1. Introduction
A reliable determination of the light-extinction parameters (particularly the coefficients of absorption κ and scattering σ) is essential for the development of comprehensive models of the complicated photocatalytic reaction processes (e.g., [
1,
2]). Such process models are necessary for research and development as well as for the scale-up from laboratory/pilot to large/commercial size photocatalytic systems intensely pursued in recent decades [
3,
4,
5]. This work deals with the catalyst slurry/suspension type photocatalytic reactors. In this area, despite fairly extensive work [
2,
6,
7,
8], significant issues remain to be resolved, particularly for the reliable and facile determination of light absorption, due to the multitude of interacting process parameters, including the light characteristics (commonly of quite a broad wavelength), the catalyst particle physico-chemical/material properties and concentration as well as the fluid conditions (pH and composition). Notable complications arise due to the well-known tendency of the catalyst particles (of a primary size in the 10 to 10
2 nm range [
9]) to form aggregates and agglomerates of a rather broad distribution, with a mean size well above 1 μm, depending on fluid pH, composition, and sonication [
10,
11].
Quite a few interesting studies have been performed [
12,
13,
14,
15,
16,
17,
18] to determine light extinction characteristics, usually under specific/idealized photocatalytic conditions, such as monochromatic UV light and controlled (through sonication) catalyst particle size. Some of these studies, briefly outlined here, have led to an improved understanding of the light extinction phenomena including the influence of key parameters.
In their seminal papers, Cabrera et al. [
12] and later Satuf et al. [
13] have used diffuse reflectance and transmittance spectrophotometric measurements of various TiO
2 dispersions to determine the variability of absorption and scattering coefficients (κ and σ) for monochromatic UV light in the range 275 nm to ~405 nm and mean agglomerate/particle sizes 300 nm to 900 nm. A fairly rigorous approach was followed involving the basic radiative transfer equation (RTE). For the determination of κ and σ from the measured extinction coefficient β, they used a model for the system under study, to describe the radiation field in the test cell and through an application of a nonlinear optimization program to adjust/fit the model predictions to the experimental data. The results obtained [
13] are considered a good reference, for the employed test conditions; however, the combined experimental and computational effort involved in this approach is quite intricate and demanding, particularly for routine use.
In papers [
14,
15,
16], appearing at about the same time, a test cell with external irradiation was used to obtain transmittance I/Io data and a simplified approach was implemented for the determination of the light-extinction parameters of photocatalyst dispersions. Specifically, making several assumptions, the Schuster–Schwarzschild approximation [
19] to the basic RTE was obtained, which models the monochromatic radiation field in terms of two oppositely directed fluxes (and respective equations), i.e., a forward flux (I) due to irradiation and a backward flux (J) due to light scattering. For these model equations, the well-known (in the field of diffuse reflectance) hyperbolic solution of Kubelka–Munk (K-M) [
20,
21] is employed to determine the extinction parameters κ and σ. Of particular interest to this work (as will be subsequently discussed), are the experimental results obtained by Yurdakal et al. [
15], by employing this approach for TiO
2 (Degussa P25) dispersions and a monochromatic UV light of 310 nm and 370 nm. In a more recent study by Calza et al. [
17], the approach based on the Κ-Μ expression was also employed to estimate the adsorption coefficient κ as well as the spatial variability of transmittance I/Io, which were further used in a simplified expression of the photocatalytic reaction rate, as will be subsequently discussed. These authors [
17] used several commercial TiO
2 catalysts and a UVA light of wavelength ~350 nm to ~400 nm. Comparing the results on β, κ, and σ, obtained in the above-mentioned typical studies [
13,
15,
17], it should be noted that there are differences that need to be clarified, particularly when different experimental techniques are used, with commercial UV lamps of a fairly broad wavelength spectrum and particles of a broad distribution.
Studies focusing on the effect of fluid medium properties on the dispersion and aggregation characteristics of photocatalysts are complementary to the work on their light extinction characteristics. Suttiponparnit et al. [
10] studied the effect of aqueous medium properties on the dispersion characteristics of TiO
2 particles, including Degussa P25. By reducing the pH below 6 (IEP for P25 at pH~6.2), their data show a significant increase of the particle-specific surface area (in cm
2/g), obviously associated with agglomerate size reduction; size reduction is also observed for pH < 8. The reported average hydrodynamic diameter tends to increase (well above 1 μm) with an increasing NaCl solution ionic strength. Also, at a fixed pH, an increase in TiO
2 mass concentration leads to a higher degree of agglomeration, which is attributed to the increased particle collision frequency. These results hold only for rather simple aqueous dispersions. However, as noted by Sharma [
22], little is known about the effect of natural organic matter (ever present in effluents to be treated) on the aggregation of nano-TiO
2. This implies that specific experimental effort should be devoted to determine the light extinction characteristics of the particular effluents under study. Along the same lines, it should be noted that the absorption by particles of the short wavelength UVC light, commonly used for the direct decontamination of water from biological species, has a negative effect as it reduces the availability of photons for this function [
23].
The preceding brief literature review suggests that experimental light extinction data for practical applications should be taken under realistic conditions, representative of the process under investigation and/or development. Furthermore, appropriate experimental tools are necessary for this task. In this paper, a novel experimental technique is presented which greatly facilitates the collection of UV light extinction data and the determination of absorption and scattering coefficients. This technique is demonstrated by employing a common commercial TiO
2 photocatalyst (Degussa P25) in dispersions of various concentrations, at three pH values and two types of irradiation, i.e., UVA and UVC. Such complete data sets, particularly with UVC light, are rarely reported in the open literature, even though UVC light has been employed successfully at a pilot scale [
4,
5,
24].
2. Theoretical Background
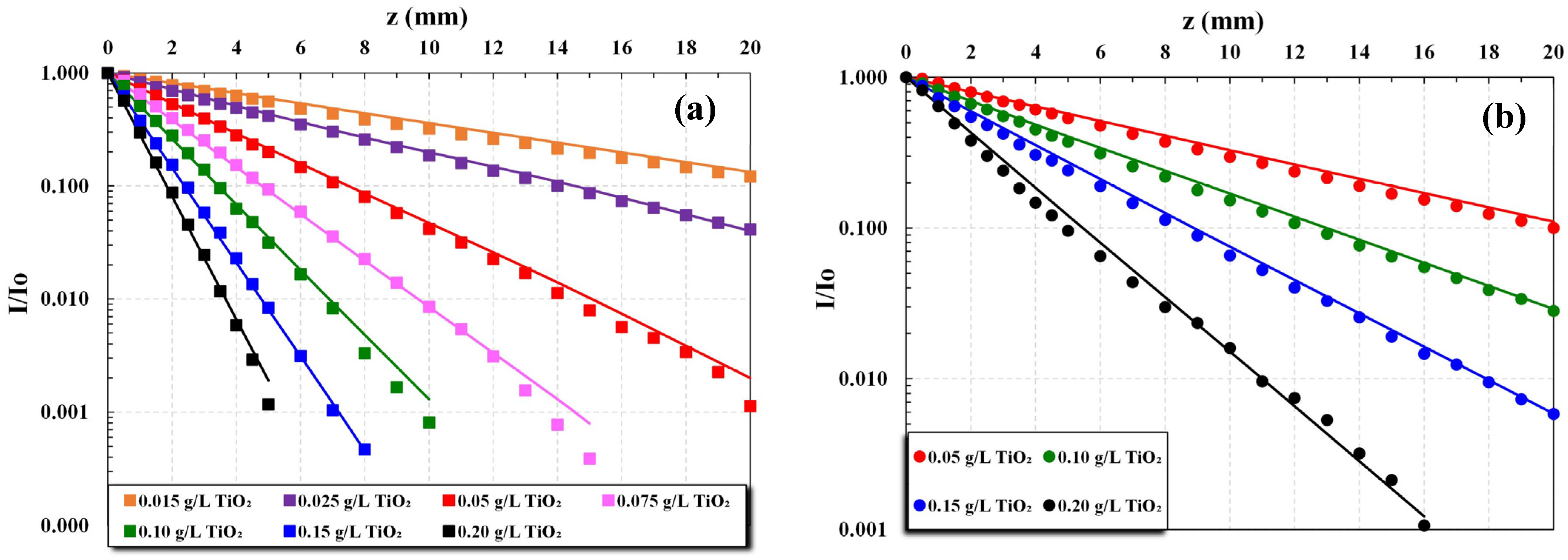
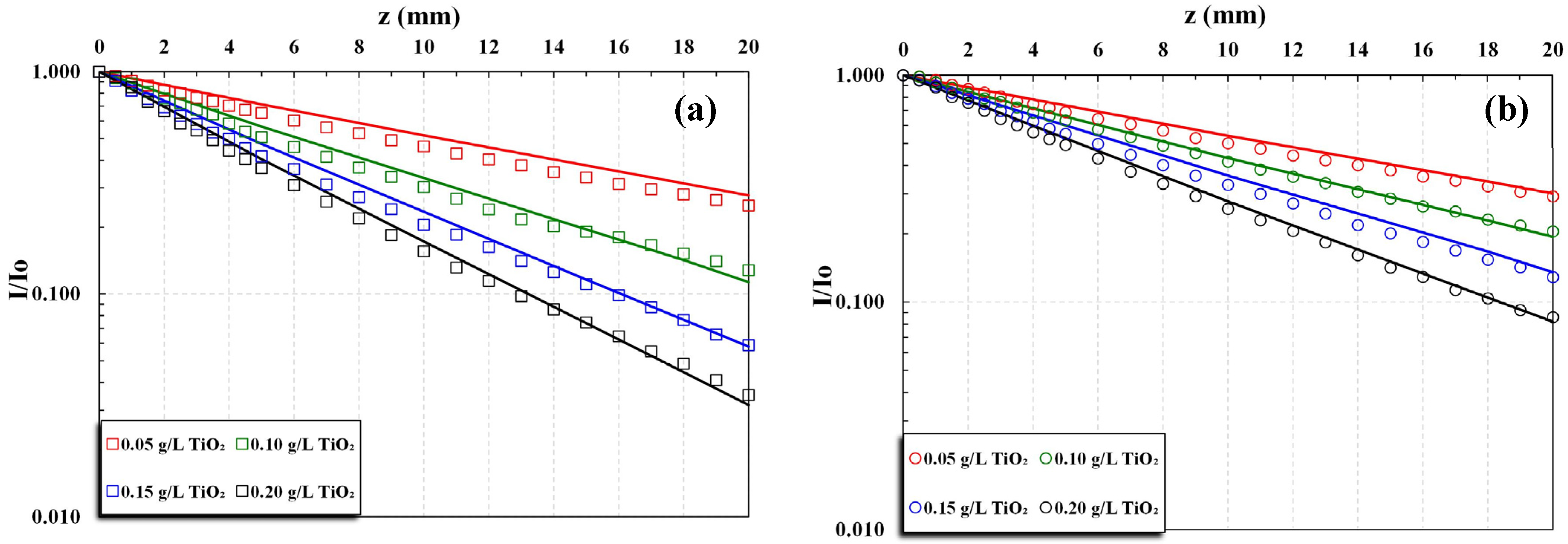
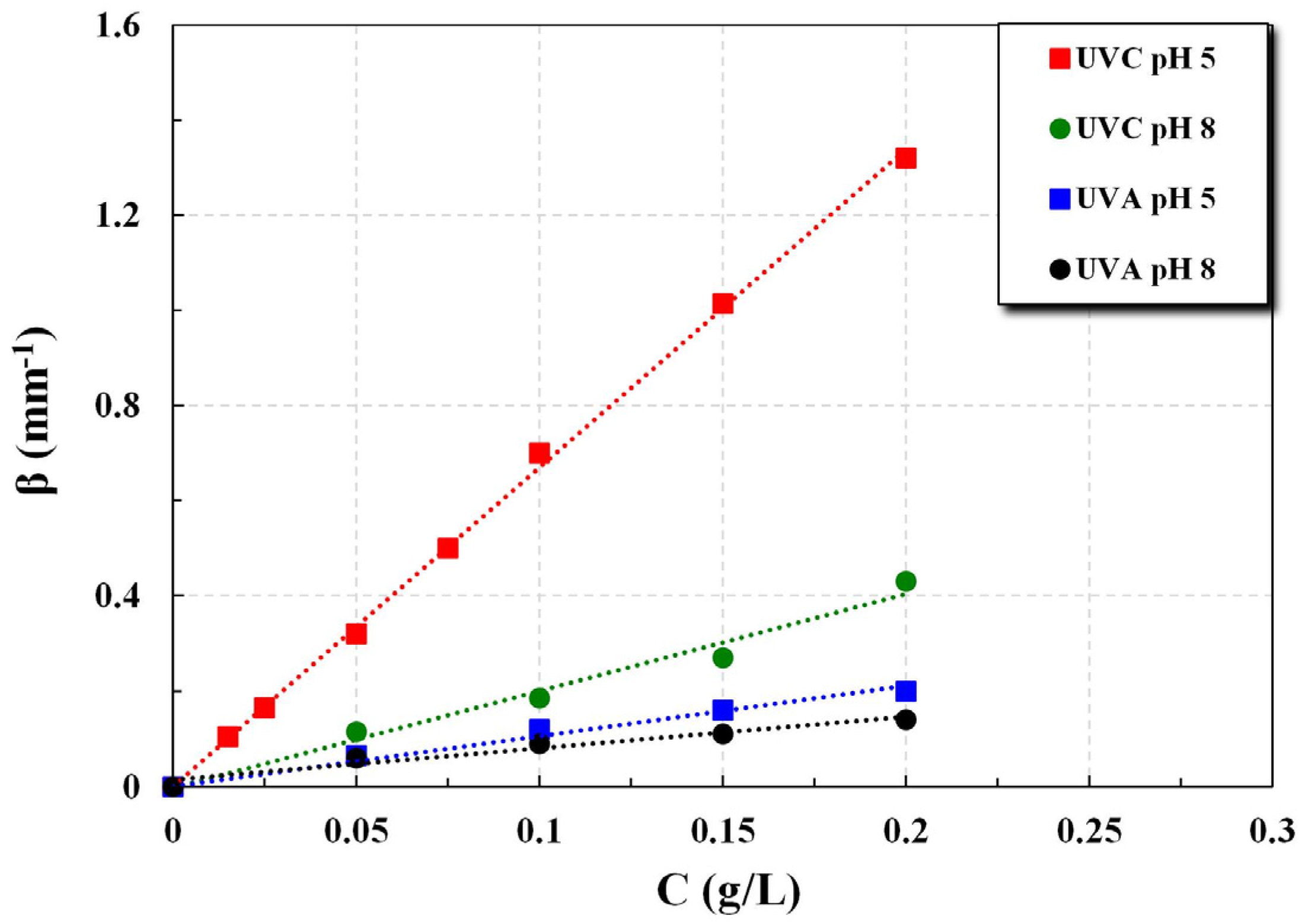
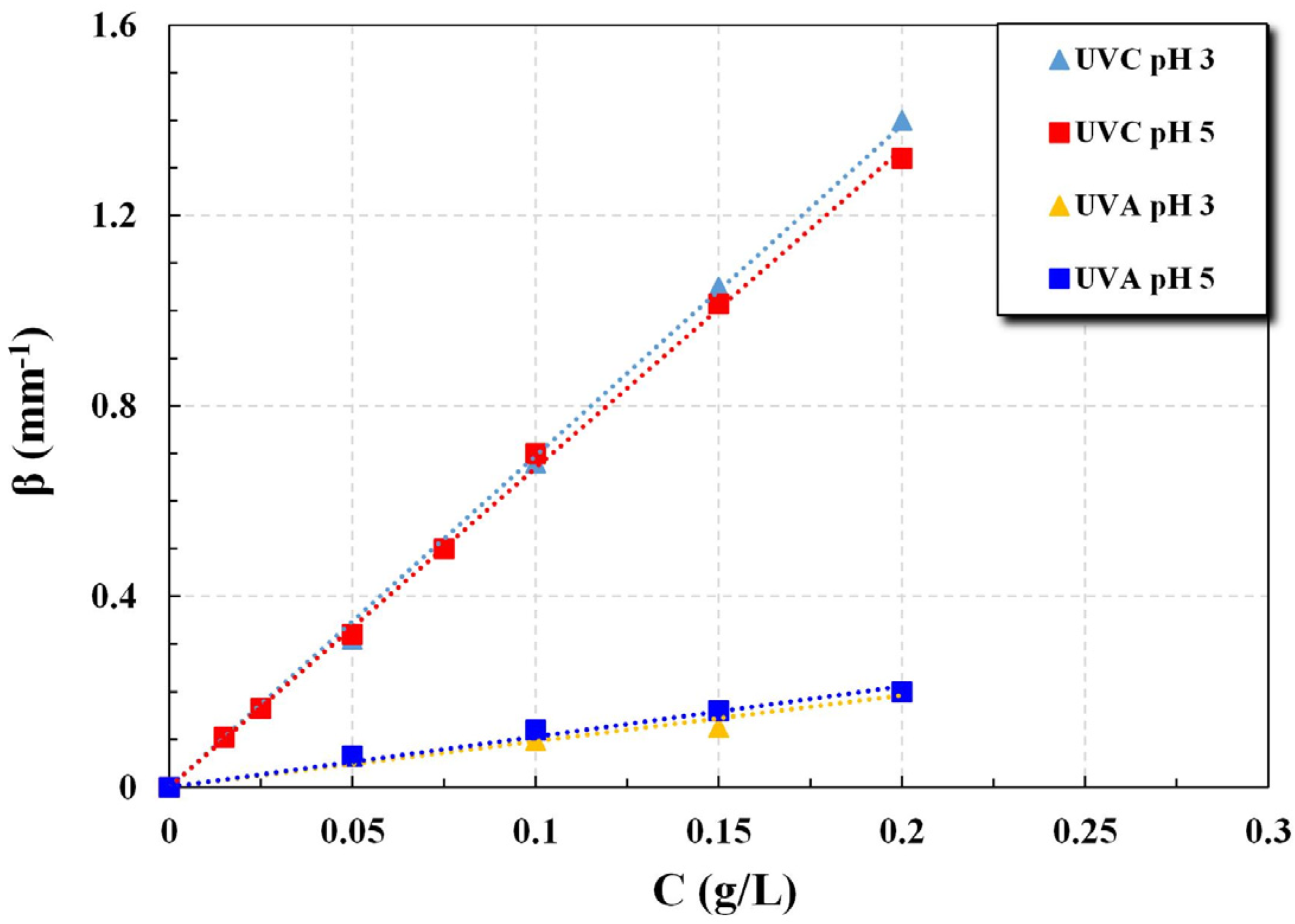
The experimental data sets of light transmittance I(z)/I
o variation with a depth of irradiation field z, for a fixed catalyst particle mass concentration C, were used to determine the total extinction coefficient β (mm
−1) in the range of concentration C = 0.015 to 0.20 g/L. Further, these data were employed to estimate the coefficients of absorption κ and scattering σ by fitting the above-mentioned hyperbolic K-M solution [
20,
21], which is of the form:
where
Further, the spectral albedo as follows:
which is strongly dependent on the wavelength of incident light [
13,
15], is commonly used in the literature to designate the fraction of scattered and absorbed light. As shown above (Equations (2)–(5)), both parameters u and v are functions of albedo ω, only.
As indicated in the Introduction, the Κ-Μ solution (Equation (1)) is applicable to the problem at hand under several simplifying assumptions discussed in the literature [
14,
21,
25]. The main assumption is that the medium, subjected to diffuse monochromatic radiation, is comprised of parallel layers (in the form of slabs), with the incident radiation impinging perpendicularly. Additionally, only a forward flux (I) due to irradiation and a backward flux (J) due to light scattering are taken into account. The experimental set-up and the data reported herein are assessed by considering these assumptions, as will be subsequently discussed.
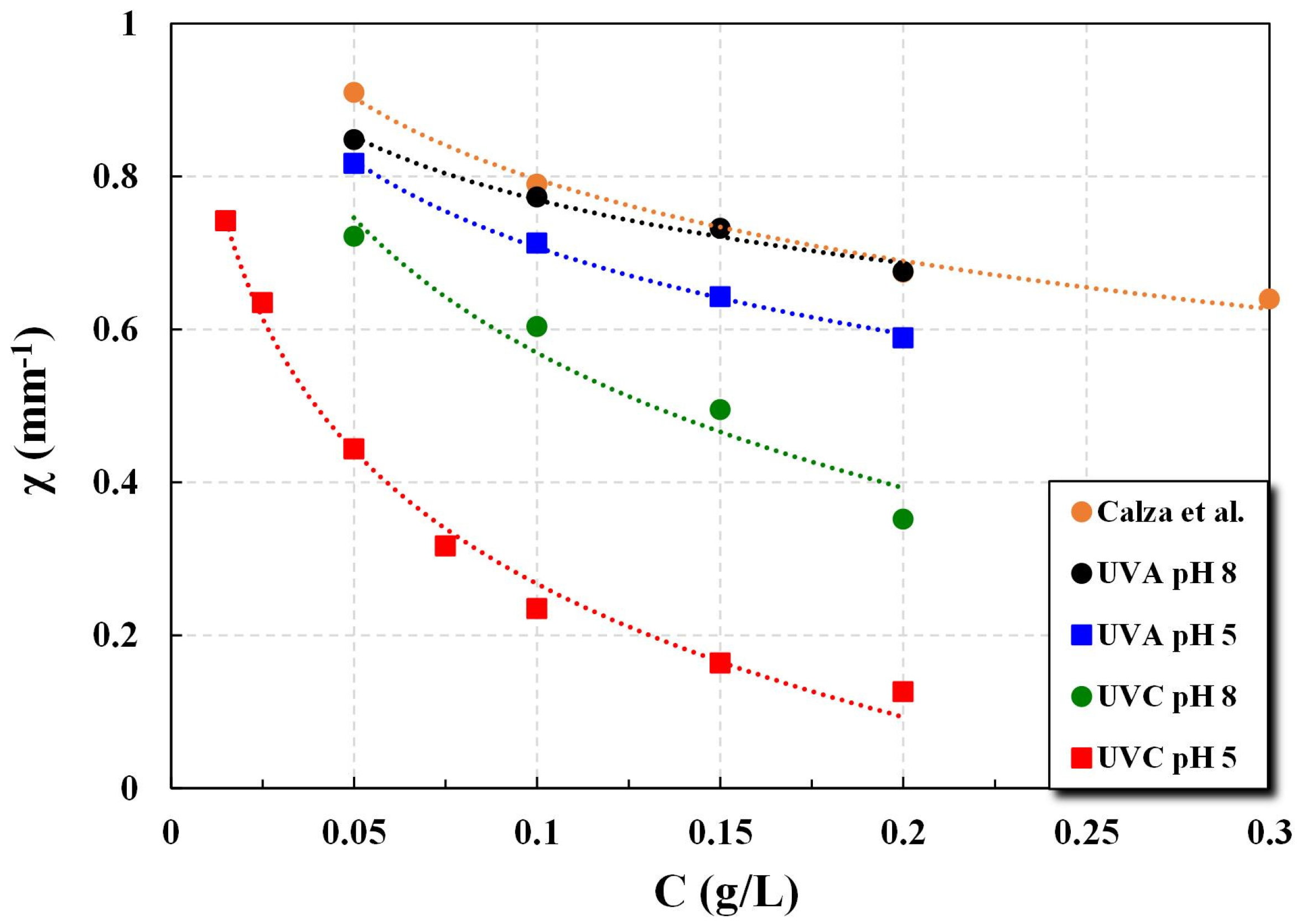
The estimated absorption coefficient κ and the associated transmittance data sets I(z)/Io can be used to determine the rate of specific photocatalytic reactions, employing various approaches proposed in the literature. Calza et al. [
17] proposed such a simplified approach and a related rate expression, which (in addition to κ) is a function of a parameter χ, defined as the average square root of light transmittance in the reactor over a depth z between zero and b, as follows:
The transmittance I(z)/Io data sets, obtained in this study under various conditions, can be used to determine the respective parameter χ values.
3. Experimental Part
3.1. Experimental Set-Up—Instrumentation
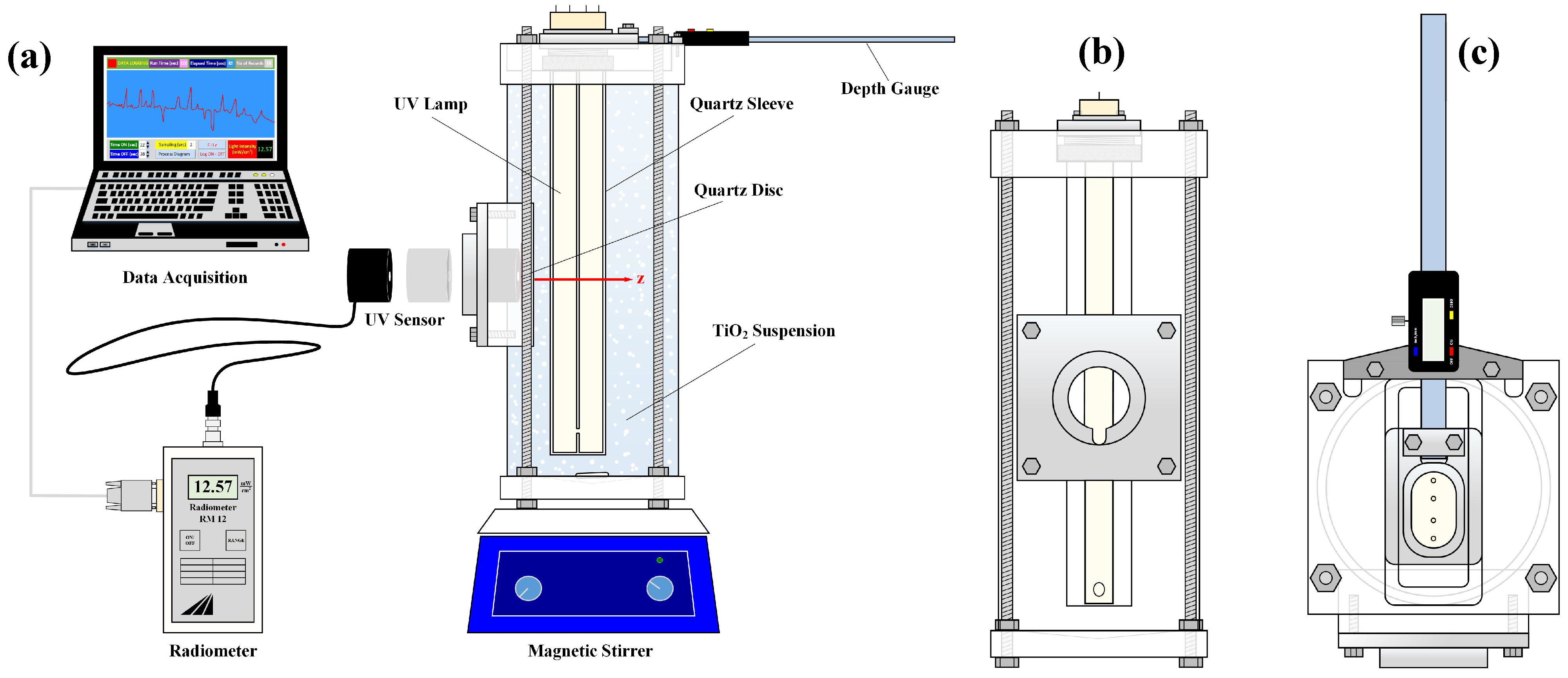
The experimental set-up employed in this study is presented in
Figure 1. The photoreactor was designed and constructed in the authors’ NRRE/CPERI/CERTH laboratory; it was made of a cylindrical Plexiglas
® vessel (33 cm high and 12 cm ID), two Plexiglas
® square end-plates (14 × 14 cm), and a square shaped “window” for the light intensity measurements. The two end-plates, of a thickness of 3 and 1.7 cm, were fixed to the top and the base of the vessel, respectively, through four holes drilled symmetrically and connected together (water-tight) with four threaded steel bars (
Figure 1a,b). The base plate was smooth (specially machined) and pan-shaped, to prevent catalyst particle deposition and facilitate cleaning. In the top-plate surface, a special channel was machined, 2 cm deep with a cross section of 12 × 5.5 cm (
Figure 1c) to accommodate the depth gauge device for light-source movement. The UV lamp, employed as a light source, was inserted in a cylindrical closed-end tube (sleeve) made of quartz glass (30 cm height and 4.5 cm OD), which was fixed/supported over this channel, and placed vertically inside the vessel, a few centimeters from the bottom. This quartz sleeve was fixed on the radially moving depth gauge bar, with a specially machined component, which was mounted on the top-plate channel. The depth gauge bar was fabricated in such a manner that it could move radially (controlled and adjusted manually), sliding over the channel surface and covering a radial distance up to 4.5 cm from its initial position. A digital gauge (E-DP15J, Kanon, Japan) with a high accuracy (10
−2 mm) was employed for the measurement of the depth of the irradiation field.
Therefore, this device allowed for the control and precise measurement of the radial distance z of the UV lamp quartz-sleeve from the flat UV sensor “window” where the UV light sensor was located (
Figure 1b). Images of the depth-gauge mechanism and of the photoreactor vessel are included in
Supplementary Figure S1. To install the window, a piece of approx. 10 × 10 cm from the cylindrical Plexiglas
® vessel was removed, where a flat Plexiglas
® piece was fixed (
Figure 1b). Another specially designed metal piece was fixed over the window’s face and perforated to support a quartz disc of a 5 cm diameter and a 0.2 cm thickness. This quartz disc (in contact with the suspension) was properly sealed on the back side part of the metal piece, ensuring smoothness. A hole of a 4 cm diameter was opened on the external side of the metal piece, where the UV sensor was placed. Geometrical details at the vessel cross-section where the measurements are made are depicted in
Supplementary Figure S2.
A continuous mixing of the catalyst suspensions was maintained with the aid of a magnetic stirring device on which the photoreactor vessel rested. The total working volume of the photoreactor was 3 L. The pH and the temperature of the catalyst dispersion were measured with sensors (744 Metrohm, Herisau, Switzerland) immersed in the vessel. The UV light intensity was recorded with a portable UV-Vis radiometer (RM-12, Dr Grobel, Ettlingen, Germany ), where suitable UVA and UVC sensors were adapted with 315–400 nm and 200–280 nm operating ranges, respectively. For each specific lump location (i.e., depth of field), five to ten light intensity measurements were taken (varying within a narrow range of ±5%) and the median value was retained. All data were monitored, recorded, and stored with the aid of a data acquisition system consisting of a PC, a microSD card, and a software (Advantech GeniDAQ, version 4.0, Germany). Finally, particle size distribution measurements were made with a particle size analyzer Mastersizer S (Malvern Instruments, Malvern, UK).
3.2. Materials
Titanium dioxide (Aeroxide® P25, Evonik, Essen, Germany) powder was used as a photocatalyst in the experiments without any pretreatment. As reported by the manufacturer, this product is a mixture of 75% anatase and 25% rutile; some of its main characteristics include the 50 m2/g BET surface area, 3.18 eV energy band gap, and a 21 nm primary average particle size. Deionized water (DI) (pH 5.8–6.8, eC < 1 μS/cm, and TOC concentration < 0.05 mg/L) was used in all of the experiments of this study, except for some preliminary tests where laboratory tap water was employed. Aqueous solutions of 1 M sodium hydroxide (NaOH) and 1 M hydrochloric acid (HCl), reagent grade (purchased from Riedel-de Haën), were used for the pH adjustment of tested catalyst suspensions.
Two lamps of different irradiation types were employed as light sources, i.e., Philips Actinic BL PL-L 24W UVA and Philips Germicidal TUV PL-L 24W UVC (Philips, Eindhoven, The Netherlands). Both lamps were of a nominal power 24 Watts, U-shaped, and single-ended, with 4 pins in the upper part for a connection to a power supply converter. As shown in
Supplementary Figure S3, the UVC lamp emits nearly monochromatic light at wavelength 253.7 nm, whereas the emission spectrum of the UVA lamp is much broader with a peak value at wavelength ~365 nm according to the manufacturer [
26].
3.3. Experimental Procedure
Experiments were carried out with several catalyst loadings, in the range of 0.015 to 0.2 g/L, three pH narrow ranges (~3, ~5, and ~8), and two types of UV irradiation, i.e., UVC and UVA. Each experiment with a fixed TiO
2 concentration lasted, at most, 45 min. The temperature of the photoreactor fluid was rising by approx. 2 °C at the end of each experiment, due to heat dissipated by the lamps, ranging from 25 to 32 °C. The main experimental conditions and extinction coefficient β values of all data sets are listed in
Table S1, Supplementary Materials.
As a first step in preparing the catalyst suspension, TiO2 powder at the desirable concentration was mixed in DI and vigorously stirred for about 4 h. Next, the pH adjustment was made by using either a NaOH or HCl solution, for preparation of the ~3 L dispersion solution, added to the photoreactor vessel. Magnetic stirring ensured a sufficient mixing and uniformity of the catalyst concentration. Afterwards, the UV lamp was turned on for approx. 15 min until the light intensity reached a maximum value and stabilized. To start the measurements, the moving lamp/quartz sleeve was placed in contact with the window quartz disc, which corresponds to zero radial distance (z = 0) and local UV light intensity Io. Then, the lamp/quartz sleeve was displaced away from the vessel wall, by manually controlling the depth gauge bar. The radial distance intervals of this movement dz were precisely controlled, i.e., by intervals dz = 0.5 mm for the first 5 mm and dz = 1 mm for the next 15 mm. The depth gauge path could be extended to z ~ 45 mm, where the light intensity is zero or negligible. The radial irradiance profile for each experiment, with a fixed TiO2 concentration, was obtained by recording value pairs of UV light intensity I and distance z from the photoreactor inside wall/window, employing the radiometer and depth gauge indications, respectively. At the end of each test, the experimental set-up was meticulously cleaned to remove catalyst deposits and other contaminants. Specifically, the reactor vessel and the quartz sleeve were first rinsed well with water and then the quartz sleeve was placed in the chamber of an ultrasonic cleaner. Finally, the window quartz disc, the UV lamp, and the quartz sleeve were wiped out with a soft tissue and an appropriate detergent.
Concerning the particle size distribution measurements, both the preparation of the catalyst dispersion and the pH adjustment were executed exactly as mentioned above. However, all the suspensions tested were of the same catalyst loading, 0.05 g/L. A suspension volume of 600 mL was placed in the instrument sampling vessel and remained there under moderate stirring (~1350 rpm) for 15 min, the recommended time for dispersion stabilization. Then, measurements for each sample after 2, 5, and 15 min were taken. With all samples, duplicate measurements were made, showing very good repeatability regarding the values of percentiles d50 (median value) and d90.
5. Conclusions
A commonly used technique to determine the extinction coefficient β (for fixed catalyst concentration C, with external irradiation) involves sequential single-point (z) measurements of transmittance I/Io through the repeated addition of a small dispersion volume to the test cell to adjust/increase the depth of field z; this is obviously tedious and prone to error. Regarding another type of set-up, with a UV lamp immersed in the test section, only single measurements are usually taken for a fixed depth z, thus greatly limiting the acquired values of a I/Io data set. However, the proposed technique, applicable to immersed UV lamps, facilitates the acquisition of complete I/Io data sets, for a fixed C, through a mechanism ensuring the precision adjustment and measurement of the depth of field z. Also, realistic photocatalytic dispersions can be assessed in this novel experimental set-up.
Regarding parameters β* and ω, the data for groups obtained with the new technique under various conditions (i.e., different irradiation types and pH values), are in fair agreement overall with literature results from several credible studies. The simplified Kubelka–Munk model, employed to estimate the albedo ω and absorption coefficient κ values, appears to perform satisfactorily. Therefore, the proposed technique is considered quite useful for implementation in photocatalytic process development and design studies. Nevertheless, additional work may be needed for possible improvements of both the K-M (or similar) model and the experimental set-up overall performance.