Kinetic Mechanisms and Efficient Leaching of Praseodymium, Neodymium, Fluorine, and Lithium from Molten-Salt Slag via Atmospheric Alkaline Leaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Methods
2.3. Equipment and Reagents
3. Results
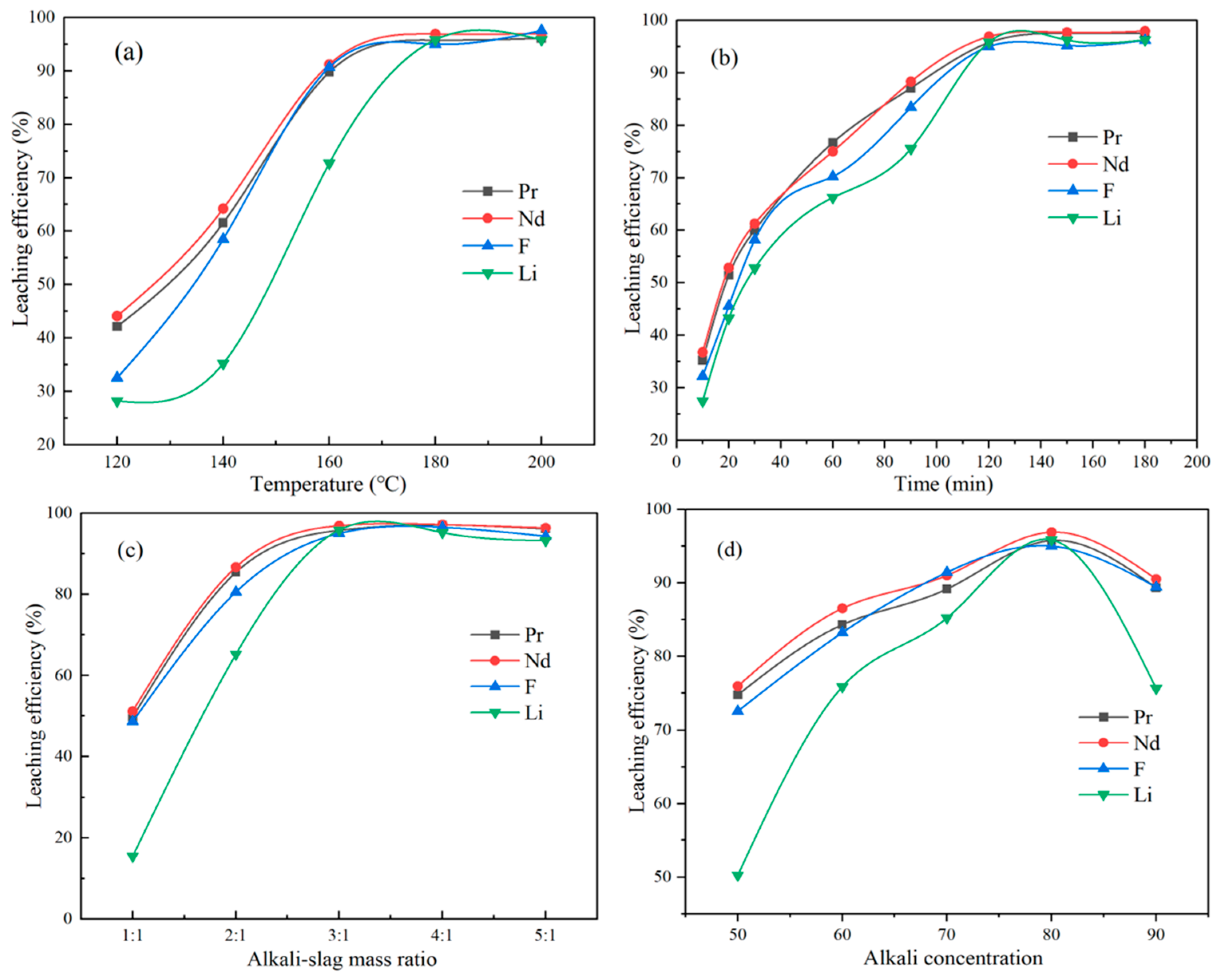
3.1. Effect of Reaction Temperature on the Leaching Efficiency of Nd, Pr, F, and Li
3.2. Effect of Reaction Time on the Leaching Efficiency of Nd, Pr, F, and Li
3.3. Effect of the Alkali-to-Slag Ratio on the Leaching Efficiency of Nd, Pr, F, and Li
3.4. Effect of NaOH Concentration on the Leaching Efficiency of Nd, Pr, F, and Li
3.5. Comparison of Energy Consumption and Environmental Benefits Between This Experiment and Current Common Methods
3.6. Mineral Phase Transformation and Morphological Analysis
4. The Kinetic Model of Leaching Reaction
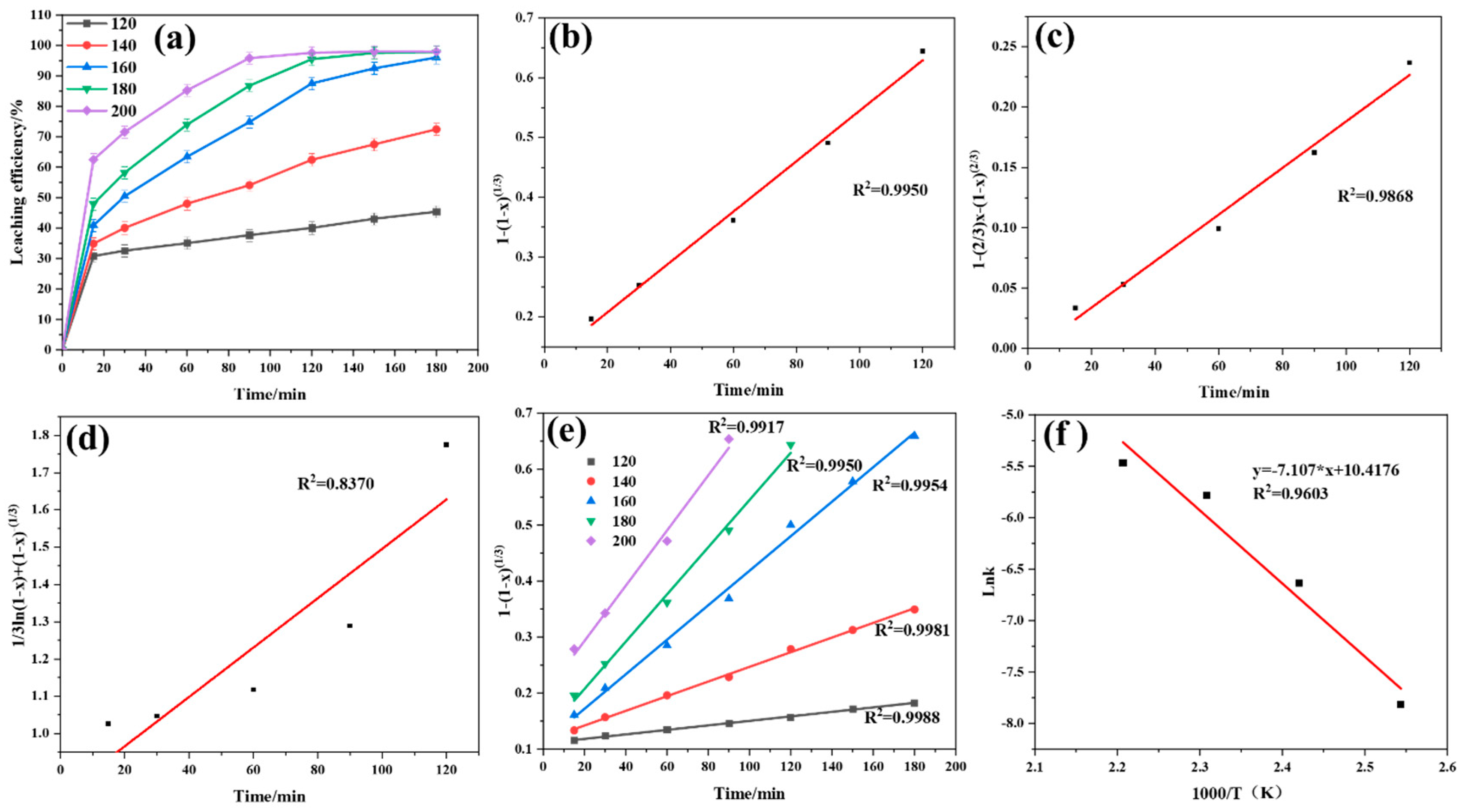
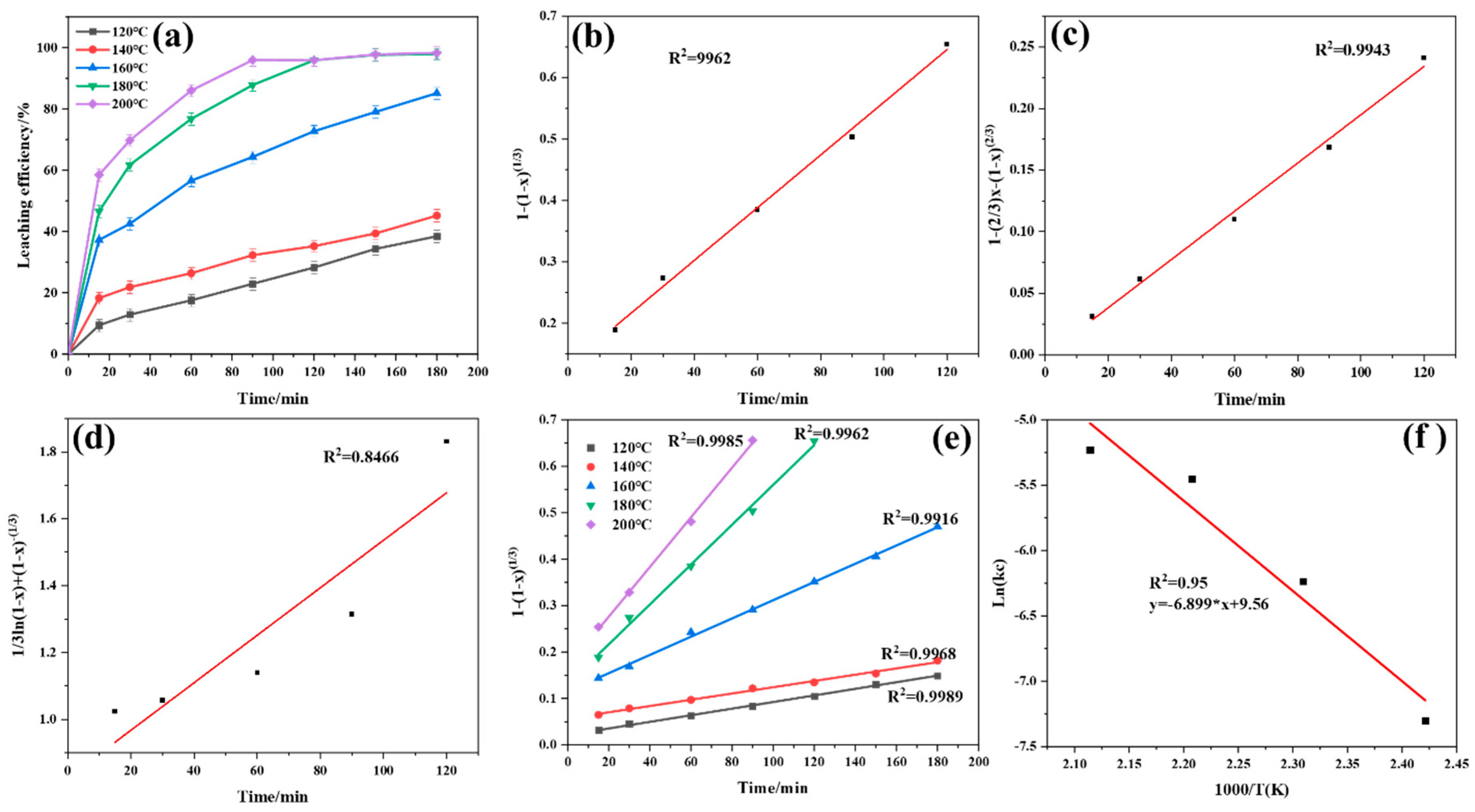
4.1. The Controlling Step and Apparent Activation Energy of Fluorine Element Conversion
4.2. The Controlling Step and Apparent Activation Energy of Li Conversion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tunsu, C.; Petranikova, M.; Gergorić, M.; Ekberg, C.; Retegan, T. Reclaiming rare earth elements from end-of-life products: A review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 2015, 156, 239–258. [Google Scholar]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar]
- Kumari, A.; Randhawa, N.S.; Sahu, S.K. Electrochemical treatment of spent NdFeB magnet in organic acid for recovery of rare earths and other metal values. J. Clean. Prod. 2021, 39, 127393. [Google Scholar]
- Liang, Y.; Li, Y.K.; Xue, L.Y.; Zou, Y. Extraction of rare earth elements from fluoride molten salt electrolytic slag by mineral phase reconstruction. J. Clean. Prod. 2018, 177, 567–572. [Google Scholar]
- Wang, J.; Hu, H. Selective extraction of rare earths and lithium from rare earth fluoride molten-salt electrolytic slag by sulfation. Miner. Eng. 2021, 160, 106711. [Google Scholar]
- Lai, Y.; Li, J.; Zhu, S.; Liu, K.; Xia, Q.; Huang, M.; Hu, G.; Zhang, H.; Qi, T. Recovery of rare earths, lithium, and fluorine from rare earth molten salt electrolytic slag by mineral phase reconstruction combined with vacuum distillation. Sep. Purif. Technol. 2023, 310, 123105. [Google Scholar]
- Yang, D.; Yu, M.; Mubula, Y.; Yuan, W.; Huang, Z.; Lin, B.; Mei, G.; Qiu, T. Recovering rare earths, lithium and fluorine from rare earth molten salt electrolytic slag using sub-molten salt method. J. Rare Earths 2023, 41, 1774–1781. [Google Scholar]
- Mubula, Y.; Yu, M.; Yang, D.; Niu, H.; Qiu, T.; Mei, G. Recovery of Rare Earths from Rare-Earth Melt Electrolysis Slag by Mineral Phase Reconstruction. JOM 2024, 76, 4732–4748. [Google Scholar]
- Qiu, T.; Wu, H.; Liang, Y.Z.; Qiu, S.; Zhou, X.; Zhu, D.; Qiu, T. Rare earth recovery from fluoride molten salt electrolytic slag by sodium hydroxide roasting-hydrochloric acid leaching. J. China Univ. Min. Technol. 2022, 51, 475–482. [Google Scholar]
- Tong, Z.; Hu, X.; Wen, H. Effect of roasting activation of rare earth molten salt slag on extraction of rare earth, lithium and fluorine. J. Rare Earths 2022, 40, 300–308. [Google Scholar]
- Wu, H.; Yan, H.; Liang, Y.; Qiu, S.; Zhou, X.; Zhu, D.; Qiu, T. Rare earth recovery from fluoride molten-salt electrolytic slag by sodium carbonate roasting-hydrochloric acid leaching. J. Rare Earths 2023, 41, 1242–1249. [Google Scholar]
- Tian, L.; Chen, L.; Gong, A.; Wu, X.; Cao, C.; Xu, Z. Recovery of rare earths, lithium and fluorine from rare earth molten salt electrolytic slag via fluoride sulfate conversion and mineral phase reconstruction. Miner. Eng. 2021, 170, 106965. [Google Scholar]
- Hu, H.; Wang, J. Selective extraction of rare earths and lithium from rare earth fluoride molten-salt electrolytic slag by nitration. Hydrometallurgy 2021, 203, 105552. [Google Scholar] [CrossRef]
- Li, S.; Kang, C.; Kim, S.; Kim, C.J.; Kang, C.J. The extraction of Ta, Nb and rare earths from fergusonite by using KOH sub-molten salt leaching. Hydrometallurgy 2021, 201, 105358. [Google Scholar]
- Sun, H.; Song, J.; Sun, S.; Qu, J.; Lü, W.; Qi, T. Decomposition kinetics of zircon sand in NaOH sub-molten salt solution. Trans. Nonferrous Met. Soc. China 2019, 29, 1948–1955. [Google Scholar] [CrossRef]
- Li, L.J.; Zheng, S.L.; Chen, D.H.; Wang, S.N.; Du, H.; Gao, M.L.; Zhang, Y. A novel method of leaching vanadium from extracted vanadium residue using sodium sub-molten salt medium. Adv. Mater. Res. 2012, 402, 253–260. [Google Scholar]
- Chen, G.; Wang, X.; Du, H.; Zhang, Y.; Wang, J.; Zheng, S.; Zhang, Y. A clean and efficient leaching process for chromite ore. Miner. Eng. 2014, 60, 60–68. [Google Scholar]
- Ma, Q.; Zhang, Y.; Cao, S.; Zhang, Y. Leaching Behavior of Diaspore Bauxite in KOH Sub-molten Salt. Chin. J. Process Eng. 2013, 13, 391–396. [Google Scholar]
- Zhou, H.; Zheng, S.; Zhang, Y. Leaching of a Low-grade Refractory Tantalum–Niobium Ore by KOH Sub-molten Salt. Chin. J. Process Eng. 2003, 3, 459–463. [Google Scholar]
- Zhao, C.; Zhai, Y.; Liu, Y.; Duan, H. Pre-desilication of laterite in NaOH sub-molten salt system. Chin. J. Nonferrous Met. 2009, 19, 949–954. [Google Scholar]






| Component | Nd | Pr | F | Li | Ca | Al |
|---|---|---|---|---|---|---|
| Content (%) | 43.58 | 12.39 | 33.23 | 3.47 | 0.91 | 0.21 |
| Conditions | Leaching Efficiency (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Alkali Concentration (%) | Alkali–Slag Mass Ratio | Time (min) | Temperature (°C) | Pr | Nd | F | Li | |
| Ex1 | 80 | 3:1 | 120 | 120 | 42.16 | 44.11 | 32.55 | 28.22 |
| Ex2 | 80 | 3:1 | 120 | 140 | 61.56 | 64.23 | 58.51 | 35.23 |
| Ex3 | 80 | 3:1 | 120 | 160 | 89.78 | 91.23 | 90.715 | 72.72 |
| Ex4 | 80 | 3:1 | 120 | 180 | 95.78 | 96.92 | 95.02 | 95.87 |
| Ex5 | 80 | 3:1 | 120 | 200 | 96.12 | 96.98 | 97.58 | 95.88 |
| Conditions | Leaching Efficiency (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Alkali Concentration (%) | Alkali–Slag Mass Ratio | Time (min) | Temperature (°C) | Pr | Nd | F | Li | |
| Ex6 | 80 | 3:1 | 10 | 180 | 35.23 | 36.78 | 32.22 | 27.45 |
| Ex7 | 80 | 3:1 | 20 | 180 | 51.45 | 52.87 | 45.57 | 43.25 |
| Ex8 | 80 | 3:1 | 30 | 180 | 60.05 | 61.28 | 58.22 | 52.77 |
| Ex9 | 80 | 3:1 | 60 | 180 | 76.73 | 75.03 | 70.26 | 66.25 |
| Ex10 | 80 | 3:1 | 90 | 180 | 87.12 | 88.35 | 83.45 | 75.55 |
| Ex11 | 80 | 3:1 | 120 | 180 | 95.78 | 96.92 | 95.02 | 95.87 |
| Ex12 | 80 | 3:1 | 150 | 180 | 97.55 | 97.72 | 95.22 | 96.25 |
| Ex13 | 80 | 3:1 | 180 | 180 | 97.56 | 97.97 | 96.22 | 96.28 |
| Conditions | Leaching Efficiency (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Alkali Concentration (%) | Alkali–Slag Mass Ratio | Time (min) | Temperature (°C) | Pr | Nd | F | Li | |
| Ex14 | 80 | 1:1 | 120 | 180 | 49.78 | 51.23 | 48.68 | 15.45 |
| Ex15 | 80 | 2:1 | 120 | 180 | 85.43 | 86.72 | 80.58 | 65.25 |
| Ex16 | 80 | 3:1 | 120 | 180 | 95.78 | 96.92 | 95.02 | 95.87 |
| Ex17 | 80 | 4:1 | 120 | 180 | 97.12 | 97.23 | 96.56 | 95.26 |
| Ex18 | 80 | 5:1 | 120 | 180 | 96.12 | 96.38 | 94.35 | 93.25 |
| Conditions | Leaching Efficiency (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Alkali Concentration (%) | Alkali–Slag Mass Ratio | Time (min) | Temperature (°C) | Pr | Nd | F | Li | |
| Ex19 | 50 | 3:1 | 120 | 180 | 74.78 | 75.97 | 72.56 | 50.25 |
| Ex20 | 60 | 3:1 | 120 | 180 | 84.33 | 86.55 | 83.25 | 75.89 |
| Ex21 | 70 | 3:1 | 120 | 180 | 89.18 | 91.04 | 91.45 | 85.25 |
| Ex22 | 80 | 3:1 | 120 | 180 | 95.78 | 96.92 | 95.02 | 95.87 |
| Ex23 | 90 | 3:1 | 120 | 180 | 89.34 | 90.54 | 89.45 | 75.65 |
| Method | Temperature | Reagent | Energy Consumption | Recovery Rate (REEs) | Product Purity | Solid/Gas Water Waste |
|---|---|---|---|---|---|---|
| Alkali-fusion method | 950 °C | Ca(OH)2 | High | 97% | Low | Yes |
| 600 °C | NaOH | High | 99% | High | Yes | |
| 600 °C | LiOH·H2O | High | 99.27 | High | No | |
| 600 °C | NaOH | High | 96% | High | Yes | |
| 300–500 °C | NaOH | High | 97% | Low | Yes | |
| Salt-roasting method | 700 °C | Na2B4O7·10H2O | High | 97% | Low | Yes |
| 630 °C | CaO, Al2(SO4)3 | High | 90% | High | Yes | |
| 850 °C | Na2SiO3 | High | 98.96% | High | No | |
| 700 °C | Na2CO3 | High | 99.13% | Low | Yes | |
| 750 °C | (NH4)2SO4 | High | 95% | High | Yes | |
| 700 °C | Na2CO3 | High | 98% | High | No | |
| Acid-leaching method | 333 °C | H2SO4 | Low | 95% | High | Yes |
| 250 °C | HNO3 | Low | 98% | High | Yes | |
| This experiment | 180 °C | NaOH | Low | 96.35% | High | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Huang, G.; Zhang, T. Kinetic Mechanisms and Efficient Leaching of Praseodymium, Neodymium, Fluorine, and Lithium from Molten-Salt Slag via Atmospheric Alkaline Leaching. Processes 2025, 13, 1025. https://doi.org/10.3390/pr13041025
Yu M, Huang G, Zhang T. Kinetic Mechanisms and Efficient Leaching of Praseodymium, Neodymium, Fluorine, and Lithium from Molten-Salt Slag via Atmospheric Alkaline Leaching. Processes. 2025; 13(4):1025. https://doi.org/10.3390/pr13041025
Chicago/Turabian StyleYu, Mingming, Guojun Huang, and Tianyong Zhang. 2025. "Kinetic Mechanisms and Efficient Leaching of Praseodymium, Neodymium, Fluorine, and Lithium from Molten-Salt Slag via Atmospheric Alkaline Leaching" Processes 13, no. 4: 1025. https://doi.org/10.3390/pr13041025
APA StyleYu, M., Huang, G., & Zhang, T. (2025). Kinetic Mechanisms and Efficient Leaching of Praseodymium, Neodymium, Fluorine, and Lithium from Molten-Salt Slag via Atmospheric Alkaline Leaching. Processes, 13(4), 1025. https://doi.org/10.3390/pr13041025





