The Effect of Agitation and the Use of Perfluorodecalin on Lipase Production by Yarrowia lipolytica in a Bioreactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism and Growth Conditions
2.3. Lipase Production in Bioreactor
2.4. Analytical Methods
2.4.1. Lipase Activity
2.4.2. Protein Assay
2.4.3. Proteolytic Activity
2.4.4. pH
2.5. Gel Electrophoresis
3. Results and Discussion
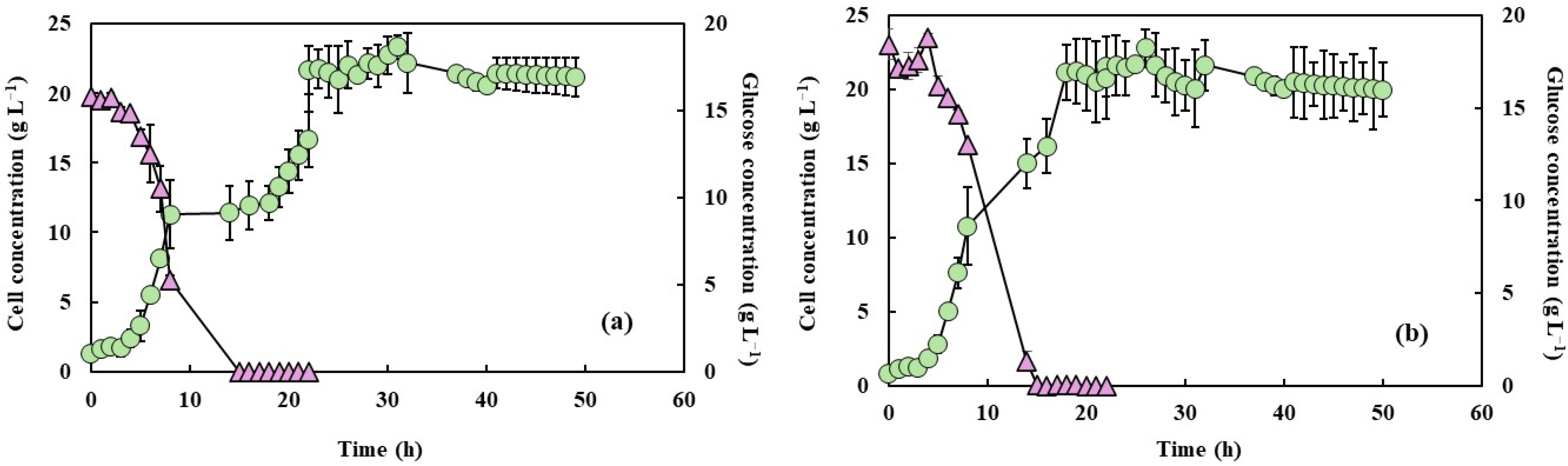
3.1. Cell Growth, Glucose Consumption, and Dissolved Oxygen Concentration
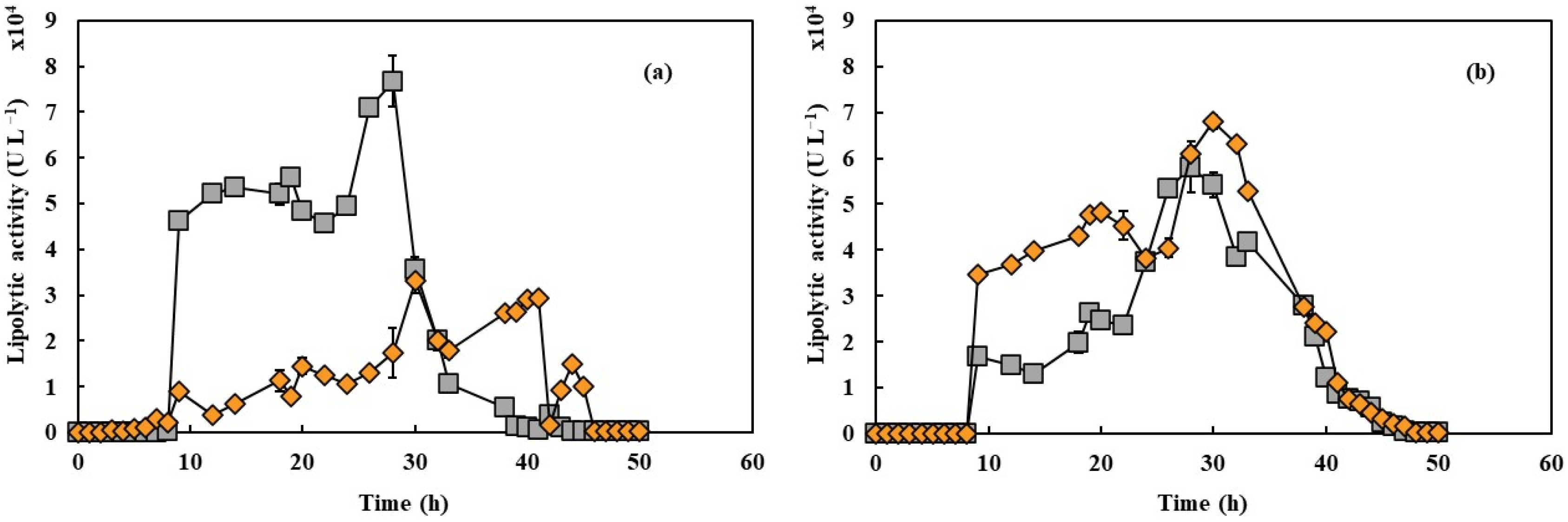
3.2. Effect of PFC on Lipase Production
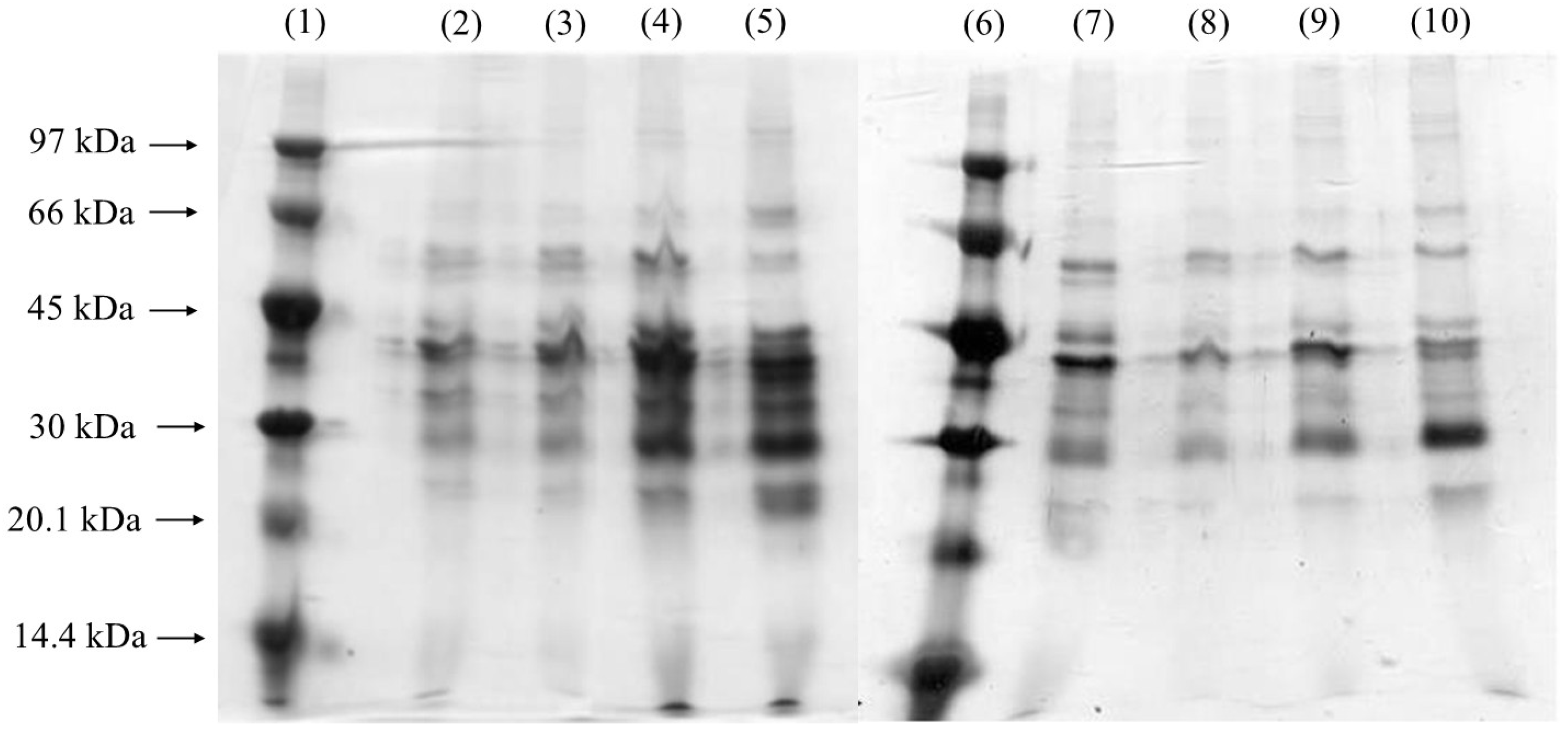
3.3. Gel Electrophoresis (SDS-PAGE)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Casas-Godoy, L.; Duquesne, S.; Bordes, F.; Sandoval, G.; Marty, A. Lipases: An overview. Methods Mol. Biol. 2012, 861, 3–30. [Google Scholar] [PubMed]
- Ge, F.; Chen, G.; Qian, M.; Xu, C.; Liu, J.; Cao, J.; Tan, Z. Artificial intelligence aided lipase production and engineering for enzymatic performance improvement. J. Agric. Food Chem. 2023, 71, 14911–14930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, M.; Zeng, Z.; Wan, D.; Yan, X.; Xia, J.; Gong, D. Production, purification, properties and current perspectives for modification and application of microbial lipases. Prep. Biochem. Biotechnol. 2024, 54, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, A.; Leow, T.C.; Rahman, M.B.A.; Oslan, S.N. Recent insight into the advances and prospects of microbial lipases and their potential applications in industry. Intern. Microbiol. 2024, 27, 1–35. [Google Scholar] [CrossRef]
- Fatima, S.; Faryad, A.; Ataa, A.; Joyia, F.A.; Parvaiz, A. Microbial lipase production: A deep insight into the recent advances of lipase production and purification techniques. Biotechnol. Appl. Biochem. 2021, 68, 445–458. [Google Scholar] [CrossRef]
- Salihu, A.; Alam, M.Z.; AbdulKarim, M.I.; Salleh, H.M. Lipase production: An insight in the utilization of renewable agricultural residues. Resour. Conserv. Recycl. 2012, 58, 36–44. [Google Scholar] [CrossRef]
- Lipase Market Outlook. Available online: https://www.futuremarketinsights.com/reports/lipase-market (accessed on 19 September 2024).
- Buarque, F.S.; Farias, M.A.; Sales, J.C.S.; Carniel, A.; Ribeiro, B.D.; Lopes, V.R.d.O.; Castro, A.M.; Coelho, M.A.Z. Valorization of Macauba (Acromia aculeata) for Integrated Production of Lipase by Yarrowia lipolytica and Biodiesel Esters. Fermentation 2023, 9, 992. [Google Scholar] [CrossRef]
- Nicaud, J.M. Yarrowia lipolytica. Yeast 2012, 29, 409–418. [Google Scholar] [CrossRef]
- Fickers, P.; Marty, A.; Nicaud, J.M. The lipases from Yarrowia lipolytica: Genetics, production, regulation, biochemical characterization and biotechnological applications. Biotechnol. Adv. 2011, 29, 632–644. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, M.; Chen, K.; Jiang, W.; Zhang, L.; Ji, X.; Lu, L. Exploiting synthetic biology platforms for enhanced biosynthesis of natural products in Yarrowia lipolytica. Bioresour. Technol. 2024, 399, 130614. [Google Scholar] [CrossRef]
- Hu, M.; Ge, J.; Jiang, Y.; Sun, X.; Guo, D.; Gu, Y. Advances and perspectives in genetic expression and operation for the oleaginous yeast Yarrowia lipolytica. Synth. Syst. Biotechnol. 2024, 9, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Bordes, F.; Barbe, S.; Escalier, P.; Mourey, L.; André, I.; Marty, A.; Tranier, S. Exploring the conformational states and rearrangements of Yarrowia lipolytica lipase. Biophys. J. 2010, 99, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.A.G.; Colen, G.; Takahashi, J.A. Yarrowia lipolytica and its multiple applications in the biotechnological industry. Sci. World J. 2014, 2014, 476207. [Google Scholar] [CrossRef] [PubMed]
- Weball, W. Production of Lipase by Yarrowia Lipolytica. Lipases from Yeasts (Review). Acta Biotechnol. 1991, 11, 159–167. [Google Scholar]
- Amaral, P.F.F.; De Almeida, A.P.R.; Peixoto, T.; Rocha-Leão, M.H.M.; Coutinho, J.A.P.; Coelho, M.A.Z. Beneficial effects of enhanced aeration using perfluorodecalin in Yarrowia lipolytica cultures for lipase production. World J. Microbiol. Biotechnol. 2007, 23, 339–344. [Google Scholar] [CrossRef]
- Posso Mendoza, H.; Pérez Salinas, R.; Tarón Dunoyer, A.; Tatis, C.C.; Morgado-Gamero, W.B.; Castillo Ramírez, M.; Parody, A. Evaluation of enzymatic extract with lipase activity of Yarrowia lipolytica. An application of data mining for the food industry wastewater treatment. In Security with Intelligent Computing and Big-Data Services: Proceedings of the Second International Conference on Security with Intelligent Computing and Big Data Services (SICBS-2018); Springer: Berlin/Heidelberg, Germany, 2020; pp. 304–313. [Google Scholar]
- Lupachev, E.V.; Voshkin, A.A.; Kisel’, A.V.; Kulov, N.N.; Zakhodyaeva, Y.A.; Polkovnichenko, A.V. Separation of an Industrial Mixture of Decalin or Naphthalene Fluorination Products: Cis-Perfluorodecalin, Trans-Perfluorodecalin and Perfluoro(butylcyclohexane): Physicochemical, Thermophysical, and Spectral Data. Processes 2023, 11, 3208. [Google Scholar] [CrossRef]
- Jawale, P.V.; Bhanage, B.M. Determination of stability and activity of immobilized lipase for transesterification reaction in fluorous solvent and deducing the reaction mechanism by molecular docking study. J. Indian Chem. Soc. 2024, 101, 101233. [Google Scholar] [CrossRef]
- Amaral, P.F.F.; Rocha-Leão, M.H.M.; Marrucho, I.M.; Coutinho, J.A.P.; Coelho, M.A.Z. Improving lipase production using a perfluorocarbon as oxygen carrier. J. Chem. Technol. Biotechnol. 2006, 81, 1368–1374. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, X.; Cai, W.; Cui, K.; Patil, S.A.; Guo, K. Recent progress on microbial electrosynthesis reactor designs and strategies to enhance the reactor performance. Biochem. Eng. J. 2023, 190, 108745. [Google Scholar] [CrossRef]
- Lambert, E.; Janjic, J.M. Quality by design approach identifies critical parameters driving oxygen delivery performance in vitro for perfluorocarbon based artificial oxygen carriers. Sci. Rep. 2021, 11, 5569. [Google Scholar] [CrossRef]
- Jägers, J.; Wrobeln, A.; Ferenz, K.B. Perfluorocarbon-based oxygen carriers: From physics to physiology. Eur. J. Physiol. 2021, 473, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Senko, O.; Maslova, O.; Aslanli, A.; Efremenko, E. Impact of Perfluorocarbons with Gas Transport Function on Growth of Phototrophic Microorganisms in a Free and Immobilized State and in Consortia with Bacteria. Appl. Sci. 2023, 13, 1868. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Amaral, P.F.F.; Coelho, M.A.Z.; Gonçalves, L.R.B. Lipase from Yarrowia lipolytica: Production, characterization and application as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2014, 101, 148–158. [Google Scholar] [CrossRef]
- Snopek, P.; Nowak, D.; Zieniuk, B.; Fabiszewska, A. Aeration and stirring in yarrowia lipolytica lipase biosynthesis during batch cultures with waste fish oil as a carbon source. Fermentation 2021, 7, 88. [Google Scholar] [CrossRef]
- Hagler, A.N.; Mendonqa-Hagler, L.C. Yeasts from Marine and Estuarine Waters with Different Levels of Pollution in the State of Rio de Janeiro, Brazil. Appl. Environ. Microbiol. 1981, 41, 173–178. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Sher, F.; Navarrete, A.A.; Américo-Pinheiro, J.H.P. Microbial adaptation to different environmental conditions: Molecular perspective of evolved genetic and cellular systems. Arch. Microbiol. 2022, 204, 144. [Google Scholar] [CrossRef]
- Elibol, M.; Mavituna, F. Effect of Perfluorodecalin as an Oxygen Carrier on Actinorhodin Production by Streptomyces Coelicolor A3(2). Appl. Microbiol. Biotechnol. 1995, 43, 206–210. [Google Scholar] [CrossRef]
- Gomes, N.; Aguedo, M.; Teixeira, J.; Belo, I. Oxygen mass transfer in a biphasic medium: Influence on the biotransformation of methyl ricinoleate into γ-decalactone by the yeast Yarrowia lipolytica. Biochem. Eng. J. 2007, 35, 380–386. [Google Scholar] [CrossRef]
- Amaral, P.F.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A. Biosurfactants from yeasts: Characteristics, production and application. In Biosurfactants; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; pp. 236–249. [Google Scholar]
- Feitosa, I.C.; de Pinho Barbosa, J.M.; Orellana, S.C.; Lima, Á.S.; Soares, C.M.F. Produção de lipase por meio de microrganismos isolados de solos com histórico de contato com petróleo. Acta Scientiarum. Technol. 2010, 32, 27–31. [Google Scholar]
- Fickers, P.; Benetti, P.H.; Waché, Y.; Marty, A.; Mauersberger, S.; Smit, M.S.; Nicaud, J.M. FEMS Yeast Research; Elsevier: Amsterdam, The Netherlands, 2005; pp. 527–543. [Google Scholar]
- Burkert, J.F.d.M.; Maldonado, R.R.; Maugeri Filho, F.; Rodrigues, M.I. Comparison of lipase production by Geotrichum candidum in stirring and airlift fermenters. J. Chem. Technol. Biotechnol. 2005, 80, 61–67. [Google Scholar] [CrossRef]
- Pereira-Meirelles, F.V.; Rocha-Leão, M.H.; Sant’Anna, G.L., Jr. Lipase location in Yarrowia lipolytica cells. Biotechnol. Lett. 2000, 11, 71–75. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Buarque, F.S.; Nogueira, V.L.R.; Melo, V.M.M.; Guisán, J.M.; Ribeiro, B.D.; Gonçalves, L.R.B.; Coelho, M.A.Z. Partial purification of crude lipase extract from Yarrowia lipolytica: Precipitation, aqueous two-phase systems (ATPS), and immobilization methods. Clean. Chem. Eng. 2023, 6, 100105. [Google Scholar] [CrossRef]
- Ju, L.-K.; Lee, J.F.; Armiger, W.B. Enhancing Oxygen Transfer in Bioreactors by Perfluorocarbon Emulsions. Biotechnol. Prog. 1991, 7, 323–329. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Freire, M.; Coutinho, J.A.P.; Marrucho, I.M. Solubility of oxygen in liquid perfluorocarbons. Fluid Phase Equilibria 2004, 222–223, 325–330. [Google Scholar] [CrossRef]
- Dalmau, E.; Montesinos, J.L.; Lotti, M.; Casas, C. Effect of Different Carbon Sources on Lipase Production by Candida Rugosa. Enzym. Microb. Technol. 2000, 26, 657–663. [Google Scholar] [CrossRef]
- Amaral, P.F.F.; da Silva, J.M.; Lehocky, M.; Barros-Timmons, A.M.V.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A.P. Production and characterization of a bioemulsifier from Yarrowia lipolytica. Process Biochem. 2006, 41, 1894–1898. [Google Scholar] [CrossRef]
- Maldonado, M.R.; Alnoch, R.C.; de Almeida, J.M.; Santos, L.A.d.; Andretta, A.T.; Ropaín, R.d.P.C.; de Souza, E.M.; Mitchell, D.A.; Krieger, N. Key mutation sites for improvement of the enantioselectivity of lipases through protein engineering. Biochem. Eng. J. 2021, 172, 108047. [Google Scholar] [CrossRef]
- Mohanty, S.; Babbal Chauhan, S.; Talwar, M.; Khasa, Y.P. Continuous cell recycling in methylotrophic yeast Pichia pastoris to enhance product yields: A case study with Yarrowia lipolytica lipase Lip2. Biotechnol. Bioprocess Eng. 2024, 30, 1–16. [Google Scholar] [CrossRef]
- Pigne, G.; Wang, H.-J.; Fudalej, F.; Seman, M.; Gaillardin, C.; Nicaud, J.-M. Autocloning and Amplification of LIP2 in Yarrowia Lipolytica. Appl. Environ. Microbiol. 2000, 66, 3283–3289. [Google Scholar] [CrossRef]


| Absence of PFC | Presence of PFC | |||||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | 550 rpm | pH | 650 rpm | pH | 550 rpm | pH | 650 rpm | pH |
| 0 | 6.7 ± 0.3 | 6.42 ± 0.10 | 8.0 ± 0.6 | 6.35 ± 0.22 | 8.3 ± 0.1 | 6.55 ± 0.10 | 7.8 ± 0.3 | 6.66 ± 0.21 |
| 1 | 6.9 ± 0.4 | 6.38 ± 0.15 | 6.5 ± 0.5 | 6.35 ± 0.27 | 6.6 ± 0.3 | 6.51 ± 0.15 | 7.2 ± 0.4 | 6.62 ± 0.26 |
| 2 | 2.4 ± 0.2 | 6.33 ± 0.14 | 7.2 ± 0.6 | 6.30 ± 0.26 | 6.8 ± 0.2 | 6.46 ± 0.14 | 7.2 ± 0.3 | 6.57 ± 0.25 |
| 3 | 3.2 ± 0.2 | 6.20 ± 0.19 | 7.1 ± 0.4 | 6.25 ± 0.31 | 6.8 ± 0.3 | 6.33 ± 0.19 | 7.1 ± 0.2 | 6.44 ± 0.30 |
| 4 | 3.3 ± 0.2 | 6.06 ± 0.27 | 6.8 ± 0.4 | 6.10 ± 0.40 | 6.4 ± 0.3 | 6.19 ± 0.27 | 6.7 ± 0.2 | 6.30 ± 0.38 |
| 5 | 3.1 ± 0.3 | 5.76 ± 0.25 | 5.4 ± 0.6 | 5.93 ± 0.37 | 5.6 ± 0.1 | 5.89 ± 0.19 | 6.1 ± 0.3 | 6.00 ± 0.36 |
| 6 | 3.4 ± 0.2 | 5.48 ± 0.19 | 4.8 ± 0.5 | 5.67 ± 0.31 | 5.2 ± 0.1 | 5.61 ± 0.25 | 5.6 ± 0.3 | 5.72 ± 0.30 |
| 7 | 3.7 ± 0.1 | 5.37 ± 0.25 | 4.2 ± 0.2 | 5.66 ± 0.39 | 5.9 ± 0.7 | 5.50 ± 0.27 | 5.2 ± 0.1 | 5.61 ± 0.36 |
| 8 | 4.2 ± 0.2 | 5.17 ± 0.27 | 3.8 ± 0.5 | 5.51 ± 0.29 | 6.3 ± 0.8 | 5.30 ± 0.17 | 5.0 ± 0.3 | 5.41 ± 0.28 |
| 14 | 4.8 ± 0.1 | 5.06 ± 0.17 | 3.9 ± 0.3 | 4.44 ± 0.46 | 5.2 ± 0.6 | 5.19 ± 0.34 | 5.3 ± 0.1 | 5.30 ± 0.45 |
| 16 | 5.4 ± 0.1 | 4.89 ± 0.34 | 4.6 ± 0.3 | 4.38 ± 0.53 | 5.0 ± 0.1 | 5.02 ± 0.51 | 7.0 ± 0.1 | 5.13 ± 0.62 |
| 18 | 6.3 ± 0.1 | 4.72 ± 0.31 | 6.3 ± 0.3 | 4.46 ± 0.60 | 4.7 ± 0.5 | 4.86 ± 0.38 | 7.8 ± 0.1 | 4.97± 0.49 |
| 19 | 6.4 ± 0.1 | 4.55 ± 0.28 | 6.7 ± 0.2 | 4.53 ± 0.47 | 4.5 ± 0.6 | 4.69 ± 0.45 | 7.5 ± 0.1 | 4.80 ± 0.36 |
| 20 | 6.8 ± 0.1 | 4.38 ± 0.25 | 6.8 ± 0.2 | 4.64 ± 0.14 | 4.3 ± 0.1 | 4.52 ± 0.20 | 7.3 ± 0.1 | 4.63 ± 0.13 |
| 21 | 7.9 ± 0.2 | 4.21 ± 0.20 | 6.8 ± 0.2 | 4.84 ± 0.31 | 4.0 ± 0.5 | 4.35 ± 0.19 | 8.0 ± 0.1 | 4.46 ± 0.30 |
| 22 | 7.0 ± 0.2 | 4.04 ± 0.19 | 6.9 ± 0.2 | 5.10 ± 0.27 | 3.8 ± 0.9 | 4.18 ± 0.15 | 7.7 ± 0.1 | 4.29 ± 0.26 |
| 22 | 7.4 ± 0.2 | 7.18 ± 0.15 | 6.9 ± 0.3 | 5.65 ± 0.31 | 7.0 ± 0.3 | 7.31 ± 0.12 | 7.4 ± 0.3 | 7.42 ± 0.30 |
| 23 | 6.8 ± 0.2 | 7.54 ± 0.19 | 7.6 ± 0.5 | 6.25 ± 0.24 | 7.0 ± 0.1 | 7.67 ± 0.21 | 7.3 ± 0.2 | 7.78 ± 0.23 |
| 24 | 6.8 ± 0.3 | 7.77 ± 0.12 | 7.6 ± 0.4 | 6.79 ± 0.34 | 7.2 ± 0.5 | 7.90 ± 0.31 | 7.4 ± 0.1 | 8.01 ± 0.32 |
| 25 | 6.7 ± 0.4 | 7.97 ± 0.21 | 7.6 ± 0.4 | 6.89 ± 0.43 | 6.6 ± 0.5 | 8.10 ± 0.25 | 7.1 ± 0.3 | 8.21 ± 0.42 |
| 26 | 6.6 ± 0.3 | 8.25 ± 0.31 | 6.8 ± 0.6 | 7.00 ± 0.37 | 6.6 ± 0.4 | 8.38 ± 0.15 | 7.1 ± 0.3 | 8.49 ± 0.36 |
| 27 | 6.7 ± 0.2 | 8.22 ± 0.25 | 6.1 ± 0.6 | 7.20 ± 0.27 | 6.6 ± 0.3 | 8.35 ± 0.16 | 7.1 ± 0.4 | 8.46 ± 0.26 |
| 28 | 6.8 ± 0.1 | 8.25 ± 0.15 | 6.3 ± 0.6 | 7.00 ± 0.28 | 6.4 ± 0.3 | 8.38 ± 0.14 | 7.0 ± 0.5 | 8.49 ± 0.27 |
| 29 | 6.8 ± 0.2 | 8.31 ± 0.16 | 6.3 ± 0.5 | 3.00 ± 0.26 | 6.1 ± 0.4 | 8.44 ± 0.10 | 6.9 ± 0.5 | 8.55 ± 0.25 |
| 30 | 5.6 ± 0.3 | 8.32 ± 0.14 | 6.4 ± 0.5 | 7.40 ± 0.22 | 6.3 ± 0.2 | 8.45 ± 0.09 | 7.0 ± 0.4 | 8.56 ± 0.21 |
| 31 | 4.3 ± 0.4 | 8.33 ± 0.10 | 6.4 ± 0.7 | 7.31 ± 0.21 | 6.2 ± 0.1 | 8.46 ± 0.01 | 7.0 ± 0.4 | 8.57 ± 0.20 |
| 32 | 2.7 ± 0.2 | 8.34 ± 0.09 | 6.6 ± 0.6 | 7.52 ± 0.12 | 6.4 ± 0.5 | 8.47 ± 0.07 | 7.1 ± 0.4 | 8.58 ± 0.11 |
| 37 | 2.3 ± 0.1 | 8.60 ± 0.02 | 6.6 ± 0.5 | 7.83 ± 0.19 | 8.5 ± 0.4 | 8.73 ± 0.15 | 7.4 ± 0.3 | 8.84 ± 0.18 |
| 38 | 1.8 ± 0.4 | 8.56 ± 0.07 | 6.1 ± 0.4 | 7.74 ± 0.28 | 8.3 ± 0.3 | 8.69 ± 0.12 | 7.3 ± 0.4 | 8.80 ± 0.26 |
| 39 | 2.0 ± 0.3 | 8.60 ± 0.15 | 5.9 ± 0.3 | 7.65 ± 0.24 | 8.3 ± 0.5 | 8.73 ± 0.01 | 7.3 ± 0.2 | 8.84 ± 0.23 |
| 40 | 6.0 ± 0.2 | 8.56 ± 0.12 | 6.5 ±0.5 | 7.72 ± 0.41 | 8.3 ± 0.4 | 8.69 ± 0.05 | 7.3 ± 0.1 | 8.80 ± 0.11 |
| 41 | 5.7 ± 0.2 | 8.45 ± 0.29 | 6.5 ± 0.6 | 7.59 ± 0.40 | 8.3 ± 0.3 | 8.58 ± 0.08 | 7.0 ± 0.6 | 8.69 ± 0.13 |
| 42 | 6.1 ± 0.3 | 8.36 ± 0.27 | 6.5 ± 0.7 | 7.89 ± 0.38 | 8.3 ± 0.2 | 8.49 ± 0.20 | 7.0 ± 0.2 | 8.60 ± 0.14 |
| 43 | 5.8 ± 0.3 | 8.24 ± 0.26 | 6.5 ± 0.6 | 7.99 ± 0.37 | 8.3 ± 0.4 | 8.37 ± 0.10 | 7.1 ± 0.4 | 8.48 ± 0.18 |
| 44 | 5.8 ± 0.2 | 8.27 ± 0.25 | 6.5 ± 0.5 | 8.09 ± 0.36 | 8.0 ± 0.6 | 8.40 ± 0.07 | 7.1 ± 0.3 | 8.51 ± 0.17 |
| 45 | 5.8 ± 0.1 | 8.22 ± 0.23 | 6.5 ± 0.5 | 8.20 ± 0.34 | 7.7 ± 0.5 | 8.35 ± 0.09 | 7.1 ± 0.5 | 8.46 ± 0.16 |
| 46 | 5.6 ± 0.3 | 8.24 ± 0.22 | 6.6 ± 0.5 | 8.27 ± 0.33 | 7.4 ± 0.1 | 8.37 ± 0.04 | 7.2 ± 0.1 | 8.48 ± 0.11 |
| 47 | 5.8 ± 0.3 | 8.21 ± 0.21 | 6.5 ± 0.6 | 8.32 ± 0.31 | 7.2 ± 0.3 | 8.29 ± 0.09 | 7.2 ± 0.4 | 8.40 ± 0.14 |
| 48 | 5.9 ± 0.2 | 8.18 ± 0.19 | 6.5 ± 0.6 | 8.34 ± 0.27 | 7.1 ± 0.4 | 8.25 ± 0.05 | 7.2 ± 0.5 | 8.36 ± 0.13 |
| 49 | 5.9 ± 0.1 | 8.11 ± 0.18 | 6.8 ± 0.4 | 8.63 ± 0.29 | 7.3 ± 0.5 | 8.20 ± 0.06 | 7.3 ± 0.3 | 8.31 ± 0.14 |
| 50 | 6.0 ± 0.3 | 8.16 ± 0.16 | 6.8 ± 0.5 | 8.63 ± 0.34 | 7.2 ± 0.2 | 8.18 ± 0.07 | 7.3 ± 0.4 | 8.51 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buarque, F.S.; da Silva, R.L.; Brígida, A.I.S.; Amaral, P.; Coelho, M.A.Z. The Effect of Agitation and the Use of Perfluorodecalin on Lipase Production by Yarrowia lipolytica in a Bioreactor. Processes 2025, 13, 865. https://doi.org/10.3390/pr13030865
Buarque FS, da Silva RL, Brígida AIS, Amaral P, Coelho MAZ. The Effect of Agitation and the Use of Perfluorodecalin on Lipase Production by Yarrowia lipolytica in a Bioreactor. Processes. 2025; 13(3):865. https://doi.org/10.3390/pr13030865
Chicago/Turabian StyleBuarque, Filipe Smith, Roseli Lopes da Silva, Ana Iraidy Santa Brígida, Priscilla Amaral, and Maria Alice Zarur Coelho. 2025. "The Effect of Agitation and the Use of Perfluorodecalin on Lipase Production by Yarrowia lipolytica in a Bioreactor" Processes 13, no. 3: 865. https://doi.org/10.3390/pr13030865
APA StyleBuarque, F. S., da Silva, R. L., Brígida, A. I. S., Amaral, P., & Coelho, M. A. Z. (2025). The Effect of Agitation and the Use of Perfluorodecalin on Lipase Production by Yarrowia lipolytica in a Bioreactor. Processes, 13(3), 865. https://doi.org/10.3390/pr13030865









