Abstract
Widespread use of pesticides in agriculture causes adverse impacts on non-target organisms and environmental pollution. Efficient and sustainable pesticide removal alternatives must be developed to reduce pesticide environmental impacts. Recently, bioremediation based on immobilized microorganisms has been proposed as an environmentally friendly and cost-effective approach for pesticide degradation in water. Agro-industrial wastes are produced in large quantities in crop fields; their high availability, low cost, and potential for reuse make them ideal support materials for microbial immobilization. This systematic review, conducted through the PRISM 2020 methodology, compiles recent research on using agro-industrial waste to immobilize microorganisms for pesticide degradation. The identified studies highlight corn straw as the most studied agro-industrial waste, while the organophosphorus insecticides, chlorpyrifos, and methyl parathion were the most representative pesticides; in the identified studies, pesticide degradation was conducted mainly by bacteria of the Acinetobacter, Bacillus, and Pseudomonas genera. Overall, microbial immobilization significantly enhanced pesticide degradation, rendering it a viable bioremediation strategy for pesticide-contaminated water.
1. Introduction
Water is a vital natural resource for all living organisms [1]. Water shortage and pollution are among the most important environmental concerns worldwide [2]. Various anthropogenic activities (e.g., extractive, productive, domestic, and industrial processes) cause water pollution due to the direct and indirect release of pollutants of different nature to water bodies [3,4]. Most relevant pollutants found in water include heavy metals [5], dyes [6], hydrocarbons [7], pesticides [8], and, more recently, emerging pollutants such as pharmaceuticals [9]. The presence of pollutants in water bodies reduce its quality and is a threat for ecosystems and human health [10].
Agricultural activities are among the more environmentally impactful economic activities due to extensive land and freshwater use and the application of different agrochemicals such as fertilizers and pesticides on a broad scale [11,12]. Pesticides are industrially produced molecules widely used in agricultural activities to protect crops from pests [13]. The use of pesticides has risen dramatically in many countries due to the need to guarantee sufficient food and raw materials supply for an ever-growing population and expanding industrial activities [14]. In 2022, the total amount of pesticides employed in the agricultural sector was estimated in 3.69 million tons, mainly in the Americas and Asia, with an average application range in crops of 2.37 kg/ha [15], while in the United States, the Environmental Protection Agency (EPA) reports that approximately 1.1 billion pounds of chemical pesticides are applied each year [16].
Pesticides often contaminate soil, water, and air, resulting in long-term environmental damage and significant risks to human health [17,18,19]. The World Health Organization (WHO) estimates that pesticides are responsible for approximately three million cases of poisoning and 200,000 deaths annually [20]. Furthermore, intensive use of pesticides has led to surface and groundwater contamination [21,22]. Water contamination is a particularly urgent issue, as pesticide residues can persist for extended periods, harming both aquatic ecosystems and human populations dependent on polluted water sources [23,24]. For example, the EPA has set a maximum contaminant level of 0.1 micrograms per liter (µg/L) for the herbicide atrazine in drinking water, underscoring concerns over pesticide residues in water supplies. Similarly, the European Union has established strict limits on pesticide residues in food for infants and young children (0.01 mg/kg) and in drinking water (0.1 µg/L for individual pesticides and 0.5 µg/L for the total pesticide presence at any given time) to safeguard public health [25,26,27]. Due to the environmental and human health impacts derived from the intensive use of pesticides, different strategies and regulations have been proposed to evaluate their environmental and human health risks and the prohibition of the use of highly toxic and persistent pesticides, regulate their commercialization, and promote the adequate management of their residues. Among the most important, the guidelines established by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO), the Stockholm Convention, the Rotterdam Convention, the European Union Regulation No. 1107/2009, the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA) of the United States, and the Environmental Protection Agency (EPA) stand out. Despite these regulatory efforts, pesticide contamination remains a global challenge, particularly in developing nations where pesticide use continues to rise without adequate regulation [28]. Therefore, developing effective and sustainable methods for pesticide degradation is essential to mitigate their harmful effects and protect water resources.
The growing problem of pesticides in aquatic environments is widespread globally, as they accumulate in sediments and aquatic organisms, causing direct adverse effects and spreading through food webs [29]. Their presence in water bodies results from agricultural runoff, industrial wastewater discharge, and their leaching into the soil [30]. Soluble pesticides are carried by water, especially during rainfall, reaching surface water, and may eventually infilter into groundwater [31]. Several methods—physical, chemical, and biological—have been developed to remove water contaminants [32]. Each method has its proper advantages and limitations, with the choice of the removal technique depending on the nature and extent of contamination, cost, and process efficiency [33].
Additionally, agro-industrial waste generation during crop production processes is a global issue, as these wastes are often inadequately processed or disposed of, contributing to environmental pollution [34]. However, agro-industrial residues have significant potential for use in processes aimed at restoring altered environmental conditions, such as serving as support for the immobilization of microorganisms capable of degrading pesticides [35]. Microbial immobilization technology has rapidly advanced in recent years for water decontamination due to its high stability, rapid reaction rate, and high activity [36]. The selection of an appropriate support material is crucial for this technology, and the materials chosen should be low cost, widely available, and efficient for pesticide removal. Consequently, using agro-industrial waste as a support for immobilizing microorganisms to degrade pesticides in water is an effective, feasible, and environmentally friendly biotechnological alternative [33].
There are several reviews in the literature discussing the potential use of agro-industrial wastes for the immobilization of microorganisms in xenobiotic degradation, such as pesticides. However, a systematic review on the immobilization of microorganisms on agro-industrial waste to enhance the efficiency of pesticide degradation in aquatic environments has not been carried out. Such a review would help identify the potential of this biological treatment technology and analyze the existing knowledge, contributing to the development of effective remediation strategies for water bodies. This study aimed to systematically collect and analyze recent scientific research about the use of agro-industrial waste as support for microorganism immobilization as an alternative to conventional water treatment technologies for the degradation of pesticide pollutants in water. The analysis of the collected data lets us answer whether the immobilization process is a feasible alternative to improve the efficiency of pesticide degradation through microbial systems. This manuscript provides a more holistic perspective on how agro-industrial residues can be efficiently utilized in pesticide bioremediation, identifying new research opportunities and helping to establish novel practical applications.
2. Materials and Methods
2.1. Systematic Literature Review
This systematic literature review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) [37] and using specialized academic databases, including Google Scholar, ScienceDirect, and SpringerLink. The search focused on identifying scientific articles using predefined keywords (microorganism + immobilization + pesticide + agro-waste + water).
2.2. Inclusion Criteria
The inclusion criteria were based on selecting articles that provided relevant and up-to-date information related to the specified keywords, including agro-industrial waste (used as supports), microorganisms involved in biodegradation processes for pesticides in aquatic environments, as well as data on the percentage and the lapse of pesticide degradation, within the established search period, 2013 to 2024.
2.3. Exclusion Criteria
Articles that did not align with the focus of the study were excluded, including those published outside the specified period of 2013–2024, those addressing xenobiotic compounds unrelated to pesticides, supports that were not agro-industrial waste, studies not involving aquatic environments, non-microbial biodegradation, or processes that did not include immobilization.
A publication period of 11 years, from 2013 to 2024, was chosen for the identification of selected articles that were included in this study due to the significant increase in research in this field in the last few years. This timeframe ensures the inclusion of the most current and pertinent information on pesticide biodegradation through microorganisms immobilized in agro-industrial waste as support.
2.4. Data Matrix from the Systematic Literature Review
From the scientific documents gathered, a database or matrix was created to outline the key points of interest for this study: the type of agro-industrial waste used and the pretreatment applied, the pesticide degraded, the pesticide type according to the target organism, the name of the microorganism, and the microorganism type (bacterium, fungus, or algae), pesticide concentration (mg/L), the degradation percentage by free cells (non-immobilized), the degradation percentage by immobilized cells, the improvement in pesticide degradation, degradation time, and the bibliographic reference. Key data extracted from identified studies are shown in Table S1.
2.5. Determination of the Improvement in the Degradation Process
The diversity of pesticide molecules, agro-industrial wastes, microorganisms, and experimental conditions used in the identified studies make it difficult to compare and identify the most efficient systems for pesticide degradation with microorganisms immobilized in agro-industrial waste. To overcome this disadvantage, it was proposed to calculate the improvement in the degradation process and degradation rate to identify the waste materials and microbial systems with higher efficiency in degradation of pesticides.
The degradation percentage of the pesticide using free cells (non-immobilized) and the degradation percentage using immobilized cells were identified for each study. To determine the improvement in the pesticide degradation process, the following formula was used:
where
%DII = ((%Dimm/%Dfree) − 1) (100)
- %DII is the degradation improvement index;
- %Dimm is the degradation percentage of immobilized cells;
- %Dfree is the degradation percentage of free cells.
Additionally, the degradation rate (DR) was calculated for each of the studies, which measures the speed at which the pesticide degrades, or its concentration decreases over time. The following formula was used to obtain this value:
where
DR = (Ci − Cf)/tD
- DR is the degradation rate;
- Ci is the initial concentration;
- Cf is the final concentration;
- tD is the degradation time.
With the obtained values, a comparison was made to identify which of the studies showed the highest degradation efficiency.
2.6. Statistical Analysis
This review identified 14 studies using agro-industrial waste as support material to immobilize microorganisms for pesticide degradation in water. These studies reported 17 pesticide degradation experiments by free and immobilized microbial cells; the dispersion data (standard deviation) of just nine experimental pesticide degradation conditions were available in the main text, tables, or graphics. Therefore, t-Student tests were used to analyze significant differences between the average degradation percentage of immobilized and free microbial cells in each study. Moreover, a t-Student test was also conducted to determine significant differences between the average pesticide degradation percentages reported for free and immobilized microbial cell systems of all nine pesticide degradation experiments [38]. Statistical analyses were conducted in GraphPad Prism v8.0.1 software (GraphPad Software Inc., San Diego, CA, USA).
3. Results
3.1. Data Matrix from the Systematic Literature Review
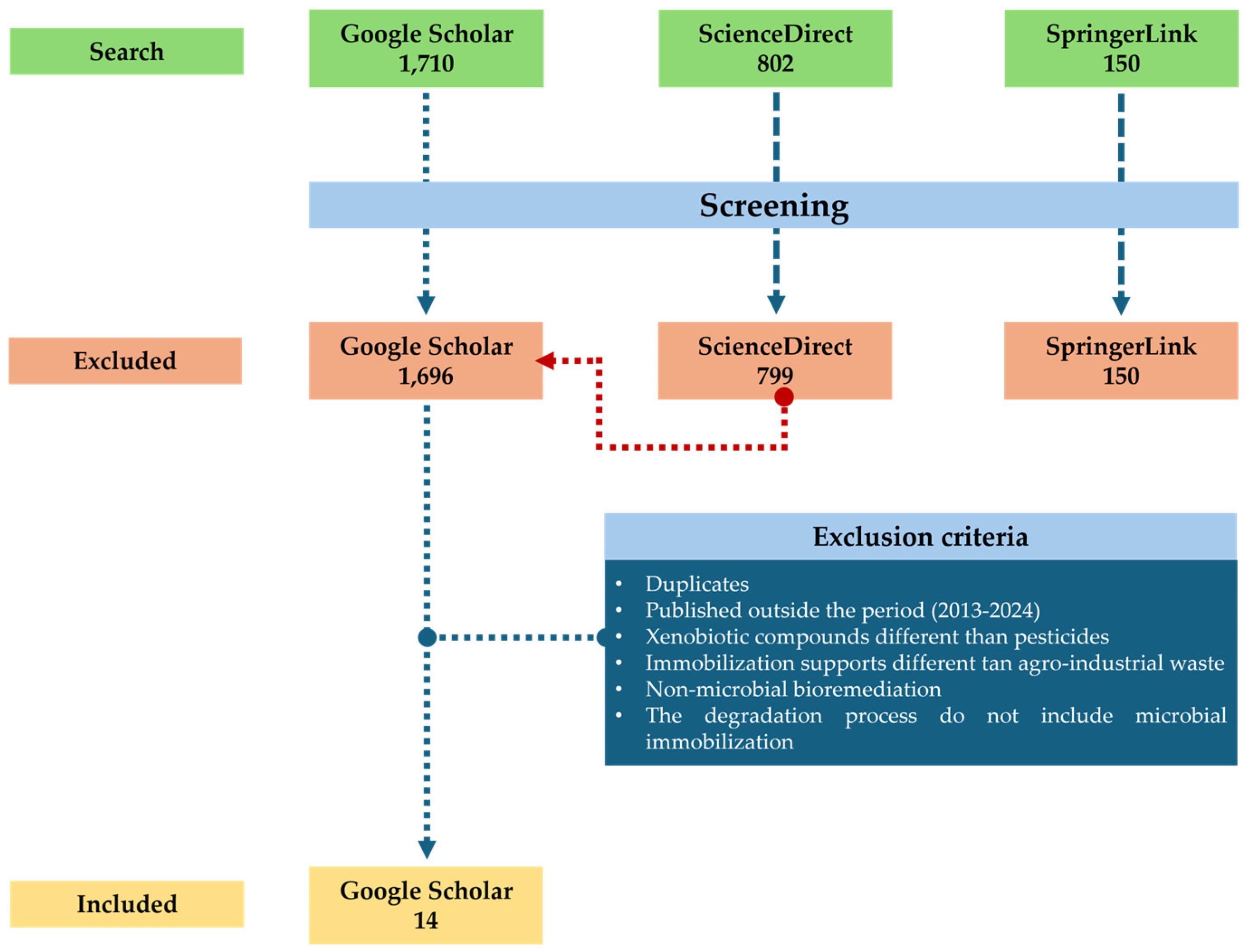
Based on the information obtained from the systematic search across three databases (Google Scholar, Science Direct, and Springer Link), using the search equation (microorganism + immobilization + pesticide + agro-waste + water) for the period 2013–2024, a total of 1710 articles were found on Google Scholar. Of these, only 14 articles (0.82%) met the inclusion criteria (Table 1), while the remaining 1695 were excluded for failing to meet the established criteria. In Springer Link, 150 articles were found. However, none of these articles met the inclusion criteria. In ScienceDirect, 802 articles were identified, of which only three met the inclusion criteria; however, these three studies had already been identified in Google Scholar, so they were discarded (Figure 1). A data matrix was generated in Windows-Excel with the information of all the identified articles. After filtering them according to the inclusion and exclusion criteria, the selected articles were reviewed by at least three authors independently.
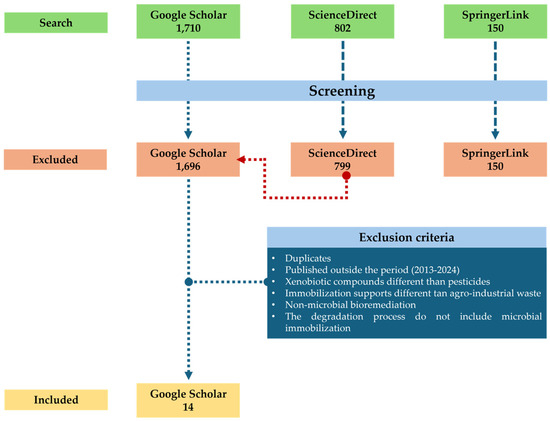
Figure 1.
Flowchart of the procedure of the systematic review.
The main characteristics of the 14 studies identified are showed in Table S1, most of the studies were conducted in China (50%), followed by Mexico (14.3%) and Thailand (14.3%), and the higher number of studies (78.6%) were published in the last five years (2020–2024), with 2024 being the year with the highest number of studies published with three studies (21.4%).
3.2. Agro-Industrial Wastes Identified as Microbial Supports in Pesticide Degradation
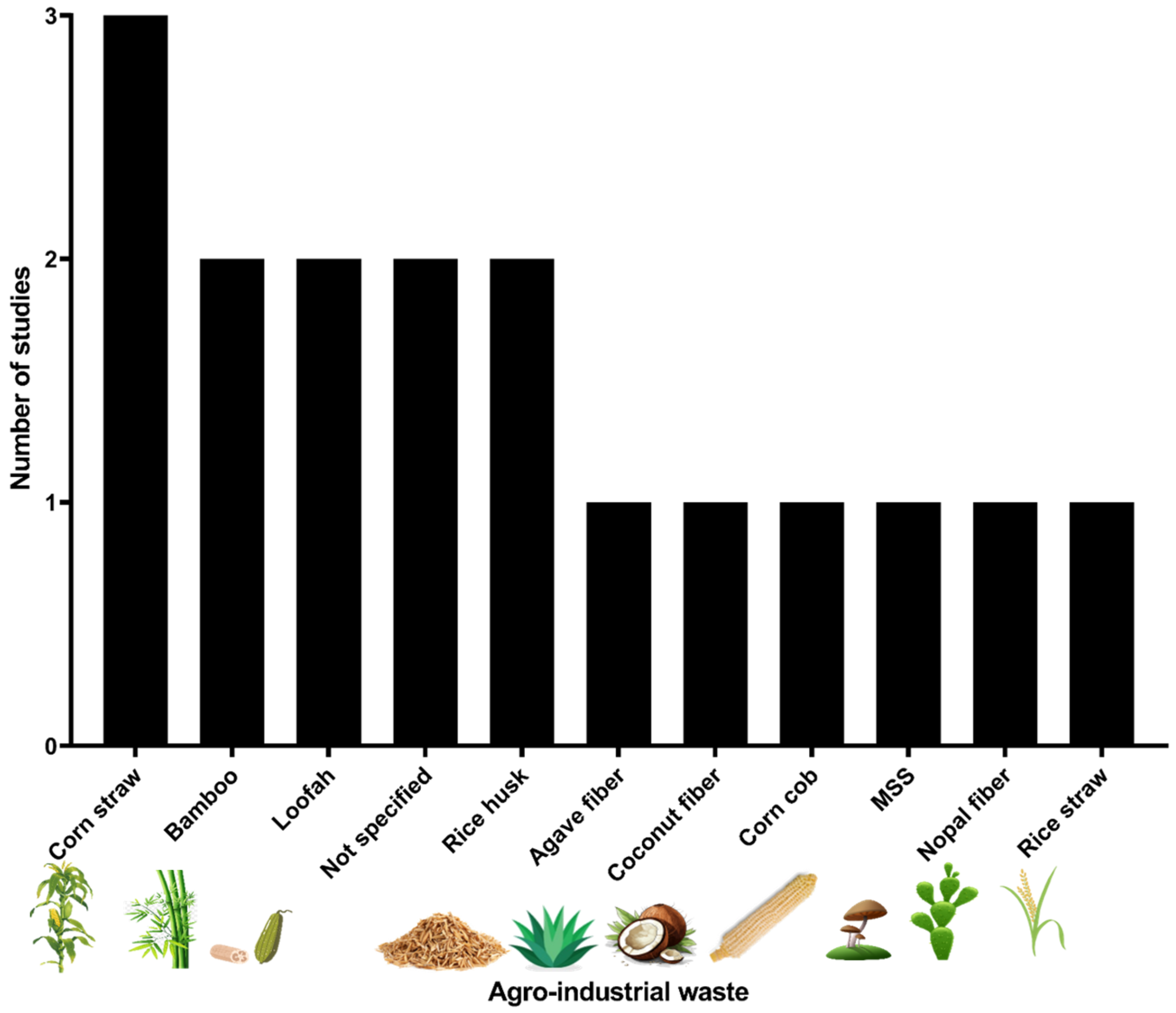
Regarding agro-industrial waste, in the 17 pesticide degradation experiments a total of ten types of materials were identified (Table S1). Corn straw was the most representative agro-industrial waste with three studies (17.6%), followed by bamboo, loofah, and rice husk; additionally, an unspecified agricultural waste was reported by Mojiri et al. [39]. Figure 2 illustrates the 11 distinct agro-industrial residues evaluated in the studies identified.
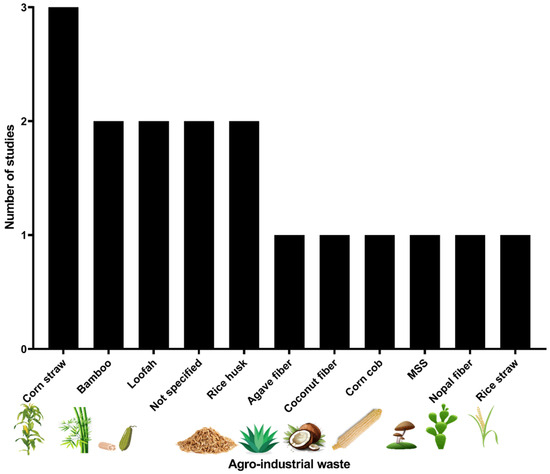
Figure 2.
Agro-industrial residues identified used to immobilize microorganisms in the degradation of pesticides. MSS: Mushroom spent substrate.
3.3. Pretreatment of Agro-Industrial Waste
It is important to note that, out of the 17 pesticide degradation experiments in the 14 studies identified, 12 used agro-industrial wastes that were subjected to a pyrolysis process to be converted into biochar as a pretreatment stage, with temperatures ranging from 450 to 850 °C. This means that 70.6% of the support materials underwent this process (Table 1).

Table 1.
Articles included in the systematic review and degradation improvement index (DII).
Table 1.
Articles included in the systematic review and degradation improvement index (DII).
| Agro-Industrial Waste (Support) | Pretreatment | Name of Pesticide | Type of Pesticide | Microorganism | Type of Microorganism | Concentration of Pesticide (mg/L) | Degradation Time (Hours) | Pesticide Degradation (%) Free Cells | Pesticide Degradation (%) Immobilized Cells | Degradation Improvement Index (DII) * | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Loofah (Luffa cylindrica) | NaOH 5% and anthraquinone at 80 °C for 3 h | Methyl parathion | Insecticide | Bacterial consortium 1 | Bacteria | 25 | 72 | 54.9 ± 3.8 | 98.5 ± 0.4 | 79.4 | [40] |
| Loofah (Luffa cylindrica) | Coumaphos | 5 | 62 ± 23 | 100 | 61.3 | ||||||
| Nopal fiber | Washed with water and dried at 50 °C | Methyl parathion | Insecticide | Burkholderia cenocepacia CEIB S5-2 1 | Bacteria | 50 | 1 | 7.4 | 100 | 1244 | [41] |
| Agave fiber | Methyl parathion | ||||||||||
| Corn straw | Pyrolysis 700 °C | Atrazine | Herbicide | Acinetobacter lwoffii DNS32 2 | Bacteria | 100 | 12 | 88.6 | 100 | 12.9 | [42] |
| Corn straw | Pyrolysis 700 °C | Atrazine | Herbicide | Arthrobacter sp. ZXY-2 3 | Bacteria | 50 | 1 | 90 ± 16.4 | 100 | 11.1 | [43] |
| Bamboo | Pyrolysis | Dimethomorph | Fungicide | Bacillus cereus WL08 1 | Bacteria | 50 | 72 | 66.9 ± 2.4 | 96.9 | 44.7 | [44] |
| Spent mushroom substrate | Pyrolysis 600 °C | Thifensulfuron-methyl | Herbicide | Serratia marcescens N80 4 | Bacteria | 50 | 48 | 83.5 ± 3.6 | 89.4 ± 3.1 | 7.1 | [45] |
| Coconut fiber | Pyrolysis | Paraquat | Herbicide | Pseudomonas putida 5 | Bacteria | 30 | 48 | 6.7 | 95.8 | 1329 | [46] |
| Not specified | Pyrolysis | Chlorpyrifos | Insecticide | Chlorella vulgaris 6 | Alga | 0.5 | 72 | 63.9 | 87.2 | 36.5 | [39] |
| Not specified | Cypermethrin | 64.2 | 93.4 | 45.5 | |||||||
| Rice straw | Washed with water and dried at 70 °C | Diuron | Herbicide | Bacillus subtilis DU1 1 Acinetobacter baumannii DU 1 Pseudomonas sp. DUK 1 | Bacteria | 20 | 36 | 70 ± 8.6 | 100 | 42.9 | [47] |
| Bamboo | Pyrolysis 850 °C | Thiamethoxam | Insecticide | Chryseobacterium spH5 7 | Bacteria | 14 | 24 | 25 | 74.0 ± 2.4 | 196.2 | [48] |
| Corn cob | Pyrolysis | Paraquat | Herbicide | Pseudomonas putida TISTR 1522 8 | Bacteria | 26 | 24 | 12.1 | 15.8 | 30.2 | [49] |
| Rice husk | Pyrolysis 450–500 °C | Chlorpyrifos | Insecticide/acaricide | Bacillus H27 1 | Bacteria | 25 | 168 | 89.6 ± 1 | 97.4 ± 0.5 | 8.7 | [50] |
| Rice husk | Pyrolysis 500 °C | Chlorpyrifos | Insecticide/acaricide | Aeromonas veronii 1 | Bacteria | 30 | 24 | 77.9 ± 1.6 | 96.5 ± 1.1 | 23.9 | [51] |
| Corn straw | Pyrolysis 500 °C | Imazethapyr | Herbicide | Bacillus cereus MZ-1 2 | Bacteria | 200 | 18 | 52.7 | 79.9 | 51.7 | [52] |
1 Isolated from soils. 2 Not specified. 3 Isolated from Jilin Pesticide Plant (China). 4 Sediment and activated sludge in a pesticide produce factory (China). 5 Purchased from the Thailand Institute of Scientific and Technological Research. 6 Obtained from a photobioreactor in the laboratory (Amin-Azma Research Center). 7 Activated sludge. 8 Purchased from Thailand Institute of Scientific and Technological Research. * This work, calculated through Equation (1).
3.4. Pesticides Identified and Degraded by Microorganisms Immobilized in Agro-Industrial Waste
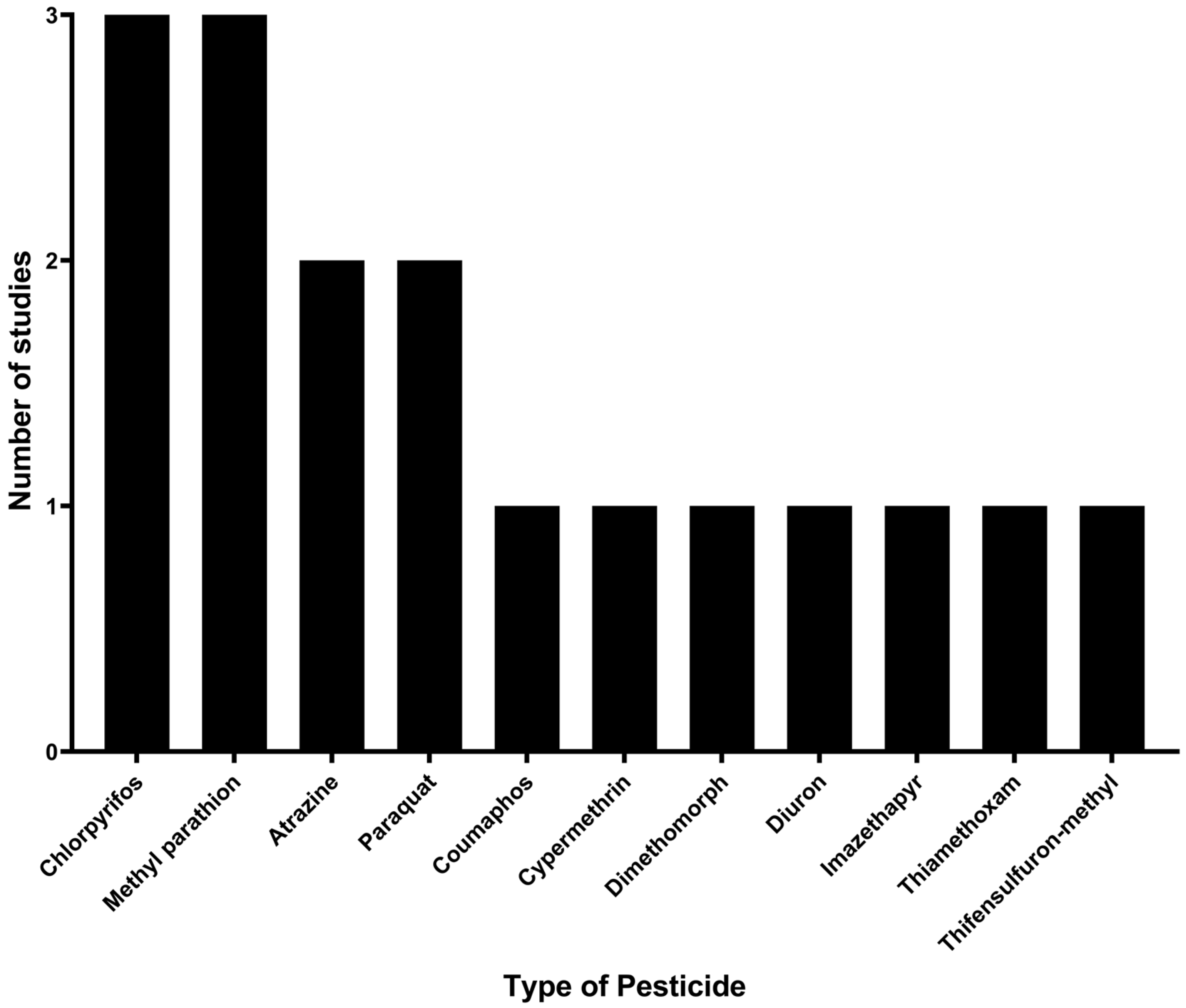
Regarding pesticides, a total of 11 types were identified based on their chemical composition: atrazine, chlorpyrifos, coumaphos, cypermethrin, dimethomorph, diuron, imazethapyr, methyl parathion, paraquat, thiamethoxam, and thifensulfuron-methyl. Among these, the pesticides with the highest number of studies on degradation by immobilized microorganisms were chlorpyrifos and methyl parathion, with three studies (17.6%) each, followed by atrazine and paraquat, with two studies (11.8%) each. The remaining pesticides (coumaphos, cypermethrin, dimethomorph, diuron, imazethapyr, thiamethoxam, and thifensulfuron-methyl) were each analyzed in only one study, as shown in Figure 3.
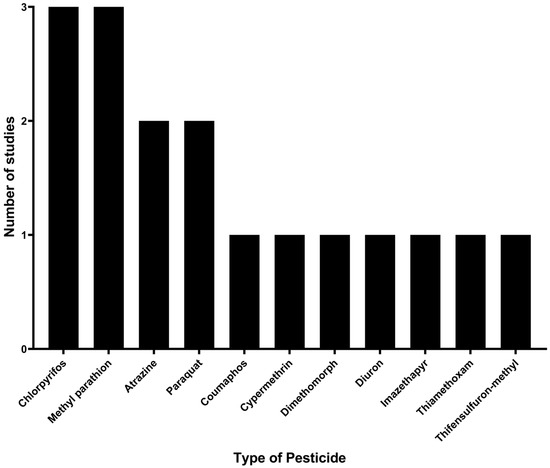
Figure 3.
Pesticides studied for their degradation by microorganisms immobilized on agro-industrial waste.
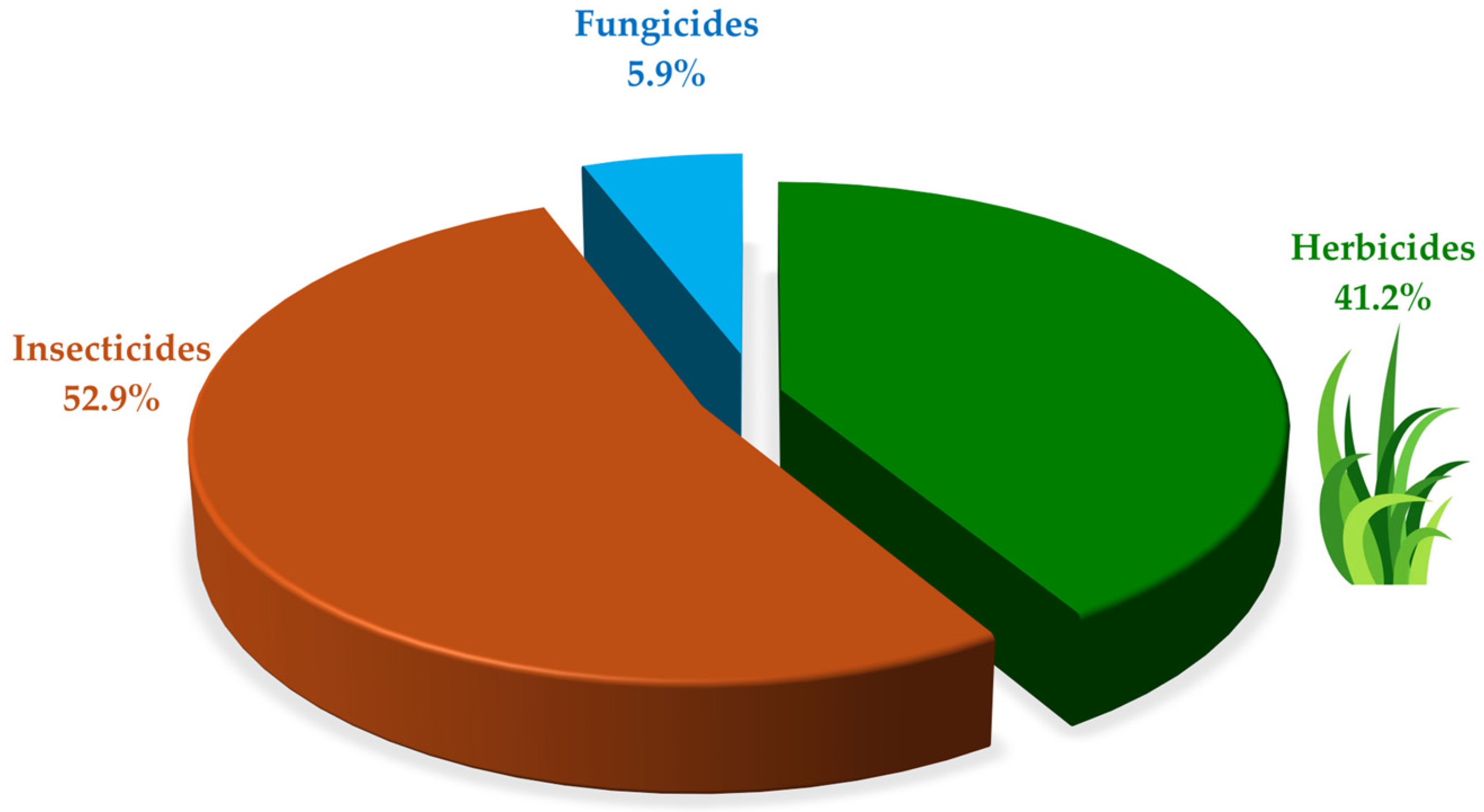
Out of the total pesticide degradation experiments categorized by target pest, in nine pesticide degradation experiments (52.9%), the pesticides under study were insecticides, encompassing methyl parathion, chlorpyrifos, coumaphos, cypermethrin, and thiamethoxam. The degradation of herbicide compounds was evaluated in seven pesticide degradation experiments (41.2%); evaluated herbicides included atrazine, paraquat, thifensulfuron-methyl, diuron, and imazethapyr. Finally, one study (5.9%) focused on the degradation of the fungicide dimethomorph (Figure 4). Regarding chemical classification, pesticides included in the identified studies belonged to nine chemical families. Organophosphorus pesticides were the most representative with seven studies (41.2%), followed by triazine and bipyridylium derivatives with two studies (11.8%) for each family. On the other hand, concerning their hazard profile, based on acute toxicity, two pesticides were classified as unlikely to present acute hazard (WHO, class U), two as slightly hazardous (WHO, class III), five as moderately hazardous (WHO, class II), and two as highly hazardous (WHO, class Ib) (Table S1).
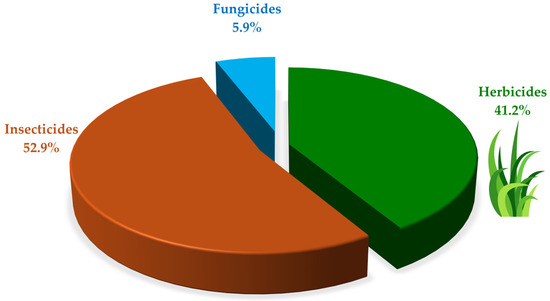
Figure 4.
Identified pesticides by target organism classification.
According to Equation (1), all herbicides showed an improvement in pesticide degradation through immobilized microorganisms systems. Showing improvements from 7.1% in the degradation of thifensulfuron-methyl (50 mg/L) by Serratia marcescens N80 immobilized on spent mushroom substrate in 48 h, to an improvement of 1329% in the degradation of paraquat (30 mg/L) by Pseudomonas putida immobilized on coconut fiber in 48 h.
Regarding the degradation of the insecticides identified in this study, Table 1 shows that improvements in the degradation efficiency varies from 8.7% in the degradation of chlorpyrifos (25 mg/L) with Bacillus H27 immobilized on rice straw as a residue over 168 h, to a 1244% increase for methyl parathion (50 mg/L) after one hour with Burkholderia cenocepacia CEIB S5-2 immobilized on nopal and agave fibers.
For fungicide degradation, only dimethomorph was identified. It was degraded from an initial concentration of 50 mg/L, with a maximum degradation of 96.9% when Bacillus cereus WL08 was immobilized on bamboo for 72 h. In contrast with free-cell experiments, in which the degradation percentage was lower (66.95%), demonstrating a 44.7% improvement in the degradation of dimethomorph when using immobilized microorganisms (Table 1).
3.5. Microorganisms Immobilized in Agro-Industrial Waste That Degrade Pesticides
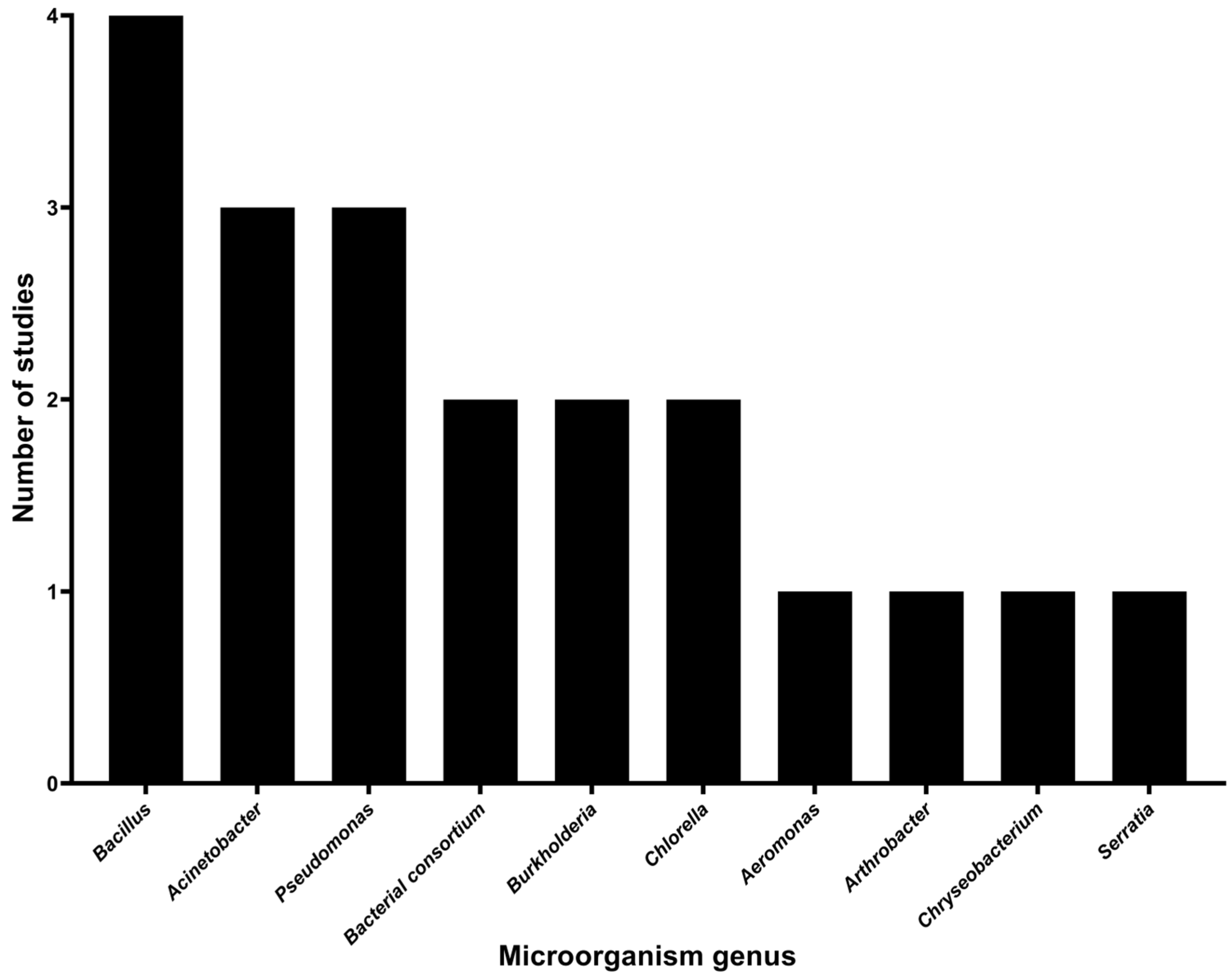
The search conducted in the present review identified bacterial and algal genera implicated in pesticide degradation, resulting in the identification of eight bacterial genera and one genus of microalgae. The bacterial genera include Aeromonas, Acinetobacter, Arthrobacter, Bacillus, Burkholderia, Chryseobacterium, Pseudomonas, Serratia, and along with a bacterial consortium as reported by Moreno-Medina et al. [40], which did not specify the individual bacterial genera involved. Of the bacterial genera, Bacillus was the most frequently studied, with four studies, followed by Acinetobacter and Pseudomonas with three studies, and the bacterial consortium and Burkholderia, each with two studies. Aeromonas, Arthrobacter, Chryseobacterium, and Serratia were each represented by one study. Chlorella was the only alga genus tested for the degradation of two pesticides in a study, as shown in Figure 5.
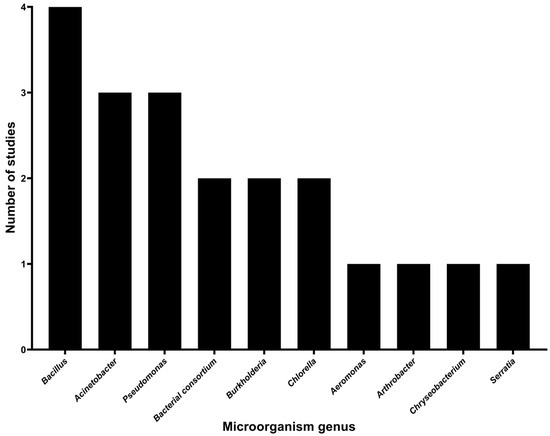
Figure 5.
Microbial genera immobilized on agro-industrial waste for pesticide degradation.
3.6. Rate Degradation of Pesticides by Free Cells and Immobilized Cells
Regarding the pesticide degradation rate, an increase in degradation rate was observed with immobilized microorganisms across all the identified studies with respect to pesticide degradation by free cells (Table 2).

Table 2.
Degradation rate of free cells and immobilized cells (mg/L∗h).
In the herbicide group, the study that showed the highest degradation rate of atrazine involved the strain Arthrobacter sp. ZXY-2 immobilized on corn straw biochar, with a pesticide concentration of 50 mg/L for one hour. In this study, free bacteria achieved a degradation rate of 45 ± 8.2 mg/L∗h, while immobilized bacteria reached 50 mg/L*h. In contrast, among insecticides, the lowest degradation rate for free cells was observed with Pseudomonas putida during the degradation of paraquat (30 mg/L) over 48 h, resulting in a degradation rate of 0.04 mg/L∗h. The lowest degradation rate for immobilized cells (0.17 mg/L∗h) was observed in a study on paraquat degradation at a concentration of 26 mg/L, using corn cob biochar and Pseudomonas putida TISTR 1522 for 24 h. Table 2 presents the degradation values for each study, comparing free and immobilized microorganisms.
Among insecticides, the highest degradation rate was achieved using nopal and agave fibers as residues, with Burkholderia cenocepacia CEIB S5-2 at a concentration of 50 mg/L of methyl parathion for one hour. The degradation rate for free cells was 3.7 mg/L∗h, while for immobilized cells it reached 50 mg/L∗h. In contrast, the lowest degradation rate for free cells among insecticides was observed with the microalga Chlorella vulgaris during the degradation of chlorpyrifos and cypermethrin (0.5 mg/L) over 72 h, resulting in a degradation rate of 0.004 mg/L∗h for both pesticides. The lowest degradation rate for cells immobilized on an unspecified agro-industrial residue was 0.006 mg/L∗h for both chlorpyrifos and cypermethrin. Regarding the degradation of the fungicide dimethomorph, a higher degradation rate was observed for immobilized cells of Bacillus cereus WL08, with a value of 0.7 mg/L∗h, compared to free cells, which exhibited a rate of 0.5 ± 0.02 mg/L∗h.
According to pesticide degradation percentages reported in the identified studies, immobilized cell systems display higher pesticide degradation, thus suggesting that the immobilization process enhances the pesticide degradation using microbial systems; to test this, unpaired t-Student tests were conducted to determine significant differences between the median values of pesticide degradation percentages reported for free and immobilized cells. In this analysis, just 9 of the 17 pesticide degradation conditions reported in the identified studies were included due to the availability of dispersion data (standard deviation). In seven conditions, pesticide degradation by immobilized cells showed higher degradation percentages with significant differences concerning the free cell systems (Table 2). Moreover, the average pesticide degradation percentages reported for free and immobilized microbial cell systems of all 17 pesticide degradation experiments showed that immobilization of microbial cells significantly enhances pesticide degradation concerning free cell systems.
However, as was mentioned previously in this manuscript, the diversity of pesticide molecules, agro-industrial wastes, microorganisms, and experimental conditions used in the identified studies make it difficult to compare and identify the most efficient systems for pesticide degradation with microorganisms immobilized in agro-industrial waste. To overcome this disadvantage, in the present review, it was proposed to calculate the improvement percentage in the pesticide degradation process and pesticide degradation rate as useful indexes to identify the waste materials and microbial systems with higher efficiency in the degradation of pesticides.
According to the results of the statistical analyses conducted in this review, it is clear that for most studies, the degradation of pesticide molecules is improved due to the microbial immobilization process in agro-industrial waste. Moreover, it is important to note that the review revealed that all studies conducted to date have been limited to the laboratory scale. The large-scale application of immobilized microorganisms, therefore, requires further exploration and development. It should be noted that the use of immobilized microorganisms on agro-industrial waste is an emerging approach in pesticide pollution remediation with significant potential for future research and practical applications.
4. Discussion
4.1. Agro-Industrial Wastes Identified as Microbial Supports in Pesticide Degradation
Corn straw, which consists of a mixture of leaves, stems, cobs, and husk tissues of Zea mays, commonly left abandoned in the crop fields without treatment after harvest, it was the most frequently found agro-industrial waste in the studies reviewed. This is due to its abundance and accessibility in agricultural areas with high corn production rates [53], as corn is the most important grain crop globally and serves as the primary food source for about one-third of the world’s population, in addition to being crucial for energy security [54,55].
In the identified studies, corn straw was then converted into biochar, a carbon-rich material obtained through biomass pyrolysis. In this process, agro-industrial waste is decomposed at high temperatures, ranging from 300 to 950 °C, in the absence of oxygen [56,57]. The resulting biochar has a porous structure, cation-exchange capacity, stability, and an abundance of functional groups [58]. Furthermore, it promotes and stabilizes microbial activity, which aids in the degradation of contaminants such as pesticides [59,60].
Utilizing agro-industrial waste for microorganism immobilization represents an innovative solution for environmental remediation with a sight of the circular economy. Unlike traditional support materials such as synthetic polymers or commercial adsorbents, agro-industrial by-products like corn straw, bamboo, rice husks, and other wastes are abundant, low-cost, and widely available, making them an attractive alternative for bioremediation [61]. The Food and Agriculture Organization (FAO) estimates that 1.3 billion tons of food are wasted annually, much of which consists of agro-industrial waste [62,63,64]. This extensive availability provides a reliable and sustainable source of materials for immobilizing microorganisms. By utilizing these wastes, bioremediation becomes more cost-effective, environmentally friendly, and offers significant advantages over conventional treatment methods [65].
4.2. Pesticides Identified and Degraded by Microorganisms Immobilized on Agro-Industrial Waste
Currently, approximately 3.6 million tons of pesticides are used worldwide, mainly in China, the United States, and Argentina, of which 52.6% are herbicides, 20.9% are insecticides, 21.5% are fungicides and bactericides, and 5% are other types of pesticides [15,30,66,67]. This aligns with the findings of this study, where insecticides and herbicides were the most representative pesticides, mainly chlorpyrifos, methyl parathion, atrazine and paraquat.
According to the data collected in this study, chlorpyrifos and methyl parathion were the most mentioned pesticides, identified in three studies each. Chlorpyrifos is one of the organophosphorus pesticides most used worldwide and widely used to control a variety of pests in agriculture and households [68]. Chlorpyrifos has been a subject of environmental and health concerns due to its potential toxicity to humans, wildlife, and aquatic organisms. Studies have shown that it can inhibit the enzyme acetylcholinesterase, which is crucial for nerve function in insects and other organisms [69,70].
Another pesticide found in this research with three studies is methyl parathion, classified as highly hazardous by the World Health Organization (WHO), which is an organophosphorus insecticide used in agriculture to control pests on crops like cotton, corn, and other vegetables [71,72]. In general, the direct exposure at organophosphorus pesticides as methyl parathion can cause symptoms like headaches, dizziness, nausea, vomiting, diarrhea, respiratory distress, and, in severe cases, seizures, because it inhibits the enzyme acetylcholinesterase, leading to an accumulation of acetylcholine in the nervous system, causing overstimulation of muscles, glands, and the central nervous system, which can lead to death [73,74]. Chronic exposure may lead to long-term health problems, including neurological damage [73]. In the environment, methyl parathion can cause pollution in the water, soil, and non-target organisms, affecting biodiversity and the health of ecosystems [75].
Atrazine was identified in two studies. Atrazine (2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine) is an herbicide from the triazine family, which is a white powder, unstable at high temperatures, and easily soluble in organic solvents [76]. This herbicide is commonly detected in both surface and groundwater due to its long half-life (ranging from 30 to 100 days) [77]. Atrazine is effective at controlling broadleaf weeds, some weeds that hinder crop growth, and certain perennial weeds [78]. Additionally, it is used as a non-selective herbicide in fallow and non-agricultural lands. Due to its effectiveness, low cost, and ease of use, it is widely applied in crops such as corn, sugarcane, and sorghum, making it the most used herbicide in corn cultivation [79].
Atrazine represents a significant environmental concern because it is one of the most detected herbicides in soil and water due to its long half-life, high mobility, and solubility, which can be influenced by various factors [80]. Atrazine enters the environment primarily through precipitation, runoff, and leaching, contributing to the contamination of soil, surface, and groundwater, and posing an additional threat to the ecosystem. Due to its effects, atrazine has been included on the list of endocrine-disrupting chemicals by various organizations and countries, including the European Community, Japan, and the United States. It is considered an environmental estrogen and a potential carcinogen, and it can persist in the environment for extended periods [80,81].
Dimethomorph is a fungicide primarily used in agriculture to control fungal diseases such as downy mildew in crops like potatoes, grapes, cucumbers, and other vegetables. It belongs to the chemical class of morpholines [82], compounds that work by inhibiting the biosynthesis of glucans, which are essential components of fungal cell walls. This disruption prevents fungal growth and spread, ultimately leading to the death of the fungus [83].
4.3. Microorganisms Immobilized in Agro-Industrial Waste That Degrade Pesticides
In the present study, microorganisms immobilized on agro-industrial waste with pesticide degradation capacity were also identified, which were primarily bacteria. Eight bacterial genera were identified: Aeromonas, Acinetobacter, Arthrobacter, Bacillus, Burkholderia, Chryseobacterium, Pseudomonas, Serratia, along with a bacterial consortium and a genus of algae (Chlorella).
Various studies have reported the presence of microorganisms capable of degrading pesticides, with bacteria being the most commonly involved organisms. This is because bacteria are the most abundant microorganisms in the biosphere and have the ability to degrade complex compounds, such as pesticides, due to the presence of genes that encode enzymes capable of transforming these molecules, utilizing pesticides as nutrient sources for their growth and metabolism [84,85,86,87].
Bacterial genera such as Acinetobacter, Bacillus, and Pseudomonas have been reported for their ability to degrade pesticides like α-endosulfan and α-cypermetrhrin [88]. Other studies have documented the ability of these bacterial genera in their immobilized form, as is the case with Pseudomonas, which has demonstrated the ability to degrade the herbicide simazine [89]; Pseudomonas and Bacillus are effective in degrading paraquat [90]; Bacillus can break down carbofuran [91]; and Acinetobacter is capable of degrading propanil [92].
On the other hand, the microalgae Chlorella was the only algae identified in this study. Several studies have indicated that microalgae have a high potential to survive in contaminated environments by activating internal defense mechanisms [93,94], and for a high production of biomass, which allows them to absorb and accumulate organic pollutants [95], contributing to the enhanced degradation of pesticides in contaminated ecosystems [96,97]. Additionally, it has been shown that various species of microalgae have been reported for their ability to degrade malathion [98] and diclofop-methyl [99]. Particularly, Chlorella presents the ability to degrade chlorpyrifos [100], diazinon and methamidophos [101], and pesticide mixtures of atrazine, molinate, simazine, isoproturon, propanil, carbofuran, dimethoate, pendimethalin, metolachlor, and pyriproxyfen [102].
Agro-industrial waste offers key properties such as high porosity, large surface area, and diverse chemical groups such as amino, hydroxyl, carboxyl, thiol, and phosphate. These features facilitate mechanisms like adsorption, ion exchange, and complexation. As a result, these wastes have been successfully employed as biosorbents for xenobiotic removal [103]. Agro-industrial waste offers a cost-effective alternative to synthetic or commercial materials in bioremediation, as it is abundant and inexpensive [104]. Compared to other methods, for example, using free-living microorganisms for contaminant degradation, immobilization offers clear advantages. Free microorganisms are vulnerable to environmental factors and may be washed away, reducing their effectiveness [105]. Biostimulation, while utilizing native microorganisms to degrade xenobiotics, is limited by environmental conditions, non-biodegradable contaminants, and its site-specific nature [106]. In contrast, immobilized microorganisms enhance cell survival and stability, providing protection from environmental threats. This approach simplifies system control, reduces operational costs, ensures higher cell density, and allows for mass production and extended storage, improving pollutant degradation and facilitating solid–liquid separation and purification [107]. Thus, microorganism immobilization offers significant potential in bioremediation, compared to other bioremediation methods.
4.4. Mechanisms of Bacteria and Algae to Degrade Pesticides
Bacteria are among the most effective organisms for the biodegradation of pesticides. They utilize mechanisms to break down pesticides as enzymatic degradation. Many bacteria have genes that code for specific enzymes such as oxidoreductases, peroxidases, transferases, and hydrolases, and translocases which can detoxify or degrade pesticides. Enzymes are powerful agents for decontamination because of their ability to catalyze biological reactions and their detoxifying characteristics. Through chemical reactions such as reduction, oxidation, hydrolysis, dehydrogenation, dehalogenation, decarboxylation, among others, bacteria can transform pesticides into less toxic metabolites. Furthermore, microorganisms, particularly bacteria, utilize a co-metabolism process in which the substance to be degraded does not serve as the primary nutrient source for the microorganism. Instead, the microorganism depends on another substrate to meet its nutritional needs while simultaneously degrading the target compound [108,109,110,111].
On the other hand, algae can absorb pesticides from their surrounding environment. Once absorbed, these chemicals are often compartmentalized in vacuoles or bound to cellular structures, reducing their toxicity, and preventing further interaction with the organism. Furthermore, algae have the capacity to metabolize pesticides through enzymatic processes. Certain species of microalgae, for instance, can use various enzymes such as esterase, transferase, and cytochrome P450, to degrade or detoxify xenobiotics, including pesticides [112,113,114,115].
4.5. Selection of Microorganisms for Pesticide Degradation
The selection of microorganisms is a critical factor in the success of bioremediation strategies, as it is influenced by several key factors, including their enzymatic capabilities, adaptability to environmental conditions, and the specific degradation pathways they possess. Microorganisms such as Arthrobacter, Pseudomonas, Bacillus, and Burkholderia species are frequently employed in bioremediation due to their broad enzymatic versatility, which enables them to degrade various pesticides [68,116,117,118,119,120,121,122,123].
Particularly in this study, due to the limited number of available studies, selecting an appropriate microorganism for the degradation of a specific pesticide remains challenging. Based on the results of this study, the microorganisms capable of achieving 100% degradation of various pesticides were identified and categorized according to their degradation time. The most efficient microorganisms include Arthrobacter sp. ZXY-2, immobilized on corn straw biochar, which demonstrated complete degradation (100%) of the herbicide atrazine at a concentration of 50 mg/L within one hour. This was followed by Burkholderia cenocepacia CEIB S5-2, immobilized on nopal or agave fibers, which achieved complete degradation (100%) of the pesticide methyl parathion at a concentration of 50 mg/L within one hour. Additionally, Acinetobacter iwoffii DNS32, immobilized on corn straw biochar, successfully degraded 100% of the herbicide atrazine at a concentration of 50 mg/L within 12 h. A bacterial consortium composed of Bacillus subtilis DU1, Acinetobacter baumannii DU, and Pseudomonas sp. DUK, immobilized on rice straw, achieved 100% degradation of the herbicide diuron at a concentration of 20 mg/L within 36 h. Furthermore, an unspecified bacterial consortium immobilized on loofah (Luffa cylindrica) was capable of degrading 100% of the insecticide coumaphos at a concentration of 5 mg/L within 72 h.
4.6. Rate Degradation of Pesticides by Free Cells and Immobilized Cells
Free microbial cells generally exhibit high pesticide degradation rates due to their active metabolic processes [124]. However, their ability to degrade pesticides can decrease over time due to factors such as environmental stress, nutrient depletion, and cell death [105].
In contrast, the immobilization of microorganisms is based on the natural properties and capabilities of several of these organisms. Many microorganisms have the ability to self-aggregate and adhere to various types of surfaces, both biotic and abiotic, or even within porous structures, using extracellular compounds they produce [125]. Agro-industrial waste includes in their composition molecules such as lignin, hemicellulose, and cellulose, as well as functional groups like hydroxyl, carbonyl, ether, and methoxy. These characteristics make them ideal for contaminant adsorption and microbial immobilization [126,127]. Various studies have reported promising results in the application of microorganism immobilization [128,129,130,131].
Agro-industrial wastes, used as support for microorganism immobilization, represent a sustainable alternative for pesticide degradation. Immobilized microbial cells, being trapped or fixed in a support or matrix, typically show a slower initial degradation rate compared to free cells. However, immobilization provides long-term stability, resistance, and protection against the toxicity of pesticides. This can lead to sustained degradation rates over time, which can be utilized in multiple cycles without a significant loss of activity, making it economically beneficial [127,130,132,133]. In this regard, the immobilization of microorganisms in agro-industrial waste constitutes a sustainable and effective strategy for long-term pesticide degradation in aquatic environments.
5. Conclusions
This study has revealed that the degradation of pesticides using microorganisms immobilized on agro-industrial waste is a promising strategy for eliminating these toxic compounds from aquatic environments. The results show that immobilizing microorganisms on these wastes not only increases the efficiency of pesticide degradation but also represents a sustainable solution by utilizing agro-industrial by-products that would otherwise be discarded as waste. Although this strategy has been developed and tested experimentally in the laboratory, there are still few studies applying this bioremediation technology, and its high efficiency makes it a promising option for the removal of pesticides in aquatic ecosystems.
Future research should focus on optimizing immobilization conditions, enhancing microorganism efficiency, and ensuring the scalability and in situ viability of the process to enable its application for remediating pesticide-contaminated aquatic ecosystems. Furthermore, pesticide degradation using microorganisms immobilized on agro-industrial waste has the potential to influence policy, agricultural practices, and environmental regulations. It could foster bioremediation research, incentivize the use of sustainable waste materials, and guide responsible waste management. Additionally, environmental agencies may update regulations on pesticide discharge and water quality standards, recognizing bioremediation as a viable solution for reducing pesticide contamination in aquatic ecosystems.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pr13041073/s1. Table S1: Frequencies and percentages of the key data extracted from the identified studies. Reference [134] is cited in Supplementary Materials.
Author Contributions
Data collection, curation, and figures design, E.A.-C.; study conception, supervision, writing—original draft, data curation, reviewing and editing final draft, M.L.C.-G.; writing—original draft, reviewing and editing final draft, P.M.-G.; writing—original draft, reviewing and editing final draft, E.T.-S.; study conception, and funding acquisition, supervision, writing—original draft, reviewing and editing final draft, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI), through the project “Sistema de tratamiento para la eliminación de glifosato, paraquat y atrazina, herbicidas altamente contaminantes, basado en biomasa residual agrícola y de especies invasoras como soporte de microorganismos degradadores”, grant number CBF2023-2024-2134.
Data Availability Statement
All relevant data are within the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kılıç, Z. The Importance of Water and Conscious Use of Water. Int. J. Hydrol. 2020, 4, 239–241. [Google Scholar] [CrossRef]
- Kordbacheh, F.; Heidari, G. Water Pollutants and Approaches for Their Removal. Mater. Chem. Horiz. 2023, 2, 139–153. [Google Scholar] [CrossRef]
- Singh, J.; Yadav, P.; Pal, A.K.; Mishra, V. Water pollutants: Origin and status. In Sensors in Water Pollutants Monitoring: Role of Material; Springer: Berlin/Heidelberg, Germany, 2020; pp. 5–20. [Google Scholar] [CrossRef]
- Ahamad, A.; Madhav, S.; Singh, A.K.; Kumar, A.; Singh, P. Types of Water Pollutants: Conventional and Emerging. In Sensors in Water Pollutants Monitoring: Role of Material, Advanced Functional Materials and Sensors; Springer: Berlin/Heidelberg, Germany, 2010; pp. 21–41. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy Metal Water Pollution: A Fresh Look About Hazards, Novel and Conventional Remediation Methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Vijayanand, M.; Ramakrishnan, A.; Subramanian, R.; Issac, P.K.; Nasr, M.; Khoo, K.S.; Rajagopal, R.; Greff, B.; Wan Azelee, N.I.; Jeon, B.-H.; et al. Polyaromatic Hydrocarbons (PAHs) in the Water Environment: A Review on Toxicity, Microbial Biodegradation, Systematic Biological Advancements, and Environmental Fate. Environ. Res. 2023, 227, 115716. [Google Scholar] [CrossRef]
- Maheshwari, K.; Agrawal, M.; Gupta, A. Dye Pollution in Water and Wastewater. In Novel Materials for Dye-Containing Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–25. [Google Scholar] [CrossRef]
- Rad, S.M.; Ray, A.K.; Barghi, S. Water Pollution and Agriculture Pesticide. Clean Technol. 2022, 4, 1088–1102. [Google Scholar] [CrossRef]
- Estrada-Almeida, A.G.; Castrejón-Godínez, M.L.; Mussali-Galante, P.; Tovar-Sánchez, E.; Rodríguez, A. Pharmaceutical Pollutants: Ecotoxicological Impacts and the Use of Agro-Industrial Waste for Their Removal from Aquatic Environments. J. Xenobiotics 2024, 14, 1465–1518. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, A.K.; Kushawaha, J.; Chauhan, J.S.; Sharma, S.; Singh, P. Water pollutants: Sources and impact on the environment and human health. In Sensors in Water Pollutants Monitoring: Role of Material; Pooja, D., Kumar, P., Singh, P., Patil, S., Eds.; Springer Nature: Singapore, 2020; pp. 43–62. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Environmental Impacts of Food Production. Our World in Data. 2020. Available online: https://ourworldindata.org/environmental-impacts-of-food (accessed on 20 January 2025).
- Fusco, G.; Campobasso, F.; Laureti, L.; Frittelli, M.; Valente, D.; Petrosillo, I. The Environmental Impact of Agriculture: An Instrument to Support Public Policy. Ecol. Indic. 2023, 147, 109961. [Google Scholar] [CrossRef]
- Zhang, W. Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 2018, 8, 1–27. [Google Scholar]
- Aryal, S.; Aryal, L.N. Pesticide Residue and Food Safety: Retrospection and Prospects. In Emerging Solutions in Sustainable Food and Nutrition Security; Ghosh, S., Kumari Panda, A., Jung, C., Singh Bisht, S., Eds.; Springer: Cham, Switzerland, 2023; pp. 183–210. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Pesticides Use. Available online: https://www.fao.org/faostat/en/#data/RP (accessed on 20 January 2025).
- Hosni, H.; Segovia, M.; Zhao, S.; Palma, M.A.; Skevas, T. Improving consumer understanding of pesticide toxicity labels: Experimental evidence. Sci. Rep. 2024, 14, 17291. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, M.I.; Sajjad, A.; Shakeel, Q.; Hussain, A. Environmental and health effects of pesticide residues. In Sustainable Agriculture Reviews 48; Sustainable Agriculture Reviews; Inamuddin, Ahamed, M.I., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021; Volume 4, pp. 311–336. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Mukherjee, P.; Banerjee, G.; Saha, N.; Mazumdar, A. Overview on the Emergence of Pesticide Contamination and Treatment Methodologies. Water Air Soil Pollut. 2024, 235, 587. [Google Scholar] [CrossRef]
- López-Benítez, A.; Guevara-Lara, A.; Domínguez-Crespo, M.A.; Andraca-Adame, J.A.; Torres-Huerta, A.M. Concentrations of Organochlorine, Organophosphorus, and Pyrethroid Pesticides in Rivers Worldwide (2014–2024): A Review. Sustainability 2024, 16, 8066. [Google Scholar] [CrossRef]
- Rana, A.K.; Mishra, Y.K.; Gupta, V.K.; Thakur, V.K. Sustainable materials in the removal of pesticides from contaminated water: Perspective on macro to nanoscale cellulose. Sci. Total Environ. 2021, 797, 149129. [Google Scholar] [CrossRef]
- Dhankhar, N.; Kumar, J. Impact of Increasing Pesticides and Fertilizers on Human Health: A Review. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumar, U.; Kumar, I.; Dwivedi, A.; Singh, P.; Mishra, S.; Seth, C.S.; Sharma, R.K. Critical review on toxic contaminants in surface water ecosystem: Sources, monitoring, and its impact on human health. Environ. Sci. Pollut. Res. 2024, 31, 56428–56462. [Google Scholar] [CrossRef]
- Hara, T.O.; Singh, B. Electrochemical biosensors for detection of pesticides and heavy metal toxicants in water: Recent trends and progress. ACS ES&T Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Medina Pastor, P. The 2021 European Union report on pesticide residues in food. EFSA J. 2023, 21, e07939. [Google Scholar] [CrossRef]
- Cossu, L.O.; De Aquino, S.F.; Mota Filho, C.R.; Smith, C.J.; Vignola, M. Review on Pesticide Contamination and Drinking Water Treatment in Brazil: The Need for Improved Treatment Methods. ACS ES&T Water 2024, 4, 3629–3644. [Google Scholar] [CrossRef]
- Zikankuba, V.L.; Mwanyika, G.; Ntwenya, J.E.; James, A. Pesticide regulations and their malpractice implications on food and environment safety. Cogent Food Agric. 2019, 5, 1601544. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A.N.; Saxena, R.; Paul, D.; Tomar, R.S. Biodiversity of pesticides degrading microbial communities and their environmental impact. Biocatal. Agric. Biotechnol. 2021, 31, 101883. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Thukral, A.K. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Kanel, S.R.; Nakarmi, A. Groundwater pollution: Occurrence, detection, and remediation of organic and inorganic pollutants. Water Environ. Res. 2020, 92, 1659–1668. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Niu, A.; Cheng, N.; Chen, M.; Zhang, X.; Wang, S. Application of biochar immobilized microorganisms for pollutants removal from wastewater: A review. Sci. Total Environ. 2022, 837, 155563. [Google Scholar] [CrossRef]
- Vilar, D.d.S.; Cruz, I.A.; Torres, N.H.; Figueiredo, R.T.; de Melo, L.; de Resende, I.T.F.; Eguiluz, K.I.B.; Bharagava, R.N.; Ferreira, L.F.R. Agro-industrial Wastes: Environmental Toxicology, Risks, and Biological Treatment Approaches. In Microorganisms for Sustainability; Bharagava, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–23. [Google Scholar] [CrossRef]
- Vargas, Y.A.; Pérez, L.I. Aprovechamiento de residuos agroindustriales en el mejoramiento de la calidad del ambiente. Rev. Fac. Cienc. Básicas 2018, 14, 59–72. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. Declaración PRISMA 2020: Una guía actualizada para la publicación de revisiones sistemáticas. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- Mojiri, A.; Zhou, J.L.; Nazari, M.; Rezania, S.; Farraji, H.; Vakili, M. Biochar enhanced the performance of microalgae/bacteria consortium for insecticides removal from synthetic wastewater. Process Saf. Environ. Prot. 2022, 157, 284–296. [Google Scholar] [CrossRef]
- Moreno-Medina, D.A.; Sánchez-Salinas, E.; Ortiz-Hernández, M.L. Removal of methyl parathion and coumaphos pesticides by a bacterial consortium immobilized in Luffa cylindrica. Rev. Int. Contam. Ambient. 2014, 30, 51–63. [Google Scholar]
- Fernández-López, M.G.; Popoca-Ursino, C.; Sánchez-Salinas, E.; Tinoco-Valencia, R.; Folch-Mallol, J.L.; Dantán-González, E.; Laura Ortiz-Hernández, M. Enhancing methyl parathion degradation by the immobilization of Burkholderia sp. isolated from agricultural soils. MicrobiologyOpen 2017, 6, e00507. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Hu, S.; Han, S.; Shi, H.; Yang, Y.; Li, H.; Jiao, Y.; Zhang, Q.; Akindolie, M.S.; Ji, M.; et al. Efficient removal of atrazine by iron-modified biochar loaded Acinetobacter lwoffii DNS32. Sci. Total Environ. 2019, 682, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, L.; Ma, F.; Wang, Y.; Bai, S. A bio-functions integration microcosm: Self-immobilized biochar-pellets combined with two strains of bacteria to remove atrazine in water and mechanisms. J. Hazard. Mater. 2020, 384, 121326. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Wu, X.; Long, Y.; An, H.; Pan, X.; Li, M.; Dong, F.; Zheng, Y. Rapid degradation of dimethomorph in polluted water and soil by Bacillus cereus WL08 immobilized on bamboo charcoal–sodium alginate. J. Hazard. Mater. 2020, 398, 122806. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, C.; Zhang, M.; Wu, Y.; Zhang, Z.; Zhang, H. Degradation characteristics and soil remediation of thifensulfuron-methyl by immobilized Serratia marcecens N80 beads. Environ. Technol. Innov. 2021, 24, 102059. [Google Scholar] [CrossRef]
- Ha, N.T.H.; Toan, N.C.; Kajitvichyanukul, P. Enhanced paraquat removal from contaminated water using cell-immobilized biochar. Clean Technol. Environ. Policy 2022, 24, 1073–1085. [Google Scholar] [CrossRef]
- Duc, H.D.; Thanh, N.T.; Thuy, N.T. Diuron degradation by a mixed culture of bacteria immobilized in rice straw. Tạp Chí Khoa Học Đại Học Đồng Tháp 2023, 12, 55–62. [Google Scholar] [CrossRef]
- Xiang, X.; Yi, X.; Zheng, W.; Li, Y.; Zhang, C.; Wang, X.; Chen, Z.; Huang, M.; Ying, G.G. Enhanced biodegradation of thiamethoxam with a novel polyvinyl alcohol (PVA)/sodium alginate (SA)/biochar immobilized Chryseobacterium sp. H5. J. Hazard. Mater. 2023, 443, 130247. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Nguyen, T.H.H.; Tungtakanpoung, D.; Tran, C.S.; Vo, T.K.Q.; Kaewlom, P. Paraquat removal by free and immobilized cells of Pseudomonas putida on corn cob biochar. Case Stud. Chem. Environ. Eng. 2023, 8, 100376. [Google Scholar] [CrossRef]
- Liu, C.; Wen, S.; Li, S.; Tian, Y.; Wang, L.; Zhu, L.; Wang, J. Enhanced remediation of chlorpyrifos-contaminated soil by immobilized strain Bacillus H27. J. Environ. Sci. 2024, 144, 172–184. [Google Scholar] [CrossRef]
- Manikandan, S.K.; Mariyam, A.; Gowda, N.K.; Singh, A.; Nair, V. Mechanistic understanding of biochar-bacteria system for enhanced chlorpyrifos bioremediation in water and soil medium. Chem. Eng. J. 2024, 483, 149119. [Google Scholar] [CrossRef]
- Miao, J.; Fan, Q.; Li, H.; Yang, Y.; Zhang, Q. Combination of the degrading bacterium Bacillus cereus MZ-1 and corn straw biochar enhanced the removal of imazethapyr from water solutions. Next Sustain. 2024, 5, 100077. [Google Scholar] [CrossRef]
- Xu, S.; Wang, F.; Fu, Y.; Li, D.; Sun, X.; Li, C.; Song, B.; Li, Y. Effects of mixed agro-residues (corn crop waste) on lignin-degrading enzyme activities, growth, and quality of Lentinula edodes. RSC Adv. 2020, 10, 9798–9807. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Liu, G.; Xie, R.; Ming, B.; Yang, Y.; Guo, X.; Wang, K.; Xue, J.; Wang, Y.; et al. Estimation of maize straw production and appropriate straw return rate in China. Agric. Ecosyst. Environ. 2022, 328, 107865. [Google Scholar] [CrossRef]
- Feng, W.; Yang, F.; Cen, R.; Liu, J.; Qu, Z.; Miao, Q.; Chen, H. Effects of straw biochar application on soil temperature, available nitrogen and growth of corn. J. Environ. Manag. 2021, 277, 111331. [Google Scholar] [CrossRef]
- Rodriguez, O.L.; Torres, E.; Zalazar, D.; Zhang, H.; Rodríguez, R.; Mazza, G. Influence of pyrolysis temperature and bio-waste composition on biochar characteristics. Renew. Energy 2020, 155, 837–847. [Google Scholar] [CrossRef]
- Landrat, M.; Abawalo, M.; Pikoń, K.; Fufa, P.A.; Seyid, S. Assessing the Potential of Teff Husk for Biochar Production through Slow Pyrolysis: Effect of Pyrolysis Temperature on Biochar Yield. Energies 2024, 17, 1988. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Zhou, Y. Novel insights into the adsorption of organic contaminants by biochar: A review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sarkar, B.; Aralappanavar, V.K.; Mukhopadhyay, R.; Basak, B.B.; Srivastava, P.; Marchut-Mikołajczyk, O.; Bhatnagar, A.; Bolan, N. Biochar-microorganism interactions for organic pollutant remediation: Challenges and perspectives. Environ. Pollut. 2022, 308, 119609. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Duan, J. Mechanisms of adsorption and functionalization of biochar for pesticides: A review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef]
- Simón, D.; Palet, C.; Costas, A.; Cristóbal, A. Agro-industrial waste as potential heavy metal adsorbents and subsequent safe disposal of spent adsorbents. Water 2022, 14, 3298. [Google Scholar] [CrossRef]
- Blakeney, M. Food Loss and Waste and Food Security. In Food Loss and Food Waste; Edward Elgar Publishing: Cheltenham, UK, 2019; pp. 1–26. [Google Scholar] [CrossRef]
- Flanagan, K.; Robertson, K.; Hanson, C. What is the food loss and waste challenge? In Reducing Food Loss and Waste; Setting the Global Action Agenda; World Resources Institute: Washington, DC, USA, 2019. [Google Scholar]
- Rohini, C.; Geetha, P.; Vijayalakshmi, R.; Mini, M.; Pasupathi, E. Global effects of food waste. J. Pharmacogn. Phytochem. 2020, 9, 690–699. [Google Scholar]
- Saravanan, A.; Swaminaathan, P.; Kumar, P.S.; Yaashikaa, P.; Kamalesh, R.; Rangasamy, G. A comprehensive review on immobilized microbes-biochar and their environmental remediation: Mechanism, challenges and future perspectives. Environ. Res. 2023, 236, 116723. [Google Scholar] [CrossRef]
- Ali, I.M. The Harmful Effects of Pesticides on the Environment and Human Health: A Review. Diyala Agric. Sci. J. 2023, 15, 114–126. [Google Scholar] [CrossRef]
- Wakhungu, C. Loss of soil biodiversity through judicious use of synthetic pesticides, A case study of Trans Nzoia county Kenya-review. Sci. Rep. Life Sci. 2023, 4, 1–24. [Google Scholar] [CrossRef]
- Gilani, R.A.; Rafique, M.; Rehman, A.; Munis, M.F.; Rehman, S.U.; Chaudhary, H.J. Biodegradation of chlorpyrifos by bacterial genus Pseudomonas. J. Basic Microbiol. 2016, 56, 105–119. [Google Scholar] [CrossRef]
- Nandi, N.K.; Vyas, A.; Akhtar, M.J.; Kumar, B. The growing concern of chlorpyrifos exposures on human and environmental health. Pestic. Biochem. Physiol. 2022, 185, 105138. [Google Scholar] [CrossRef]
- Wolejko, E.; Lozowicka, B.; Jablonska-Trypuc, A.; Pietruszynska, M.; Wydro, U. Chlorpyrifos Occurrence and Toxicological Risk Assessment: A Review. Int. J. Environ. Res. Public Health 2022, 19, 12209. [Google Scholar] [CrossRef]
- Celik, I.; Suzek, H. The hematological effects of methyl parathion in rats. J. Hazard. Mater. 2008, 153, 1117–1121. [Google Scholar] [CrossRef]
- Kashni, M.; Arora, R.; Jain, R. An expository note on notorious methyl parathion engendering risk evaluation and its redressal. In Hazardous Chemicals: Overview, Toxicological Profile, Challenges, and Future Perspectives; Chawla, M., Singh, J., Kaushik, R.D., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 105–118. [Google Scholar]
- Jokanović, M.; Oleksak, P.; Kuca, K. Multiple neurological effects associated with exposure to organophosphorus pesticides in man. Toxicology 2023, 484, 153407. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Darabi, R.; Baghayeri, M.; Karimi, F.; Fu, L.; Rouhi, J.; Niculina, D.E.; Gündüz, E.S.; Dragoi, E.N. Recent developments in carbon nanomaterials-based electrochemical sensors for methyl parathion detection. J. Food Meas. Charact. 2023, 17, 5371–5389. [Google Scholar] [CrossRef]
- Gill, H.K.; Garg, H. Pesticide: Environmental impacts and management strategies. Pestic. Toxic Asp. 2014, 8, 10–5772. [Google Scholar] [CrossRef]
- Jari, Y.; Roche, N.; Necibi, M.C.; El Hajjaji, S.; Dhiba, D.; Chehbouni, A. Emerging pollutants in Moroccan wastewater: Occurrence, impact, and removal technologies. J. Chem. 2022, 2022, 9727857. [Google Scholar] [CrossRef]
- Taverna, M.E.; Busatto, C.A.; Lescano, M.R.; Nicolau, V.V.; Zalazar, C.S.; Meira, G.R.; Estenoz, D.A. Microparticles based on ionic and organosolv lignins for the controlled release of atrazine. J. Hazard. Mater. 2018, 359, 139–147. [Google Scholar] [CrossRef]
- Muhammad, F.; Khan, R.; Shafique, M. Optimizing Atrazine Application Rates for Efficacious Weed Control in Maize Cultivation. Indus J. Agric. Biol. 2024, 3, 15–22. [Google Scholar]
- He, H.; Liu, Y.; You, S.; Liu, J.; Xiao, H.; Tu, Z. A review on recent treatment technology for herbicide atrazine in contaminated environment. Int. J. Environ. Res. Public Health 2019, 16, 5129. [Google Scholar] [CrossRef] [PubMed]
- Abd Rani, N.F.; Ahmad Kamil, K.; Aris, F.; Mohamed Yunus, N.; Zakaria, N.A. Atrazine-degrading bacteria for bioremediation strategy: A review. Biocatal. Biotransform. 2022, 40, 233–247. [Google Scholar] [CrossRef]
- Urseler, N.; Bachetti, R.; Morgante, C.; Agostini, E. Bioremediation Strategies to Mitigate the Impact of Atrazine on the Environment: Recent Advances and Prospects. In Agrochemicals in Soil and Environment; Naeem, M., Bremont, J.F.J., Ansari, A.A., Gill, S.S., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Wang, Y.; Ruan, H.; Zhang, J.; Wang, Y.; Guo, M.; Ke, T.; Luo, J.; Yang, M. CHA-based dual signal amplification immunofluorescence biosensor for ultrasensitive detection of dimethomorph. Anal. Chim. Acta 2022, 1227, 340323. [Google Scholar] [CrossRef]
- Hao, K.; Lin, B.; Nian, F.; Gao, X.; Wei, Z.; Luo, G.; Lu, Y.; Lan, M.; Yang, J.; Wu, G. RNA-seq analysis of the response of plant-pathogenic oomycete Phytophthora parasitica to the fungicide dimethomorph. Rev. Argent. Microbiol. 2019, 51, 268–277. [Google Scholar] [CrossRef]
- Parte, S.G.; Mohekar, A.D.; Kharat, A.S. Microbial degradation of pesticide: A review. Afr. J. Microbiol. Res. 2017, 11, 992–1012. [Google Scholar] [CrossRef]
- Umadevi, S.; Ayyasamy, P.M.; Rajakumar, S. Biological Perspective and Role of Bacteria in Pesticide Degradation. In Bioremediation and Sustainable Technologies for Cleaner Environment; Prashanthi, M., Sundaram, R., Jeyaseelan, A., Kaliannan, T., Eds.; Environmental Science and Engineering; Springer: Cham, Switzerland, 2017; pp. 3–12. [Google Scholar] [CrossRef]
- Paul, D.; Mandal, S.M. Microbial Adaptation and Resistance to Pesticides. In Bacterial Adaptation to Co-Resistance; Mandal, S., Paul, D., Eds.; Springer: Singapore, 2019; pp. 233–249. [Google Scholar] [CrossRef]
- Miglani, R.; Parveen, N.; Kumar, A.; Ansari, M.A.; Khanna, S.; Rawat, G.; Panda, A.K.; Bisht, S.S.; Upadhyay, J.; Ansari, M.N. Degradation of xenobiotic pollutants: An environmentally sustainable approach. Metabolites 2022, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Gur Ozdal, O.; Algur, O. Biodegradation α-endosulfan and α-cypermethrin by Acinetobacter schindleri B7 isolated from the microflora of grasshopper (Poecilimon tauricola). Arch. Microbiol. 2022, 204, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ni, Y.; Liu, J.; Yan, T.; Zhu, X.; Li, Q.X.; Hua, R.; Pan, D.; Wu, X. Bead-immobilized Pseudomonas stutzeri Y2 prolongs functions to degrade s-triazine herbicides in industrial wastewater and maize fields. Sci. Total Environ. 2020, 731, 139183. [Google Scholar] [CrossRef]
- Jindakaraked, M.; Khan, E.; Kajitvichyanukul, P. Biodegradation of paraquat by Pseudomonas putida and Bacillus subtilis immobilized on ceramic with supplemented wastewater sludge. Environ. Pollut. 2021, 286, 117307. [Google Scholar] [CrossRef] [PubMed]
- Duc, H.D. Enhancement of carbofuran degradation by immobilized Bacillus sp. strain DT1. Environ. Eng. Res. 2022, 27, 210158. [Google Scholar] [CrossRef]
- Ha, D.D.; Nguyen, T.O. Degradation of propanil by Acinetobacter baumannii DT immobilized in alginate. Vietnam J. Sci. Technol. Eng. 2022, 64, 8–12. [Google Scholar] [CrossRef]
- Torres, E.M.; Hess, D.; McNeil, B.T.; Guy, T.; Quinn, J.C. Impact of inorganic contaminants on microalgae productivity and bioremediation potential. Ecotoxicol. Environ. Saf. 2017, 139, 367–376. [Google Scholar] [CrossRef]
- Kumar, V.S.; Sarkar, D.J.; Sarkar, S.D.; Das, B.K. Microalgal Remediation in the Aquiatic Environment. In Handbook of Aquatic Microbiology; Pandey, P.K., Mallik, S.K., Yumnam, R., Eds.; CRC Press: Boca Raton, FL, USA; Taylos & Francis Group: Abingdon, UK, 2024; pp. 260–270. [Google Scholar]
- Touliabah, H.E.S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A review of microalgae-and cyanobacteria-based biodegradation of organic pollutants. Molecules 2022, 27, 1141. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Ismail, A.F.; Samawati, Z.; Liang, Y.Y.; Kanakaraju, D. Microalgae-enabled wastewater treatment: A sustainable strategy for bioremediation of pesticides. Water 2022, 15, 70. [Google Scholar] [CrossRef]
- Fayaz, T.; Rana, S.S.; Goyal, E.; Ratha, S.K.; Renuka, N. Harnessing the potential of microalgae-based systems for mitigating pesticide pollution and its impact on their metabolism. J. Environ. Manag. 2024, 357, 120723. [Google Scholar] [CrossRef]
- Abdel-Razek, M.A.; Abozeid, A.M.; Eltholth, M.M.; Abouelenien, F.A.; El-Midany, S.A.; Moustafa, N.Y.; Mohamed, R.A. Bioremediation of a pesticide and selected heavy metals in wastewater from various sources using a consortium of microalgae and cyanobacteria. Slov. Vet. Res. 2019, 56, 61–73. [Google Scholar] [CrossRef]
- Cai, X.; Liu, W.; Jin, M.; Lin, K. Relation of diclofop-methyl toxicity and degradation in algae cultures. Environ. Toxicol. Chem. Int. J. 2007, 26, 970–975. [Google Scholar] [CrossRef]
- Habibah, R.; Iswanto, B.; Rinanti, A. The significance of tropical microalgae Chlorella sorokiniana as a remediate of polluted water caused by chlorpyrifos. Int. J. Sci. Technol. Res. 2020, 9, 4460–4463. [Google Scholar]
- Trejo-Carrizalez, I.; Cervantes-González, E. Potential degradation efficiency of Chlorella vulgaris towards methamidophos and diazinon. Algal Res. 2024, 81, 103566. [Google Scholar] [CrossRef]
- Hussein, M.H.; Abdullah, A.M.; Badr El-Din, N.I.; Mishaqa, E.S.I. Biosorption potential of the microchlorophyte Chlorella vulgaris for some pesticides. J. Fertil. Pestic. 2017, 8, 1000177. [Google Scholar] [CrossRef]
- Girelli, A.M.; Astolfi, M.L.; Scuto, F.R. Agro-Industrial Wastes as Potential Carriers for Enzyme Immobilization: A Review. Chemosphere 2020, 244, 125368. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Kuo, C.-H.; Sun, P.-P.; Dong, C.-D. Agro-Industrial Food Waste as a Low-Cost Substrate for Sustainable Production of Industrial Enzymes: A Critical Review. Catalysts 2022, 12, 1373. [Google Scholar] [CrossRef]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of immobilized bacteria for environmental bioremediation: A review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Goswami, M.; Chakraborty, P.; Mukherjee, K.; Mitra, G.; Bhattacharyya, P.; Dey, S.; Tribedi, P. Bioaugmentation and biostimulation: A potential strategy for environmental remediation. J. Microbiol. Exp. 2018, 6, 62–65. [Google Scholar]
- Wu, C.; Zhi, D.; Yao, B.; Zhou, Y.; Yang, Y.; Zhou, Y. Immobilization of microbes on biochar for water and soil remediation: A review. Environ. Res. 2022, 212, 113226. [Google Scholar]
- Bhandari, S.; Poudel, D.K.; Marahatha, R.; Dawadi, S.; Khadayat, K.; Phuyal, S.; Shrestha, S.; Gaire, S.; Basnet, K.; Khadka, U.; et al. Microbial Enzymes Used in Bioremediation. J. Chem. 2021, 1, 8849512. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.V.N.; Rajamohan, N.; Saravanan, R. Microbial degradation of recalcitrant pesticides: A review. Environ. Chem. Lett. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Sehrawat, A.; Phour, M.; Kumar, R.; Sindhu, S.S. Bioremediation of Pesticides: An Eco-Friendly Approach for Environment Sustainability. In Microbial Rejuvenation of Polluted Environment; Microorganisms for Sustainability; Panpatte, D.G., Jhala, Y.K., Eds.; Springer: Singapore, 2021; Volume 1, pp. 23–84. [Google Scholar] [CrossRef]
- Chia, X.K.; Hadibarata, T.; Kristanti, R.A.; Jusoh, M.N.H.; Tan, I.S.; Foo, H.C.Y. The Function of Microbial Enzymes in Breaking down Soil Contaminated with Pesticides: A Review. Bioprocess Biosyst. Eng. 2024, 47, 597–620. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Sun, Y.; Zhou, Y.; Kumar, M.; Usman, M.; Li, J.; Shao, J.; Wang, L.; Tsang, D.C. Bioremediation of water containing pesticides by microalgae: Mechanisms, methods, and prospects for future research. Sci. Total Environ. 2020, 707, 136080. [Google Scholar] [CrossRef] [PubMed]
- Singhal, M.; Jadhav, S.; Sonone, S.S.; Sankhla, M.S.; Kumar, R. Microalgae based sustainable bioremediation of water contaminated by pesticides. Biointerface Res. Appl. Chem. 2021, 12, 149–169. [Google Scholar] [CrossRef]
- de Morais, M.G.; Zaparoli, M.; Lucas, B.F.; Costa, J.A.V. Microalgae for bioremediation of pesticides: Overview, challenges, and future trends. In Algal Biotechnology. Integrated Algal Engineering for Bioenergy, Bioremediation, and Biomedical Applications; Ahmad, A., Banat, F., Taher, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 63–78. [Google Scholar] [CrossRef]
- Narayanan, M.; Devarayan, K.; Verma, M.; Selvaraj, M.; Ghramh, H.A.; Kandasamy, S. Assessing the Ecological Impact of Pesticides/Herbicides on Algal Communities: A Comprehensive Review. Aquat. Toxicol. 2024, 268, 106851. [Google Scholar] [CrossRef]
- Li, R.; Guo, X.; Chen, K.; Zhu, J.; Li, S.; Jiang, J. Isolation of an isocarbophos-degrading strain of Arthrobacter sp. scl-2 and identification of the degradation pathway. J. Microbiol. Biotechnol. 2009, 19, 1439–1446. [Google Scholar]
- Liu, Z.Y.; Chen, X.; Shi, Y.; Su, Z.C. Bacterial Degradation of Chlorpyrifos by Bacillus cereus. In Advanced Materials Research; Trans Tech Publications Ltd.: Bach, Switzerland, 2012; pp. 676–680. [Google Scholar] [CrossRef]
- El-Helow, E.R.; Badawy, M.E.I.; Mabrouk, M.E.M.; Mohamed, E.A.H.; El-Beshlawy, Y.M. Biodegradation of Chlorpyrifos by a Newly Isolated Bacillus subtilis Strain, Y242. Bioremediation J. 2013, 17, 113–123. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, C.X.; Luo, X.J.; Lai, Q.L.; Xu, J.H. Burkholderia jiangsuensis sp. nov., a methyl parathion degrading bacterium, isolated from methyl parathion contaminated soil. Int. J. Syst. Evol. Microbiol. 2014, 64, 3247–3253. [Google Scholar] [CrossRef]
- Zuo, Z.; Gong, T.; Che, Y.; Liu, R.; Xu, P.; Jiang, H.; Qiao, C.; Song, C.; Yang, C. Engineering Pseudomonas putida KT2440 for simultaneous degradation of organophosphates and pyrethroids and its application in bioremediation of soil. Biodegradation 2015, 26, 223–233. [Google Scholar] [CrossRef]
- Gao, J.; Song, P.; Wang, G.; Wang, J.; Zhu, L.; Wang, J. Responses of atrazine degradation and native bacterial community in soil to Arthrobacter sp. strain HB-5. Ecotoxicol. Environ. Saf. 2018, 159, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Morya, R.; Salvachua, D.; Thakur, I.S. Burkholderia: An untapped but promising bacterial genus for the conversion of aromatic compounds. Trends Biotechnol. 2020, 38, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Mali, H.; Shah, C.; Patel, D.H.; Trivedi, U.; Subramanian, R.B. Degradation Insight of Organophosphate Pesticide Chlorpyrifos through Novel Intermediate 2,6-Dihydroxypyridine by Arthrobacter sp. HM01. Bioresour. Bioprocess. 2022, 9, 31. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Han, M.; Zhang, C.; Ho, S.H. Immobilized microalgal system: An achievable idea for upgrading current microalgal wastewater treatment. Environ. Sci. Ecotechnol. 2023, 14, 100227. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.J.; Saravanan, A. Advances in production and application of biochar from lignocellulosic feedstocks for remediation of environmental pollutants. Bioresour. Technol. 2019, 292, 122030. [Google Scholar] [CrossRef]
- Ponnuchamy, M.; Kapoor, A.; Kumar, P.S.; Vo, D.V.N.; Balakrishnan, A.; Jacob, M.M.; Sivaraman, P. Sustainable adsorbents for the removal of pesticides from water: A review. Environ. Chem. Lett. 2021, 19, 2425–2463. [Google Scholar] [CrossRef]
- Fuentes, M.S.; Briceño, G.E.; Saez, J.M.; Benimeli, C.S.; Diez, M.C.; Amoroso, M.J. Enhanced removal of a pesticides mixture by single cultures and consortia of free and immobilized Streptomyces strains. BioMed Res. Int. 2013, 2013, 392573. [Google Scholar] [CrossRef]
- Talwar, M.P.; Ninnekar, H.Z. Biodegradation of pesticide profenofos by the free and immobilized cells of Pseudoxanthomonas suwonensis strain HNM. J. Basic Microbiol. 2015, 55, 1094–1103. [Google Scholar] [CrossRef]
- Mustapha, M.U.; Halimoon, N.; Johari, W.L.W.; Shukor, M.Y.A. Enhanced carbofuran degradation using immobilized and free cells of Enterobacter sp. isolated from soil. Molecules 2020, 25, 2771. [Google Scholar] [CrossRef]
- Najim, A.A.; Radeef, A.Y.; al-Doori, I.; Jabbar, Z.H. Immobilization: The promising technique to protect and increase the efficiency of microorganisms to remove contaminants. J. Chem. Technol. Biotechnol. 2024, 99, 1707–1733. [Google Scholar] [CrossRef]
- Ortiz-Hernández, M.L.; Sánchez-Salinas, E.; Dantán-González, E.; Castrejón-Godínez, M.L. Pesticide biodegradation: Mechanisms, genetics and strategies to enhance the process. In Biodegradation-Life of Science; Chamy, R., Rosenkranz, F., Eds.; IntechOpen: Rijeka, Croatia, 2013; Volume 10, pp. 251–287. [Google Scholar] [CrossRef]
- Conde-Avila, V.; Ortega-Martínez, L.D.; Loera, O.; El Kassis, E.G.; Dávila, J.G.; Valenzuela, C.M.; Armendáriz, B.P. Pesticides degradation by immobilised microorganisms. Int. J. Environ. Anal. Chem. 2021, 101, 2975–3005. [Google Scholar] [CrossRef]
- World Health Organization. Inter-Organization Programme for the Sound Management of Chemicals. In The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019; World Health Organization: Geneva, Switzerland, 2020; 92p, Available online: https://www.who.int/publications/i/item/9789240005662 (accessed on 26 January 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).