Green Synthesis, Characterization, and Optimization of Chitosan Nanoparticles Using Blumea balsamifera Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of B. balsamifera Extract
2.2. Total Phenolic Content Assay of B. balsamifera Extract
2.3. Preparation of Chitosan Nanoparticles
2.4. Characterization of Optimized CNPs
2.5. Optimization of the Green Synthesized Chitosan Nanoparticles
2.6. Statistical Analysis
2.7. Antibacterial Activity Against E. coli
3. Results
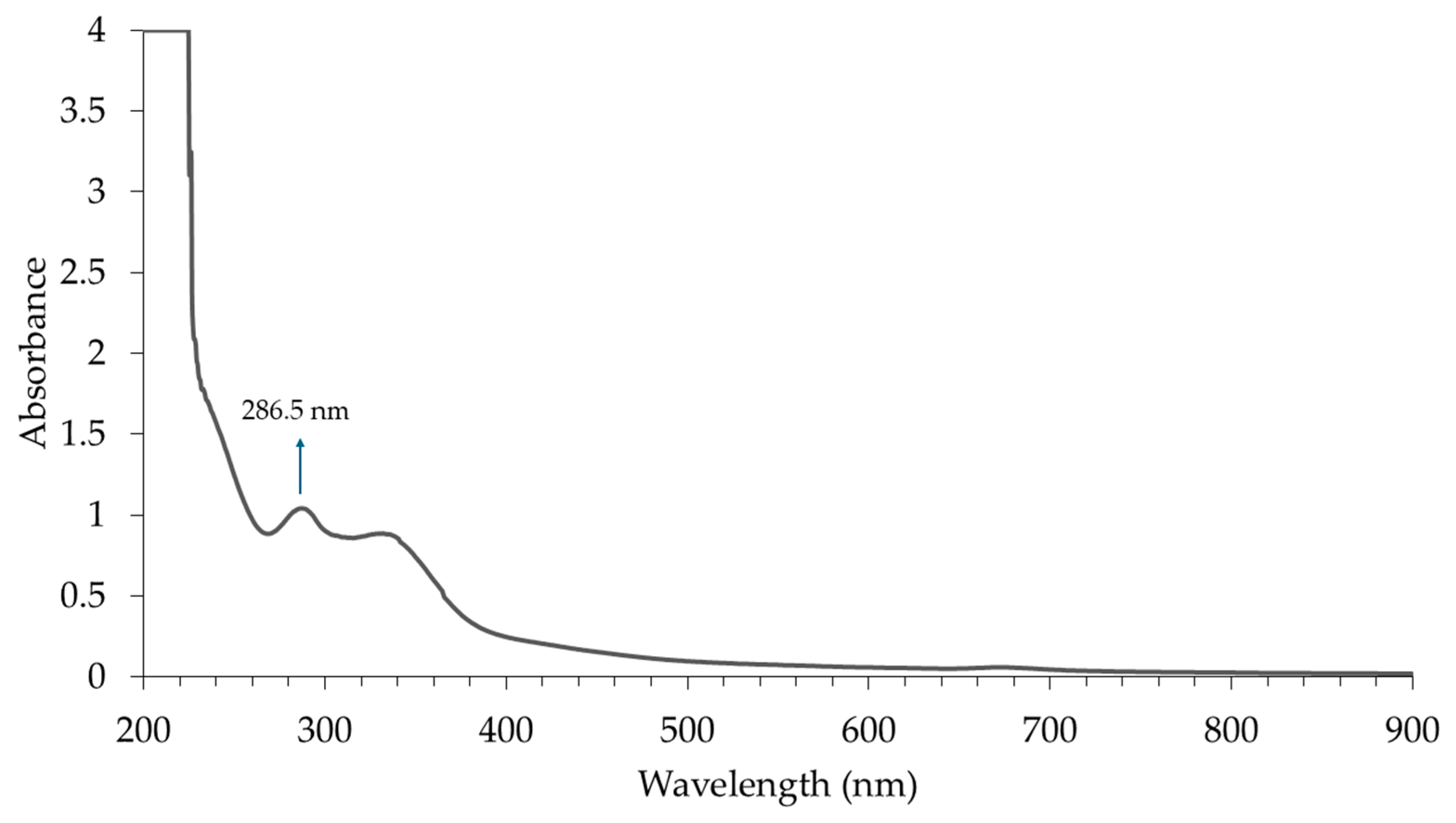
3.1. B. balsamifera Extract Preparation
3.2. Green Synthesis of Chitosan Nanoparticles (CNPs)
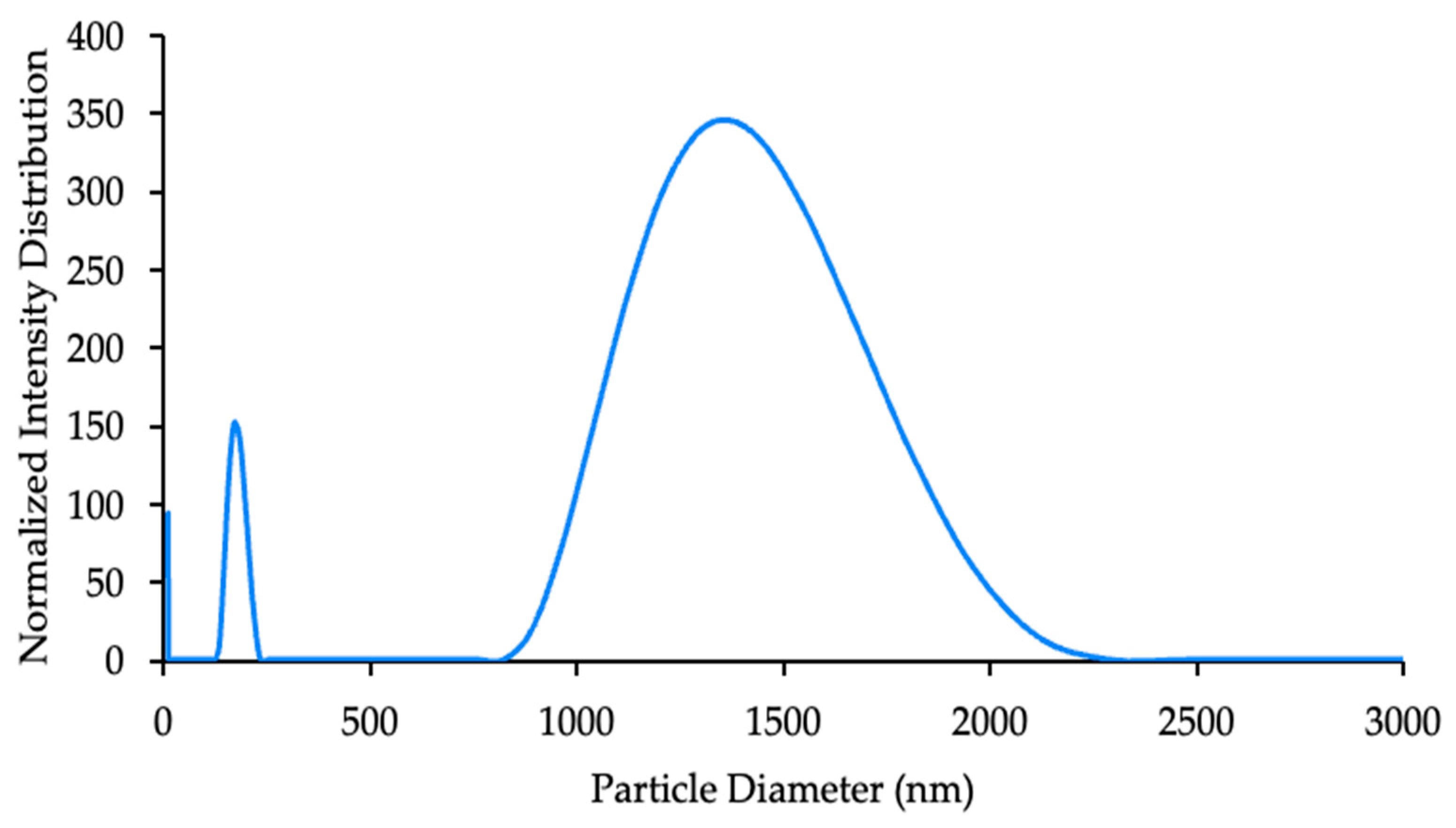
3.3. Characterization of CNPs
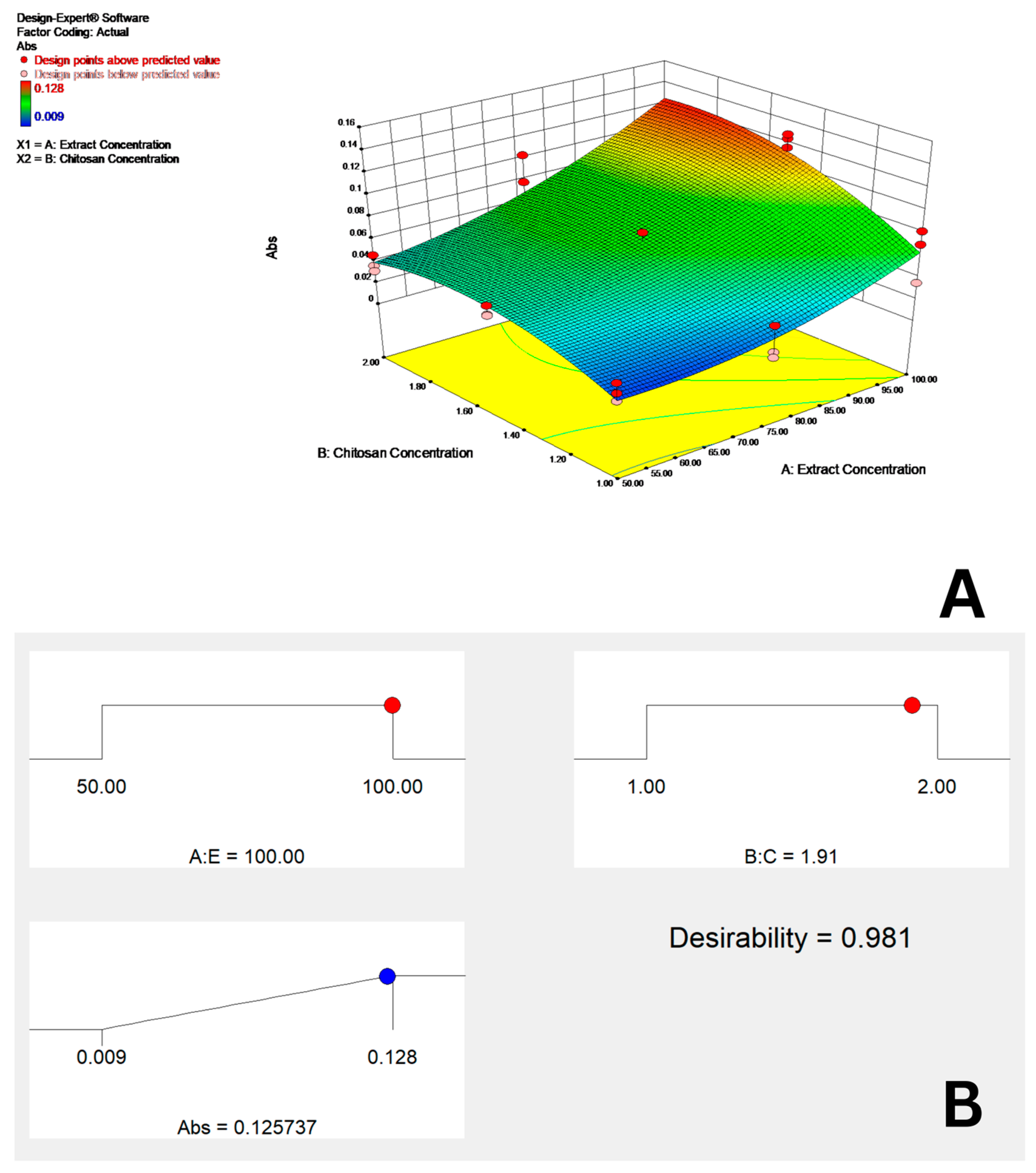
3.4. Optimization of CNPs Synthesis
3.4.1. Response Surface Approach for Optimization of CNP Synthesis
3.4.2. Multiple Regression Analysis
3.4.3. Impact of Variable Levels (Analysis of Variance)
3.4.4. Model Validation
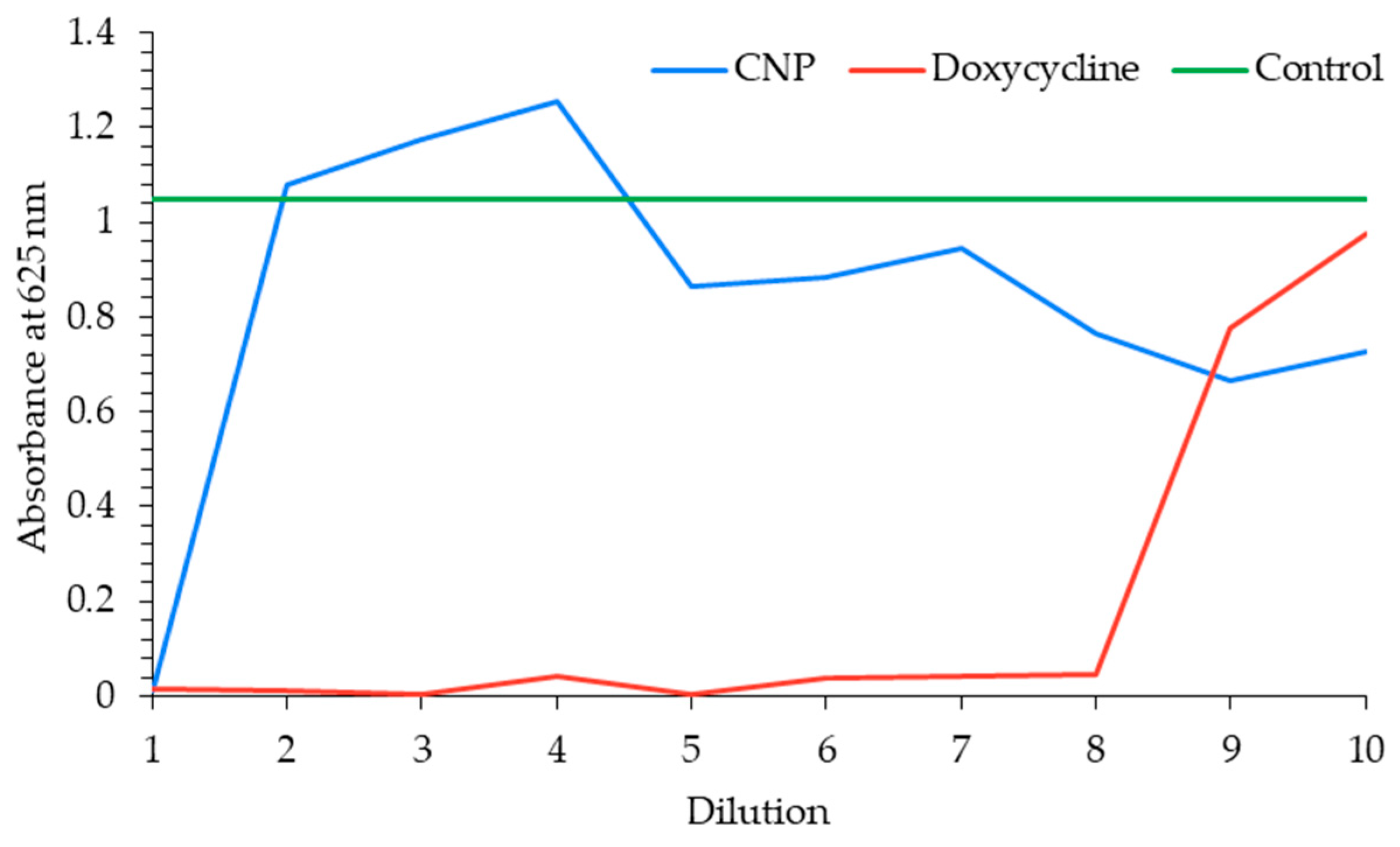
3.5. Antibacterial Activity of B. balsamifera CNPs Against E. coli
4. Discussion
4.1. B. balsamifera Extract
4.2. Green Synthesis of Chitosan Nanoparticles (CNPs)
4.3. Characterization of CNPs
4.4. Optimization of CNPs Synthesis
4.4.1. Multiple Regression Analysis
4.4.2. Impact of Variable Levels (Analysis of Variance)
4.4.3. Model Validation
4.5. Antibacterial Activity of B. balsamifera -CNPs Against E. coli
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TPC | Total Phenolic Content |
| SEM | Scanning Electron Microscopy |
| UV-vis | Ultraviolet-visible Spectroscopy |
| CNP | Chitosan Nanoparticles |
| DLS | Dynamic Light Scattering |
| FCCCD | Face-Centered Central Composite Design |
| MIC | Minimum Inhibitory Concentration |
| SRM | Surface Response Methodology |
References
- Jha, R.; Mayanovic, R.A. A Review of the Preparation, Characterization, and Applications of Chitosan Nanoparticles in Nanomedicine. Nanomaterials 2023, 13, 1302. [Google Scholar] [CrossRef]
- Rahman, A.; Kafi, A.; Beak, G.; Saha, S.K.; Roy, K.J.; Habib, A.; Faruqe, T.; Siddique, M.P.; Islam, S.; Hossain, K.S.; et al. Green Synthesized Chitosan Nanoparticles for Controlling Multidrug-Resistant mecA- and blaZ-Positive Staphylococcus aureus and aadA1-Positive Escherichia coli. Int. J. Mol. Sci. 2024, 25, 4746. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Gonçalves, I.C.; Henriques, P.C.; Seabra, C.L.; Martins, M.C.L. The potential utility of chitosan micro/nanoparticles in the treatment of gastric infection. Expert Rev. Anti-infective Ther. 2014, 12, 981–992. [Google Scholar] [CrossRef]
- Widhiantara, I.G.; Jawi, I.M. Phytochemical composition and health properties of Sembung plant (Blumea balsamifera): A review. Veter- World 2021, 14, 1185–1196. [Google Scholar] [CrossRef]
- Ma, C.; Liu, B.; Du, L.; Liu, W.; Zhu, Y.; Chen, T.; Wang, Z.; Chen, H.; Pang, Y. Green Preparation and Antibacterial Activity Evaluation of AgNPs-Blumea balsamifera Oil Nanoemulsion. Molecules 2024, 29, 2009. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Zheng, W.; Han, L.; Huang, J.; He, Z.; Kang, J. Nanoemulsion of the Essential Oil from Blumea balsamifera (L.) DC. and Its Effect on Trauma Repair. J. Oleo Sci. 2023, 72, 869–879. [Google Scholar] [CrossRef]
- Ginting, B.; Maulana, I.; Karnila, I. Biosynthesis Copper Nanoparticles using Blumea balsamifera Leaf Extracts: Characterization of its Antioxidant and Cytotoxicity Activities. Surfaces Interfaces 2020, 21, 100799. [Google Scholar] [CrossRef]
- Aryasa, I.W.T.; Artini, N.P.R. Green Synthesis Silver Nanoparticles Using Sembung (Blumea balsamifera) Leaf Extract as an Antibacterial and Antioxidant. J. Penelit. Pendidik. IPA 2023, 9, 11877–11886. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Ali, M.R.; Smuda, S.S.; Abedelmaksoud, T.G. Utilization of sugarcane bagasse aqueous extract as a natural preservative to extend the shelf life of refrigerated fresh meat. Braz. J. Food Technol. 2021, 24, e2020167. [Google Scholar] [CrossRef]
- Burapan, S.; Kim, M.; Paisooksantivatana, Y.; Eser, B.E.; Han, J. Thai Curcuma Species: Antioxidant and Bioactive Compounds. Foods 2020, 9, 1219. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; Shiha, A.M.; Mahrous, H.; Mohammed, A.B.A. Green synthesis of chitosan nanoparticles, optimization, characterization and antibacterial efficacy against multi drug resistant biofilm-forming Acinetobacter baumannii. Sci. Rep. 2022, 12, 1–19. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Saber, W.I.A.; Zweil, A.M.; Bashir, S.I. An innovative green synthesis approach of chitosan nanoparticles and their inhibitory activity against phytopathogenic Botrytis cinerea on strawberry leaves. Sci. Rep. 2022, 12, 3515. [Google Scholar] [CrossRef] [PubMed]
- Jirakitticharoen, S.; Wisuitiprot, W.; Jitareerat, P.; Wongs-Aree, C. Phenolics, Antioxidant and Antibacterial Activities of Immature and Mature Blumea balsamifera Leaf Extracts Eluted with Different Solvents. J. Trop. Med. 2022, 2022, 7794227. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Prabaharan, M. Review Paper: Chitosan Derivatives as Promising Materials for Controlled Drug Delivery. J. Biomater. Appl. 2008, 23, 5–36. [Google Scholar] [CrossRef]
- Popa, M.-I.; Aelenei, N.; Popa, V.I.; Andrei, D. Study of the interactions between polyphenolic compounds and chitosan. React. Funct. Polym. 2000, 45, 35–43. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, S.-H.; Eun, C.-J.; Yoo, J.; Seo, Y.-S. Dispersion of chitosan nanoparticles stable over a wide pH range by adsorption of polyglycerol monostearate. Nanomater. Nanotechnol. 2020, 10, 184798042091726. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef]
- Luo, D.; Yan, C.; Wang, T. Interparticle Forces Underlying Nanoparticle Self-Assemblies. Small 2015, 11, 5984–6008. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Eltarahony, M.; Hafez, E.E.; Bashir, S.I. Green fabrication of chitosan nanoparticles using Lavendula angustifolia, optimization, characterization and in-vitro antibiofilm activity. Sci. Rep. 2023, 13, 11127. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, N.; Dhayalan, S.; Shaik, M.R.; Shaik, A.H.; Shaik, J.P.; Shaik, B. Green Synthesis of Chitosan Nanoparticles Using of Martynia annua L. Ethanol Leaf Extract and Their Antibacterial Activity. Crystals 2022, 12, 1550. [Google Scholar] [CrossRef]
- El-Shafei, H.A.; Asaad, G.F.; Elkhateeb, Y.A.M.; El-Dakroury, W.A.; Hamed, H.A.; Hassan, A.; Nomier, Y.A. Antimicrobial and Hepatoprotective Effect of Chitosan Nanoparticles: In-vitro and In-vivo Study. J. Pharm. Res. Int. 2021, 33, 244–264. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Dalal, S.R.; Zweil, A.M.; Eltarahony, M. Artificial intelligence-based optimization for chitosan nanoparticles biosynthesis, characterization and in-vitro assessment of its anti-biofilm potentiality. Sci. Rep. 2023, 13, 4401. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; Shiha, A.M.; Mahrous, H.; Mohammed, A.B.A. A sustainable green-approach for biofabrication of chitosan nanoparticles, optimization, characterization, its antifungal activity against phytopathogenic Fusarium culmorum and antitumor activity. Sci. Rep. 2024, 14, 11336. [Google Scholar] [CrossRef]
- Rasonabe, Z.M.P.; Cruz, J.D.; Tiausas, C.G.; Areza, F. Chemical composition and antimicrobial activity of the extracts and essential oil of Blumea balsamifera from the Philippines. Int. J. Herb. Med. 2023, 11, 6–14. [Google Scholar] [CrossRef]



| Run | Extract Concentration | Chitosan Concentration | Experimental | Predicted | Residual |
|---|---|---|---|---|---|
| 7 | 50 | 1 | 0.017 | 0.018 | −0.001 |
| 15 | 50 | 1 | 0.024 | 0.018 | 0.006 |
| 24 | 50 | 1 | 0.033 | 0.018 | 0.015 |
| 19 | 100 | 1 | 0.082 | 0.063 | 0.019 |
| 26 | 100 | 1 | 0.07 | 0.063 | 0.007 |
| 8 | 100 | 1 | 0.035 | 0.063 | −0.028 |
| 10 | 50 | 2 | 0.036 | 0.041 | −0.005 |
| 5 | 50 | 2 | 0.031 | 0.041 | −0.010 |
| 21 | 50 | 2 | 0.046 | 0.041 | 0.005 |
| 3 | 100 | 2 | 0.116 | 0.127 | −0.011 |
| 23 | 100 | 2 | 0.119 | 0.127 | −0.008 |
| 13 | 100 | 2 | 0.113 | 0.127 | −0.014 |
| 27 | 50 | 1.5 | 0.048 | 0.048 | 0.000 |
| 12 | 50 | 1.5 | 0.04 | 0.048 | −0.008 |
| 2 | 50 | 1.5 | 0.039 | 0.048 | −0.009 |
| 17 | 100 | 1.5 | 0.124 | 0.114 | 0.010 |
| 9 | 100 | 1.5 | 0.116 | 0.114 | 0.002 |
| 14 | 100 | 1.5 | 0.128 | 0.114 | 0.014 |
| 20 | 75 | 1 | 0.014 | 0.027 | −0.013 |
| 25 | 75 | 1 | 0.009 | 0.027 | −0.018 |
| 6 | 75 | 1 | 0.038 | 0.027 | 0.011 |
| 18 | 75 | 2 | 0.103 | 0.070 | 0.033 |
| 1 | 75 | 2 | 0.057 | 0.070 | −0.013 |
| 16 | 75 | 2 | 0.078 | 0.070 | 0.008 |
| 22 | 75 | 1.5 | 0.048 | 0.067 | −0.019 |
| 4 | 75 | 1.5 | 0.063 | 0.067 | −0.004 |
| 11 | 75 | 1.5 | 0.074 | 0.067 | 0.007 |
| Sum of Source | Mean Squares | F df | p-Value Square | Value | Prob > F | |
|---|---|---|---|---|---|---|
| Mean vs. Total | 0.11 | 1 | 0.11 | |||
| Linear vs. Mean | 0.027 | 2 | 0.014 | 35.09 | * <0.0001 | |
| 2FI vs. Linear | 1.24 × 10−3 | 1 | 1.24 × 10−3 | 3.54 | 0.0725 | |
| Quadratic vs. 2FI | 3.28 × 10−3 | 2 | 1.64 × 10−3 | 7.21 | * 0.0041 | Suggested |
| Cubic vs. Quadratic | 1.16 × 10−3 | 2 | 5.79 × 10−4 | 3.04 | 0.0715 | Aliased |
| Residual | 3.62 × 10−3 | 19 | 1.90 × 10−4 | |||
| Total | 0.14 | 27 | 5.32 × 10−3 |
| Sum of Source | Mean Squares | F df | p-Value Square | Value | Prob f | |
|---|---|---|---|---|---|---|
| Linear | 5.82 × 10−3 | 6 | 9.70 × 10−4 | 5.03 | 0.0034 | |
| 2FI | 4.58 × 10−3 | 5 | 9.16 × 10−4 | 4.75 | 0.0061 | |
| Quadratic | 1.31 × 10−3 | 3 | 4.35 × 10−4 | 2.26 | * 0.1167 | Suggested |
| Pure Error | 3.47 × 10−3 | 18 | 1.93 × 10−4 |
| Std. | Adjusted | Predicted | ||||
|---|---|---|---|---|---|---|
| Source | Dev. | R-Squared | R-Squared | R-Squared | PRESS | |
| Linear | 0.02 | 0.7452 | 0.724 | 0.6775 | 0.012 | |
| 2FI | 0.019 | 0.7792 | 0.7504 | 0.7076 | 0.011 | |
| Quadratic | 0.015 | * 0.8691 | * 0.8379 | * 0.7842 | a 7.87 × 10−3 | Suggested |
| Source of Variance | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Coefficient Factor |
|---|---|---|---|---|---|---|
| Model | 0.032 | 5 | 6.34 × 10−3 | 27.88 | * <0.0001 | 0.066 |
| E-Extract Concentration | 0.019 | 1 | 0.019 | 84.78 | * <0.0001 | 0.033 |
| C-Chitosan Concentration | 7.90 × 10−3 | 1 | 7.90 × 10−3 | 34.73 | * <0.0001 | 0.021 |
| E * C | 1.24 × 10−3 | 1 | 1.24 × 10−3 | 5.46 | * 0.0295 | 0.01 |
| E2 | 1.15 × 10−3 | 1 | 1.15 × 10−3 | 5.05 | * 0.0355 | 0.014 |
| C2 | 2.13 × 10−3 | 1 | 2.13 × 10−3 | 9.36 | * 0.006 | −0.019 |
| Residual | 4.77 × 10−3 | 21 | 2.27 × 10−4 | |||
| Lack of Fit | 1.31 × 10−3 | 3 | 4.35 × 10−4 | 2.26 | 0.1167 | |
| Pure Error | 3.47 × 10−3 | 18 | 1.93 × 10−4 | |||
| CorTotal | 0.036 | 26 | ||||
| Source of Variance | Sum of Squares | df | Mean Square | F Value | p-value Prob > F | Coefficient Factor |
| Model | 0.032 | 5 | 6.34 × 10−3 | 27.88 | * <0.0001 | 0.066 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarta, J.D.A.; Paylago, F.J.C.; Poldo, J.C.H.; Santos, J.S.R.; Escordial, T.A.M.M.; Montealegre, C.M. Green Synthesis, Characterization, and Optimization of Chitosan Nanoparticles Using Blumea balsamifera Extract. Processes 2025, 13, 804. https://doi.org/10.3390/pr13030804
Villarta JDA, Paylago FJC, Poldo JCH, Santos JSR, Escordial TAMM, Montealegre CM. Green Synthesis, Characterization, and Optimization of Chitosan Nanoparticles Using Blumea balsamifera Extract. Processes. 2025; 13(3):804. https://doi.org/10.3390/pr13030804
Chicago/Turabian StyleVillarta, Johann Dominic A., Fernan Joseph C. Paylago, Janne Camille H. Poldo, Jalen Stephen R. Santos, Tricia Anne Marie M. Escordial, and Charlimagne M. Montealegre. 2025. "Green Synthesis, Characterization, and Optimization of Chitosan Nanoparticles Using Blumea balsamifera Extract" Processes 13, no. 3: 804. https://doi.org/10.3390/pr13030804
APA StyleVillarta, J. D. A., Paylago, F. J. C., Poldo, J. C. H., Santos, J. S. R., Escordial, T. A. M. M., & Montealegre, C. M. (2025). Green Synthesis, Characterization, and Optimization of Chitosan Nanoparticles Using Blumea balsamifera Extract. Processes, 13(3), 804. https://doi.org/10.3390/pr13030804








