Effect of Cr Doping on Microstructure and Hydrogen Storage Properties of Zr0.8Ti0.2CrxCo1−x (x = 0, 0.05, 0.1, 0.15) Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
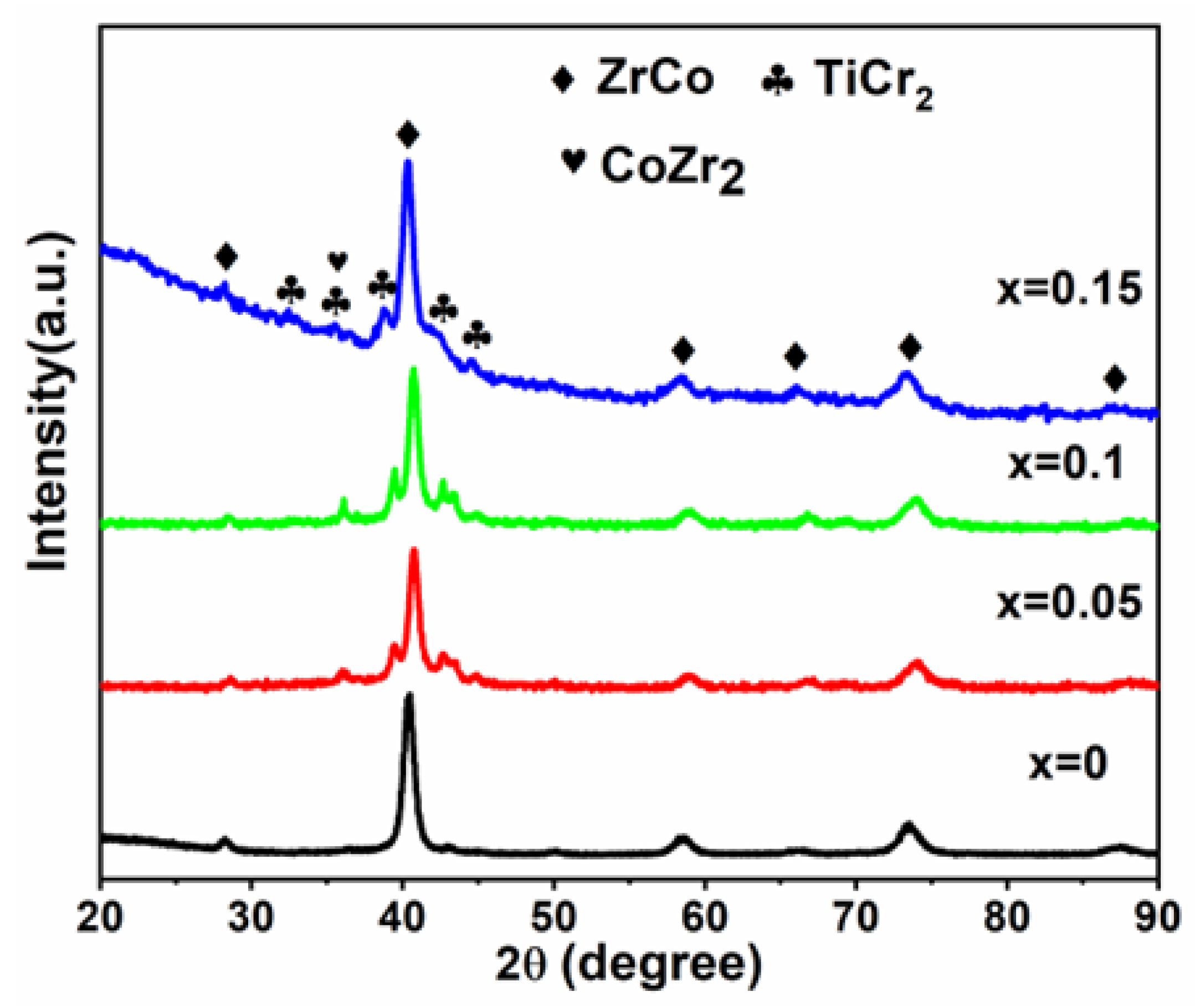
3.1. Structural Characterizations
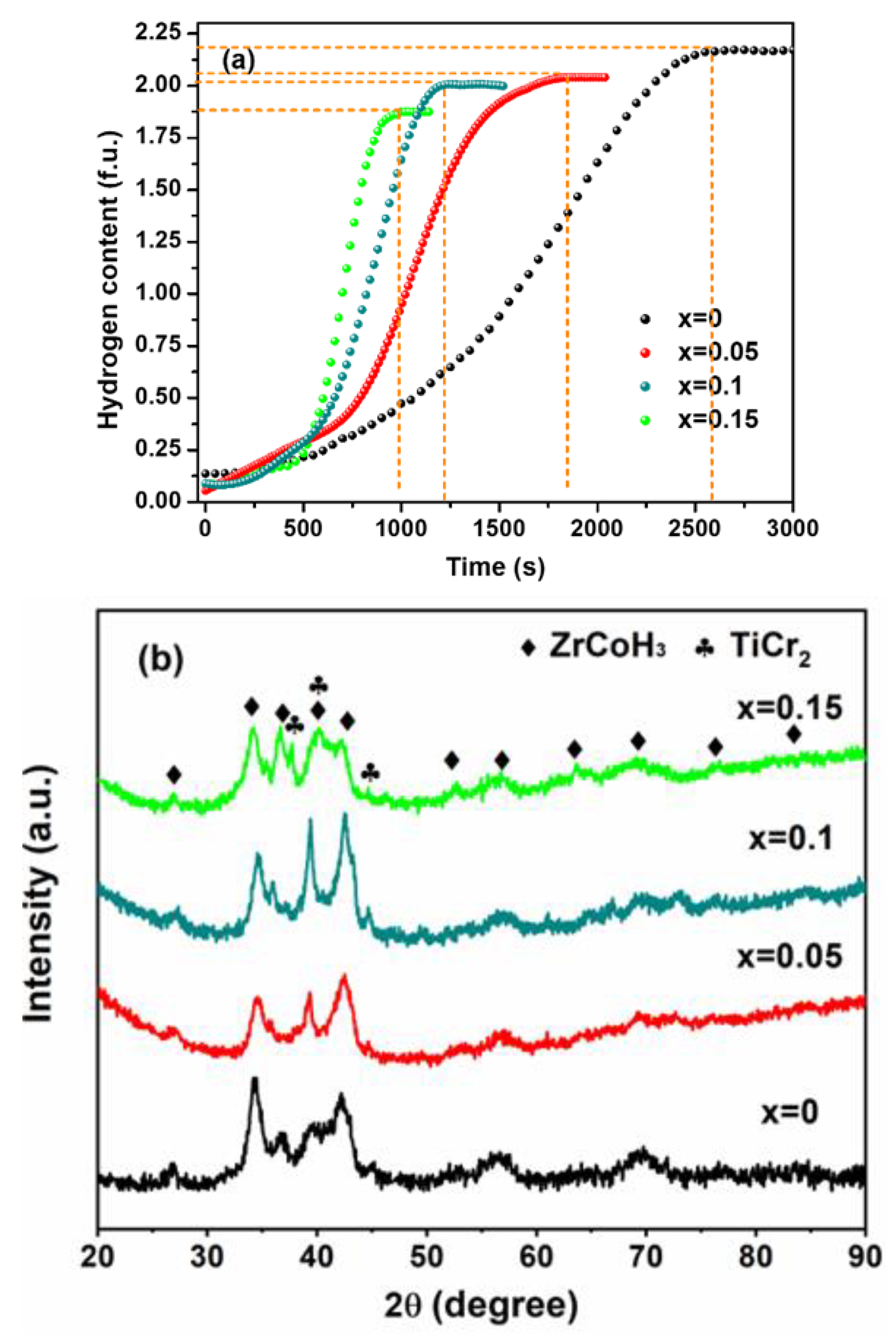
3.2. Hydriding Kinetics of Zr0.8Ti0.2CrxCo1−x Alloy
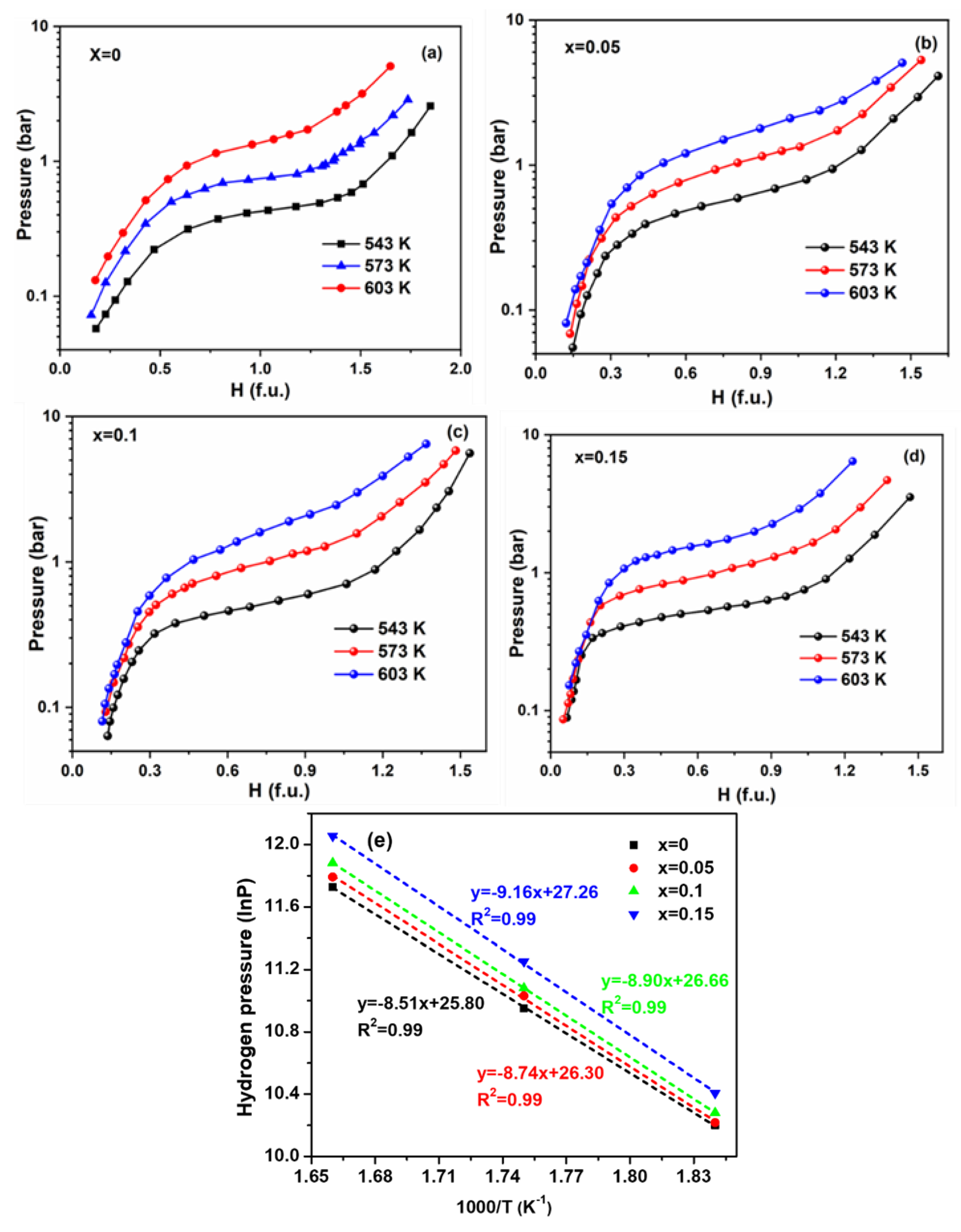
3.3. Thermodynamics Property of the Zr0.8Ti0.2CrxCo1−x Alloy
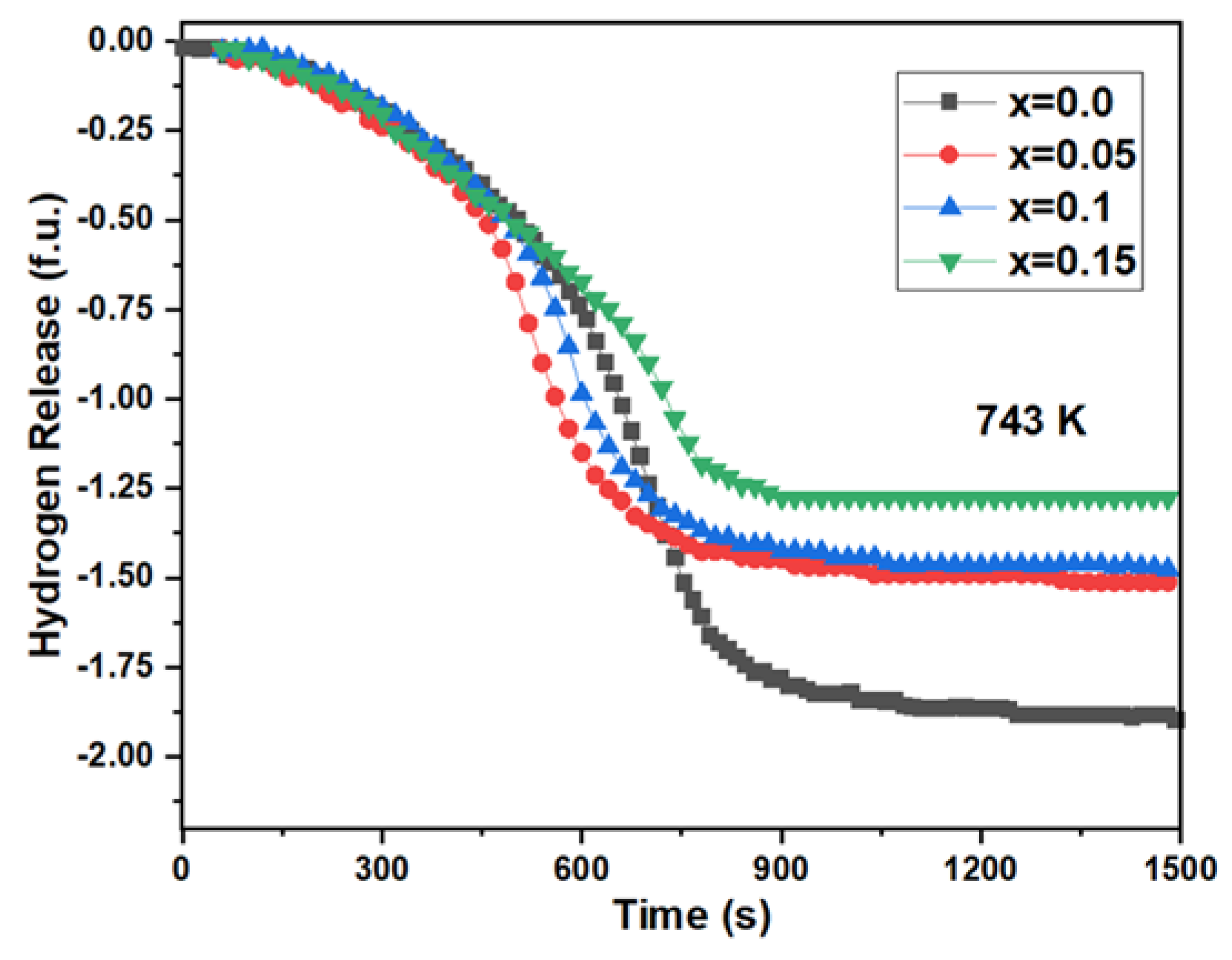
3.4. Dehydrogenation Properties
3.5. Disproportionation Property
4. Conclusions
- (1)
- The ZrTiCo-based alloy is composed of the ZrCo main phase. After Cr substitution, two Laves phases appear in Zr0.8Ti0.2CrxCo1−x (x = 0.05, 0.1, 0.15), namely CoZr2 phase and TiCr2 phase. With increasing Cr content, the lattice constant and unit cell volume increase, and the amount of the secondary phases also increases;
- (2)
- Cr doping improves the initial activation kinetics of Zr0.8Ti0.2Co alloy. The hydriding time is reduced from 2600 s to 990 s for Zr0.8Ti0.2Cr0.15Co0.85. This improvement is attributed to the catalytic effect of the TiCr2 Laves phase on the dissociation and diffusion of hydrogen molecules;
- (3)
- A certain amount of Cr doping enhances the hydrogen desorption kinetics. The time required for Zr0.8Ti0.2Cr0.05Co0.95 to achieve 90% of the desorption progress is reduced to 748 s compared with Zr0.8Ti0.2Co of 852 s. This is due to the decreased desorption activation energy resulting from Cr doping;
- (4)
- With the addition of Cr, the hydrogen absorption enthalpy of Zr0.8Ti0.2CrxCo1−x (x = 0, 0.05, 0.1, 0.15) increases, leading to enhanced stability of the hydride phase. Meanwhile, the alloys maintain good anti-disproportionation performance, which is beneficial for the further application of ZrTiCo-based alloys.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Laworska, L.; Skrzekut, T.; Stępień, M.; Pałka, P.; Boczkal, G.; Zwoliński, A.; Noga, P.; Podsiadło, M.; Wnuk, R.; Ostachowski, P. The Pressure Compaction of Zr-Nb Powder Mixtures and Selected Properties of Sintered and KOBO-Extruded Zr-xNb Materials. Materials 2021, 14, 3172. [Google Scholar] [CrossRef]
- Qi, J.; Liang, Z.; Xiao, X.; Yao, Z.; Zhou, P.; Li, R.; Lv, L.; Zhang, X.; Kou, H.; Huang, X.; et al. Effect of isostructural phase transition on cycling stability of ZrCo-based alloys for hydrogen isotopes storage. Chem. Eng. J. 2023, 455, 140571. [Google Scholar] [CrossRef]
- Devillers, M.; Sirch, M.; Penzhorn, R.D. Hydrogen-induced disproportionation of the intermetallic zirconium-cobalt compound ZrCo. Chem. Mater. 1992, 4, 631–639. [Google Scholar] [CrossRef]
- Bekris, N.; Besserer, U.; Sirch, M.; Penzhorn, R.D. On the thermal stability of the zirconium/cobalt–hydrogen system. Fusion Eng. Des. 2000, 49–50, 781–789. [Google Scholar] [CrossRef]
- Yao, Z.; Xiao, X.; Liang, Z.; Huang, X.; Kou, H.; Luo, W.; Chen, C.; Chen, L. An in-depth study on the thermodynamics and kinetics of disproportionation behaviors in ZrCoH system. J. Mater. Chem. A 2020, 8, 9322–9330. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Pu, Y.; Huang, G.; Yuan, Y.; Zhu, R.; Li, X.; Chen, C.; Deng, G.; Zou, H. Single-crystal ZrCo nanoparticle for advanced hydrogen and H-isotope storage. Nat. Commun. 2023, 14, 7966. [Google Scholar] [CrossRef]
- Yao, Z.; Liang, Z.; Xiao, X.; Qi, J.; He, J.; Huang, X.; Kou, H.; Luo, W.; Chen, C.; Chen, L. Achieving excellent cycle stability in Zr–Nb–Co–Ni based hydrogen isotope storage alloys by controllable phase transformation reaction. Renew. Energy 2022, 187, 500–507. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, R.; Tang, R.; Li, B.; Yu, R.; Liu, W.; Kou, H.; Meng, J. Effect of Ti substitution on hydrogen storage properties of Zr1−xTixCo (x = 0, 0.1, 0.2, 0.3) alloys. J. Energy Chem. 2014, 23, 9–14. [Google Scholar] [CrossRef]
- Kou, H.; Sang, G.; Luo, W.; Huang, Z.; Meng, D.; Zhang, G. Comparative study of full-scale thin double-layered annulus beds loaded with ZrCo, Zr0.8Hf0.2Co and Zr0.8Ti0.2Co for recovery and delivery of hydrogen isotopes. Int. J. Hydrogen Energy 2015, 40, 10923–10933. [Google Scholar] [CrossRef]
- Yao, Z.; Xiao, X.; Liang, Z.; Kou, H.; Luo, W.; Chen, C.; Jiang, L.; Chen, L. Improvement on the kinetic and thermodynamic characteristics of Zr1-xNbxCo (x = 0–0.2) alloys for hydrogen isotope storage and delivery. J. Alloys Compd. 2019, 784, 1062–1070. [Google Scholar] [CrossRef]
- Weng, C.; Xiao, X.; Huang, X.; Jiang, F.; Yao, Z.; Li, S.; Ge, H.; Chen, L. Effect of Mn substitution for Co on the structural, kinetic, and thermodynamic characteristics of ZrCo1-xMnx (x = 0–0.1) alloys for tritium storage. Int. J. Hydrogen Energy 2017, 42, 28498–28506. [Google Scholar] [CrossRef]
- Luo, L.; Ye, X.; Zhao, C.; Zhang, G.; Kou, H.; Xiong, R.; Sang, G.; Han, T. Effects of Mo substitution on the kinetic and thermodynamic characteristics of ZrCo1-xMox (x = 0–0.2) alloys for hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 2989–2998. [Google Scholar] [CrossRef]
- Jat, R.A.; Singh, R.; Parida, S.; Das, A.; Agarwal, R.; Mukerjee, S.; Ramakumar, K. Structural and hydrogen isotope storage properties of Zr-Co-Fe alloy. Int. J. Hydrogen Energy 2015, 40, 5135–5143. [Google Scholar] [CrossRef]
- Jat, R.A.; Singh, R.; Parida, S.; Das, A.; Agarwal, R.; Ramakumar, K. Determination of deuterium site occupancy in ZrCoD3 and its role in improved durability of Zr-Co-Ni deuterides against disproportionation. Int. J. Hydrogen Energy 2014, 39, 15665–15669. [Google Scholar] [CrossRef]
- Zhang, G.; Sang, G.; Xiong, R.; Kou, H.; Liu, K.; Luo, W. Effects and mechanism of Ti, Ni, Sc, Fe substitution on the thermal stability of zirconium cobalt-hydrogen system. Int. J. Hydrogen Energy 2015, 40, 6582–6593. [Google Scholar] [CrossRef]
- Liang, Z.; Yao, Z.; Xiao, X.; Wang, X.; Kou, H.; Luo, W.; Chen, C.; Chen, L. Positive impacts of tuning lattice on cyclic performance in ZrCo-based hydrogen isotope storage alloys. Mater. Today Energy 2021, 20, 100645. [Google Scholar] [CrossRef]
- Jat, R.A.; Pati, S.; Parida, S.; Agarwal, R.; Mukerjee, S. Synthesis, characterization and hydrogen isotope storage properties of Zr-Ti-Co ternary alloys. Int. J. Hydrogen Energy 2017, 42, 2248–2256. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Z.; Yang, Y.; Hu, X.; Tian, X.; Yue, Y.; Song, J. The mechanism of anti-disproportionation for Ti-doped ZrCo alloys. Appl. Energy 2022, 328, 120236. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, W.; Sun, H.; Guo, S.; Hou, Z.; Mu, X.; Xu, L.; Zhao, D. Effects and mechanism of Ti, Cu, Y, La, Ce, Pr, Nd and Sm substitutions on the anti-disproportionation performance of ZrCo alloy. Int. J. Hydrogen Energy 2024, 56, 562–569. [Google Scholar] [CrossRef]
- Liang, Z.; Yao, Z.; Xiao, X.; Kou, H.; Luo, W.; Chen, C.; Chen, L. The functioning mechanism of Al valid substitution for Co in improving the cycling performance of Zr-Co-Al based hydrogen isotope storage alloys. J. Alloys Compd. 2020, 848, 156618. [Google Scholar] [CrossRef]
- Huang, X.; Wang, D.; Kou, H.; Bao, J.; Ye, R.; Chen, C.; Luo, W. Kinetic hydrogen isotope effects and flow compensation strategy of the ZrCo-based chemical beds in full-scale SDS demo-system. Fusion Eng. Des. 2023, 192, 113748. [Google Scholar] [CrossRef]
- Wan, J.; Li, R.; Wang, F.; Ding, C.; Yu, R.; Wu, Y. Effect of Ni substitution on hydrogen storage properties of Zr0.8Ti0.2Co1−xNix (x = 0, 0.1, 0.2, 0.3) alloys. Int. J. Hydrogen Energy 2016, 41, 7408–7418. [Google Scholar] [CrossRef]
- Xu, S.; Wang, F.; Tan, W.; Wang, Y.; Yu, R. Microstructure and hydrogen storage properties of Zr0. 8Ti0. 2Co1-xFex (x = 0, 0.1, 0.2, 0.3) alloys. Int. J. Hydrogen Energy 2018, 43, 839–847. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, W.; Ye, X.; Sang, G. First-principles investigation on the effect of Nb and Ta doping on the hydrogen storage performance of ZrCo. Comput. Theor. Chem. 2024, 1238, 114688. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, B.; Luo, W.; Zhou, L.; Kou, H.; Li, P.; Sang, G. The synergistic effect of co-doping with Ti, Nb, and Ni on the hydrogen storage capacity of ZrCo. Int. J. Hydrogen Energy 2024, 86, 899–912. [Google Scholar] [CrossRef]
- Luo, L.; Ye, X.; Zhang, G.; Kou, H.; Xiong, R.; Sang, G.; Yu, R.; Zhao, D. Enhancement of hydrogenation kinetics and thermodynamic properties of ZrCo1-xCrx(x = 0–0.1) alloys for hydrogen storage. Chin. Phys. B 2020, 29, 088801. [Google Scholar]
- Tregubov, I.A.; Evseeva, L.N.; Ivanov, O.S. The zirconium corner of the Zr-Cr-Cu phase diagram (in Russian). Russ. Metall. 1977, 5, 183–186. [Google Scholar]
- Bulbich, A.A. Effect of dissolved hydrogen on the ductile properties of metals as a result of nucleation on dislocations. J. Alloys Compd. 1993, 196, 29–36. [Google Scholar] [CrossRef]
- Sleiman, S.; Huot, J. Microstructure and Hydrogen Storage Properties of Ti1V0.9Cr1.1 Alloy with Addition of x wt.% Zr (x = 0, 2, 4, 8, and 12). Inorganics 2017, 5, 86–98. [Google Scholar] [CrossRef]
- Liang, J.; Li, G.; Ding, X.; Li, Y.; Wen, Z.; Zhang, T.; Qu, Y. Effect of C14 Laves/BCC on microstructure and hydrogen storage properties of (Ti32.5V27.5Zr7.5Nb32.5) 1-xFex (x = 0.03, 0.06, 0.09) high entropy hydrogen storage alloys. J. Energy Storage 2023, 73, 108852. [Google Scholar] [CrossRef]
- Hang, Z.; Shi, L.; Feng, Y.; Dong, H.; Yang, L.; Chen, L. Experimental and theoretical insights into vanadium-based alloys for room temperature hydrogen storage on the example of Ti16Cr22Zr5V55-xFe2Mnx (x = 0–3) alloys. J. Alloys Compd. 2024, 988, 174315. [Google Scholar] [CrossRef]
- Liang, L.; Wang, F.; Wang, J.; Wu, C.; Wang, Z.; Rong, M.; Zhou, H. Effects of V doping on microstructure, kinetic, and thermodynamic characteristics of Zr50-xVxCo50 (x = 0, 2.5, 3.5, and 5.0) hydrogen storage alloys. Int. J. Energy Res. 2022, 46, 6833–6846. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Liu, H.; He, T.; Yan, M. Effects of nano-composites (FeB, FeB/CNTs) on hydrogen storage properties of MgH2. J. Power Sources 2019, 438, 227006. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Yu, H.; Lu, Z.; Chen, L. Achieving superior hydrogen storage properties of MgH2 by the effect of TiFe and carbon nanotubes. Chem. Eng. J. 2021, 422, 130101. [Google Scholar] [CrossRef]
- Li, X.; Yuan, Z.; Li, Z.; Liu, C.; Zhai, T.; Zhang, Y.; Li, T. Rapid hydrogen energy storage of self-supporting VS2/NC modified MgH2. Energy 2025, 314, 134284. [Google Scholar] [CrossRef]




| Sample | Lattice Parameters (Å) | Cell Volume (Å3) |
|---|---|---|
| Cr000 | 3.156 (4) | 31.43 |
| Cr005 | 3.163 (1) | 31.85 |
| Cr010 | 3.168 (5) | 32.14 |
| Cr015 | 3.177 (8) | 32.86 |
| Samples | ΔH (kJ/mol H2) | ΔS (kJ/mol·K H2) |
|---|---|---|
| ZrCo-H [10] | 89.71 | 226.16 |
| ZrCo-H [32] | 90.12 | 226.14 |
| Cr000 | 70.75 | 214.50 |
| Cr005 | 72.66 | 218.66 |
| Cr010 | 73.99 | 221.65 |
| Cr015 | 76.16 | 226.64 |
| Samples | The Hydriding Incubation Period (s) | The Maximum Hydriding Capacity (f.u.) | The Desorption Capacity (f.u.) | The Desorption Ratio (%) |
|---|---|---|---|---|
| Cr000 | 1000 | 2.20 | 1.90 | 86.49 |
| Cr005 | 700 | 2.06 | 1.54 | 74.62 |
| Cr010 | 540 | 2.02 | 1.49 | 73.51 |
| Cr015 | 470 | 1.87 | 1.28 | 68.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liu, W.; Liang, L.; Liu, Y.; Huang, Z.; Rong, M.; Liu, J.; Lv, W.; Ji, S.; Wang, J. Effect of Cr Doping on Microstructure and Hydrogen Storage Properties of Zr0.8Ti0.2CrxCo1−x (x = 0, 0.05, 0.1, 0.15) Alloys. Processes 2025, 13, 1026. https://doi.org/10.3390/pr13041026
Wang F, Liu W, Liang L, Liu Y, Huang Z, Rong M, Liu J, Lv W, Ji S, Wang J. Effect of Cr Doping on Microstructure and Hydrogen Storage Properties of Zr0.8Ti0.2CrxCo1−x (x = 0, 0.05, 0.1, 0.15) Alloys. Processes. 2025; 13(4):1026. https://doi.org/10.3390/pr13041026
Chicago/Turabian StyleWang, Feng, Wenting Liu, Lina Liang, Yue Liu, Zhengru Huang, Maohua Rong, Jiageng Liu, Wei Lv, Shuai Ji, and Jiang Wang. 2025. "Effect of Cr Doping on Microstructure and Hydrogen Storage Properties of Zr0.8Ti0.2CrxCo1−x (x = 0, 0.05, 0.1, 0.15) Alloys" Processes 13, no. 4: 1026. https://doi.org/10.3390/pr13041026
APA StyleWang, F., Liu, W., Liang, L., Liu, Y., Huang, Z., Rong, M., Liu, J., Lv, W., Ji, S., & Wang, J. (2025). Effect of Cr Doping on Microstructure and Hydrogen Storage Properties of Zr0.8Ti0.2CrxCo1−x (x = 0, 0.05, 0.1, 0.15) Alloys. Processes, 13(4), 1026. https://doi.org/10.3390/pr13041026








