Compositional Analysis and Bioactivity Assessment of the Anemone baicalensis Rhizome: Exploring the Potential for Substituting Anemones raddeanae Rhizoma
Abstract
1. Introduction
2. Results and Discussion
2.1. Qualitative Phytochemical Analysis
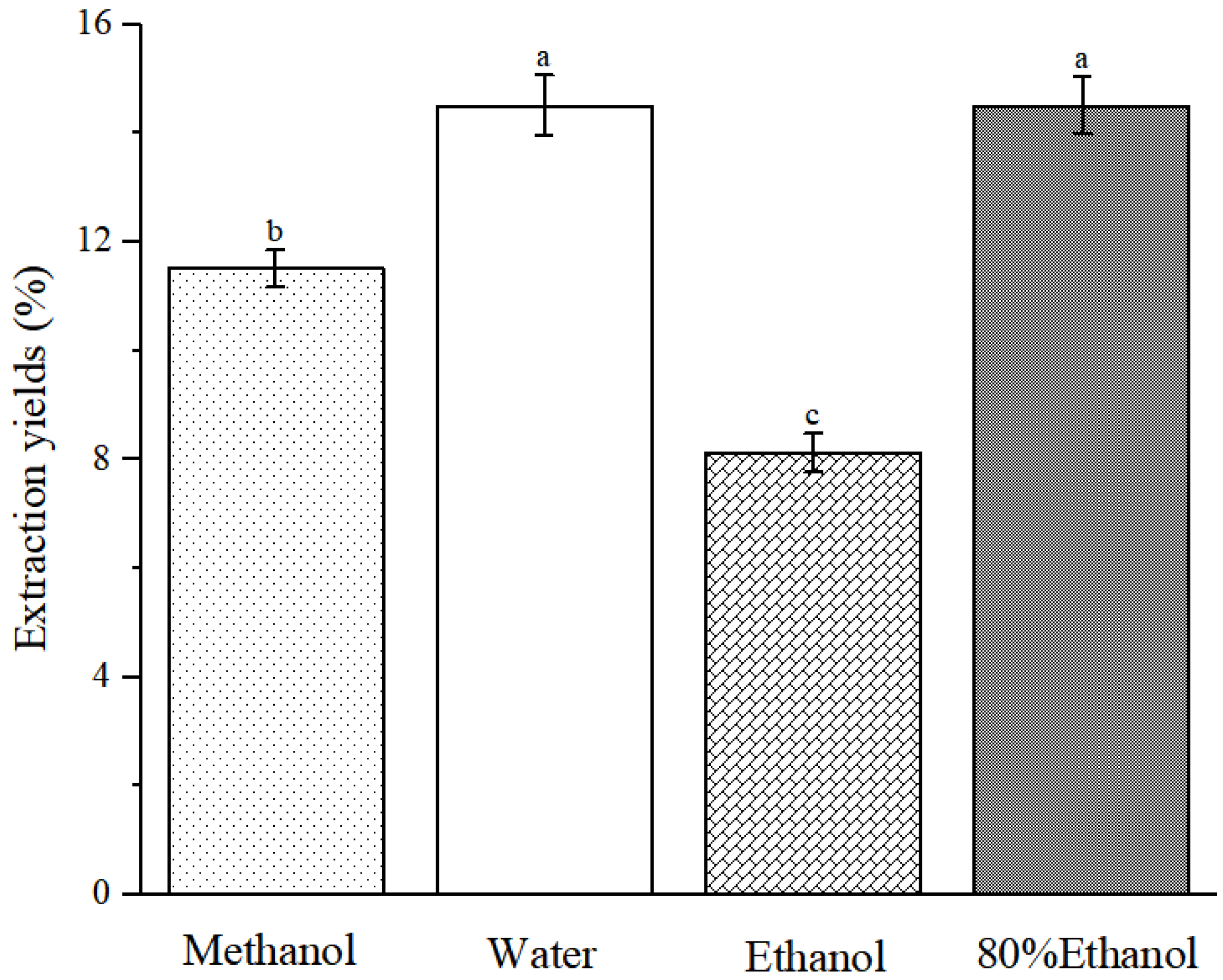
2.2. Extraction Yields
2.3. Quantitative Phytochemical Analysis
2.3.1. Total Carbohydrate Content (TCC)
2.3.2. Total Protein Content (TProC)
2.3.3. Total Alkaloid Content (TAC)
2.3.4. Total Phenolic Content (TPheC)
2.3.5. Total Phenolic Acid Content (TPAC)
2.3.6. Total Flavonoid Content (TFC)
2.3.7. Total Tannin Content (TTanC), Gallotannin Content (GC) and Condensed Tannin Content (CTC)
2.3.8. Total Triterpenoid Content (TTriC)
2.4. Antioxidant Activity In Vitro
2.4.1. Free Radical Scavenging Ability
2.4.2. Ferric-Reducing Antioxidant Power (FRAP) and Cupric Ion Reducing Antioxidant Capacity (CUPRAC)
2.4.3. Metal Chelation
2.4.4. Hydrogen Peroxide (H2O2), Singlet Oxygen and Hypochlorous Acid (HClO)
2.4.5. β-Carotene Bleaching and Nitric Oxide (NO)
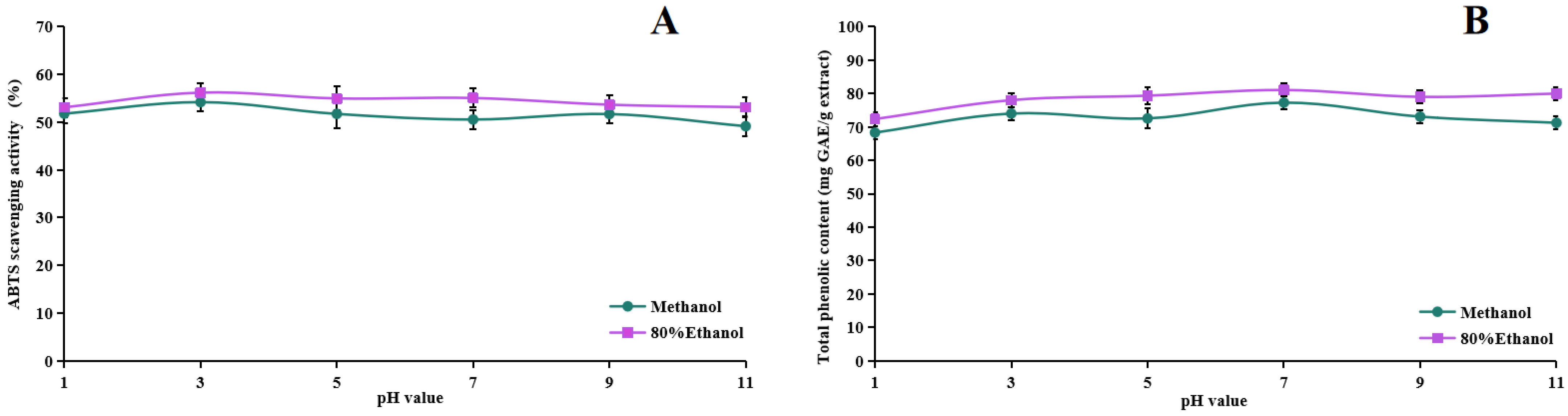
2.5. Stability Studies of Methanol Extract and 80% Ethanol Extract of ABR
2.6. UHPLC-MS Analysis
2.7. Oral Acute Toxicity Study
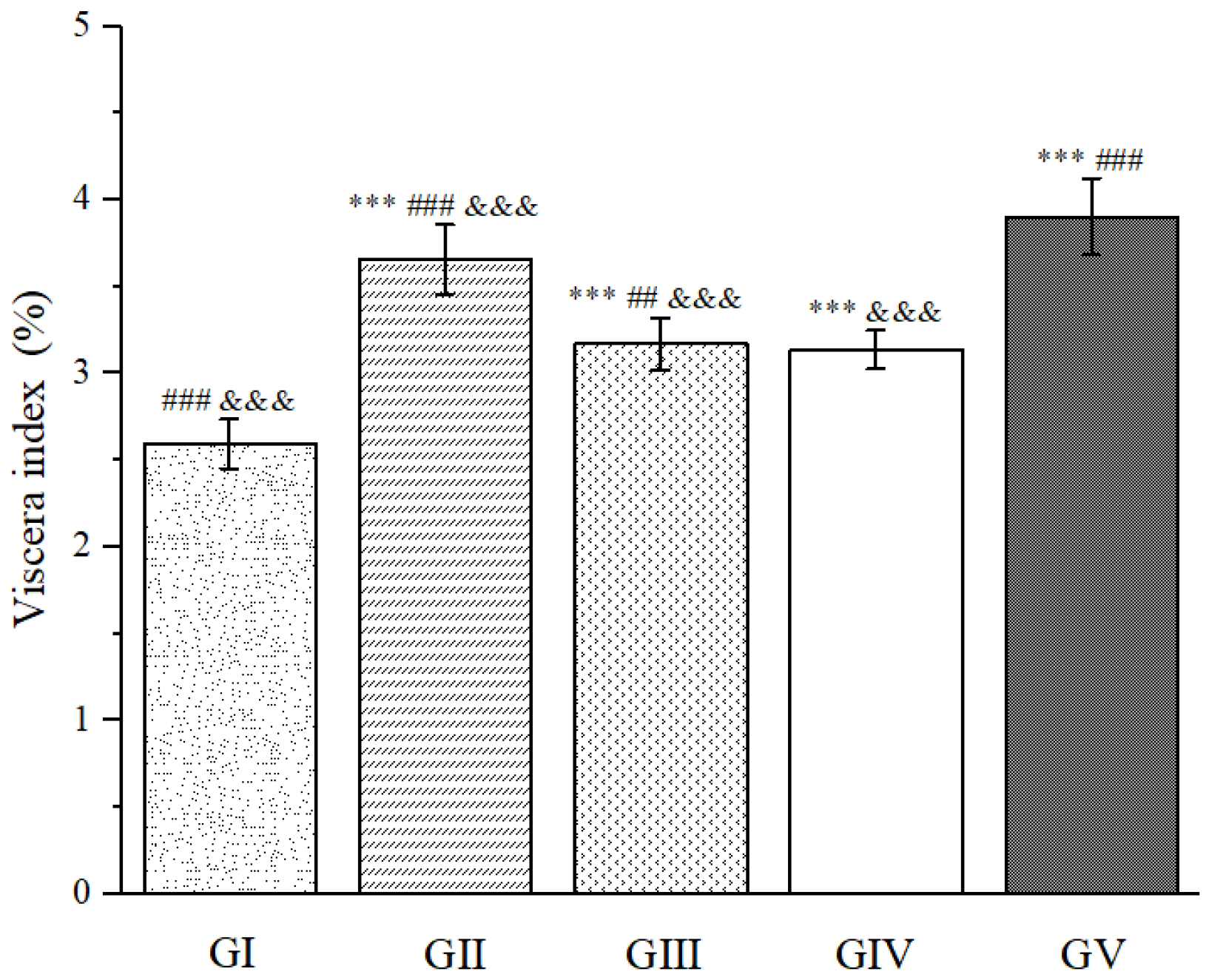
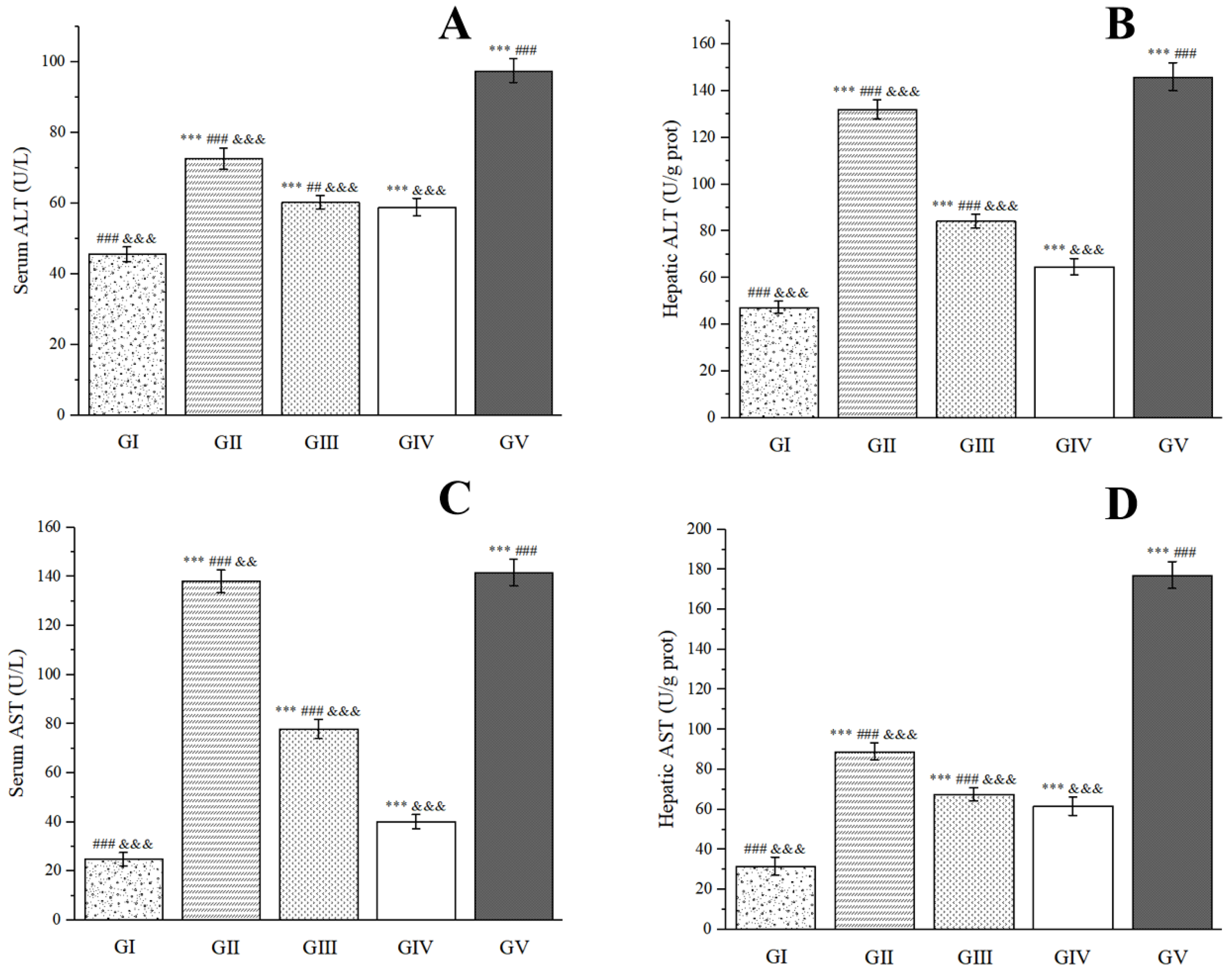
2.8. Hepatoprotective Activity
3. Material and Methods
3.1. Materials
3.2. Methods
3.2.1. Qualitative Phytochemical Analysis
3.2.2. Preparation of Four Extracts of ABR
3.2.3. Quantitative Phytochemical Analysis
3.2.4. Antioxidant Activity Assays
3.2.5. Stability Studies of Methanol and 80% Ethanol Extract
3.2.6. UHPLC-MS Analysis
3.2.7. Oral Acute Toxicity Study
3.2.8. Hepatoprotective Experiments
3.2.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABR | Anemone baicalensis rhizome |
| ABAP | Anemone baicalensis aerial parts |
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt |
| ALB | Albumin |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BHT | Butylated hydroxytoluene |
| BSAE | Bovine serum albumin equivalents |
| BHE | Berberine hydrochloride equivalents |
| CAT | Catalase |
| CAE | Caffeic acid equivalents |
| CTC | Condensed tannin content |
| CUPRAC | Cupric ion reducing antioxidant capacity |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| d-GalN | d-Galactosamine |
| EDTANa2 | Ethylenediaminetetraacetic acid disodium salt |
| FRAP | Ferric-reducing antioxidant power |
| GE | Glucose equivalents |
| GAE | Gallic acid equivalents |
| GRE | Ginsenoside Re equivalents |
| GSH | Glutathione |
| GC | Gallotannin content |
| GI | Control group |
| GII | d-GalN + ABR150 group |
| GIII | d-GalN + ABR300 group |
| GIV | d-GalN + SMN group |
| GV | d-GalN group |
| H2O2 | Hydrogen peroxide |
| HClO | Hypochlorous acid |
| IC50 | Half maximal inhibitory concentration |
| MDA | Malondialdehyde |
| QE | Quercetin equivalents |
| SMN | Silymarin |
| Trolox | 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
| TBHQ | Tertiary butylhydroquinone |
| TCC | Total carbohydrate content |
| TProC | Total protein content |
| TAC | Total alkaloid content |
| TPheC | Total phenolic content |
| TPAC | Total phenolic acid content |
| TFC | Total flavonoid content |
| TTanC | Total tannin content |
| TTriC | Total triterpenoid content |
| TAE | Tannic acid equivalents |
| UHPLC-ESI-Q-TOF-MS | Ultra-high-performance liquid chromatography–electrospray ionization–quadrupole–time of flight–mass spectrometry |
| UHPLC–MS | Ultra-high-performance liquid chromatography–mass spectrometry |
| γ-GT | γ-Glutamyl transpeptidase |
References
- Flora of China Editorial Committee of Chinese Academy of Sciences. The Flora of China; Science Press: Beijing, China, 1980; Volume 28, p. 20. [Google Scholar]
- Liu, Y.; Liu, L.; Tian, C.K.; Zhou, D.Z. Research progress of studies on chemical constituents and biologic activities of Anemone species. China J. Chin. Mater. Med. 2019, 44, 912–919. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume 1, p. 175. [Google Scholar]
- Wang, S.L.; Zhao, Z.K.; Sun, J.F.; Sun, Y.T.; Pang, X.Q.; Zeng, Z.W.; Xie, T. Review of Anemone raddeana Rhizome and its pharmacological effects. Chin. J. Integr. Med. 2018, 24, 72–79. [Google Scholar] [CrossRef]
- Li, C.; Cao, F.C.; Lv, C.N.; Qin, R.L.; Jia, L.Y.; Lu, J.C. Pharmacognostic identification of six species of medicinal plant of Anemone from northeast of China. Shenyang Pharm. Univ. 2015, 32, 548–555. [Google Scholar] [CrossRef]
- Sun, S.; Xia, G.Q.; Pang, H.; Zhu, J.Y.; Li, L.; Zang, H. Phytochemical analysis and antioxidant activities of various extracts from the aerial part of Anemone baicalensis Turcz.: In vitro and in vivo studies. Molecules 2024, 29, 4602. [Google Scholar] [CrossRef]
- Chen, X.; Lu, J.C.; He, W.F.; Chi, H.D.; Yamashita, K.; Manabe, M.; Kodama, H. Antiperoxidation activity of triterpenoids from rhizome of Anemone raddeana. Fitoterapia 2009, 80, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.S. Carbohydrate drugs: Current status and development prospect. Drug Discov. Ther. 2015, 9, 79–87. [Google Scholar] [CrossRef]
- Ni, T.J.; Zhang, S.; Rao, J.; Zhao, J.Q.; Huang, H.Q.; Liu, Y.; Ding, Y.; Liu, Y.Q.; Ma, Y.C.; Zhang, S.J.; et al. Phlorizin, an important glucoside: Research progress on its biological activity and mechanism. Molecules 2024, 29, 741. [Google Scholar] [CrossRef]
- Tang, W.W.; Chen, Y.; Guo, F.X. Effects of topping on rhizome, and analysis of chemical composition, antioxidant activity and α-amylase and α-glucosidase inhibition of the aerial parts in Polygonatum cyrtonema. PLoS ONE 2023, 18, e0287894. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Fernández-Fernández, C.; Carneiro-Freire, N.; Vila-Altesor, M.; Ameneiros-Rodríguez, E. The differential effect of animal versus vegetable dietary protein on the clinical manifestations of diabetic kidney disease in humans. Clin. Nutr. ESPEN 2022, 48, 21–35. [Google Scholar] [CrossRef]
- Yang, N.N.; Guo, J.F.; Zhang, J.; Gao, S.; Xiang, Q.W.; Wen, J.Y.; Huang, Y.; Rao, C.L.; Chen, Y. A toxicological review of alkaloids. Drug Chem. Toxicol. 2024, 47, 1267–1281. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Quitete, F.T.; Almeida Santos, G.M.; de Oliveira Ribeiro, L.; Aguiar da Costa, C.; Freitas, S.P.; Martins da Matta, V.; Daleprane, J.B. Phenolic-rich smoothie consumption ameliorates non-alcoholic fatty liver disease in obesity mice by increasing antioxidant response. Chem. Biol. Interact. 2021, 336, 109369. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Sun, P.; Feng, J.; Yuan, J.; Wang, Y.; Shang, Y.F.; Niu, X.L.; Yang, S.H.; Wei, Z.J. Solvent effect on phenolics and antioxidant activity of Huangshan Gongju (Dendranthema morifolium (Ramat) Tzvel. cv. Gongju) extract. Food Chem. Toxicol. 2021, 147, 111875. [Google Scholar] [CrossRef]
- Chipaca-Domingos, H.S.; Ferreres, F.; Fornari, T.; Gil-Izquierdo, A.; Pessela, B.C.; Villanueva-Bermejo, D. Pressurized liquid extraction for the production of extracts with antioxidant activity from Borututu (Cochlospermum angolense Welw.). Foods 2023, 12, 1186. [Google Scholar] [CrossRef]
- Ozen, T.; Demir, M.; Marah, S.; Korkmaz, H. In vitro and in silico assessment of bioactivity potencies with components of Aristolochia bodamae Dingler extracts from Turkey. Chem. Sel. 2024, 9, e202403703. [Google Scholar] [CrossRef]
- Ibitoye, O.B.; Ajiboye, T.O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch. Physiol. Biochem. 2018, 124, 410–417. [Google Scholar] [CrossRef]
- Singh, B.; Semwal, B.C. A compressive review on source, toxicity and biological activity of flavonoid. Curr. Top. Med. Chem. 2024, 24, 2093–2116. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.L.; Ma, Y.X. Seasonal changes and response to stress of total flavonoids content of Farfugium japonicum. J. Zhejiang Univ. Med. Sci. 2013, 42, 319–325. [Google Scholar]
- Sultan, S.; Hussain, M.; Ashfaq, S.; Ullah, A.; Shabir, T.; Khan, N.; Husna, A.U. Microscopic investigation and pharmacognostic evaluation of leaves of Anemone rupicola Camb. Microsc. Res. Tech. 2024, 87, 1904–1911. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Gong, W.; Wang, Q.; Xu, D.; Liu, M.; Bi, K.; Song, Y. Anticancer, antioxidant, and antimicrobial activities of anemone (Anemone cathayensis). Food. Sci. Biotechnol. 2012, 21, 551–557. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Li, Y.Y.; Lin, B.; Guan, P.P.; Mu, Y.; Qiao, W.J.; Zhang, J.S.; Huang, X.S.; Han, L. New phenolic glycosides from Anemone chinensis Bunge and their antioxidant activity. Nat. Prod. Res. 2022, 36, 5009–5015. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Recent overview of potent antioxidant activity of coordination compounds. Antioxidants 2023, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Pirvu, L.; Stefaniu, A.; Neagu, G.; Pintilie, L. Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies. Open Chem. 2022, 20, 299–312. [Google Scholar] [CrossRef]
- Ghaffari, H.; Venkataramana, M.; Jalali Ghassam, B.; Chandra Nayaka, S.; Nataraju, A.; Geetha, N.P.; Prakash, H.S. Rosmarinic acid mediated neuroprotective effects against H2O2-induced neuronal cell damage in N2A cells. Life Sci. 2014, 113, 7–13. [Google Scholar] [CrossRef]
- Gülçin, I.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Antioxidant activity of saponins isolated from ivy: Alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F. Planta Med. 2004, 70, 561–563. [Google Scholar] [CrossRef]
- Dahmani, W.; Akissi, Z.L.E.; Elaouni, N.; Bouanani, N.E.; Mekhfi, H.; Bnouham, M.; Legssyer, A.; Sahpaz, S.; Ziyyat, A. Carob leaves: Phytochemistry, antioxidant properties, vasorelaxant effect and mechanism of action. J. Ethnopharmacol. 2024, 340, 119226. [Google Scholar] [CrossRef]
- Haida, Z.; Hakiman, M. A comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 2019, 7, 1555–1563. [Google Scholar] [CrossRef]
- Cho, S.; Choi, H.J.; Song, G.Y.; Bae, J.S. Therapeutic effects of hederacolchiside A1 on particulate matter-induced pulmonary injury. Toxicon 2024, 241, 107650. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.C.; Gu, X.; Xiao, P. Anemone medicinal plants: Ethnopharmacology, phytochemistry and biology. Acta Pharm. Sin. B 2017, 7, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, X.; Chi, H.; Li, Z.; Wang, C.; Wang, Q.; Feng, H.; Li, P. A qualitative analysis of cultured adventitious ginseng root’s chemical composition and immunomodulatory effects. Molecules 2023, 29, 111. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Xu, Q.; Yu, Z.; Song, F.; Luo, Q.; Wang, S.; Tang, H.; Li, H. Exploring the underlying mechanisms of Danshen-Shanzha decoction on coronary heart disease: An integrated analysis combining pharmacoinformatics and experimental validation. J. Ethnopharmacol. 2025, 337, 118779. [Google Scholar] [CrossRef]
- Tu, J.; Guo, J.; Dong, H.; Cheng, P.; Brennan, C.; Bai, W.; Zeng, X.; Yang, J. Novel umami-, salty-, and kokumi-enhancing γ-glutamyl tripeptides synthesized with the bitter dipeptides from defatted peanut meal protein hydrolysate. J. Agric. Food Chem. 2023, 71, 7812–7819. [Google Scholar] [CrossRef]
- Liu, D.Y.; Deng, H.; Liu, Y.J.; Chen, H.X. Analysis and determination of amino acids and trace elements from Anemones raddeana Rhizoma. Spec. Wild Econ. Anim. Plant Res. 1991, 15, 53–55. [Google Scholar]
- Wen, Y.Y.; Zhang, S.J.; Meng, X.Y.; Zhao, C.Y.; Hou, B.; Zhu, X.X.; Cai, W.W.; Zhou, Y.T.; Qiu, L.Y.; Sun, H.J. Water extracts of Tibetan medicine Wuweiganlu attenuates experimental arthritis via inducing macrophage polarization towards the M2 type. J. Ethnopharmacol. 2024, 318, 116934. [Google Scholar] [CrossRef]
- Zhou, S.; Gao, X.; Chen, C.; Zhang, J.; Zhang, Y.; Zhang, L.; Yan, X. Porcine cardiac blood—Salvia miltiorrhiza root alleviates cerebral ischemia reperfusion injury by inhibiting oxidative stress induced apoptosis through PI3K/AKT/Bcl-2/Bax signaling pathway. J. Ethnopharmacol. 2023, 316, 116698. [Google Scholar] [CrossRef]
- Mróz, M.; Parchem, K.; Jóźwik, J.; Domingues, M.R.; Kusznierewicz, B. The impact of different drying methods on the metabolomic and lipidomic profiles of Arthrospira platensis. Molecules 2024, 29, 1747. [Google Scholar] [CrossRef]
- Hayes, C.; Nurkolis, F.; Laksemi, D.A.A.S.; Chung, S.; Park, M.N.; Choi, M.; Choi, J.; Darmaputra, I.G.N.; Gunawan, W.B.; Lele, J.A.J.M.N.; et al. Coffee silverskin phytocompounds as a novel anti-aging functional food: A pharmacoinformatic approach combined with in vitro study. Molecules 2023, 28, 7037. [Google Scholar] [CrossRef]
- Wang, Y.; Han, L.Y.; Sun, C.D.; Cao, J.P. Golden-Edge Fructus forsythiae Peel Alcohol Extract as Well as Preparation Method and Application. C.N. Patent 117,323,377, 2 January 2024. [Google Scholar]
- Zhang, D.; Miyase, T.; Kuroyanagi, M.; Umehara, K.; Ueno, A. Five new triterpene saponins, polygalasaponins XXVIII-XXXII from the root of Polygala japonica houtt. Chem. Pharm. Bull. 1996, 44, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Q.; Zheng, Y.; Lu, J. Anti-breast cancer and toxicity studies of total secondary saponin from Anemone raddeana rhizome on MCF-7 cells via ROS generation and PI3K/AKT/mTOR inactivation. J. Ethnopharmacol. 2020, 259, 112984. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Miao, X.; Ge, M.; Zhang, M.; Lv, Z.; Wang, W.; Chang, Y.; Ouyang, H.; He, J. Exploration of habitat-related chemical markers for Stephania tetrandra applying multiple chromatographic and chemometric analysis. Molecules 2022, 27, 7224. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Mohammad, F.V.; Noorwala, M.; Sener, B. A new bidesmosidic triterpenoidal saponin from the roots of Symphytum officinale. Planta Med. 1993, 59, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; He, W.; Lu, J.; Wang, Z.; Yamashita, K.; Yokoyama, M.; Kodama, H. Effects of five oleanolic acid triterpenoid saponins from the rhizome of Anemone raddeana on stimulus-induced superoxide generation, phosphorylation of proteins and translocation of cytosolic compounds to cell membrane in human neutrophils. Fitoterapia 2012, 83, 402–407. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Jiang, Y.; Qi, Y.; Guan, H.; Bai, L.; Chen, P.; Gao, W.; Zhuang, G.D.; Lu, T.; Yan, G. Comparative study on Angelica sinensis after different processing with yellow rice wine in color, aromas, chemical components, and antioxidant activities. Food Chem. X 2023, 19, 100822. [Google Scholar] [CrossRef]
- Lai, Y.; Hua, L.; Yang, J.; Xu, J.; Chen, J.; Zhang, S.; Zhu, S.; Li, J.; Shi, S. The effect of chinese agarwood essential oil with cyclodextrin inclusion against PCPA-induced insomnia rats. Molecules 2023, 28, 635. [Google Scholar] [CrossRef]
- Zhao, Z.K. Quality Control of Anemone raddeana Rhizome and Its Toxicity. Master’s Thesis, Hangzhou Normal University, Hangzhou, China, 2013. [Google Scholar]
- Wang, S.S.; Zhou, S.Y.; Xie, X.Y.; Zhao, L.; Fu, Y.; Cai, G.Z.; Gong, J.Y. Comparison of the acute toxicity, analgesic and anti-inflammatory activities and chemical composition changes in Rhizoma Anemones raddeanae caused by vinegar processing. BMC Complement. Med. Ther. 2020, 20, 7. [Google Scholar] [CrossRef]
- Xu, G.B.; Xiao, Y.H.; Zhang, Q.Y.; Zhou, M.; Liao, S.G. Hepatoprotective natural triterpenoids. Eur. J. Med. Chem. 2018, 145, 691–716. [Google Scholar] [CrossRef]
- Islam Shawon, S.; Nargis Reyda, R.; Qais, N. Medicinal herbs and their metabolites with biological potential to protect and combat liver toxicity and its disorders: A review. Heliyon 2024, 10, e25340. [Google Scholar] [CrossRef]
- Jaffar, H.M.; Al-Asmari, F.; Khan, F.A.; Rahim, M.A.; Zongo, E. Silymarin: Unveiling its pharmacological spectrum and therapeutic potential in liver diseases-A comprehensive narrative review. Food Sci. Nutr. 2024, 12, 3097–3111. [Google Scholar] [CrossRef]
- Benić, M.S.; Nežić, L.; Vujić-Aleksić, V.; Mititelu-Tartau, L. Novel therapies for the treatment of drug-induced liver injury: A systematic review. Front. Pharmacol. 2022, 12, 785790. [Google Scholar] [CrossRef]
- Ali, S.A.; Sharief, N.H.; Mohamed, Y.S. Hepatoprotective activity of some medicinal plants in Sudan. J. Evid.-Based Complement. Altern. Med. 2019, 2019, 2196315. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W.; Zhang, X.L.; Wu, Q.H.; Jin, Y.B.; Ning, C.T.; Wang, R.; Mao, J.X.; Chen, M. The hepatoprotective effects of Sedum sarmentosum extract and its isolated major constituent through Nrf2 activation and NF-κB inhibition. Phytomedicine 2019, 53, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.F.; Chen, Y.H.; Shi, X.P.; Ye, C.; Wang, J.W.; Huang, J.Z.; Zhang, B.; Deng, Z.Y. Hepatoprotective effects of different mulberry leaf extracts against acute liver injury in rats by alleviating oxidative stress and inflammatory response. Food Funct. 2022, 13, 8593–8604. [Google Scholar] [CrossRef]
- Ahmed Abdel-Reheim, M.; Messiha, B.A.S.; Abo-Saif, A.A. Quillaja saponaria bark saponin protects Wistar rats against ferrous sulphate-induced oxidative and inflammatory liver damage. Pharm. Biol. 2017, 55, 1972–1983. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Wei, J.H.; Fang, S.Q.; Tang, Y.P.; Cheng, H.B.; Wang, T.L.; Li, C.Y.; Peng, G.P. Hepatoprotective triterpene saponins from the roots of Glycyrrhiza inflata. Molecules 2015, 20, 6273–6283. [Google Scholar] [CrossRef]
- Shilpa, V.S.; Shams, R.; Dash, K.K.; Pandey, V.K.; Dar, A.H.; Ayaz Mukarram, S.; Harsányi, E.; Kovács, B. Phytochemical properties, extraction, and pharmacological benefits of naringin: A review. Molecules 2023, 28, 5623. [Google Scholar] [CrossRef]
- Xue, H.; Wei, M.; Ji, L. Chlorogenic acids: A pharmacological systematic review on their hepatoprotective effects. Phytomedicine 2023, 118, 154961. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Habtemariam, S. Hepatoprotective effects of rosmarinic acid: Insight into its mechanisms of action. Biomed. Pharmacother. 2019, 112, 108600. [Google Scholar] [CrossRef]
- Gepdiremen, A.; Mshvildadze, V.; Süleyman, H.; Elias, R. Acute anti-inflammatory activity of four saponins isolated from ivy: Alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw edema. Phytomedicine 2005, 12, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna Okoro, C.; Aloke, C.; Ibiam, U.A.; Obasi, N.A.; Orji, O.U.; Ogbonnia, E.C.; Ogbu, P.N.; Emelike, C.U.; Ufebe, G.O.; Nwamaka Ezeani, N. Studies on ethanol extracts of Olax subscorpioidea against carbon tetrachloride-induced hepatotoxicity in rats. Pak. J. Biol. Sci. 2021, 24, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Li, D.L.; Men, J.Z. Clinical observation of herbal extract of Anemone raddeana Regel in the treatment of hepatic fibrosis in 67 cases of chronic hepatitis B. World J. Integr. Tradit. Wes. Med. 2010, 5, 219–221. [Google Scholar] [CrossRef]
- Liu, X. Study on Chemical Composition and Anti-Liver Fibrosis Effect of Anemones raddeanae Rhizoma Before and After Processing. Ph.D. Thesis, Changchun University of Chinese Medicine, Changchun, China, 2022. [Google Scholar] [CrossRef]
- Zhang, D.; Lei, T.; Lv, C.; Zhao, H.; Xu, H.; Lu, J. Pharmacokinetic studies of active triterpenoid saponins and the total secondary saponin from Anemone raddeana Regel. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1044–1045, 54–62. [Google Scholar] [CrossRef]
- Feng, J.X.; Sun, Y.; Wei, Z.B.; Sun, H.; Li, L.; Zhu, J.Y.; Xia, G.Q.; Zang, H. Screening the extract of Laportea bulbifera (Sieb. et Zucc.) Wedd. based on active component content, its antioxidant capacity and exploration of hepatoprotective activity in rats. Molecules 2023, 28, 6256. [Google Scholar] [CrossRef]








| Medicament Portions | Extracting Solvents | TCC (mg GE/g Extract) | TProC (mg BSAE/g Extract) | TAC (mg BHE/g Extract) | TPheC (mg GAE/g Extract) | TPAC (mg CAE/g Extract) | TFC (mg QE/g Extract) | TTanC (mg TAE/g Extract) | GC (mg GAE/g Extract) | CTC (mg GAE/g Extract) | TTriC (mg GRE/g Extract) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABAP | Methanol | 424.65 ± 3.08 f | N.T. | 4.36 ± 0.03 b | 30.92 ± 0.15 c | 15.38 ± 0.86 b | 13.73 ± 0.47 b | 22.72 ± 0.11 c | NONE | NONE | 86.85 ± 2.43 a |
| Water | 355.38 ± 2.86 h | 493.08 ± 3.15 a | 4.12 ± 0.03 c | 34.53 ± 0.24 a | 11.62 ± 0.79 c | 4.04 ± 0.21 d | 27.25 ± 0.38 a | 4.33 ± 0.11 f | NONE | 12.30 ± 1.05 f | |
| Ethanol | 433.95 ± 5.17 e | N.T. | 5.71 ± 0.01 a | 19.06 ± 0.43 f | 8.97 ± 0.53 d | 6.14 ± 0.12 c | 16.31 ± 0.20 d | NONE | NONE | 73.20 ± 1.62 b | |
| 80% Ethanol | 389.32 ± 3.24 g | N.T. | 3.00 ± 0.00 d | 31.57 ± 0.58 b | 16.45 ± 0.20 a | 15.44 ± 0.31 a | 25.93 ± 0.18 b | 6.29 ± 0.15 d | NONE | 42.97 ± 0.75 c | |
| ABR | Methanol | 700.30 ± 5.02 a | N.T. | NONE | 19.80 ± 0.13 e | 7.79 ± 0.23 f | NONE | 15.70 ± 0.12 e | 7.37 ± 0.34 b | NONE | 36.01 ± 1.31 d |
| Water | 522.50 ± 3.37 d | 191.04 ± 1.80 b | NONE | 16.04 ± 0.14 h | 3.14 ± 0.11 h | NONE | 15.39 ± 0.09 f | 4.50 ± 0.14 e | NONE | NONE | |
| Ethanol | 525.36 ± 4.37 c | N.T. | NONE | 17.11 ± 0.08 g | 8.59 ± 0.44 e | NONE | 14.63 ± 0.15 g | 7.27 ± 0.15 c | NONE | 1.24 ± 0.16 g | |
| 80% Ethanol | 587.48 ± 3.18 b | N.T. | NONE | 21.76 ± 0.32 d | 7.53 ± 0.19 g | NONE | 14.39 ± 0.08 h | 8.53 ± 0.28 a | NONE | 29.27 ± 0.55 e |
| Medicament Portions | Extracting Solvents | DPPH (IC50, μg/mL) | ABTS (IC50, μg/mL) | Hydroxyl Radicals (%, 2500 μg/mL) | Superoxide Radicals (%, 2143 μg/mL) |
|---|---|---|---|---|---|
| ABAP | Methanol | 37.21 ± 1.66 c | 71.21 ± 2.05 e | 47.28 ± 0.43 c | 55.01 ± 0.45 b |
| Water | 93.53 ± 2.35 g | 52.36 ± 0.54 c | 41.12 ± 1.21 e | 20.81 ± 0.34 f | |
| Ethanol | 99.76 ± 2.45 h | 125.03 ± 2.40 j | 40.74 ± 1.62 f | 32.09 ± 0.54 d | |
| 80% Ethanol | 45.14 ± 0.88 d | 58.10 ± 1.36 d | 42.15 ± 1.08 d | 48.72 ± 0.55 c | |
| ABR | Methanol | 91.36 ± 2.49 f | 86.13 ± 4.42 h | 14.26 ± 0.60 h | 11.76 ± 1.21 h |
| Water | 376.72 ± 5.00 j | 113.58 ± 2.09 i | 29.24 ± 1.94 g | 8.56 ± 0.81 i | |
| Ethanol | 81.58 ± 1.89 e | 84.30 ± 1.85 g | NONE | 21.38 ± 0.85 e | |
| 80% Ethanol | 115.61 ± 5.22 i | 74.48 ± 0.46 f | NONE | 14.43 ± 0.34 g | |
| Standard Antioxidant | Trolox * | 2.19 ± 0.23 a | 3.16 ± 0.17 a | 86.48 ± 2.68 a | N.T. |
| BHT * | 10.06 ± 0.32 b | 5.18 ± 0.14 a | 72.33 ± 1.06 b | N.T. | |
| Curcumin * | N.T. | N.T. | N.T. | 74.97 ± 0.53 a |
| Medicament Portions | Extracting Solvents | TEACFRAP | TEACCUPRAC | Iron Chelation (IC50, μg/mL) | Copper Chelation (IC50, μg/mL) |
|---|---|---|---|---|---|
| ABAP | Methanol | 0.22 ± 0.00 b | 0.14 ± 0.00 c | 921.30 ± 4.04 b | 473.20 ± 4.85 d |
| Water | 0.20 ± 0.00 d | 0.13 ± 0.00 d | 2019.96 ± 13.63 f | 347.42 ± 3.84 c | |
| Ethanol | 0.18 ± 0.00 f | 0.06 ± 0.00 f | 1481.40 ± 11.92 d | 1243.17 ± 5.87 h | |
| 80% Ethanol | 0.21 ± 0.00 c | 0.17 ± 0.00 b | 1065.22 ± 10.94 c | 317.04 ± 3.12 b | |
| ABR | Methanol | 0.17 ± 0.00 g | 0.02 ± 0.00 h | 2388.66 ± 14.81 g | 1547.81 ± 21.16 i |
| Water | 0.17 ± 0.00 g | 0.06 ± 0.00 f | >2500 d | 585.59 ± 23.27 f | |
| Ethanol | 0.18 ± 0.00 f | 0.05 ± 0.00 g | >2500 d | 1164.63 ± 35.17 g | |
| 80% Ethanol | 0.19 ± 0.00 e | 0.09 ± 0.00 e | 2096.62 ± 20.14 e | 519.30 ± 16.35 e | |
| Standard Antioxidant | Trolox * | 0.91 ± 0.02 a | 0.90 ± 0.02 a | N.T. | N.T. |
| EDTANa2 * | N.T. | N.T. | 2.85 ± 0.07 a | 32.26 ± 0.16 a |
| Medicament Portions | Extracting Solvents | H2O2 (IC50, μg/mL) | Singlet Oxygen (%, 2000 μg/mL) | HClO (IC50, μg/mL) | β-Carotene Bleaching AAC |
|---|---|---|---|---|---|
| ABAP | Methanol | 1143.52 ± 6.17 c | 15.30 ± 1.29 g | NONE | 496.45 ± 2.59 c |
| Water | 970.74 ± 8.13 b | 5.91 ± 0.39 i | NONE | 443.68 ± 2.12 g | |
| Ethanol | 1522.10 ± 10.39 e | 25.40 ± 1.46 f | NONE | 482.21 ± 2.29 e | |
| 80% Ethanol | 1393.38 ± 8.05 d | 40.20 ± 1.18 b | NONE | 483.12 ± 2.37 d | |
| ABR | Methanol | 2035.32 ± 22.95 h | 29.45 ± 1.64 e | 812.45 ± 6.60 b | 135.98 ± 1.67 i |
| Water | 1801.87 ± 31.30 f | 7.79 ± 0.74 h | >990 e | 65.80 ± 4.41 j | |
| Ethanol | 1970.25 ± 30.83 g | 37.71 ± 0.46 d | 852.51 ± 7.29 c | 282.88 ± 5.63 g | |
| 80% Ethanol | 2491.80 ± 26.05 i | 39.36 ± 0.28 c | 956.60 ± 8.49 d | 458.58 ± 5.43 f | |
| Standard Antioxidant | Trolox * | N.T. | N.T. | 15.62 ± 0.46 a | N.T. |
| Lipoic acid * | N.T. | N.T. | 26.58 ± 0.52 a | N.T. | |
| Gallic acid * | 36.62 ± 0.97 a | N.T. | N.T. | N.T. | |
| Ferulic acid * | N.T. | 88.25 ± 1.30 a | N.T. | N.T. | |
| BHT * | N.T. | N.T. | N.T. | 875.53 ± 6.33 a | |
| TBHQ * | N.T. | N.T. | N.T. | 799.85 ± 3.88 b |
| No. | RT (min) | Identification | Molecular Formula | Selective Ion | Full Scan MS (m/z) | MS/MS Fragments (m/z) | |
|---|---|---|---|---|---|---|---|
| Theory | Measured | ||||||
| 1 | 0.84 | Sucrose | C12H22O11 | [M + NH4]+ | 360.1506 | 360.1419 | 342.1313, 325.1067, 312.1210 |
| 2 | 0.93 | Biotin | C10H16N2O3S | [M + NH4]+ | 262.1226 | 262.1221 | 245.1075, 229.1481 |
| 3 | 1.28 | L-Pyroglutamic acid | C5H7NO3 | [M + H]+ | 130.0504 | 130.0461 | 113.0310, 101.0754 |
| 4 | 1.35 | L-Saccharopine | C11H20N2O6 | [M]+ | 276.1321 | 276.1376 | 294.1475, 132.0980 |
| 5 | 2.43 | N-Fructosyl phenylalanine | C15H21NO7 | [M + H]+ | 328.1396 | 328.1311 | 310.1208, 178.1176, 250.1586 |
| 6 | 3.37 | γ-Glutamyltyrosine | C14H18N2O6 | [M + Na]+ | 333.1063 | 333.1080 | 166.0819, 145.0028 |
| 7 | 3.79 | Histidine | C6H9N3O2 | [M]+ | 155.0695 | 155.0659 | 137.0558 |
| 8 | 4.37 | Valine | C5H11NO2 | [M + H]+ | 118.0868 | 118.0828 | 135.0415, 100.0727 |
| 9 | 4.51 | l-Phenylalanine | C9H11NO2 | [M + Na]+ | 188.0688 | 188.0655 | 150.0534 |
| 10 | 4.53 | Rosmarinic acid | C18H16O8 | [M + NH4]+ | 378.1189 | 378.1312 | 361.1048, 163.0148, 135.0403 |
| 11 | 5.52 | L-Threonyl-L-leucine | C10H20N2O4 | [M + H]+ | 233.1501 | 233.1441 | 216.1182, 169.1294, 118.0827 |
| 12 | 7.10 | Chlorogenic acid | C16H18O9 | [M + H]+ | 355.1029 | 355.0946 | 310.1693, 163.0347, 135.0405 |
| 13 | 7.43 | Naringin | C27H32O14 | [M + H]+ | 581.1870 | 581.1954 | 603.1769, 163.0346 |
| 14 | 8.83 | 6-O-Feruloylglucose | C16H20O9 | [M + Na]+ | 379.1005 | 379.0940 | 194.1131, 177.0500 |
| 15 | 8.94 | 1-O-Feruloyl-β-d-glucose | C16H20O9 | [M + NH4]+ | 374.1451 | 374.1361 | 194.1126, 163.0134 |
| 16 | 9.08 | Benzyl β-primeveroside | C18H26O10 | [M + NH4]+ | 420.1870 | 420.1778 | 313.1698, 149.0566 |
| 17 | 17.68 | Unknown | 205.0557 | 183.0741, 155.0433 | |||
| 18 | 22.61 | Unknown | 701.4847 | 679.5028, 340.2544 | |||
| 19 | 24.53 | Polygalasaponin XXIX | C64H102O33 | [M + NH4]+ | 1416.6647 | 1416.6727 | 737.5043, 575.4238 |
| 20 | 26.08 | Unknown | 905.6681 | 569.4659, 453.3367 | |||
| 21 | 26.77 | Pulsatiloside A | C47H76O18 | [M + H]+ | 929.5110 | 929.4915 | 913.5043, 767.4475, 751.4464 |
| 22 | 27.64 | Leonloside D | C59H96O27 | [M + H]+ | 1237.6217 | 1237.5965 | 1075.5557, 960.5368, 767.4448 |
| 23 | 31.67 | Hederacolchiside F | C65H106O31 | [M + NH4]+ | 1400.7062 | 1400.6804 | 1335.5651, 340.2525 |
| 24 | 34.42 | Raddeanoside Ra | C41H66O13 | [M + NH4]+ | 784.4847 | 784.4686 | 455.3449, 437.3328 |
| 25 | 36.35 | 3-O-β-d-glucopyranosyl-(1-2)-β-d-glucopyranosyl-(1-6)-β-d-galactopyranosyl-hederagenin | C48H78O19 | [M + H]+ | 959.5215 | 959.5059 | 797.4584 |
| 26 | 37.12 | Phytosphingosine | C18H39NO3 | [M + H]+ | 318.3008 | 318.2930 | 340.2535, 183.0741, 135.0415 |
| 27 | 37.23 | Leontoside B | C41H66O13 | [M + H]+ | 767.4581 | 767.4433 | 587.3847, 455.3447 |
| 28 | 37.78 | Lauryldiethanolamine | C16H35NO2 | [M + H]+ | 274.2746 | 274.2672 | 155.0431, 118.0831, 100.0730 |
| 29 | 38.04 | 27-Hydroxyoleanolic acid 3-O-β-D-glucopyranosyl (1-2)-α-L-arabinopyranoside | C41H66O13 | [M + H]+ | 767.4581 | 767.4410 | 789.4286, 455.3447, 437.3341 |
| 30 | 39.23 | Raddeanoside R22b | C47H76O17 | [M + NH4]+ | 930.5427 | 930.5240 | 589.3988, 571.3921, 439.3496 |
| 31 | 39.79 | Raddeanoside R13 | C47H76O17 | [M + NH4]+ | 930.5427 | 930.5239 | 788.4270, 603.2123 |
| 32 | 40.96 | Dehydrophytosphingosine | C18H37NO3 | [M + H]+ | 316.2851 | 316.2774 | 140.1149, 135.0405, 127.0123 |
| 33 | 41.53 | Gingerglycolipid A | C33H56O14 | [M + NH4]+ | 694.4014 | 694.3871 | 353.2628, 295.2204 |
| 34 | 41.89 | 1-Palmitoylglycerophosphoinositol | C25H49O12P | [M + H]+ | 573.3040 | 573.2914 | 555.2839, 537.2757 |
| 35 | 42.02 | 9-Eicosenedioic acid | C20H36O4 | [M]+ | 340.2614 | 340.2525 | 295.2210, 281.2423 |
| 36 | 42.44 | Sphinganine | C18H39NO2 | [M + H]+ | 302.3059 | 302.2980 | 230.8856, 127.0124, 100.0730 |
| 37 | 45.97 | Linolenic acid | C18H30O2 | [M + H]+ | 279.2324 | 279.2245 | 183.0739, 149.1036 |
| 38 | 47.21 | Stearidonic acid | C18H28O2 | [M + H]+ | 277.2167 | 277.2086 | 155.0428 |
| 39 | 47.60 | Palmitoleic acid | C16H30O2 | [M + Na]+ | 277.2144 | 277.2088 | 196.9613, 140.1150, 130.1555 |
| 40 | 48.10 | 13-HOTrE | C18H30O3 | [M + H]+ | 295.2273 | 295.2190 | 277.2105, 235.1869, 222.1077 |
| 41 | 48.60 | Erucamide | C22H43NO | [M + H]+ | 338.3423 | 338.3330 | 279.1533, 155.0429 |
| No. | RT (min) | Ingredient | ABR | ABAP | Anemones raddeanae Rhizoma |
|---|---|---|---|---|---|
| 1 | 0.84 | Sucrose | + | − | − |
| 2 | 0.93 | Biotin | + | + | − |
| 3 | 1.28 | L-Pyroglutamic acid | + | − | − |
| 4 | 1.35 | L-Saccharopine | + | + | − |
| 5 | 2.43 | N-Fructosyl phenylalanine | + | + | − |
| 6 | 3.37 | γ-Glutamyltyrosine | + | − | − |
| 7 | 3.79 | Histidine | + | − | + |
| 8 | 4.37 | Valine | + | − | + |
| 9 | 4.51 | L-Phenylalanine | + | + | − |
| 10 | 4.53 | Rosmarinic acid | + | − | − |
| 11 | 5.52 | L-Threonyl-L-leucine | + | − | − |
| 12 | 6.79 | Thymine | − | + | − |
| 13 | 7.10 | Chlorogenic acid | + | + | − |
| 14 | 7.43 | Naringin | + | − | − |
| 15 | 7.62 | 4-O-Caffeoylquinic acid | − | + | − |
| 16 | 8.83 | 6-O-Feruloylglucose | + | − | − |
| 17 | 8.94 | 1-O-Feruloyl-β-d-glucose | + | + | − |
| 18 | 9.08 | Benzyl β-primeveroside | + | + | − |
| 19 | 9.39 | 1-O-Caffeoylquinic acid | − | + | − |
| 20 | 9.80 | 4-p-Coumaroylquinic acid/3-p-Coumaroylquinic acid | − | + | − |
| 21 | 11.55 | 3-O-Feruloylquinic acid | − | + | − |
| 22 | 13.05 | Kaempferol 3-O-sophoroside 7-O-rhamnoside | − | + | − |
| 23 | 14.85 | Rutin | − | + | − |
| 24 | 15.30 | Kaempferol 3-O-sophoroside | − | + | − |
| 25 | 15.97 | Kaempferol 3-glucuronide | − | + | − |
| 26 | 18.88 | Apigenin 7-glucuronide | − | + | − |
| 27 | 19.86 | Diosmetin 7-glucuronide | − | + | − |
| 28 | 24.53 | Polygalasaponin XXIX | + | − | − |
| 29 | 25.84 | 1-O-{3-[(3-O-Hexopyranosylhexopyranosyl)oxy]-28-oxoolean-12-en-28-yl}hexopyranose | − | + | − |
| 30 | 26.37 | Hederagenin 28-O-β-D-glucopyranosyl-(1-3)-α-L-rhamnopyranosyl-(1-4)-β-D-glucopyranosyl-(1-6)-β-D-glucopyranosyl ester | − | + | − |
| 31 | 26.77 | Pulsatiloside A | + | + | + |
| 32 | 26.95 | Oleanolic acid 28-O-β-D-glucopyranosyl-(1-3)-α-L-rhamnopyranosyl-(1-4)-β-D-glucopyranosyl-(1-6)-β-D-glucopyranosyl ester | − | + | − |
| 33 | 27.64 | Leonloside D | + | + | + |
| 34 | 28.41 | Raddeanoside R18 | − | + | + |
| 35 | 29.98 | Cussonoside B | − | + | + |
| 36 | 31.67 | Hederacolchiside F | + | + | + |
| 37 | 32.37 | Anhuienoside E | − | + | − |
| 38 | 32.52 | Hederacolchiside E | − | + | + |
| 39 | 32.94 | Hederacoside C | − | + | − |
| 40 | 33.17 | Raddeanoside R14 | − | + | + |
| 41 | 34.42 | Raddeanoside Ra | + | − | + |
| 42 | 36.35 | 3-O-β-d-glucopyranosyl-(1-2)-β-d-glucopyranosyl-(1-6)-β-d-galactopyranosyl-hederagenin | + | + | + |
| 43 | 37.12 | Phytosphingosine | + | − | − |
| 44 | 37.23 | Leontoside B | + | − | + |
| 45 | 37.78 | Lauryldiethanolamine | + | − | − |
| 46 | 38.04 | 27-Hydroxyoleanolic acid 3-O-β-D-glucopyranosyl (1-2)-α-L-arabinopyranoside | + | − | + |
| 47 | 39.23 | Raddeanoside R22b | + | − | + |
| 48 | 39.79 | Raddeanoside R13 | + | + | + |
| 49 | 40.96 | Dehydrophytosphingosine | + | − | − |
| 50 | 41.53 | Gingerglycolipid A | + | + | − |
| 51 | 41.89 | 1-Palmitoylglycerophosphoinositol | + | + | − |
| 52 | 42.02 | 9-Eicosenedioic acid | + | − | − |
| 53 | 42.44 | Sphinganine | + | + | − |
| 54 | 45.97 | Linolenic acid | + | + | + |
| 55 | 47.21 | Stearidonic acid | + | + | − |
| 56 | 47.60 | Palmitoleic acid | + | + | − |
| 57 | 48.10 | 13-HOTrE | + | − | − |
| 59 | 48.60 | Erucamide | + | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Xia, G.; Pang, H.; Li, L.; Zang, H. Compositional Analysis and Bioactivity Assessment of the Anemone baicalensis Rhizome: Exploring the Potential for Substituting Anemones raddeanae Rhizoma. Processes 2025, 13, 844. https://doi.org/10.3390/pr13030844
Sun S, Xia G, Pang H, Li L, Zang H. Compositional Analysis and Bioactivity Assessment of the Anemone baicalensis Rhizome: Exploring the Potential for Substituting Anemones raddeanae Rhizoma. Processes. 2025; 13(3):844. https://doi.org/10.3390/pr13030844
Chicago/Turabian StyleSun, Shuang, Guangqing Xia, Hao Pang, Li Li, and Hao Zang. 2025. "Compositional Analysis and Bioactivity Assessment of the Anemone baicalensis Rhizome: Exploring the Potential for Substituting Anemones raddeanae Rhizoma" Processes 13, no. 3: 844. https://doi.org/10.3390/pr13030844
APA StyleSun, S., Xia, G., Pang, H., Li, L., & Zang, H. (2025). Compositional Analysis and Bioactivity Assessment of the Anemone baicalensis Rhizome: Exploring the Potential for Substituting Anemones raddeanae Rhizoma. Processes, 13(3), 844. https://doi.org/10.3390/pr13030844






