Evaporation of Non-Isothermal Wall Microlayer Based on the Lattice Boltzmann Method
Abstract
1. Introduction
2. Numerical Model
2.1. Single-Component Multiphase Pseudopotential Lattice Boltzmann Method
2.2. Energy Transfer and Vapor–Liquid Phase Change
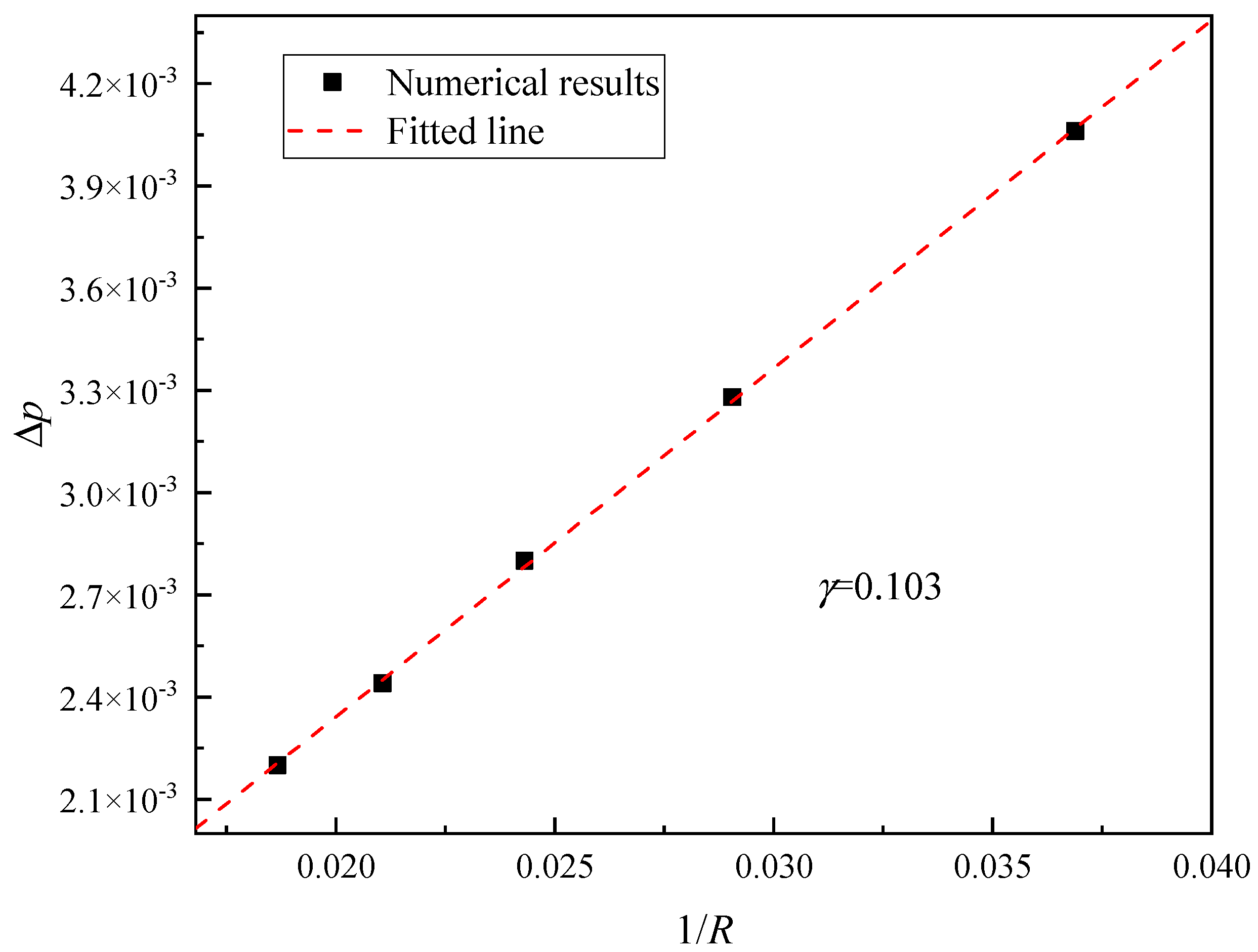
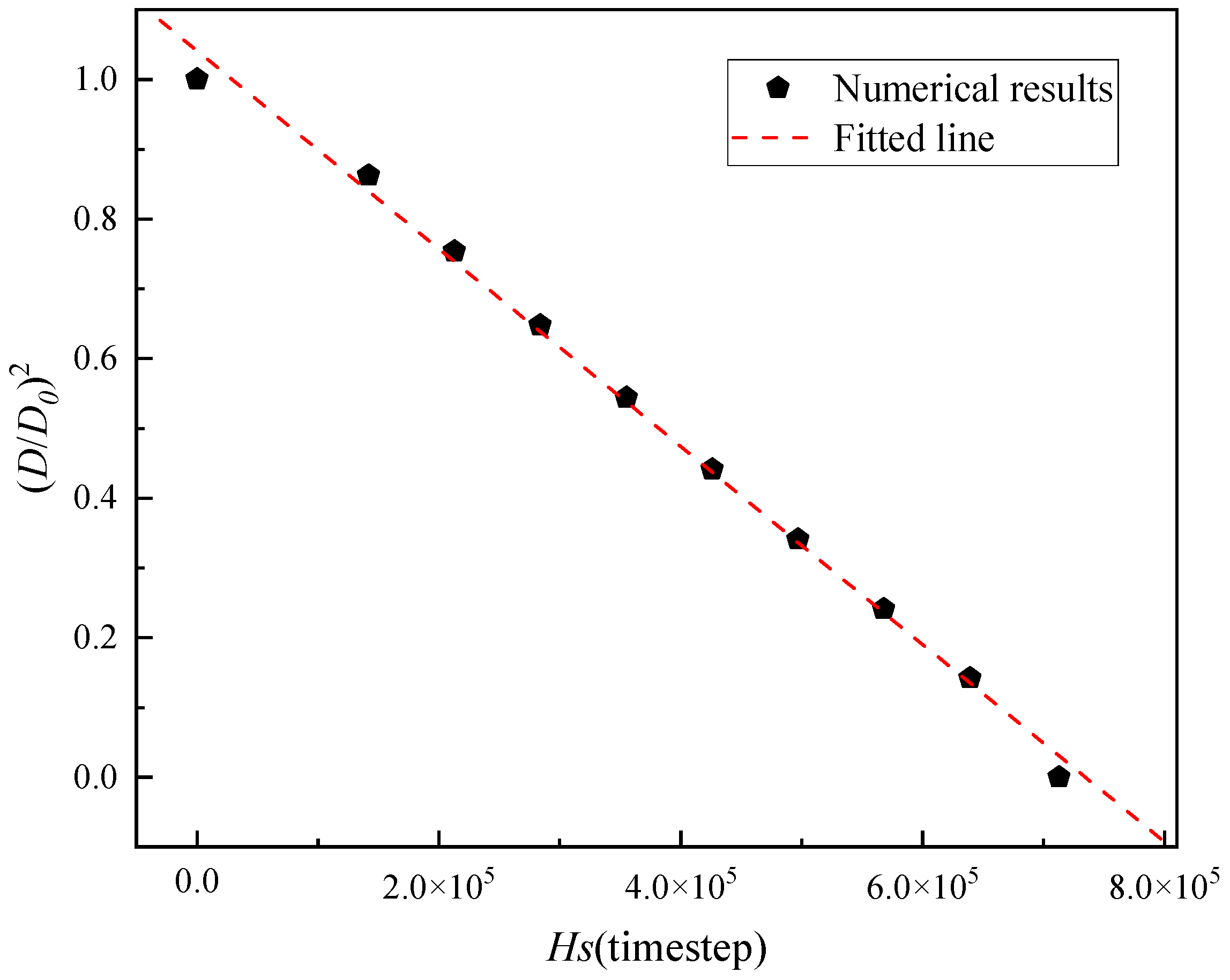
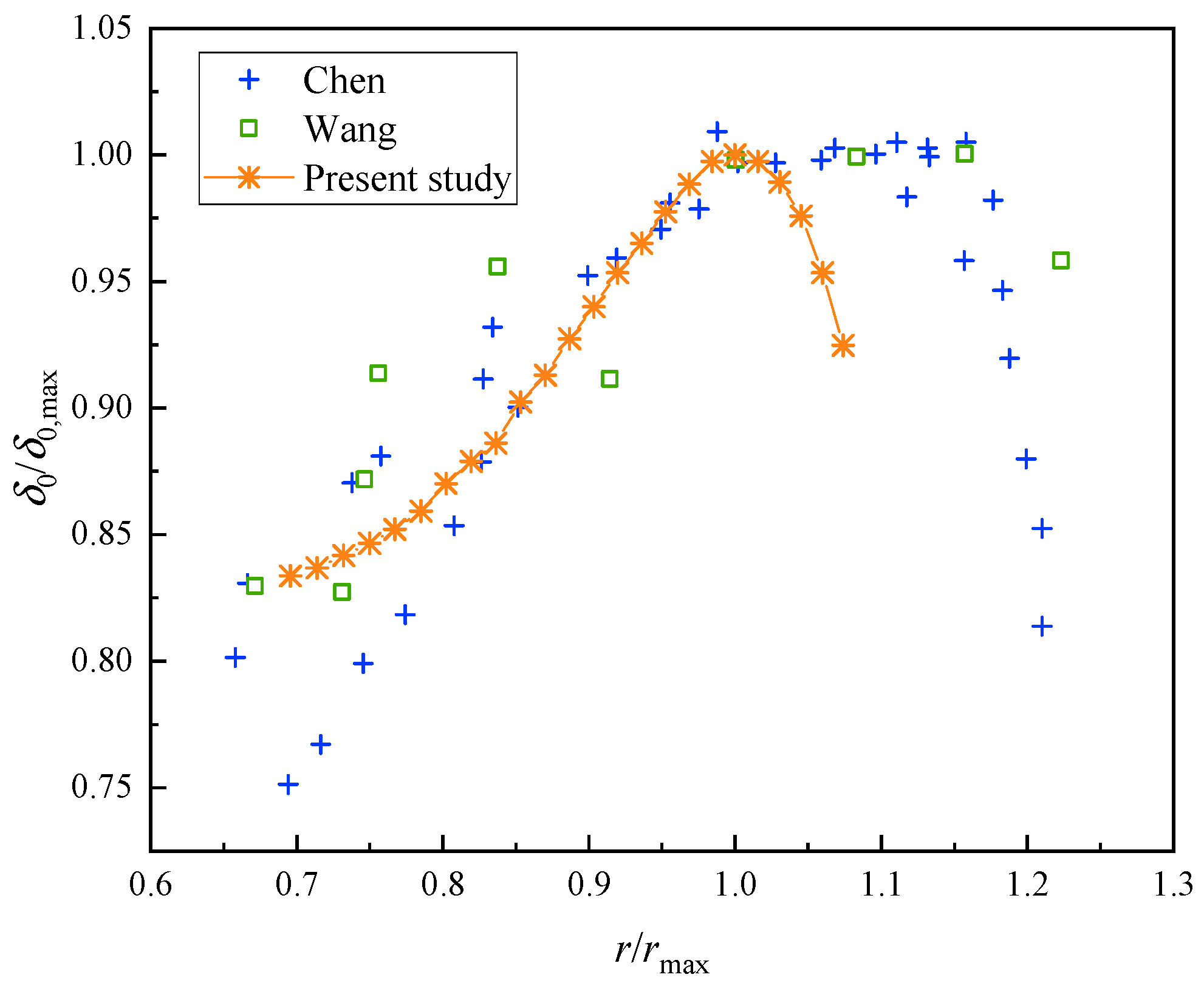
2.3. Model Validation
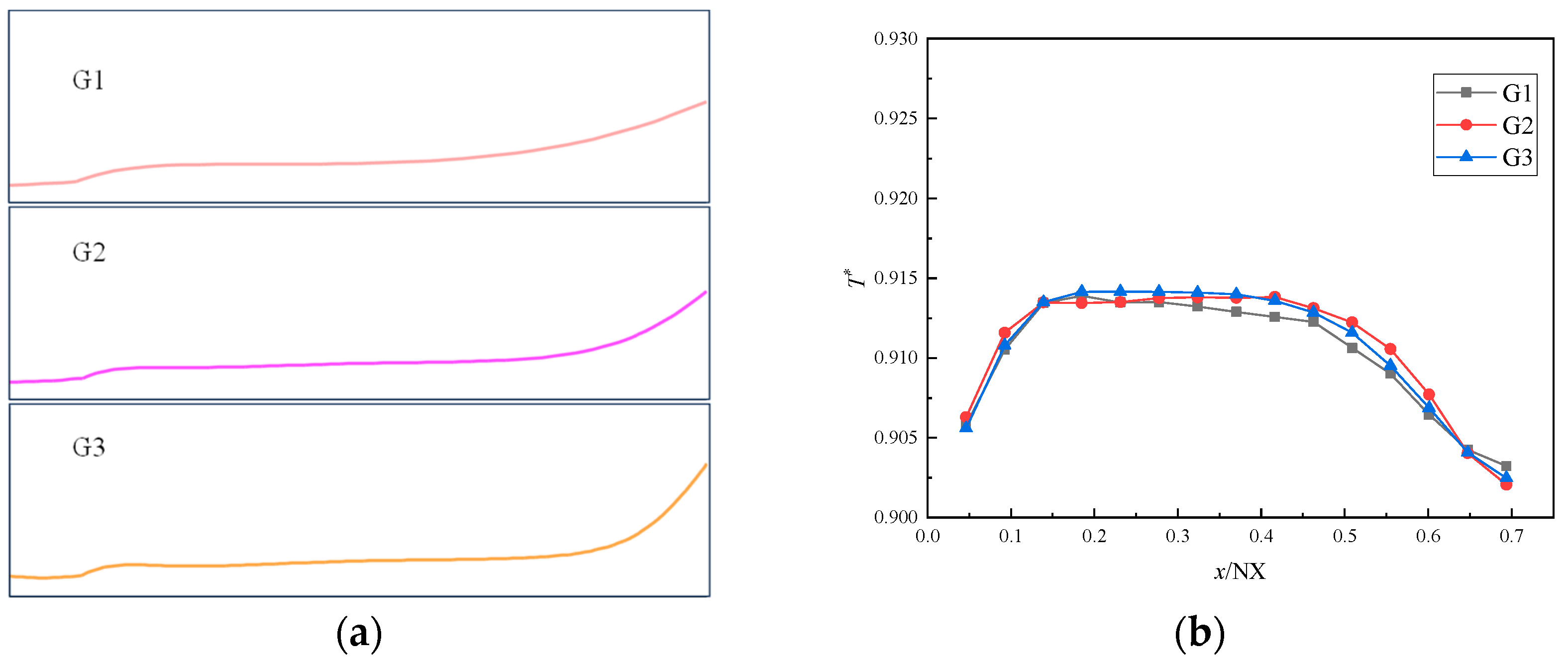
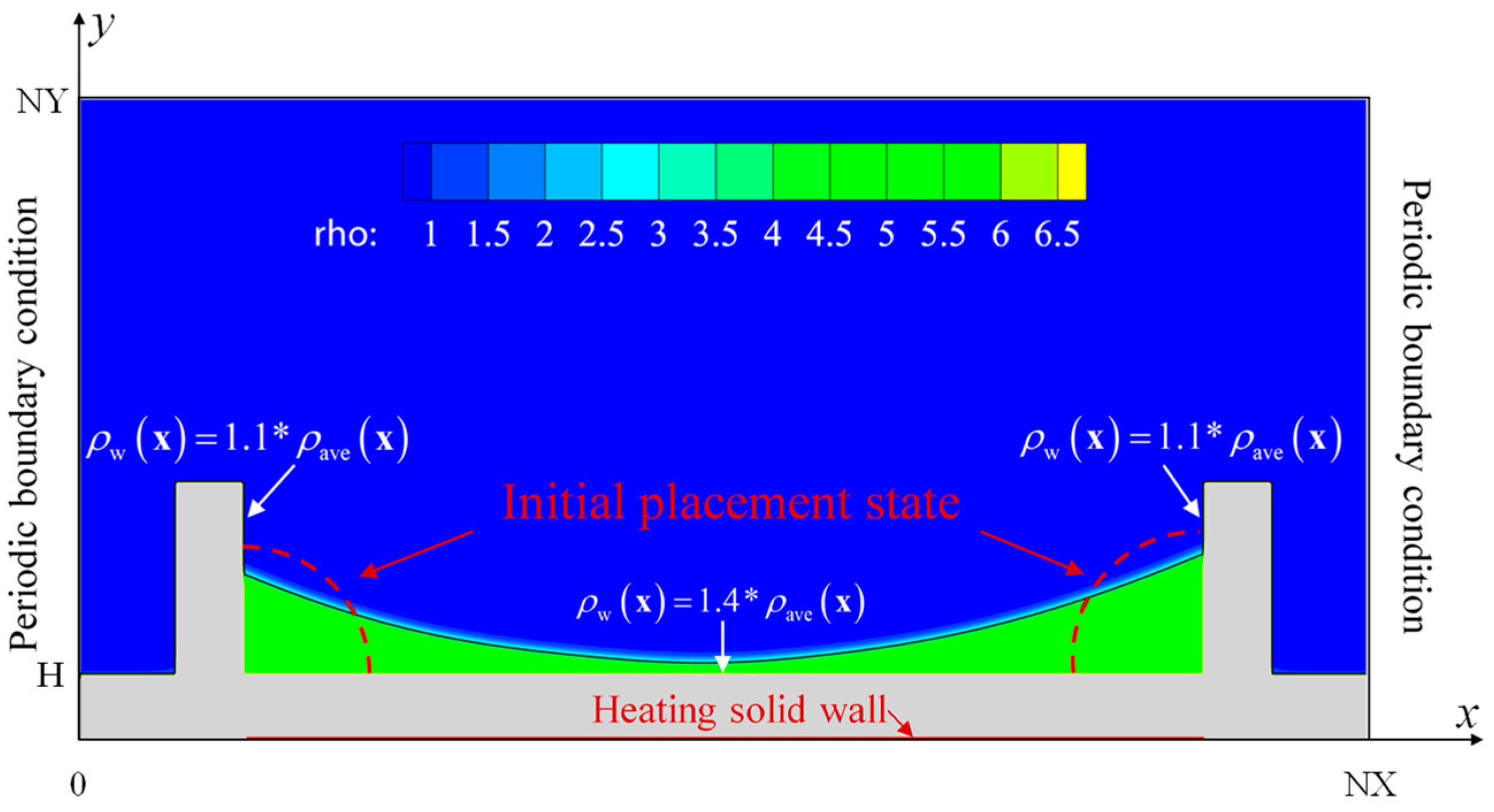
2.4. Computational Domains and Models
3. Results and Discussion
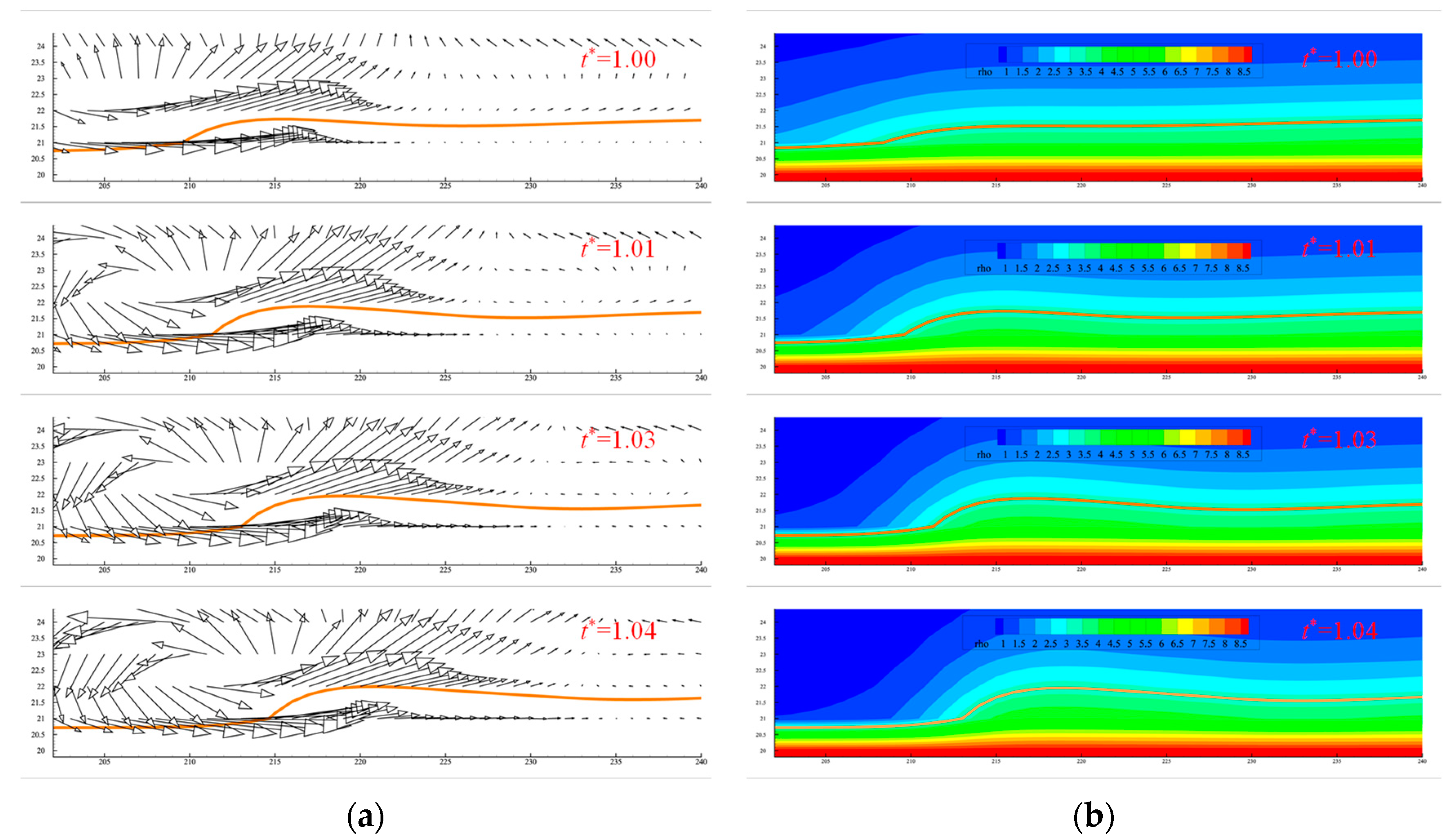
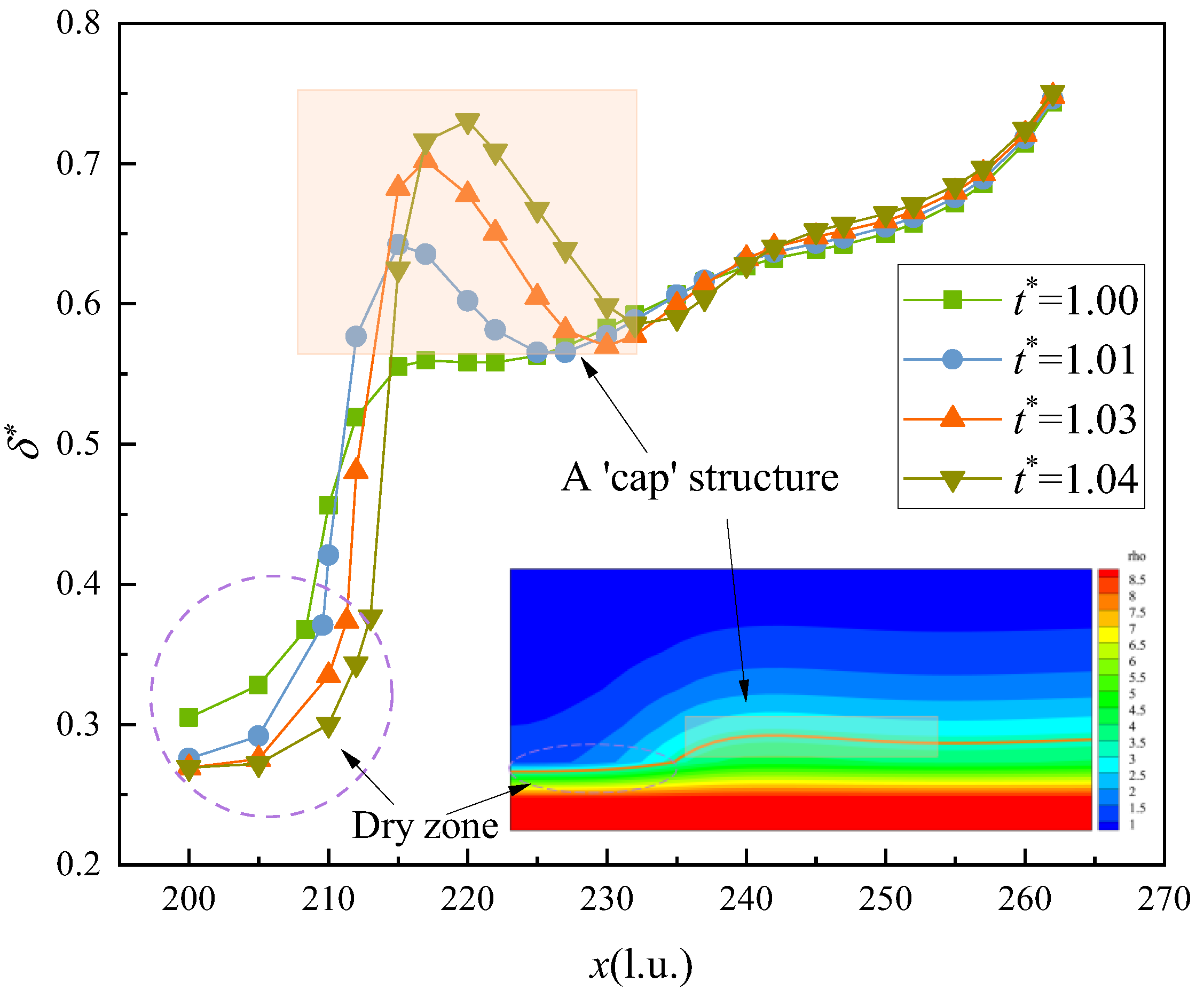
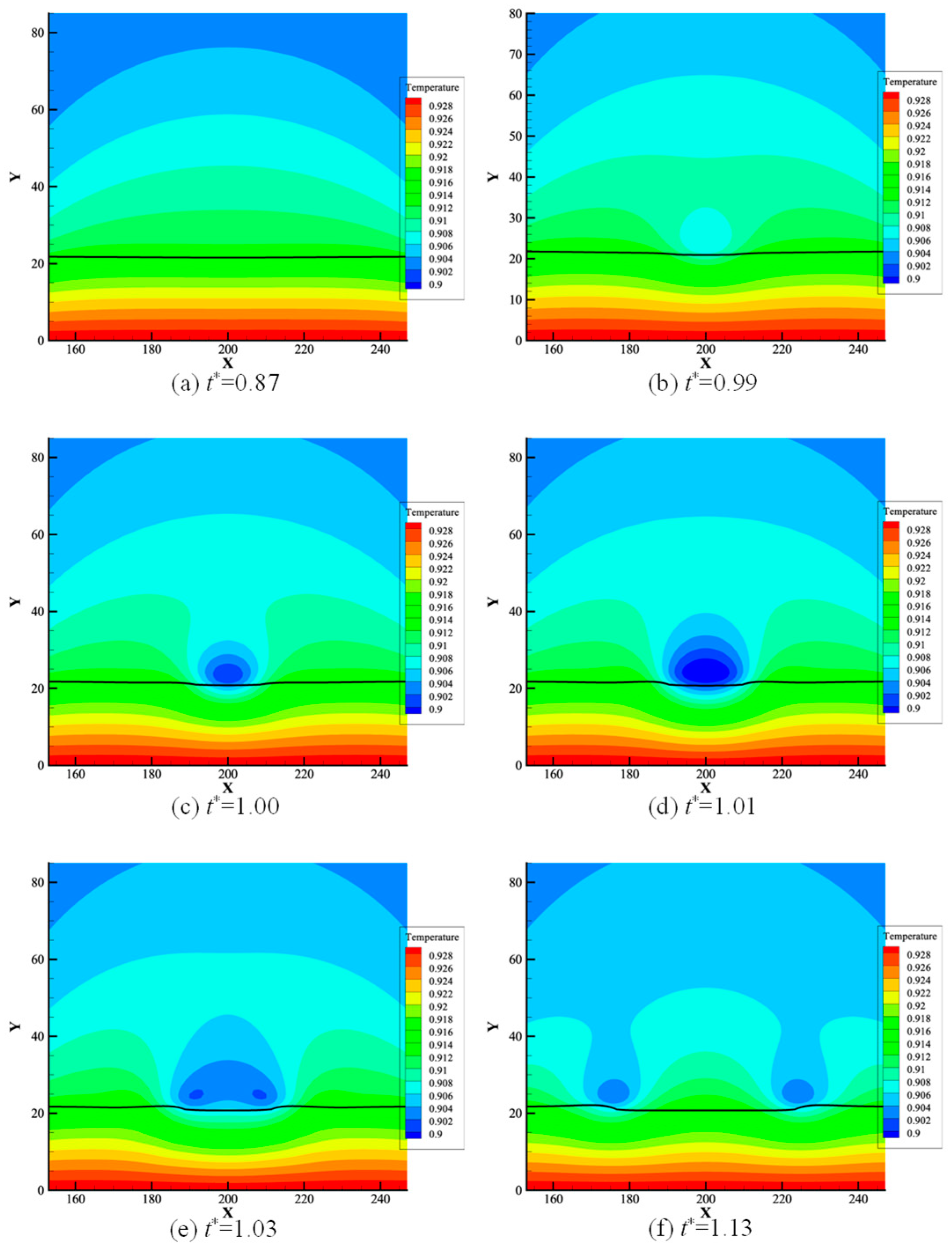
3.1. Microlayer Dynamic Characteristics
3.2. Heat Flux and Temperature
3.3. Effects of Heating Conditions
4. Conclusions
- (1)
- During the evaporation process, the microlayer displays a contraction behavior due to the differences in flow rate in different areas, especially at the three-phase contact point, where the difference in flow rate is larger than that in other places, leading to the accumulation of microlayer heads and the formation of a “cap-like” structure. The growth of the dry zone shows a linear pattern at the beginning; then, with the increase in the microlayer’s thickness, the growth of the dry spot slows down, and the change starts to become flat. Due to the effect of the density of the near-wall surface, there is also a certain thickness of the dry zone. The initial microlayer thickness was compared with previous studies, showing a consistent trend. However, some differences exist due to the inherent disparities between experimental and numerical simulations.
- (2)
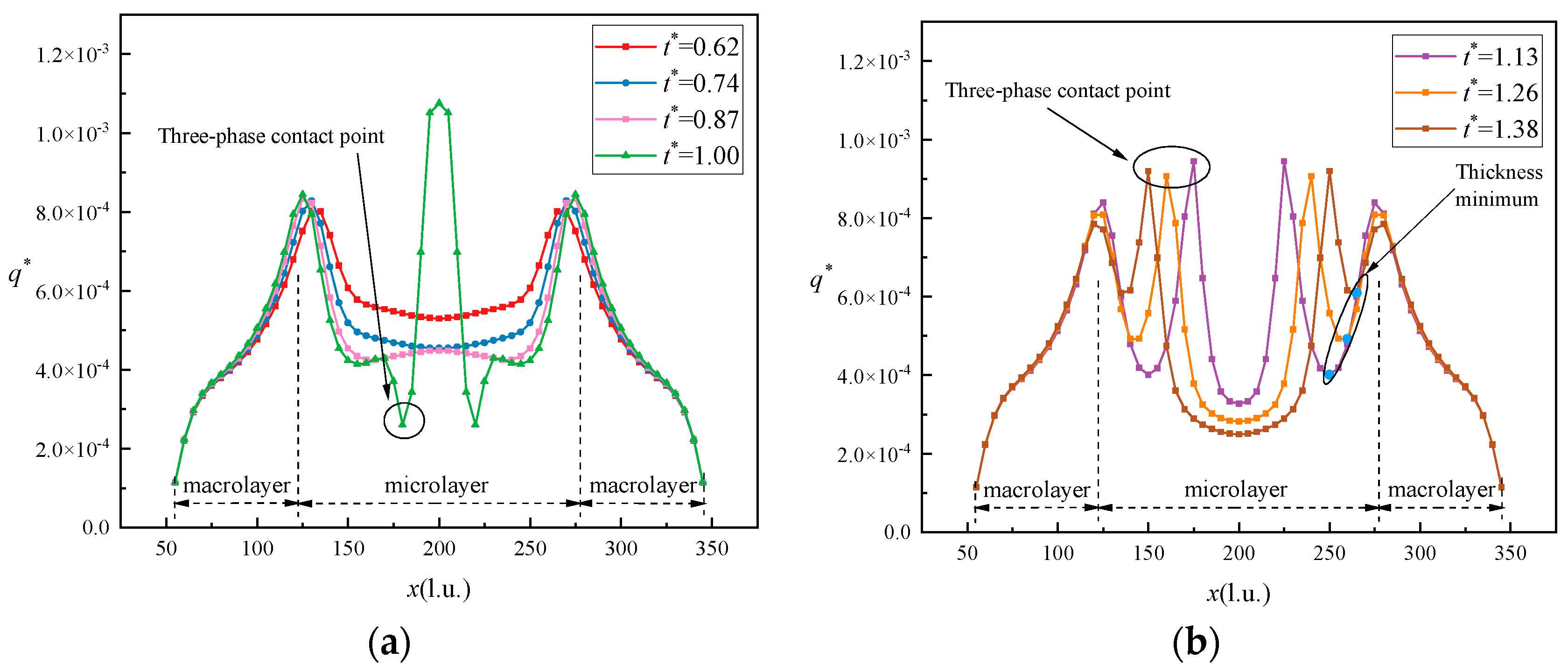
- During the study of heat flux, the lowest heat flux at the three-phase contact point occurred when dry spots appeared and there was a sudden increase in heat flux. After the appearance of the dry spot, the heat flux at the three-phase contact point was the lowest. At the time of the dry spot, a cold air ring appeared above the dry zone, and it expanded and then split, moving with the three-phase contact point.
- (3)
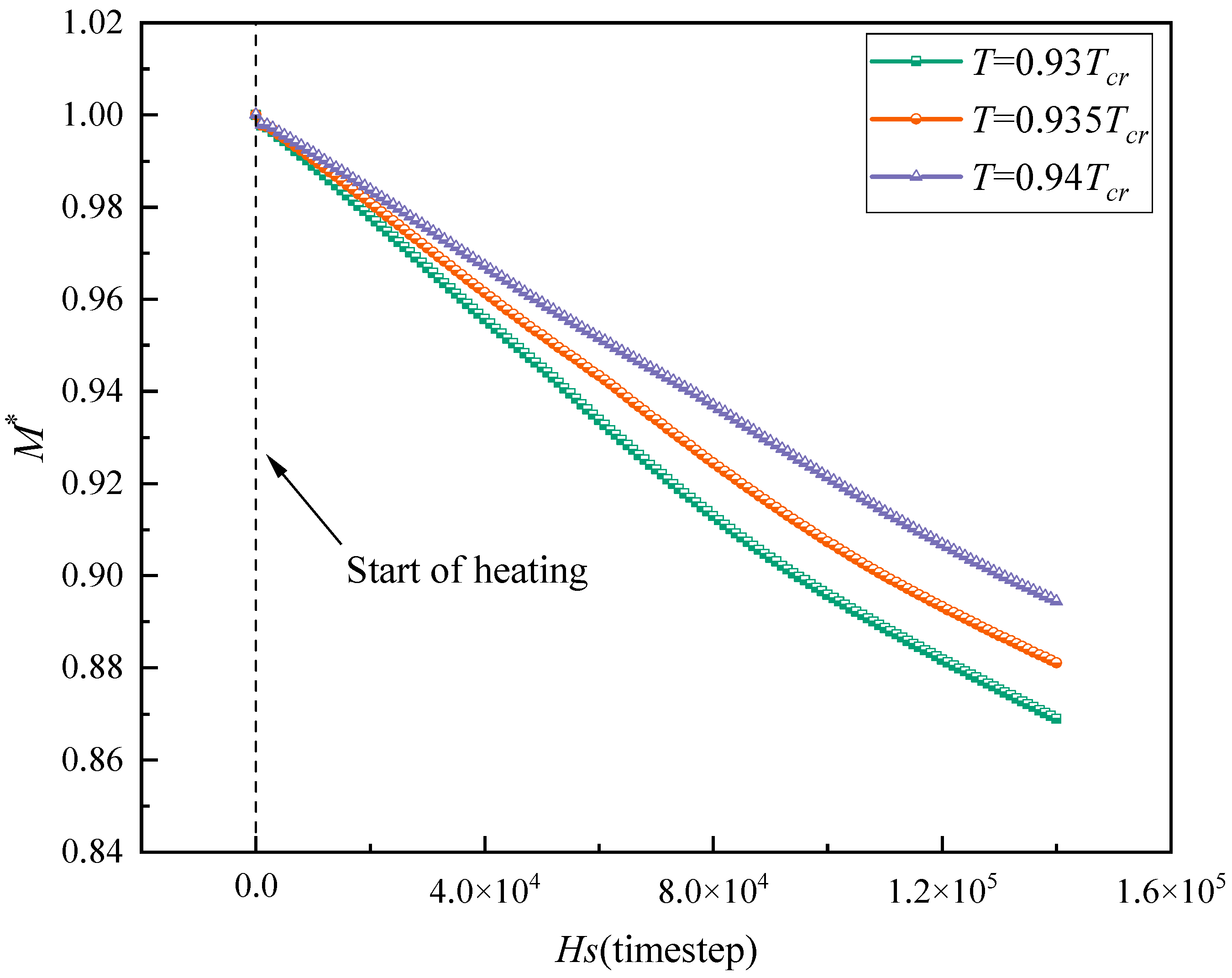
- Raising the temperature is beneficial to the microlayer evaporation process. Under the condition of Hs = 1,400,000 and the same heating duration, increasing the temperature from T = 0.93 Tcr to T = 0.94 Tcr raises the evaporation volume by 3.2% and 6.7% compared to T = 0.935 Tcr and T = 0.93 Tcr, respectively, reduces the total fluid mass to 0.868 (compared to 0.881 and 0.894, respectively), and, through the synergistic action of internal and external Marangoni effects, leads to an approximately linear increase in evaporation volume and a linear decrease in total mass.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| Nomenclature | |
| f | Density distribution function |
| g | Temperature distribution function |
| cp | Specific heat capacity |
| cs | Lattice sound speed |
| c | The speed of sound |
| v | Velocity |
| Gs | Solid–fluid interaction strength coefficient |
| t | Lattice time |
| td | Characteristic time |
| t0 | Timestep for onset of evaporation |
| t* | Dimensionless time |
| k | Thermal conductivity |
| q* | Dimensionless heat flux |
| r* | Dimensionless dry spot radius |
| r | Dry spot radius |
| rd | Characteristic dry spot radius |
| minitial | Initial liquid phase mass |
| m | Liquid phase mass |
| m*evap | Dimension evaporation |
| M | Fluid mass |
| Minitial | Initial fluid mass |
| M* | Dimension fluid mass |
| T | Temperature |
| Tcr | Critical temperature |
| T* | Dimensionless temperature |
| Greek symbols | |
| ξ | Discrete lattice velocity |
| α | Thermal diffusivity |
| ρ | Density |
| υ | Kinematic viscosity |
| Ψ | Effective mass |
| ω | Weighting coefficient |
| σ | Surface tension |
| τ | Dimensionless relaxation time |
| ε | Deviation degree |
| δ | Thickness |
| τg | Single relaxation time |
| Subscripts | |
| l | Liquid |
| v | Vapor |
| s | Heating surface |
| eq | Equilibrium |
| i | Velocity direction |
References
- Zhao, Y.H.; Masuoka, T.; Tsuruta, T.J.I.J.o.H.; Transfer, M. Unified theoretical prediction of fully developed nucleate boiling and critical heat flux based on a dynamic microlayer model. Int. J. Heat Mass Transf. 2002, 45, 3189–3197. [Google Scholar] [CrossRef]
- Utaka, Y.; Kashiwabara, Y.; Ozaki, M.; Chen, Z. Heat transfer characteristics based on microlayer structure in nucleate pool boiling for water and ethanol. Int. J. Heat Mass Transf. 2014, 68, 479–488. [Google Scholar] [CrossRef]
- Utaka, Y.; Kashiwabara, Y.; Ozaki, M. Microlayer structure in nucleate boiling of water and ethanol at atmospheric pressure. Int. J. Heat Mass Transf. 2013, 57, 222–230. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, J.; Gong, S.; Cheng, P. High fidelity simulations of single bubble nucleate boiling heat transfer considering microscale effects. Int. J. Heat Mass Transf. 2025, 239, 126584. [Google Scholar] [CrossRef]
- Sodtke, C.; Kern, J.; Schweizer, N.; Stephan, P. High resolution measurements of wall temperature distribution underneath a single vapour bubble under low gravity conditions. Int. J. Heat Mass Transf. 2006, 49, 1100–1106. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, L.; Cheng, P.; Quan, X. An investigation of microlayer beneath nucleation bubble by laser interferometric method. Int. J. Heat Mass Transf. 2013, 57, 183–189. [Google Scholar] [CrossRef]
- Chen, Z.; Haginiwa, A.; Utaka, Y. Detailed structure of microlayer in nucleate pool boiling for water measured by laser interferometric method. Int. J. Heat Mass Transf. 2017, 108, 1285–1291. [Google Scholar] [CrossRef]
- Tecchio, C.; Cariteau, B.; Houedec, C.L.; Bois, G.; Saikali, E.; Zalczer, G.; Vassant, S.; Roca i Cabarrocas, P.; Bulkin, P.; Charliac, J.; et al. Microlayer evaporation during bubble growth in nucleate boiling. Int. J. Heat Mass Transf. 2024, 231, 125860. [Google Scholar] [CrossRef]
- Han, J.; Kim, I.; Choi, J.; Kim, H. Contribution of wall heat transfer in the transition region between microlayer and macroscopic regions to the growth of a single boiling bubble. Int. J. Heat Mass Transf. 2024, 235, 126213. [Google Scholar] [CrossRef]
- Sato, Y.; Niceno, B. A depletable micro-layer model for nucleate pool boiling. J. Comput. Phys. 2015, 300, 20–52. [Google Scholar] [CrossRef]
- Giustini, G.; Jung, S.; Kim, H.; Ardron, K.H.; Walker, S.P. Microlayer evaporation during steam bubble growth. Int. J. Therm. Sci. 2019, 137, 45–54. [Google Scholar] [CrossRef]
- Gao, M.; Dong, W.h.; Shen, Z.X.; Zhang, L.x. Theoretical model of microlayer and macrolayer evaporation considering heating surface temperature radial distribution at the bottom of the bubble. Int. Commun. Heat Mass Transf. 2023, 140, 106515. [Google Scholar] [CrossRef]
- Chen, Z.; Utaka, Y. On heat transfer and evaporation characteristics in the growth process of a bubble with microlayer structure during nucleate boiling. Int. J. Heat Mass Transf. 2015, 81, 750–759. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, F.; Utaka, Y. Numerical simulation of thermal property effect of heat transfer plate on bubble growth with microlayer evaporation during nucleate pool boiling. Int. J. Heat Mass Transf. 2018, 118, 989–996. [Google Scholar] [CrossRef]
- Zhang, J.; Rafique, M.; Ding, W.; Bolotnov, I.A.; Hampel, U. Direct numerical simulation of microlayer formation and evaporation underneath a growing bubble driven by the local temperature gradient in nucleate boiling. Int. J. Therm. Sci. 2023, 193, 108551. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; He, S.; Li, B.; Wang, J. Marangoni effect on the size of dry spot and CHF in saturated pool boiling of pure water and RB solution. Int. Commun. Heat Mass Transf. 2023, 141, 106601. [Google Scholar] [CrossRef]
- Girimaji, S. Lattice Boltzmann Method: Fundamentals and Engineering Applications with Computer Codes. AIAA J. 2013, 51, 278–279. [Google Scholar] [CrossRef]
- Yang, J.h.; Gao, M.; Wang, D.m.; Dong, W.h.; Zuo, Q.r. Lattice Boltzmann simulation of the effect for adding a ghost fluid layer on the simulation accuracy of a sessile droplet on a heated surface. Int. J. Therm. Sci. 2023, 193, 108525. [Google Scholar] [CrossRef]
- He, X.; Luo, L.S. Theory of the lattice Boltzmann method: From the Boltzmann equation to the lattice Boltzmann equation. Phys. Rev. E 1997, 56, 6811–6817. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, T.S.; Guo, Z.L. Thermal lattice Bhatnagar-Gross-Krook model for flows with viscous heat dissipation in the incompressible limit. Phys. Rev. E 2004, 70, 066310. [Google Scholar] [CrossRef] [PubMed]
- Kupershtokh, A.L.; Medvedev, D.A. Lattice Boltzmann equation method in electrohydrodynamic problems. J. Electrost. 2006, 64, 581–585. [Google Scholar] [CrossRef]
- Gong, S.; Cheng, P. Numerical investigation of droplet motion and coalescence by an improved lattice Boltzmann model for phase transitions and multiphase flows. Comput. Fluids 2012, 53, 93–104. [Google Scholar] [CrossRef]
- Yuan, P.; Schaefer, L. Equations of state in a lattice Boltzmann model. Phys. Fluids 2006, 18, 42101–42111. [Google Scholar] [CrossRef]
- Hu, A.; Li, L.; Uddin, R.; Liu, D. Contact angle adjustment in equation-of-state-based pseudopotential model. Phys. Rev. E 2016, 93, 053307. [Google Scholar] [CrossRef]
- Li, Q.; Luo, K.H.; Kang, Q.J.; Chen, Q. Contact angles in the pseudopotential lattice Boltzmann modeling of wetting. Phys. Rev. E 2014, 90, 053301. [Google Scholar] [CrossRef]
- Li, Q.; Yu, Y.; Luo, K.H. Implementation of contact angles in pseudopotential lattice Boltzmann simulations with curved boundaries. Phys. Rev. E 2019, 100, 053313. [Google Scholar] [CrossRef]
- Guo, Z.; Shi, B.; Zheng, C. A coupled lattice BGK model for the Boussinesq equations. Int. J. Numer. Methods Fluids 2002, 39, 325–342. [Google Scholar] [CrossRef]
- Gong, S.; Cheng, P. A lattice Boltzmann method for simulation of liquid–vapor phase-change heat transfer. Int. J. Heat Mass Transf. 2012, 55, 4923–4927. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, P.; Yan, H.J. Improved thermal lattice Boltzmann model for simulation of liquid-vapor phase change. Phys. Rev. E 2017, 96, 063303. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, P. Mesoscale simulations of boiling curves and boiling hysteresis under constant wall temperature and constant heat flux conditions. Int. J. Heat Mass Transf. 2017, 110, 319–329. [Google Scholar] [CrossRef]
- Nemati, M.; Shateri Najaf Abady, A.R.; Toghraie, D.; Karimipour, A. Numerical investigation of the pseudopotential lattice Boltzmann modeling of liquid–vapor for multi-phase flows. Phys. A Stat. Mech. Its Appl. 2018, 489, 65–77. [Google Scholar] [CrossRef]
- Lakew, E.; Sarchami, A.; Giustini, G.; Kim, H.; Bellur, K. Thin Film Evaporation Modeling of the Liquid Microlayer Region in a Dewetting Water Bubble. Fluids 2023, 8, 126. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Xiong, J. Experimental investigation on microlayer behavior and bubble growth based on laser interferometric method. Front. Energy Res. 2023, 11, 1130459. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, M.; Gao, M.; Yang, J.; Dong, W.; Zhang, L. Evaporation of Non-Isothermal Wall Microlayer Based on the Lattice Boltzmann Method. Processes 2025, 13, 872. https://doi.org/10.3390/pr13030872
Dang M, Gao M, Yang J, Dong W, Zhang L. Evaporation of Non-Isothermal Wall Microlayer Based on the Lattice Boltzmann Method. Processes. 2025; 13(3):872. https://doi.org/10.3390/pr13030872
Chicago/Turabian StyleDang, Mengyuan, Ming Gao, Jianhua Yang, Wuhan Dong, and Lixin Zhang. 2025. "Evaporation of Non-Isothermal Wall Microlayer Based on the Lattice Boltzmann Method" Processes 13, no. 3: 872. https://doi.org/10.3390/pr13030872
APA StyleDang, M., Gao, M., Yang, J., Dong, W., & Zhang, L. (2025). Evaporation of Non-Isothermal Wall Microlayer Based on the Lattice Boltzmann Method. Processes, 13(3), 872. https://doi.org/10.3390/pr13030872






