Abstract
Lipase production by the strictly aerobic yeast Yarrowia lipolytica is closely related to the content of dissolved oxygen in the culture medium. Some strategies to improve oxygen transfer to microorganisms have already been used, such as the use of perfluorocarbons (PFCs). The present work investigates the influence of agitation speed and the use of perfluorodecalin (PFC) on the profile of the produced lipases. Lipase production increased 2.5-fold with a higher agitation speed (550 to 650 rpm) without PFCs in the medium. The presence of an oxygen carrier led to a significant 91% increase in lipase production at lower shaking speeds compared to the assay without PFC; however, an increase in lipase production was not detected with PFC at 650 rpm. The protein profiles exhibited typical bands for two lipases produced (near 40 and 60 kDa), and these bands became more intense when PFC was added during production, as a result of the large enhancement in lipolytic activity. Additionally, the protein profiles obtained from extracts at 650 rpm were clearer and more selective regardless of the presence of PFC, suggesting an enhancement in specific activity associated with increased shaking. These findings highlight the significant impact of oxygen availability on lipase production, offering valuable insights for industrial applications.
1. Introduction
Lipase production by microorganisms has received significant attention due to its vast industrial applications in sectors such as detergents, food, biofuels, and pharmaceuticals [1,2]. These enzymes display broad biotechnological potential due to their remarkable catalytic versatility, being capable of catalyzing an extensive range of chemical reactions, including the complete or partial hydrolysis of triacylglycerols and several lipid modifications through esterification, interesterification, and transesterification [3,4]. Moreover, lipases exhibit significant regioselectivity and enantioselectivity, overcoming traditional chemical catalysts [5,6]. Lipases are extensively utilized in industrial applications, and their market value reflects their broad utility. As of 2023, the enzyme market, including lipases, was valued at approximately USD 613 million. Projections indicate a significant growth trajectory, with the market expected to nearly triple (USD 1.63 billion) by 2033, growing at a compound annual growth rate (CAGR) of 10.3%. This expansion is driven by increasing applications of microbial lipases in diverse industries, including biofuels, pharmaceuticals, and food processing [7]. Moreover, studies have explored the valorization of agro-industrial residues, such as macauba pulp [8], as substrates for lipase production, further contributing to the cost-effectiveness and sustainability of industrial enzyme production.
Among the various microbial sources, the yeast Yarrowia lipolytica has been highlighted as a promising model for protein secretion and enzyme production studies. This yeast is renowned for its high extracellular production and exhibits relevant catalytic properties, including high substrate selectivity and remarkable stability across a broad range of pHs and temperatures [9,10,11,12]. Y. lipolytica exhibits a strong preference for lipid substrates, making it highly efficient in lipase production. This yeast has a robust extracellular lipase secretion system, with lipase biosynthesis typically associated with nitrogen limitation and the stationary growth phase. Furthermore, Y. lipolytica possesses a highly active β-oxidation pathway, enabling it to metabolize hydrophobic substrates more efficiently than other microbial lipase producers. It is also capable of synthesizing bioemulsifiers, which enhance substrate availability for enzymatic action, thereby increasing overall lipase productivity [13,14]. Y. lipolytica is considered unconventional and strictly aerobic, which means that controlling oxygen transfer is essential for maximizing its productivity under submerged cultivation conditions. In aerobic fermentation processes, the dissolved oxygen limitation in the culture medium is one of the most critical challenges. The low solubility of oxygen in water under standard cultivation conditions (around 7 ppm at 1 atm and 35 °C) frequently hampers the process’s efficiency. Strategies such as intense agitation and using bioreactors equipped with efficient aeration systems are employed to overcome this limitation [8,15,16,17]. However, these solutions can be limited by mechanical or energy restrictions, opening the way for innovative alternatives such as using perfluorocarbons (PFCs) to increase oxygen transfer [18].
PFCs are compounds characterized by their good ability to dissolve gases and have been widely studied for their application in biotechnological processes. PFCs are notable for their high oxygen solubility, chemical and thermal stability, and biological inertness, allowing them to be recovered after processes [16,18,19,20]. However, the specific impact of these compounds on lipase production by Y. lipolytica under different agitation conditions is still poorly investigated. Agitation conditions in bioreactors play a crucial role in the production efficiency of enzymes. Agitation speed directly influences oxygen transfer and nutrient dispersion in the culture medium, which are critical factors for microbial growth and the biosynthesis of extracellular products. Oxygen solubility in PFCs is 10–20 times higher than in pure water [21,22]. Studies have shown that incorporating PFCs into bioreactors successfully enhances the oxygen transfer rate and overall productivity [23,24]. Moreover, the use of PFCs as a second liquid phase in fermentation media has been demonstrated to increase both the oxygen uptake rate and the oxygen transfer coefficient (KLa). The amount of oxygen available to the microorganisms also seems to be an important parameter, since many authors have shown the dependence of lipase productivity on system aeration and agitation [21,22,23,24]. Brígida et al. [25] investigated lipase production in a multiphase bioreactor from Yarrowia lipolytica 50682 in a medium containing perfluorodecalin. However, this study focused on the characterization of the extracellular lipase produced, reporting increased aeration with the use of PFC. It was found that, above 350 rpm, the addition of PFC promotes a great increase in the oxygen transfer rate, reflecting great enhancements in the specific growth rate, glucose consumption rate, DO level, and lipase production. However, the study indicated that the best lipase activity was observed at 650 rpm with the addition of 20% (v/v) perfluorodecalin (PFC), reaching a maximum activity of 95,580 U L−1 h−1 and a productivity of 3724 U L−1 h−1. Moreover, previous studies have shown that Y. lipolytica responds to different agitation speeds with significant variations in its enzymatic productivity [26]. Snopek et al. [26] observed that an increase in aeration intensity limited the period of oxygen deficit in the medium. Simultaneously, an increase in lipolytic activity was observed from 2.09 U L−1 h−1 to 14.21 U L−1 h−1. However, the interaction between agitation and using PFCs in lipase production is still not completely understood. The aim of this manuscript is to evaluate the combined influence of agitation speed and the use of perfluorodecalin (a perfluorocarbon oxygen carrier) on lipase production by Yarrowia lipolytica 50682 in bioreactors. The study focuses on optimizing lipase productivity and specific activity by improving oxygen transfer in the culture medium.
2. Materials and Methods
2.1. Materials
Peptone, yeast extract, glucose, and agar-agar were purchased from Oxoid (Hampshire, UK) and Vetec (Rio de Janeiro, Brasil). Antifoam 204 and 4-Nitrophenyl dodecanoate were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Perfluorodecalin was obtained from Apollo Scientific Ltd. (Manchester, UK) with the following important physical properties at 25 °C and 1.01 × 105 Pa: density 1.917 g·cm−3, vapor pressure 810 Pa, and oxygen solubility 127.8 mg dm−3 [16].
2.2. Microorganism and Growth Conditions
The strain used in this study was wild-type Yarrowia lipolytica (IMUFRJ 50682), selected from an estuary in the Guanabara Bay in Rio de Janeiro, Brazil [27]. The strain was preserved at 4 °C on YPD agar medium. Pre-cultures were prepared in 500 mL shake flasks containing 200 mL YPD medium (w/v: yeast extract 1%; peptone 2%; glucose 2%) and placed in a rotary shaker (160 rpm) at 28 °C for 72 h. Cell concentration was determined by collecting a sample and measuring its absorbance at 570 nm, converted to g dry cell weight L−1 using a conversion factor determined on a dry weight curve. To obtain the dry weight curve, a cell suspension was generated in an aqueous saline solution (NaCl—0.9 wt%). Solutions with different cell concentrations were filtered through Millipore filter paper (0.45 µm) and dried under infrared light until they reached constant weight. Subsequently, an aliquot of the culture containing the biomass required for an initial cell concentration in the production medium of 1 g dry cell weight L−1 was centrifuged and resuspended for inoculation [3].
2.3. Lipase Production in Bioreactor
After the pre-cultivation step, the culture was transferred to a 2 L bioreactor (Multigen, New Brunswick Scientific Co., Edison, NJ, USA) with a working volume of 1.5 L. The cells (1 g cells dm−3) were inoculated in YPD medium containing the following: yeast extract 1% (w/v); peptone 0.64% (w/v); glucose 2% (w/v); and 1 mL of antifoam. Batch reactions in the bioreactor were performed at 28 °C with 1.5 vvm air flow rate and two shaking speeds: 550 and 650 rpm. For assays using PFC, perfluorodecalin at a concentration of 20% (v/v) was added to the medium. This concentration was based on the study by Amaral et al. [20], who investigated the effect of PFC concentration (ranging from 10% to 50%) on lipase production by yeast; their findings identified 20% as the optimal condition. Dissolved oxygen was continuously monitored with an oxygen probe (Lutron DO-5510, Lutron Electronics Co., Inc., Coopersburg, PA, USA). Samples were removed periodically at intervals of 1 h for a total of 50 h and analyzed for lipase production, residual glucose, and biomass estimation. Glucose concentration in the culture supernatant was determined using a glucose oxidase kit (In Vitro- Human, Minas Gerais, Brazil), following the manufacturer’s recommendations.
2.4. Analytical Methods
2.4.1. Lipase Activity
Lipase activity was determined in the culture supernatant using a spectrophotometric method by the oxidation of p-nitrophenyl laurate (p-NFL) at a concentration of 0.162 mg mL−1 in potassium phosphate buffer (0.05 M) at pH 7.0. Samples (20 μL) were incubated in the microtiter plate for five minutes, and the reaction was started by the addition of 180 μL of p-NFL solution [4]. All the experiments were carried out in triplicate, and the mean and standard deviation were calculated for each analysis in this work. The activity was determined according to Equation (1), in which one unit (U) of lipase activity is defined as the amount of enzyme that produces 1 μmol min−1 of product.
where A = the enzyme activity (U L−1); ∆Abs = the absorbance variation in the time interval ∆t (in minutes); D = the dilution of the enzymatic solution; Vr = the reaction volume (L); Vs = the volume of the enzymatic solution (L); and f = the conversion factor.
2.4.2. Protein Assay
Protein concentration was determined by the Bradford [28] method using bovine serum albumin as standard.
2.4.3. Proteolytic Activity
Protease activity was determined by the hydrolysis of azocasein. The 0.5% (w/v) azocasein solution was prepared in a 50 mM acetate buffer, pH 5.0. A total of 1 mL of the enzyme extract was added to 1 mL of the azocasein solution, and the mixture was incubated for 40 min at 32 °C. Then, 1 mL of trichloroacetic acid (15% w/v) was added to precipitate the protein molecules not hydrolyzed by the proteases. Afterward, the mixture was centrifuged for 15 min at 3000 rpm and 25 °C, then 2 mL of the mixture was added to 2 mL of 5 M KOH [3]. The absorbance reading of the sample was performed at λ = 428 nm, and the proteolytic activity was calculated according to Equation (2). All the experiments were carried out in triplicate, and the mean and standard deviation were calculated for each analysis in this work.
where A = enzyme activity (U L−1); Abs = absorbance; ∆t = analysis time (in minutes); Vs = volume of the enzymatic solution (0.001 L).
2.4.4. pH
The pH of the cell-free enzyme extract was determined using a Digimed DM-22 (São Paulo, Brazil) benchtop digital pH meter at room temperature.
2.5. Gel Electrophoresis
Gel electrophoresis (SDS-PAGE) was performed with a 10% polyacrylamide gel on a vertical gel apparatus (GSR) at 150 V for 1 h. The molecular weight marker was purchased from GE Healthcare (Chalfont St Giles, United Kingdom). The following low-range-pertaining SDS-PAGE standards were used as molecular mass markers: phosphorylase B (94 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (30 kDa), soybean trypsin inhibitor (20.1 kDa), and α-lactalbumin (22 kDa). Proteins were stained with Coomassie Brilliant Blue R-250 (Merck Millipore, São Paulo, Brazil) and aqueous silver nitrate.
3. Results and Discussion
3.1. Cell Growth, Glucose Consumption, and Dissolved Oxygen Concentration
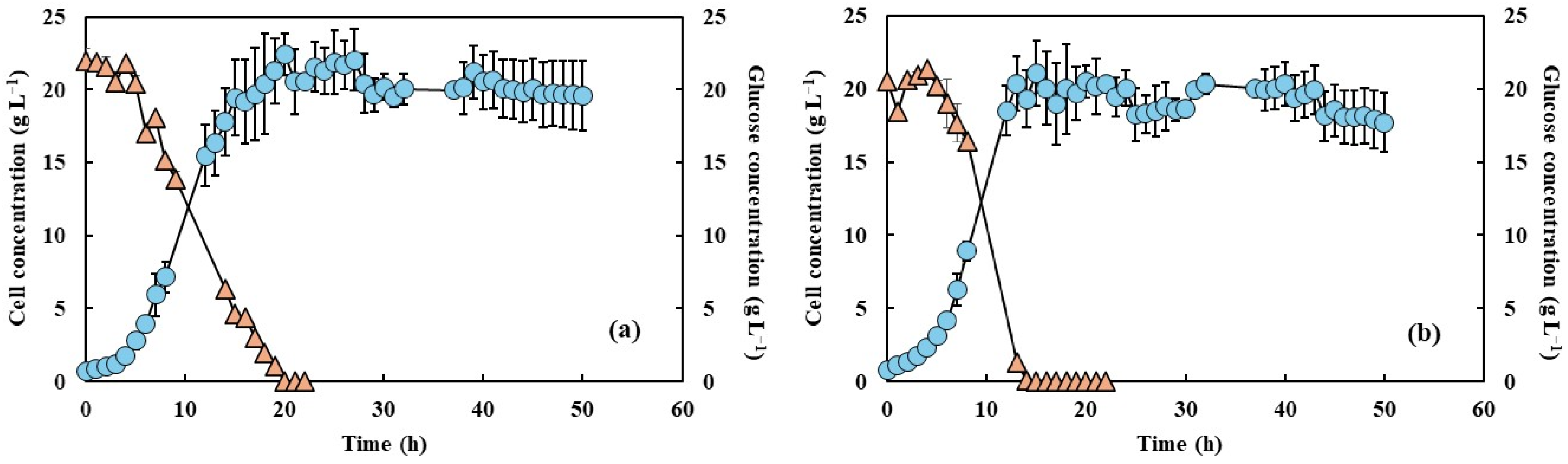
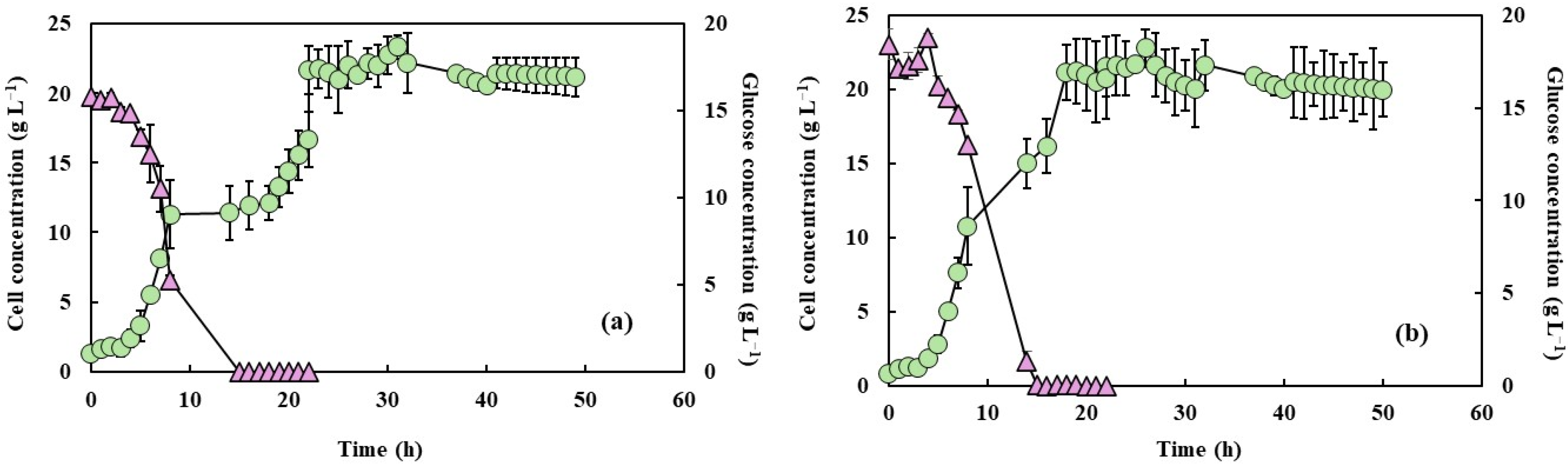
The cell growth and glucose consumption measurements under the two agitation speeds, both with and without PFC, are shown in Figure 1 and Figure 2. Microorganism growth is often quantified by increases in biomass or cell numbers, which result from a series of enzymatically catalyzed and highly coordinated metabolic events. This growth depends on nutrient availability and environmental parameters such as pH and temperature, which must be maintained at optimal levels to ensure robust cellular activity [29]. In this study, the cell growth profiles were similar under both agitation conditions, irrespective of the presence of PFC, but differences in growth rates can be noted. Also, increased agitation at 650 rpm without PFC led to a faster glucose consumption rate, likely due to enhanced oxygen transfer and increased metabolic activity. The addition of PFC further accelerated glucose depletion, with complete consumption observed within 15 h of fermentation. This acceleration was higher in the presence of PFC, and under these conditions glucose concentration was fully depleted after 15 h of fermentation. This is in accordance with the results of Elibol and Mavituna [30], who showed that the use of perfluorodecalin in Streptomyces coelicolor growth media improved the glucose consumption rate. This can be attributed to the relief of O2 transfer limitation, the cells being able to easily consume the glucose and the additional oxygen supplied. Amaral et al. [16,20] indicated that increased agitation and PFC supplementation accelerated glucose depletion, suggesting an intensified metabolic flux towards growth and energy generation. In addition, Brígida et al. [25] reported that the metabolic shift induced by glucose depletion and the transition to nitrogenous compound metabolism, as indicated by pH variations, could suggest an increase in byproducts such as ammonium ions or organic acids.
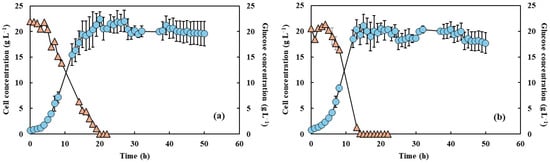
Figure 1.
Cell concentration (light blue circles) and glucose consumption (light orange triangles) of Y. lipolytica grown in 2 L bioreactor (working volume 1.5 L) in YPD medium and aeration of 1.5 L min−1 without PFC and with shaking at 550 rpm (a) and 650 rpm (b).
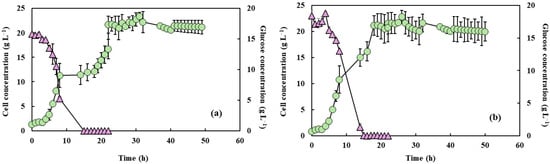
Figure 2.
Cell concentration (light green circles) and glucose consumption (light purple triangles) of Y. lipolytica grown in 2 L bioreactor (working volume 1.5 L) in YPD medium and aeration of 1.5 L min−1 with PFC and with shaking at 550 rpm (a) and 650 rpm (b).
Table 1 illustrates the dissolved oxygen (DO) profiles during cultivation under the experimental conditions. Dissolved oxygen concentrations decreased sharply within the first hour, indicating high metabolic activity during the exponential growth phase. At 650 rpm, the DO content remained consistently higher throughout the experiment compared to 550 rpm, likely due to improved oxygen transfer dynamics at higher shaking speeds. The minimum DO values observed at 550 rpm (1.8 L min−1) and 650 rpm (3.8 L min−1) without PFC reflect the oxygen demand during peak cell activity. Although the profiles presented in both conditions are practically identical, at 650 rpm the dissolved oxygen content remained higher throughout the experiment, especially between 22 and 24 h (7.6 L min−1). This indicates that higher agitation speeds provide greater oxygen dissolution in the medium during the process. A minimum oxygen concentration was reached at the peak cell activity, at which point there was an increase in dissolved oxygen concentration, stabilizing the system. The dissolved oxygen concentration then became constant until the end of the assay, with average values of 6.0 and 6.6 L min−1 at 550 and 650 rpm, respectively. The dissolved oxygen profile is similar to the one presented by Gomes et al. [31] when studying the biotransformation of flavoring lipids by Yarrowia lipolytica W29 (ATCC20460: CLIB89) in a 2 L bioreactor at 400 rpm and 0.9 vvm, as well as the profile presented by Amaral et al. [32] in a study of lipase production by Y. lipolytica in a multiphase bioreactor. Minimum oxygen concentrations were higher in the PFC-supplemented systems (Table 1), with values of 4.5 mg L−1 at 550 rpm and 5.5 mg L−1 at 650 rpm.

Table 1.
Dissolved oxygen and pH during cultivation of Y. lipolytica in 2 L bioreactor (1.5 L working volume) in YPD medium with aeration of 1.5 L min−1 in absence and presence of PFC with shaking at 550 rpm and 650 rpm.
The mechanism behind this improvement is primarily based on the biphasic nature of the system, where PFCs act as a secondary, non-miscible liquid phase. Oxygen dissolves preferentially in the PFC phase due to its higher affinity, creating an oxygen-rich reservoir. As the fermentation progresses, dissolved oxygen from the PFC phase is continuously transferred to the aqueous phase via diffusion, thereby mitigating oxygen limitations, especially at high cell densities. Furthermore, the enhancement in oxygen transfer reduces oxygen-limiting conditions, which are crucial in aerobic bioprocesses where oxygen is often a limiting factor for microbial metabolism. The increased dissolved oxygen concentration ensures a more stable and sustained supply, reducing the need for excessive mechanical agitation, which can cause shear stress and energy inefficiencies [21,22].
These results demonstrate the effectiveness of PFC as an oxygen carrier, increasing oxygen availability by 2.5 and 1.44 times at low and high agitation speeds, respectively, compared to systems without PFC. These observations are consistent with the findings of Feitosa et al. [33], who highlighted the role of PFCs in enhancing oxygen solubility and transfer efficiency in bioreactors.
The pH of the culture medium plays a critical role in influencing cell growth, enzyme secretion, and product stability. Variations in pH during fermentation are often linked to metabolic byproducts, particularly organic acids secreted by microorganisms. In this study, the pH of the culture medium decreased from 6.4 to 4.4 during the first hours of fermentation, corresponding to the exponential growth phase (Table 1). This drop can be attributed to the production of organic acids such as citric, isocitric, and pyruvic acids, commonly secreted by Yarrowia lipolytica [34]. The gradual increase in pH observed after approximately 18 h can be attributed to metabolic shifts associated with glucose depletion. During the exponential phase, Yarrowia lipolytica primarily utilizes glucose as a carbon source, leading to the production of organic acids such as citric acid which contribute to maintaining a relatively lower pH. However, as glucose becomes limited after approximately 18 h, the metabolism shifts towards the utilization of nitrogenous substrates. The catabolism of nitrogenous compounds results in the release of ammonium ions, which can contribute to an increase in pH by neutralizing the acidic byproducts. Additionally, the reduction in organic acid production, due to the shift away from glycolysis-driven metabolism, further decreases the acid load in the medium. This metabolic transition is well documented in microbial fermentations, where nitrogen metabolism becomes dominant under carbon-limited conditions, leading to an alkaline shift in pH [35].
This profile of pH variation was also observed by Burkert et al. [35] in a study of lipase production by NRRL-Y522 Geotrichum candidum using a 3 L benchtop bioreactor with a 2.2 L working volume under the following conditions: 30 °C, 300 rpm, and 1 vvm aeration. In summary, the combination of increased shaking and PFC supplementation demonstrated significant improvements in glucose consumption and oxygen availability. These results highlight the potential of PFCs to enhance oxygen transfer in aerobic fermentation processes, making them valuable tools for optimizing biotechnological applications involving strict aerobes such as Yarrowia lipolytica.
3.2. Effect of PFC on Lipase Production
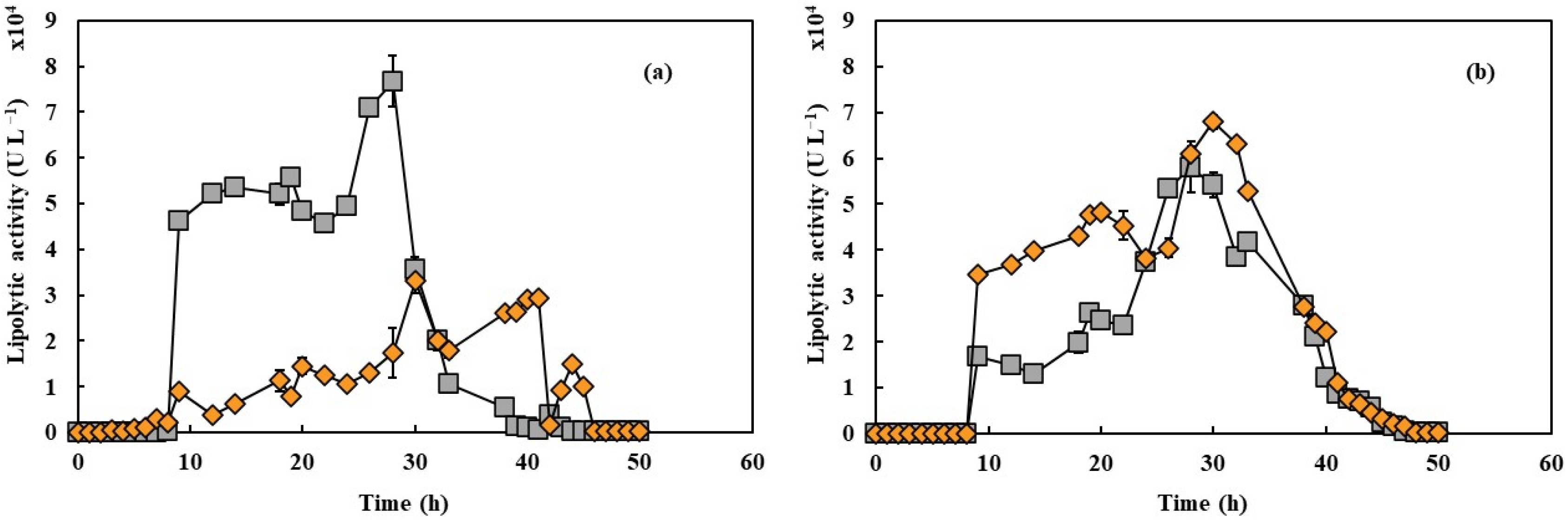
In Figure 3a, lipase production initiated in the low glucose concentrations present in the medium and reached its peak at 25 h for 550 rpm and 23 h for 650 rpm, achieving maximum activities of approximately 33,000 and 76,000 U L−1, respectively. An increase of 100 rpm in shaking speed resulted in a 130% improvement in peak activity. The recorded lipase productivity was 1327.4 U L−1h−1 at 550 rpm and 3338.5 U L−1h−1 at 650 rpm, indicating a 2.5-fold improvement in productivity with the higher shaking speed. As shown in Figure 3b, lipase production in the presence of PFC exhibited a distinct pattern, with both conditions displaying similar trends, reaching approximately 60,000 U L−1 of hydrolytic activity. The value achieved at 650 rpm was lower than that observed in fermentations without PFC. In contrast, at 550 rpm, there was a significant 91% increase in activity compared to the assay without PFC. However, this value remained lower than the activity recorded at 650 rpm under the same conditions.
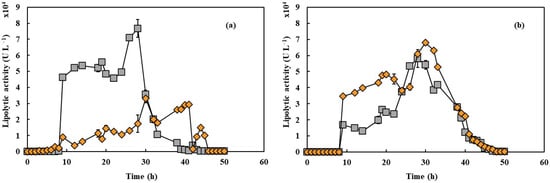
Figure 3.
Lipase activity during cultivation of Y. lipolytica in 2 L bioreactor (working volume 1.5 L) in YPD with aeration of 1.5 L min−1 in absence (a) and presence (b) of PFC under shaking speeds of 550 rpm (orange diamonds) and 650 rpm (gray squares).
These findings align with Amaral et al. [20], who reported a 23-fold increase in lipase yield with the addition of 20% PFC at 350 rpm in 500 mL shaker flasks containing 200 mL of working volume. Lipase production generally reaches its maximum when the cell culture enters the stationary phase [3], which was also observed in this study. Additionally, Pereira-Meirelles [36] found that Y. lipolytica lipase production occurs while the enzyme substrate is abundant, with enzymes being released to hydrolyze the remaining substrate as its levels decline. The values obtained suggest the presence of hydrolytic activity, as Y. lipolytica has a strictly aerobic metabolism, making agitation and aeration critical factors influencing enzyme production. The presence of PFC significantly impacts enzyme production, as demonstrated by Brígida et al. [37], who observed maximum lipolytic activities of 6797 ± 884 U L−1 at 550 rpm and 32,115 ± 3854 U L−1 at 650 rpm in bioreactors without PFC. The corresponding maximum productivities were 288 ± 21 U L−1h−1 (550 rpm) and 1191 ± 155 U L−1h−1 (650 rpm). These results suggest that PFC enhances oxygen transfer to the aqueous phase, leading to a higher specific growth rate. Furthermore, as noted by Ju et al. [38], the reduction in PFC droplet size appears to be associated with the increased production of other compounds, such as bioemulsifiers. The literature also indicates that Y. lipolytica produces bioemulsifiers, and that their production increases at higher impeller speeds [39].
The rapid decline in lipase hydrolytic activity after reaching its peak is often attributed to the action of proteases. These proteases, secreted during all phases of cell growth, degrade extracellular proteins, including lipases, thus reducing their overall activity [40]. In this study, protease production was observed under both shaking conditions, with the content remaining steady at approximately 1.0 U mL−1 regardless of the presence of the oxygen carrier. This consistent protease activity across different conditions suggests that protease production is relatively independent of agitation speed and oxygen availability. Similar findings were reported by Amaral et al. [41], who noted that protease secretion in Y. lipolytica was more strongly influenced by the metabolic state of the cells than external environmental conditions. Moreover, the presence of the oxygen carrier (PFC) in this study did not significantly alter protease production, which is consistent with the findings of Maldonado et al. [42]. They demonstrated that the use of oxygen carriers enhances oxygen transfer without directly affecting the secretion of proteolytic enzymes.
3.3. Gel Electrophoresis (SDS-PAGE)
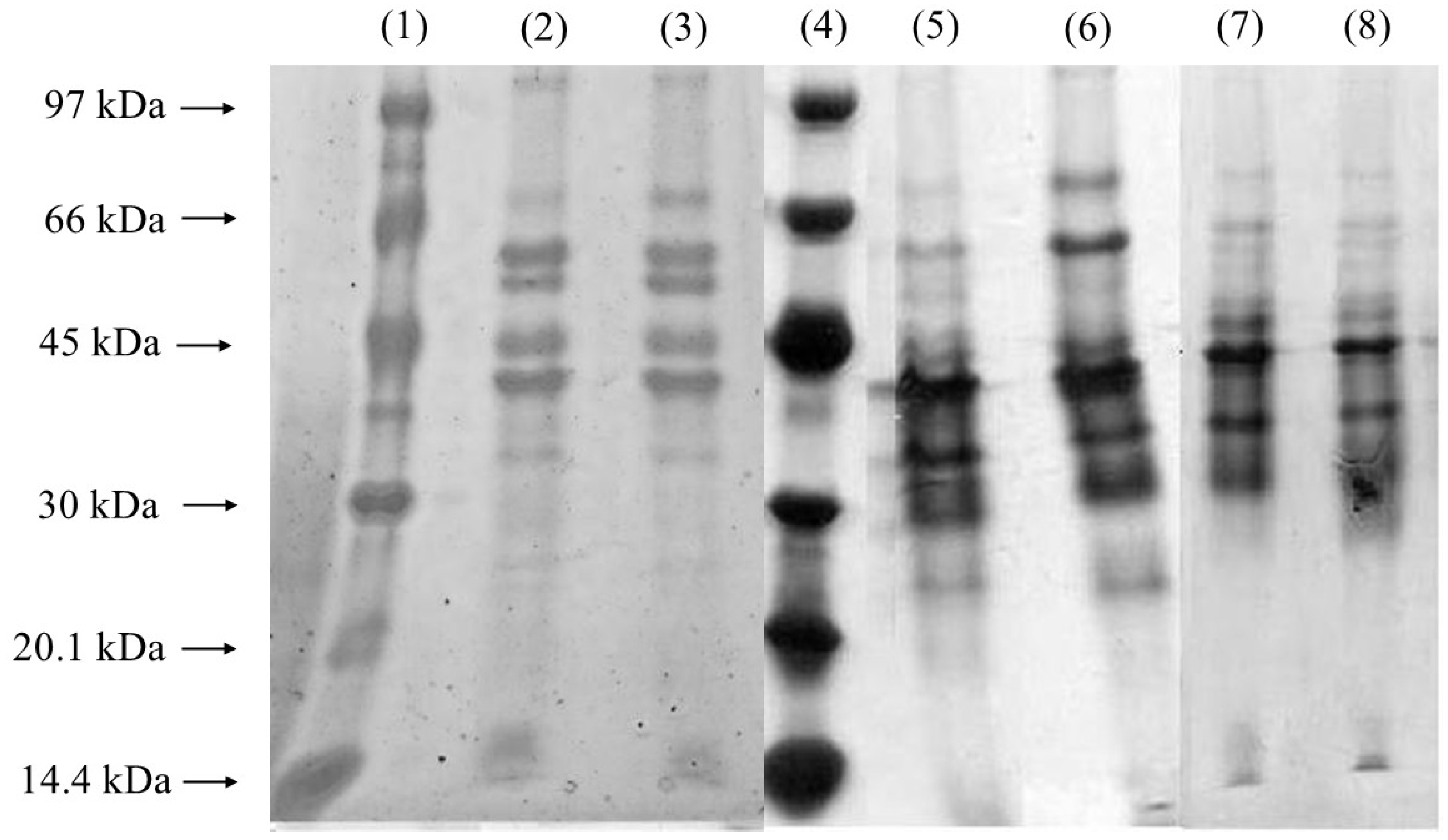
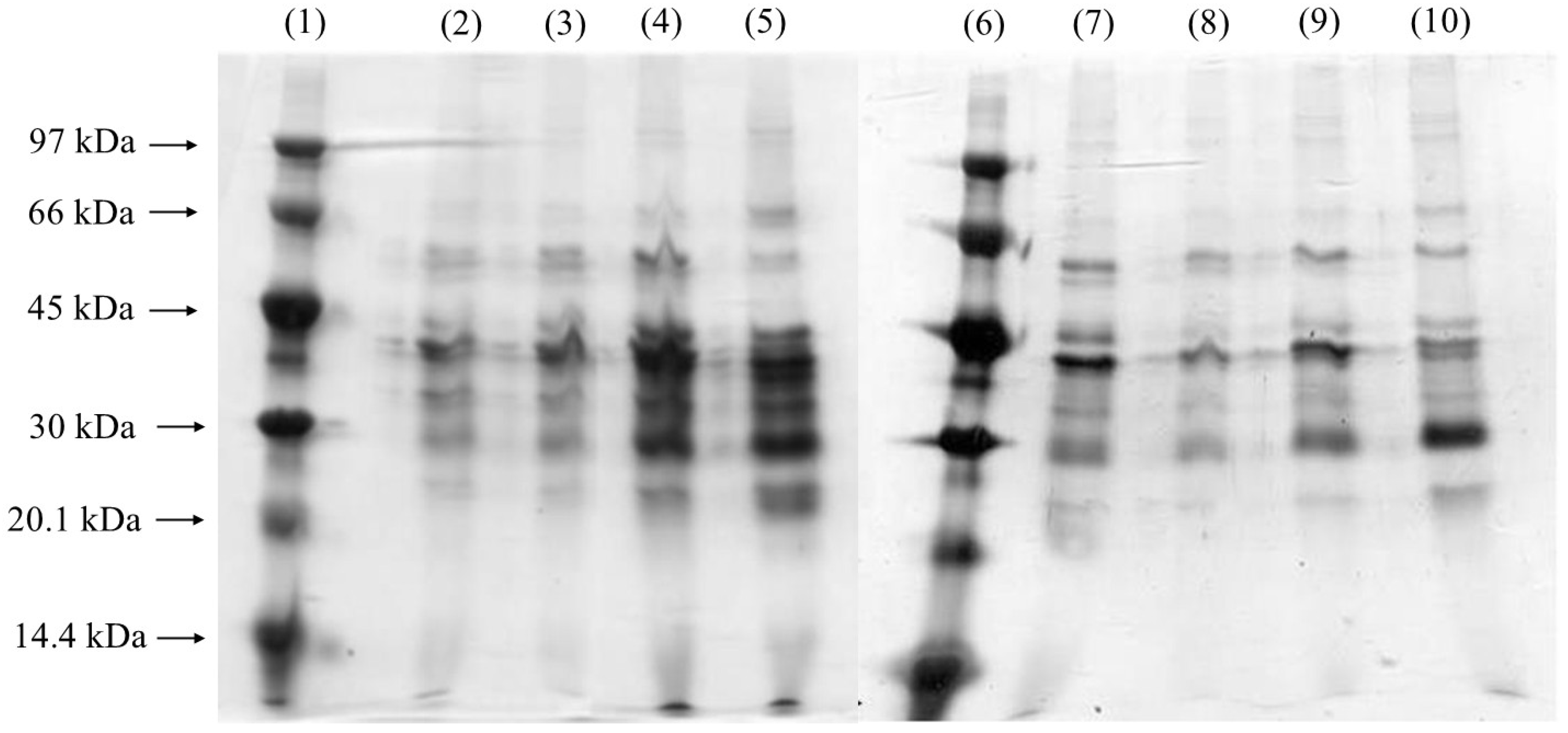
The Coomassie Blue-stained gel (Figure 4) displayed an increase in band intensity near 40 kDa, aligning with the molecular weight expected for lipases produced by Yarrowia lipolytica [43]. This observation is consistent with prior studies highlighting the prominent production of lipases by this yeast under specific growth conditions. The enhanced visualization of bands with higher protein concentrations was achieved using silver staining (Figure 4, Lanes 4–8), a technique known for its superior sensitivity in detecting low-abundance proteins. Interestingly, the presence of a band around 32 kDa, potentially indicative of alkaline proteases, was noted, corroborating earlier findings by Brígida et al. [25], who reported that Y. lipolytica produces proteases with similar molecular weights. This protease activity could represent a secondary enzymatic activity linked to the yeast’s metabolic response during fermentation, emphasizing the organism’s enzymatic versatility. Figure 4 shows an increase in the intensity of the protein bands near 40 kDa in the samples obtained after 22 and 24 h of fermentation, especially in the 650 rpm condition. This molecular weight range corresponds to the lipases previously characterized for Y. lipolytica, suggesting an increase in enzyme production in response to the higher agitation rate. Stirring at 650 rpm favored the transfer of oxygen in the culture medium, which may have stimulated the biosynthesis of lipases, since the species is strictly aerobic. A comparative analysis of the different agitation conditions revealed an increase in the intensity of the lipase-associated band after 24 h of cultivation at 550 rpm and after 20 h at 650 rpm. This temporal shift suggests that higher agitation speeds may accelerate lipase production, potentially by enhancing oxygen transfer and nutrient availability, both of which are critical for lipase synthesis. Gene expression analyses further support these findings, indicating that the primary extracellular lipase produced under these conditions corresponds to LIP2. This glycoprotein, with a molecular weight of approximately 38 kDa, exhibits optimal catalytic activity at 37 °C and pH 7 and is characterized by its robust hydrolytic, esterification, and transesterification activities. This enzyme plays a significant role in industrial applications due to its versatility and stability under varying conditions. Additionally, other lipases from Y. lipolytica with molecular weights close to 41 kDa and distinct optimum temperatures and substrate specificities have been reported [3]. These findings underscore the complexity of the lipolytic system in Y. lipolytica, which comprises multiple lipase isoforms tailored to different functional roles. The dynamic expression and activity of these enzymes highlight the potential of Y. lipolytica as a valuable microbial cell factory for biotechnological applications, including biodiesel production, bioremediation, and the synthesis of fine chemicals.
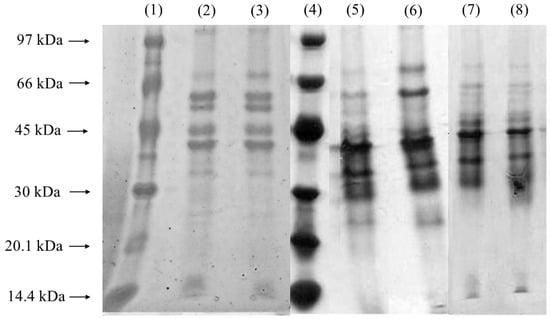
Figure 4.
Coomassie Blue-stained and silver-stained SDS-PAGE gel for samples without PFC. Lanes 1 and 4: low-molecular-weight markers. Lanes 2 and 3 (Coomassie Blue-stained): after 24 h of fermentation at 550 and 650 rpm, respectively; Lanes 5 and 6 (silver-stained): after 22 and 24 h of fermentation, respectively, at 550 rpm; Lanes 7 and 8 (silver-stained): after 22 and 24 h of fermentation, respectively, at 650 rpm.
Figure 5, which shows the results for the fermentations supplemented with PFC, shows an intensification of the bands at 40 kDa and 60 kDa, particularly between 18 and 24 h of cultivation. The greater intensity of these bands suggests that the addition of PFC improved oxygen availability, promoting a greater synthesis of lipases in a shorter period of time when compared to conditions without PFC. These results contrast sharply with the assays conducted without PFC, where lanes around 40 kDa became prominent only after 24 h of fermentation. This suggests that the presence of PFC influences the banding profile, enhancing band intensity earlier in the process due to increased lipase production during this period. In SDS-PAGE, the increased intensity of the bands corresponding to lipases in the presence of PFC indicates a significant enhancement in enzyme production and activity. This is because PFC improves oxygen transfer in the culture medium, favoring the growth of Yarrowia lipolytica and, consequently, the expression of lipases. As a result, there is a higher concentration of the enzyme in the sample, reflected in the intensification of the bands, suggesting greater enzymatic efficiency and optimization of the fermentation conditions.
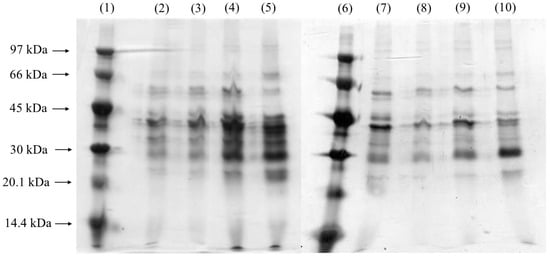
Figure 5.
Silver-stained SDS-PAGE gel of samples with PFC. Lanes 1 and 6 are low-molecular-weight markers. Lanes 2 to 5 refer to 18, 20, 22, and 24 h of fermentation at 550 rpm, respectively; Lanes 7 to 10 refer to 18, 20, 22, and 24 h of fermentation at 650 rpm, respectively.
In the silver-stained gels, low-molecular-weight bands are also visible, with greater intensity observed between 22 and 24 h. Many extracellular enzymes produced by yeast, such as LIP2, are glycosylated. Pignède et al. [44] identified two LIP2 enzymes (pro-Lip2p and Lip2p) with low molecular weights, where glycosylation (containing 10–15% sugar) accounted for the apparent reduction in molecular weight. Importantly, these enzymes showed no differences in specific activity compared to their deglycosylated forms, indicating that glycosylation does not affect enzyme activity. At 650 rpm, lanes near 60 kDa were present at all the time points analyzed, while bands around 30 kDa became more intense but appeared only after 24 h of fermentation. Bands near 40 kDa, typically associated with lipases, were more prominent between 18 and 22 h of fermentation. In the gel from the 650 rpm assay without PFC, lipase-related lanes (40 kDa) showed greater intensities between 20 and 22 h, whereas in the 650 rpm assay with PFC, these bands were more intense between 18 and 22 h. Additionally, both with and without PFC, the protein profiles extracted after fermentation at 650 rpm appeared clearer and more selective compared to those obtained at lower shaking speeds. This indicates that increased agitation speeds enhanced specific enzyme activity.
4. Conclusions
The present study demonstrated that perfluorodecalin significantly enhances Yarrowia lipolytica growth and lipase production by improving oxygen availability in the culture medium. The highest lipase productivity was observed at 650 rpm, where peak enzyme production occurred after 23 h, compared to 25 h at 550 rpm. The maximum lipase productivity reached 3338.5 U L−1h−1 at 650 rpm, representing a 2.5-fold increase over the productivity obtained at 550 rpm. Electrophoretic analysis confirmed that lipase production was enhanced in the presence of perfluorodecalin, as evidenced by the increased intensity of protein bands near 40 kDa, characteristic of Y. lipolytica lipases. These results highlight the importance of oxygen bioavailability in optimizing the production of microbial enzymes and highlight the potential of perfluorodecalin as an oxygen carrier to enhance bioprocess performance. However, the interaction between agitation speed, PFC concentration, and metabolic activity requires further investigation to fully understand the underlying mechanisms governing lipase synthesis. Future studies should explore alternative oxygen carriers with environmentally sustainable profiles, such as fluoropolymers, organofluorines, phosphocholines, metal–organic frameworks (MOFs), and metal oxides. Additionally, optimizing controlled oxygen release, biocompatibility, and system stability will be crucial for further improving lipase production. The integration of multiple oxygen-enriching strategies holds promise for advancing biotechnological applications and enhancing enzyme productivity in industrial settings.
Author Contributions
Conceptualization: P.A. and M.A.Z.C.; Methodology: F.S.B. and R.L.d.S.; Formal Analysis: R.L.d.S. and A.I.S.B.; Data Curation: F.S.B. Writing—Original Draft Preparation: F.S.B.; Supervision: P.A. and M.A.Z.C.; Funding Acquisition: M.A.Z.C. and P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by national funds through the National Council for Scientific and Technological Development (CNPq); Coordination of Improvement of Higher-Level Personnel (CAPES); and Foundation for Research Support and Technological Innovation of the State of Rio de Janeiro (FAPERJ). Buarque, F.S. acknowledges the scholarship grant from FAPERJ: E-26/204.344/2021.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Casas-Godoy, L.; Duquesne, S.; Bordes, F.; Sandoval, G.; Marty, A. Lipases: An overview. Methods Mol. Biol. 2012, 861, 3–30. [Google Scholar] [PubMed]
- Ge, F.; Chen, G.; Qian, M.; Xu, C.; Liu, J.; Cao, J.; Tan, Z. Artificial intelligence aided lipase production and engineering for enzymatic performance improvement. J. Agric. Food Chem. 2023, 71, 14911–14930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, M.; Zeng, Z.; Wan, D.; Yan, X.; Xia, J.; Gong, D. Production, purification, properties and current perspectives for modification and application of microbial lipases. Prep. Biochem. Biotechnol. 2024, 54, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, A.; Leow, T.C.; Rahman, M.B.A.; Oslan, S.N. Recent insight into the advances and prospects of microbial lipases and their potential applications in industry. Intern. Microbiol. 2024, 27, 1–35. [Google Scholar] [CrossRef]
- Fatima, S.; Faryad, A.; Ataa, A.; Joyia, F.A.; Parvaiz, A. Microbial lipase production: A deep insight into the recent advances of lipase production and purification techniques. Biotechnol. Appl. Biochem. 2021, 68, 445–458. [Google Scholar] [CrossRef]
- Salihu, A.; Alam, M.Z.; AbdulKarim, M.I.; Salleh, H.M. Lipase production: An insight in the utilization of renewable agricultural residues. Resour. Conserv. Recycl. 2012, 58, 36–44. [Google Scholar] [CrossRef]
- Lipase Market Outlook. Available online: https://www.futuremarketinsights.com/reports/lipase-market (accessed on 19 September 2024).
- Buarque, F.S.; Farias, M.A.; Sales, J.C.S.; Carniel, A.; Ribeiro, B.D.; Lopes, V.R.d.O.; Castro, A.M.; Coelho, M.A.Z. Valorization of Macauba (Acromia aculeata) for Integrated Production of Lipase by Yarrowia lipolytica and Biodiesel Esters. Fermentation 2023, 9, 992. [Google Scholar] [CrossRef]
- Nicaud, J.M. Yarrowia lipolytica. Yeast 2012, 29, 409–418. [Google Scholar] [CrossRef]
- Fickers, P.; Marty, A.; Nicaud, J.M. The lipases from Yarrowia lipolytica: Genetics, production, regulation, biochemical characterization and biotechnological applications. Biotechnol. Adv. 2011, 29, 632–644. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, M.; Chen, K.; Jiang, W.; Zhang, L.; Ji, X.; Lu, L. Exploiting synthetic biology platforms for enhanced biosynthesis of natural products in Yarrowia lipolytica. Bioresour. Technol. 2024, 399, 130614. [Google Scholar] [CrossRef]
- Hu, M.; Ge, J.; Jiang, Y.; Sun, X.; Guo, D.; Gu, Y. Advances and perspectives in genetic expression and operation for the oleaginous yeast Yarrowia lipolytica. Synth. Syst. Biotechnol. 2024, 9, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Bordes, F.; Barbe, S.; Escalier, P.; Mourey, L.; André, I.; Marty, A.; Tranier, S. Exploring the conformational states and rearrangements of Yarrowia lipolytica lipase. Biophys. J. 2010, 99, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.A.G.; Colen, G.; Takahashi, J.A. Yarrowia lipolytica and its multiple applications in the biotechnological industry. Sci. World J. 2014, 2014, 476207. [Google Scholar] [CrossRef] [PubMed]
- Weball, W. Production of Lipase by Yarrowia Lipolytica. Lipases from Yeasts (Review). Acta Biotechnol. 1991, 11, 159–167. [Google Scholar]
- Amaral, P.F.F.; De Almeida, A.P.R.; Peixoto, T.; Rocha-Leão, M.H.M.; Coutinho, J.A.P.; Coelho, M.A.Z. Beneficial effects of enhanced aeration using perfluorodecalin in Yarrowia lipolytica cultures for lipase production. World J. Microbiol. Biotechnol. 2007, 23, 339–344. [Google Scholar] [CrossRef]
- Posso Mendoza, H.; Pérez Salinas, R.; Tarón Dunoyer, A.; Tatis, C.C.; Morgado-Gamero, W.B.; Castillo Ramírez, M.; Parody, A. Evaluation of enzymatic extract with lipase activity of Yarrowia lipolytica. An application of data mining for the food industry wastewater treatment. In Security with Intelligent Computing and Big-Data Services: Proceedings of the Second International Conference on Security with Intelligent Computing and Big Data Services (SICBS-2018); Springer: Berlin/Heidelberg, Germany, 2020; pp. 304–313. [Google Scholar]
- Lupachev, E.V.; Voshkin, A.A.; Kisel’, A.V.; Kulov, N.N.; Zakhodyaeva, Y.A.; Polkovnichenko, A.V. Separation of an Industrial Mixture of Decalin or Naphthalene Fluorination Products: Cis-Perfluorodecalin, Trans-Perfluorodecalin and Perfluoro(butylcyclohexane): Physicochemical, Thermophysical, and Spectral Data. Processes 2023, 11, 3208. [Google Scholar] [CrossRef]
- Jawale, P.V.; Bhanage, B.M. Determination of stability and activity of immobilized lipase for transesterification reaction in fluorous solvent and deducing the reaction mechanism by molecular docking study. J. Indian Chem. Soc. 2024, 101, 101233. [Google Scholar] [CrossRef]
- Amaral, P.F.F.; Rocha-Leão, M.H.M.; Marrucho, I.M.; Coutinho, J.A.P.; Coelho, M.A.Z. Improving lipase production using a perfluorocarbon as oxygen carrier. J. Chem. Technol. Biotechnol. 2006, 81, 1368–1374. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, X.; Cai, W.; Cui, K.; Patil, S.A.; Guo, K. Recent progress on microbial electrosynthesis reactor designs and strategies to enhance the reactor performance. Biochem. Eng. J. 2023, 190, 108745. [Google Scholar] [CrossRef]
- Lambert, E.; Janjic, J.M. Quality by design approach identifies critical parameters driving oxygen delivery performance in vitro for perfluorocarbon based artificial oxygen carriers. Sci. Rep. 2021, 11, 5569. [Google Scholar] [CrossRef]
- Jägers, J.; Wrobeln, A.; Ferenz, K.B. Perfluorocarbon-based oxygen carriers: From physics to physiology. Eur. J. Physiol. 2021, 473, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Senko, O.; Maslova, O.; Aslanli, A.; Efremenko, E. Impact of Perfluorocarbons with Gas Transport Function on Growth of Phototrophic Microorganisms in a Free and Immobilized State and in Consortia with Bacteria. Appl. Sci. 2023, 13, 1868. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Amaral, P.F.F.; Coelho, M.A.Z.; Gonçalves, L.R.B. Lipase from Yarrowia lipolytica: Production, characterization and application as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2014, 101, 148–158. [Google Scholar] [CrossRef]
- Snopek, P.; Nowak, D.; Zieniuk, B.; Fabiszewska, A. Aeration and stirring in yarrowia lipolytica lipase biosynthesis during batch cultures with waste fish oil as a carbon source. Fermentation 2021, 7, 88. [Google Scholar] [CrossRef]
- Hagler, A.N.; Mendonqa-Hagler, L.C. Yeasts from Marine and Estuarine Waters with Different Levels of Pollution in the State of Rio de Janeiro, Brazil. Appl. Environ. Microbiol. 1981, 41, 173–178. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Sher, F.; Navarrete, A.A.; Américo-Pinheiro, J.H.P. Microbial adaptation to different environmental conditions: Molecular perspective of evolved genetic and cellular systems. Arch. Microbiol. 2022, 204, 144. [Google Scholar] [CrossRef]
- Elibol, M.; Mavituna, F. Effect of Perfluorodecalin as an Oxygen Carrier on Actinorhodin Production by Streptomyces Coelicolor A3(2). Appl. Microbiol. Biotechnol. 1995, 43, 206–210. [Google Scholar] [CrossRef]
- Gomes, N.; Aguedo, M.; Teixeira, J.; Belo, I. Oxygen mass transfer in a biphasic medium: Influence on the biotransformation of methyl ricinoleate into γ-decalactone by the yeast Yarrowia lipolytica. Biochem. Eng. J. 2007, 35, 380–386. [Google Scholar] [CrossRef]
- Amaral, P.F.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A. Biosurfactants from yeasts: Characteristics, production and application. In Biosurfactants; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; pp. 236–249. [Google Scholar]
- Feitosa, I.C.; de Pinho Barbosa, J.M.; Orellana, S.C.; Lima, Á.S.; Soares, C.M.F. Produção de lipase por meio de microrganismos isolados de solos com histórico de contato com petróleo. Acta Scientiarum. Technol. 2010, 32, 27–31. [Google Scholar]
- Fickers, P.; Benetti, P.H.; Waché, Y.; Marty, A.; Mauersberger, S.; Smit, M.S.; Nicaud, J.M. FEMS Yeast Research; Elsevier: Amsterdam, The Netherlands, 2005; pp. 527–543. [Google Scholar]
- Burkert, J.F.d.M.; Maldonado, R.R.; Maugeri Filho, F.; Rodrigues, M.I. Comparison of lipase production by Geotrichum candidum in stirring and airlift fermenters. J. Chem. Technol. Biotechnol. 2005, 80, 61–67. [Google Scholar] [CrossRef]
- Pereira-Meirelles, F.V.; Rocha-Leão, M.H.; Sant’Anna, G.L., Jr. Lipase location in Yarrowia lipolytica cells. Biotechnol. Lett. 2000, 11, 71–75. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Buarque, F.S.; Nogueira, V.L.R.; Melo, V.M.M.; Guisán, J.M.; Ribeiro, B.D.; Gonçalves, L.R.B.; Coelho, M.A.Z. Partial purification of crude lipase extract from Yarrowia lipolytica: Precipitation, aqueous two-phase systems (ATPS), and immobilization methods. Clean. Chem. Eng. 2023, 6, 100105. [Google Scholar] [CrossRef]
- Ju, L.-K.; Lee, J.F.; Armiger, W.B. Enhancing Oxygen Transfer in Bioreactors by Perfluorocarbon Emulsions. Biotechnol. Prog. 1991, 7, 323–329. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Freire, M.; Coutinho, J.A.P.; Marrucho, I.M. Solubility of oxygen in liquid perfluorocarbons. Fluid Phase Equilibria 2004, 222–223, 325–330. [Google Scholar] [CrossRef]
- Dalmau, E.; Montesinos, J.L.; Lotti, M.; Casas, C. Effect of Different Carbon Sources on Lipase Production by Candida Rugosa. Enzym. Microb. Technol. 2000, 26, 657–663. [Google Scholar] [CrossRef]
- Amaral, P.F.F.; da Silva, J.M.; Lehocky, M.; Barros-Timmons, A.M.V.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A.P. Production and characterization of a bioemulsifier from Yarrowia lipolytica. Process Biochem. 2006, 41, 1894–1898. [Google Scholar] [CrossRef]
- Maldonado, M.R.; Alnoch, R.C.; de Almeida, J.M.; Santos, L.A.d.; Andretta, A.T.; Ropaín, R.d.P.C.; de Souza, E.M.; Mitchell, D.A.; Krieger, N. Key mutation sites for improvement of the enantioselectivity of lipases through protein engineering. Biochem. Eng. J. 2021, 172, 108047. [Google Scholar] [CrossRef]
- Mohanty, S.; Babbal Chauhan, S.; Talwar, M.; Khasa, Y.P. Continuous cell recycling in methylotrophic yeast Pichia pastoris to enhance product yields: A case study with Yarrowia lipolytica lipase Lip2. Biotechnol. Bioprocess Eng. 2024, 30, 1–16. [Google Scholar] [CrossRef]
- Pigne, G.; Wang, H.-J.; Fudalej, F.; Seman, M.; Gaillardin, C.; Nicaud, J.-M. Autocloning and Amplification of LIP2 in Yarrowia Lipolytica. Appl. Environ. Microbiol. 2000, 66, 3283–3289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).