, F.; Noorpoor, Z.; Rudd, J. Preparation of Fiber-like Nanoporous Carbon from Jute Thread Waste for Superior CO2 and H2S Removal from Natural Gas: Experimental and DFT Study. Chem. Eng. J. 2021, 415, 129076. [Google Scholar] [CrossRef]
- Wang, S.; Nam, H.; Nam, H. Preparation of Activated Carbon from Peanut Shell with KOH Activation and Its Application for H2S Adsorption in Confined Space. J. Environ. Chem. Eng. 2020, 8, 103683. [Google Scholar] [CrossRef]
- He, X.; Kumakiri, I.; Hillestad, M. Conceptual Process Design and Simulation of Membrane Systems for Integrated Natural Gas Dehydration and Sweetening. Sep. Purif. Technol. 2020, 247, 116993. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Liu, G.; Qiu, W.; Bhuwania, N.; Chinn, D.; Koros, W.J. Surprising Plasticization Benefits in Natural Gas Upgrading Using Polyimide Membranes. J. Membr. Sci. 2020, 593, 117430. [Google Scholar] [CrossRef]
- Sassi, M.; Gupta, A.K. Sulfur Recovery from Acid Gas Using the Claus Process and High Temperature Air Combustion (HiTAC) Technology. Am. J. Environ. Sci. 2008, 4, 502–511. [Google Scholar] [CrossRef]
- Piéplu, A.; Saur, O.; Lavalley, J.C.; Legendre, O.; Nédez, C. Claus Catalysis and H2S Selective Oxidation. Catal. Rev. 1998, 40, 409–450. [Google Scholar]
- George, Z.M. Advances in Claus Reaction and Related Reactions. Phosphorus Sulfur Relat. Elem. 1976, 1, 315–322. [Google Scholar] [CrossRef]
- de Angelis, A.; Bellussi, G.; Pollesel, P.; Perego, C. New Method for H2S Removal in Acid Solutions. ChemSusChem 2010, 3, 829–833. [Google Scholar] [PubMed]
- Zhang, X.; Xiong, W.; Shi, M.; Wu, Y.; Hu, X. Task-Specific Ionic Liquids as Absorbents and Catalysts for Efficient Capture and Conversion of H2S into Value-Added Mercaptan Acids. Chem. Eng. J. 2021, 408, 127866. [Google Scholar]
- Kennes, C.; Veiga, M.C.; Alloway, B.J.; Trevors, J.T. (Eds.) Bioreactors for Waste Gas Treatment; Environmental Pollution; Springer: Dordrecht, The Netherlands, 2001; Volume 4. [Google Scholar]
- Devinny, J.S.; Deshusses, M.A.; Webster, T.S. Biofiltration for Air Pollution Control, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Iranpour, R.; Cox, H.H.; Deshusses, M.A.; Schroeder, E.D. Literature Review of Air Pollution Control Biofilters and Biotrickling Filters for Odor and Volatile Organic Compound Removal. Environ. Prog. 2005, 24, 254–267. [Google Scholar]
- Swanson, W.J.; Loehr, R.C. Biofiltration: Fundamentals, Design and Operations Principles, and Applications. J. Environ. Eng. 1997, 123, 538–546. [Google Scholar]
- Wani, A.H.; Lau, A.K.; Branion, R.M. Biofiltration Control of Pulping Odors—Hydrogen Sulfide: Performance, Macrokinetics and Coexistence Effects of Organo-Sulfur Species. J. Chem. Technol. Biotechnol. 1999, 74, 9–16. [Google Scholar] [CrossRef]
- Li, H.; Crittenden, J.C.; Mihelcic, J.R.; Hautakangas, H. Optimization of Biofiltration for Odor Control: Model Development and Parameter Sensitivity. Water Environ. Res. 2002, 74, 5–16. [Google Scholar]
- Alonso, C.; Suidan, M.T.; Kim, B.R.; Kim, B.J. Dynamic Mathematical Model for the Biodegradation of VOCs in a Biofilter: Biomass Accumulation Study. Environ. Sci. Technol. 1998, 32, 3118–3123. [Google Scholar]
- Alinezhad, E.; Haghighi, M.; Rahmani, F.; Keshizadeh, H.; Abdi, M.; Naddafi, K. Technical and economic investigation of chemical scrubber and bio-filtration in removal of H2S and NH3 from wastewater treatment plant. J. Environ. Manag. 2019, 241, 32–43. [Google Scholar] [CrossRef]
- Wi, J.; Lee, S.; Kim, E.; Lee, M.; Koziel, J.A.; Ahn, H. Evaluation of semi-continuous pit manure recharge system performance on mitigation of ammonia and hydrogen sulfide emissions from a swine finishing barn. Atmosphere 2019, 10, 170. [Google Scholar] [CrossRef]
- Castro, Y.E.; Viloria, A. New Trends for Hydrogen Sulfide Scavenging Using Natural Compounds as Biogenic Amines. ACS Omega 2024, 9, 10671–10679. [Google Scholar] [PubMed]
- Choudhury, A.; Lansing, S. Adsorption of hydrogen sulfide in biogas using a novel iron-impregnated biochar scrubbing system. J. Environ. Chem. Eng. 2021, 9, 104837. [Google Scholar]
- Fini, M.N.; Montesantos, N.; Maschietti, M.; Muff, J. Performance evaluation of membrane filtration for treatment of H2S scavenging wastewater from offshore oil and gas production. Sep. Purif. Technol. 2021, 277, 119641. [Google Scholar] [CrossRef]
- Vikrant, K.; Kailasa, S.K.; Tsang, D.C.; Lee, S.S.; Kumar, P.; Giri, B.S.; Singh, R.S.; Kim, K.H. Biofiltration of hydrogen sulfide: Trends and challenges. J. Clean. Prod. 2018, 187, 131–147. [Google Scholar] [CrossRef]
- Nhut, H.H.; Le, L.T. Removal of H2S in biogas using biotrickling filter: Recent development. Process Saf. Environ. Prot. 2020, 144, 297–309. [Google Scholar] [CrossRef]
- Khairulin, S.; Kerzhentsev, M.; Salnikov, A.; Ismagilov, Z.R. Direct selective oxidation of hydrogen sulfide: Laboratory, pilot and industrial tests. Catalysts 2021, 11, 1109. [Google Scholar] [CrossRef]
- Zhu, W.; Ye, H.; Zou, X.; Yang, Y.; Dong, H. Analysis and optimization for chemical absorption of H2S/CO2 system: Applied in a multiple gas feeds sweetening process. Sep. Purif. Technol. 2021, 276, 119301. [Google Scholar] [CrossRef]
- Jafarinejad, S. 5-control and treatment of air emissions. In Petroleum Waste Treatment and Pollution Control; Butterworth-Heinemann: Oxford, UK, 2017; pp. 149–183. [Google Scholar]
| Category | Examples of Sources | Main Emitted Gases | Typical Emission Levels | Impact on Air Quality |
|---|---|---|---|---|
| Natural | Swamps, wetlands, thermal springs, volcanic activity, anaerobic decomposition | H2S, NH3, VSC (dimethyl sulfide, methyl mercaptan) | 0.00011–0.00033 ppm (H2S in wetland air); up to 7–8 ppm near geothermal sources | Low to moderate; depends on environmental conditions |
| Anthropogenic | Wastewater treatment plants, agriculture (livestock, fertilizers), chemical industry, oil refineries | H2S, NH3, VSC, volatile organic compounds (VOCs) | 5–18 ppm (NH3 in pig farms); 200–1300 mg/m3 (H2S in wastewater treatment plants) | High; contributes to strong odors in urban and rural areas |
| Hybrid | Rivers polluted by urban and industrial discharges, waste treatment in landfills, biogas production | H2S, NH3, VSC, gas mixtures depending on contamination | Up to 16 ppm NH3 in polluted rivers; VSC in tens of ppb in landfills | Variable; depends on pollution levels and proximity to emission sources |
| Reference | Application | Exposure Time/Concentration |
|---|---|---|
| Odor perception threshold in Toxicological profile AFTS [31] | International reference | 0.0005 to 0.13 ppm |
| WHO guideline for tolerable concentration in air | International reference | 150 µg/m3 (108 ppm) |
| Odor perception threshold in Spain NTP 320 [61] | Spain | 0.0081 ppm |
| TA Luft Technical Instruction on Air Quality Control | Germany | 8 µg/m3 (0.006 ppm) |
| Air Quality Guide | New Zealand | 7 µg/m3 (0.005 ppm) |
| Maximum permissible level in Resolution 610 2010 [57] | Colombia | 7 µg/m3 (0.005 ppm) |
| Maximum permissible level in Resolution 1541 de 2013 [58] | Colombia | 1 h average exposure/30 µg/m3 (0.021 ppm) |
| 24 h average exposure/7 µg/m3 (0.005 ppm) |
| Study | Dominant VSCs | Concentration Ranges | Key Formation Factors |
|---|---|---|---|
| [154] | H2S, MeSH | H2S: 7 to 39,000 g/m3 | Organic matter, pH, Fe content |
| [148] | DMDS, DMS | Total VSCs: 561.89 mg/dry kg (summer) | Higher ambient temperatures |
| [149] | H2S, MT | H2S: 361 ± 101 g/ton, MT: 82 ± 76 g/ton | Higher ambient temperatures, aeration |
| [155] | MT | MT: 4 to 40 nM in sediment | Anaerobic mineralization, H2S concentration |
| [156] | H2S | Maximum 250 ppm | Higher ambient temperatures |
| [157] | DMS, , DMDS | DMS: 608.5 g/m3, : 658.5 g/m3, DMDS: 857.8 g/m3 | Higher ambient temperatures |
| Formation Mechanism | Contributing Factors | Seasonal Influence | Control Measures |
|---|---|---|---|
| Anaerobic degradation | Organic matter content, sulfate concentration | Higher in warmer seasons | Aeration, chemical dosing |
| Methylation of H2S | H2S concentration, methyl donors | Temperature dependent | Sulfide control, pH adjustment |
| Biological sulfate reduction | Sulfate concentration, organic substrate | Enhanced in warmer temperatures | Sulfate removal, bioaugmentation |
| Thermal decomposition of sulfur compounds | Temperature, sulfur-containing organics | More significant in dry, hot seasons | Temperature control, feedstock management |
| Method | Cost | Sensitivity | Practicality | References |
|---|---|---|---|---|
| GC-SCD | High | 120 ppt–2 ppb | Laboratory-based, specialized equipment | [64,194,195] |
| TD-GC-SCD | High | 0.10–5.26 µg/m3 | Laboratory-based, broader VSC analysis | [64] |
| TD-GC-MS | High | Trace level (µg/m3 to sub-ppb) | Laboratory-based, rapid, precise, integrated olfactory evaluation | [157,196,197,204,205] |
| UPLC-MS | High | Submicromolar (below µM range) | Laboratory based, rapid, accurate, ideal for complex samples | [199,206,207] |
| Satellite Remote Sensing | Moderate–High | Indirect NH3/H2S estimation | Large-scale coverage, good for regional assessments but indirect measurement | [200,201] |
| UV-visible Chemiluminescence | Moderate–High | High (ppb–ppm range) | Specialized instrumentation, limited field portability | [202] |
| Open-path Tunable Diode Laser Absorption | Moderate–High | High temporal resolution (ppb) | Excellent field applicability, good temporal resolution, field deployable | [203] |
| Air Dispersion Models | Moderate | Variable (depends on input data) | Computational approach, requires input data, useful for scenario modeling | [198] |
| Method | Cost | Sensitivity | Practicality | References |
|---|---|---|---|---|
| Mid-infrared Sensor (Hollow Waveguide with UV) | High | ~207 ppbv (H2S, SO2) | Portable, rapid, suitable for simultaneous multi-gas detection | [208] |
| Cavity-enhanced Near-infrared Laser Absorption Spectroscopy | High | ppbv level | Fast response, minimal interference, precise for fuel streams | [209] |
| Quartz-enhanced Photoacoustic Spectroscopy (QEPAS) | High | 10–450 ppb | Ultra fast, highly sensitive, suitable for real-time analysis, minimal cross-interference | [210,211] |
| Cavity Ring-Down Spectroscopy (CRDS) | High | ppb level | Real-time monitoring, highly sensitive, minimal VOC and humidity interference | [212] |
| Method Category | Method | Cost | Sensitivity (Detection Limit) | Practicality | References |
|---|---|---|---|---|---|
| Electrochemical Sensors | Triple-pulse amperometry | Moderate–Low | ppb to ppm levels | Portable, rapid detection, resistant to sulfur poisoning | [214] |
| Electrochemical impedance spectroscopy | Moderate–Low | ppb levels | Highly sensitive, suitable for toxic gases, portable | [215] | |
| Flame Photometric Detectors (FPD) | Multiple flame FPD (mFPD) | High | 4 × 10−11 g S/s | High sensitivity, resistant to interference | [217] |
| Quartz rod-enhanced FPD (qFPD) | High | 0.3–0.5 pg S/s | Exceptionally sensitive, stable signal | [218] | |
| Microcountercurrent FPD (microcc-FPD) | High | 3 ng S/s | Ultrafast chromatographic separations, high precision | [219] | |
| Pulsed FPD (PFPD) | High | 2.37–4.89 pg | Excellent performance, stable at varying concentrations | [220] | |
| Colorimetric and Smartphone Methods | 3D-printed paper sensors | Low | ppm levels (NH3, H2S) | Affordable, portable, suitable for rapid field analysis | [221] |
| QR code-integrated sensors (QRsens) | Low | ppm levels (NH3, H2S) | Quick field measurements, accessible via smartphones | [222] | |
| Nanocatalyst-enhanced assays | Moderate–Low | 7.5 nM (H2S) | Ultrasensitive, rapid response, field compatible | [223] | |
| Smartphone-based silver nanoparticles | Low | 200 mg/L (NH3) | User friendly, portable | [224] |
| Methodology | Working Principle | Application | Advantages | Limitations | Effectiveness |
|---|---|---|---|---|---|
| GC-SCD | Gas chromatography coupled with sulfur chemiluminescence detection. | H2S, VSC | High selectivity and sensitivity in various matrices [236].Suitable for low concentrations [195]. | Requires advanced equipment prone to frequent maintenance and component corrosion [236].Non-linear responses at low concentrations for some compounds [195]. | High selectivity and sensitivity for sulfur-containing compounds; very low detection limits. |
| TD-GC-SCD | Gas chromatography with thermal desorption and chemiluminescence detection. | H2S, VSC | Detects a wide range of sulfur compounds [237].High sensitivity [64]. | For complex matrices like straight-run gas oils, high-resolution mass spectrometry may be necessary [238]. | Similar to GC-SCD, optimized for volatile compounds using thermal desorption; excellent for trace-level sulfur compounds. |
| GC-MS | Gas chromatography coupled with mass spectrometry. | H2S, VSC | Qualitative and quantitative analysis capabilities [239].Suitable for odor studies with simultaneous olfactory evaluation [196]. | Long analysis times [240].Requires specialized equipment and expertise [241]. | Broad-spectrum identification and quantification; high precision. |
| TD-GC-MS | Gas chromatography with thermal desorption and mass spectrometry. | H2S, VSC | Optimized detection limits for trace volatile organic compounds [204].Short collection times [205]. | Susceptible to contamination risk [242].May be more affected by matrix effects than other methods [204]. | Enhanced sensitivity for highly volatile compounds; short sampling times. |
| Laser Spectroscopy (NIR, QEPAS) | Measurement based on laser light interaction with gaseous compounds, enhancing sensitivity with optical cavities. | H2S, NH3 | Real-time continuous monitoring [243].High sensitivity and low detection limits [210]. | Gas flow and temperature effects at high excitation powers [244]. Requires frequent calibration and long response times [243]. | Capable of ppb or sub-ppb detection in real time; ideal for continuous monitoring of one/few specific compounds. |
| Electrochemical Sensors (EIS, Amperometric) | Based on detecting changes in current or impedance in response to gas presence. | H2S, NH3 | Compact, portable, and cost-effective [245].Ease of operation and simplicity of construction [245]. | Sensitive to electrode contamination [246]. Potential for nonspecific impedance changes [246]. | Portable and inexpensive, but less accurate and prone to drift under complex conditions. |
| FPD and mFPD Detectors | Based on light emission from flames interacting with sulfur compounds. | H2S, VSC | High sensitivity for sulfur compounds [219].Low detection limits [219]. | Requires controlled combustion conditions, which can be challenging in unstable systems [247].Flame stability issues in certain environments [247]. | High sensitivity to sulfur species; excellent detection limits, though they require stable flame conditions. |
| UPLC-MS | Ultra-performance liquid chromatography coupled with mass spectrometry for NH3. | NH3 | High precision for volatile nitrogenous compounds [206].Rapid analysis with high sensitivity [207]. | Detection of other compounds may obscure the detection of NH3 [207]. | Highly accurate for volatile nitrogen compounds (especially ammonia); very fast and sensitive. Less common for volatile sulfur. |
| TDLAS | Tunable diode laser absorption spectroscopy to measure spectral absorption of specific gases. | NH3, H2S | High precision in monitoring continuous emissions [248].Suitable for harsh environmental conditions [248]. | Inherent sensitivity limitations due to infrared absorption principles [249]. | Excellent for continuous measurements, high sensitivity, robust in industrial environments. |
| Colorimetric Techniques and Smartphone Detection | Color changes induced by chemical reactions detected via optical platforms like smartphone cameras. | NH3, H2S | Affordable and portable technology [221].Suitable for rapid, low-cost measurements [221]. | Limited selectivity and detection limits compared to laboratory instruments [250].Detection limits ranging from 1 to 200 mg/L for NH3 and H2S [224]. | Very accessible and portable; lower selectivity and detection limits compared to advanced instrumentation. |
| CRDS (Cavity Ring-Down Spectroscopy) | Laser absorption through multiple reflections in a closed cavity for NH3 and H2S analysis. | NH3, H2S | Ideal for real-time monitoring with high sensitivity [251].Excellent linearity over a wide range of NH3 concentrations [212]. | Slight humidity effects at extreme conditions [212]. | Highly sensitive real-time measurements; ideal for trace gases. |
| Wind Tunnels and Passive Samplers | Devices that capture gases in open fields analyzed using dispersion models. | NH3 | Cost-effective and easy to implement over large areas [252].High precision and fast response [253]. | Dependent on climatic conditions such as wind speed and temperature [252].May underestimate NH3 concentrations under low wind speed conditions [254]. | Useful to estimate emissions over large areas; heavily dependent on weather (wind speed, temperature). Less precise for low wind speeds. |
| H2S Removal Method | Principle | Medium Used | Advantages | Disadvantages |
|---|---|---|---|---|
| Absorption | Removal through solubility or reaction capability in a liquid medium. | Physical: sulfolane. Chemical: alkylamines. | High efficiency in systems with high gas flow rates. | Requires solvent regeneration. |
| Adsorption | Separation by attachment to the surface of a solid through weak intermolecular interactions. | Physical: activated carbon. Chemical: activated carbon impregnated with strong bases (NaOH, KOH). | Low operating cost. Wide availability of adsorbents. | Adsorbent saturation requires frequent replacement. Chemical adsorption implies additional costs. |
| Membrane Separation | Segregation by forcing passage through a porous membrane due to differences in solubility and diffusion. | Polymeric membranes: polysulfones and polyamides, | High modularity and ability to operate in remote areas. Low environmental impact. | Lower efficiency compared to other methods. High installation costs. |
| Claus Process | Conversion to elemental sulfur through partial combustion and the catalytic reaction of resulting products. | Oxygen, catalyst (Al2O3). | High recovery of elemental sulfur. | Requires controlled operating conditions. Secondary emissions need to be controlled. |
| Liquid Catalytic Oxidation | Conversion to sulfuric acid in a liquid medium using an oxidizing agent and a catalyst. | Oxidizing agent (O2), catalyst (Fe2O3). | High selectivity and efficiency in liquid streams. | Risk of corrosion. Difficult operation handling. |
| Biofilter | Conversion to sulfuric acid or elemental sulfur through aerobic biological oxidation by sulfur-oxidizing microorganisms. | Packed-bed bioreactor. | Eco-friendly and low operating cost. Low energy consumption. | Sensitivity to environmental conditions. Requires constant monitoring. Can collapse. |
| Biopercolator | Conversion to sulfuric acid or elemental sulfur through aerobic biological oxidation in a three-phase system. | Packed-bed bioreactor. | Improves biofilter efficiency by maintaining operating conditions. | Complexity in design and operation. Requires liquid effluent management. Can collapse. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meléndez-Plata, G.; Mastrogiacomo, J.R.A.; Castellanos, M.L.; Romero, J.P.; Hincapié, V.; Lizcano, H.; Acero, J.D.; Villegas-Torres, M.F.; Gómez, J.M.; Cruz, J.C.; et al. Malodorous Gases in Aquatic Environments: A Comprehensive Review from Microbial Origin to Detection and Removal Techniques. Processes 2025, 13, 1077. https://doi.org/10.3390/pr13041077
Meléndez-Plata G, Mastrogiacomo JRA, Castellanos ML, Romero JP, Hincapié V, Lizcano H, Acero JD, Villegas-Torres MF, Gómez JM, Cruz JC, et al. Malodorous Gases in Aquatic Environments: A Comprehensive Review from Microbial Origin to Detection and Removal Techniques. Processes. 2025; 13(4):1077. https://doi.org/10.3390/pr13041077
Chicago/Turabian StyleMeléndez-Plata, Gabriela, Jesus R. A. Mastrogiacomo, Martha L. Castellanos, Juan P. Romero, Victor Hincapié, Héctor Lizcano, Juan D. Acero, María Francisca Villegas-Torres, Jorge M. Gómez, Juan C. Cruz, and et al. 2025. "Malodorous Gases in Aquatic Environments: A Comprehensive Review from Microbial Origin to Detection and Removal Techniques" Processes 13, no. 4: 1077. https://doi.org/10.3390/pr13041077
APA StyleMeléndez-Plata, G., Mastrogiacomo, J. R. A., Castellanos, M. L., Romero, J. P., Hincapié, V., Lizcano, H., Acero, J. D., Villegas-Torres, M. F., Gómez, J. M., Cruz, J. C., & Reyes, L. H. (2025). Malodorous Gases in Aquatic Environments: A Comprehensive Review from Microbial Origin to Detection and Removal Techniques. Processes, 13(4), 1077. https://doi.org/10.3390/pr13041077








