Fe3+ and Mn2+ Removal from Water Solutions by Clinoptilolite Zeolites as a Potential Treatment for Groundwater Wells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Zeolite Activation
2.3. Groundwater Wells: Collect and Characterization
2.4. Adsorption Experiments
2.5. Kinetic and Isotherm Studies
2.5.1. Adsorption Kinetic Modeling
2.5.2. Adsorption Isotherm Modeling
2.5.3. Statistical and Computational Details
3. Results and Discussion
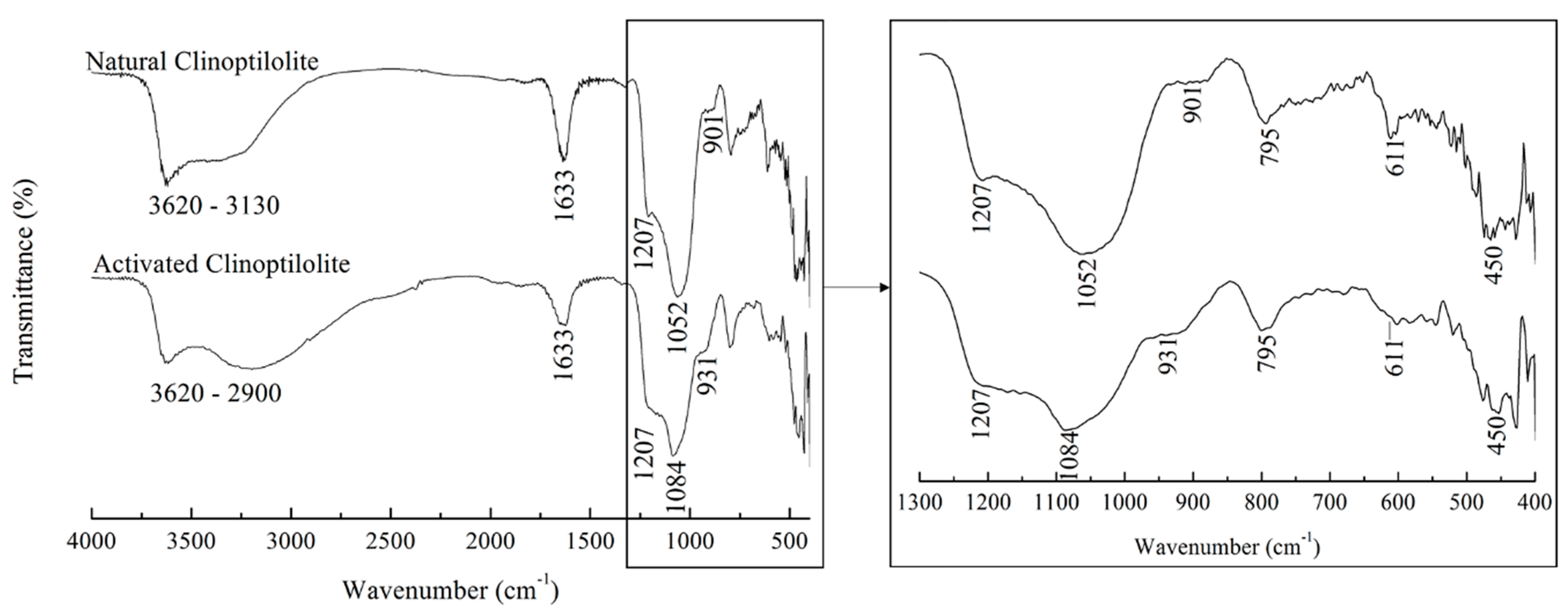
3.1. Clinoptilolites’ Functional Groups
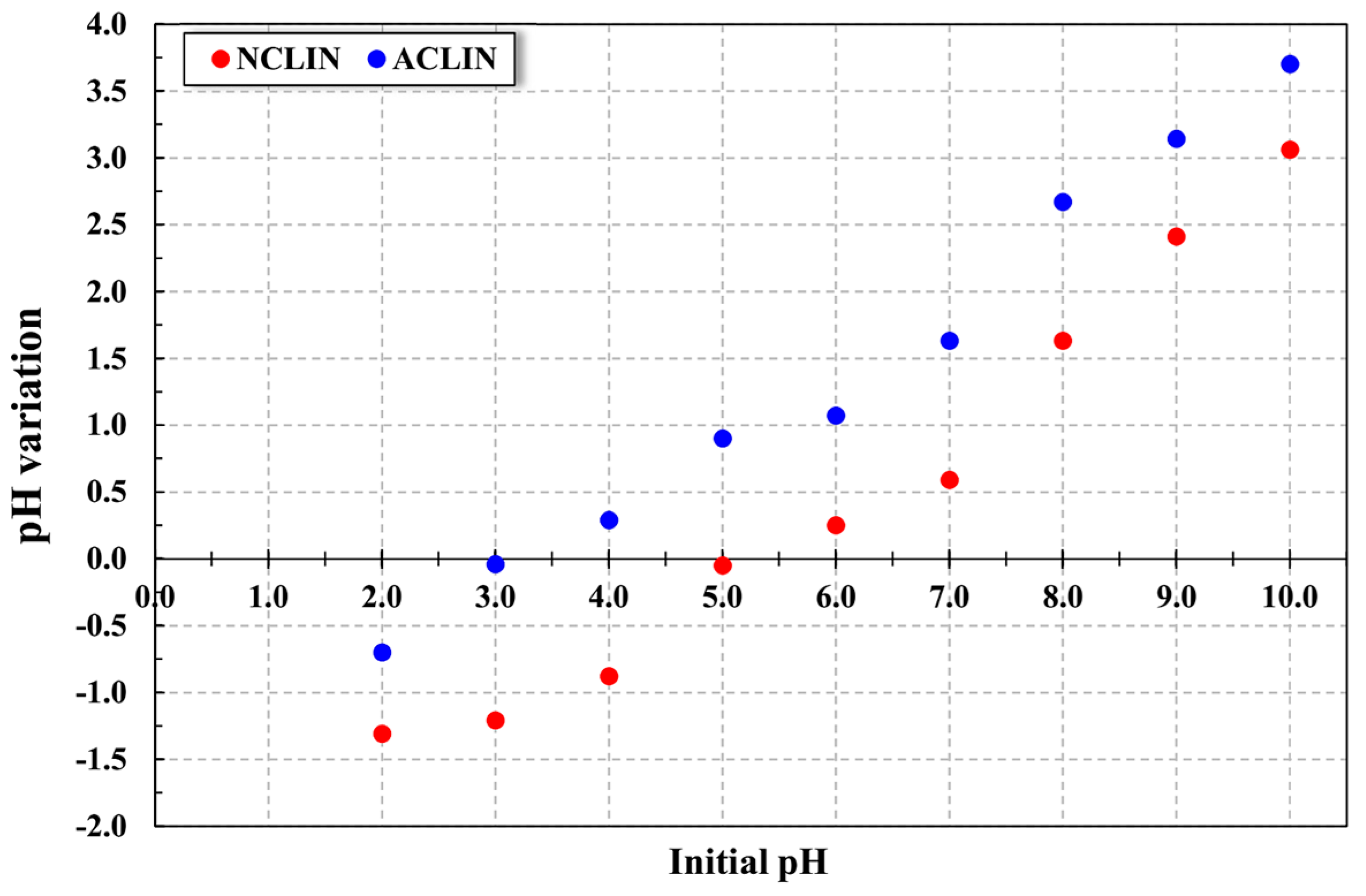
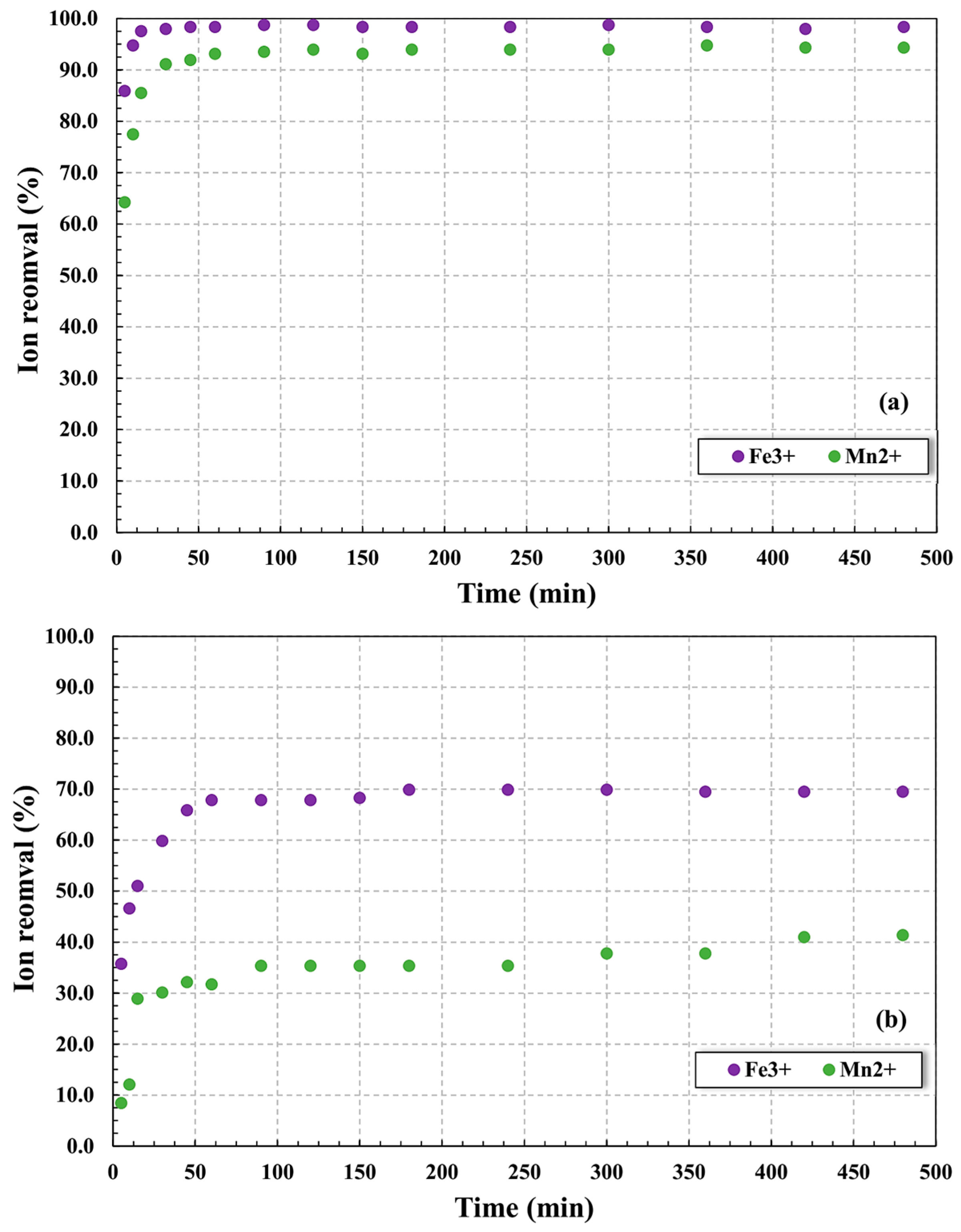
3.2. Adsorptive Process
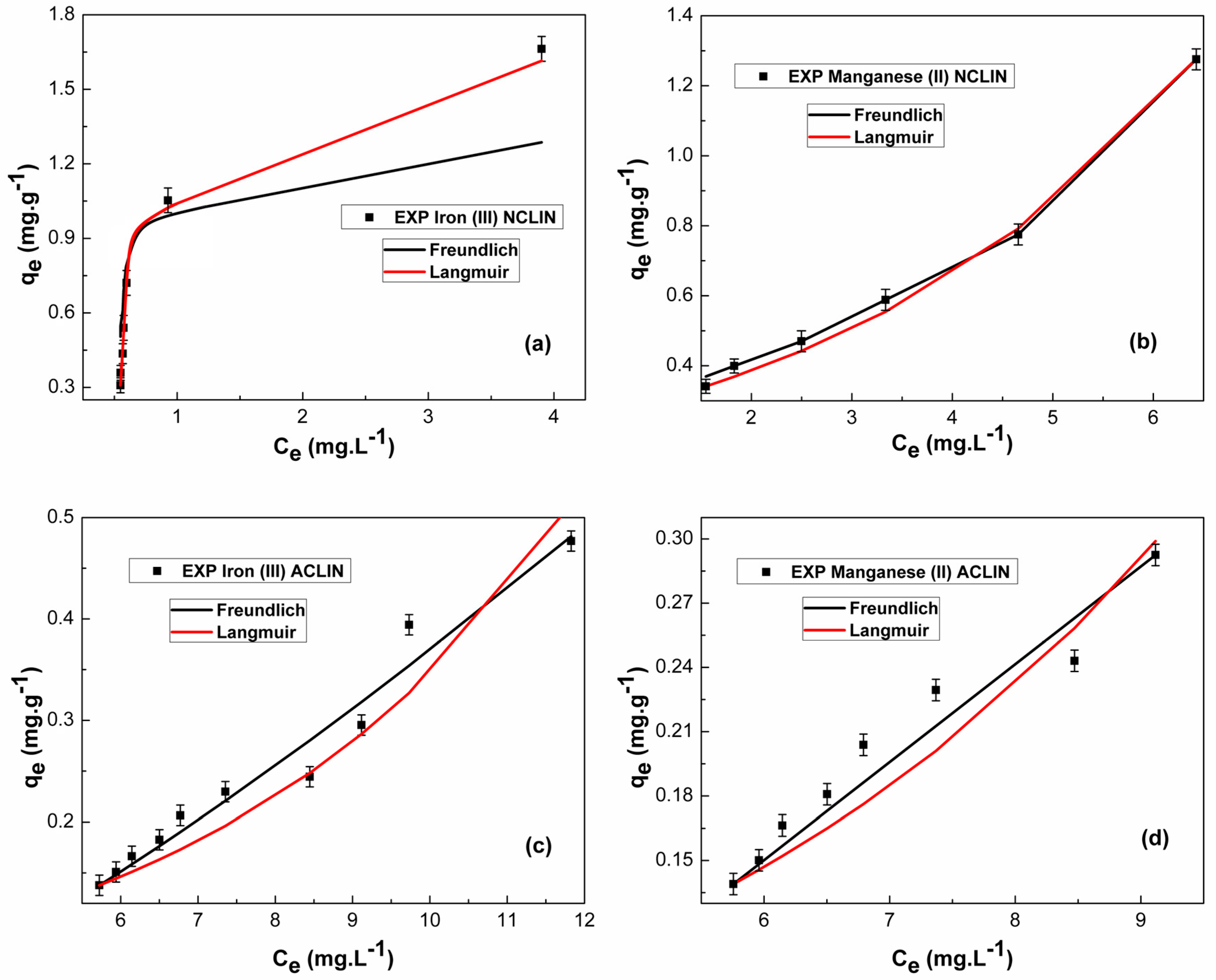
3.3. Process Modeling
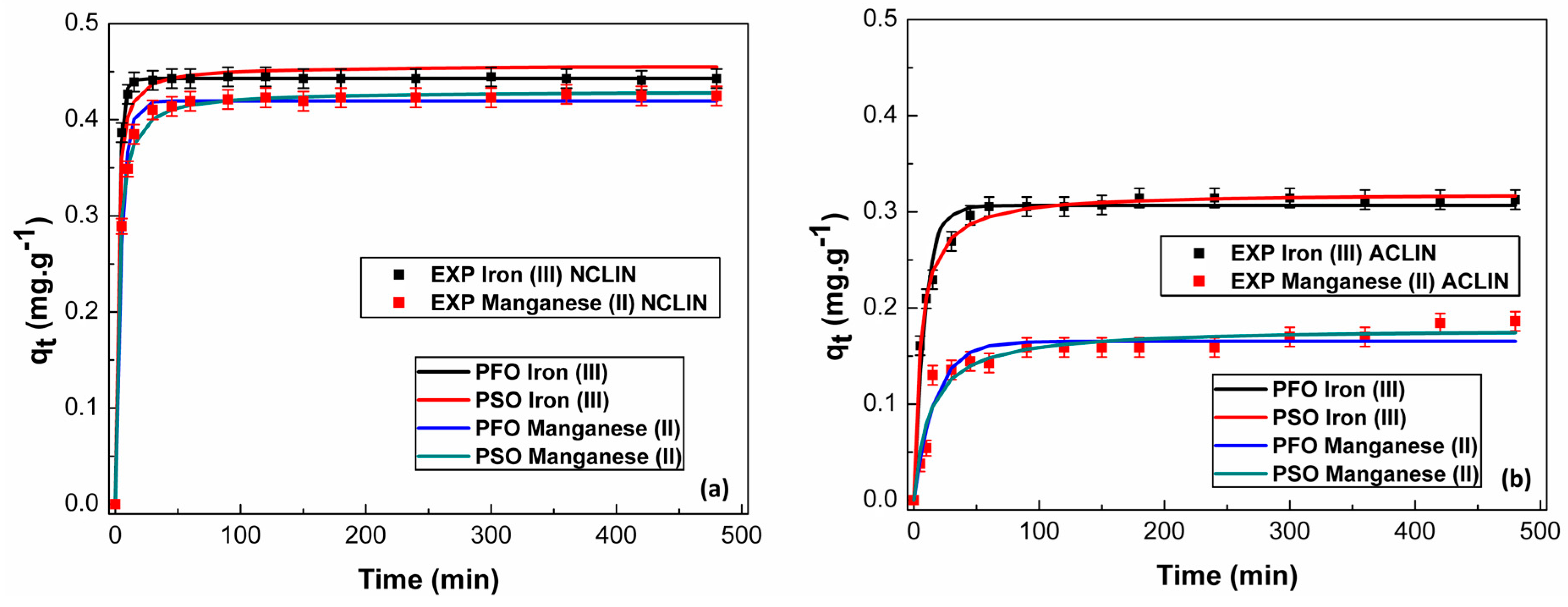
3.3.1. Adsorption Kinetics
3.3.2. Adsorption Isotherms
3.4. The Guarani Aquifer: A Preliminary Analysis of Its Groundwater Wells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNESCO. The United Nations World Water Development Report: Valuing Water; UNESCO: Perugia, Italy, 2022. [Google Scholar]
- Biswas, A.K.; Tortajada, C. Water Crisis and Water Wars: Myths and Realities. Int. J. Water Resour. Dev. 2019, 35, 727–731. [Google Scholar] [CrossRef]
- UNESCO Groundwater: Making the Invisible Visible. Available online: https://www.unesco.org/en/articles/groundwater-making-invisible-visible (accessed on 12 December 2024).
- Che Nordin, N.F.; Mohd, N.S.; Koting, S.; Ismail, Z.; Sherif, M.; El-Shafie, A. Groundwater Quality Forecasting Modelling Using Artificial Intelligence: A Review. Groundw. Sustain. Dev. 2021, 14, 100643. [Google Scholar] [CrossRef]
- Padikkal, S.; Sumam, K.S.; Sajikumar, N. Sustainability Indicators of Water Sharing Compacts. Environ. Dev. Sustain. 2018, 20, 2027–2042. [Google Scholar] [CrossRef]
- Teramoto, E.H.; Gonçalves, R.D.; Chang, H.K. Hydrochemistry of the Guarani Aquifer System Modulated by Mixing with Underlying and Overlying Hydrostratigraphic Units. J. Hydrol. Reg. Stud. 2020, 30, 100713. [Google Scholar] [CrossRef]
- Hirata, R.; Kirchheim, R.E.; Manganelli, A. Diplomatic Advances and Setbacks of the Guarani Aquifer System in South America. Environ. Sci. Policy 2020, 114, 384–393. [Google Scholar] [CrossRef]
- Noor, A.E.; Fatima, R.; Aslam, S.; Hussain, A.; un Nisa, Z.; Khan, M.; Mohammed, A.A.A.; Sillanpaa, M. Health Risks Assessment and Source Admeasurement of Potentially Dangerous Heavy Metals (Cu, Fe, and Ni) in Rapidly Growing Urban Settlement. Environ. Res. 2024, 242, 117736. [Google Scholar] [CrossRef]
- Wessling-Resnick, M. Excess Iron: Considerations Related to Development and Early Growth. Am. J. Clin. Nutr. 2017, 106, 1600S–1605S. [Google Scholar] [CrossRef]
- Ghosh, G.C.; Khan, M.J.H.; Chakraborty, T.K.; Zaman, S.; Kabir, A.H.M.E.; Tanaka, H. Human Health Risk Assessment of Elevated and Variable Iron and Manganese Intake with Arsenic-Safe Groundwater in Jashore, Bangladesh. Sci. Rep. 2020, 10, 5206. [Google Scholar] [CrossRef]
- Yuce, G.; Alptekin, C. In Situ and Laboratory Treatment Tests for Lowering of Excess Manganese and Iron in Drinking Water Sourced from River–Groundwater Interaction. Environ. Earth Sci. 2013, 70, 2827–2837. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of Heavy Metals on Natural Zeolites: A Review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Dehmani, Y.; Ba Mohammed, B.; Oukhrib, R.; Dehbi, A.; Lamhasni, T.; Brahmi, Y.; El-Kordy, A.; Franco, D.S.P.; Georgin, J.; Lima, E.C.; et al. Adsorption of Various Inorganic and Organic Pollutants by Natural and Synthetic Zeolites: A Critical Review. Arab. J. Chem. 2024, 17, 105474. [Google Scholar] [CrossRef]
- Das, S.; Singh, C.K.; Sodhi, K.K.; Singh, V.K. Circular Economy Approaches for Water Reuse and Emerging Contaminant Mitigation: Innovations in Water Treatment. Environ. Dev. Sustain. 2023, 27, 5753–5794. [Google Scholar] [CrossRef]
- El-habacha, M.; Miyah, Y.; Lagdali, S.; Mahmoudy, G.; Dabagh, A.; Chiban, M.; Sinan, F.; Iaich, S.; Zerbet, M. General Overview to Understand the Adsorption Mechanism of Textile Dyes and Heavy Metals on the Surface of Different Clay Materials. Arab. J. Chem. 2023, 16, 105248. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural Zeolites as Effective Adsorbents in Water and Wastewater Treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Schiavo, L.; Cammarano, A.; Carotenuto, G.; Longo, A.; Palomba, M.; Nicolais, L. An Overview of the Advanced Nanomaterials Science. Inorg. Chim. Acta 2024, 559, 121802. [Google Scholar] [CrossRef]
- Zanin, E.; Scapinello, J.; de Oliveira, M.; Rambo, C.L.; Franscescon, F.; Freitas, L.; de Mello, J.M.M.; Fiori, M.A.; Oliveira, J.V.; Dal Magro, J. Adsorption of Heavy Metals from Wastewater Graphic Industry Using Clinoptilolite Zeolite as Adsorbent. Process Saf. Environ. Prot. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Vaezi, M.; Noormohammadbeigi, M.; Cruciani, G.; Zendehdel, M. Ion-Exchange of Copper into Mordenite and Clinoptilolite Zeolites by Molecular Dynamics Simulations and Experimental Investigations. Microporous Mesoporous Mater. 2025, 382, 113397. [Google Scholar] [CrossRef]
- Munir, T.; Zhou, J.; Liu, M.; Bai, S.; Sun, J. Interfacial Adsorption for Efficient Removal of Fe3+, Mn2+, Ni2+, Cd2+, and Sr2+ from Wastewater Using Synthetic Clinoptilolite via Single-Treatment Approach. Chem. Eng. Sci. 2025, 305, 121098. [Google Scholar] [CrossRef]
- Chuesutham, T.; Sirivat, A.; Paradee, N.; Changkhamchom, S.; Wattanakul, K.; Anumart, S.; Krathumkhet, N.; Khampim, J. Improvement of Sulfonated Poly(Ether Ether Ketone)/Y Zeolite -SO3H via Organo-Functionalization Method for Direct Methanol Fuel Cell. Renew. Energy 2019, 138, 243–249. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater; SIDALC: Washington DC, USA, 2017. [Google Scholar]
- Aziz, K.; Aziz, F.; Mamouni, R.; Aziz, L.; Anfar, Z.; Azrrar, A.; Kjidaa, B.; Saffaj, N.; Laknifli, A. High Thiabendazole Fungicide Uptake Using Cellana Tramoserica Shells Modified by Copper: Characterization, Adsorption Mechanism, and Optimization Using CCD-RSM Approach. Environ. Sci. Pollut. Res. 2022, 29, 86020–86035. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the Adsorption Kinetics Models for the Removal of Contaminants from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Hodaifa, G.; Ochando-Pulido, J.M.; Driss Alami, S.B.; Rodriguez-Vives, S.; Martinez-Ferez, A. Kinetic and Thermodynamic Parameters of Iron Adsorption onto Olive Stones. Ind. Crops Prod. 2013, 49, 526–534. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo-First Order and Pseudo-Second Order Rate Laws: A Review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Gelöster Stoff. K. Sven. Vetenskapasakademiens Handl. 1989, 4, 1–39. [Google Scholar]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Gemici, B.T.; Ozel, H.U.; Ozel, H.B. Removal of Methylene Blue onto Forest Wastes: Adsorption Isotherms, Kinetics and Thermodynamic Analysis. Environ. Technol. Innov. 2021, 22, 101501. [Google Scholar] [CrossRef]
- Bensalah, J. Removal of the Textile Dyes by a Resin Adsorbent Polymeric: Insight into Optimization, Kinetics and Isotherms Adsorption Phenomenally. Inorg. Chem. Commun. 2024, 161, 111975. [Google Scholar] [CrossRef]
- Garba, Z.N.; Imam, M.; Adamu, H.; Agbaji, E.B. Optimization of Adsorption Conditions for Acid Chrome Blue K Removal from Aqueous Solution Using Sugar-Based Activated Carbon: Equilibrium Isotherms and Kinetics Modeling. Sustain. Chem. One World 2024, 1, 100001. [Google Scholar] [CrossRef]
- Freundlich, H. Colloid & Capillary Chemistry, 3rd ed.; Methuen & Company Limited: Malton, UK, 1926. [Google Scholar]
- Ketzer, F.; Wancura, J.H.C.; Tres, M.V.; de Oliveira, J.V. Kinetic and Thermodynamic Study of Enzymatic Hydroesterification Mechanism to Fatty Acid Methyl Esters Synthesis. Bioresour. Technol. 2022, 356, 127335. [Google Scholar] [CrossRef]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Akpa, O.M.; Unuabonah, E.I. Small-Sample Corrected Akaike Information Criterion: An Appropriate Statistical Tool for Ranking of Adsorption Isotherm Models. Desalination 2011, 272, 20–26. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Hubadillah, S.K.; Abd Aziz, M.H.; Jamalludin, M.R. Application of Natural Zeolite Clinoptilolite for the Removal of Ammonia in Wastewater. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Bellaj, M.; Aziz, K.; El Achaby, M.; El Haddad, M.; Gebrati, L.; Kurniawan, T.A.; Chen, Z.; Yap, P.-S.; Aziz, F. Cationic and Anionic Dyes Adsorption from Wastewater by Clay-Chitosan Composite: An Integrated Experimental and Modeling Study. Chem. Eng. Sci. 2024, 285, 119615. [Google Scholar] [CrossRef]
- Aghadavoud, A.; Rezaee Ebrahim Saraee, K.; Shakur, H.R.; Sayyari, R. Removal of Uranium Ions from Synthetic Wastewater Using ZnO/Na-Clinoptilolite Nanocomposites. Radiochim. Acta 2016, 104, 809–819. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Qiu, X.; Meng, Y.; Wang, H.; Zhou, S.; Qiao, Q.; Yan, C. Clinoptilolite Based Zeolite-Geopolymer Hybrid Foams: Potential Application as Low-Cost Sorbents for Heavy Metals. J. Environ. Manag. 2023, 330, 117167. [Google Scholar] [CrossRef]
- Salvestrini, S.; Sagliano, P.; Iovino, P.; Capasso, S.; Colella, C. Atrazine Adsorption by Acid-Activated Zeolite-Rich Tuffs. Appl. Clay Sci. 2010, 49, 330–335. [Google Scholar] [CrossRef]
- Giraldo-Bareño, Y.Y.; Pinzón-García, A.D.; Sousa, D.V.M.; Bomfim Filho, L.F.O.; Lopes, D.H.A.; Cortés, N.S.; Morávia, M.C.S.A.; Sinisterra, R.D.; Orlando, R.M. Efficient and Easily Scaled-up Biosorbent Based on Natural and Chemically Modified Macauba (Acrocomia Aculeata) to Remove Al3+, Mn2+ and Fe3+ from Surface Water Contaminated with Iron Mining Tailings. Talanta 2023, 256, 124273. [Google Scholar] [CrossRef]
- Gomaa, H.; Shenashen, M.A.; Yamaguchi, H.; Alamoudi, A.S.; Abdelmottaleb, M.; Cheira, M.F.; Seaf El-Naser, T.A.; El-Safty, S.A. Highly-Efficient Removal of AsV, Pb2+, Fe3+, and Al3+ Pollutants from Water Using Hierarchical, Microscopic TiO2 and TiOF2 Adsorbents through Batch and Fixed-Bed Columnar Techniques. J. Clean. Prod. 2018, 182, 910–925. [Google Scholar] [CrossRef]
- Biblioteca, I.; Sambucci, M.; Valente, M. Zeolite-Clinoptilolite Conditioning for Improved Heavy Metals Ions Removal: A Preliminary Assessment. Ceram. Int. 2023, 49, 39649–39656. [Google Scholar] [CrossRef]
- Ketzer, F.; de Castilhos, F. An Assessment on Kinetic Modeling of Esterification Reaction from Oleic Acid and Methyl Acetate over USY Zeolite. Microporous Mesoporous Mater. 2021, 314, 110890. [Google Scholar] [CrossRef]
- Xie, F.; Fan, R.; Yi, Q.; Zhang, Q.; Luo, Z. NaOH Modification of Persimmon Powder-Formaldehyde Resin to Enhance Cu2+ and Pb2+ Removal from Aqueous Solution. Procedia Environ. Sci. 2016, 31, 817–826. [Google Scholar] [CrossRef]
- Carvalho, F.L.; Pinto, D.; Schio, R.R.; dos Santos, J.P.; Ketzer, F.; Silva, L.F.O.; Dotto, G.L. Polishing of Painting Process Effluents through Adsorption with Biochar from Winemaking Residues. Environ. Sci. Pollut. Res. 2022, 29, 66348–66358. [Google Scholar] [CrossRef]
- Emre, D.; Zorer, Ö.S.; Bilici, A.; Budak, E.; Yilmaz, S.; Kilic, N.C.; Sogut, E.G. Adsorption of Uranium (VI) from Aqueous Solutions Using Boron Nitride/Polyindole Composite Adsorbent. J. Appl. Polym. Sci. 2024, 141, 54856. [Google Scholar] [CrossRef]
- Nizam, T.; Krishnan, K.A.; Joseph, A.; Krishnan, R.R. Isotherm, Kinetic and Thermodynamic Modelling of Liquid Phase Adsorption of the Heavy Metal Ions Zn(II), Pb(II) and Cr(VI) onto MgFe2O4 Nanoparticles. Groundw. Sustain. Dev. 2024, 25, 101120. [Google Scholar] [CrossRef]
- Robati, D. Pseudo-Second-Order Kinetic Equations for Modeling Adsorption Systems for Removal of Lead Ions Using Multi-Walled Carbon Nanotube. J. Nanostructure Chem. 2013, 3, 55. [Google Scholar] [CrossRef]
- Srivastava, V.; Iftekhar, S.; Wang, Z.; Babu, I.; Sillanpää, M. Synthesis and Application of Biocompatible Nontoxic Nanoparticles for Reclamation of Ce3+ from Synthetic Wastewater: Toxicity Assessment, Kinetic, Isotherm and Thermodynamic Study. J. Rare Earths 2018, 36, 994–1006. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Wu, P.; Zhang, J.; Zhang, X.; Chen, M.; Cao, X.; Feng, Q. Revealing the Role of Calcium Alginate-Biochar Composite for Simultaneous Removing SO42− and Fe3+ in AMD: Adsorption Mechanisms and Application Effects. Environ. Pollut. 2023, 329, 121702. [Google Scholar] [CrossRef] [PubMed]
- Abouzayed, F.I.; El-nassr, N.T.A.; Abouel-Enein, S.A. Synthesis, Characterization of Functionalized Grafted Cellulose and Its Environmental Application in Uptake of Copper (II), Manganese (II) and Iron (III) Ions. J. Mol. Struct. 2022, 1270, 133907. [Google Scholar] [CrossRef]
- Shukla, V.; Panchal, D.; Prakash, O.; Mondal, P.; Hiwrale, I.; Dhodapkar, R.S.; Pal, S. Magnetically Engineered Sulfurized Peat-Based Activated Carbon for Remediation of Emerging Pharmaceutical Contaminants. Bioresour. Technol. 2023, 369, 128399. [Google Scholar] [CrossRef]
- Sami Abboud, A.; Ghaffarinejad, A.; Mollahosseini, A. Removal of Lead and Cadmium Ions from Wastewater Using Magnetic Alginate Impregnated Sulfur. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100874. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Nawa, N.; Miyazaki, K. The Analysis of Saltwater Intrusion through Komesu Underground Dam and Water Quality Management for Salinity. Paddy Water Environ. 2009, 7, 71–82. [Google Scholar] [CrossRef]
- Brazilian Ministry of Health. Ordinance n. 888 of 4th May 2021; Brazilian Ministry of Health: Brasilia, Brazil, 2021; p. 21.
| Pseudo-First-Order | |||||
| Element/Catalyst | (min−1) | (mg·g−1) | R2 | ||
| Fe3+/NCLIN | 0.3841 ± 0.0017 | 0.443 ± 0.002 | 0.9991 | −172.24 | 0.6328 |
| Mn2+/NCLIN | 0.2073 ± 0.0217 | 0.418 ± 0.006 | 0.9933 | −186.92 | 0.7416 |
| Fe3+/ACLIN | 0.1132 ± 0.0085 | 0.307 ± 0.009 | 0.9782 | −175.45 | 0.3488 |
| Mn2+/ACLIN | 0.0595 ± 0.0189 | 0.165 ± 0.001 | 0.9349 | −172.13 | 0.2378 |
| Pseudo-Second-Order | |||||
| (g.mg−1.min−1) | (mg·g−1) | R2 | |||
| Fe3+/NCLIN | 1.6349 ± 0.0204 | 0.456 ± 0.008 | 0.9853 | −217.37 | 0.6981 |
| Mn2+/NCLIN | 1.0356 ± 0.063 | 0.430 ± 0.003 | 0.9979 | −207.17 | 0.7643 |
| Fe3+/ACLIN | 0.6051 ± 0.0066 | 0.320 ± 0.003 | 0.9967 | −205.49 | 0.4722 |
| Mn2+/ACLIN | 0.4473 ± 0.1876 | 0.179 ± 0.001 | 0.9436 | −174.92 | 0.2273 |
| Element | Isotherm Model | Parameters | Statistics | ||
|---|---|---|---|---|---|
| Fe3+/NCLIN | Freundlich | = 1.8199 ± 3.4541 | R2 = 0.6843 | = −13.55 | = 0.3433 |
| Langmuir | = 172.4457 ± 27.1223 | R2 = 0.9425 | = −124.43 | = 0.7828 | |
| Mn2+/NCLIN | Freundlich | = 1.1171 ± 0.4919 | R2 = 0.9953 | = −34.83 | = 0.5748 |
| Langmuir | = 406.5852 ± 32.2788 | R2 = 0.9947 | = −36.95 | = 0.8287 | |
| Fe3+/ACLIN | Freundlich | = 4.6559 ± 2.2774 | R2 = 0.9647 | = −89.28 | = 0.4540 |
| Langmuir | = 751.2998 ± 43.2478 | R2 = 0.9202 | = −82.30 | = 0.7931 | |
| Mn2+/ACLIN | Freundlich | = 0.5741 ± 0.3742 | R2 = 0.9402 | = −74.44 | = 0.6769 |
| Langmuir | = 872.8606 ± 9.1299 | R2 = 0.8782 | = −68.75 | = 0.8199 |
| Parameter | Limit a | SJO | ABV | CCO |
|---|---|---|---|---|
| Total iron (mg L−1) | 0.30 | 0.10 | 0.40 | 0.01 |
| Manganese (mg L−1) | 0.10 | 0.08 | 0.03 | 0.13 |
| Aluminum (mg L−1) | 0.20 | 0.00 | 0.00 | 0.00 |
| Total alkalinity (mg L−1) | 200.00 | 142.00 | 366.00 | 66.00 |
| Chlorides (mg L−1) | 250.00 | 187.15 | 115.18 | 169.55 |
| Fluoride (mg L−1) | 1.50 | 1.84 | 1.58 | 1.76 |
| Sulfate (mg L−1) | 250.00 | 161.50 | 141.98 | 126.63 |
| Total hardness (mg L−1) | 300.00 | 208.00 | 190.00 | 146.00 |
| Apparent color (uH) | 15.00 | 0.00 | 2.40 | 0.00 |
| pH | 6.0 to 9.5 | 9.30 | 9.50 | 9.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arenhardt, W.D.; Ketzer, F.; Wancura, J.H.C.; Seraglio, J.; Carasek, F.L.; Zin, G.; Calisto, J.F.F.; Rodrigues, C.A.; de Meneses, A.C.; Oliveira, J.V.; et al. Fe3+ and Mn2+ Removal from Water Solutions by Clinoptilolite Zeolites as a Potential Treatment for Groundwater Wells. Processes 2025, 13, 1060. https://doi.org/10.3390/pr13041060
Arenhardt WD, Ketzer F, Wancura JHC, Seraglio J, Carasek FL, Zin G, Calisto JFF, Rodrigues CA, de Meneses AC, Oliveira JV, et al. Fe3+ and Mn2+ Removal from Water Solutions by Clinoptilolite Zeolites as a Potential Treatment for Groundwater Wells. Processes. 2025; 13(4):1060. https://doi.org/10.3390/pr13041060
Chicago/Turabian StyleArenhardt, William D., Felipe Ketzer, João H. C. Wancura, Janaina Seraglio, Fabio L. Carasek, Guilherme Zin, Jean F. F. Calisto, Clovis A. Rodrigues, Alessandra C. de Meneses, José Vladimir Oliveira, and et al. 2025. "Fe3+ and Mn2+ Removal from Water Solutions by Clinoptilolite Zeolites as a Potential Treatment for Groundwater Wells" Processes 13, no. 4: 1060. https://doi.org/10.3390/pr13041060
APA StyleArenhardt, W. D., Ketzer, F., Wancura, J. H. C., Seraglio, J., Carasek, F. L., Zin, G., Calisto, J. F. F., Rodrigues, C. A., de Meneses, A. C., Oliveira, J. V., & Dal Magro, J. (2025). Fe3+ and Mn2+ Removal from Water Solutions by Clinoptilolite Zeolites as a Potential Treatment for Groundwater Wells. Processes, 13(4), 1060. https://doi.org/10.3390/pr13041060






