Synthesis and Property Characterization of AM/AMPS/C18DMAAC/NVP Tetrameric Temperature-Sensitive Thickening Copolymer
Abstract
1. Introduction
2. Experimental Design
2.1. Experimental Raw Materials
2.2. Polymer Synthesis Process
- ①
- To dissolve and prepare the reaction system, 100 milliliters of acetonitrile was used to dissolve 29.7 g of C18DMA. The resulting solution was then transferred to a 250 milliliter three-necked flask to establish the reaction system. To ensure that the temperature of the reaction remained within the desired range of 0–5 °C, the flask was immersed in an ice bath.
- ②
- For chloropropene dropwise addition, 9.2 g of C3H5Cl was slowly added dropwise under ice bath conditions, controlling the drop rate to ensure that the reaction temperature remained stable between 0–5 °C. Stirring was continued during the dropwise addition to ensure uniform mixing of the reactants.
- ③
- For temperature increase and reaction, upon completion of the addition, the ice bath was removed, the reaction temperature was raised to 45 °C, and stirring was continued for 24 h. The temperature was monitored throughout the reaction to ensure that it did not exceed 45 °C to prevent side reactions or overreactions.
- ④
- For solvent removal, when the reaction was complete, vacuum distillation was used to remove the acetonitrile solvent and concentrate the reaction product. This process was performed under mild conditions to avoid decomposition of the product.
- ⑤
- For recrystallization, a quantity of 200 milliliters of anhydrous ether was used to dissolve the crude product, which was then recrystallized. This step helped to remove unreacted raw materials and by-products. The recrystallized solid was separated by suction filtration, washed, and collected.
- ⑥
- For drying, to ensure the complete drying of the product and the removal of the residual solvent, the recrystallized solid product was placed in a vacuum drying oven and dried under vacuum at 40 °C for 12 h.
- ①
- For the preparation of monomer solution, AM, AMPS, and NVP were dissolved in 80 mL of deionized water, and C18DMAAC was dissolved separately in 20 mL of deionized water; 20% by mass NaOH was added, and the pH was adjusted to 6.0~7.0.
- ②
- For the reactor preparation, a 250 mL four-port flask equipped with a mechanical stirrer, thermometer, nitrogen gas line, and dropping funnel was placed in a water bath.
- ③
- For the reaction procedure, the monomer solution was added to the reactor, nitrogen was introduced to the deoxygenate for 30 min, the temperature was raised to 50 °C, the freshly prepared (NH4)2S2O8 and NaHSO3 solution (1.2:1) was quickly added, and it was reacted at 50 °C for 6 h at a stirring rate of 300 rpm.
- ④
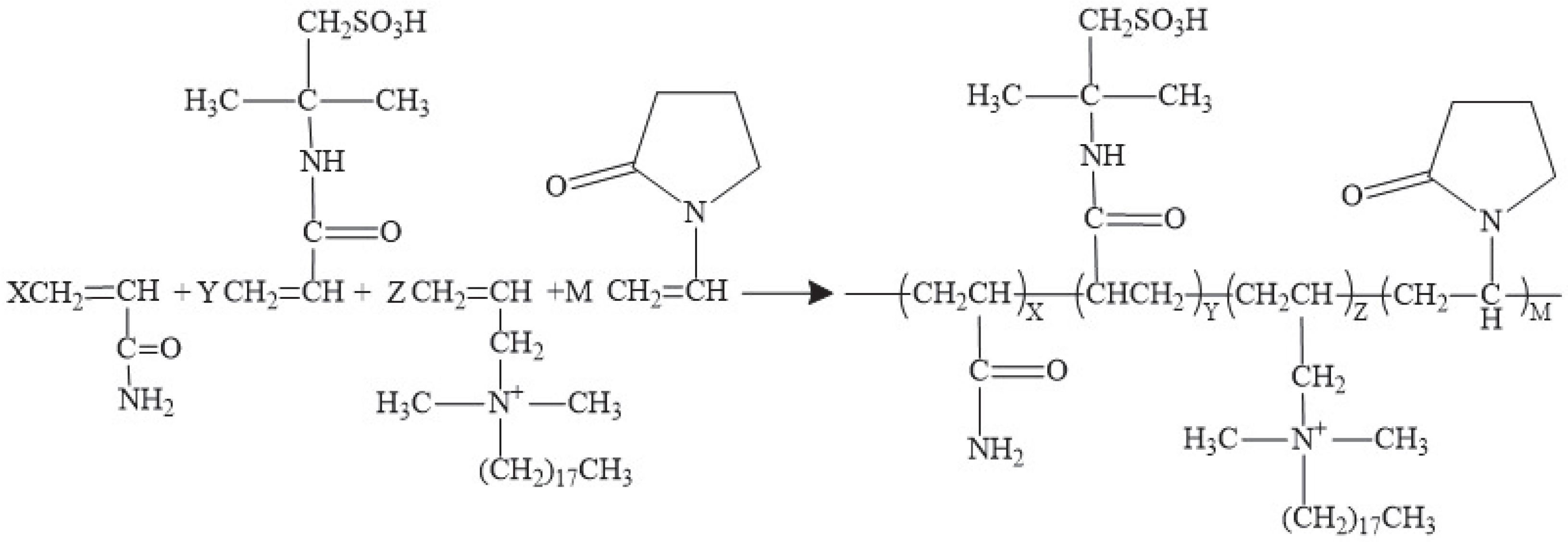
- Post-treatment, upon completion of the reaction, the product solution was transferred to an excess of acetone, resulting in precipitation of the target compound. The precipitate was then collected, thoroughly washed three times with acetone, and dried under vacuum at 40 °C for 24 h. This process was repeated until a consistent weight was achieved, resulting in a white powdered temperature-sensitive thickening polymer. As shown in Figure 1, the polymerization grafting process was characterized by the following molar ratios: X, Y, Z, and M, corresponding to AM, AMPS, C18DMAAC, and NVP, respectively.
2.3. Synthesis Conditions Optimization
2.4. Characterization of Polymers
2.5. Polymer Property Testing
2.6. Testing the Effect of Polymers on the Properties of Cement Paste
3. Results and Discussion
3.1. Structural Characterization of the Polymers
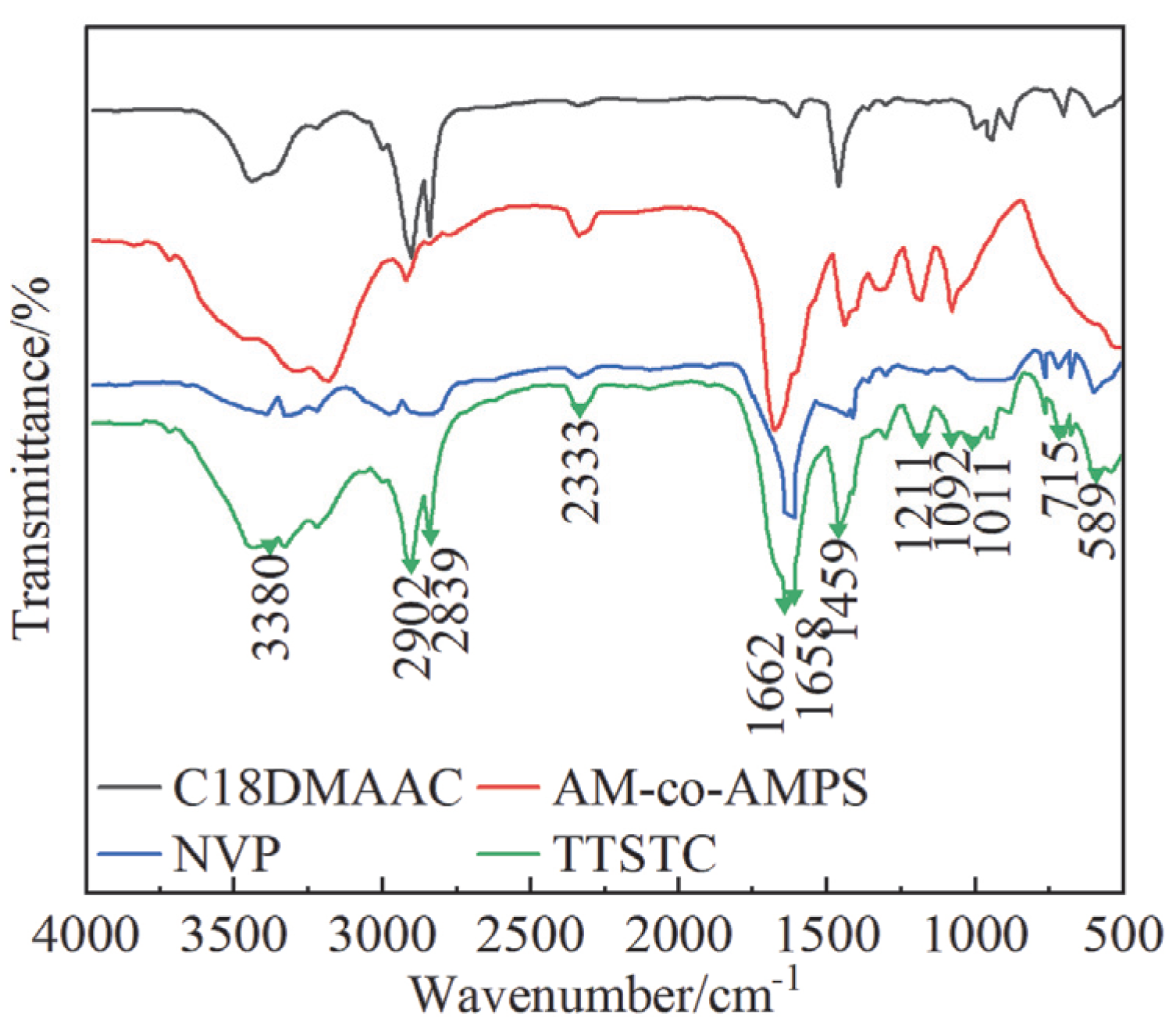
3.1.1. FT-IR Analysis Results
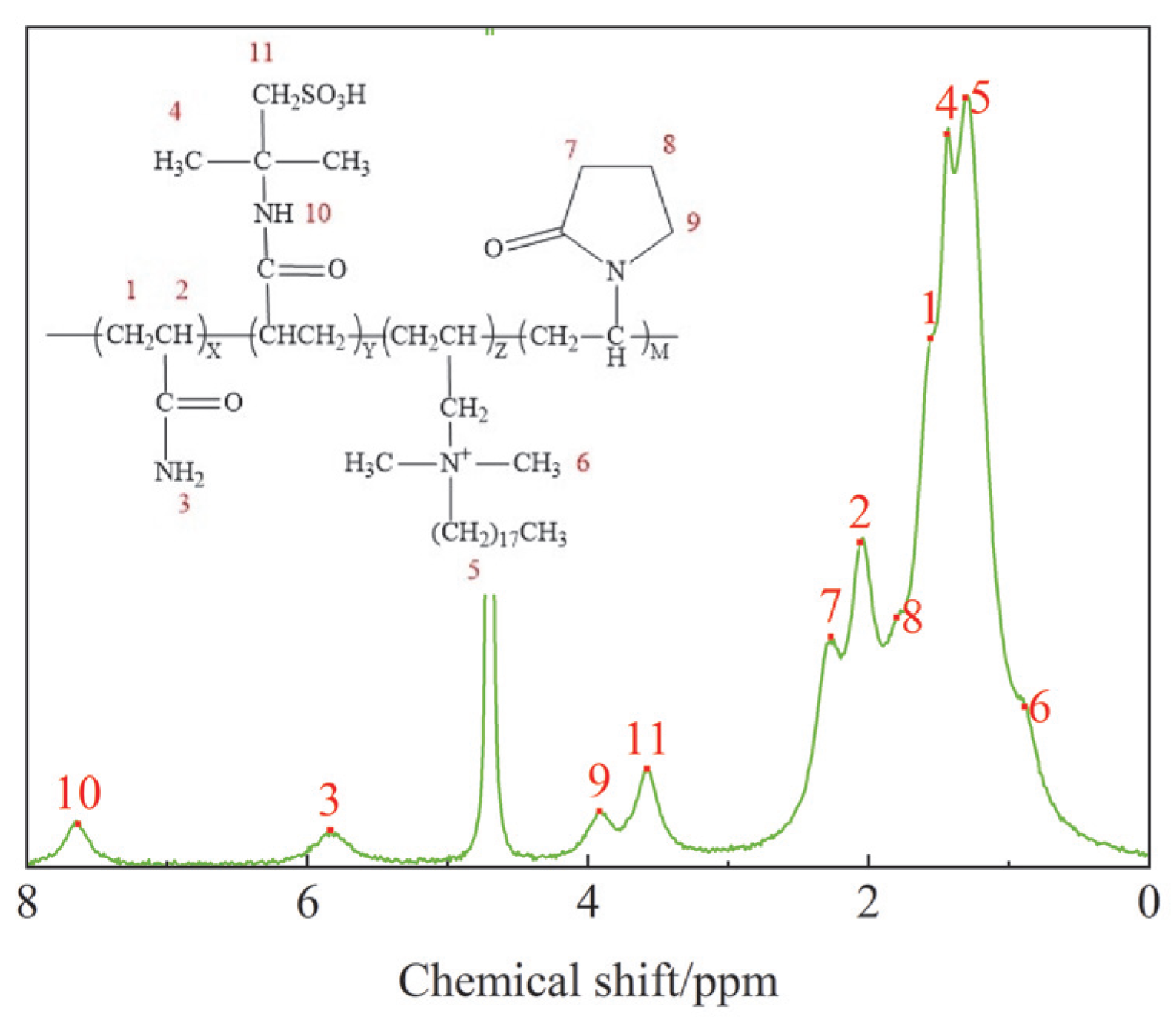
3.1.2. 1H NMR Analysis Results
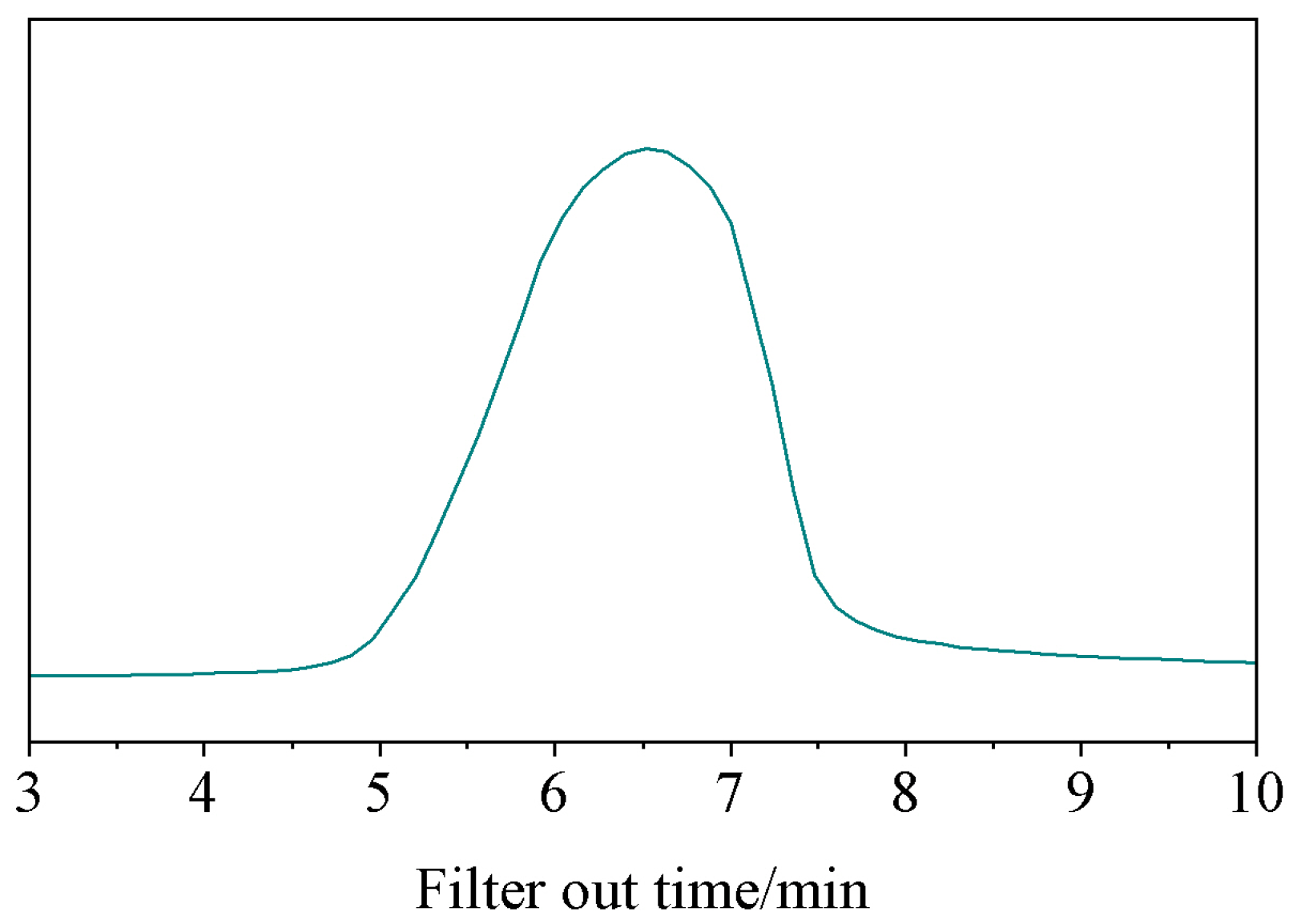
3.1.3. Gel Permeation Chromatography Analysis Results
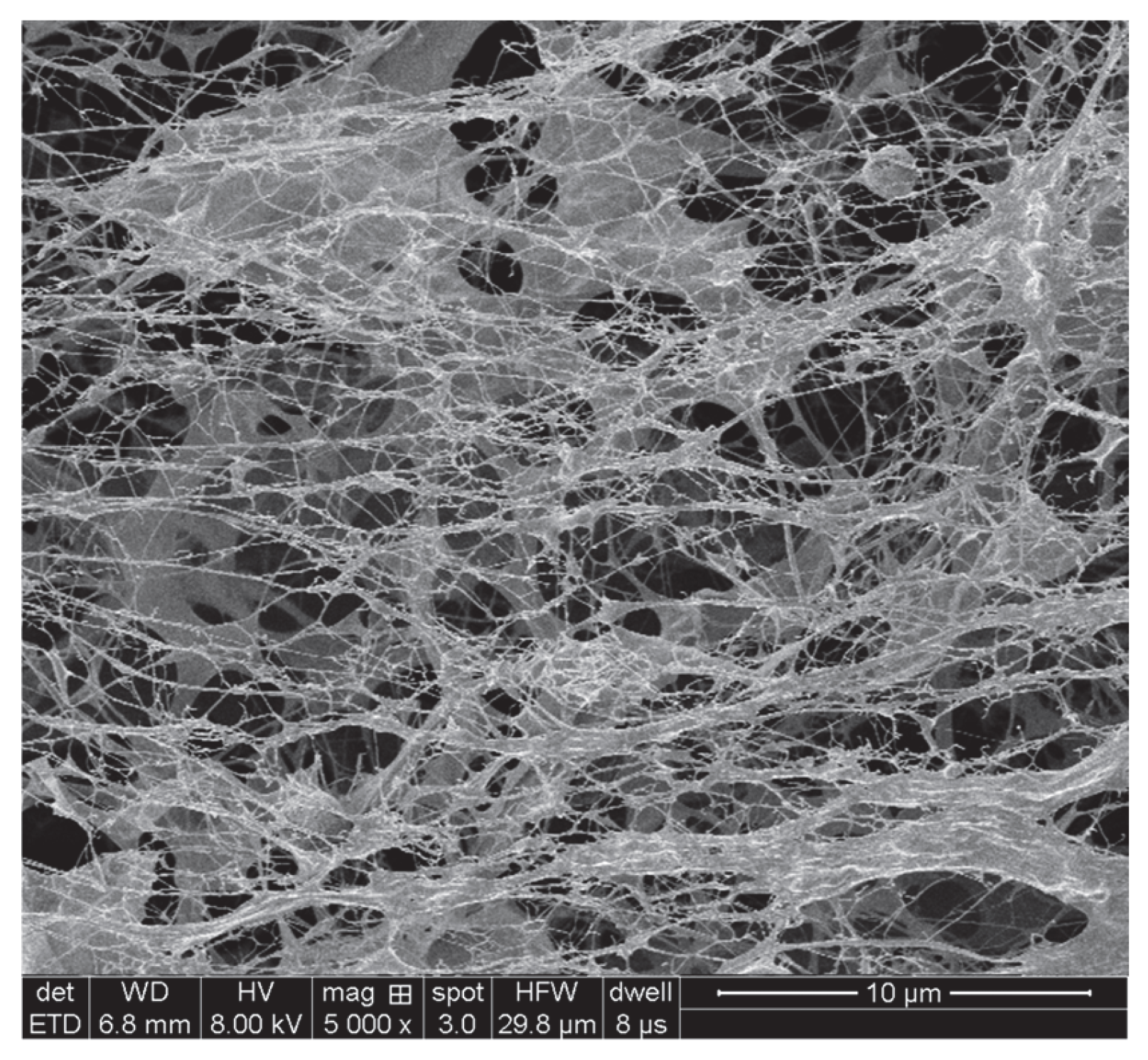
3.1.4. Scanning Electron Microscopy Analysis Results
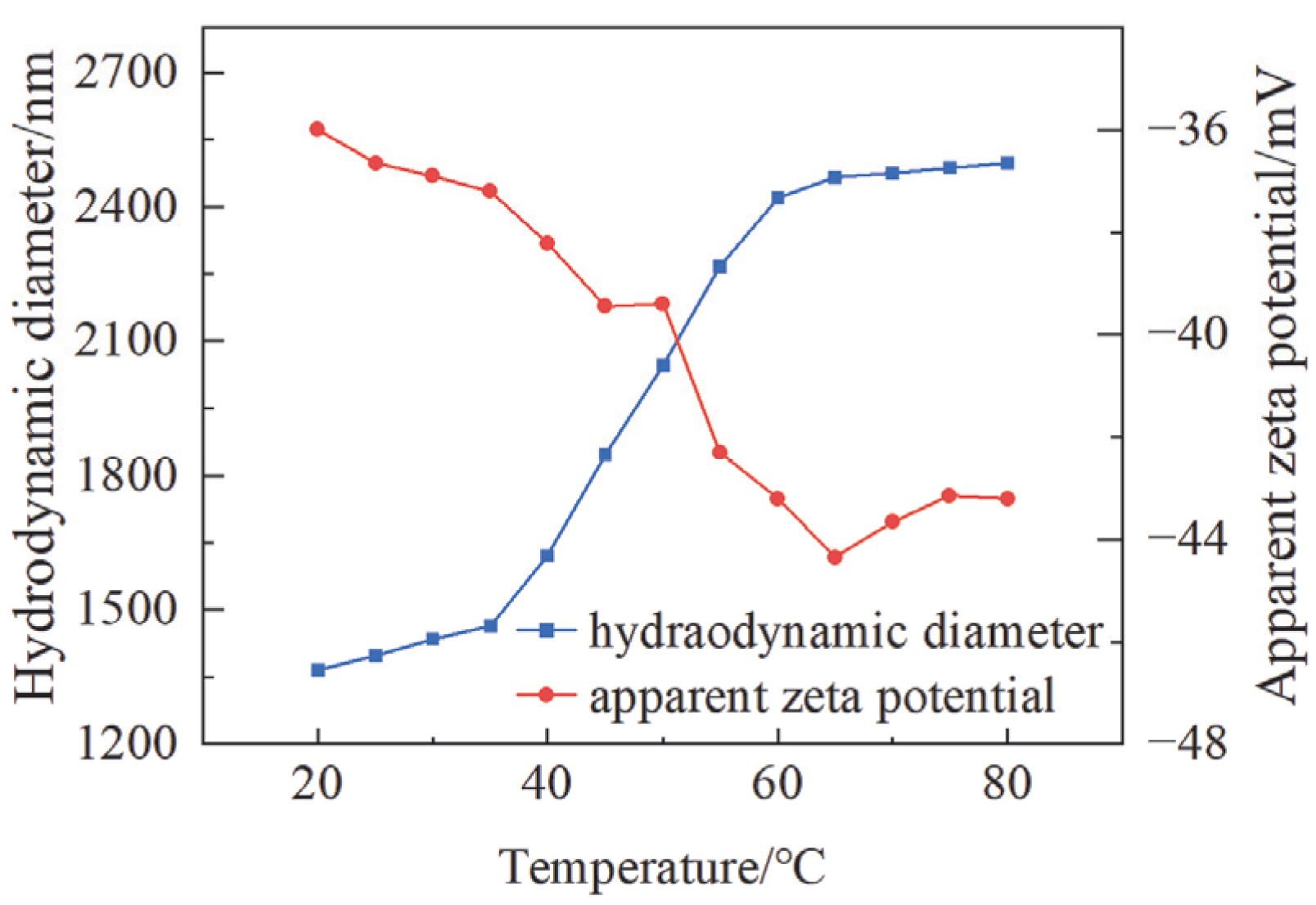
3.1.5. Zeta Potential and Hydrated Dynamic Diameter of Polymer Analysis Results
3.2. Properties of Polymers
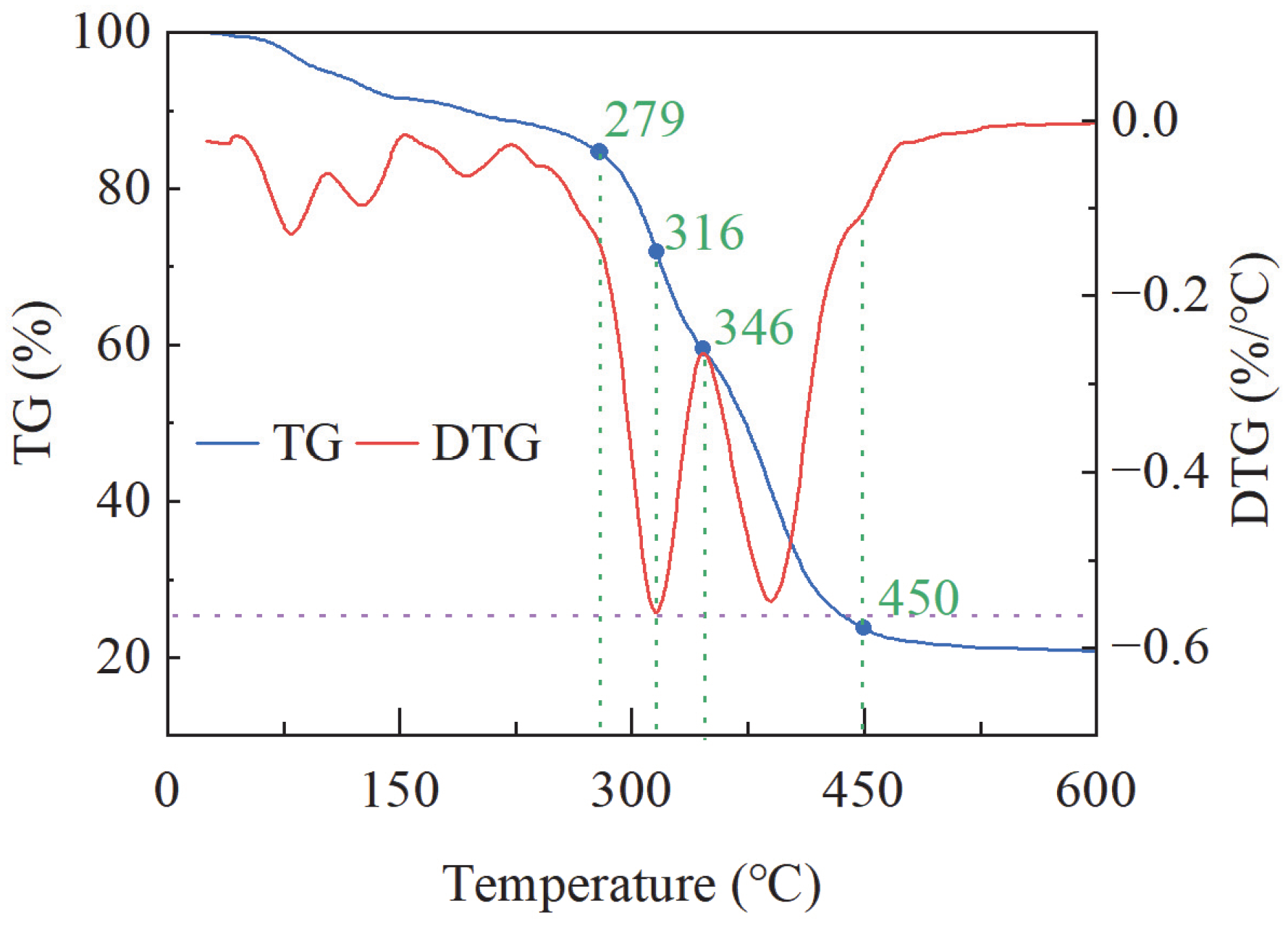
3.2.1. Thermogravimetric Analysis
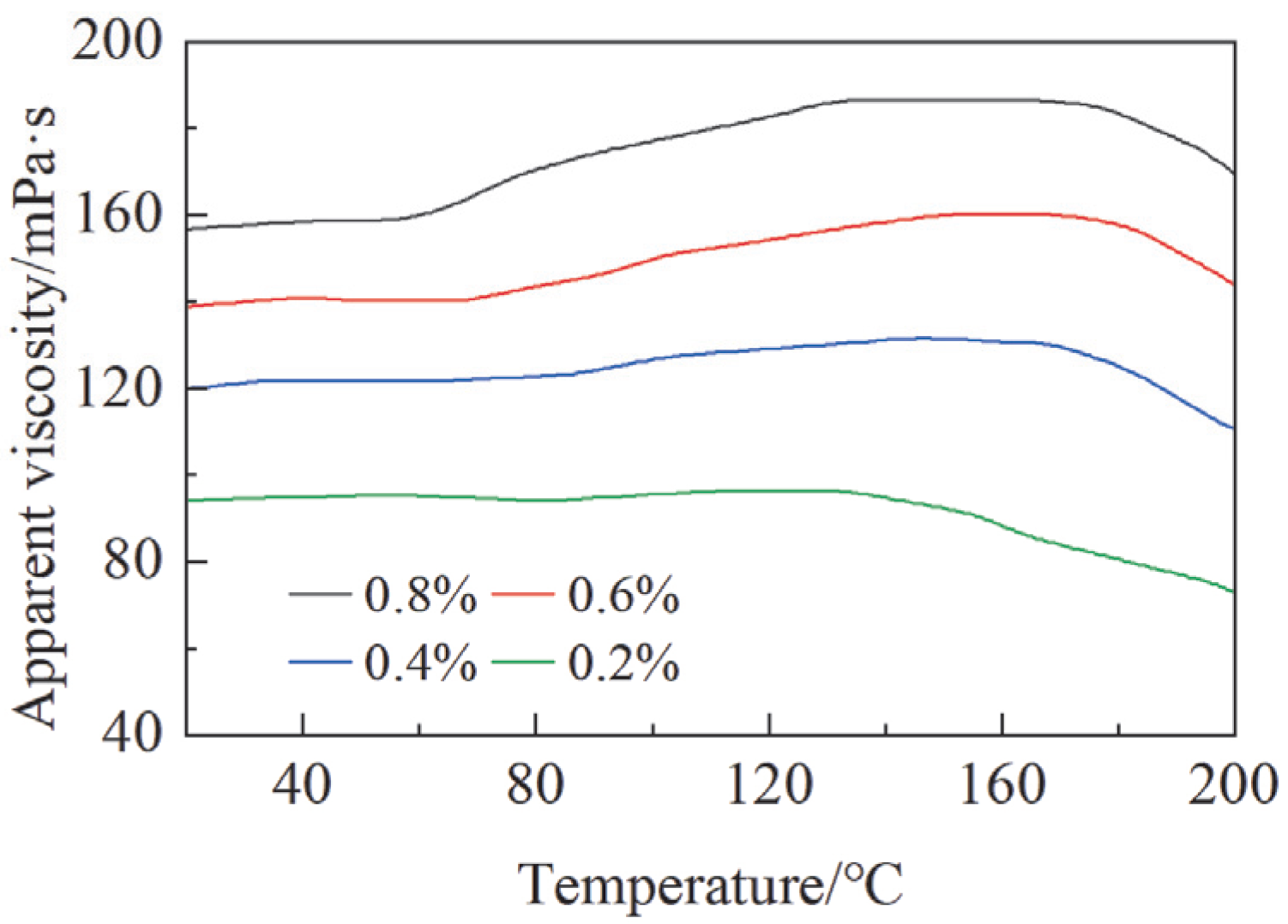
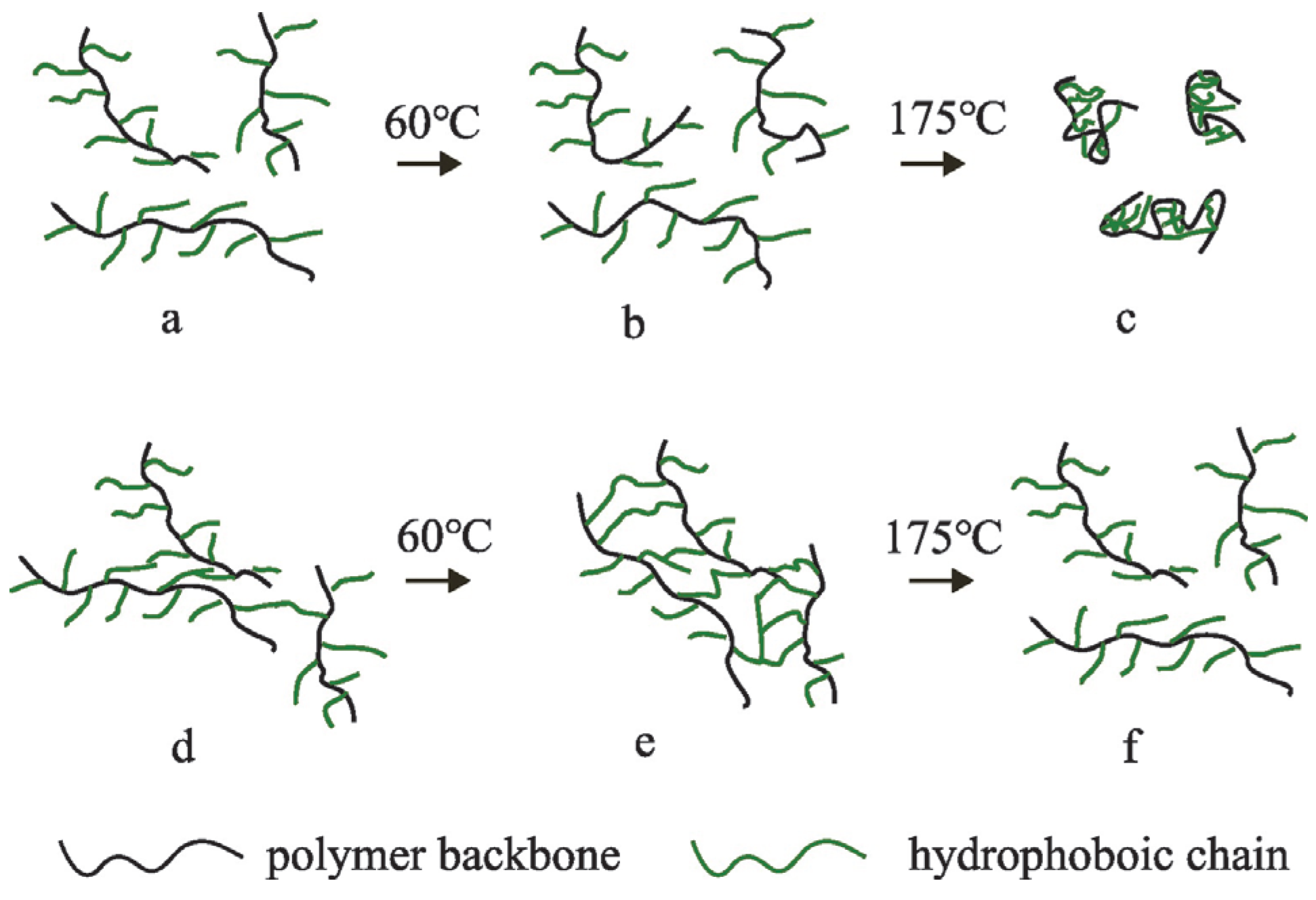
3.2.2. Temperature-Sensitive Thickening Properties
3.2.3. Salt Tolerance
3.2.4. Alkali Resistance
3.3. Influence of Polymers on the Properties of Cement Slurries
3.3.1. Sedimentation Stability
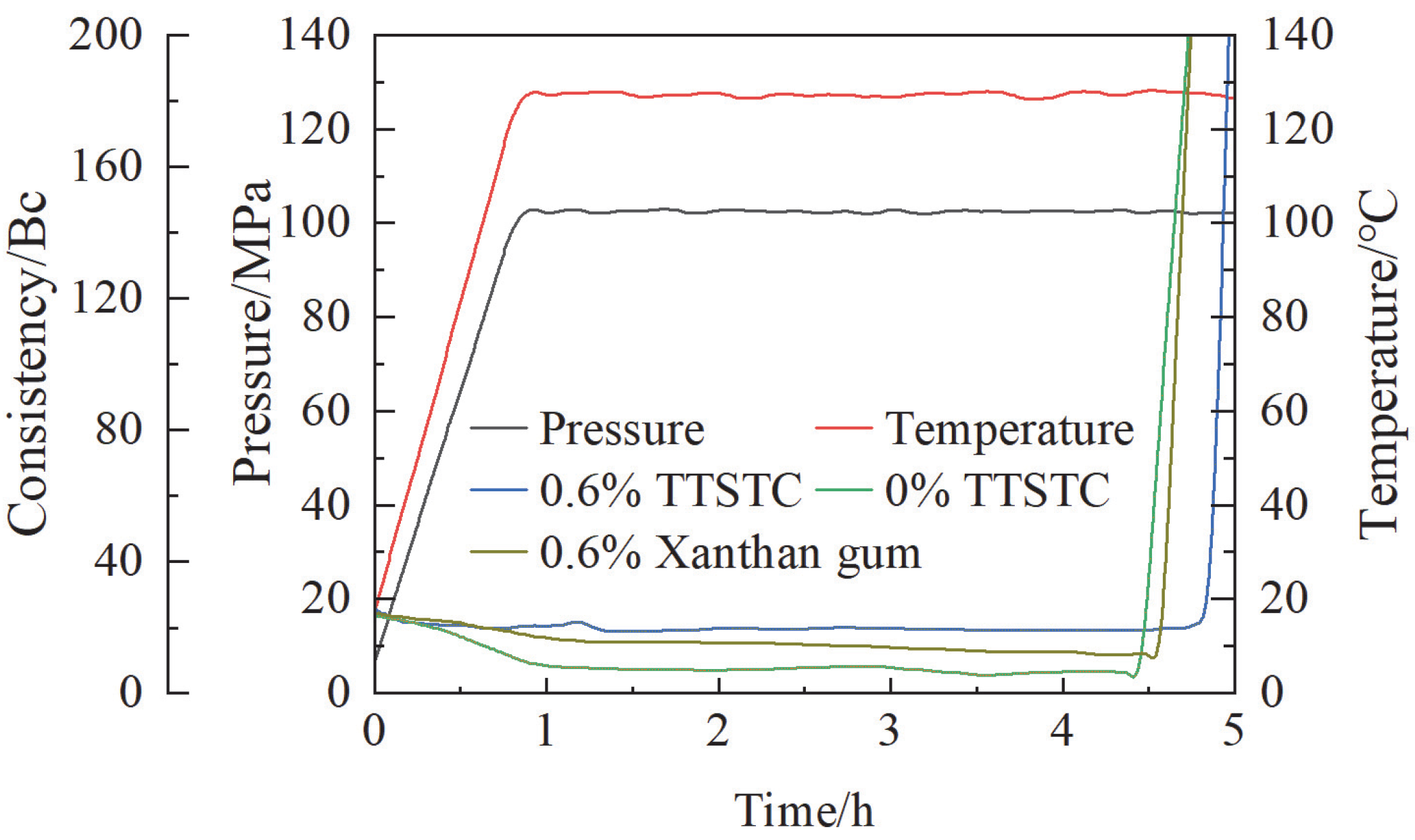
3.3.2. Thickening Properties
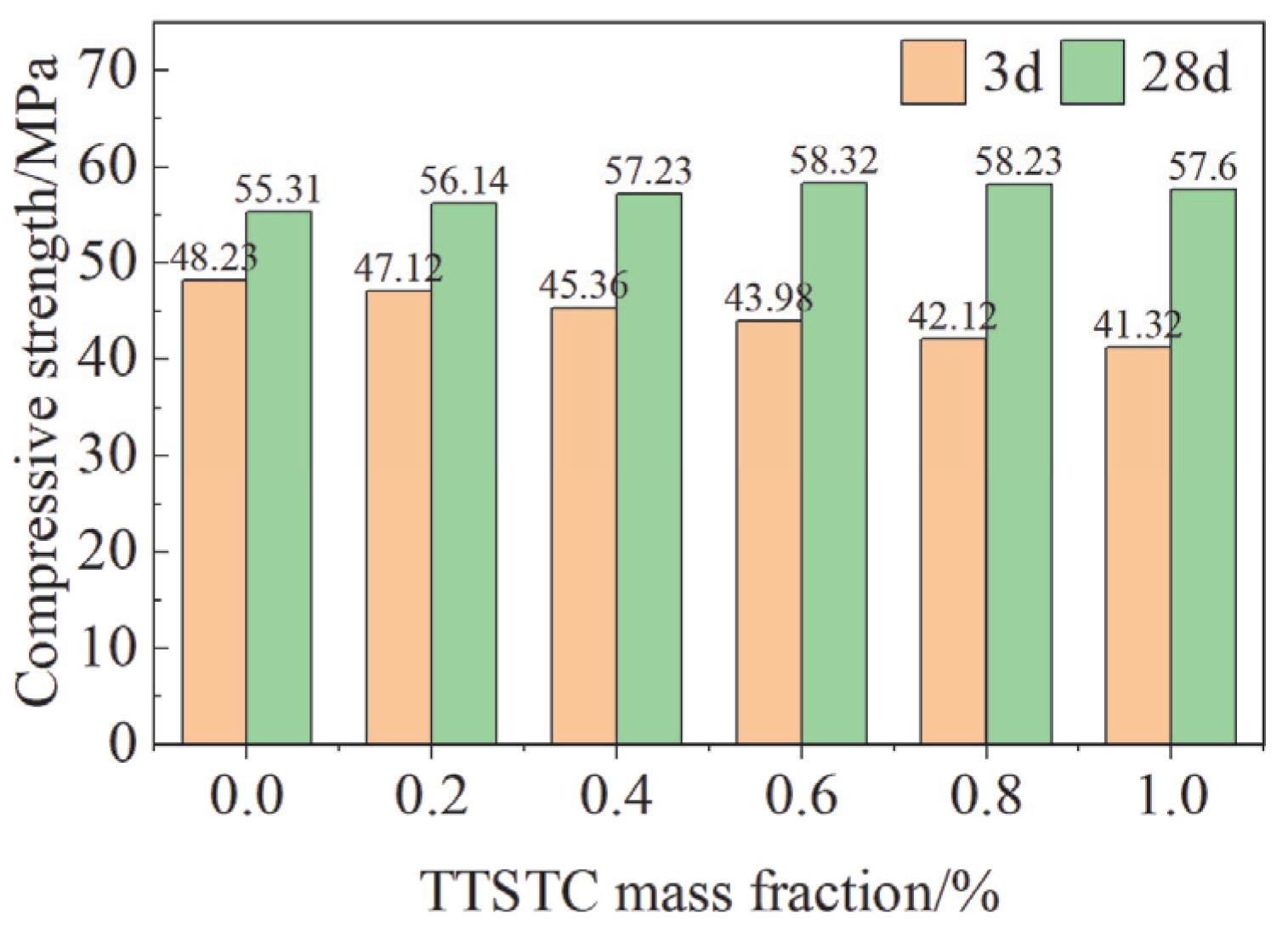
3.3.3. Compressive Strength of Cement Paste
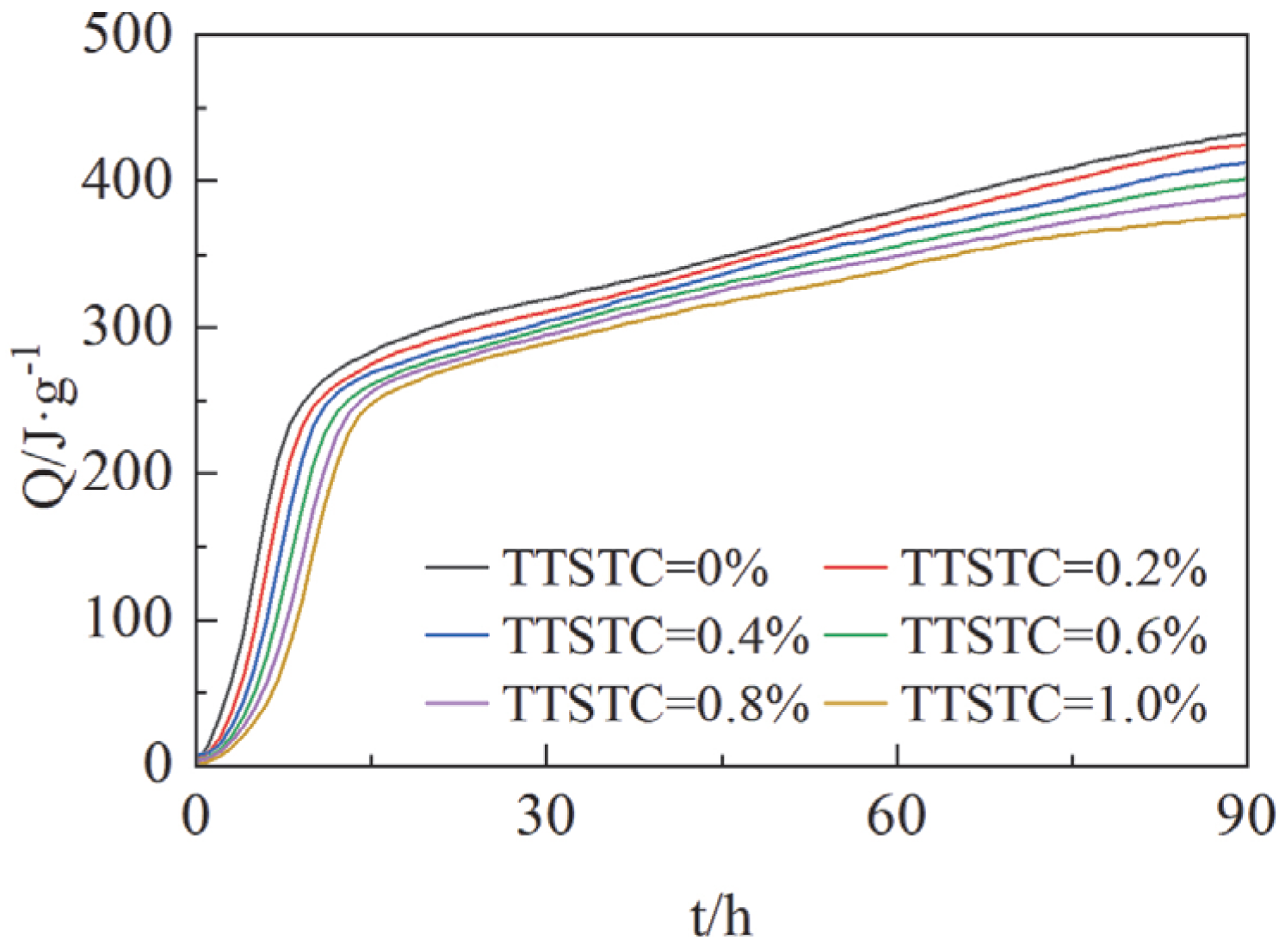
3.3.4. Hydration Kinetics Analyses Results
4. Conclusions
- (1)
- The target polymer TTSTC was successfully synthesized by aqueous solution radical polymerization using AM, AMPS, long-chain C18DMAAC, and NVP as monomers. The polymerization conditions were optimized by orthogonal experiments, and the optimal monomer molar ratio was determined to be 15:10:5:5, the initiator concentration was 16 wt%, the cross-linking agent concentration was 0.45 wt%, the pH was 6, and the polymerization temperature was 60 °C.
- (2)
- In-depth characterization of the chemical structure of TTSTC using Fourier transform infrared spectroscopy (FT-IR), proton nuclear magnetic resonance (1H-NMR), gel permeation chromatography, scanning electron microscopy (SEM), Zeta potential, and particle size tests confirmed the successful synthesis of the target polymer and validated its molecular structural properties containing thermosensitive monomers and functional groups.
- (3)
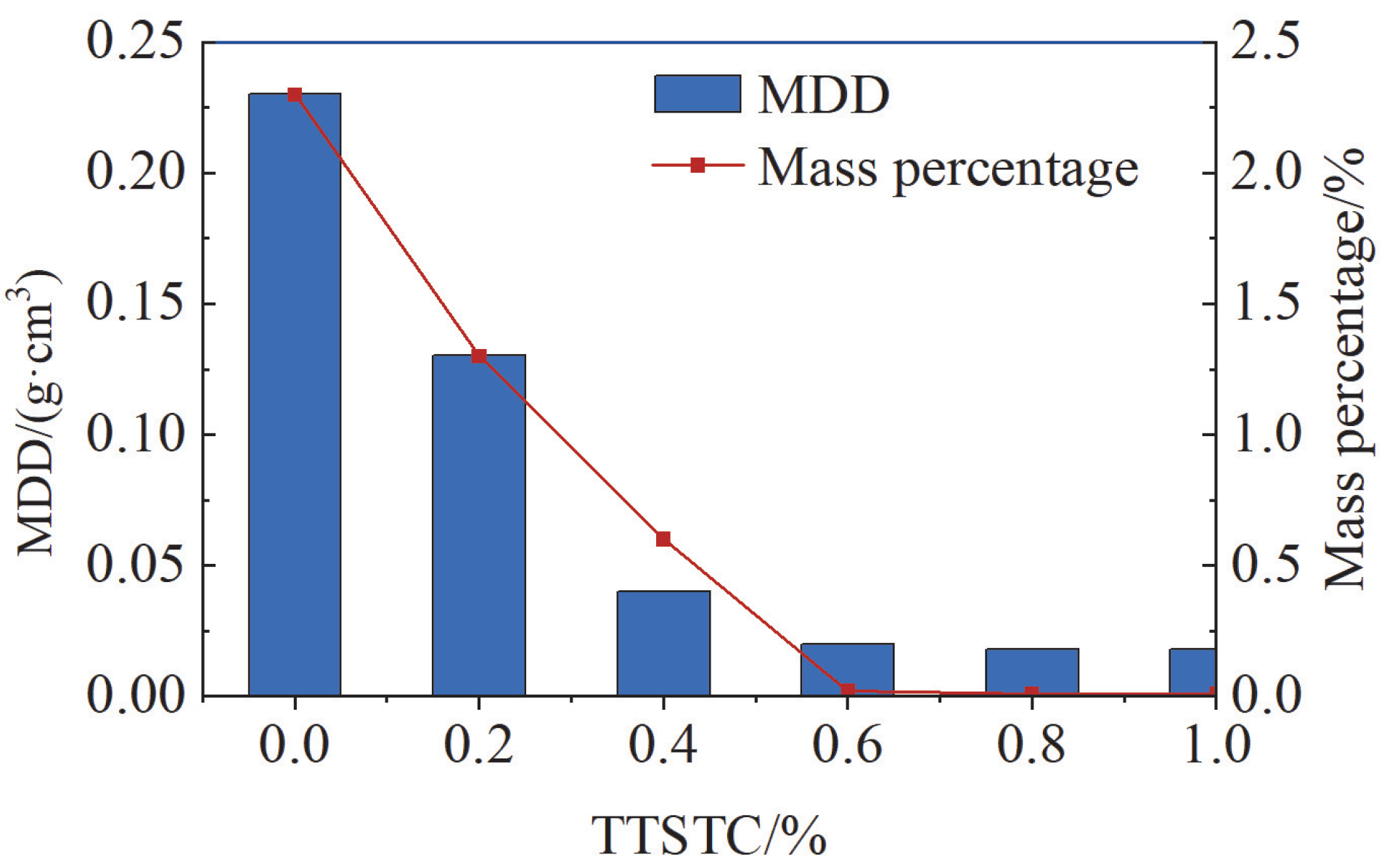
- TTSTC demonstrated remarkable thermosensitive thickening properties under elevated temperatures (up to 279 °C) and high alkaline conditions (pH 11–13) and was stable in a saline environment with a NaCl/CaCl2 concentration of 0.05–0.5 g/L. The thickening performance of TTSTC was optimal when the mass fraction was 0.6–0.8 wt%.
- (4)
- The incorporation of TTSTC was demonstrated to enhance the sedimentation stability of the cement slurry, thereby mitigating the risk of delamination under elevated temperatures and promoting enhanced slurry homogeneity.
- (5)
- The compressive strength of the cement stone was significantly increased with the addition of TTSTC at 0.6–1.0 wt%. The experimental results showed that TTSTC could effectively improve the 28d mechanical properties of cementite with less influence on the hydration kinetics, which provided a reliable guarantee for the integrity of the wellbore under a high-temperature and high-pressure environment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, P.; Wang, L.; Lai, X.; Gao, J.; Dang, Z.; Wang, R.; Mao, F.; Li, Y.; Jia, G. Two-Level Self-Thickening Mechanism of a Novel Acid Thickener with a Hydrophobic-Associated Structure during High-Temperature Acidification Processes. Polymers 2024, 16, 679. [Google Scholar] [CrossRef] [PubMed]
- Fakher, S.; El-Sayed, A.; Sameh, L.; Abdeltawab, B. Evaluating a Novel Fly Ash Resin-Reinforced Cement’s Interactions under Acidic, Basic, High-Salinity, and High-Temperature Conditions. Polymers 2023, 15, 3404. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Jia, F.; Peng, Z.; Zheng, Y. Development of temperature-responsive suspension stabilizer and its application in cementing slurry system. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130734. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Huang, X.; Lv, K.; Geng, Y. A temperature-sensitive polymer with thinner effect as a rheology modifier in deepwater water-based drilling fluids. J. Mol. Liq. 2024, 393, 123536. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Z.; Su, X.; Mianowicz, K.; Jia, H.; Wu, K. Slurry pumps in deep-sea mining: A review of numerical and experimental studies. Ocean Eng. 2022, 251, 111150. [Google Scholar] [CrossRef]
- Zhou, P.; Li, M.; Zhang, C.; Xia, X.; Yu, Y.; Qi, F.; Wu, Y. Ultra-high temperature suspending stabilizer and cement slurry interface interaction law and mechanism study for ultra-deep well cementing. Constr. Build. Mater. 2023, 409, 133979. [Google Scholar] [CrossRef]
- Lv, K.; Huang, Z.; Ling, X.; Xia, X. Analysis of the weight loss of high temperature cement slurry. Fluid Dyn. Mater. Process. 2022, 18, 1307–1318. [Google Scholar] [CrossRef]
- Djouonkep, L.D.W.; Xie, B.; Tao, H.; Zhuo, L.; Tchameni, A.P.; Zhao, L. Investigating the structure-to-property of thermo-thickening polymers capped by different structural side chains. React. Funct. Polym. 2023, 186, 105569. [Google Scholar] [CrossRef]
- Chen, F.; Lu, G.; Yuan, H.; Li, R.; Nie, J.; Zhao, Y.; Shu, X.; Zhu, X.; Chen, F. Mechanism and regulation of LCST behavior in poly(hydroxypropyl acrylate)-based temperature-sensitive hydrogels. J. Mater. Chem. A 2022, 10, 18235–18247. [Google Scholar] [CrossRef]
- Bai, X.; Yong, X.; Luo, Y.; Deng, L.; Li, K.; Zhou, Y. Synthesis and application of temperature-sensitive polymer as a novel plugging agent for water-based drilling fluids synthesis and application of temperature-sensitive polymer as a novel plugging agent for water-based drilling fluids. J. Appl. Polym. Sci. 2022, 139, e52524. [Google Scholar] [CrossRef]
- Yang, G.; Liu, T.; Zhu, H.; Zhang, Z.; Feng, Y.; Leusheva, E.; Morenov, V. Heat control effect of phase change microcapsules upon cement slurry applied to hydrate-bearing sediment. Energies 2022, 15, 4197. [Google Scholar] [CrossRef]
- Cai, W.; Deng, J.; Luo, M.; Feng, Y.; Li, J.; Liu, Q. Recent advances of cementing technologies for ultra-HTHP formations. Int. J. Oil Gas Coal Technol. 2022, 29, 27–51. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, L.; Wu, X.; Wu, J.; Yu, Y.; Li, L.; Li, M. Preparation and characterization of polymer retarder for plugging cement slurry. J. Dispers. Sci. Technol. 2023, 44, 2239–2246. [Google Scholar] [CrossRef]
- Zou, Q.; Chen, X.; Zhong, S.; Yi, D.; Liu, L. Performance experiment of ultra high temperature cementing slurry system. Front. Mater. 2024, 11, 1383286. [Google Scholar] [CrossRef]
- Shi, J.; Wu, Z.; Deng, Q.; Liu, L.; Zhang, X.; Wu, X.; Wang, Y. Synthesis of hydrophobically associating polymer: Temperature resistance and salt tolerance properties. Polym. Bull. 2022, 79, 4581–4591. [Google Scholar] [CrossRef]
- Hou, Y.; Guo, Y.; Qian, S.; Khan, H.; Han, G.; Zhang, W. A new thermoresponsive polymer of poly(N-acetoxylethyl acrylamide). Polymer 2019, 167, 159–166. [Google Scholar] [CrossRef]
- Papadakis, C.M.; Niebuur, B.-J.; Schulte, A. Thermoresponsive polymers under pressure with a focus on poly (n-isopropylacrylamide) (pnipam). Langmuir 2023, 40, 1–20. [Google Scholar] [CrossRef]
- Wang, W.; Gao, C.; Qu, Y.; Song, Z.; Zhang, W. In Situ Synthesis of Thermoresponsive Polystyrene-b-poly(N-isopropylacrylamide)-b-polystyrene Nanospheres and Comparative Study of the Looped and Linear Poly(N-isopropylacrylamide)s. Macromolecules 2016, 49, 2772–2781. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Jiang, Z.; Gao, S.; Xiao, X.; Xiang, C.; Tao, J. Formulation and characterization of surfactants with antibacterial and corrosion-inhibiting properties for enhancing shale gas drainage and production. Sci. Rep. 2025, 15, 2376. [Google Scholar] [CrossRef]
- Yan, Y.; Xue, Z.; Wu, L.; Luo, Y.; Bai, X. Synthesis and application of a temperature sensitive poly (acrylamide-co-n-isopropylacrylamide-co-sodium p-styrene sulfonate) as a new water-based drilling fluid plugging agent. J. Appl. Polym. Sci. 2025, e56733. [Google Scholar] [CrossRef]
- Ran, Y.; Zhang, G.; Jiang, P.; Pei, H. Preparation method and performance evaluation of a gel based on am/amps copolymer. Gels 2022, 8, 802. [Google Scholar] [CrossRef]
- Przesławski, G.; Szcześniak, K.; Gajewski, P.; Marcinkowska, A. Influence of Initiator Concentration on the Polymerization Course of Methacrylate Bone Cement. Polymers 2022, 14, 5005. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Dong, M.; Yu, Y. Synthesis and property of thermo-thickening oil well cement additives. J. Tianjin Univ. Sci. Technol. 2016, 49, 597–602. [Google Scholar]
- Majumder, L.; Bera, K.; Khamaru, K.; Pal, U.; Maiti, N.C.; Banerji, B. NMR and vibrational spectroscopic studies on the structure and self-assembly of Two de novo dipeptides in methanol. J. Mol. Struct. 2022, 1266, 133455. [Google Scholar] [CrossRef]
- Shi, S.; Sun, J.; Lv, K.; Liu, J.; Bai, Y.; Wang, J.; Huang, X.; Jin, J.; Li, J. Comparative studies on thickeners as hydraulic fracturing fluids: Suspension versus powder. Gels 2022, 8, 722. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, M.; Luo, Z.; Zhang, S. Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications. e-Polymers 2024, 24, 20240016. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, C.; Zhang, H.; Zhang, X.; Wen, X.; Bai, J.; Mao, H. Preparation of low-molecular-weight polyacrylamide as the delayed crosslinking plugging agent for drilling fluid. Gels 2024, 10, 112. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Xue, Y.; Yang, L.; Wang, R.; Jin, J.; Li, Y. Development and performance of thermo-viscosifying polymer for high temperature sedimentation control of high density cement slurry. Acta Pet. Sin. 2020, 41, 1416. [Google Scholar]
- Wu, Y.; Duan, M.; Wang, Q.; Chen, B.; Chen, H.; Li, X.; Fang, S. Dispersion polymerization of am-amps-dam-doh and its sand control property. Geoenergy Sci. Eng. 2024, 241, 213190. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Jiang, Z.; Xian, L.; Liu, J.; Chen, J. Development and characterization of a surfactant responsive to redox conditions for gas recovery in foam drainage. Sci. Rep. 2025, 15, 511. [Google Scholar] [CrossRef]
- Xiaolin, L.; Jianhua, L.; Hongbin, Y.; Wenming, L.; Ben, Q.; Zhe, L.; Jingyu, Y. Study on thermally viscosifying copolymer as a high temperature stabilizer for high density cement slurries. Drill. Fluid Complet. Fluid 2022, 39, 76–81. [Google Scholar]
- Reddy, B.R.R.; Patil, R.; Patil, S. Chemical modification of biopolymers to design cement slurries with temperature-activated viscosification—A laboratory study. SPE Drill. Complet. 2012, 27, 94–102. [Google Scholar] [CrossRef]
- Tchameni, A.P.; Xie, B.; Zhang, H.; Zhao, L.; Luo, M.; Wen, J. Thermo-associating polymers based on cross-linked 2-acrylamido-methylpropane sulfonic acid, Part A: Synthesis and solution behavior. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124611. [Google Scholar] [CrossRef]
- Su, X.; Feng, Y. Thermoviscosifying smart polymers for oil and gas production: State of the art. ChemPhysChem 2018, 19, 1941–1955. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; Wang, Y.; Wang, H.; Wang, R. Prevention strategy of cement slurry sedimentation under high temperature. Part 1: A polymer with continuous thermo-thickening behavior from 48 to 148 C. J. Phys. Chem. C 2019, 123, 18573–18584. [Google Scholar] [CrossRef]
- Xu, S. Research progress on viscosity reducing agents for water-based drilling fluids. Stand. Qual. China’s Pet. Chem. Ind. 2023, 43, 99–101. [Google Scholar]
- Liu, Y.; Huang, X.; Zhang, X.; Dai, Z.; Li, T. Study on temperature and salt resistance plugging agent and characterization of its structure. Appl. Chem. Ind. 2023, 52, 3392–3396. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Yang, J.; Liu, Y.; Jiang, Z.; Chen, J. Synthesis and analysis of magnetic nanoparticles within foam matrix for foam drainage gas production. Geoenergy Sci. Eng. 2024, 238, 212887. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, J.; Lin, R.; Wang, J.; Zhu, H.; Xie, Y. Analysis on retarding effect of amps retarder at high temperature. Contemp. Chem. Ind. 2024, 53, 1–6. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, L.; Guo, Y.; Luo, P.; Xiong, G.; Li, Z.; Ao, H. Study on high-temperature degradation of acrylamide-based polymer ZP1 in aqueous solution. Polym. Degrad. Stab. 2023, 217, 110533. [Google Scholar] [CrossRef]
- Lai, X.; Liu, G.; Liu, Y.; Dong, X.; Liu, X.; Mukhtar, Y.; Wang, L.; Wen, X.; Lu, L. Preparation and viscoelasticity of novel hydrophobic associating polymer with salt stimulation responsiveness by functional monomer modification for fracturing fluids. Colloid Polym. Sci. 2023, 301, 1271–1283. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, B.; Hou, W.; Shi, L.; Dong, Z.; Zou, S. Research progress of oil well cement suspension. Oilfield Chem. 2023, 40, 736–742. [Google Scholar] [CrossRef]
- Shang, L. Research on quality evaluation methods of ultra-low density cementing in oil well cementing. Petrochem. Ind. Technol. 2024, 31, 215–217. [Google Scholar]


| Levels | Factors | ||||
|---|---|---|---|---|---|
| r | w1 (%) | w2 (%) | pH | T (°C) | |
| 1 | 10:15:5:5 | 10 | 0.40 | 5 | 40 |
| 2 | 15:10:5:5 | 13 | 0.45 | 6 | 50 |
| 3 | 13:13:6:3 | 16 | 0.50 | 7 | 60 |
| 4 | 13:13:3:6 | 19 | 0.55 | 8 | 70 |
| No | r | r | w1 (%) | w2 (%) | pH | T (°C) | Density Difference (g/cm−3) |
|---|---|---|---|---|---|---|---|
| T1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.194 |
| T2 | 1 | 1 | 2 | 2 | 2 | 2 | 0.128 |
| T3 | 1 | 1 | 3 | 3 | 3 | 3 | 0.113 |
| T4 | 1 | 1 | 4 | 4 | 4 | 4 | 0.203 |
| T5 | 2 | 2 | 1 | 2 | 3 | 4 | 0.177 |
| T6 | 2 | 2 | 2 | 1 | 4 | 3 | 0.164 |
| T7 | 2 | 2 | 3 | 4 | 1 | 2 | 0.107 |
| T8 | 2 | 2 | 4 | 3 | 2 | 1 | 0.13 |
| T9 | 3 | 3 | 1 | 3 | 4 | 2 | 0.211 |
| T10 | 3 | 3 | 2 | 4 | 3 | 1 | 0.181 |
| T11 | 3 | 3 | 3 | 1 | 2 | 4 | 0.123 |
| T12 | 3 | 3 | 4 | 2 | 1 | 3 | 0.135 |
| T13 | 4 | 4 | 1 | 2 | 2 | 3 | 0.235 |
| T14 | 4 | 4 | 2 | 1 | 1 | 4 | 0.247 |
| T15 | 4 | 4 | 3 | 4 | 4 | 1 | 0.252 |
| T16 | 4 | 4 | 4 | 3 | 3 | 2 | 0.256 |
| K1 | 0.160 | 0.160 | 0.204 | 0.182 | 0.171 | 0.189 | |
| K2 | 0.145 | 0.145 | 0.180 | 0.169 | 0.154 | 0.176 | |
| K3 | 0.163 | 0.163 | 0.149 | 0.178 | 0.182 | 0.162 | |
| K4 | 0.248 | 0.248 | 0.181 | 0.186 | 0.208 | 0.188 | |
| R | 0.103 | 0.103 | 0.056 | 0.017 | 0.054 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhu, X.; Gan, C.; Li, Y.; Liu, D. Synthesis and Property Characterization of AM/AMPS/C18DMAAC/NVP Tetrameric Temperature-Sensitive Thickening Copolymer. Processes 2025, 13, 922. https://doi.org/10.3390/pr13030922
Chen X, Zhu X, Gan C, Li Y, Liu D. Synthesis and Property Characterization of AM/AMPS/C18DMAAC/NVP Tetrameric Temperature-Sensitive Thickening Copolymer. Processes. 2025; 13(3):922. https://doi.org/10.3390/pr13030922
Chicago/Turabian StyleChen, Xu, Xiangpeng Zhu, Cheng Gan, Yigang Li, and Diren Liu. 2025. "Synthesis and Property Characterization of AM/AMPS/C18DMAAC/NVP Tetrameric Temperature-Sensitive Thickening Copolymer" Processes 13, no. 3: 922. https://doi.org/10.3390/pr13030922
APA StyleChen, X., Zhu, X., Gan, C., Li, Y., & Liu, D. (2025). Synthesis and Property Characterization of AM/AMPS/C18DMAAC/NVP Tetrameric Temperature-Sensitive Thickening Copolymer. Processes, 13(3), 922. https://doi.org/10.3390/pr13030922







