Abstract
Arsenic is a well-known, highly toxic carcinogen element that is widely found in nature, with numerous studies highlighting its hazardous impact on human health and the environment. Therefore, considering its toxicity and adverse health effects on mammals and the environment, rapid, sensitive, and effective methods for the recognition of arsenic are necessary. Over the past decade, a variety of fluorescent probes, such as small molecules, nanomaterials, gold nanoparticles (AuNPs), carbon dots (CDs), quantum dots (QDs), and more, have been designed and successfully employed for the recognition of lethal arsenic. Compared to other conventional sensor materials, sensors based on metal-organic frameworks (MOFs) are advantageous due to their simple preparation, easy functional group modulation, large specific surface area, and excellent chemical stability. In recent years, MOFs have been utilized as dual-functional materials for the detection and adsorptive removal of arsenic from water. This unique functionality distinguishes MOF-based materials from conventional sensors and arsenic adsorbents. Herein, we provide an overview of the state-of-the-art knowledge on the current development of MOFs for the fluorogenic detection of arsenic in aqueous media. Furthermore, the underlying detection mechanisms are also summarized in this review. The existing challenges in this field and potential remedial strategies for improving detection are elaborated upon in the relevant sections.
1. Introduction
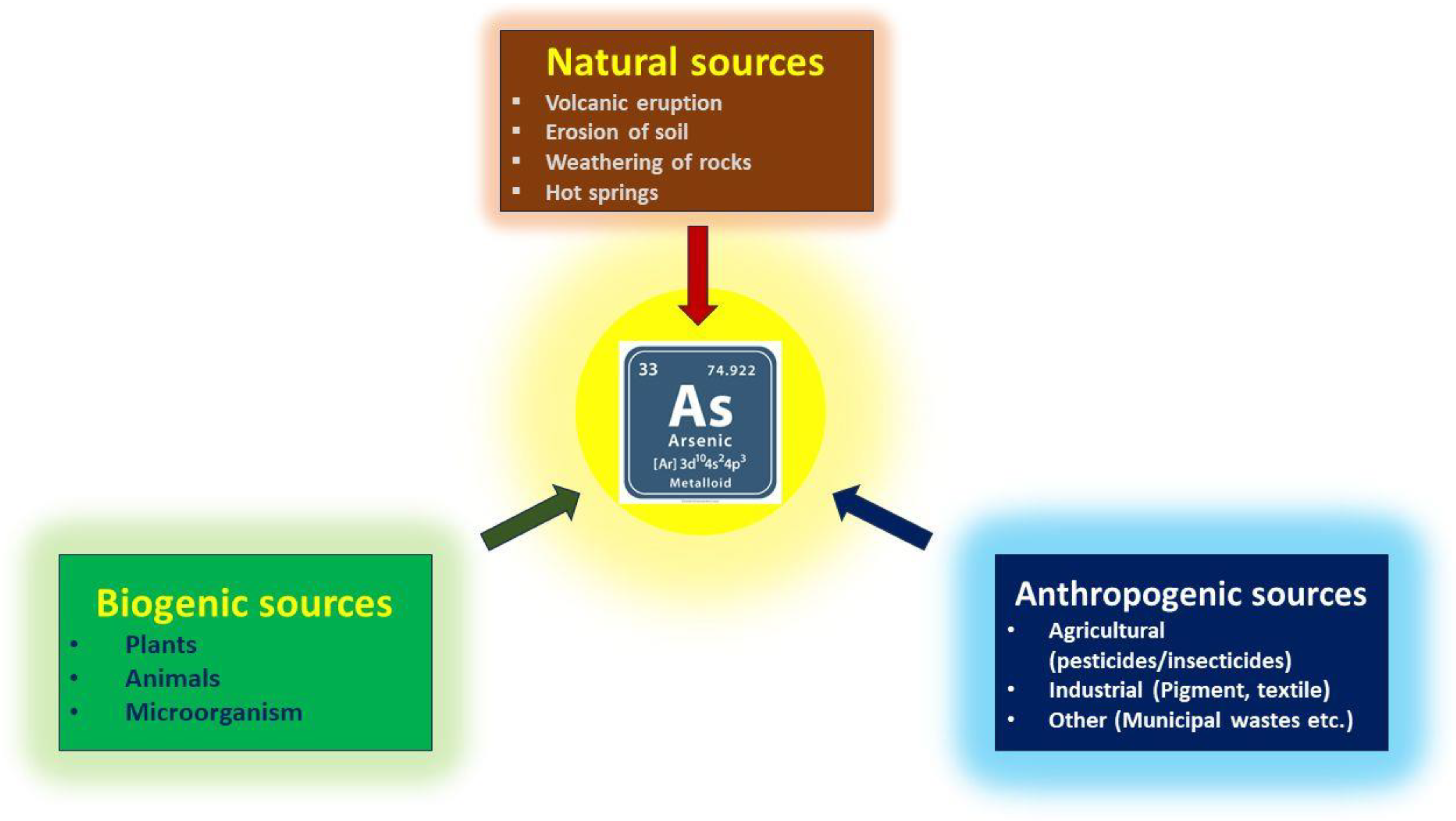
Arsenic exists in four oxidation states in nature: As(V), As(III), As(0), and As(−III). Recent research shows that the consumption of arsenic, particularly arsenic (III) and arsenic (V) ions, through food and drink has significantly increased [1]. Arsenic has already been classified by the World Health Organization (WHO) as a class I human carcinogen [2]. In response to emerging evidence on the chronic health effects of arsenic, the WHO revised its guidelines in 1993, reducing the recommended arsenic concentration in drinking water from 50 μg/L to 10 μg/L [3]. The U.S. Environmental Protection Agency (EPA) also set the same maximum contaminant level (MCL) of total arsenic in drinking water as recommended by WHO [4]. The primary sources of arsenic in surface and groundwater are multifaceted, encompassing both natural processes and human activities. Natural contributions include soil erosion and magmatic activity, which introduce arsenic into water bodies [5]. However, anthropogenic factors such as agricultural practices, industrial discharges, and mining operations significantly amplify arsenic contamination. For instance, the operation of mines can release harmful pollutants, including arsenic, threatening local health and the environment [6]. Unfortunately, arsenic is also widely used as a dietary supplement for animals, primarily to prevent diseases, promote growth, and improve the pigmentation of poultry meat, among other purposes [7]. The extensive use of agricultural chemicals has resulted in the prevalence of organic arsenic, including methylated organic arsenic (MOAs) and aromatic organic arsenic (AOCs), in the water ecological environment. Within 30 days, biotic and abiotic processes may convert the water-soluble AOCs in manure into extremely deadly inorganic arsenic species (such as arsenate), which contaminate soil and groundwater [8]. Among all four possible oxidation states for arsenic, plants and animals are mostly harmed by the trivalent and pentavalent forms of arsenic [9]. It has also been found that trivalent arsenic species exhibit greater toxicity compared to pentavalent arsenic species [10]. Therefore, the detection of arsenic content is critical for biological, environmental, medicinal, agricultural, and other relevant sectors, considering the health concerns. Figure 1 summarizes the different sources of arsenic in the environment.
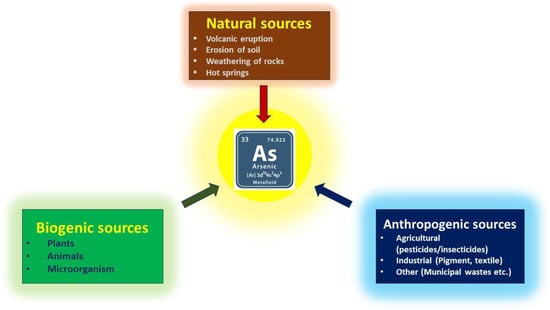
Figure 1.
Different potential sources of arsenic.
This review provides a comprehensive and focused analysis of the recent advancements in the fluorescent metal-organic framework (MOF)-based detection of arsenic in aqueous environments. While several excellent reviews have been published in the past decade on arsenic detection [11,12,13,14,15,16], there is a noticeable gap in the literature specifically addressing fluorescence sensors for arsenic, particularly in aquatic systems. Although the detection and removal of arsenic using MOFs have been extensively summarized, a dedicated review on the fluorometric detection of arsenic by MOFs remains limited. Given the rapid growth and heightened interest in this area, this review stands out by combining the most recent developments in fluorescent MOF sensors for arsenic. It offers a detailed classification of various MOF-based fluorescence sensors, exploring their types, specific mechanisms, and the advantages of each approach in arsenic detection, thereby providing a more focused and up-to-date resource compared to other reviews on this research field.
2. The Adverse Impacts of Arsenic
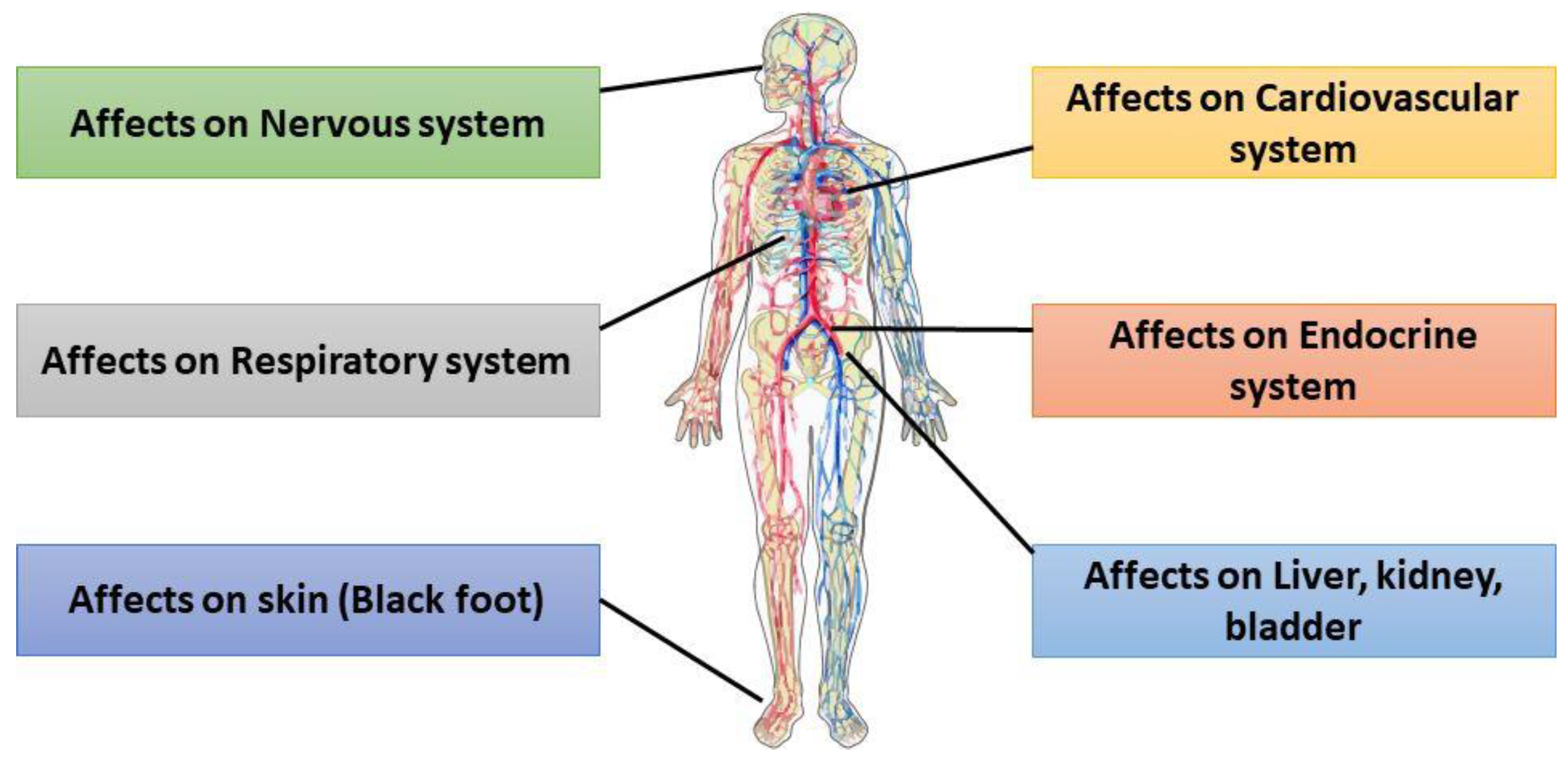
Arsenic is one of the most prevalent metalloids in nature and is known to be carcinogenic to living organisms. Chronic exposure to arsenic-contaminated drinking water can result in both immediate and long-term adverse health effects. (Figure 2). Acute arsenic intoxication has been reported to cause acute gastrointestinal syndrome (AGS) and acute paralytic syndrome (APS) [17]. In APS, cardiovascular failure occurs, and central nervous system depression is observed, partly due to the necrosis of both red blood cells (RBCs) and white blood cells (WBCs) [18]. Arsenic (III) affects blood vessels, leading to impaired circulation and symptoms such as tingling or numbness in the hands and feet. The early manifestations of AGS include dysphagia, dry mouth, burning lips, and a characteristic garlic-like taste [19]. Chronic arsenic exposure is associated with Blackfoot disease, a well-documented vascular disorder [20]. Additionally, arsenic undergoes biomethylation in the body, converting it into more toxic and carcinogenic forms [21]. Arsenic undergoes enzymatic methylation in the body, primarily facilitated by arsenic (III) methyltransferase (AS3MT). This process converts inorganic arsenic into methylated metabolites, including monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA). However, arsenic exposure can disrupt DNA methylation patterns, leading to the hypermethylation of tumor suppressor genes such as p16 and p53, thereby increasing the risk of carcinogenesis. Additionally, arsenic interferes with DNA repair enzymes, further contributing to genomic instability and the potential development of cancer [22]. Chronic arsenic toxicity is commonly associated with specific skin patches in pigmentation and keratosis [23]. Several studies have established a link between elevated arsenic levels in drinking water and an increased risk of cancer in the skin, bladder, lungs, and other organs [20]. Global public health is greatly affected by arsenic-induced sickness, including cancer, black foot, etc. [17]. Therefore, accurately determining arsenic concentrations in drinking and environmental water is crucial for identifying arsenic contamination. Prompt detection of arsenic in water can aid in mitigating arsenic-induced health disorders and prevent long-term exposure risks.
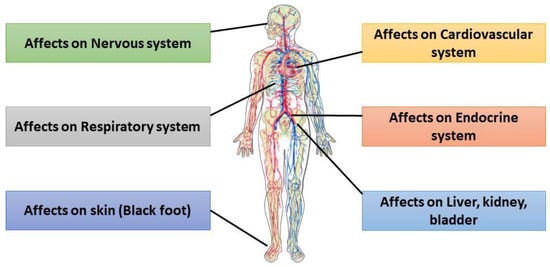
Figure 2.
Multiorgan effects of chronic arsenic exposure, impacting the nervous, cardiovascular, endocrine, respiratory, hepatic-renal systems, and skin.
3. Conventional Techniques and Fluorometric Recognition of Arsenic
Various analytical techniques have been employed for the detection and quantification of arsenic in aqueous media. These include Raman spectroscopy (RS), atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectrometry (ICP-MS), atomic fluorescence spectrometry (AFS), atomic emission spectrometry (AES), total reflection X-ray fluorescence (TXRF) spectrometry, surface-enhanced Raman spectroscopy (SERS), and laser-induced breakdown spectroscopy (LIBS), among others [24,25,26,27,28,29,30]. As discussed in the previous section, it has been observed that an excessive utilization of arsenic may cause several environmental and health hazards. In this context several conventional techniques were employed for the detection and removal of lethal arsenic. However, these methods are inherently expensive, complex, and dependent on large-scale machinery. While they offer high detection sensitivity, several drawbacks hinder their further development, including costly equipment, complicated operating procedures, labor-intensive sample preparation, and the need for skilled personnel [16]. Owing to these constraints, a wide range of electrochemical and optical techniques have been utilized for arsenic detection in recent years [31,32,33]. Among these, optical detection technology—especially the fluorescent probe—has emerged as an effective method for the rapid detection of arsenic due to its ease of use, affordability, portability, accuracy, repeatability, selectivity, and ability to provide quick, real-time biological and environmental monitoring [34]. Over the past decade, various nanoparticles, quantum dots, carbon dots, small organic molecules, aptamers, and MOFs have been extensively used for fluorescence-based detection of arsenic and arsenic-derived compounds [14].
Fluorescence-based detection is non-destructive, providing rapid responses, high selectivity, and enhanced sensitivity over a wide concentration range, making it an ideal method for arsenic detection [31,35]. The interaction between the analyte and probe can induce changes in the optoelectronic properties of the probe material, which demonstrates changes in its emission spectrum. These interactions can be categorized into long-range, short-range, and complex/collision-driven types [36]. Long-range interactions include radiative energy transfer, where the target analyte absorbs emission from the probe, and competitive absorption, where the analyte competes with the probe for excitation energy, both of which lead to emission quenching [37]. In Förster resonance energy transfer (FRET), dipole-dipole coupling between an excited donor and a ground-state acceptor chromophore allows the transfer of energy without an electron exchange [38]. Depending on the system, FRET may either quench the probe’s emission or enhance its fluorescence. Short-range interactions, such as orbital overlap or collision between the analyte and probe, are also important for certain sensing mechanisms. Additionally, complexation between the analyte and the probe can result in various sensing interactions [39]. In cases where the fluorescent probe consists of emitter and antenna moieties, the presence of an analyte may disrupt the antenna effect, leading to quenching of the probe’s emission [40,41].
4. Key Features of MOFs for Fluorometric Detection
Desired selectivity, sensitivity, quick response time, material stability, and reusability are required for a fluorescence sensor [42]. Among various fluorescent probes, next-generation porous fluorescent MOFs have garnered significant attention due to their unique properties. These materials are composed of metal-containing units and suitable organic linkers, which contribute to their high versatility as sensors. MOFs possess unique properties that make them highly effective as chemical sensors. MOFs possess a highly porous architecture that facilitates the preconcentration of analytes, thereby enhancing sensitivity toward specific target molecules [34,43]. Several key characteristics are essential for MOFs to function effectively as fluorescent sensor materials. Firstly, the well-defined, crystalline, and tunable structure of MOFs significantly influences their emission properties. The spatial arrangement and intermolecular interactions of the framework components play a crucial role in energy transfer processes, thereby affecting the fluorescence behavior [44]. Secondly, biocompatibility is a fundamental requirement for the application of MOFs in biomedical fields, particularly for in vivo studies [45]. A biocompatible material is one that does not cause harm to living tissues [46]. When designing MOFs for biomedical applications, a careful selection of non-toxic and biocompatible organic linkers is necessary [47]. Equally important is the choice of metal ions, as certain metals—such as Ca, Mn, Fe, Zn, Mg, and Zr—have been identified as biocompatible due to their relatively high lethal-dose thresholds (≥1 g/kg), making them suitable for biological applications [48,49]. Thirdly, luminescent properties in MOFs arise from multiple sources, including organic linkers, metal centers, metal-organic charge transfer, and interactions with guest molecules [50,51,52,53]. These diverse luminescence mechanisms enable the development of MOFs as highly efficient luminescent materials for sensing applications.
5. Origin of Fluorescence in MOFs
Fluorescence in MOFs arises from various mechanisms, including the intrinsic emission of conjugated organic linkers (π-π* or n-π* transitions), metal-centered luminescence (e.g., lanthanide f-f transitions), metal-to-ligand (MLCT) or ligand-to-metal charge transfer (LMCT), and ligand-to-ligand charge transfer (LLCT). Additionally, guest-induced fluorescence and framework interactions can further enhance or modulate the fluorescence properties, making MOFs highly versatile for sensing and optoelectronic applications.
5.1. Ligand Based Fluorescence
The π-conjugated rigid organic compounds containing heterocyclic or multi-carboxylate groups are frequently used as ligands in fluorescent MOFs. The fluorescence properties of these ligands, such as lifetime and maximum emission wavelength intensities, often change upon MOF formation compared to their free ligand state. The molecules in the solid state are brought closer together by molecular interactions, which permits charge transfer among the organic ligands and causes a change in the spectrum, a broadening of the emission band, and a loss of fine structure. Additionally, the overall coordination environment, along with the spatial orientation and arrangement of the ligands within the MOF framework, can influence their fluorescence properties [54]. These factors may induce various interactions between or within organic ligand molecules, allowing for the tunability of MOF luminescence by controlling these interactions.
5.2. Metal-Centered Fluorescence
Lanthanide-based MOFs predominantly exhibit metal-centered fluorescence due to the progressive electron filling of the 4f orbitals in lanthanide ions. These ions display characteristic narrow 4f–4f transitions, leading to luminescence across the ultraviolet (UV), visible, and near-infrared (NIR) spectral regions. Specifically, Yb3⁺, Nd3⁺, and Er3⁺ emit in the NIR range, while Eu3⁺, Tb3⁺, Sm3⁺, and Tm3⁺ produce red, green, orange, and blue emissions, respectively [55]. However, due to the forbidden nature of f-f transitions, lanthanide ions exhibit inherently weak absorption, often necessitating high-power laser excitation. This limitation can be mitigated by incorporating energy transfer mechanisms, such as “luminescence sensitization” or the “antenna effect,” where organic ligands act as sensitizers to efficiently transfer energy to lanthanide ions [56]. Leveraging the antenna effect in organic ligands enables the development of highly efficient fluorescent lanthanide MOFs for advanced sensing applications.
5.3. Charge Transfer Fluorescence
Charge transfer fluorescence in MOFs arises from permitted electronic transitions between excited and ground states. MOFs are known to exhibit two distinct forms of charge transfer: metal-to-ligand (MLCT) and ligand-to-metal (LMCT) charge transfer [57,58]. In LMCT, electronic excitation results in charge transfer from the confined orbital of the organic ligand to the metal-centered orbital, whereas MLCT involves the opposite process, where a charge is transferred from the metal-centered orbital to an organic ligand. These transitions enable efficient energy transfer within the MOF framework, significantly influencing its photophysical properties. Additionally, the emission characteristics of MOFs can be modulated by the interplay between ligand-centered fluorescence and LMCT/MLCT transitions, leading to diverse fluorescent behaviors.
5.4. Guest Induced Fluorescence
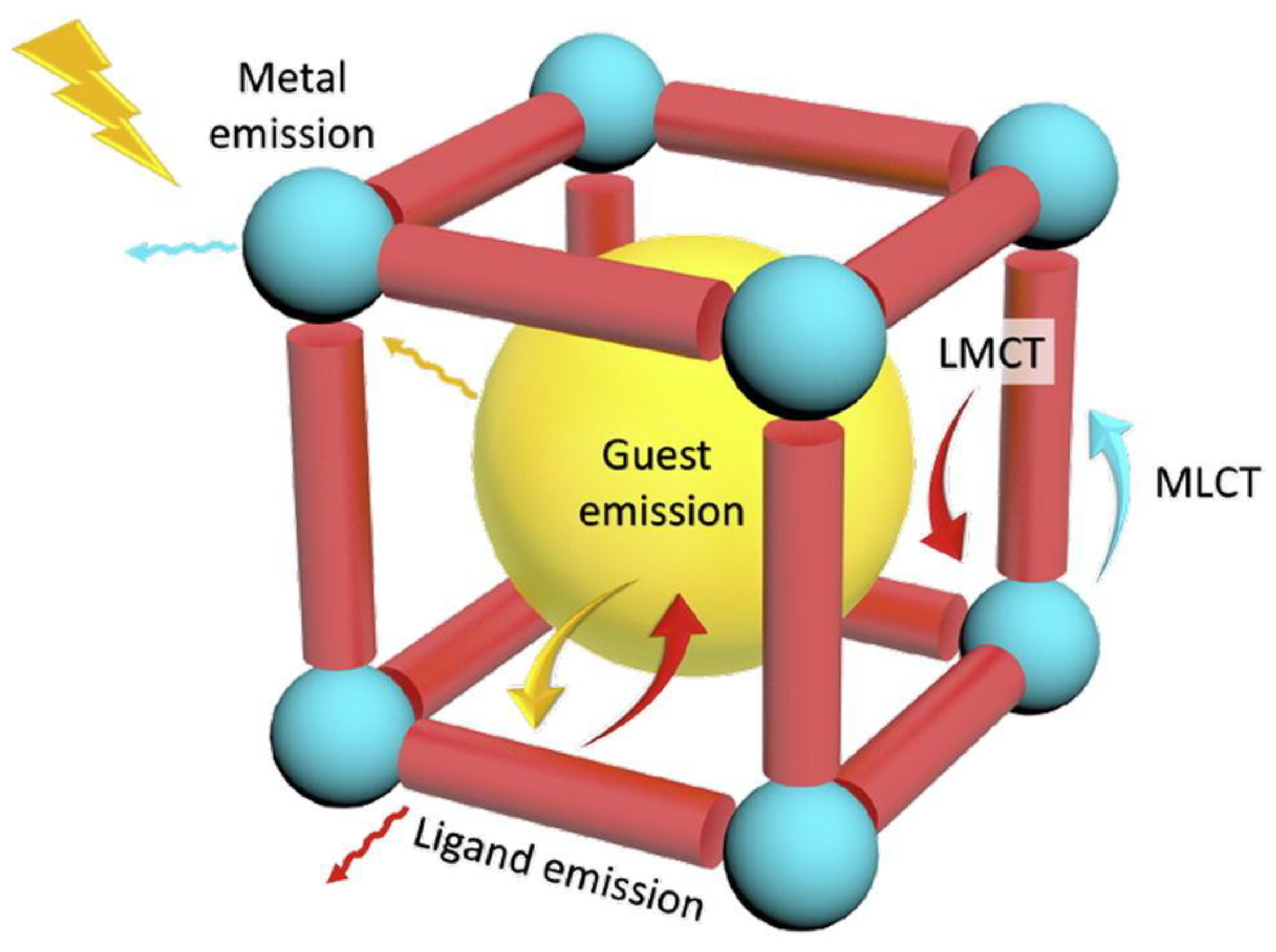
MOFs, with their highly ordered structures and tunable pore sizes, serve as exceptional hosts for encapsulating luminescent guest species such as fluorescent dyes and lanthanide ions [59]. Guest encapsulation often leads to significant modifications in the photophysical properties of MOFs, broadening their potential applications in sensing, bioimaging, and optoelectronics. Lanthanide-doped MOFs, synthesized via cation exchange, exhibit enhanced luminescence due to efficient energy transfer mechanisms [41]. Similarly, the encapsulation of fluorescent dye molecules within MOFs has been widely explored, yielding hybrid materials with superior optical properties [60]. Compared to their unmodified counterparts, guest-loaded MOFs demonstrate intensified and tunable fluorescence, making them highly promising for molecular detection and advanced sensor applications. The various possible modes of emission present in the MOF are shown in Figure 3.
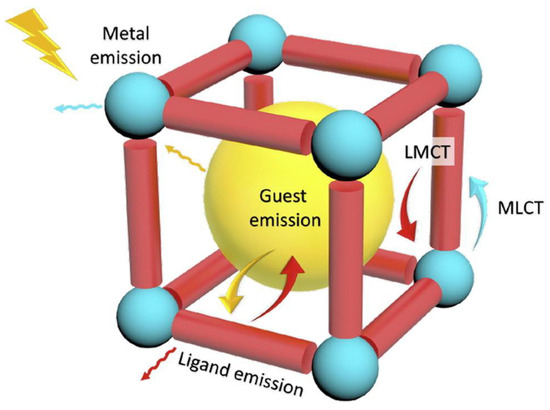
Figure 3.
Representation of possible emission modes in MOFs. Inorganic SBUs, blue sphere; organic ligands, red cylinders; guest chromophores, yellow sphere inside. Reproduced with permission from ref. [61] Copyright 2018, Elsevier.
6. Fluorometric Detection of Arsenic by MOFs
MOFs represent a rapidly developing class of materials of great interest, with a wide range of applications in chemical and physical sensing, optoelectronics, and imaging [61,62,63,64,65,66,67,68,69,70]. Since several MOFs exhibit high stability in water and tunable fluorescence properties, extensive research has been conducted on their use as sensing materials in aqueous environments. This fluorescence property can be modulated upon analyte interaction, leading to either fluorescence quenching (“turn-off” mechanism) or enhancement (“turn-on” mechanism). Such fluorescence changes—whether in intensity or emission wavelength—enable both the qualitative and quantitative detection of target analytes. Over the past decade, MOFs have been extensively utilized for sensing a wide range of substances, including metal ions, nitro explosives, volatile organic compounds, hazardous chemicals, gases, biomolecules, temperature, pH, and humidity [71,72]. In the following section, we will focus on the fluorometric detection of arsenic and its derivatives using MOFs, as this forms the core of our focus.
6.1. “Turn On” Sensing of Arsenic
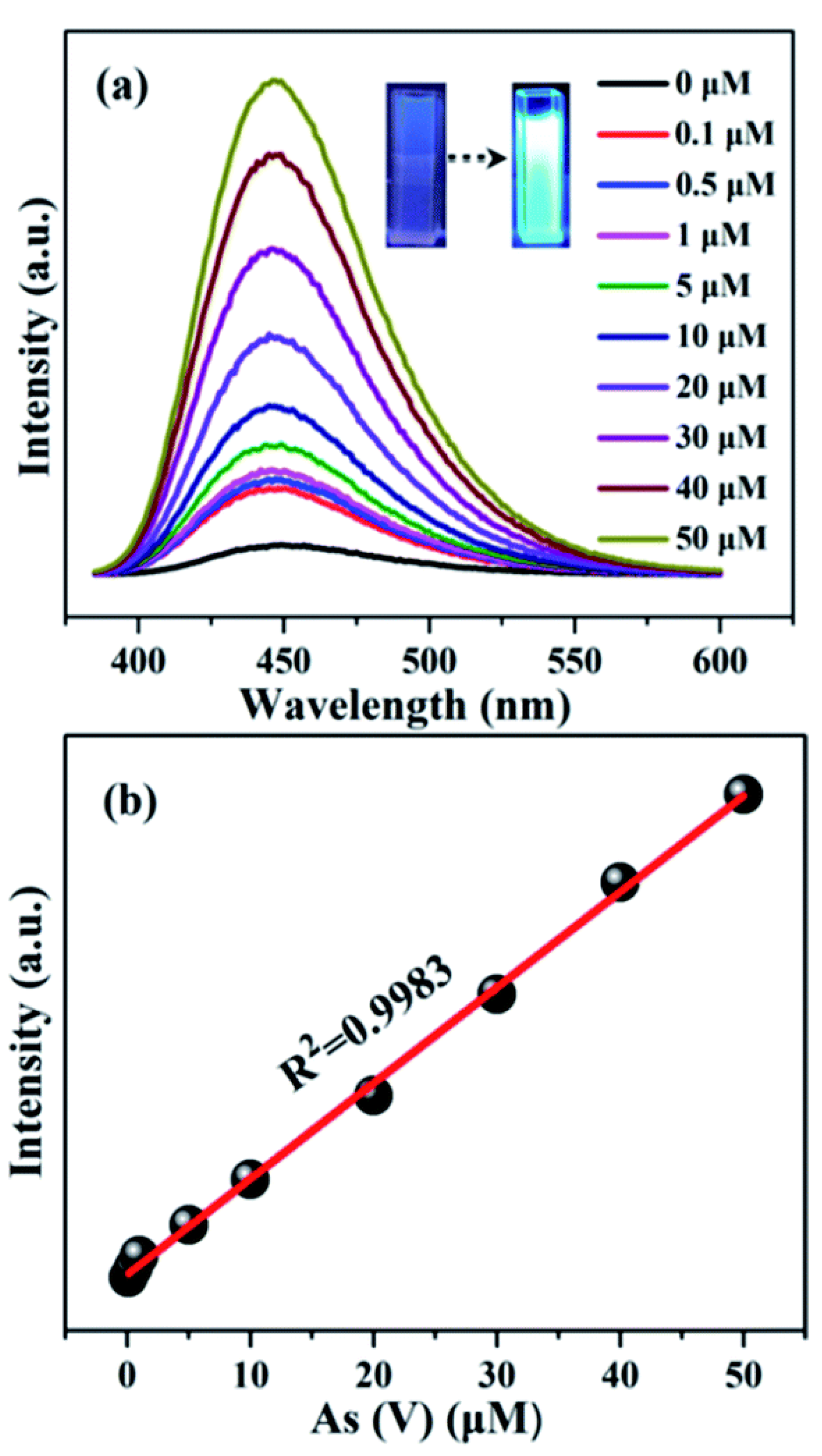
It has been observed that the emission intensity of MOFs is significantly enhanced upon the introduction of arsenic ions, a phenomenon known as “turn-on” sensing for arsenic detection. Zhao et al. developed NH2-MIL-88(Fe) nano-octahedra, which was successfully employed for the “turn-on” detection of As(V) ion [73] (Figure 4). The fluorescence intensity of NH2-MIL-88(Fe) is observed to be around 20 times lower than that of the parent NH2-BDC ligand. The phenomenon of fluorescence quenching can be attributed to the ligand-to-metal charge transfer (LMCT) action that occurs when the BDC-NH2 ligand is incorporated into the NH2-MIL-88(Fe) framework. It is interesting to note that after the incubation of 6.6 µM As(V), the fluorescence signal of NH2-MIL-88(Fe) was enhanced significantly. Additionally, a strong linear connection (R2 = 0.9983) exists between the concentration of arsenate in the range of 0.1–50 µM and the fluorescence intensity of NH2-MIL-88(Fe). The limit of detection (LOD) value towards As(V) for this material is around 56 nM (approximately 4.2 ppb), which is remarkably lower than the arsenic threshold value of 10 ppb (133 nM) in drinking water recommended by the WHO. Along with great sensitivity, the material is also highly selective towards As(V) over several possible interfering ions. The material is also capable to detect As(V) from practical environmental samples i.e., tap water and lake water samples. The exceptional enhancement effect of NH2-MIL-88(Fe) towards arsenate results from the metal centers (Fe3-µ3-oxo clusters) inside the compound competingly coordinating with arsenate. This leads to the release of the organic ligand and an increase in fluorescence, which in turn enables the highly sensitive and selective detection of As(V) [74]. This possible mechanism was validated by reliable analytical techniques like 1H nuclear magnetic resonance spectra (NMR), X-ray powder diffraction (XRPD), and scanning electron microscopy (SEM).
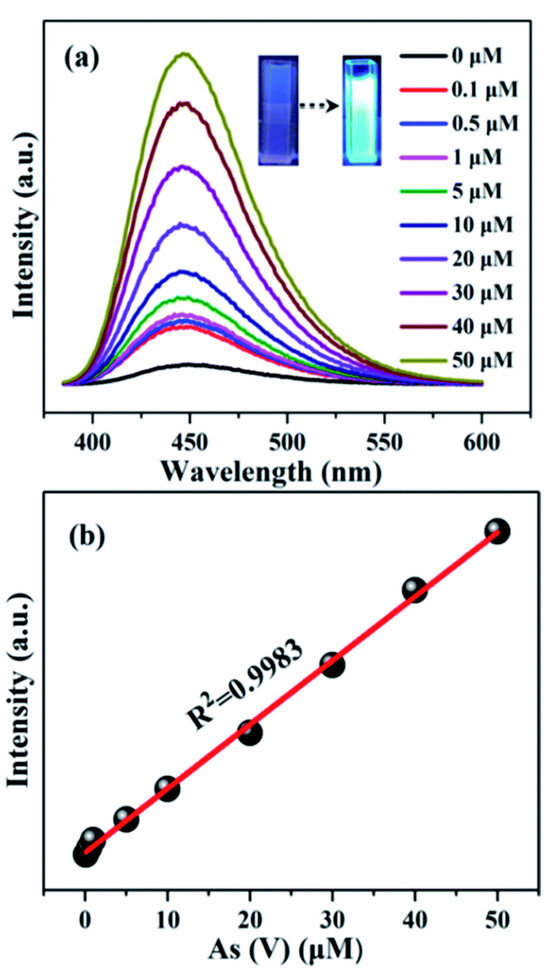
Figure 4.
(a) Fluorescence spectra of the NH2-MIL-88(Fe) aqueous suspension (50 mg L−1) upon the addition of different concentrations of As(V) [0.1 to 50 µM] under excitation at 350 nm; (b) linear fitting plot between the fluorescence intensity of NH2-MIL-88(Fe) and the concentration of As(V). Reproduced with permission from ref. [73] Copyright 2017, RSC.
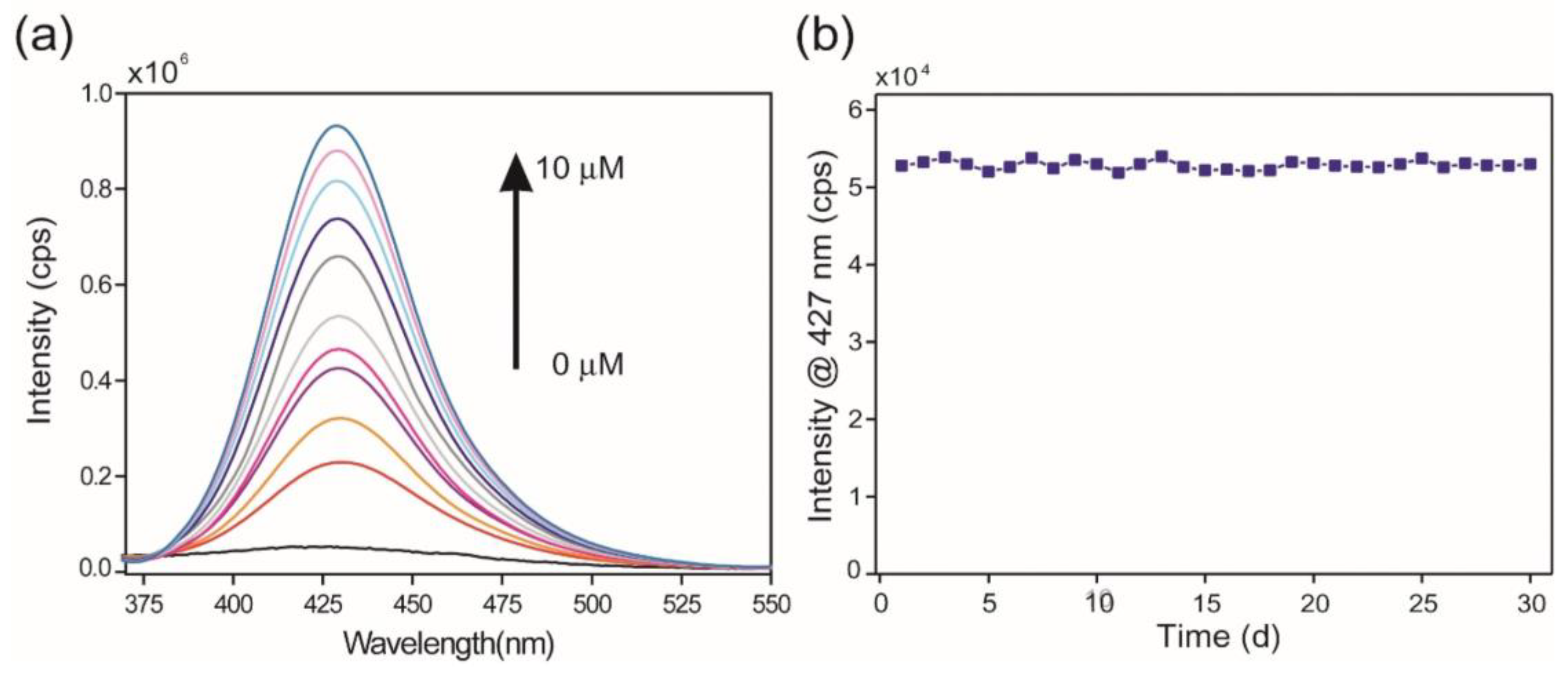
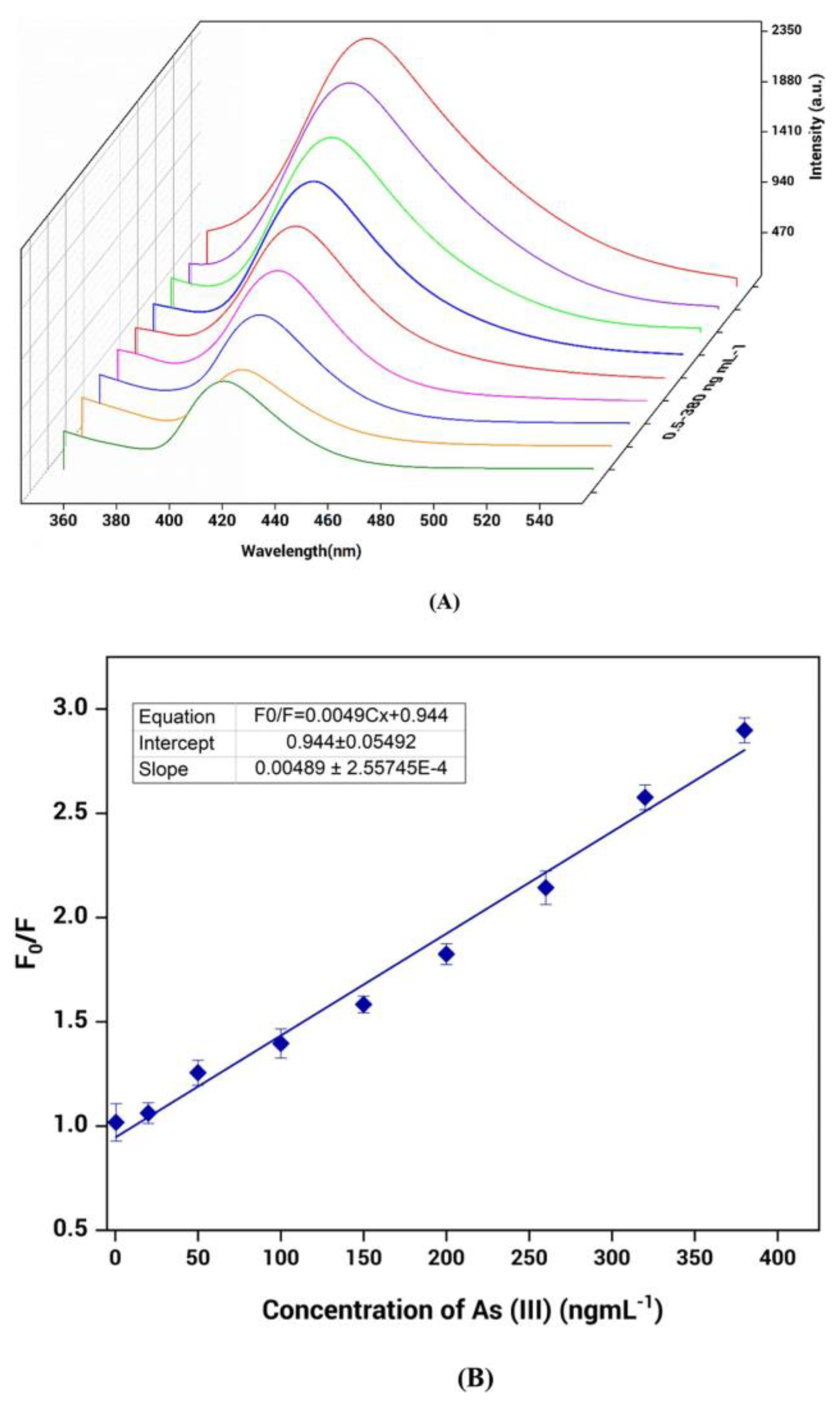
Tian et al. prepared an Ln-MOFs-based probe by employing europium as a metal center and condensation product of 4-formylbenzoic acid and 4-amino-3-hydroxybenzoic acid as ligands for the fluorogenic recognition of arsenate ion (AsO43−) [75]. Without arsenate, the aqueous suspension of the sample showed a weak fluorescence near about 427 nm. On the other hand, when trace amounts of arsenate were added, the sample’s emission intensity increased certainly, and the emission band progressively enhanced with increasing concentration of arsenate (Figure 5). The specificity of the arsenate ion of the probe was also examined by adding several possible interfering ions. Upon the addition of the congeners, minimal effect was observed in the luminescence property of the probe. The LOD value for the probe towards the detection of the probe is 17.8 nM. The periodic density functional theory (DFT) calculation predicts that twisted features of the linker of Ln-MOFs, as well as energy transfer from the linker to Eu ion, were mainly responsible for the weak fluorescence property of the Eu-MOFs in the absence of the arsenate ion. In the presence of arsenate ions, the twisting of the ligand unit is likely reduced due to hydrogen bonding interactions between the arsenate anion, the phenolic hydroxyl group, and the imine nitrogen. This interaction is predicted to enhance the rigidity of the unit and restrict the C–N isomerization of the linker [76,77]. Thus, it was found that Ln-MOF’s fluorescence was enhanced in the presence of arsenate as opposed to its fluorescence in its absence.
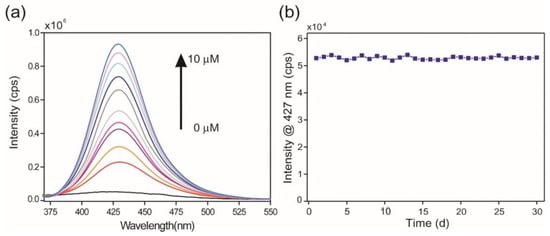
Figure 5.
(a) Emission spectra (excited at 350 nm) of aqueous suspension of Eu-MOF sample in the presence of arsenate ion with concentration in the range of 0 μM (black line) 10 μM (blue line). (b) Time courses of the photoluminescence intensity change of Eu-MOF upon storage at room temperature for up to 30 days. Reproduced with permission from ref. [75] Copyright 2021, Elsevier.
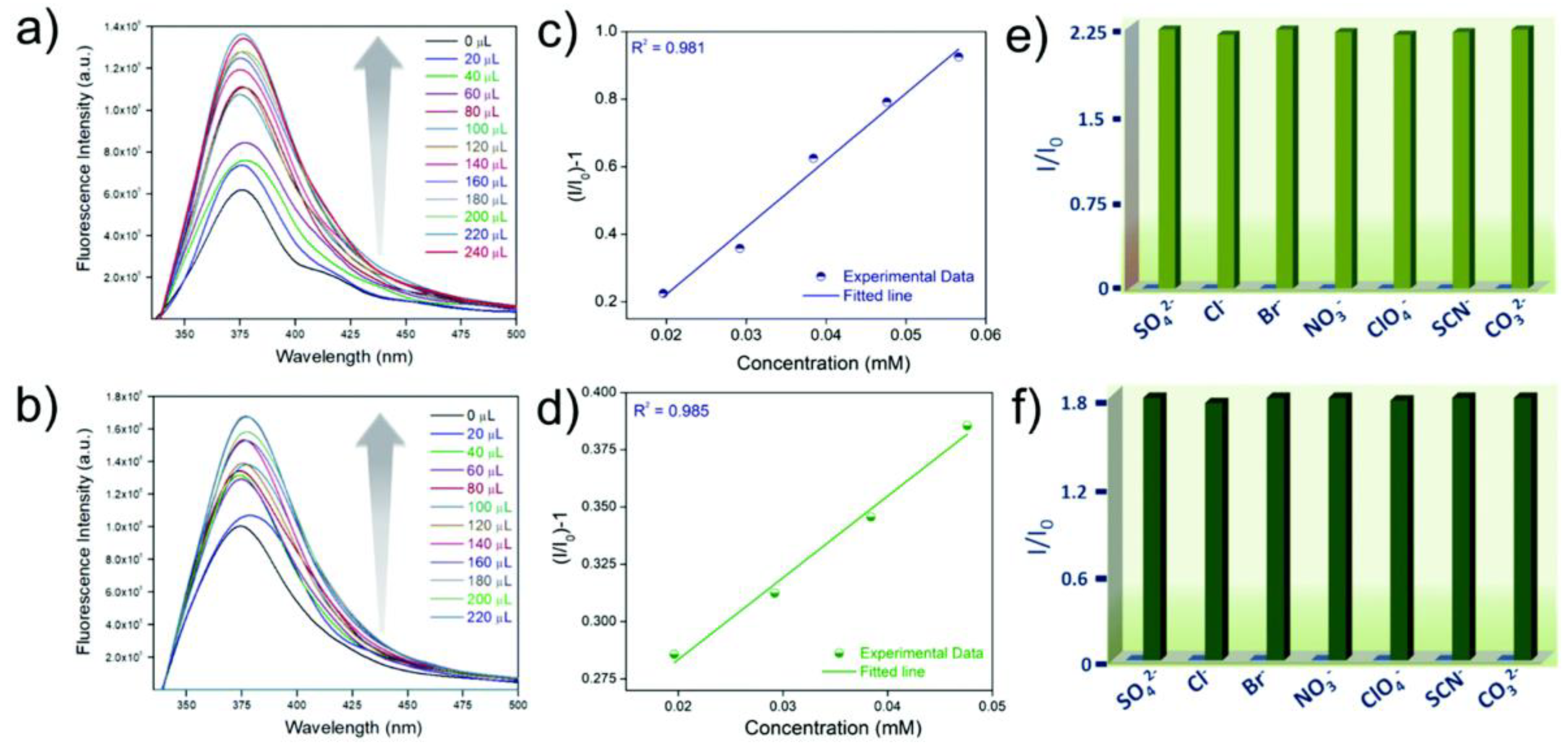
A highly water-stable fluorescent cationic MOF (iMOF-4C) for the “turn-on” detection of HAsO42−and HAsO32− in an aqueous medium was reported by Ghosh et al., which was synthesized by a d10 metal ion (Zn2+) system and tripodal neutral N-donor linker (1,1′-(5′ (4 (1H-imidazol-1-yl)phenyl)-[1,1′:3′,1″-terphenyl]-4,4″-diyl)bis(1H-imidazole) [78]. When HAsO42− and HAsO32− were added incrementally, iMOF-4C showed an increase in fluorescence intensity under UV vis light as well as a fluorescence “turn-on” response. In the presence of other competing anions, the probe is greatly selective towards HAsO42−and HAsO32− ions (Figure 6). The LOD values of the material towards HAsO42− and HAsO32− were calculated to be as low as 8.3 µM and 9.9 µM, respectively. The DFT calculations provide mechanistic insights into the sensing behavior of the material. Compared to iMOF-4C, the ΔE (energy gap between HOMO and LUMO) values for iMOF-4C@HAsO42− and iMOF-4C@HAsO32− are notably lower, which may facilitate electron transfer and enhance emission intensity [79]. Hence, a good correlation was observed between the experimentally obtained patterns and the theoretically predicted fluorescence emission patterns.
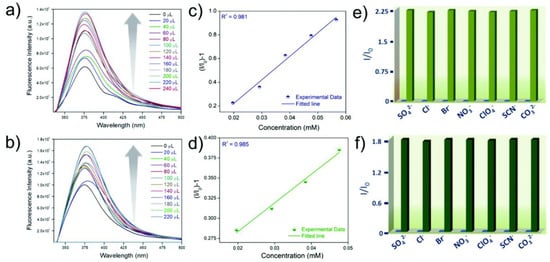
Figure 6.
Fluorescence turn-on response of iMOF-4C with the inclusion of (a) HAsO42− (0 to 240 µL) and (b) HAsO32− (0 to 220 µL); a linear fit of (I0/I −1) vs. concentration of the analyte for iMOF-4C in a low concentration region for (c) HAsO42− and (d) HAsO32−; comparison of the fluorescence response of iMOF-4C in the presence of other competing anions toward (e) HAsO42− and (f) HAsO32−. Reproduced with permission from ref. [78] Copyright 2021, RSC.
High-performance arsenate (As (V)) sensing using a ratiometric fluorescence biosensor based on acid phosphatase and hemin loaded multifunctional Zn-based MOF (ACP/hemin@Zn-MOF) was reported by Pan et al. [80]. Hemin demonstrates peroxidase-like activity in ACP/hemin@Zn-MOF, and the 2-aminoterephthalic acid ligand brings the inherent fluorescence at 452 nm in ACP/hemin@Zn-MOF. Due to the inner filter effect (IFE), the intrinsic fluorescence of ACP/hemin@Zn-MOF (452 nm) was weakened when it catalyzed the oxidation of o-phenylenediamine (OPD), resulting in the production of fluorescent 2,3-diaminophenazine (DAP) with an emission signal at 564 nm [81]. The ascorbic acid 2-phosphate (AAP) can be hydrolyzed by the loaded ACP to produce ascorbic acid (AA), which has some reducibility and can inhibit OPD oxidation competitively. This results in the recovery of the ACP/hemin@Zn-MOF one (452 nm) and the suppression of the DAP signal (564 nm). Upon the addition of As (V), ACP becomes irreversibly poisoned, preventing it from hydrolyzing AAP. This results in the recovery of the fluorescence signal at 564 nm and the suppression of the one at 452 nm [82]. The LOD of this probe for As (V) is 1.05 μg/L, which can fully satisfy the requirements for harmful As(V) detection in numerous matrices. Furthermore, ACP/hemin@Zn-MOF + H2O2 + OPD + AAP system’s ability to detect As(V) selectively is mostly unaffected by 16 potential interfering ions, including phosphate ion (Pi). Practical food and environmental assessments could benefit from the manufactured sensor’s capability, as evidenced by its analysis of rice samples.
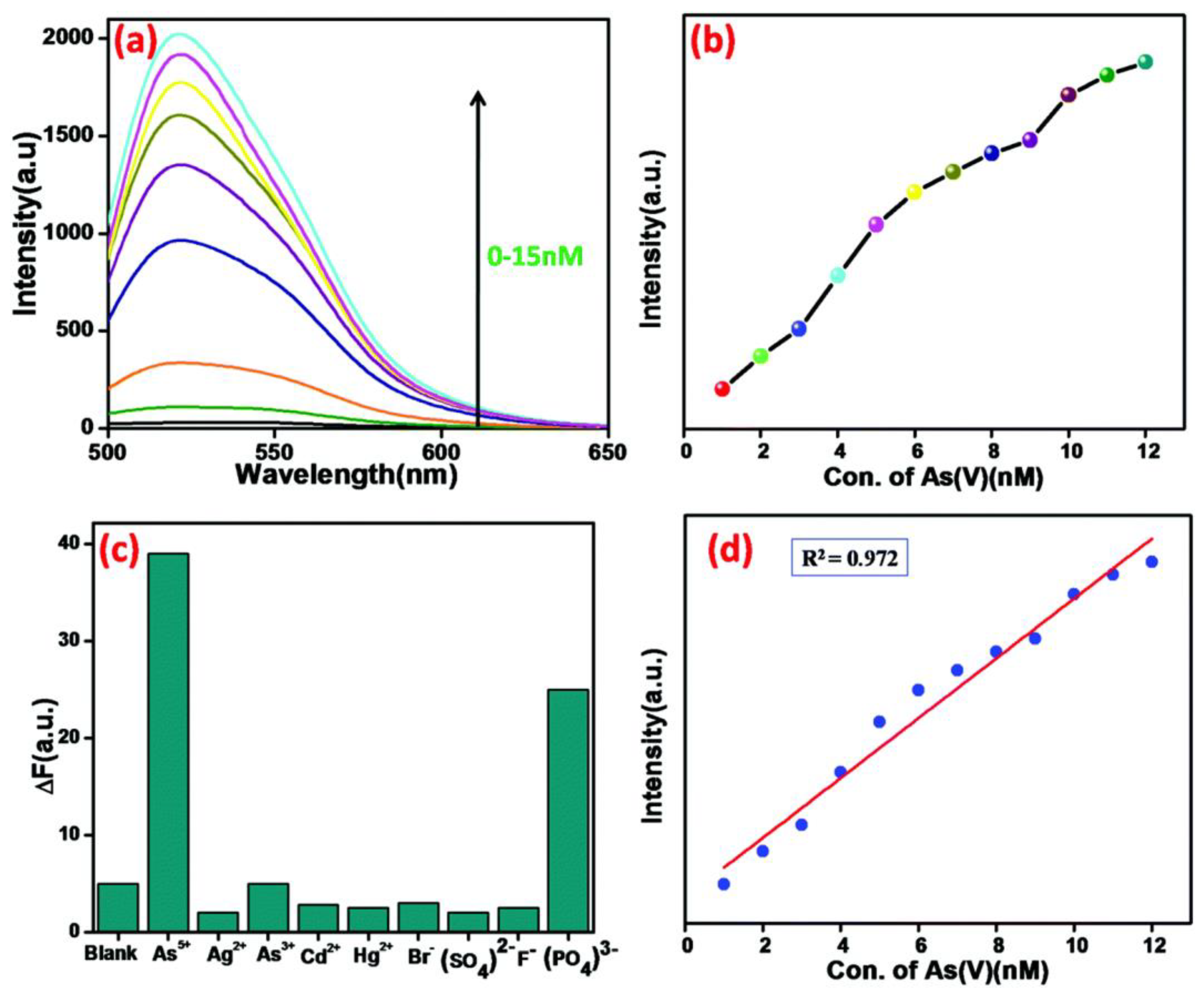
Subramanian et al. fabricated a MOF derived magnetic porous carbon (MPC) composite by dissolving FeCl3·6H2O and fumaric acid in mili-Q water and followed by a hydrothermal reaction [83]. It was discovered that when the MPC composite attaches to FAM-ssDNA, an MPC/FAM-ssDNA complex is formed, which significantly quenches the fluorescence [84,85]. When As(V) was introduced into the sensing system, the immobilized DNA was displaced, and arsenate ions bound to the surface of the MPC composite, resulting in an increase in fluorescence intensity. (Figure 7). This result demonstrates that the probe is highly sensitive enough towards As(V), with a detection limit of 630 pM. DNA single strands linked to FAM are used in this sensing device. The fluorescence is totally quenched when As(V) is not present because the FAM-labeled single-strand DNA attaches to the MPC composite through π–π stacking interactions between the MPC composite’s surface and the nucleobases in ssDNA. When added to this sensing system, As(V) forms a strong bond with the Fe3O4 nanoparticles on the MPC composite’s surface. In the meantime, FAM-labeled ssDNA is liberated from the MPC composite’s surface, which results in an increase in the fluorescence intensity [86]. Furthermore, the primary benefit of this sensing system is the formation of MPC/As(V), which can be separated by a powerful magnet.
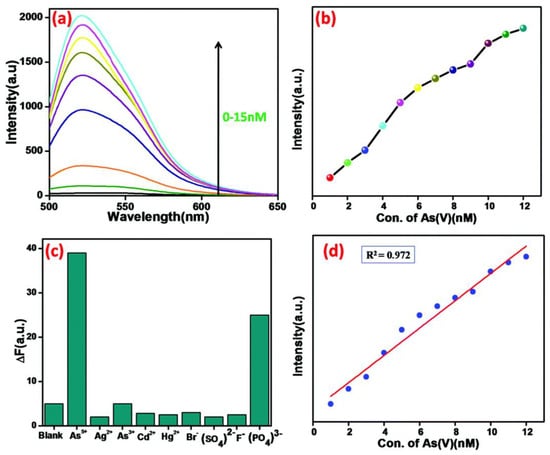
Figure 7.
(a) Detection of different concentrations (0–15 nM) of As(V) in Tris-HCl buffer solution (pH = 7.4) by 10 µM FAM-ssDNA and a 20 µg mL−1 suspension of the MOF@Fe3O4 composite (λex= 490 nm, λem = 520 nm) (b) the relative fluorescence intensity of the MPC composite plotted against As(V) concentration, (c) selectivity studies with other anions and cations, (d) sensitivity of arsenate detection by this arsenate de-adsorption method. Reproduced with permission from ref. [83] Copyright 2019, RSC.
Yang et al. designed and synthesized two new Cu-MOFs, Cu(I)-tpt and Cu(II)-tpt (Htpt = 5-[4(1H-1,2,4-triazol-1-yl)]phenyl-2H-tetrazole), with distinct valences and structures through solvothermal route [87]. Cu(II)-tpt-on-Cu(I)-tpt membrane was prepared by a layer-by-layer approach. Meanwhile, the first Cu(I)-tpt layer acts as a fluorescent “Turn on” sensing platform for p-Arsanilic acid. Although p-ASA is a less hazardous form of organic arsenate acid, it has been shown to inflict significant harm to cells and is readily converted into extremely poisonous inorganic arsenic compounds that may be harmful to species in the environment genetically. The detection limit is 0.0556 μg/L towards p-ASA by the material. Cr2O72− is reduced to Cr3+ by the electron-donor groups (e.g.,−NH2) of p-ASA due to the strong redox potential of Cr2O72− [88,89]. After the adsorbent repelled the produced Cr 3+ ions, the benzenoid amine (−NH2) in p-ASA was oxidized to the quinoid imine, which resulted in an emission enhancement of the material. The mechanism was well tested by the XPS analysis [90,91,92].
6.2. “Turn Off” Sensing of Arsenic
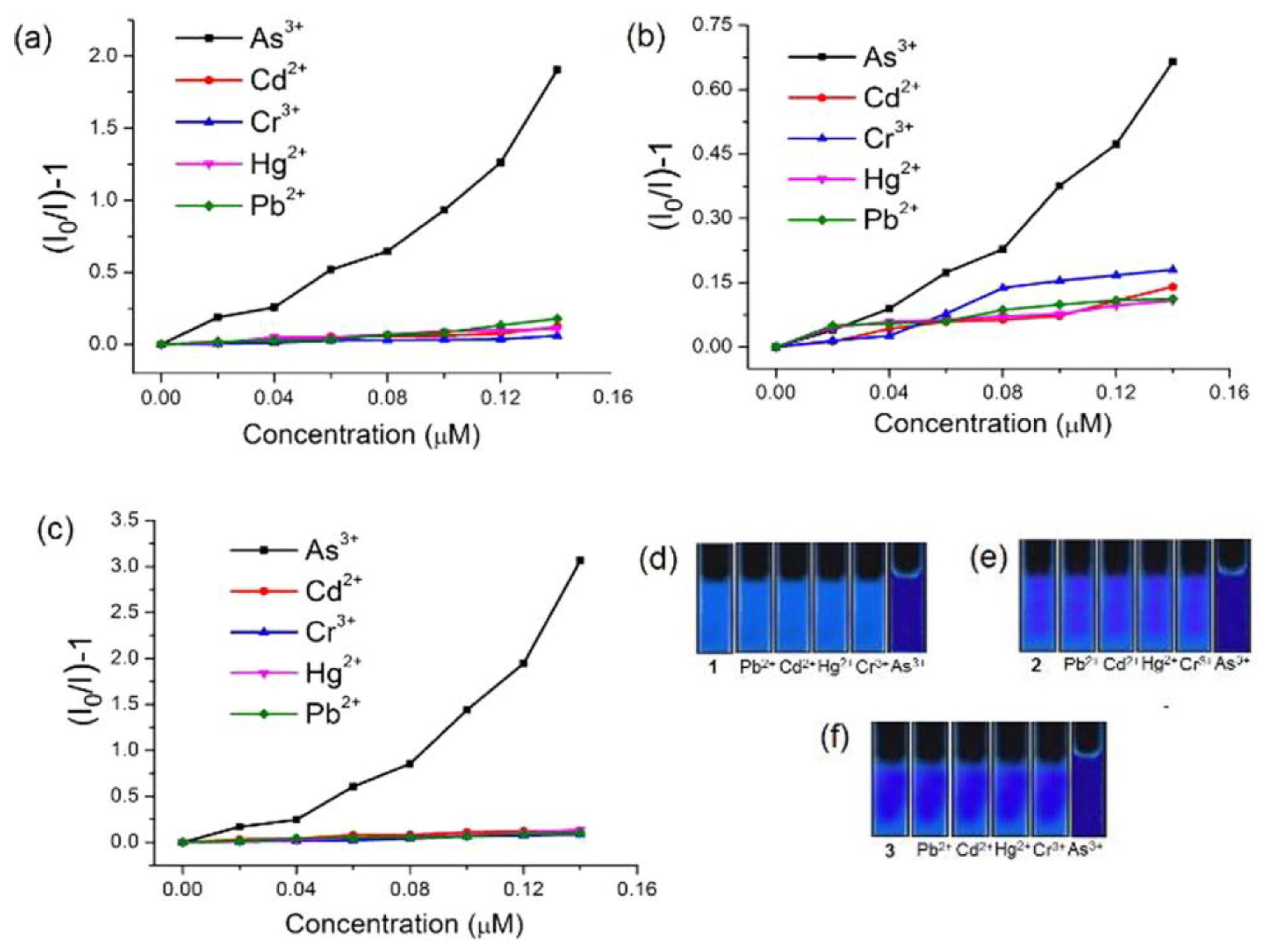
During the “turn-off” detection of a targeted analyte, the emission property of the probe is significantly diminished. In the year 2018, Siddiqi et al. synthesized three lanthanide MOFs (Ln–MOFs) [Ln3(PDC)3Cl3(H2O)]n [Ln = La (1), Nd (2) or Pr (3), PDCH2 = pyridine-2,6-dicarboxylic acid] [93]. These Ln-MOFs were successfully applied for the detection of As3+ in a water medium. Specificity towards As3+ was also examined for all three MOFs, and it has been observed that the said MOFs showed great selectivity towards As3+ over possible interfering metal ions (Fe3+, Cr3+, Cd2+, Hg2+, Pb2+, etc.) (Figure 8). The calculated detection limit of all three Ln–MOFs (1–3) is significantly lower than the permissible limit set by the WHO and U.S. EPA for As3⁺ ions in drinking water (10 ppb). The mechanism underlying the selectivity for As3⁺ ions was investigated using emission and absorption spectra. The As (lone pair)–π(aryl) interactions between As3⁺ ions and PDC²⁻ moieties may lead to the formation of a ground-state non-fluorescent complex, which the authors suggested as the responsible mechanism for the observed static quenching [94,95]. Thus, the Ln-MOFs could be utilized as a promising sensing platform for As3+.
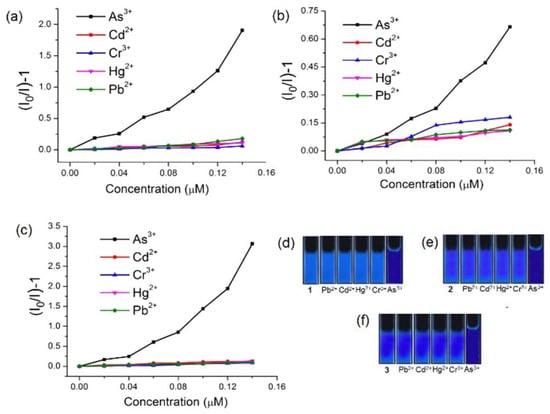
Figure 8.
Stern–Volmer (SV) plots for various toxic elements with the emission intensities under UV light for different ions in water of 1 (a,d), 2 (b,e), and 3 (c,f) upon excitation at 268 (1), 280 (2), and 310 (3) nm [Ln3(PDC)3Cl3(H2O)]n [Ln = La (1), Nd (2) or Pr (3), PDCH2= pyridine-2,6-dicarboxylic acid]. Reproduced with permission from ref. [93] Copyright 2018, Elsevier.
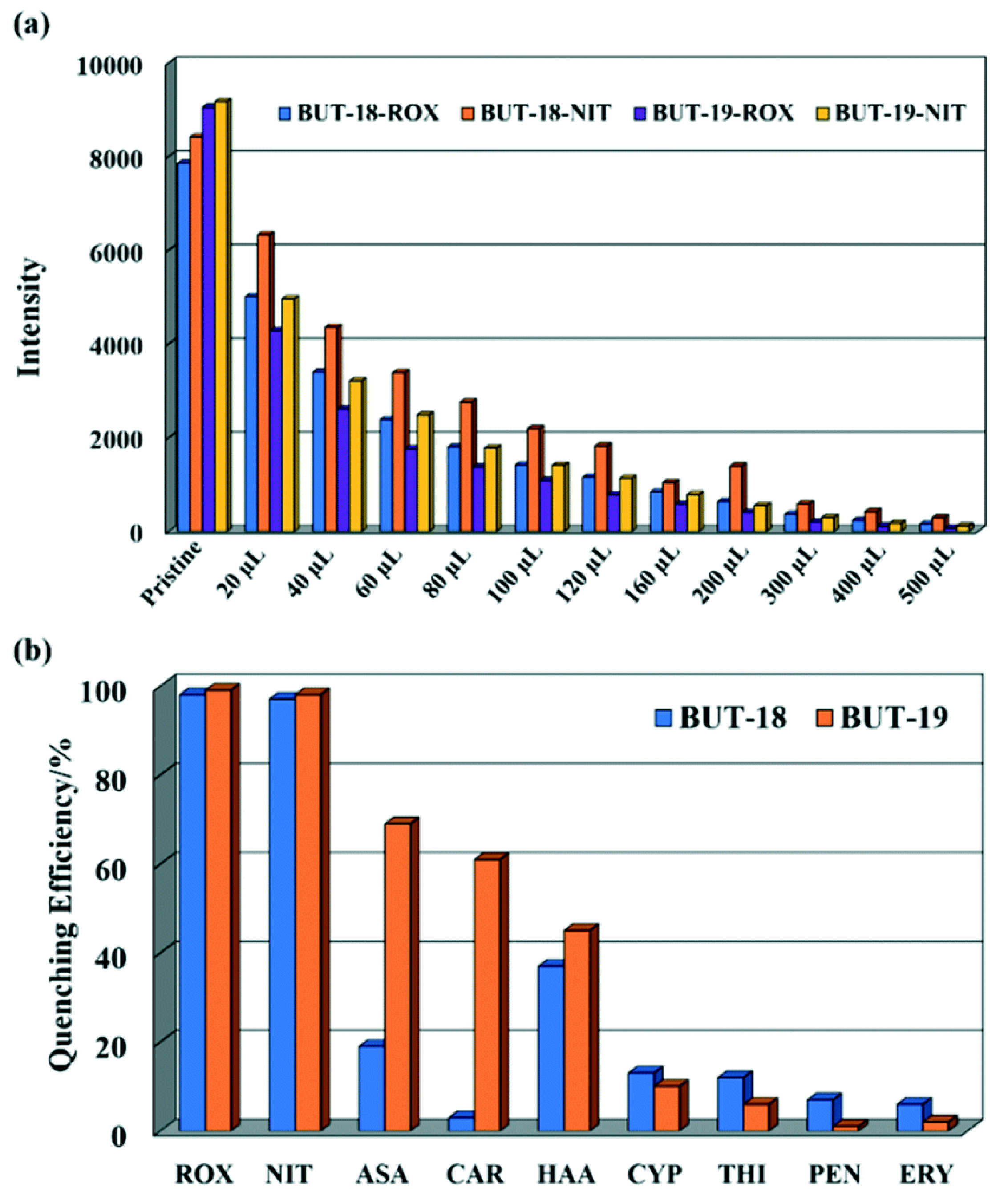
Li et al. prepared two stable isostructural Al-MOFs, Al(CTTA) (BUT-18) and Al(CETA) (BUT-19), by employing two tritopic organic carboxylic acids functionalized with methyl and ethyl groups, dimethyl-5′-(4-(methoxycarbonyl)phenyl)-2′,4′,6′-trimethyl-[1,1′:3′,1″-terphenyl]-4,4″-dicarboxylic acid (H3CTTA), and dimethyl-2′,4′,6′-triethyl-5′-(4-(methoxycarbonyl)phenyl)-[1,1′,3′,1″-terphenyl]-4,4″-dicarboxylic acid (H3CETA) [96]. These two Al-MOFs represent the first reported MOFs designed for the detection of organic arsenic compounds, specifically roxarsone (ROX) and nitarsone (NIT). Notably, they demonstrate remarkable stability across a wide pH range. Both BUT-18 and BUT-19 exhibit exceptional quenching efficiency, achieving nearly 98% quenching in the presence of ROX and NIT. (Figure 9). The limit of detection towards organic arsenic compounds is found to be at the ppb level by the present MOFs. To check the selective sensing behavior towards the roxarsone (ROX) and nitarsone (NIT), other possible organic arsenic compounds, including p-arsanilic acid (ASA), carbarsone (CAR), and 4-hydroxybenzenearsonic acid (HAA) are utilized for detection, but it has been observed that none of them are able to quench the emission intensity of BUT-18 and BUT-19 as like the targeted analytes. It is strongly assumed that a FRET process is the primary mechanism for the fluorescence quenching of ROX because the LUMO energy level of ROX is very close to those of the two MOFs, whereas a photoinduced electron transfer (PET) process is responsible for NIT detection because the LUMO energy of NIT is below those of the two MOFs [62,97]. Hence, the present Al-MOFs may be helpful for organic arsenic sensing applications in wastewater treatment.
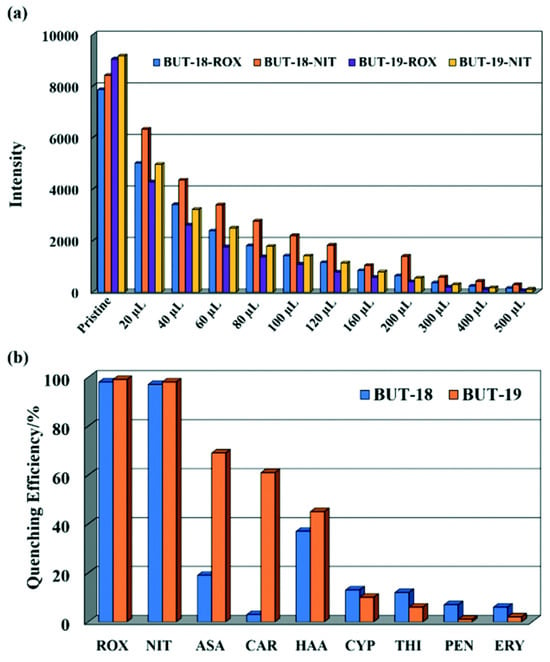
Figure 9.
(a) Fluorescence changes of BUT-18 and -19 dispersed in water upon incremental addition of ROX and NIT (100 ppm) and (b) fluorescence quenching of BUT-18 and BUT-19 by different analytes (100 ppm) (λex = 280 nm). Reproduced with permission from ref. [96] Copyright 2019, RSC.
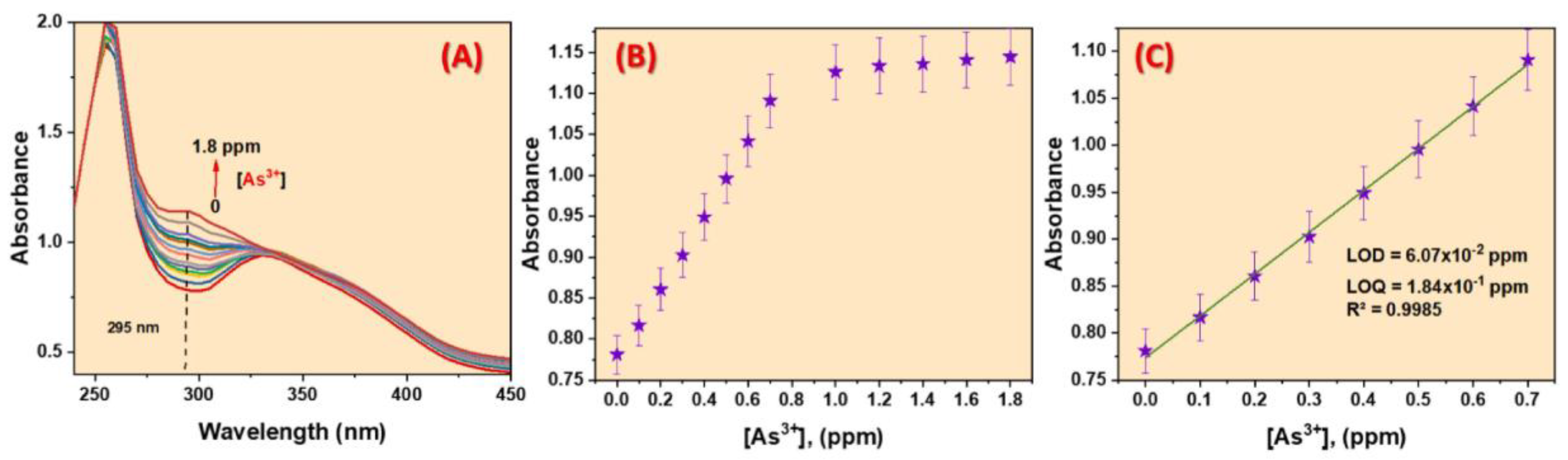
A novel zirconium-based fluorescence MOF was synthesized by Shahat et al. by the condensation reaction between 5-Chloro-2-hydroxyacetophenone and NH2-UiO-66(Zr) [98]. Using spectrophotometric and fluorometric techniques, the authors successfully achieved a selective and sensitive detection of ultra-trace amounts of As3⁺ ions in real water samples. (Figure 10). Furthermore, the effects of several ions, including K+, Na+, Al3+, Mg2+, Cd2+, Hg2+, Mn2+, Ni2+, Ca2+, Cu2+, and SiO42−, on the sensor were evaluated, demonstrating that these ions had no significant impact on the detection of As3⁺. This suggests that the 5Cl2HA = (Zr) MOF sensor effectively discriminates against other tested ions and exhibits excellent selectivity for As3⁺ detection. The LOD value of these reported MOFs was found to be 88.3 ppb towards As3+ ions. It is interesting that material could be recycled several times for the detection of As3+. Furthermore, the material was employed for the detection of As3+ from groundwater and city water. As3⁺ ions interact with the active sites of the 5Cl2HA = (Zr) MOF sensor, specifically targeting the lone pairs of electrons present on the hydroxyl group and nitrogen atom. This interaction leads to the formation of stable chelates between the functional groups of the sensor and As3⁺ ions. In this process, the excited-state intramolecular proton transfer (ESIPT) of the methyl protons in 5-chloro-2-hydroxyacetophenone is inhibited, while the isomerization of the C=N bond is also restricted due to chelation [99]. As a result of ESIPT inhibition and the strong binding interaction, the sensor’s fluorescence is effectively quenched, enabling the selective detection of As3⁺ ions [100]. Thus, the reported probe has great potential for the efficient detection of As3+ ions in aqueous media.
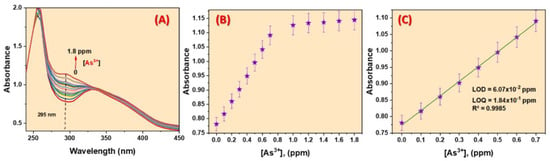
Figure 10.
(A) Spectrophotometric spectra, (B) calibration plot, and (C) calibration curve of the 5Cl2HA = (Zr) MOF sensor with various As3+ ions concentrations in aqueous medium. Reproduced with permission from ref. [98] Copyright 2023, Elsevier.
Pourreza et al. developed a novel turn-off fluorescent platform based on a MOF. Gold nanoparticles (AuNPs) were loaded into a standard Fe-BTC (BTC = 1,3,5-benzenetricarboxylate or trimesic acid) MOF using the porous template method [101]. A fluorescence emission peak was observed at 410 nm by the embedded label-free gold nanoparticles in MOF, and this emission gradually diminished as the amount of As3+ increased (Figure 11). The LOD value for this reported probe was calculated to be 0.2 ng mL−1, which is remarkably below the WHO permissible level (10 μg/L). The spectral overlap between AuNPs@Fe-BTC’s emission spectrum and UV-vis absorption suggests that the fluorescence resonance energy transfer (FRET) process is most likely present [102,103]. Real water samples taken from various rivers and wastewaters were tested for As3+ to determine the validity and applicability of the proposed procedure. The specificity of the method was great, and there was no interference effect in the presence of interfering analytes.
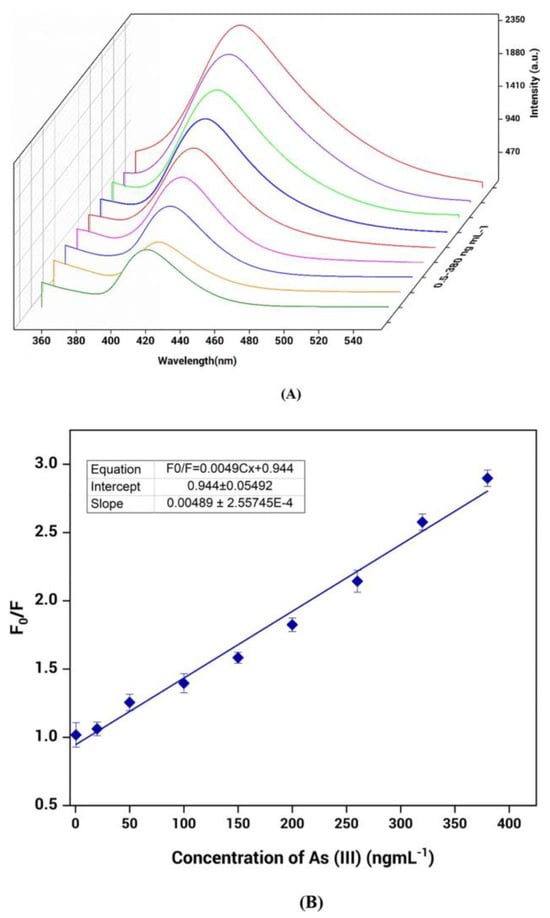
Figure 11.
(A) Fluorescence emission spectra of aqueous suspension of AuNPs@Fe-BTC MOF (stabilized in citrate buffer) in the presence of a different concentration of As (III) under optimal conditions and (B) calibration curve for As(III) based on AuNPs@Fe-BTC sensing system. Reproduced with permission from ref. [101] Copyright 2023, Elsevier.
Very recently, Ghosh et al. demonstrated an aqua-stable luminescent cationic MOF viz., iMOF-12C, which was applied for the precise detection of organic arsenic drugs such as roxarsone(ROX) and nitarsone(NIT) [104]. The iMOF-12C is a Zn MOF having Tris(4-(1H-imidazol-1-yl)phenyl)amine (TIPA) and 1,5-naphthalenedisulfonic acid (1,5-NDSA) organic linker molecules. In iMOF-12C, a progressive decrease in fluorescence intensity was observed with increasing concentrations of ROX and NIT. The MOF exhibited significant fluorescence quenching, with efficiencies of 78% and 82% for ROX and NIT, respectively. Notably, potential interfering analytes such as 4-hydroxyphenylarsonic acid (HAA), 4-aminophenyl arsenic acid (ASA), and phenyl arsenic acid (PAA) had negligible effects on its emission spectra, highlighting its selectivity. Furthermore, the excellent recyclability of iMOF-12C demonstrated its potential for the real-time and long-term detection of organic arsenic compounds. The LOD for ROX and NIT was determined to be 3.95 ppb and 1.35 ppb, respectively—both well below the WHO’s permissible limit. The PET and FRET were found to be the main mechanisms influencing the fluorescence quenching of iMOF-12C toward ROX [105], whereas PET was found to be the major mechanism for NIT [106,107]. Theoretical investigations into the fluorescence quenching process revealed that the electronic properties of iMOF-12C are significantly altered when exposed to ROX and NIT. To further explore its potential, iMOF-12C-based mixed-matrix membranes (MMMs) were developed for practical, on-site use. The results were promising, showing effective detection of organic arsenic in water. These findings position iMOF-12C as a highly promising candidate for real-time, on-site monitoring of organic arsenic in aqueous environments.
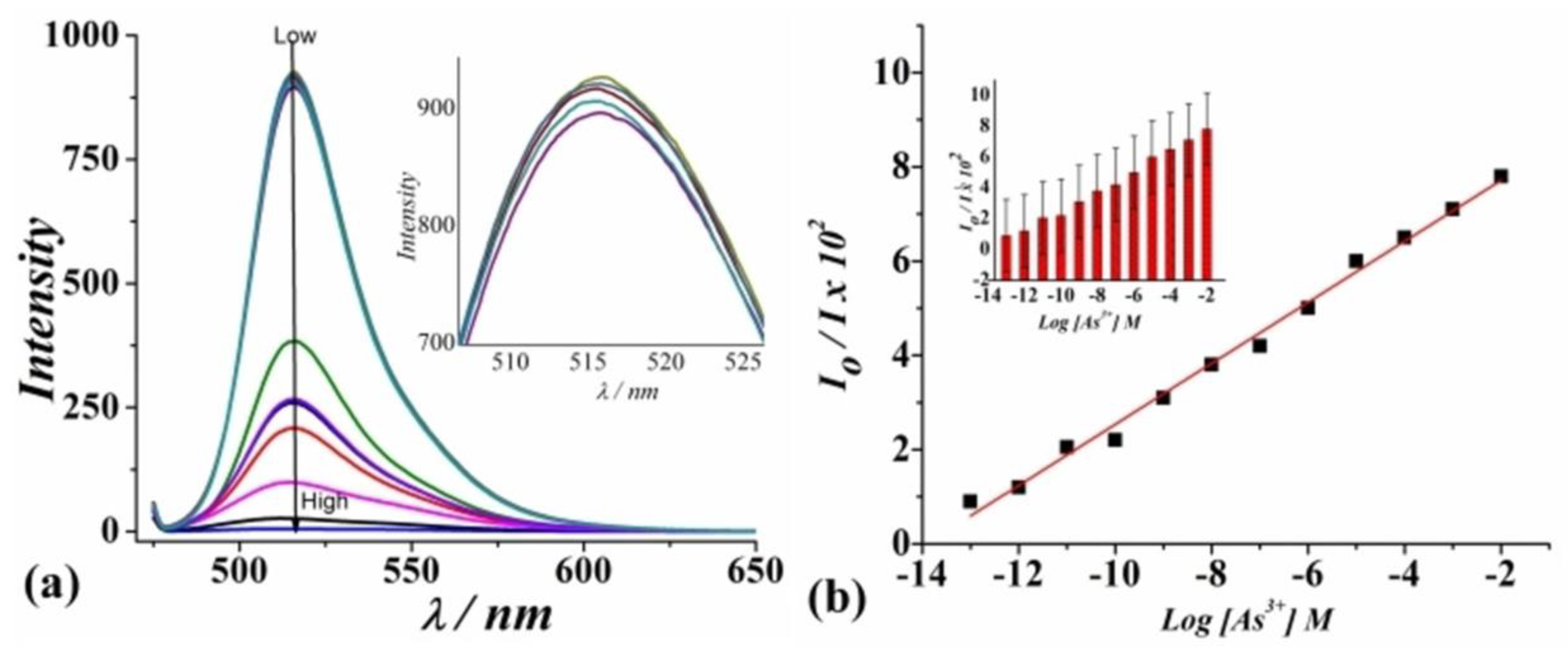
A novel turn-off fluorescent sensor for the selective detection of As3⁺ was developed by Shukla et al. This sensing method utilizes a fluorescein (Flu) molecule as the emitting center, polyhedral micellized silver colloids (AgNPs) to stimulate the excitation rate, and a nanoscaled Prussian blue metal-organic framework (PB-MOF) as the light sensitizer. [108]. The Fluorescein/AgNPs/PB-MOF system serves as a fluorescent sensor for the selective detection of As3⁺. Upon the addition of As3⁺, a noticeable decrease in the emission intensity of the fluorescent system is observed (Figure 12a). Furthermore, a linear relationship between As3⁺ concentration and signal intensity, with a sensitivity of Kv = 2.305 vol–1, is evident (Figure 12b). The detection limit is estimated to be 0.150 ppm. The incorporation of AgNPs and PB-MOF facilitates intermolecular activation, leading to a sequential amplification of fluorescein’s emission maxima (λem = 520 nm). Interaction with As3⁺ induces non-radiative energy transfer, resulting in the quenching of the emission spectra [109,110]. Notably, the sensor exhibits high selectivity for As3⁺ over other potential interfering ions, including Na+, K +, Cs+, Ca2+, Mg2+, Cr 3+, Mn2+, Zn 2+, Cd 2+, and Pb2+.
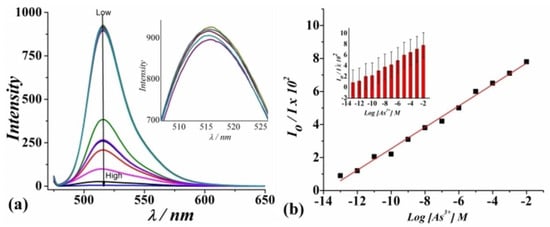
Figure 12.
(a) Fluorescence spectra of Fluorescein/AgNPs/PB-MOF for analyzing different concentrations of As3+ (from top to bottom: 0.0001 nM to 107 nM) (λem = 520 nm). (b) The dependence of I520 on the concentration of As3+ or SternVolmer plot shows the fluorescence quenching. The inset to (b) shows the error bars representing the standard deviation. Reproduced with permission from ref. [108] Copyright 2020, Wiley-VCH.
El-Bindary et al. demonstrated that the condensation of NH2-Al-MOF and 2-hydroxyacetophenone ligand via a Schiff base reaction resulted in a covalent linkage, creating a novel fluorescence sensor for As(III) [111]. The successful formation of the Schiff base was confirmed using several analytical techniques, including Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET) surface area analysis, and X-ray photoelectron spectroscopy (XPS). In aqueous suspension, the Schiff base material exhibited a significant reduction in fluorescence intensity upon the addition of As3⁺, highlighting its sensing capability. Importantly, interfering ions had no effect on the fluorescence intensity of the 2HA=N-MIL-53(Al) sensor, indicating its strong selectivity and excellent anti-interference ability for As3⁺ detection. Moreover, the sensor demonstrated effective arsenic detection in real water samples. The sensor’s fluorescence is attributed to the C=N isomerization at the excited state, which occurs in optical sensors with a covalently bridged C=N structure. The hydroxyl group and the lone electron pair of nitrogen atoms serve as the two active sites in the 2HA=N-MIL-53(Al) sensor. The fluorescence quenching observed is a result of stable chelation between the 2HA=N-MIL-53(Al) sensor and As3⁺, which inhibits both the C=N isomerization and the ESIPT of the phenolic protons in the 2-hydroxyacetophenone moiety [112,113]. The fluorescence intensity of the 2HA=N-MIL-53(Al) sensor decreased with the sequential addition of As3⁺ ions. The analytical performance of the MOFs discussed above is summarized in Table 1.

Table 1.
List of MOFs showing fluorescent sensing of different arsenic species via “Turn on” and “Turn off” mode.
7. Conclusions and Prospects for the Future
Arsenic contamination in groundwater remains a critical global challenge, affecting millions of people, particularly in regions with limited access to clean water. While traditional field kits have been routinely used for arsenic detection, concerns about their sensitivity and selectivity have prompted a shift towards more advanced approaches. The development of ultrasensitive sensors for arsenic detection requires that the target analyte and the sensing probe operate according to the lock-and-key principle, which has led to a surge in research focused on designing materials with enhanced selectivity for arsenic ions.
This review has highlighted recent advancements in the fluorometric detection of arsenic using MOFs over the past decade. These fluorometric approaches combine chemical, biological, and nanotechnological techniques to significantly improve the sensitivity of arsenic detection compared to conventional methods. We have covered notable studies that explore various aspects of MOF-based sensors, including their sensing mechanisms, signal processing strategies, and detection performance. MOFs such as Zr-MOFs, Ln-MOFs, Zn-MOFs, and Fe-MOFs have demonstrated excellent efficacy in the fluorogenic detection of arsenic. These materials have not only enabled quantitative detection in controlled environments but also shown promise in real-world applications. In some cases, MOFs have even been successfully employed for naked-eye detection, making them accessible and user-friendly for everyday use.
The unique properties of MOFs, including their high porosity, large surface area, and ease of functionalization, make them ideal candidates for the selective and sensitive detection of arsenic. Despite significant progress, the field of MOF-based arsenic sensors is still in its early stages, and several challenges must be addressed to realize their full potential.
Looking forward, there are several key areas where further research is needed. First, as arsenic detection often occurs in environmental samples, MOFs with broad pH stability are essential to ensure reliable performance in diverse conditions. Second, improving the water stability of these materials is critical for ensuring their long-term effectiveness in aqueous environments. Third, simplifying the synthesis processes for MOFs will be crucial to enable large-scale, cost-effective production, making these materials more accessible for widespread use. Lastly, while fluorometric detection methods are promising, there is an increasing demand for portable, user-friendly, and highly specific on-site detection kits that can provide rapid results. By utilizing luminescent MOFs, such kits could facilitate real-time monitoring of arsenic contamination, even in remote or underserved areas.
As research in this field progresses, we are optimistic that ongoing advancements in MOF design, sensor development, and manufacturing techniques will lead to the creation of highly effective, affordable, and practical arsenic detection solutions. The future of arsenic sensing lies in the development of MOF-based sensors that are not only more sensitive and selective but also more adaptable to real-world conditions. With continued innovation, we hope to see the widespread adoption of these technologies, contributing to the global effort to mitigate arsenic contamination and protect public health.
Author Contributions
Conceptualization, writing—original draft preparation, S.N.; writing—reviewing and editing, R.D.; visualization, R.D.; supervision, R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data used in this study are included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yoshida, T.; Yamauchi, H.; Sun, G.F. Chronic health effects in people exposed to arsenic via the drinking water: Dose–response relationships in review. Toxicol. Appl. Pharmacol. 2004, 198, 243–252. [Google Scholar] [PubMed]
- Richardson, S.D.; Ternes, T.A. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2005, 77, 3807–3838. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Bhattacharya, P. Arsenic in Drinking Water: Is 10 µg/L a Safe Limit? Curr. Pollut. Rep. 2019, 5, 1–3. [Google Scholar]
- Olavarria-Fullerton, J.; Wells, S.; Ortiz-Rivera, W.; Sepaniak, M.J.; DeJesus, M.A. Surface-enhanced Raman scattering (SERS) characterization of trace organoarsenic antimicrobials using silver/polydimethylsiloxane nanocomposites. Appl. Spectrosc. 2011, 65, 423–428. [Google Scholar]
- Gamboa-Loira, B.; Cebrián, M.E.; Franco-Marina, F.; López-Carrillo, L. Arsenic metabolism and cancer risk: A meta-analysis. Environ. Res. 2017, 156, 551–558. [Google Scholar]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar]
- Mangalgiri, K.P.; Adak, A.; Blaney, L. Organo arsenicals in Poultry litter: Detection, fate, and toxicity. Environ. Int. 2015, 75, 68–80. [Google Scholar]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar]
- Lee, J.-J.; Kim, Y.-K.; Cho, S.-H.; Park, K.-S.; Chung, I.-J.; Cho, D.; Ryang, D.-W.; Kim, H.-J. Hemolytic anemia as a sequela of arsenic intoxication following long-term ingestion of traditional chinese medicine. J. Korean Med. Sci. 2004, 19, 127–129. [Google Scholar]
- Scott, N.; Hatlelid, K.M.; MacKenzie, N.E.; Carter, D.E. Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem. Res. Toxicol. 1993, 6, 102–106. [Google Scholar]
- Ding, W.-Q.; Labiadh, L.; Xu, L.; Li, X.-Y.; Chen, C.; Fu, M.-L.; Yuan, B. Current advances in the detection and removal of organic arsenic by metal-organic frameworks. Chemosphere 2023, 339, 139687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.-R.; Wen, S.-H.; Liang, R.-P.; Qiu, J.-D. Optical sensors for inorganic arsenic detection. Trends Anal. Chem. 2019, 118, 869–879. [Google Scholar]
- Kolya, H.; Hashitsume, K.; Kang, C.-W. Recent advances in colorimetric detection of arsenic using metal-based nanoparticles. Toxics 2021, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yu, S.; Li, L. Research progress in fluorescent probes for arsenic species. Molecules 2022, 27, 8497. [Google Scholar] [CrossRef]
- Thakkar, S.; Dumée, L.F.; Gupta, M.; Singh, B.R.; Yang, W.; Gamboa-Loira, B. Nano–Enabledsensorsfor detectionofarsenicin water. Water Research 2021, 188, 116538. [Google Scholar]
- Devi, P.; Thakur, A.; Lai, R.Y.; Saini, S.; Jain, R.; Kumar, P. Progress in the materials for optical detection of arsenic in water. Trends Anal. Chem. 2019, 110, 97–115. [Google Scholar] [CrossRef]
- Abernathy, C.O.; Thomas, D.J.; Calderon, R.L. Health effects and risk assessment of Arsenic. J. Nutr. 2003, 133, 1536S–1538S. [Google Scholar] [CrossRef]
- Brouwer, O.; Onkenhout, W.; Edelbroek, P.; Kom, J.D.; Wolff, F.D.; Peters, A. Increased neurotoxicity of arsenic in methylenetetrahydrofolate reductase deficiency. Clin. Neurol. Neurosurg. 1992, 94, 307–310. [Google Scholar]
- Civantos, D.P.; Rodríguez, A.L.; Aguado-Borruey, J.M.; Narvaez, J.A.J. Fulminant malignant arrythmia and multiorgan failure in acute arsenic poisoning. Chest 1995, 108, 1774–1775. [Google Scholar]
- Tseng, W.P.; Chu, H.M.; How, S.W.; Fong, J.M.; Yeh, S.; Lin, C.S. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J. Natl. Cancer Inst. 1968, 40, 453–463. [Google Scholar]
- Thomas, D.J.; Styblob, M.; Linc, S. The cellular metabolism and systemic toxicity of arsenic. Toxicol. Appl. Pharmacol. 2001, 176, 127–144. [Google Scholar] [PubMed]
- Cullen, W.; McBride, B.; Reglinski, J. The reduction of trimethylarsine oxide to trimethylarsine by thiols: A mechanistic model for the biological reduction of arsenicals. J. Inorg. Biochem. 1984, 21, 45–60. [Google Scholar] [CrossRef]
- Mazumder, D.N.G. Chronic arsenic toxicity & human health. Indian J. Med. Res 2008, 128, 436–447. [Google Scholar]
- Zhang, Q.; Minami, H.; Inoue, S.; Atsuya, I. Differential determination of trace amounts of arsenic (III) and arsenic (V) in seawater by solid sampling atomic absorption spectrometry after preconcentration by coprecipitation with a nickel–pyrrolidine dithiocarbamate complex. Anal. Chim. Acta 2004, 508, 99–105. [Google Scholar] [CrossRef]
- Hung, D.Q.; Nekrassova, O.; Comptons, R.G. Analytical methods for inorganic arsenic in water: A review. Talanta 2004, 64, 269–277. [Google Scholar] [CrossRef]
- Mao, X.; Qi, Y.; Huang, J.; Liu, J.; Chen, G.; Na, X.; Wang, M.; Qian, Y. Ambient-temperature trap/release of arsenic by dielectric barrier discharge and its application to ultratrace arsenic determination in surface water followed by atomic fluorescence spectrometry. Anal. Chem. 2016, 88, 4147–4152. [Google Scholar] [CrossRef]
- Colon, M.; Hidalgo, M.; Iglesias, M. Arsenic determination by ICP-QMS with octopole collision/reaction cell. Overcome of matrix effects under vented and pressurized cell conditions. Talanta 2011, 85, 1941–1947. [Google Scholar] [CrossRef]
- Diesel, E.; Schreiber, M.; Meer, J.R.v.d. Development of bacteria-based bioassays for arsenic detection in natural waters. Anal. Bioanal. Chem. 2009, 394, 687–693. [Google Scholar] [CrossRef]
- Barros, H.; Parra, L.-M.M.; Bennun, L.; Greaves, E.D. Determination of arsenic in water samples by Total Reflection X-Ray Fluorescence using pre-concentration with alumina. Spectrochim. Acta Part B 2010, 65, 489–492. [Google Scholar]
- Jiang, M.; Ma, M.-J.; Yang, M.; Fang, L.; Li, Y.-X.; Zhao, N.-J.; Huang, X.-J. Highly sensitive and stable analysis of trace arsenic(III) and mercury(II) in water by Low-pulse-energy (15 mJ) laser-induced breakdown spectroscopy assisted by active controllable spark discharge and electrochemical enrichment. Sens. Actuators B 2020, 305, 127486. [Google Scholar] [CrossRef]
- Banik, D.; Manna, S.K.; Mahapatra, A.K. Recent development of chromogenic and fluorogenic chemosensors for the detection of arsenic species: Environmental and biological applications. Spectrochim. Acta A 2021, 246, 119047. [Google Scholar]
- Samanta, T.; Shunmugam, R. Colorimetric and fluorometric probes for the optical detection of environmental Hg(II) and As(III) ions. Mater. Adv. 2021, 2, 64–95. [Google Scholar]
- Luong, J.H.T.; Lam, E.; Male, K.B. Recent advances in electrochemical detection of arsenic in drinking and ground waters. Anal. Methods 2014, 6, 6157–6169. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Tatsu, Y.; Lu, Z.-H.; Xu, Q. Non-, Micro-, and Mesoporous Metal–Organic Framework Isomers: Reversible Transformation, Fluorescence Sensing, and Large Molecule Separation. J. Am. Chem. Soc. 2010, 132, 5586–5587. [Google Scholar]
- Lee, M.H.; Yoon, B.; Kim, J.S.; Sessler, J.L. Naphthalimide trifluoroacetylacetonate: A hydrazine-selective chemodosimetric sensor. Chem. Sci. 2013, 4, 4121–4126. [Google Scholar]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar]
- Fang, X.; Zong, B.; Mao, S. Metal–organic framework-based sensors for environmental contaminant sensing. Nano-Micro Lett. 2018, 10, 64. [Google Scholar]
- Zhang, Z.; Zhuang, Z.; Song, L.L.; Lin, X.; Zhang, S.; Zheng, G.; Zhan, F. A FRET-based ratiometric fluorescent probe for hydrazine and its application in living cells. J. Photochem. Photobiol. A 2018, 358, 10–16. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, J.; Wang, L.; Li, H.; Yang, F.; Yang, X. Inner filter effect-based sensor for horseradish peroxidase and its application to fluorescence immunoassay. ACS Sens. 2018, 3, 183–190. [Google Scholar]
- Abdelhamid, H.N.; B.-Gómez, A.; M.-Matute, B.; Zou, X. A water-stable lanthanide metal-organic framework for fluorimetric detection of ferric ions and tryptophan. Microchim. Acta 2017, 184, 3363–3371. [Google Scholar]
- Luo, F.; Batten, S.R. Metal–organic framework (MOF): Lanthanide(iii)-doped approach for luminescence modulation and luminescent sensing. Dalton Trans. 2010, 39, 4485–4488. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Mondal, A.; Reinsch, H.; Biswas, S. An ultra-robust luminescent CAU-10 MOF acting as a fluorescent turn-off sensor for Cr2O72− in aqueous medium. Inorg. Chim. Acta 2019, 497, 119078. [Google Scholar] [CrossRef]
- Dalapati, R.; Nandi, S.; Reinsch, H.; Bhunia, B.K.; Mandal, B.B.; Stock, N.; Biswas, S. Fluorogenic naked-eye sensing and live-cell imaging of cyanide by a hydrazine-functionalized CAU-10 metal–organic framework. CrystEngComm 2018, 20, 4194–4201. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–Organic Framework Nanoparticles. Adv. Mater. 2018, 30, 1800202. [Google Scholar] [CrossRef]
- Tibbetts, I.; Kostakis, G.E. Recent Bio-Advances in Metal-Organic Frameworks. Molecules 2020, 25, 1291. [Google Scholar] [CrossRef]
- Gulcay, E.; Erucar, I. Biocompatible MOFs for Storage and Separation of O2: A Molecular Simulation Study. Ind. Eng. Chem. Res. 2019, 58, 3225–3237. [Google Scholar] [CrossRef]
- Huxford, R.C.; Rocca, J.D.; Lin, W. Metal-organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef]
- Leng, X.; Dong, X.; Wang, W.; Sai, N.; Yang, C.; You, L.; Huang, H.; Yin, X.; Ni, J. Biocompatible Fe-based micropore metal-organic frameworks as sustained-release anticancer drug carriers. Molecules 2018, 23, 2490. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, X.-Y.; Cheng, P.; Liao, D.-Z.; Yan, S.-P.; Jiang, Z.-H. Coordination Polymers Containing 1D Channels as Selective Luminescent Probes. J. Am. Chem. Soc. 2004, 126, 15394–15395. [Google Scholar] [PubMed]
- Xu, H.; Liu, F.; Cui, Y.; Chen, B.; Qian, G. A luminescent nanoscale metal–organic framework for sensing of nitroaromatic explosives. Chem. Commun. 2011, 47, 3153–3155. [Google Scholar]
- Rieter, W.J.; Taylor, K.M.L.; An, H.; Lin, W.; Lin, W. Nanoscale metal–organic frameworks as potential multimodal contrast enhancing agents. J. Am. Chem. Soc. 2006, 128, 9024–9025. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.-W.; Zhang, Y.-H. Blue photoluminescent 3D Zn(II) metal-organic framework constructing from pyridine-2,4,6-tricarboxylate. Inorg. Chem. Commun. 2008, 11, 832–834. [Google Scholar]
- Eliseeva, S.V.; B€unzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar]
- Moore, E.G.; Samuel, A.P.S.; Raymond, K.N. From Antenna to assay: Lessons learned in lanthanide luminescence. Acc. Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, Y.; Wu, K. Highly Stable Five-Coordinated Mn(II) Polymer [Mn(Hbidc)]n (Hbidc=1H-Benzimidazole-5,6-dicarboxylate): Crystal Structure, Antiferromegnetic Property, and Strong Long-Lived Luminescence. Cryst. Growth Des. 2008, 8, 2087–2089. [Google Scholar]
- Wang, X.W.; Chen, J.-Z.; Liu, J.-H. Photoluminescent Zn(II) Metal–Organic Frameworks Built from Tetrazole Ligand: 2D Four-Connected Regular Honeycomb (4363)-net. Cryst. Growth Des. 2007, 7, 1227–1229. [Google Scholar]
- An, J.; Shade, C.M.; Chengelis-Czegan, D.A.; Petoud, S.; Rosi, N.L. Zinc-Adeninate Metal–Organic Framework for Aqueous Encapsulation and Sensitization of Near-infrared and Visible Emitting Lanthanide Cations. J. Am. Chem. Soc. 2011, 133, 1220–1223. [Google Scholar]
- Karmakar, A.; Joarder, B.; Mallick, A.; Samanta, P.; Desai, A.V.; Basu, S.; Ghosh, S.K. Aqueous phase sensing of cyanide ions using a hydrolytically stable metal–organic framework. Chem. Commun. 2017, 53, 1253–1256. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H.-C. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Müller-Buschbaum, K.; Beuerle, F.; Feldmann, C. MOF based luminescence tuning and chemical/physical sensing. Microporous Mesoporous Mater. 2015, 216, 171–199. [Google Scholar]
- Taylor, J.M.; Dawson, K.W.; Shimizu, G.K.H. A water-stable metal–organic framework with highly acidic pores for proton-conducting applications. J. Am. Chem. Soc. 2013, 135, 1193–1196. [Google Scholar]
- Cao, X.; Lin, W.; Zheng, K.; He, L. A near-infrared fluorescent turn-on probe for fluorescence imaging of hydrogen sulfide in living cells based on thiolysis of dinitrophenyl ether. Chem. Commun. 2012, 48, 10529–10531. [Google Scholar]
- Chen, W.; Liu, W.; Liu, X.-J.; Kuang, Y.-Q.; Yu, R.-Q.; Jiang, J.-H. A novel fluorescent probe for sensitive detection and imaging of hydrazine in living cells. Talanta 2017, 162, 225–231. [Google Scholar]
- Alezi, D.; Belmabkhout, Y.; Suyetin, M.; Bhatt, P.M.; Weselinski, L.J.; Solovyeva, V.; Adil, K.; Spanopoulos, I.; Trikalitis, P.N.; Emwas, A.H.; et al. MOF Crystal Chemistry Paving the Way to Gas Storage Needs: Aluminum-Based soc-MOF for CH4, O2, and CO2 Storage. J. Am. Chem. Soc. 2015, 137, 13308–13318. [Google Scholar]
- Li, B.; Wen, H.-M.; Zhou, W.; Chen, B. Porous metal–organic frameworks for gas storage and separation: What, how, and why? J. Phys. Chem. Lett. 2014, 5, 3468–3479. [Google Scholar] [CrossRef]
- Dalapati, R.; Sakthivel, B.; Dhakshinamoorthy, A.; Buragohain, A.; Bhunia, A.; Jainak, C.; Biswas, S. A highly stable dimethyl-functionalized Ce(IV)-based UiO-66 metal-organic framework material for gas sorption and redox catalysis. CrystEngComm 2016, 18, 7855–7864. [Google Scholar]
- Li, R.; Ren, X.; Ma, H.; Feng, X.; Lin, Z.; Li, X.; Hu, C.; Wang, B. Nickel-substituted zeolitic imidazolate frameworks for time-resolved alcohol sensing and photocatalysis under visible light. J. Mater. Chem. A 2014, 2, 5724–5729. [Google Scholar] [CrossRef]
- Liu, D.; Lu, K.; Poon, C.; Lin, W. Metal–organic frameworks as sensory materials and imaging agents. Inorg. Chem. 2014, 53, 1916–1924. [Google Scholar] [PubMed]
- Kumar, N.; Bhalla, V.; Kumar, M. Recent developments of fluorescent probes for the detection of gasotransmitters (NO, CO and H2S). Coord. Chem. Rev. 2013, 257, 2335–2347. [Google Scholar] [CrossRef]
- Xie, D.; Ma, Y.; Gu, Y.; Zhou, H.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Bifunctional NH2-MIL-88(Fe) metal–organic framework nanooctahedra for highly sensitive detection and efficient removal of arsenate in aqueous media. Mater. Chem. A 2017, 5, 23794–23804. [Google Scholar]
- Yang, J.; Dai, Y.; Zhu, X.; Wang, Z.; Li, Y.; Zhuang, Q.; Shi, J.; Gu, J. Metal–organic frameworks with inherent recognition sites for selective phosphate sensing through their coordination-induced fluorescence enhancement effect. J. Mater. Chem. A 2015, 3, 7445–7452. [Google Scholar]
- Liu, S.; Liu, M.; Guo, M.; Wang, Z.; Wang, X.; Cui, W.; Tian, Z. Development of Eu-based metal-organic frameworks (MOFs) for luminescence sensing and entrapping of arsenate ion. J. Lumin. 2021, 236, 118102. [Google Scholar]
- Maity, D.; Mandal, S.K.; Guha, B.; Roy, P. A salicylaldehyde based dual chemosensor for zinc and arsenate ion detection: Biological application. Inorg. Chim. Acta. 2021, 519, 120258. [Google Scholar] [CrossRef]
- Lohar, S.; Sahana, A.; Banerjee, A.; Banik, A.; Mukhopadhyay, S.K.; Matalobos, J.S.; Das, D. Antipyrine based arsenate selective fluorescent probe for living cell imaging. Anal. Chem. 2013, 85, 1778–1783. [Google Scholar]
- Dutta, S.; Let, S.; Shirolkar, M.M.; Desai, A.V.; Samanta, P.; Fajal, S.; More, Y.D.; Ghosh, S.K. A luminescent cationic MOF for bimodal recognition of chromium and arsenic based oxo-anions in water. Dalton Trans. 2021, 50, 10133–10141. [Google Scholar]
- Shirolkar, M.M.; Athavale, R.; Ravindran, S.; Rale, V.; Kulkarni, A.; Deokar, R. Antibiotics functionalization intervened morphological, chemical and electronic modifications in chitosan nanoparticles. Nano-Struct. Nano-Obj. 2021, 25, 100657. [Google Scholar]
- Xu, X.; Luo, Z.; Ye, K.; Zou, X.; Niu, X.; Pan, J. One-pot construction of acid phosphatase and hemin loaded multifunctional metal–organic framework nanosheets for ratiometric fluorescent arsenate sensing. J. Hazard. Mater. 2021, 412, 124407. [Google Scholar] [CrossRef]
- Wen, S.-H.; Liang, R.-P.; Zeng, H.-H.; Zhang, L.; Qiu, J.-D. CdSe/ZnS quantum dots coated with carboxy-PEG and modified with the terbium(III) complex of guanosine 5′-monophosphate as a fluorescent nanoprobe for ratiometric determination of arsenate via its inhibition of acid phosphatase activity. Microchim. Acta 2019, 186, 45. [Google Scholar]
- Tong, Y.-J.; Yu, L.-D.; Wu, L.-L.; Cao, S.-P.; Liang, R.-P.; Zhang, L.; Xia, X.-H.; Qiu, J.-D. Aggregation-induced emission of luminol: A novel strategy for fluorescence ratiometric detection of ALP and As(v) with high sensitivity and selectivity. Chem. Commun. 2018, 54, 7487–7490. [Google Scholar]
- Muppidathi, M.; Perumal, P.; Ayyanu, R.; Subramanian, S. Immobilization of ssDNA on a metal–organic framework derived magnetic porous carbon (MPC) composite as afluorescent sensing platform for the detection of arsenate ions. Analyst 2019, 144, 3111–3118. [Google Scholar] [PubMed]
- Tan, H.; Tang, G.; Wang, Z.; Li, Q.; Gao, J.; Wu, S. Magnetic porous carbon nanocomposites derived from metal-organic frameworks as a sensing platform for DNA fluorescent detection. Anal. Chim. Acta 2016, 940, 136–142. [Google Scholar]
- Kim, J.S.; Quang, D.T. Calixarene-Derived Fluorescent Probes. Chem. Rev. 2007, 107, 3780–3799. [Google Scholar]
- Zheng, D.; Zou, R.; Lou, X. Label-Free Fluorescent Detection of Ions, Proteins, and Small Molecules Using Structure-Switching Aptamers, SYBR Gold, and Exonuclease I. Anal. Chem. 2021, 8, 3554–3560. [Google Scholar] [CrossRef]
- Zhu, K.; Fan, R.; Wu, J.; Wang, B.; Lu, H.; Zheng, X.; Sun, T.; Gai, S.; Zhou, X.; Yang, Y. MOF-on-MOF membrane with cascading functionality for capturing dichromate ions and p-Arsanilic Acid turn-on sensing. ACS Appl. Mater. Interfaces 2020, 12, 58239–58251. [Google Scholar]
- Huang, Q.S.; Wang, C.; Wei, W.; Ni, B.J. Magnetic poly(anilineco-5-sulfo-2-anisidine) as multifunctional adsorbent for highly effective co-removal of aqueous Cr(VI) and 2,4-Dichlophenol. Chem. Eng. J. 2020, 387, 124152. [Google Scholar]
- Gu, H.; Rapole, S.B.; Sharma, J.; Huang, Y.; Cao, D.; Colorado, H.A.; Luo, Z.; Haldolaarachchige, N.; Young, D.P.; Walters, B.B.; et al. Magnetic polyaniline nanocomposites toward toxichexavalent chromium removal. RSC Adv. 2012, 2, 11007–11018. [Google Scholar]
- Yang, R.; Wang, Y.; Li, M.; Hong, Y. A new carbon/ferrous sulfide/iron composite prepared by an in situ carbonization reduction method from hemp (Cannabis sativa L.) stems and its Cr(VI) removal abilitIty. ACS Sustain. Chem. Eng. 2014, 2, 1270–1279. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Li, C.; Yang, W.; Song, T.; Tang, C.; Meng, Y.; Dai, S.; Wang, H.; Chai, L.; et al. Synthesis of core–shell magnetic Fe3O4@poly(m-Phenylenediamine) particles for chromium reduction and adsorption. Environ. Sci. Technol 2015, 49, 5654–5662. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, S.R.; Afeworki, M.; Gorbaty, M.L.; Kwiatek, P.J.; Solum, M.S.; Hu, J.Z.; Pugmire, R.J. XPS and15N NMR study of nitrogen forms in carbonaceous solids. Energy Fuels 2002, 16, 1507–1515. [Google Scholar] [CrossRef]
- Raizada, M.; Sama, F.; Ashafaq, M.; Shahid, M.; Khalid, M.; Ahmad, M.; Siddiqi, Z.A. Synthesis, structure and magnetic studies of lanthanide metal–organic frameworks (Ln–MOFs): Aqueous phase highly selective sensors for picric acid as well as the arsenic ion. Polyhedron 2018, 139, 131–141. [Google Scholar] [CrossRef]
- Caracelli, I.; Schpector, J.Z.; Haiduc, I.; Tiekink, E.R.T. Main group metal lone-pair⋯π(arene) interactions: A new bonding mode for supramolecular associations. CrystEngComm 2016, 18, 6960–6978. [Google Scholar] [CrossRef]
- Zukerman-Schpector, J.; Otero-de-la-Roza, A.; Luaña, V.; Tiekink, E.R.T. Supramolecular architectures based on As(lone pair)⋯π(aryl) interactions. Chem. Commun 2011, 47, 7608–7610. [Google Scholar] [CrossRef]
- Lv, J.; Wang, B.; Xie, Y.; Wang, P.; Shu, L.; Su, X.; Li, J.-R. Selective detection of two representative organic arsenic compounds in aqueous medium with metal–organic frameworks. Environ. Sci. Nano 2019, 6, 2759–2766. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Alshammari, K.F.; Subaihi, A.; Alharbi, A.; Khalil, M.A.; Shahat, A. Efficient dual sensor based on modified NH2-UiO-66(Zr) MOF for sensitive and rapid monitoring of ultra-trace arsenic (III) in aqueous media. J. Mol. Liq. 2023, 389, 122787. [Google Scholar] [CrossRef]
- Liu, G.; Li, B.; Shao, J. Ratiometric fluorescence anion sensor based on inhibition of excited-state intramolecular proton transfer (ESIPT). J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 97–102. [Google Scholar] [CrossRef]
- Roy, S.B.; Maity, A.; Rajak, K.K. A turn-off fluorescence sensor for cyanide detection which in turn inhibit 2-way ESIPT investigated by experimental and theoretical study. Inorg. Chem. Commun. 2017, 76, 81–86. [Google Scholar] [CrossRef]
- Hassani, F.; Larki, A.; Ghomi, M.; Pourreza, N. Gold nanoparticles embedded Fe-BTC (AuNPs@Fe-BTC) metal-organic framework as a fluorescence sensor for the selective detection of As(III) in contaminated waters. Spectrochim. Acta Part A 2023, 302, 123104. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Han, Z.; Min, H.; Chen, Z.; Sun, T.; Wang, L.; Shi, W.; Cheng, P. Bilanthanide Metal-Organic Frameworks for Instant Detection of 17β-Estradiol, a Vital hysiological Index. Small Struct. 2022, 3, 2100113. [Google Scholar]
- Zhao, Y.; Zeng, H.; Zhu, X.-W.; Lu, W.; Li, D. Metal–organic frameworks as photoluminescent biosensing platforms: Mechanisms and applications. Chem. Soc. Rev 2021, 50, 4484–4513. [Google Scholar] [PubMed]
- Dam, G.K.; Fajal, S.; Dutta, S.; Let, S.; Desai, A.V.; Ghosh, S.K. Hydrolytically stable luminescent cationic MOF for selective detection of toxic organic arsenic in water. ACS Appl. Opt. Mater. 2023, 1, 1217–1226. [Google Scholar]
- Salinas, Y.; Martinez-Manez, R.; Marcos, M.D.; Sancenon, F.; Costero, A.M.; Parra, M.; Gil, S. Optical chemo sensors and reagents to detect explosives. Chem. Soc. Rev. 2021, 41, 1261–1296. [Google Scholar]
- Zhao, D.; Swager, T.M. Sensory responses in solution vs solid state: A fluorescence quenching study of poly (iptycenebutadiynylene)s. Macromolecules 2005, 38, 9377–9384. [Google Scholar]
- Chen, S.; Yu, Y.L.; Wang, J.H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar]
- Shukla, S.; Singh, S.; Mitra, M.D. Photosensitizer Modulated Turn–off Fluorescence System and Molecula rLogic Functions for Selective Detection of Arsenic(III). Chemistry Select 2020, 5, 13609–13618. [Google Scholar]
- Wang, J.-X.; Yin, J.; Shekhah, O.; Bakr, O.M.; Eddaoudi, M.; Mohammed, O.F. Energy Transfer in Metal–Organic Frameworks for Fluorescence Sensing. ACS Appl. Mater. Interfaces 2022, 14, 9970–9986. [Google Scholar]
- Haldar, R.; Bhattacharyya, S.; Maji, T.K. Luminescent metal–organic frameworks and their potential applications. J. Chem. Sci. 2020, 132, 99. [Google Scholar] [CrossRef]
- El-Bindary, M.A.; Shahat, A.; El-Deen, I.M.; El-Afify, M.A.M.; Hassan, N. Synthesis of novel fluorescent sensor based on a modified amino Al-MOF for rapid, sensitive, and selective detection of arsenic in aqueous solution. Appl. Organomet Chem. 2023, 37, e7102. [Google Scholar] [CrossRef]
- Bag, R.; Sikdar, Y.; Sahu, S.; Islam, M.M.; Mandal, S.; Goswami, S. Experimental and Theoretical Exploration of ESIPT in a Systematically Constructed Series of Benzimidazole Based Schiff Base Probes: Application as Chemosensors. Chem. Eur. J. 2023, 29, e2022. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Sahana, A.; Sarkar, B.; Mukhopadhyay, S.K.; Das, D. Pyridine Based Fluorescence Probe: Simultaneous Detection and Removal of Arsenate from Real Samples with Living Cell Imaging Properties. J. Fluoresc. 2015, 25, 1191–1201. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).