Pesticides Degradation Through Microorganisms Immobilized on Agro-Industrial Waste: A Promising Approach for Their Elimination from Aquatic Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Literature Review
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Matrix from the Systematic Literature Review
2.5. Determination of the Improvement in the Degradation Process
- %DII is the degradation improvement index;
- %Dimm is the degradation percentage of immobilized cells;
- %Dfree is the degradation percentage of free cells.
- DR is the degradation rate;
- Ci is the initial concentration;
- Cf is the final concentration;
- tD is the degradation time.
2.6. Statistical Analysis
3. Results
3.1. Data Matrix from the Systematic Literature Review
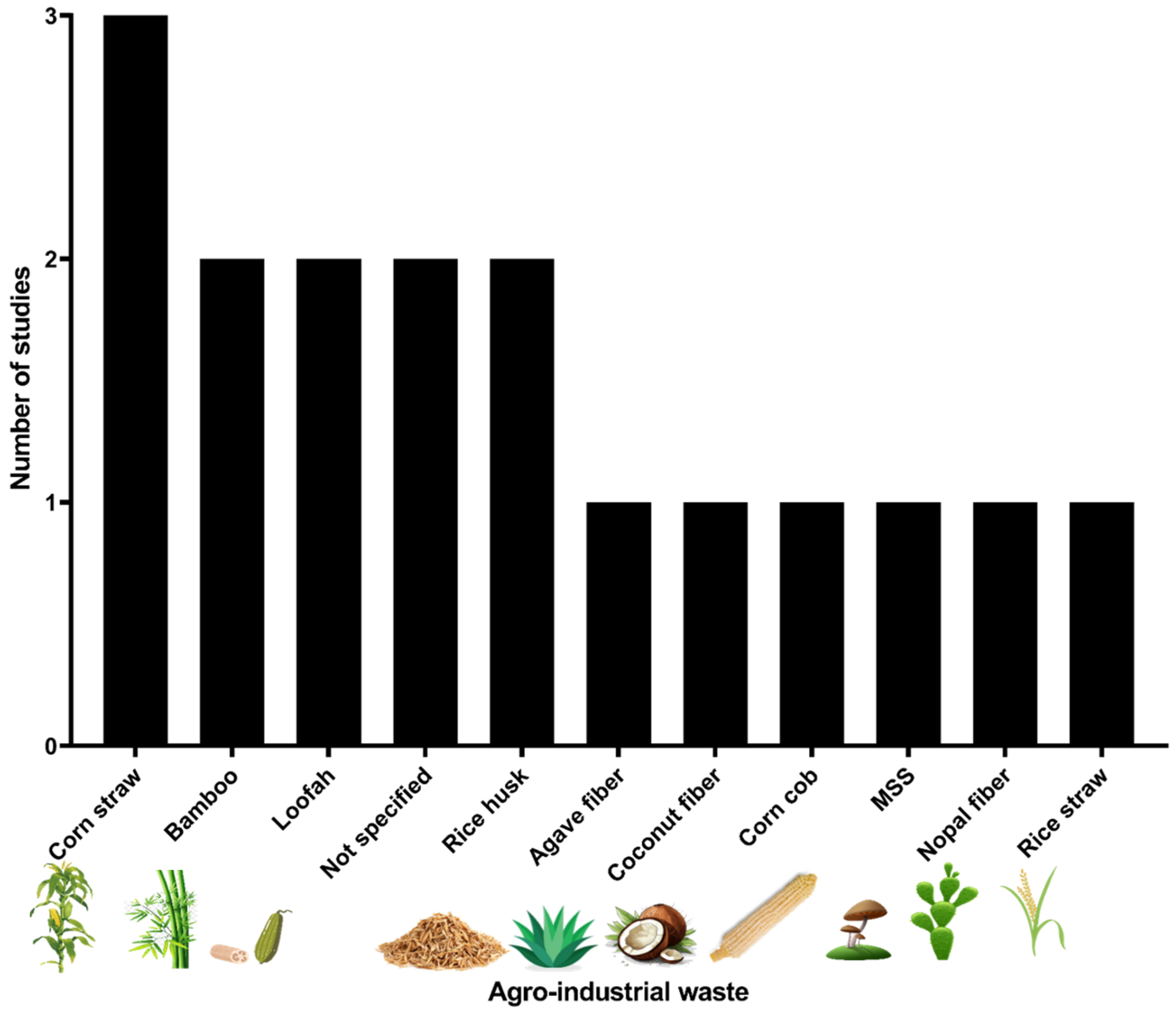
3.2. Agro-Industrial Wastes Identified as Microbial Supports in Pesticide Degradation
3.3. Pretreatment of Agro-Industrial Waste
| Agro-Industrial Waste (Support) | Pretreatment | Name of Pesticide | Type of Pesticide | Microorganism | Type of Microorganism | Concentration of Pesticide (mg/L) | Degradation Time (Hours) | Pesticide Degradation (%) Free Cells | Pesticide Degradation (%) Immobilized Cells | Degradation Improvement Index (DII) * | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Loofah (Luffa cylindrica) | NaOH 5% and anthraquinone at 80 °C for 3 h | Methyl parathion | Insecticide | Bacterial consortium 1 | Bacteria | 25 | 72 | 54.9 ± 3.8 | 98.5 ± 0.4 | 79.4 | [40] |
| Loofah (Luffa cylindrica) | Coumaphos | 5 | 62 ± 23 | 100 | 61.3 | ||||||
| Nopal fiber | Washed with water and dried at 50 °C | Methyl parathion | Insecticide | Burkholderia cenocepacia CEIB S5-2 1 | Bacteria | 50 | 1 | 7.4 | 100 | 1244 | [41] |
| Agave fiber | Methyl parathion | ||||||||||
| Corn straw | Pyrolysis 700 °C | Atrazine | Herbicide | Acinetobacter lwoffii DNS32 2 | Bacteria | 100 | 12 | 88.6 | 100 | 12.9 | [42] |
| Corn straw | Pyrolysis 700 °C | Atrazine | Herbicide | Arthrobacter sp. ZXY-2 3 | Bacteria | 50 | 1 | 90 ± 16.4 | 100 | 11.1 | [43] |
| Bamboo | Pyrolysis | Dimethomorph | Fungicide | Bacillus cereus WL08 1 | Bacteria | 50 | 72 | 66.9 ± 2.4 | 96.9 | 44.7 | [44] |
| Spent mushroom substrate | Pyrolysis 600 °C | Thifensulfuron-methyl | Herbicide | Serratia marcescens N80 4 | Bacteria | 50 | 48 | 83.5 ± 3.6 | 89.4 ± 3.1 | 7.1 | [45] |
| Coconut fiber | Pyrolysis | Paraquat | Herbicide | Pseudomonas putida 5 | Bacteria | 30 | 48 | 6.7 | 95.8 | 1329 | [46] |
| Not specified | Pyrolysis | Chlorpyrifos | Insecticide | Chlorella vulgaris 6 | Alga | 0.5 | 72 | 63.9 | 87.2 | 36.5 | [39] |
| Not specified | Cypermethrin | 64.2 | 93.4 | 45.5 | |||||||
| Rice straw | Washed with water and dried at 70 °C | Diuron | Herbicide | Bacillus subtilis DU1 1 Acinetobacter baumannii DU 1 Pseudomonas sp. DUK 1 | Bacteria | 20 | 36 | 70 ± 8.6 | 100 | 42.9 | [47] |
| Bamboo | Pyrolysis 850 °C | Thiamethoxam | Insecticide | Chryseobacterium spH5 7 | Bacteria | 14 | 24 | 25 | 74.0 ± 2.4 | 196.2 | [48] |
| Corn cob | Pyrolysis | Paraquat | Herbicide | Pseudomonas putida TISTR 1522 8 | Bacteria | 26 | 24 | 12.1 | 15.8 | 30.2 | [49] |
| Rice husk | Pyrolysis 450–500 °C | Chlorpyrifos | Insecticide/acaricide | Bacillus H27 1 | Bacteria | 25 | 168 | 89.6 ± 1 | 97.4 ± 0.5 | 8.7 | [50] |
| Rice husk | Pyrolysis 500 °C | Chlorpyrifos | Insecticide/acaricide | Aeromonas veronii 1 | Bacteria | 30 | 24 | 77.9 ± 1.6 | 96.5 ± 1.1 | 23.9 | [51] |
| Corn straw | Pyrolysis 500 °C | Imazethapyr | Herbicide | Bacillus cereus MZ-1 2 | Bacteria | 200 | 18 | 52.7 | 79.9 | 51.7 | [52] |
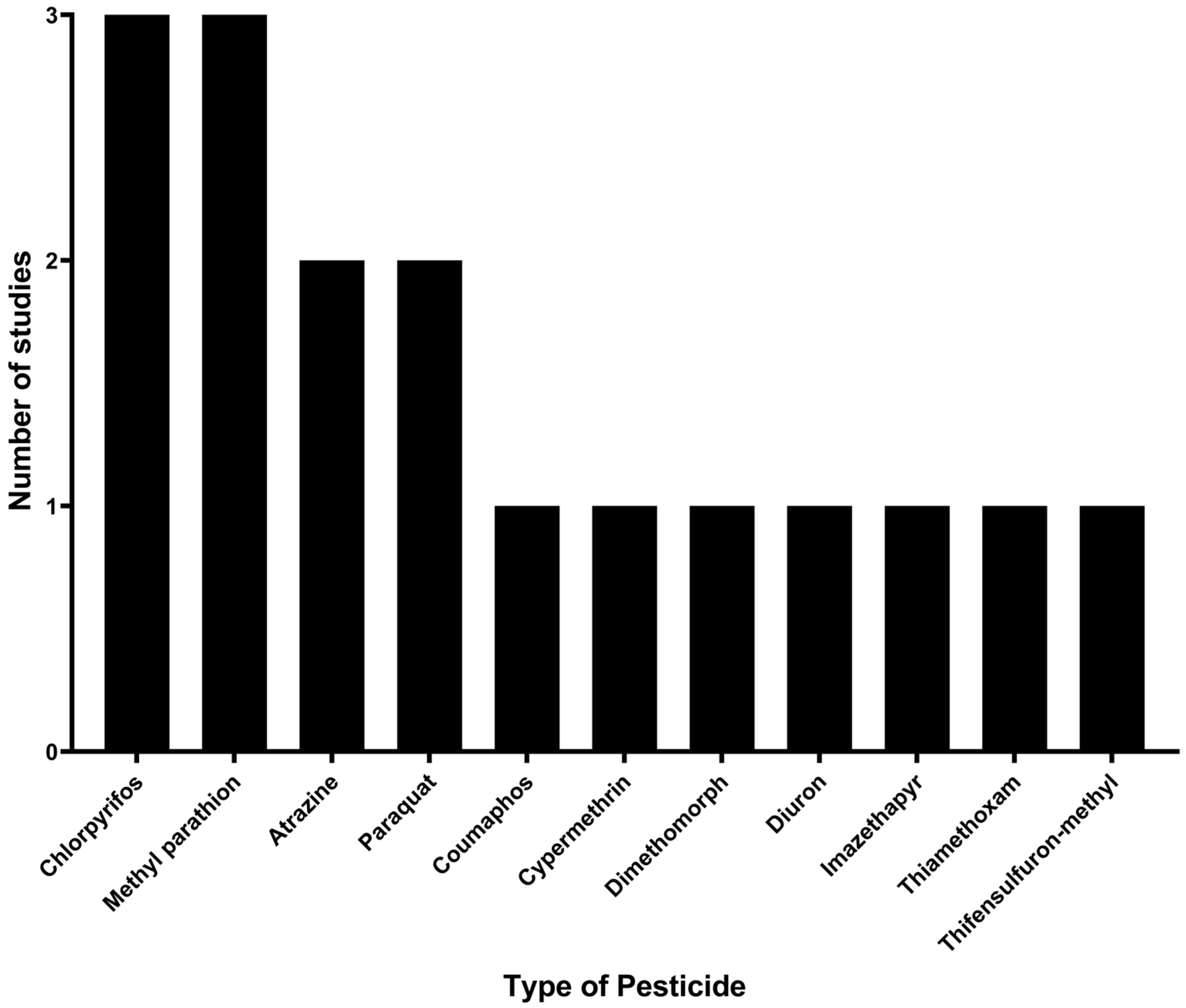
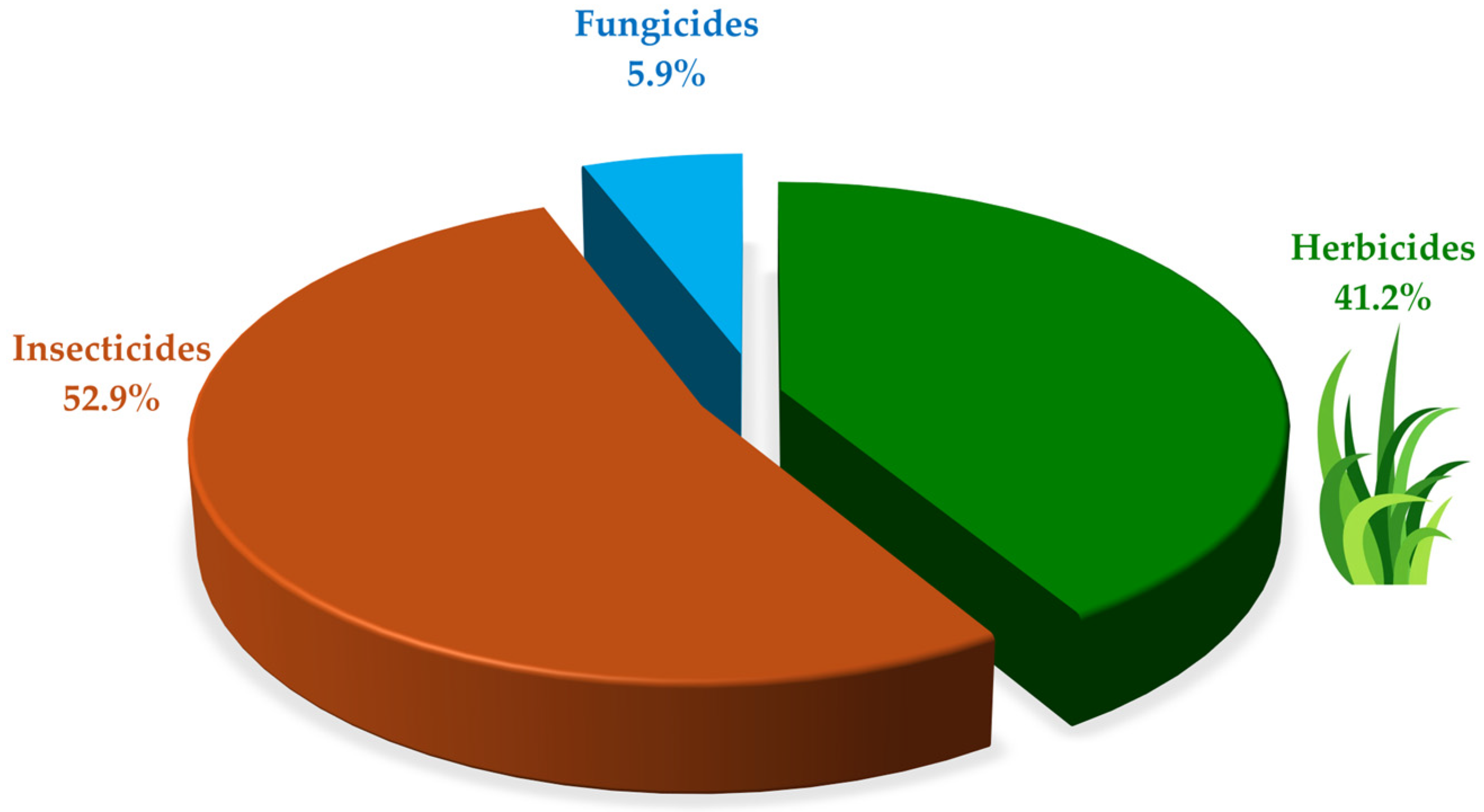
3.4. Pesticides Identified and Degraded by Microorganisms Immobilized in Agro-Industrial Waste
3.5. Microorganisms Immobilized in Agro-Industrial Waste That Degrade Pesticides
3.6. Rate Degradation of Pesticides by Free Cells and Immobilized Cells
4. Discussion
4.1. Agro-Industrial Wastes Identified as Microbial Supports in Pesticide Degradation
4.2. Pesticides Identified and Degraded by Microorganisms Immobilized on Agro-Industrial Waste
4.3. Microorganisms Immobilized in Agro-Industrial Waste That Degrade Pesticides
4.4. Mechanisms of Bacteria and Algae to Degrade Pesticides
4.5. Selection of Microorganisms for Pesticide Degradation
4.6. Rate Degradation of Pesticides by Free Cells and Immobilized Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kılıç, Z. The Importance of Water and Conscious Use of Water. Int. J. Hydrol. 2020, 4, 239–241. [Google Scholar] [CrossRef]
- Kordbacheh, F.; Heidari, G. Water Pollutants and Approaches for Their Removal. Mater. Chem. Horiz. 2023, 2, 139–153. [Google Scholar] [CrossRef]
- Singh, J.; Yadav, P.; Pal, A.K.; Mishra, V. Water pollutants: Origin and status. In Sensors in Water Pollutants Monitoring: Role of Material; Springer: Berlin/Heidelberg, Germany, 2020; pp. 5–20. [Google Scholar] [CrossRef]
- Ahamad, A.; Madhav, S.; Singh, A.K.; Kumar, A.; Singh, P. Types of Water Pollutants: Conventional and Emerging. In Sensors in Water Pollutants Monitoring: Role of Material, Advanced Functional Materials and Sensors; Springer: Berlin/Heidelberg, Germany, 2010; pp. 21–41. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy Metal Water Pollution: A Fresh Look About Hazards, Novel and Conventional Remediation Methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Vijayanand, M.; Ramakrishnan, A.; Subramanian, R.; Issac, P.K.; Nasr, M.; Khoo, K.S.; Rajagopal, R.; Greff, B.; Wan Azelee, N.I.; Jeon, B.-H.; et al. Polyaromatic Hydrocarbons (PAHs) in the Water Environment: A Review on Toxicity, Microbial Biodegradation, Systematic Biological Advancements, and Environmental Fate. Environ. Res. 2023, 227, 115716. [Google Scholar] [CrossRef]
- Maheshwari, K.; Agrawal, M.; Gupta, A. Dye Pollution in Water and Wastewater. In Novel Materials for Dye-Containing Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–25. [Google Scholar] [CrossRef]
- Rad, S.M.; Ray, A.K.; Barghi, S. Water Pollution and Agriculture Pesticide. Clean Technol. 2022, 4, 1088–1102. [Google Scholar] [CrossRef]
- Estrada-Almeida, A.G.; Castrejón-Godínez, M.L.; Mussali-Galante, P.; Tovar-Sánchez, E.; Rodríguez, A. Pharmaceutical Pollutants: Ecotoxicological Impacts and the Use of Agro-Industrial Waste for Their Removal from Aquatic Environments. J. Xenobiotics 2024, 14, 1465–1518. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, A.K.; Kushawaha, J.; Chauhan, J.S.; Sharma, S.; Singh, P. Water pollutants: Sources and impact on the environment and human health. In Sensors in Water Pollutants Monitoring: Role of Material; Pooja, D., Kumar, P., Singh, P., Patil, S., Eds.; Springer Nature: Singapore, 2020; pp. 43–62. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Environmental Impacts of Food Production. Our World in Data. 2020. Available online: https://ourworldindata.org/environmental-impacts-of-food (accessed on 20 January 2025).
- Fusco, G.; Campobasso, F.; Laureti, L.; Frittelli, M.; Valente, D.; Petrosillo, I. The Environmental Impact of Agriculture: An Instrument to Support Public Policy. Ecol. Indic. 2023, 147, 109961. [Google Scholar] [CrossRef]
- Zhang, W. Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 2018, 8, 1–27. [Google Scholar]
- Aryal, S.; Aryal, L.N. Pesticide Residue and Food Safety: Retrospection and Prospects. In Emerging Solutions in Sustainable Food and Nutrition Security; Ghosh, S., Kumari Panda, A., Jung, C., Singh Bisht, S., Eds.; Springer: Cham, Switzerland, 2023; pp. 183–210. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Pesticides Use. Available online: https://www.fao.org/faostat/en/#data/RP (accessed on 20 January 2025).
- Hosni, H.; Segovia, M.; Zhao, S.; Palma, M.A.; Skevas, T. Improving consumer understanding of pesticide toxicity labels: Experimental evidence. Sci. Rep. 2024, 14, 17291. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, M.I.; Sajjad, A.; Shakeel, Q.; Hussain, A. Environmental and health effects of pesticide residues. In Sustainable Agriculture Reviews 48; Sustainable Agriculture Reviews; Inamuddin, Ahamed, M.I., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021; Volume 4, pp. 311–336. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Mukherjee, P.; Banerjee, G.; Saha, N.; Mazumdar, A. Overview on the Emergence of Pesticide Contamination and Treatment Methodologies. Water Air Soil Pollut. 2024, 235, 587. [Google Scholar] [CrossRef]
- López-Benítez, A.; Guevara-Lara, A.; Domínguez-Crespo, M.A.; Andraca-Adame, J.A.; Torres-Huerta, A.M. Concentrations of Organochlorine, Organophosphorus, and Pyrethroid Pesticides in Rivers Worldwide (2014–2024): A Review. Sustainability 2024, 16, 8066. [Google Scholar] [CrossRef]
- Rana, A.K.; Mishra, Y.K.; Gupta, V.K.; Thakur, V.K. Sustainable materials in the removal of pesticides from contaminated water: Perspective on macro to nanoscale cellulose. Sci. Total Environ. 2021, 797, 149129. [Google Scholar] [CrossRef]
- Dhankhar, N.; Kumar, J. Impact of Increasing Pesticides and Fertilizers on Human Health: A Review. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumar, U.; Kumar, I.; Dwivedi, A.; Singh, P.; Mishra, S.; Seth, C.S.; Sharma, R.K. Critical review on toxic contaminants in surface water ecosystem: Sources, monitoring, and its impact on human health. Environ. Sci. Pollut. Res. 2024, 31, 56428–56462. [Google Scholar] [CrossRef]
- Hara, T.O.; Singh, B. Electrochemical biosensors for detection of pesticides and heavy metal toxicants in water: Recent trends and progress. ACS ES&T Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Medina Pastor, P. The 2021 European Union report on pesticide residues in food. EFSA J. 2023, 21, e07939. [Google Scholar] [CrossRef]
- Cossu, L.O.; De Aquino, S.F.; Mota Filho, C.R.; Smith, C.J.; Vignola, M. Review on Pesticide Contamination and Drinking Water Treatment in Brazil: The Need for Improved Treatment Methods. ACS ES&T Water 2024, 4, 3629–3644. [Google Scholar] [CrossRef]
- Zikankuba, V.L.; Mwanyika, G.; Ntwenya, J.E.; James, A. Pesticide regulations and their malpractice implications on food and environment safety. Cogent Food Agric. 2019, 5, 1601544. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A.N.; Saxena, R.; Paul, D.; Tomar, R.S. Biodiversity of pesticides degrading microbial communities and their environmental impact. Biocatal. Agric. Biotechnol. 2021, 31, 101883. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Thukral, A.K. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Kanel, S.R.; Nakarmi, A. Groundwater pollution: Occurrence, detection, and remediation of organic and inorganic pollutants. Water Environ. Res. 2020, 92, 1659–1668. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Niu, A.; Cheng, N.; Chen, M.; Zhang, X.; Wang, S. Application of biochar immobilized microorganisms for pollutants removal from wastewater: A review. Sci. Total Environ. 2022, 837, 155563. [Google Scholar] [CrossRef]
- Vilar, D.d.S.; Cruz, I.A.; Torres, N.H.; Figueiredo, R.T.; de Melo, L.; de Resende, I.T.F.; Eguiluz, K.I.B.; Bharagava, R.N.; Ferreira, L.F.R. Agro-industrial Wastes: Environmental Toxicology, Risks, and Biological Treatment Approaches. In Microorganisms for Sustainability; Bharagava, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–23. [Google Scholar] [CrossRef]
- Vargas, Y.A.; Pérez, L.I. Aprovechamiento de residuos agroindustriales en el mejoramiento de la calidad del ambiente. Rev. Fac. Cienc. Básicas 2018, 14, 59–72. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. Declaración PRISMA 2020: Una guía actualizada para la publicación de revisiones sistemáticas. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- Mojiri, A.; Zhou, J.L.; Nazari, M.; Rezania, S.; Farraji, H.; Vakili, M. Biochar enhanced the performance of microalgae/bacteria consortium for insecticides removal from synthetic wastewater. Process Saf. Environ. Prot. 2022, 157, 284–296. [Google Scholar] [CrossRef]
- Moreno-Medina, D.A.; Sánchez-Salinas, E.; Ortiz-Hernández, M.L. Removal of methyl parathion and coumaphos pesticides by a bacterial consortium immobilized in Luffa cylindrica. Rev. Int. Contam. Ambient. 2014, 30, 51–63. [Google Scholar]
- Fernández-López, M.G.; Popoca-Ursino, C.; Sánchez-Salinas, E.; Tinoco-Valencia, R.; Folch-Mallol, J.L.; Dantán-González, E.; Laura Ortiz-Hernández, M. Enhancing methyl parathion degradation by the immobilization of Burkholderia sp. isolated from agricultural soils. MicrobiologyOpen 2017, 6, e00507. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Hu, S.; Han, S.; Shi, H.; Yang, Y.; Li, H.; Jiao, Y.; Zhang, Q.; Akindolie, M.S.; Ji, M.; et al. Efficient removal of atrazine by iron-modified biochar loaded Acinetobacter lwoffii DNS32. Sci. Total Environ. 2019, 682, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, L.; Ma, F.; Wang, Y.; Bai, S. A bio-functions integration microcosm: Self-immobilized biochar-pellets combined with two strains of bacteria to remove atrazine in water and mechanisms. J. Hazard. Mater. 2020, 384, 121326. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Wu, X.; Long, Y.; An, H.; Pan, X.; Li, M.; Dong, F.; Zheng, Y. Rapid degradation of dimethomorph in polluted water and soil by Bacillus cereus WL08 immobilized on bamboo charcoal–sodium alginate. J. Hazard. Mater. 2020, 398, 122806. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, C.; Zhang, M.; Wu, Y.; Zhang, Z.; Zhang, H. Degradation characteristics and soil remediation of thifensulfuron-methyl by immobilized Serratia marcecens N80 beads. Environ. Technol. Innov. 2021, 24, 102059. [Google Scholar] [CrossRef]
- Ha, N.T.H.; Toan, N.C.; Kajitvichyanukul, P. Enhanced paraquat removal from contaminated water using cell-immobilized biochar. Clean Technol. Environ. Policy 2022, 24, 1073–1085. [Google Scholar] [CrossRef]
- Duc, H.D.; Thanh, N.T.; Thuy, N.T. Diuron degradation by a mixed culture of bacteria immobilized in rice straw. Tạp Chí Khoa Học Đại Học Đồng Tháp 2023, 12, 55–62. [Google Scholar] [CrossRef]
- Xiang, X.; Yi, X.; Zheng, W.; Li, Y.; Zhang, C.; Wang, X.; Chen, Z.; Huang, M.; Ying, G.G. Enhanced biodegradation of thiamethoxam with a novel polyvinyl alcohol (PVA)/sodium alginate (SA)/biochar immobilized Chryseobacterium sp. H5. J. Hazard. Mater. 2023, 443, 130247. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Nguyen, T.H.H.; Tungtakanpoung, D.; Tran, C.S.; Vo, T.K.Q.; Kaewlom, P. Paraquat removal by free and immobilized cells of Pseudomonas putida on corn cob biochar. Case Stud. Chem. Environ. Eng. 2023, 8, 100376. [Google Scholar] [CrossRef]
- Liu, C.; Wen, S.; Li, S.; Tian, Y.; Wang, L.; Zhu, L.; Wang, J. Enhanced remediation of chlorpyrifos-contaminated soil by immobilized strain Bacillus H27. J. Environ. Sci. 2024, 144, 172–184. [Google Scholar] [CrossRef]
- Manikandan, S.K.; Mariyam, A.; Gowda, N.K.; Singh, A.; Nair, V. Mechanistic understanding of biochar-bacteria system for enhanced chlorpyrifos bioremediation in water and soil medium. Chem. Eng. J. 2024, 483, 149119. [Google Scholar] [CrossRef]
- Miao, J.; Fan, Q.; Li, H.; Yang, Y.; Zhang, Q. Combination of the degrading bacterium Bacillus cereus MZ-1 and corn straw biochar enhanced the removal of imazethapyr from water solutions. Next Sustain. 2024, 5, 100077. [Google Scholar] [CrossRef]
- Xu, S.; Wang, F.; Fu, Y.; Li, D.; Sun, X.; Li, C.; Song, B.; Li, Y. Effects of mixed agro-residues (corn crop waste) on lignin-degrading enzyme activities, growth, and quality of Lentinula edodes. RSC Adv. 2020, 10, 9798–9807. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Liu, G.; Xie, R.; Ming, B.; Yang, Y.; Guo, X.; Wang, K.; Xue, J.; Wang, Y.; et al. Estimation of maize straw production and appropriate straw return rate in China. Agric. Ecosyst. Environ. 2022, 328, 107865. [Google Scholar] [CrossRef]
- Feng, W.; Yang, F.; Cen, R.; Liu, J.; Qu, Z.; Miao, Q.; Chen, H. Effects of straw biochar application on soil temperature, available nitrogen and growth of corn. J. Environ. Manag. 2021, 277, 111331. [Google Scholar] [CrossRef]
- Rodriguez, O.L.; Torres, E.; Zalazar, D.; Zhang, H.; Rodríguez, R.; Mazza, G. Influence of pyrolysis temperature and bio-waste composition on biochar characteristics. Renew. Energy 2020, 155, 837–847. [Google Scholar] [CrossRef]
- Landrat, M.; Abawalo, M.; Pikoń, K.; Fufa, P.A.; Seyid, S. Assessing the Potential of Teff Husk for Biochar Production through Slow Pyrolysis: Effect of Pyrolysis Temperature on Biochar Yield. Energies 2024, 17, 1988. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Zhou, Y. Novel insights into the adsorption of organic contaminants by biochar: A review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sarkar, B.; Aralappanavar, V.K.; Mukhopadhyay, R.; Basak, B.B.; Srivastava, P.; Marchut-Mikołajczyk, O.; Bhatnagar, A.; Bolan, N. Biochar-microorganism interactions for organic pollutant remediation: Challenges and perspectives. Environ. Pollut. 2022, 308, 119609. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Duan, J. Mechanisms of adsorption and functionalization of biochar for pesticides: A review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef]
- Simón, D.; Palet, C.; Costas, A.; Cristóbal, A. Agro-industrial waste as potential heavy metal adsorbents and subsequent safe disposal of spent adsorbents. Water 2022, 14, 3298. [Google Scholar] [CrossRef]
- Blakeney, M. Food Loss and Waste and Food Security. In Food Loss and Food Waste; Edward Elgar Publishing: Cheltenham, UK, 2019; pp. 1–26. [Google Scholar] [CrossRef]
- Flanagan, K.; Robertson, K.; Hanson, C. What is the food loss and waste challenge? In Reducing Food Loss and Waste; Setting the Global Action Agenda; World Resources Institute: Washington, DC, USA, 2019. [Google Scholar]
- Rohini, C.; Geetha, P.; Vijayalakshmi, R.; Mini, M.; Pasupathi, E. Global effects of food waste. J. Pharmacogn. Phytochem. 2020, 9, 690–699. [Google Scholar]
- Saravanan, A.; Swaminaathan, P.; Kumar, P.S.; Yaashikaa, P.; Kamalesh, R.; Rangasamy, G. A comprehensive review on immobilized microbes-biochar and their environmental remediation: Mechanism, challenges and future perspectives. Environ. Res. 2023, 236, 116723. [Google Scholar] [CrossRef]
- Ali, I.M. The Harmful Effects of Pesticides on the Environment and Human Health: A Review. Diyala Agric. Sci. J. 2023, 15, 114–126. [Google Scholar] [CrossRef]
- Wakhungu, C. Loss of soil biodiversity through judicious use of synthetic pesticides, A case study of Trans Nzoia county Kenya-review. Sci. Rep. Life Sci. 2023, 4, 1–24. [Google Scholar] [CrossRef]
- Gilani, R.A.; Rafique, M.; Rehman, A.; Munis, M.F.; Rehman, S.U.; Chaudhary, H.J. Biodegradation of chlorpyrifos by bacterial genus Pseudomonas. J. Basic Microbiol. 2016, 56, 105–119. [Google Scholar] [CrossRef]
- Nandi, N.K.; Vyas, A.; Akhtar, M.J.; Kumar, B. The growing concern of chlorpyrifos exposures on human and environmental health. Pestic. Biochem. Physiol. 2022, 185, 105138. [Google Scholar] [CrossRef]
- Wolejko, E.; Lozowicka, B.; Jablonska-Trypuc, A.; Pietruszynska, M.; Wydro, U. Chlorpyrifos Occurrence and Toxicological Risk Assessment: A Review. Int. J. Environ. Res. Public Health 2022, 19, 12209. [Google Scholar] [CrossRef]
- Celik, I.; Suzek, H. The hematological effects of methyl parathion in rats. J. Hazard. Mater. 2008, 153, 1117–1121. [Google Scholar] [CrossRef]
- Kashni, M.; Arora, R.; Jain, R. An expository note on notorious methyl parathion engendering risk evaluation and its redressal. In Hazardous Chemicals: Overview, Toxicological Profile, Challenges, and Future Perspectives; Chawla, M., Singh, J., Kaushik, R.D., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 105–118. [Google Scholar]
- Jokanović, M.; Oleksak, P.; Kuca, K. Multiple neurological effects associated with exposure to organophosphorus pesticides in man. Toxicology 2023, 484, 153407. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Darabi, R.; Baghayeri, M.; Karimi, F.; Fu, L.; Rouhi, J.; Niculina, D.E.; Gündüz, E.S.; Dragoi, E.N. Recent developments in carbon nanomaterials-based electrochemical sensors for methyl parathion detection. J. Food Meas. Charact. 2023, 17, 5371–5389. [Google Scholar] [CrossRef]
- Gill, H.K.; Garg, H. Pesticide: Environmental impacts and management strategies. Pestic. Toxic Asp. 2014, 8, 10–5772. [Google Scholar] [CrossRef]
- Jari, Y.; Roche, N.; Necibi, M.C.; El Hajjaji, S.; Dhiba, D.; Chehbouni, A. Emerging pollutants in Moroccan wastewater: Occurrence, impact, and removal technologies. J. Chem. 2022, 2022, 9727857. [Google Scholar] [CrossRef]
- Taverna, M.E.; Busatto, C.A.; Lescano, M.R.; Nicolau, V.V.; Zalazar, C.S.; Meira, G.R.; Estenoz, D.A. Microparticles based on ionic and organosolv lignins for the controlled release of atrazine. J. Hazard. Mater. 2018, 359, 139–147. [Google Scholar] [CrossRef]
- Muhammad, F.; Khan, R.; Shafique, M. Optimizing Atrazine Application Rates for Efficacious Weed Control in Maize Cultivation. Indus J. Agric. Biol. 2024, 3, 15–22. [Google Scholar]
- He, H.; Liu, Y.; You, S.; Liu, J.; Xiao, H.; Tu, Z. A review on recent treatment technology for herbicide atrazine in contaminated environment. Int. J. Environ. Res. Public Health 2019, 16, 5129. [Google Scholar] [CrossRef] [PubMed]
- Abd Rani, N.F.; Ahmad Kamil, K.; Aris, F.; Mohamed Yunus, N.; Zakaria, N.A. Atrazine-degrading bacteria for bioremediation strategy: A review. Biocatal. Biotransform. 2022, 40, 233–247. [Google Scholar] [CrossRef]
- Urseler, N.; Bachetti, R.; Morgante, C.; Agostini, E. Bioremediation Strategies to Mitigate the Impact of Atrazine on the Environment: Recent Advances and Prospects. In Agrochemicals in Soil and Environment; Naeem, M., Bremont, J.F.J., Ansari, A.A., Gill, S.S., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Wang, Y.; Ruan, H.; Zhang, J.; Wang, Y.; Guo, M.; Ke, T.; Luo, J.; Yang, M. CHA-based dual signal amplification immunofluorescence biosensor for ultrasensitive detection of dimethomorph. Anal. Chim. Acta 2022, 1227, 340323. [Google Scholar] [CrossRef]
- Hao, K.; Lin, B.; Nian, F.; Gao, X.; Wei, Z.; Luo, G.; Lu, Y.; Lan, M.; Yang, J.; Wu, G. RNA-seq analysis of the response of plant-pathogenic oomycete Phytophthora parasitica to the fungicide dimethomorph. Rev. Argent. Microbiol. 2019, 51, 268–277. [Google Scholar] [CrossRef]
- Parte, S.G.; Mohekar, A.D.; Kharat, A.S. Microbial degradation of pesticide: A review. Afr. J. Microbiol. Res. 2017, 11, 992–1012. [Google Scholar] [CrossRef]
- Umadevi, S.; Ayyasamy, P.M.; Rajakumar, S. Biological Perspective and Role of Bacteria in Pesticide Degradation. In Bioremediation and Sustainable Technologies for Cleaner Environment; Prashanthi, M., Sundaram, R., Jeyaseelan, A., Kaliannan, T., Eds.; Environmental Science and Engineering; Springer: Cham, Switzerland, 2017; pp. 3–12. [Google Scholar] [CrossRef]
- Paul, D.; Mandal, S.M. Microbial Adaptation and Resistance to Pesticides. In Bacterial Adaptation to Co-Resistance; Mandal, S., Paul, D., Eds.; Springer: Singapore, 2019; pp. 233–249. [Google Scholar] [CrossRef]
- Miglani, R.; Parveen, N.; Kumar, A.; Ansari, M.A.; Khanna, S.; Rawat, G.; Panda, A.K.; Bisht, S.S.; Upadhyay, J.; Ansari, M.N. Degradation of xenobiotic pollutants: An environmentally sustainable approach. Metabolites 2022, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Gur Ozdal, O.; Algur, O. Biodegradation α-endosulfan and α-cypermethrin by Acinetobacter schindleri B7 isolated from the microflora of grasshopper (Poecilimon tauricola). Arch. Microbiol. 2022, 204, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ni, Y.; Liu, J.; Yan, T.; Zhu, X.; Li, Q.X.; Hua, R.; Pan, D.; Wu, X. Bead-immobilized Pseudomonas stutzeri Y2 prolongs functions to degrade s-triazine herbicides in industrial wastewater and maize fields. Sci. Total Environ. 2020, 731, 139183. [Google Scholar] [CrossRef]
- Jindakaraked, M.; Khan, E.; Kajitvichyanukul, P. Biodegradation of paraquat by Pseudomonas putida and Bacillus subtilis immobilized on ceramic with supplemented wastewater sludge. Environ. Pollut. 2021, 286, 117307. [Google Scholar] [CrossRef] [PubMed]
- Duc, H.D. Enhancement of carbofuran degradation by immobilized Bacillus sp. strain DT1. Environ. Eng. Res. 2022, 27, 210158. [Google Scholar] [CrossRef]
- Ha, D.D.; Nguyen, T.O. Degradation of propanil by Acinetobacter baumannii DT immobilized in alginate. Vietnam J. Sci. Technol. Eng. 2022, 64, 8–12. [Google Scholar] [CrossRef]
- Torres, E.M.; Hess, D.; McNeil, B.T.; Guy, T.; Quinn, J.C. Impact of inorganic contaminants on microalgae productivity and bioremediation potential. Ecotoxicol. Environ. Saf. 2017, 139, 367–376. [Google Scholar] [CrossRef]
- Kumar, V.S.; Sarkar, D.J.; Sarkar, S.D.; Das, B.K. Microalgal Remediation in the Aquiatic Environment. In Handbook of Aquatic Microbiology; Pandey, P.K., Mallik, S.K., Yumnam, R., Eds.; CRC Press: Boca Raton, FL, USA; Taylos & Francis Group: Abingdon, UK, 2024; pp. 260–270. [Google Scholar]
- Touliabah, H.E.S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A review of microalgae-and cyanobacteria-based biodegradation of organic pollutants. Molecules 2022, 27, 1141. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Ismail, A.F.; Samawati, Z.; Liang, Y.Y.; Kanakaraju, D. Microalgae-enabled wastewater treatment: A sustainable strategy for bioremediation of pesticides. Water 2022, 15, 70. [Google Scholar] [CrossRef]
- Fayaz, T.; Rana, S.S.; Goyal, E.; Ratha, S.K.; Renuka, N. Harnessing the potential of microalgae-based systems for mitigating pesticide pollution and its impact on their metabolism. J. Environ. Manag. 2024, 357, 120723. [Google Scholar] [CrossRef]
- Abdel-Razek, M.A.; Abozeid, A.M.; Eltholth, M.M.; Abouelenien, F.A.; El-Midany, S.A.; Moustafa, N.Y.; Mohamed, R.A. Bioremediation of a pesticide and selected heavy metals in wastewater from various sources using a consortium of microalgae and cyanobacteria. Slov. Vet. Res. 2019, 56, 61–73. [Google Scholar] [CrossRef]
- Cai, X.; Liu, W.; Jin, M.; Lin, K. Relation of diclofop-methyl toxicity and degradation in algae cultures. Environ. Toxicol. Chem. Int. J. 2007, 26, 970–975. [Google Scholar] [CrossRef]
- Habibah, R.; Iswanto, B.; Rinanti, A. The significance of tropical microalgae Chlorella sorokiniana as a remediate of polluted water caused by chlorpyrifos. Int. J. Sci. Technol. Res. 2020, 9, 4460–4463. [Google Scholar]
- Trejo-Carrizalez, I.; Cervantes-González, E. Potential degradation efficiency of Chlorella vulgaris towards methamidophos and diazinon. Algal Res. 2024, 81, 103566. [Google Scholar] [CrossRef]
- Hussein, M.H.; Abdullah, A.M.; Badr El-Din, N.I.; Mishaqa, E.S.I. Biosorption potential of the microchlorophyte Chlorella vulgaris for some pesticides. J. Fertil. Pestic. 2017, 8, 1000177. [Google Scholar] [CrossRef]
- Girelli, A.M.; Astolfi, M.L.; Scuto, F.R. Agro-Industrial Wastes as Potential Carriers for Enzyme Immobilization: A Review. Chemosphere 2020, 244, 125368. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Kuo, C.-H.; Sun, P.-P.; Dong, C.-D. Agro-Industrial Food Waste as a Low-Cost Substrate for Sustainable Production of Industrial Enzymes: A Critical Review. Catalysts 2022, 12, 1373. [Google Scholar] [CrossRef]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of immobilized bacteria for environmental bioremediation: A review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Goswami, M.; Chakraborty, P.; Mukherjee, K.; Mitra, G.; Bhattacharyya, P.; Dey, S.; Tribedi, P. Bioaugmentation and biostimulation: A potential strategy for environmental remediation. J. Microbiol. Exp. 2018, 6, 62–65. [Google Scholar]
- Wu, C.; Zhi, D.; Yao, B.; Zhou, Y.; Yang, Y.; Zhou, Y. Immobilization of microbes on biochar for water and soil remediation: A review. Environ. Res. 2022, 212, 113226. [Google Scholar]
- Bhandari, S.; Poudel, D.K.; Marahatha, R.; Dawadi, S.; Khadayat, K.; Phuyal, S.; Shrestha, S.; Gaire, S.; Basnet, K.; Khadka, U.; et al. Microbial Enzymes Used in Bioremediation. J. Chem. 2021, 1, 8849512. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.V.N.; Rajamohan, N.; Saravanan, R. Microbial degradation of recalcitrant pesticides: A review. Environ. Chem. Lett. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Sehrawat, A.; Phour, M.; Kumar, R.; Sindhu, S.S. Bioremediation of Pesticides: An Eco-Friendly Approach for Environment Sustainability. In Microbial Rejuvenation of Polluted Environment; Microorganisms for Sustainability; Panpatte, D.G., Jhala, Y.K., Eds.; Springer: Singapore, 2021; Volume 1, pp. 23–84. [Google Scholar] [CrossRef]
- Chia, X.K.; Hadibarata, T.; Kristanti, R.A.; Jusoh, M.N.H.; Tan, I.S.; Foo, H.C.Y. The Function of Microbial Enzymes in Breaking down Soil Contaminated with Pesticides: A Review. Bioprocess Biosyst. Eng. 2024, 47, 597–620. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Sun, Y.; Zhou, Y.; Kumar, M.; Usman, M.; Li, J.; Shao, J.; Wang, L.; Tsang, D.C. Bioremediation of water containing pesticides by microalgae: Mechanisms, methods, and prospects for future research. Sci. Total Environ. 2020, 707, 136080. [Google Scholar] [CrossRef] [PubMed]
- Singhal, M.; Jadhav, S.; Sonone, S.S.; Sankhla, M.S.; Kumar, R. Microalgae based sustainable bioremediation of water contaminated by pesticides. Biointerface Res. Appl. Chem. 2021, 12, 149–169. [Google Scholar] [CrossRef]
- de Morais, M.G.; Zaparoli, M.; Lucas, B.F.; Costa, J.A.V. Microalgae for bioremediation of pesticides: Overview, challenges, and future trends. In Algal Biotechnology. Integrated Algal Engineering for Bioenergy, Bioremediation, and Biomedical Applications; Ahmad, A., Banat, F., Taher, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 63–78. [Google Scholar] [CrossRef]
- Narayanan, M.; Devarayan, K.; Verma, M.; Selvaraj, M.; Ghramh, H.A.; Kandasamy, S. Assessing the Ecological Impact of Pesticides/Herbicides on Algal Communities: A Comprehensive Review. Aquat. Toxicol. 2024, 268, 106851. [Google Scholar] [CrossRef]
- Li, R.; Guo, X.; Chen, K.; Zhu, J.; Li, S.; Jiang, J. Isolation of an isocarbophos-degrading strain of Arthrobacter sp. scl-2 and identification of the degradation pathway. J. Microbiol. Biotechnol. 2009, 19, 1439–1446. [Google Scholar]
- Liu, Z.Y.; Chen, X.; Shi, Y.; Su, Z.C. Bacterial Degradation of Chlorpyrifos by Bacillus cereus. In Advanced Materials Research; Trans Tech Publications Ltd.: Bach, Switzerland, 2012; pp. 676–680. [Google Scholar] [CrossRef]
- El-Helow, E.R.; Badawy, M.E.I.; Mabrouk, M.E.M.; Mohamed, E.A.H.; El-Beshlawy, Y.M. Biodegradation of Chlorpyrifos by a Newly Isolated Bacillus subtilis Strain, Y242. Bioremediation J. 2013, 17, 113–123. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, C.X.; Luo, X.J.; Lai, Q.L.; Xu, J.H. Burkholderia jiangsuensis sp. nov., a methyl parathion degrading bacterium, isolated from methyl parathion contaminated soil. Int. J. Syst. Evol. Microbiol. 2014, 64, 3247–3253. [Google Scholar] [CrossRef]
- Zuo, Z.; Gong, T.; Che, Y.; Liu, R.; Xu, P.; Jiang, H.; Qiao, C.; Song, C.; Yang, C. Engineering Pseudomonas putida KT2440 for simultaneous degradation of organophosphates and pyrethroids and its application in bioremediation of soil. Biodegradation 2015, 26, 223–233. [Google Scholar] [CrossRef]
- Gao, J.; Song, P.; Wang, G.; Wang, J.; Zhu, L.; Wang, J. Responses of atrazine degradation and native bacterial community in soil to Arthrobacter sp. strain HB-5. Ecotoxicol. Environ. Saf. 2018, 159, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Morya, R.; Salvachua, D.; Thakur, I.S. Burkholderia: An untapped but promising bacterial genus for the conversion of aromatic compounds. Trends Biotechnol. 2020, 38, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Mali, H.; Shah, C.; Patel, D.H.; Trivedi, U.; Subramanian, R.B. Degradation Insight of Organophosphate Pesticide Chlorpyrifos through Novel Intermediate 2,6-Dihydroxypyridine by Arthrobacter sp. HM01. Bioresour. Bioprocess. 2022, 9, 31. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Han, M.; Zhang, C.; Ho, S.H. Immobilized microalgal system: An achievable idea for upgrading current microalgal wastewater treatment. Environ. Sci. Ecotechnol. 2023, 14, 100227. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.J.; Saravanan, A. Advances in production and application of biochar from lignocellulosic feedstocks for remediation of environmental pollutants. Bioresour. Technol. 2019, 292, 122030. [Google Scholar] [CrossRef]
- Ponnuchamy, M.; Kapoor, A.; Kumar, P.S.; Vo, D.V.N.; Balakrishnan, A.; Jacob, M.M.; Sivaraman, P. Sustainable adsorbents for the removal of pesticides from water: A review. Environ. Chem. Lett. 2021, 19, 2425–2463. [Google Scholar] [CrossRef]
- Fuentes, M.S.; Briceño, G.E.; Saez, J.M.; Benimeli, C.S.; Diez, M.C.; Amoroso, M.J. Enhanced removal of a pesticides mixture by single cultures and consortia of free and immobilized Streptomyces strains. BioMed Res. Int. 2013, 2013, 392573. [Google Scholar] [CrossRef]
- Talwar, M.P.; Ninnekar, H.Z. Biodegradation of pesticide profenofos by the free and immobilized cells of Pseudoxanthomonas suwonensis strain HNM. J. Basic Microbiol. 2015, 55, 1094–1103. [Google Scholar] [CrossRef]
- Mustapha, M.U.; Halimoon, N.; Johari, W.L.W.; Shukor, M.Y.A. Enhanced carbofuran degradation using immobilized and free cells of Enterobacter sp. isolated from soil. Molecules 2020, 25, 2771. [Google Scholar] [CrossRef]
- Najim, A.A.; Radeef, A.Y.; al-Doori, I.; Jabbar, Z.H. Immobilization: The promising technique to protect and increase the efficiency of microorganisms to remove contaminants. J. Chem. Technol. Biotechnol. 2024, 99, 1707–1733. [Google Scholar] [CrossRef]
- Ortiz-Hernández, M.L.; Sánchez-Salinas, E.; Dantán-González, E.; Castrejón-Godínez, M.L. Pesticide biodegradation: Mechanisms, genetics and strategies to enhance the process. In Biodegradation-Life of Science; Chamy, R., Rosenkranz, F., Eds.; IntechOpen: Rijeka, Croatia, 2013; Volume 10, pp. 251–287. [Google Scholar] [CrossRef]
- Conde-Avila, V.; Ortega-Martínez, L.D.; Loera, O.; El Kassis, E.G.; Dávila, J.G.; Valenzuela, C.M.; Armendáriz, B.P. Pesticides degradation by immobilised microorganisms. Int. J. Environ. Anal. Chem. 2021, 101, 2975–3005. [Google Scholar] [CrossRef]
- World Health Organization. Inter-Organization Programme for the Sound Management of Chemicals. In The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019; World Health Organization: Geneva, Switzerland, 2020; 92p, Available online: https://www.who.int/publications/i/item/9789240005662 (accessed on 26 January 2025).


| Agro-Industrial Waste (Support) | Pesticide | Microorganism | Pesticide Concentration (mg/L) | Degradation Time (h) | Pesticide Degradation (%) Free Cells | Pesticide Degradation (%) Immobilized Cells | Free Cells Pesticide Degradation Rate (mg/L∗h) § | Immobilized Cells Pesticide Degradation Rate (mg/L∗h) § | t-Student Test | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Herbicides | ||||||||||
| Corn straw | Atrazine | Arthrobacter sp. ZXY-2 | 50 | 1 | 90 ± 16.4 | 100 ± 0.0 | 45 ± 8.2 | 50 | 1.056 ns | [43] |
| Spent mushroom substrate | Thifensulfuron-methyl | Serratia marcescens N80 | 50 | 48 | 83.5 ± 3.6 | 89.4 ± 3.1 | 0.9 ± 0.04 | 0.9 ± 0.03 | 2.151 ns | [45] |
| Rice straw | Diuron | Bacillus subtilis DU1 | 20 | 36 | 70 ± 8.6 | 100 | 0.4 ± 0.05 | 0.6 | 6.042 *** | [47] |
| Acinetobacter baumannii DU | ||||||||||
| Pseudomonas sp. DUK | ||||||||||
| Corn cob | Paraquat | Pseudomonas putida TISTR 1522 | 26 | 24 | 12.1 | 15.8 | 0.1 | 0.2 | - | [49] |
| Corn straw | Atrazine | Acinetobacter lwoffii DNS32 | 100 | 12 | 88.6 | 100 | 7.4 | 8.3 | - | [42] |
| Coconut fiber | Paraquat | Pseudomonas putida | 30 | 48 | 6.7 | 95.8 | 0.04 | 0.6 | - | [46] |
| Corn straw | Imazethapyr | Bacillus cereus MZ-1 | 200 | 18 | 52.7 | 79.9 | 5.9 | 8.9 | - | [52] |
| Insecticides | ||||||||||
| Loofah (Luffa cylindrica) | Methyl parathion | Undefined bacterial consortium | 25 | 72 | 54.9 ± 3.8 | 98 ± 0.4 | 0.2 ± 0.1 | 0.3 ± 0.0 | 19.54 *** | [40] |
| Loofah (Luffa cylindrica) | Coumaphos | 5 | 62 ± 2 | 100 ± 0.0 | 0.04 ± 0.2 | 0.07 ± 0.0 | 32.91 *** | |||
| Bamboo | Thiamethoxam | Chryseobacterium spH5 | 14 | 24 | 25 ± 0.0 | 74.0 ± 2.4 | 0.2 ± 0.0 | 0.4 ± 0.01 | 35.36 *** | [48] |
| Rice husk | Chlorpyrifos | Bacillus H27 | 25 | 168 | 89.6 ± 1 | 97.4 ± 0.5 | 0.1 ± 0.0 | 0.1 ± 0.0 | 6.042 ** | [50] |
| Rice husk | Chlorpyrifos | Aeromonas veronii | 30 | 24 | 77.9 ± 1.6 | 96.3 ± 1.1 | 1.0 ± 0.02 | 1.2 ± 0.01 | 21.13 *** | [51] |
| Nopal fiber | Methyl parathion | Burkholderia cenocepacia CEIB S5-2 | 50 | 1 | 7.4 | 100 | 3.7 | 50 | - | [41] |
| Agave fiber | - | |||||||||
| Not specified ‡ | Chlorpyrifos | Chlorella vulgaris | 0.5 | 72 | 63.9 | 87.2 | 0.004 | 0.006 | - | [39] |
| Not specified ‡ | Cypermethrin | 64.2 | 93.4 | 0.004 | 0.006 | - | ||||
| Fungicide | ||||||||||
| Bamboo | Dimethomorph | Bacillus cereus WL08 | 50 | 72 | 66.9 ± 2.4 | 96.9 | 0.5 ± 0.02 | 0.7 | 32.91 *** | [45] |
| Average pesticide degradation | 61.8±26.8 | 92.2 ± 15.4 | - | - | 5.819 *** | - | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Castro, E.; Castrejón-Godínez, M.L.; Mussali-Galante, P.; Tovar-Sánchez, E.; Rodríguez, A. Pesticides Degradation Through Microorganisms Immobilized on Agro-Industrial Waste: A Promising Approach for Their Elimination from Aquatic Environments. Processes 2025, 13, 1073. https://doi.org/10.3390/pr13041073
Arias-Castro E, Castrejón-Godínez ML, Mussali-Galante P, Tovar-Sánchez E, Rodríguez A. Pesticides Degradation Through Microorganisms Immobilized on Agro-Industrial Waste: A Promising Approach for Their Elimination from Aquatic Environments. Processes. 2025; 13(4):1073. https://doi.org/10.3390/pr13041073
Chicago/Turabian StyleArias-Castro, Esmeralda, María Luisa Castrejón-Godínez, Patricia Mussali-Galante, Efraín Tovar-Sánchez, and Alexis Rodríguez. 2025. "Pesticides Degradation Through Microorganisms Immobilized on Agro-Industrial Waste: A Promising Approach for Their Elimination from Aquatic Environments" Processes 13, no. 4: 1073. https://doi.org/10.3390/pr13041073
APA StyleArias-Castro, E., Castrejón-Godínez, M. L., Mussali-Galante, P., Tovar-Sánchez, E., & Rodríguez, A. (2025). Pesticides Degradation Through Microorganisms Immobilized on Agro-Industrial Waste: A Promising Approach for Their Elimination from Aquatic Environments. Processes, 13(4), 1073. https://doi.org/10.3390/pr13041073










