Experimental Design Modelization and Optimization of Pickling Process Parameters for Corrosion Inhibition in Steel Construction
Abstract
1. Introduction
- (i)
- Organic compounds with aromatic rings containing electronegative functional groups and π-electrons in conjugated double bonds.
- (ii)
- Specific interactions between functional groups containing nitrogen heteroatoms with free lone pairs of electrons and the metal surface, which can play an important role in inhibition.
- (i)
- Conducting experiments at different points in this region and collecting data;
- (ii)
- Predicting the results at other points by analyzing the reliability predictions.
2. Materials and Methods
2.1. Practical Conditions
2.2. Design of Experiment Approach
2.3. Study Vicinity
2.4. Experimental Response
2.5. Design of Experiment Matrix
3. Findings
3.1. Weight Loss Investigation
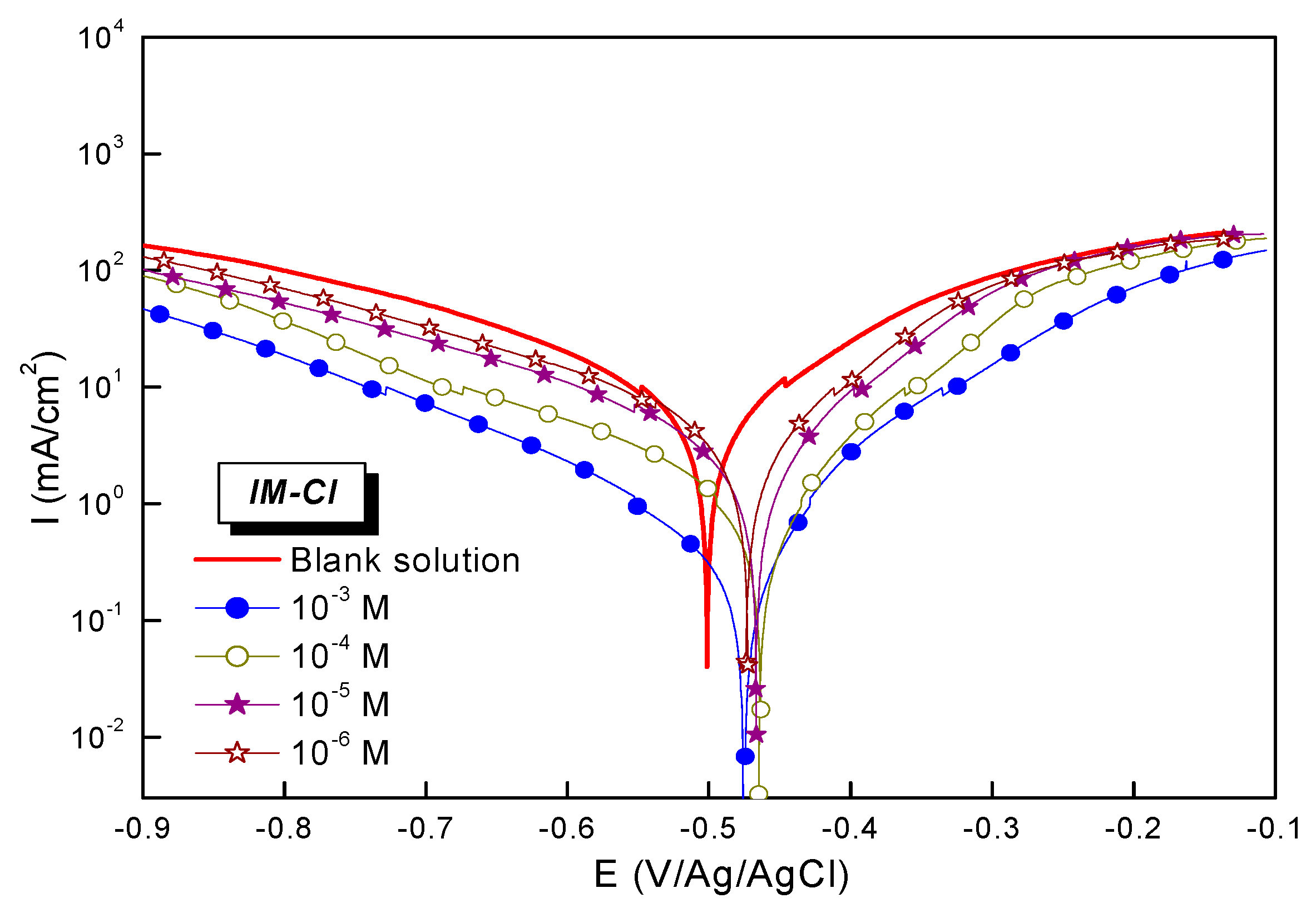
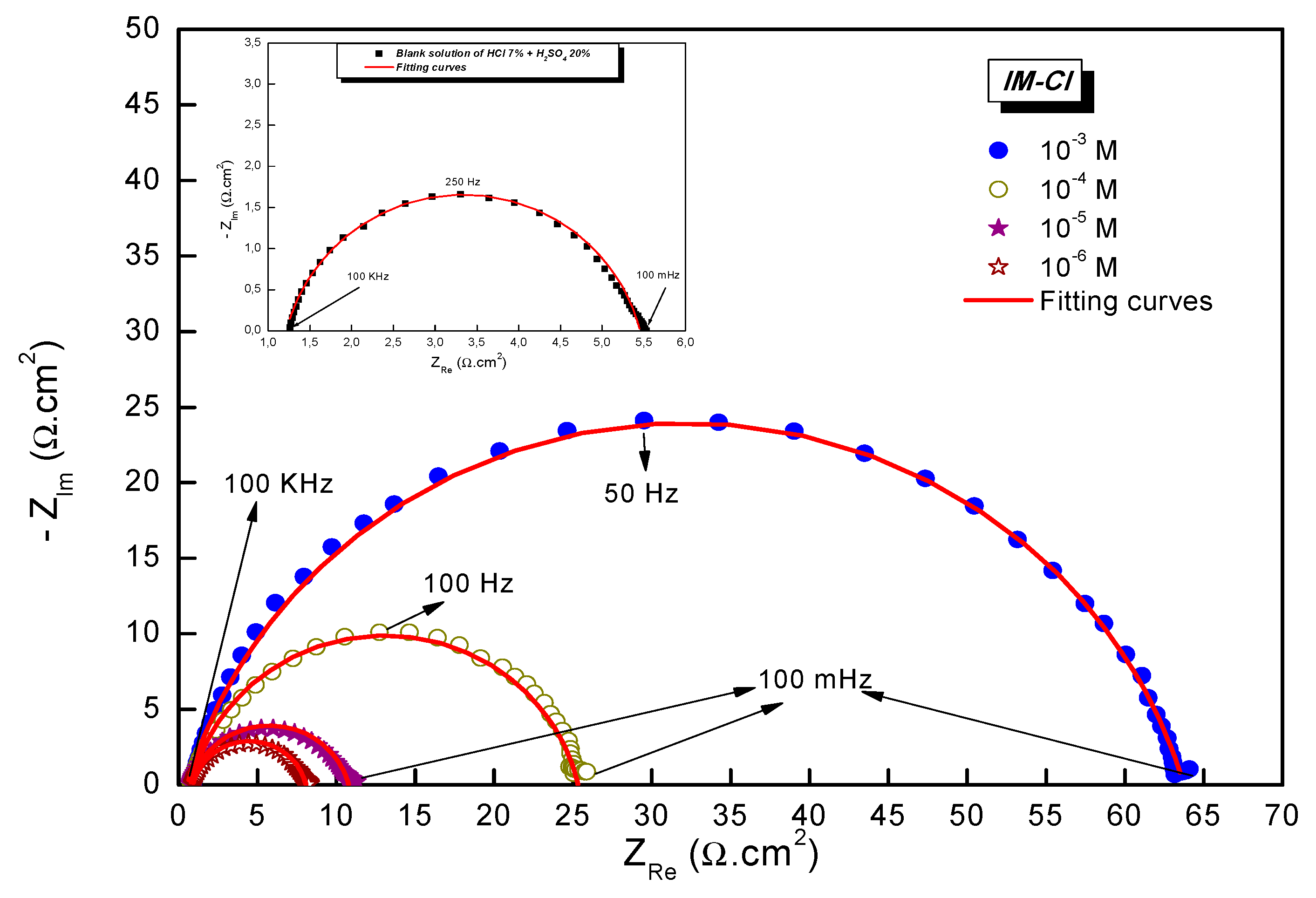
3.2. Electrochemical Investigation—Current Potential
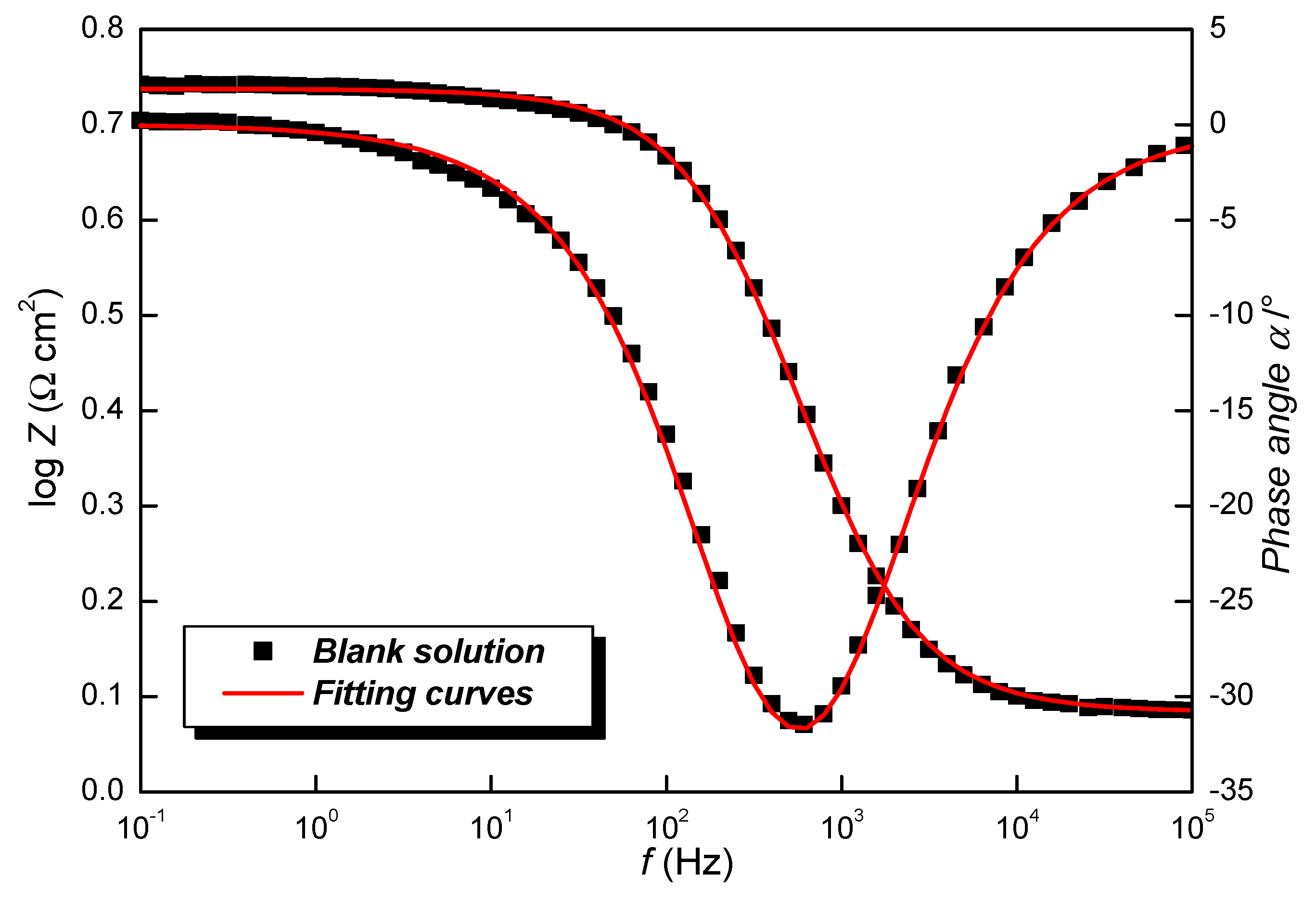
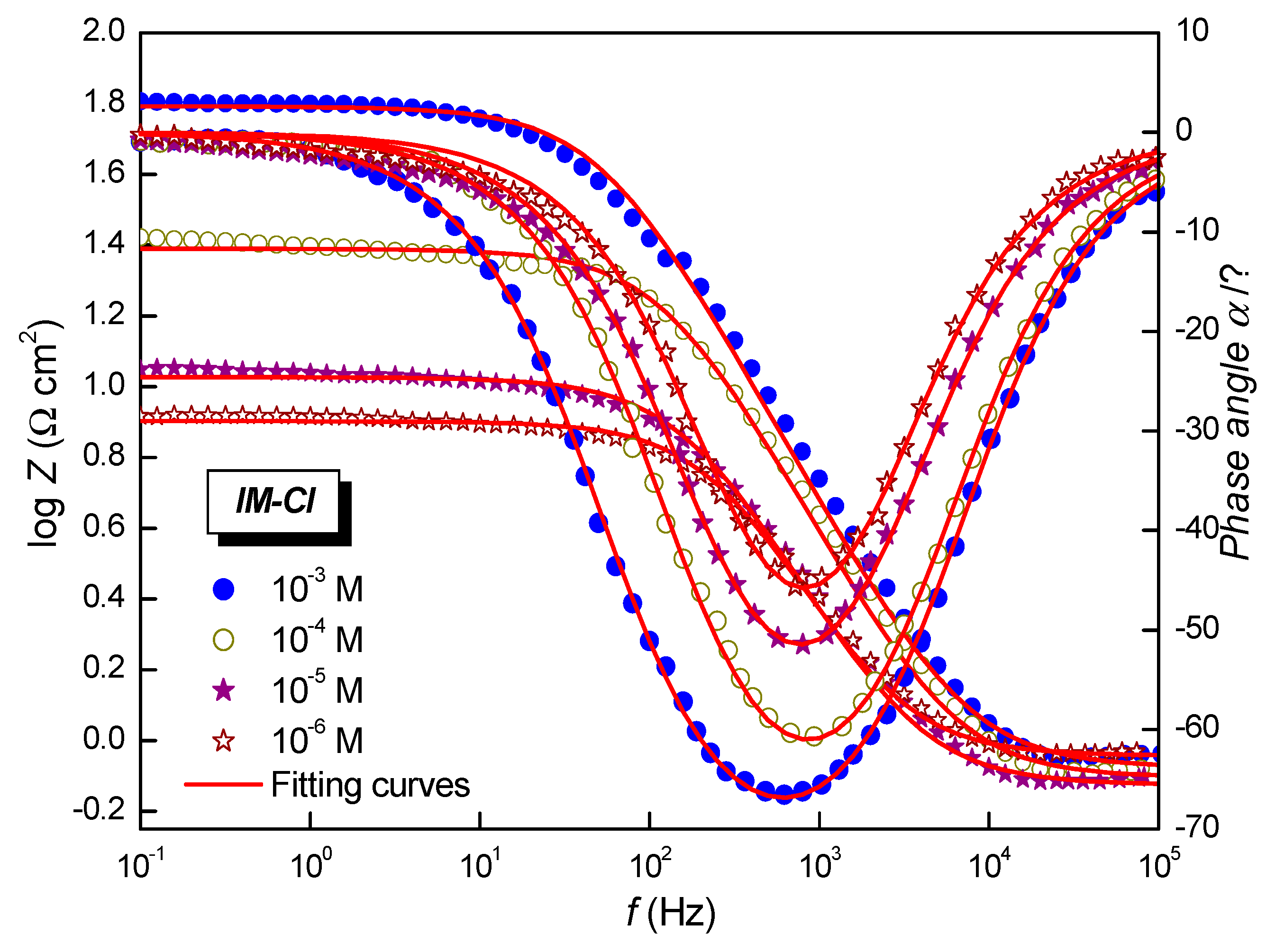
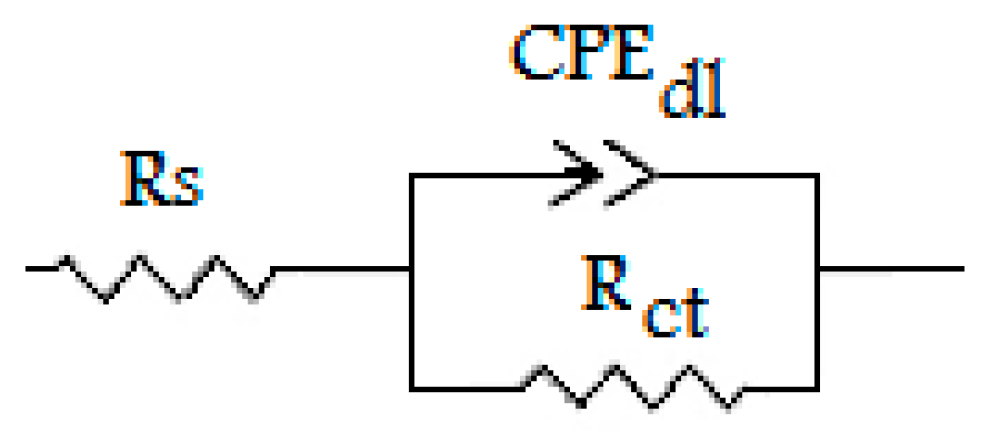
3.3. Electrochemical Impedance Spectroscopy
3.4. Statistical Treatment
3.5. Validity of the Model
3.6. Residual Analysis
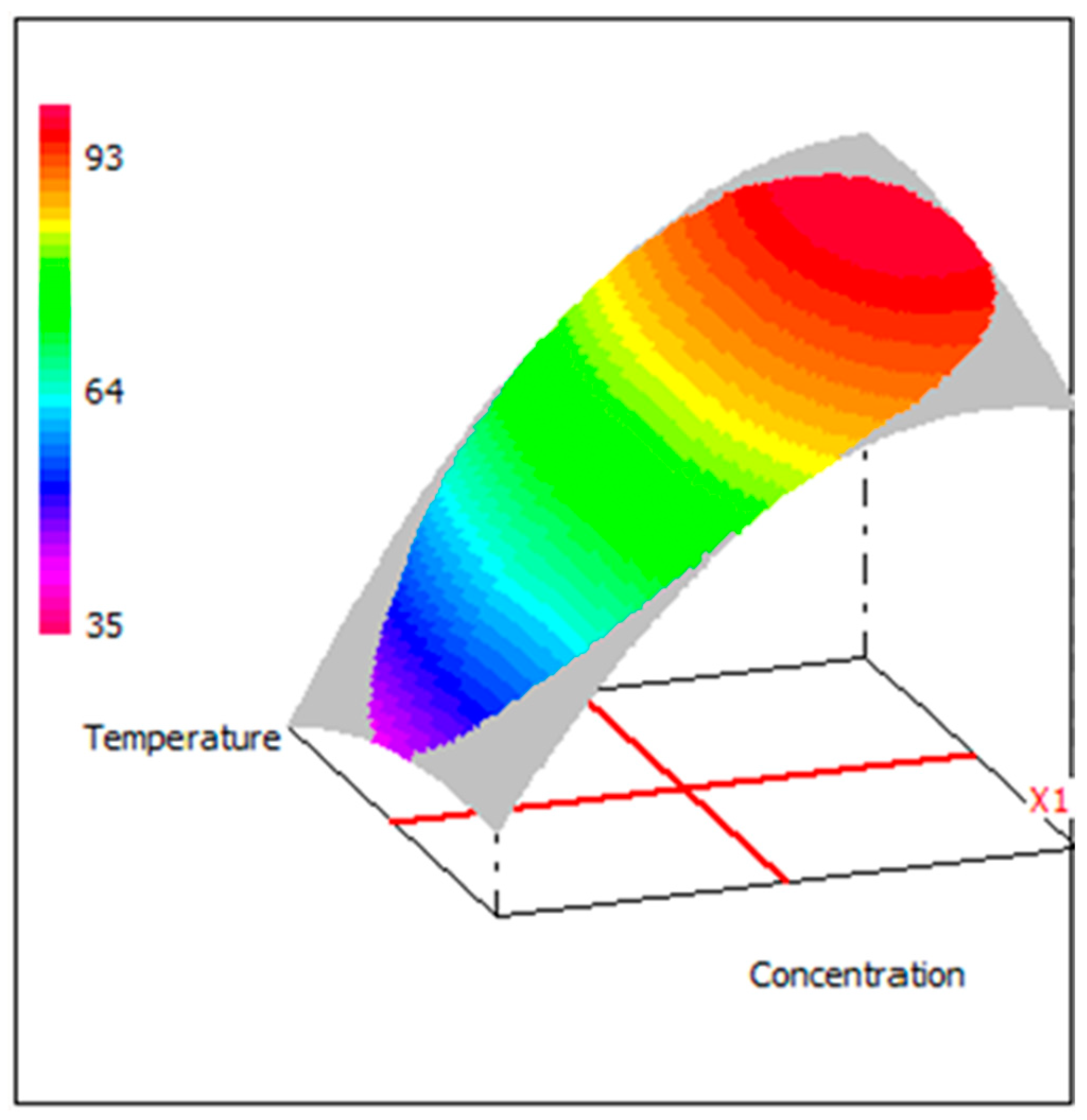
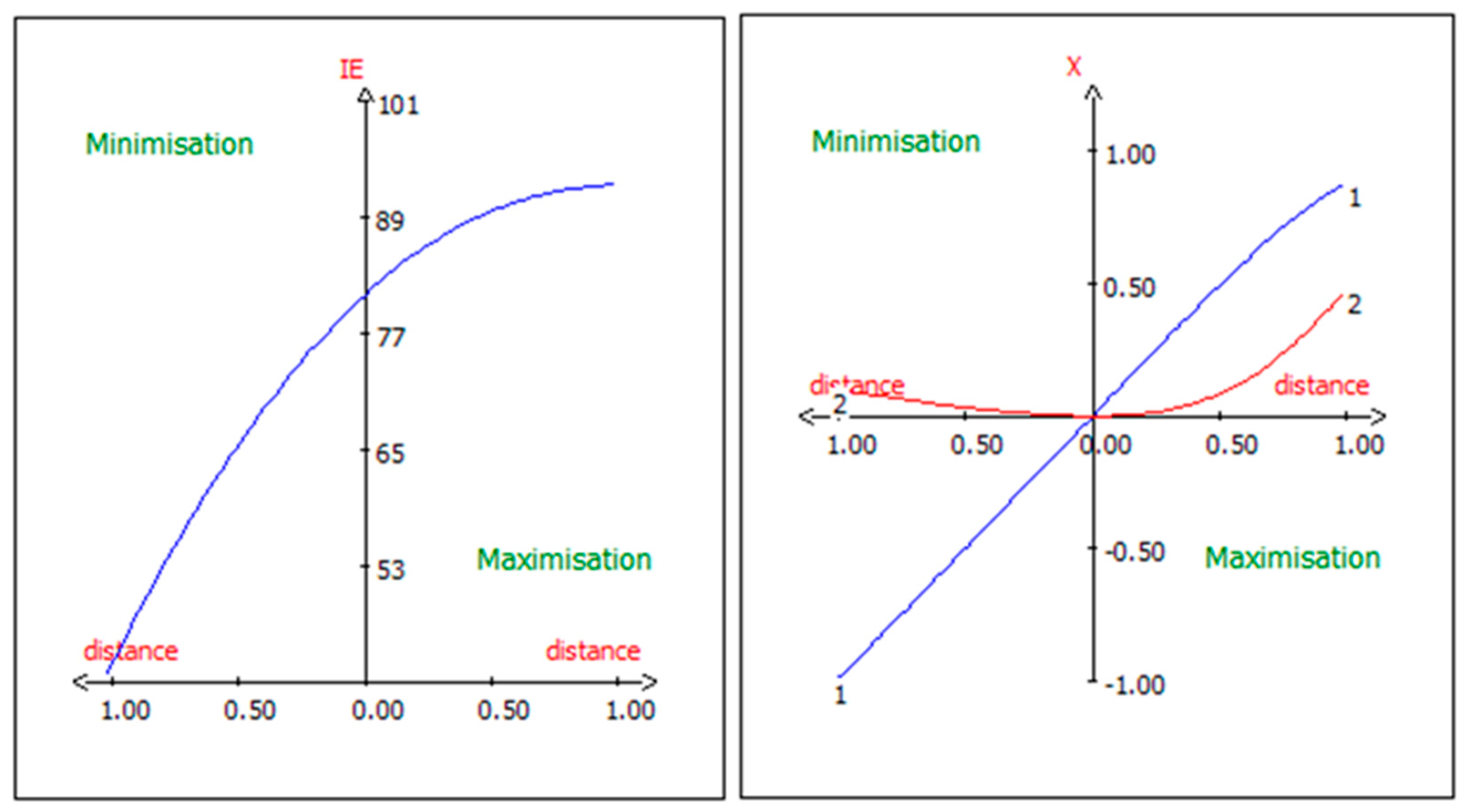
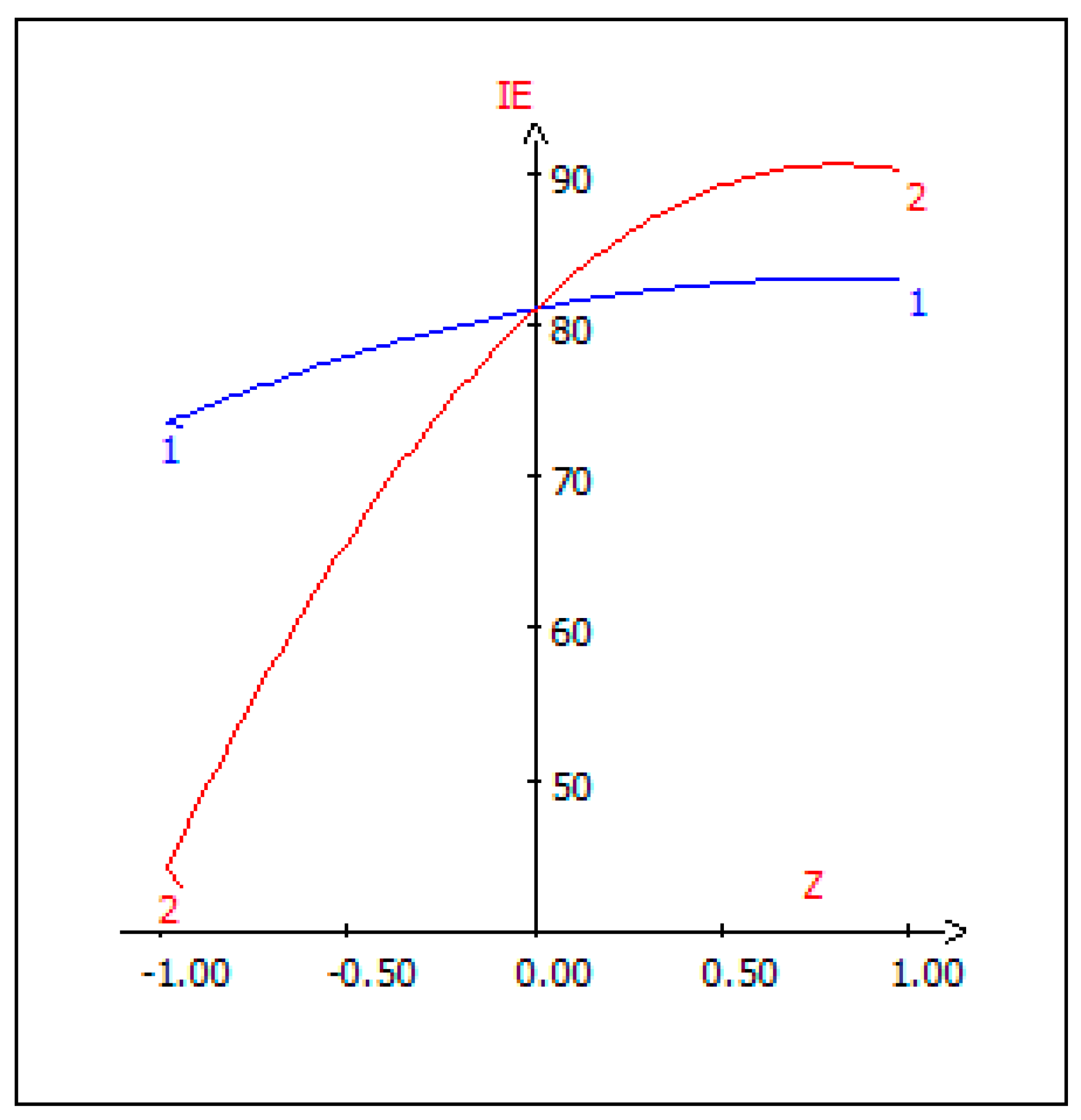
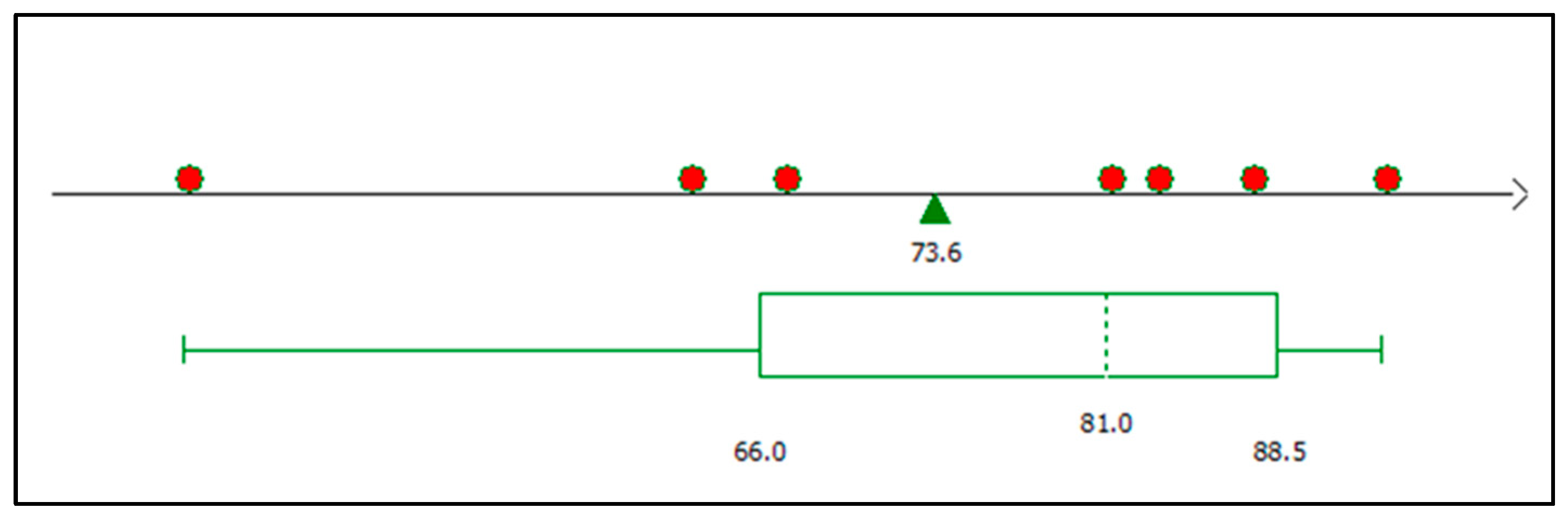
3.7. Graphic Analysis of the Model
3.8. Evolution of Efficiency as a Function of Inhibitor Concentration and Temperature
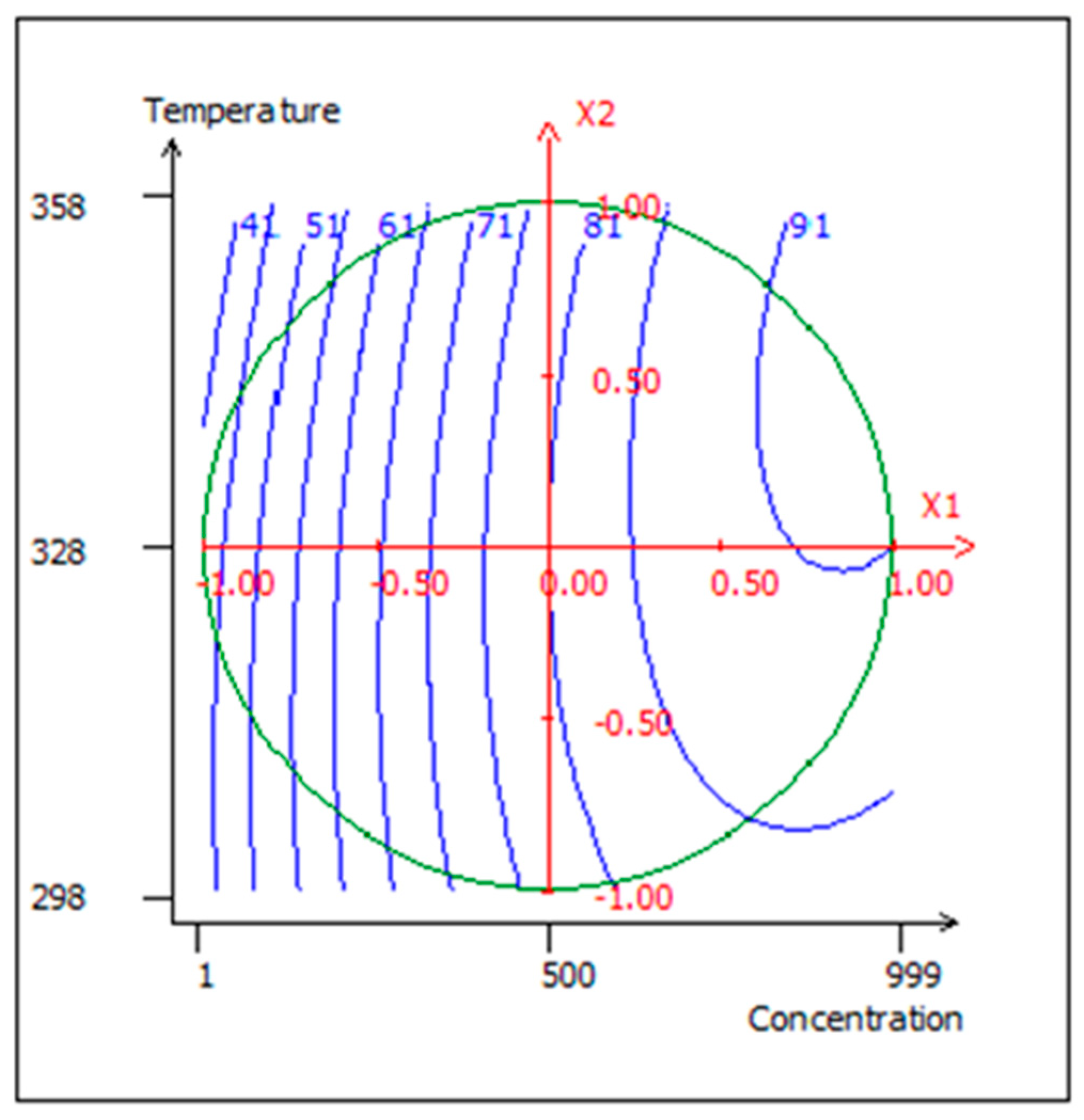
3.9. Canonical Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alaoui, K.; Kaya, S.; Salim, R.; Kamal, A.; Moussaoui, A.; Habsaoui, A.; Touhami, M.E.; El Kacimi, Y. Correlation between halogens atoms elements, their positions on the main chain of organic compounds, and corrosion inhibition performance. In Handbook of Research on Corrosion Sciences and Engineering; IGI Global: Hershey, PA, USA, 2023; pp. 65–84. [Google Scholar] [CrossRef]
- Hussin, M.H.; Kassim, M.J.; Razali, N.; Dahon, N.; Nasshorudin, D. The effect of Tinospora crispa extracts as a natural mild steel corrosion inhibitor in 1M HCl solution. Arab. J. Chem. 2016, 9, S616–S624. [Google Scholar] [CrossRef]
- Abboud, Y.; Abourriche, A.; Saffaj, T.; Berrada, M.; Charrouf, M.; Bennamara, A.; Al Himidi, N.; Hannache, H. 2,3-Quinoxalinedione as a novel corrosion inhibitor for mild steel in 1M HCl. Mater. Chem. Phys. 2007, 105, 1–5. [Google Scholar] [CrossRef]
- James, A.O.; Oforka, N.C.; Abiola, O.K. Inhibition of acid corrosion of mild steel by pyridoxal and pyridoxol hydrochlorides. Int. J. Electrochem. Sci. 2007, 2, 278–284. [Google Scholar] [CrossRef]
- Ebenso, E.E. Inhibition of corrosion of mild steel hydrochloric acid by some azo dyes. Niger. J. Chem. Res. 2001, 6, 8–12. [Google Scholar] [CrossRef]
- Elkacimi, Y.; Achnin, M.; Aouine, Y.; E Touhami, M.; Alami, A.; Touir, R.; Sfaira, M.; Chebabe, D.; Elachqar, A.; Hammouti, B. Inhibition of Mild Steel Corrosion by some Phenyltetrazole Substituted Compounds in Hydrochloric Acid. Port. Electrochim. Acta 2012, 30, 53–65. [Google Scholar] [CrossRef]
- Ouakki, M.; Galai, M.; Rbaa, M.; Abousalem, A.S.; Lakhrissi, B.; Rifi, E.H.; Cherkaoui, M. Investigation of imidazole derivatives as corrosion inhibitors for mild steel in sulfuric acidic environment: Experimental and theoretical studies. Ionics 2020, 26, 5251–5272. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Chen, B.; Ren, X. Investigation of three betaine surfactants and KI compounds as a corrosion inhibitor on carbon steel in industrial pickling. J. Mol. Struct. 2025, 1328, 141269. [Google Scholar] [CrossRef]
- Li, W.; He, Q.; Pei, C.; Hou, B. Experimental and theoretical investigation of the adsorption behaviour of new triazole derivatives as inhibitors for mild steel corrosion in acid media. Electrochim. Acta 2007, 52, 6386–6394. [Google Scholar] [CrossRef]
- Chaouche, R.; Tiskar, M.; Larhlid, I.; Ihamdane, R.; El Amri, A.; Mansour, A.A.; Hsissou, R.; Salghi, R.; Cheikhi, N.; Chaouch, A.; et al. Exploring of the Origanum Compactum essential oil as an ecofriendly corrosion inhibitor for mild steel in 1M HCl environment: Experimental, DFT, MD, DFTB and PDOS approaches. J. Mol. Struct. 2025, 1327, 141112. [Google Scholar] [CrossRef]
- Zerga, B.; Attayibat, A.; Sfaira, M.; Taleb, M.; Hammouti, B.; Touhami, M.E.; Radi, S.; Rais, Z. Effect of some tripodal bipyrazolic compounds on C38 steel corrosion in hydrochloric acid solution. J. Appl. Electrochem. 2010, 40, 1575–1582. [Google Scholar] [CrossRef]
- Scendo, M.; Hepel, M. Inhibiting properties of benzimidazole films for Cu(II)/Cu(I) reduction in chloride media studied by RDE and EQCN techniques. Corros. Sci. 2007, 49, 3381–3407. [Google Scholar] [CrossRef]
- Larabi, L.; Benali, O.; Mekelleche, S.; Harek, Y. 2-Mercapto-1-methylimidazole as corrosion inhibitor for copper in hydrochloric acid. Appl. Surf. Sci. 2006, 253, 1371–1378. [Google Scholar] [CrossRef]
- Belhadi, M.; Roby, O.; Chafi, M.; Lgaz, H.; Lee, H.-S.; Alzahrani, A.Y.; Tighadouini, S. Mechanistic Insights and Performance of Pyrazole-Based Corrosion Inhibitors for Carbon Steel in Acidic Media: Experimental and Computational Approaches. J. Bio- Tribo-Corros. 2025, 11, 16. [Google Scholar] [CrossRef]
- Chugh, B.; Singh, A.K.; Chaouiki, A.; Salghi, R.; Thakur, S.; Pani, B. A comprehensive study about anti-corrosion behaviour of pyrazine carbohydrazide: Gravimetric, electrochemical, surface and theoretical study. J. Mol. Liq. 2020, 299, 112160. [Google Scholar] [CrossRef]
- Verma, C.; Obot, I.; Bahadur, I.; Sherif, E.-S.M.; Ebenso, E.E. Choline based ionic liquids as sustainable corrosion inhibitors on mild steel surface in acidic medium: Gravimetric, electrochemical, surface morphology, DFT and Monte Carlo simulation studies. Appl. Surf. Sci. 2018, 457, 134–149. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M.; Motamedi, M. Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: Experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 2018, 255, 185–198. [Google Scholar] [CrossRef]
- Stern, M.; Geaby, A.L. Electrochemical Polarization. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Benoist, D.; Tourbier, Y.; Germain-Tourbier, S. Designs D’expériences: Construction et Analyse; Technique & Documentation, Lavoisier: Paris, France, 1994; ISBN 978-2-8520-6988-6. [Google Scholar]
- Goupy, J. Pratiquer les Designs D’experiences; Dunod: Paris, France, 2017; ISBN 2-10-004217-3. [Google Scholar]
- M’hanni, N.; Galai, M.; Anik, T.; Touhami, M.E.; Rifi, E.H.; Asfari, Z.; Touir, R. Influence of additives selected calix[4]arenes on electroless copper plating using hypophosphite as reducing agent. Surf. Coat. Technol. 2017, 310, 8–16. [Google Scholar] [CrossRef]
- Galai, M.; Ouassir, J.; Ebn Touhami, M.; Nassali, H.; Benqlilou, H.; Belhaj, T.; Berrami, K.; Mansouri, I.; Oauki, B. α-Brass and (α+ β) brass degradation processes in Azrou soil medium used in plumbing devices. J. Bio- Tribo-Corros. 2017, 3, 30. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, S.; Li, W.; Yin, L.; He, J.; Wu, J. Investigation of 1-butyl-3-methyl-1H-benzimidazolium iodide as inhibitor for mild steel in sulfuric acid solution. Corros. Sci. 2014, 80, 383–392. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abdallah, M.; Awad, M.K.; Rezk, M. Three novel di-quaternary ammonium salts as corrosion inhibitors for API X65 steel pipeline in acidic solution. Part I: Experimental results. Corros. Sci. 2014, 81, 54–64. [Google Scholar] [CrossRef]
- Mathieu, D.; Nony, J.; Phan-Tan-Luu, R. NEMROD-W Software; LPRAI: Marseille, France, 2000. [Google Scholar]
- Heakal, F.E.T.; Fouda, A.S.; Radwan, M.S. Some new thiadiazole derivatives as corrosion inhibitors for 1018 carbon steel dissolution in sodium chloride solution. Int. J. Electrochem. Sci. 2011, 6, 3140–3163. [Google Scholar] [CrossRef]
- ASTM G 81-97a; Standard Test Method for Jaw Crusher Gouging Abrasion Test. ASTM International: West Conshohocken, PA, USA, 2007.
- Murakawa, T.; Hackerman, N. The double layer capacity at the interface between iron and acid solutions with and without organic materials. Corros. Sci. 1964, 4, 387–396. [Google Scholar] [CrossRef]
- Allabergenov, K.D.; Kurbanov, F.K. Acetylene Compounds—Inhibitors of Steel Corrosion in Sulfuric Acid. Zashch. Met. 1979, 15, 472–473. [Google Scholar]
- Rengamani, S.; Muralidharan, S.; Anbu Kulandainathan, M.; Venkatakrishna Iyer, S. Inhibiting and accelerating effects of aminophenols on the corrosion and permeation of hydrogen through mild steel in acidic solutions. J. Appl. Electrochem. 1994, 24, 355–360. [Google Scholar] [CrossRef]
- Bockris, J.M.; Yang, B. The mechanism of corrosion inhibition of iron in acid solution by acetylenic alcohols. J. Electrochem. Soc. 1991, 138, 2237. [Google Scholar] [CrossRef]
- Hackerman, N.; Snavely, E.S., Jr.; Fiel, L.D. The anodic polarization behaviour of metals in hydrogen fluoride. Corrosion Science 1967, 7, 39–50. [Google Scholar] [CrossRef]
- Oskes, G.; Vest, J.M. Influence of Thiourea on the Dissolution of Mild Steel in Strong Hydrochloric Acid. Br. Corros. J. 1969, 4, 66–73. [Google Scholar]
- MAmin, M.A.; Abd El-Rehim, S.S.; El-Sherbini, E.E.F.; Bayoumi, R.S. The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid: Part I. Weight loss, polarization, EIS, PZC, EDX and SEM studies. Electrochim. Acta 2007, 52, 3588–3600. [Google Scholar]
- Lenderink, H.J.W.; Linden, M.V.D.; De Wit, J.H.W. Corrosion of aluminium in acidic and neutral solutions. Electrochim. Acta 1993, 38, 1989–1992. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F.; Mohsen, Q.; Arida, H.A. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Alaoui, K.; El Kacimi, Y.; Galai, M.; Serrar, H.; Touir, R.; Kaya, S.; Kaya, C.; Touhami, M.E. New triazepine carboxylate derivatives: Correlation between corrosion inhibition property and chemical structure. Int. J. Ind. Chem. 2020, 11, 23–42. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.H.; Mugila, N.; Hemapriya, V.; Parameswari, K.; Chitra, S.; Chung, I.M. Aster koraiensis as nontoxic corrosion inhibitor for mild steel in sulfuric acid. J. Ind. Eng. Chem. 2017, 52, 235–242. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Erasmus, R.M.; Comins, J.D. Inhibition of copper corrosion in acidic chloride pickling solutions by 5-(3-aminophenyl)-tetrazole as a corrosion inhibitor. Corros. Sci. 2008, 50, 3439–3445. [Google Scholar] [CrossRef]
- El Mehdi, B.; Mernari, B.; Traisnel, M.; Bentiss, F.; Lagrenee, M. Synthesis and comparative study of the inhibitive effect of some new triazole derivatives towards corrosion of mild steel in hydrochloric acid solution. Mater. Chem. Phys. 2003, 77, 489–496. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Messali, M.; Aljuhani, A.; Aouad, M.; Hammouti, B.; Belghiti, M.; Chauhan, D.; Quraishi, M. Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acid medium: Electrochemical and molecular dynamics simulation studies. J. Mol. Liq. 2018, 249, 997–1008. [Google Scholar] [CrossRef]
- Singh, A.K.; Quraishi, M.A. Effect of 2, 2′ benzothiazolyl disulfide on the corrosion of mild steel in acid media. Corros. Sci. 2009, 51, 2752–2760. [Google Scholar] [CrossRef]
- Prabhu, R.; Venkatesha, T.; Shanbhag, A.; Kulkarni, G.; Kalkhambkar, R. Inhibition effects of some Schiff’s bases on the corrosion of mild steel in hydrochloric acid solution. Corros. Sci. 2008, 50, 3356–3362. [Google Scholar] [CrossRef]
- El Caid, Z.A.; Left, D.B.; Thoume, A.; Kellal, R.; Zertoubi, M. A Comprehensive Computational Study of N-Phenylacetamide Derivatives as Corrosion Inhibitors for Copper: Insights from DFT and Molecular Dynamics. J. Bio- Tribo-Corros. 2023, 9, 83. [Google Scholar] [CrossRef]
- Singh, A.K.; Quraishi, M.A. The effect of some bis-thiadiazole derivatives on the corrosion of mild steel in hydrochloric acid. Corros. Sci. 2010, 52, 1373–1385. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Seifzadeh, D.; Hosseini, M.G. EN, EIS and polarization studies to evaluate the inhibition effect of 3H-phenothiazin-3-one, 7-dimethylamin on mild steel corrosion in 1 M HCl solution. Corros. Sci. 2008, 50, 3363–3370. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, S.; Li, W.; Hou, B. Corrosion inhibition of mild steel in acidic solution by some oxo-triazole derivatives. Corros. Sci. 2009, 51, 2588–2595. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Obot, I.B.; Ebenso, E.E.; Quraishi, M.A. 5-Arylpyrimido-[4,5-b]quinoline-diones as new and sustainable corrosion inhibitors for mild steel in 1 M HCl: A combined experimental and theoretical approach. RSC Adv. 2016, 6, 15639–15654. [Google Scholar] [CrossRef]
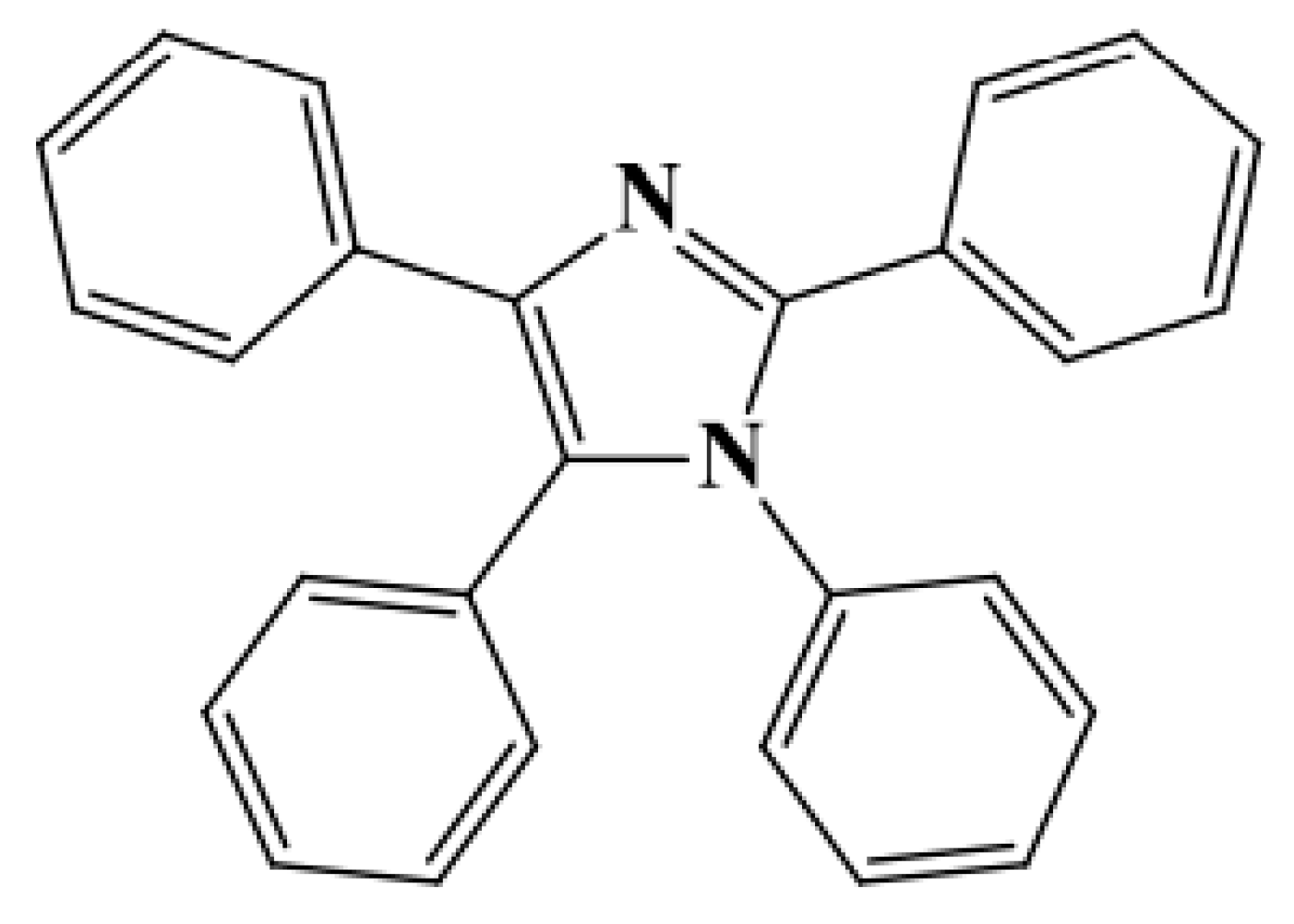
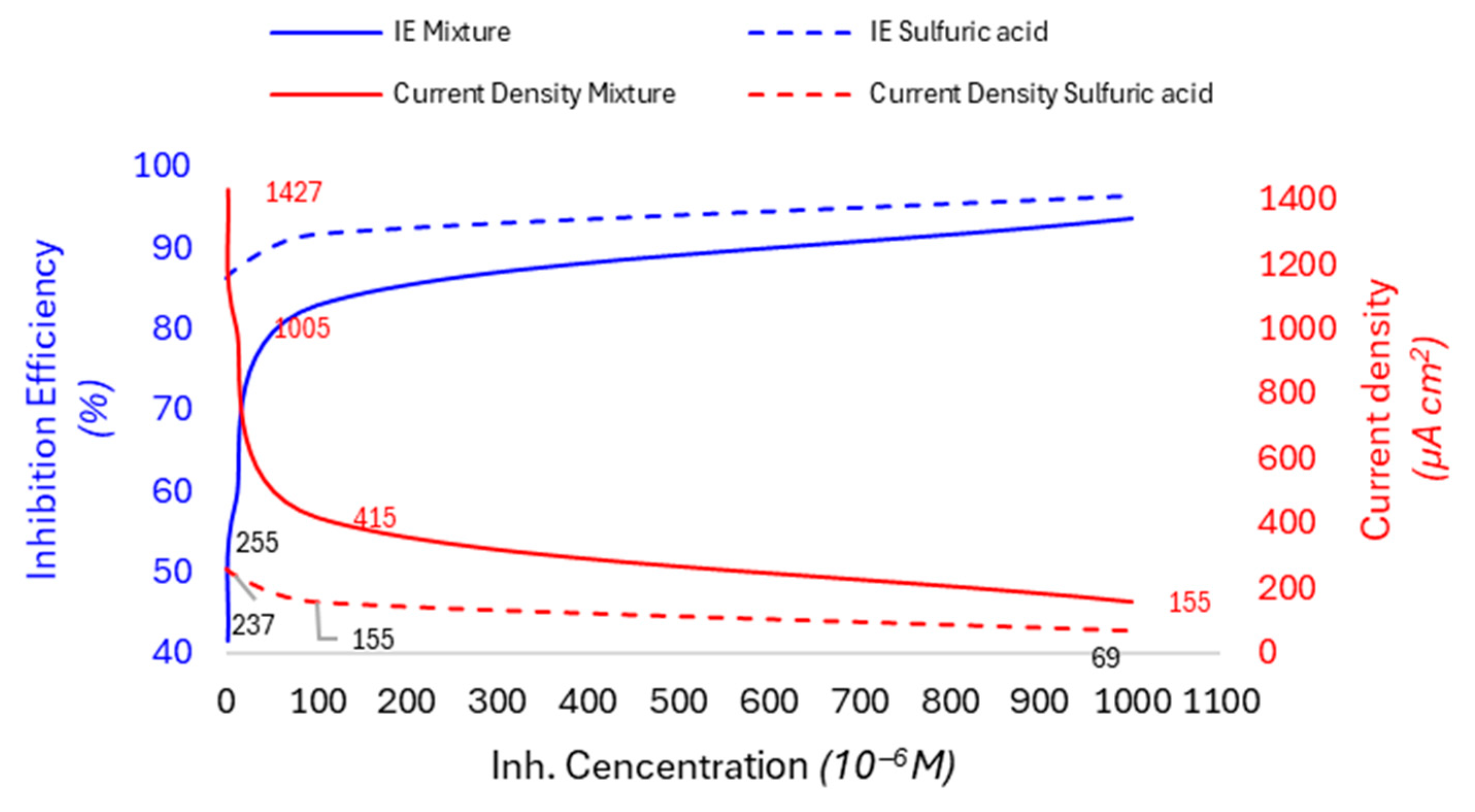
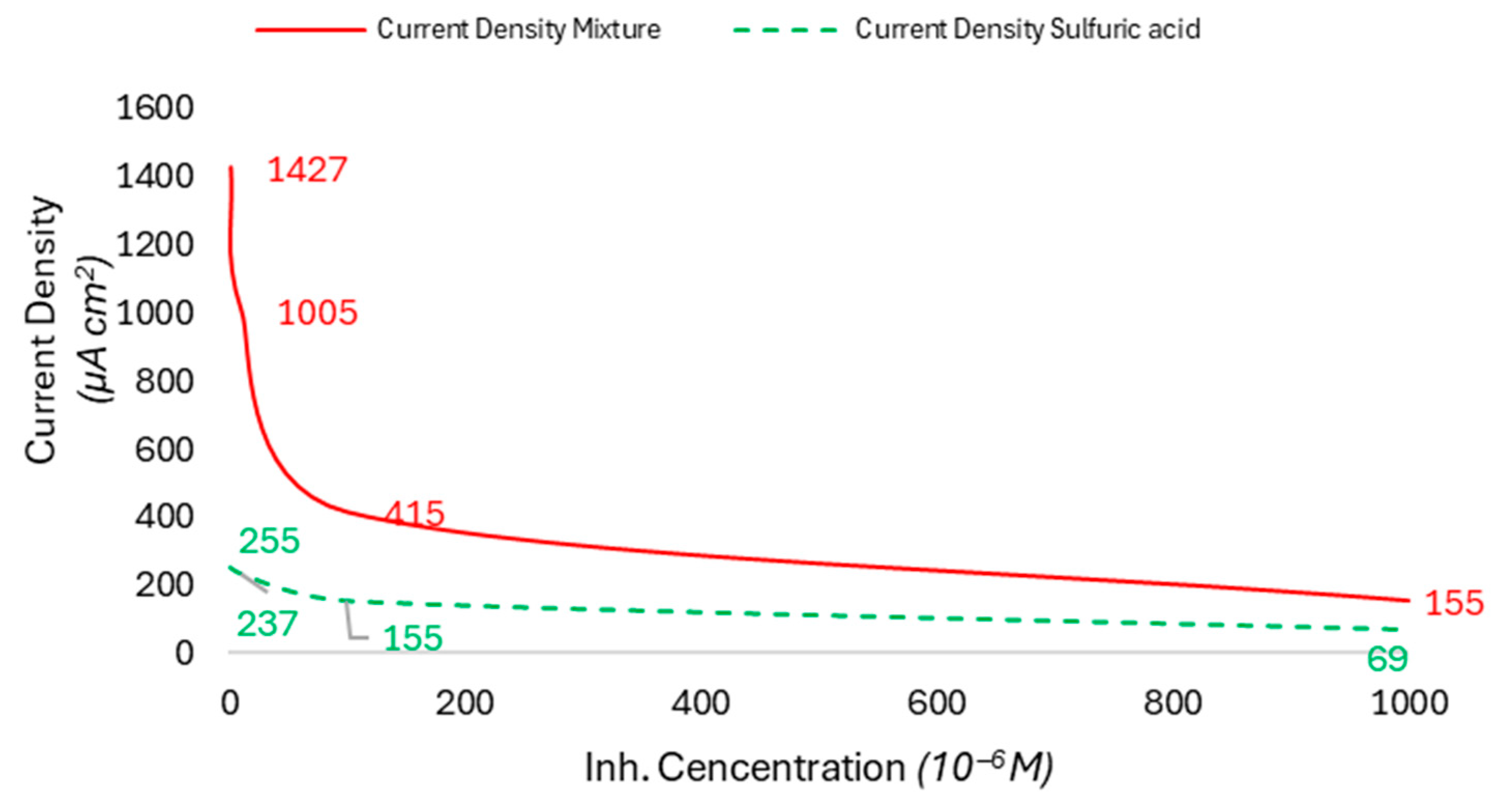
| Factor | Unit | Range | Centre | Variation Step | |
|---|---|---|---|---|---|
| U1 | Inhibitor concentration | µM | 1 to 1000 | 500 | 500 |
| U2 | Pickling bath temperature | K | 298 ± 2 to 328 ± 2 | 318 | 10 |
| N° Exp | X1 | X2 | X1-1 | X2-2 | X1-2 |
|---|---|---|---|---|---|
| 1 | 1.00000 | 0.00000 | 1.00000 | 0.00000 | 0.00000 |
| 2 | −1.00000 | 0.00000 | 1.00000 | 0.00000 | 0.00000 |
| 3 | 0.50000 | 0.86603 | 0.25000 | 0.74996 | 0.43300 |
| 4 | −0.50000 | −0.86603 | 0.25000 | 0.74996 | 0.43300 |
| 5 | 0.50000 | −0.86603 | 0.25000 | 0.74996 | −0.43300 |
| 6 | −0.50000 | 0.86603 | 0.25000 | 0.74996 | −0.43300 |
| 7 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| Solution | Conc (10−6 M) | Ecorr (mV/Ag-AgCl) | icorr (µA cm2) | -βc (mV dec−1) | βa (mV dec−1) | Epp (%) |
|---|---|---|---|---|---|---|
| Blank | 0 | 499 | 2436 | 125 | 136 | -- |
| Inhibited | 1 | 470 | 1427 | 131 | 136 | 41.4 |
| Inhibited | 10 | 463 | 1005 | 129 | 121 | 58.7 |
| Inhibited | 100 | 461 | 415 | 126 | 108 | 82.9 |
| Inhibited | 1000 | 474 | 155 | 127 | 120 | 93.6 |
| Solution | Conc (10−6 M) | Rs (Ω cm2) | Rct (Ω cm2) | Cdc (µF cm−2) | ndl | Q (µF Sn−1) | ƞEIS (%) |
|---|---|---|---|---|---|---|---|
| Blank | 0 | 1.2 ± 0.3 | 4.2 ± 0.1 | 157.0 | 0.844 ± 0.03 | 489.8 ± 0.5 | - |
| Inhibited | 1 | 0.9 ± 0.3 | 7.2 ± 0.2 | 94.3 | 0.863 ± 0.02 | 255.9 ± 0.4 | 40.8 |
| Inhibited | 10 | 0.7 ± 0.3 | 10.0 ± 0.3 | 93.1 | 0.845 ± 0.03 | 250.3 ± 0.3 | 57.7 |
| Inhibited | 100 | 0.7 ± 0.3 | 24.5 ± 0.4 | 53.8 | 0.863 ± 0.01 | 133.0 ± 0.4 | 82.7 |
| Inhibited | 1000 | 0.5 ± 0.2 | 63.2 ± 0.5 | 45.0 | 0.826 ± 0.02 | 127.4 ± 0.3 | 93.3 |
| Source of Variation | Sum of Squares | Degree of Freedom | Mean Square | Ratio | Significance of Regression Coefficients |
|---|---|---|---|---|---|
| Regression | 1.8937 × 103 | 5 | 2.7874 × 102 | 51.20 | <0.001 |
| Residues | 20.4 | 1 | 20.4 | - | - |
| Validity | 112.3 | 1 | 31.21 | 12.25 | <0.01 |
| Error | 2.13 | 2 | 1.24 | - | - |
| Total | 1.9177 × 103 | 9 | - | - | - |
| N° Experience | Yexp. (%) | Ycal. (%) | Deviation | Standard Deviation | dU |
|---|---|---|---|---|---|
| 1 | 93.1 | 91.2 | 1.9 | −0.103 | 0.180 |
| 2 | 41.3 | 43.7 | −2.4 | −0.120 | 0.236 |
| 3 | 87.2 | 89.1 | −1.9 | −0.283 | 0.152 |
| 4 | 67.6 | 65.6 | 2.0 | 0.244 | 0.352 |
| 5 | 83.4 | 85.4 | −2.0 | −0.771 | 0.311 |
| 6 | 63.1 | 61.3 | 1.8 | −0.010 | 0.710 |
| 7 | 81.7 | 81.5 | 0.2 | 0.182 | 0.560 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouakki, M.; Alaoui, K.; Lachhab, R.; Rbaa, M.; Cherkaoui, M.; Ebn Touhami, M.; El Kacimi, Y. Experimental Design Modelization and Optimization of Pickling Process Parameters for Corrosion Inhibition in Steel Construction. Processes 2025, 13, 796. https://doi.org/10.3390/pr13030796
Ouakki M, Alaoui K, Lachhab R, Rbaa M, Cherkaoui M, Ebn Touhami M, El Kacimi Y. Experimental Design Modelization and Optimization of Pickling Process Parameters for Corrosion Inhibition in Steel Construction. Processes. 2025; 13(3):796. https://doi.org/10.3390/pr13030796
Chicago/Turabian StyleOuakki, Moussa, Khaoula Alaoui, Radouane Lachhab, Mohamed Rbaa, Mohamed Cherkaoui, Mohamed Ebn Touhami, and Younes El Kacimi. 2025. "Experimental Design Modelization and Optimization of Pickling Process Parameters for Corrosion Inhibition in Steel Construction" Processes 13, no. 3: 796. https://doi.org/10.3390/pr13030796
APA StyleOuakki, M., Alaoui, K., Lachhab, R., Rbaa, M., Cherkaoui, M., Ebn Touhami, M., & El Kacimi, Y. (2025). Experimental Design Modelization and Optimization of Pickling Process Parameters for Corrosion Inhibition in Steel Construction. Processes, 13(3), 796. https://doi.org/10.3390/pr13030796








