Abstract
Water pollution caused by landfill leachate, which contains high concentrations of heavy metals and organic contaminants, poses a serious environmental threat. Among the potential remediation strategies, phytoremediation using Miscanthus x giganteus (giant miscanthus) has gained attention due to its strong resistance to harsh conditions and its capacity to accumulate heavy metals. This study evaluates the effectiveness of Miscanthus x giganteus in treating landfill leachate, with a focus on removing key pollutants such as zinc (Zn), nickel (Ni), and copper (Cu) by simulating wetland conditions. A pilot-scale experiment conducted at the Grebača landfill site assessed the plant’s ability to enhance metal bioavailability, stabilize contaminants, and limit their mobility within the leachate system. The results demonstrated that Miscanthus x giganteus effectively mobilized Zn and Ni through rhizospheric activity, whereas Cu remained largely immobile, indicating potential for phytostabilization. Sequential extraction analysis further confirmed that the plant significantly reduced the mobile fractions of Zn and Ni in the soil, highlighting its dual role in both phytoremediation and phytostabilization. These findings suggest that Miscanthus x giganteus offers a sustainable and cost-effective approach to landfill leachate treatment, serving as a viable alternative to conventional methods. By integrating this nature-based solution into industrial and municipal waste management, it promotes environmental sustainability while enhancing remediation efficiency.
1. Introduction
Water pollution caused by industrial, agricultural, and municipal waste is an increasingly serious issue in modern society [1]. Landfills, as one of the largest sources of pollution, generate significant amounts of leachate, which can contain heavy metals, organic contaminants, and high concentrations of nutrients such as nitrogen and phosphorus [2,3,4,5]. Treating this type of wastewater presents a major challenge. Recently, phytoremediation—the use of plants to remove pollutants from water and soil—has gained attention as an environmentally friendly and sustainable approach to wastewater treatment [6]. Miscanthus x giganteus (giant miscanthus) is a tall grass primarily used in biomass production, but its potential for wastewater treatment remains largely unexplored [7,8]. This study explores the feasibility of using Miscanthus x giganteus for landfill leachate treatment, mimicking wetland conditions as one of the most promising nature-based solutions for contaminant removal. While various conventional wastewater treatment technologies, such as coagulation and flocculation, are commonly used, they have several limitations. As a result, there is a growing interest in alternative approaches, including bioremediation technologies [9].
Certain phytoremediation plants can accumulate high levels of heavy metals in their roots, tolerate various metal contaminants, and adapt to diverse environmental conditions. The energy crop Miscanthus x giganteus (hereafter referred to as miscanthus) has been identified as a promising candidate for the phytoremediation of contaminated soils and landfill leachate. Miscanthus exhibits high resistance to various diseases and pests and is characterized by high productivity, even under minimal nutrient availability and extreme weather conditions [7,9]. Additionally, its long-term cultivation contributes to carbon sequestration in the soil, enhances cation exchange capacity, and improves water retention. Over the past decade, a growing body of research has demonstrated the potential of this species for biomass production on soils contaminated with trace elements (TE), further supporting its role in sustainable land management and bioenergy production [10,11,12].
The comparison of Miscanthus x giganteus with other phytoremediation species, such as Phragmites australis and Typha spp., reveals distinct advantages and capabilities in addressing soil contamination. Miscanthus has demonstrated significant potential in reducing heavy metal concentrations and improving soil quality, often outperforming other species in specific contexts. It effectively reduced mobile forms of heavy metals, with reductions of 1.06 mg/kg for chromium and 5.25 mg/kg for pesticides, surpassing Phragmites australis in similar conditions [13]. It exhibited a high tolerance to trace elements like cadmium, lead, and zinc, maintaining growth and physiological functions even in contaminated soils [14]. The cultivation of Miscanthus improved soil biological parameters, such as microbial biomass and enzymatic activities, more effectively than Typha spp., which typically show lower resilience in mixed contaminant scenarios [11]. Long-term studies indicated that Miscanthus can accumulate heavy metals in its root system, enhancing its phytostabilization capabilities compared to Phragmites australis [15]. In contrast, while Phragmites australis and Typha spp. are also effective in phytoremediation, they may not match the comprehensive soil quality improvements and heavy metal tolerance exhibited by Miscanthus x giganteus, particularly in complex contamination scenarios.
Although previous studies have provided valuable insights into this topic, there is still a gap in knowledge regarding the accumulation of trace elements (TEs) in Miscanthus x giganteus cultivated on TE-polluted soils. Further research is needed to determine the plant’s phytoremediation potential under such conditions. Additionally, the impact of miscanthus cultivation on soil parameters and metal mobility in field contamination studies remains insufficiently explored. To date, no studies have specifically investigated the phytoremediation of landfill leachate using Miscanthus x giganteus, highlighting the need for research in this area to obtain scientifically valid results. Wetland-based treatment systems have been widely used for wastewater treatment over the past few decades due to their reliability, cost-effectiveness, and simple design [16]. These systems have proven effective in removing a range of environmental pollutants, including phosphorus, nitrogen, heavy metals, organic compounds, and suspended solids, through an integrated mechanism involving plants, substrate media, and microbial activity.
From an environmental pollution perspective, the key components of landfill leachate include organic pollutants (e.g., volatile acids, humic substances, aromatic hydrocarbons, and halogenated organic compounds) and inorganic pollutants (e.g., heavy metals, ammonia nitrogen, sulfates, sodium, magnesium, and calcium) [17]. In most landfills, leachate comes into direct contact with surface and groundwater, posing a significant environmental risk [18].
Various advanced leachate treatment technologies have been developed, with coagulation–flocculation being the most widely used method for removing suspended particles from wastewater. This process works by neutralizing negatively charged suspended particles through the addition of coagulants and flocculants [19]. Commonly used coagulants, such as aluminum sulfate (alum) and poly-aluminum chloride (PAC), are favored due to their high coagulation efficiency and availability. However, their use presents potential environmental concerns, particularly the release of residual aluminum, which can accumulate in the human body—affecting organs such as the brain, bones, and liver—and pose broader ecological risks.
Given these challenges, alternatives to conventional treatment methods should be explored through the lens of bioremediation technologies. Bioremediation, a branch of biotechnology, encompasses processes that facilitate the recovery of contaminated environments using living organisms [20]. This approach offers a sustainable and environmentally friendly alternative to chemical-based treatment systems.
Bioremediation can be categorized into three main types: (i) enzymatic degradation, which involves the use of industrially produced enzymes to break down toxic compounds; (ii) microbial remediation, which utilizes indigenous or inoculated bacteria and fungi capable of converting toxic substances into less harmful forms; and (iii) phytoremediation, which relies on plants for environmental decontamination [21,22,23].
Certain phytoremediation plants have demonstrated the ability to accumulate high levels of heavy metals in their roots, tolerate a variety of metal contaminants, and adapt to different environmental conditions. Studies have shown that Phragmites australis can accumulate the highest concentrations of cadmium and nickel, while Echinochloa stagnina is particularly effective at absorbing lead into its tissues [24]. In addition to phytoextraction, Echinochloa stagnina and Ludwigia stolonifera have shown potential for the phytostabilization of heavy metals [24,25]. Given these capabilities, phytoremediation is often considered a cost-effective and environmentally sustainable technique for removing heavy metals from water and soil.
One specialized phytoremediation technique is rhizofiltration, in which plant roots absorb and remove pollutants from wastewater. Root exudates play a crucial role in this process by altering the pH of the rhizosphere and facilitating heavy metal uptake. Research by Benavides et al. [26] demonstrated that Zea mays has significant potential for mercury extraction and bioaccumulation. Additionally, Robinson and Robinson [27] showed that reed bed systems can be successfully applied for landfill leachate treatment, with a focus on monitoring chemical oxygen demand (COD), biochemical oxygen demand (BOD), chloride, ammonia, and nitrate nitrogen. However, given that both heavy metals and organic pollutants are commonly found in groundwater, further research is needed to expand these studies and incorporate additional monitoring parameters.
Despite its advantages, phytoremediation also presents several challenges, particularly from an economic perspective. One of the primary limitations is the long treatment duration compared to conventional technologies. The efficiency of phytoremediation depends on plant growth rates, environmental conditions, and pollutant bioavailability, which can prolong the remediation process. Additionally, large land areas are often required to achieve meaningful contaminant removal, making implementation difficult in urban or space-limited settings. The costs associated with land acquisition, maintenance, and potential biomass disposal or treatment further complicate the economic feasibility of large-scale phytoremediation projects. While phytoremediation offers a lower-cost alternative to chemical and physical remediation methods, its application must be carefully evaluated in terms of cost-effectiveness, land use, and long-term sustainability.
At the KwaZulu-Natal wastewater treatment plant in South Africa, a rhizofiltration system planted with Phragmites australis and Kyllinga nemoralis was tested for its efficiency in removing heavy metals from municipal wastewater. The results showed varying concentrations of heavy metal deposition in both the planted and reference sections of the rhizofilter. Notably, cadmium levels increased by 33% and 21% in the root system of Kyllinga nemoralis, indicating the system’s effectiveness in extracting heavy metals from wastewater [28].
Miscanthus x giganteus has shown strong resistance to diseases, harmful insects, and extreme environmental conditions. It maintains high productivity even in nutrient-poor soils and harsh climates, while its cultivation contributes to carbon sequestration, cation exchange, and water retention in the soil. Over the past decade, research has increasingly demonstrated the ability of this species to produce biomass on trace element-contaminated soils for bioenergy applications [29]. Recent studies have explored trace element (TE) accumulation in miscanthus cultivated in Poland, France, Serbia, and other regions worldwide [15,30,31]. However, TE distribution patterns within different plant organs (roots, rhizomes, stems, and leaves) have been primarily studied in hydroponic systems with cadmium (Cd) and in mining waste (Technosols) polluted with arsenic (As) and lead (Pb) [32].
While previous studies have investigated the phytoremediation potential of various plant species, there is limited knowledge regarding the ability of Miscanthus x giganteus to treat landfill leachate. Existing research has primarily focused on its biomass potential and phytoremediation of trace element-contaminated soils, but its capacity to remove heavy metals and nutrients from landfill leachate remains unexplored. This study aims to fill this gap by assessing the feasibility of Miscanthus x giganteus for landfill leachate treatment under wetland-like conditions. Specifically, this research aims to: (1) assess the plant’s capacity to remove pollutants such as heavy metals from leachate; and (2) explore the potential application of this technology for wastewater bioremediation.
2. Materials and Methods
Location of the experiment. Obrenovac is a municipality within the administrative jurisdiction of the city of Belgrade, Serbia. It is located approximately 30 km southwest of the central area of Belgrade, positioned near the meandering course of the Sava River to the north. Additionally, the Kolubara River flows to the east of the municipality before converging with the Sava. The geographical coordinates (GPS location) of Obrenovac are 44°39′ N latitude and 20°12′ E longitude.
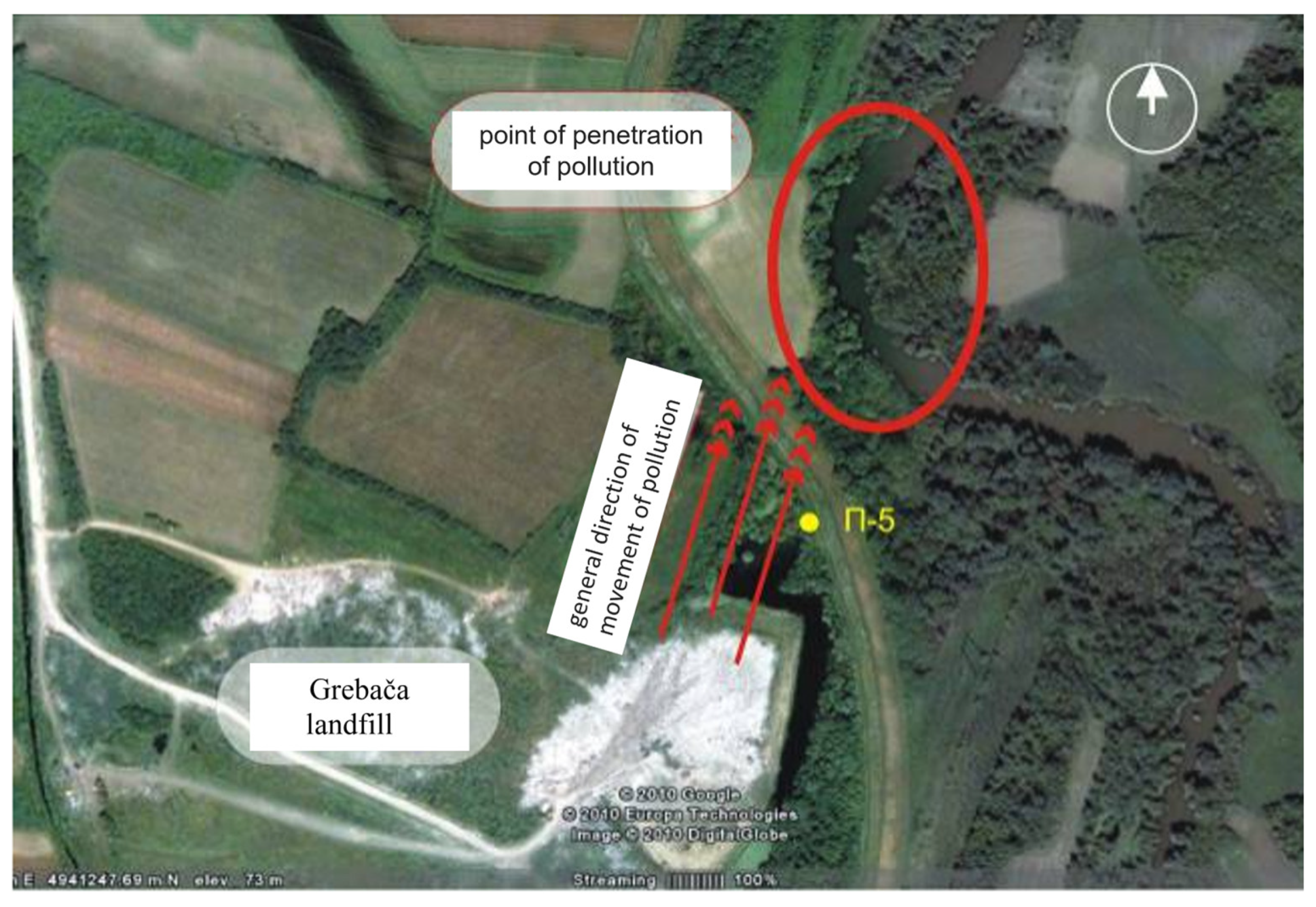
One of the most pressing environmental challenges in the municipality of Obrenovac is the proliferation of illegal waste dumps along the banks of the Kolubara River. Due to the highly permeable gravelly substrate in this area, there is a significant risk of leachate from these dumps infiltrating both surface and groundwater systems. Such contamination could have severe adverse effects on water quality and pose long-term environmental risks [33]. A particularly critical concern is the municipal landfill “Grebača”, which is in a former oxbow of the Kolubara River (Figure 1). This positioning further exacerbates the risk of groundwater contamination, highlighting the urgent need for effective waste management and remediation strategies.
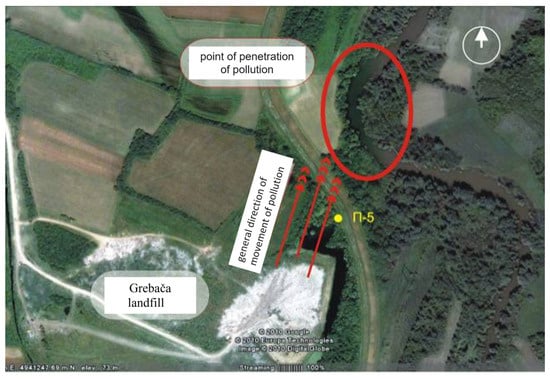
Figure 1.
General direction of movement of pollution from Grebaca landfill to Kolubara River.
The microclimatic conditions are illustrated in Table 1, providing data on precipitation levels and average temperatures at the study site, which are particularly relevant for understanding the flow of leachate from the Grebača landfill. This information is based on available analyses from the Republic Hydrometeorological Institute of Serbia (RHMZ) and regional climate models. Throughout the year, average temperatures increased from 2 °C in January to a peak of 25 °C in July, followed by a decline to approximately 4 °C in December. The warmest months are June, July, and August. The highest precipitation levels are recorded in May (70 mm), while September and February are the driest months, with precipitation ranging between 30 and 35 mm. This climate follows a typical temperate continental pattern, characterized by warm and relatively dry summers and colder, wet winters.

Table 1.
Average temperature and precipitation values for Obrenovac in 2023 (RHMZ 2024).
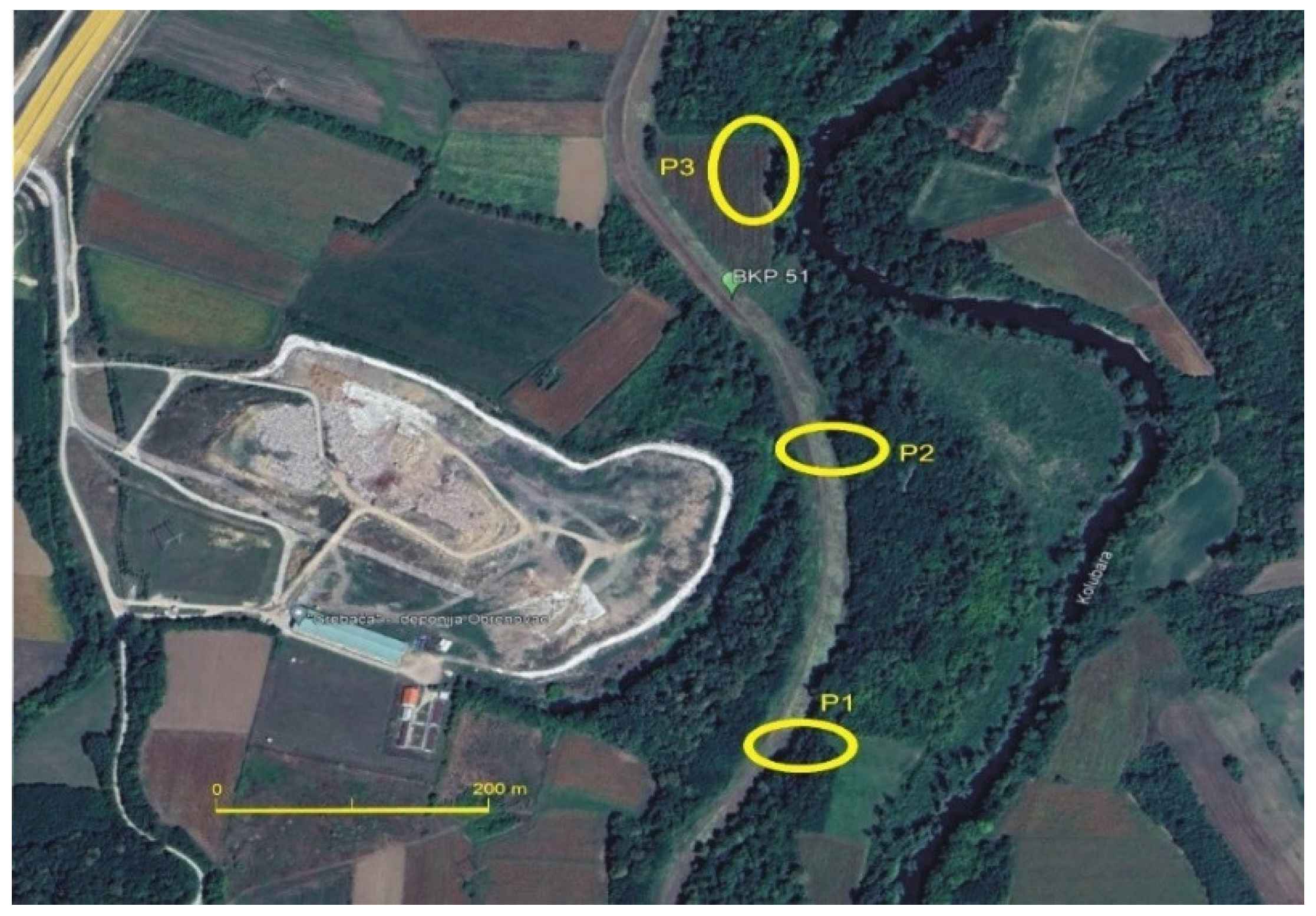
To establish the necessary conditions for the successful implementation of this experiment, three exploration boreholes were drilled to a depth of 7–8 m. At each location, piezometers were installed, with particular emphasis on the piezometer positioned at the periphery of the oxbow lake (Figure 2). These specific piezometers are of primary importance for the study, as they extract groundwater that is in direct hydraulic connection with the water in the oxbow lake, which constitutes leachate. As highlighted in previous sections, leachate from the Grebača landfill directly discharges into the oxbow lake.
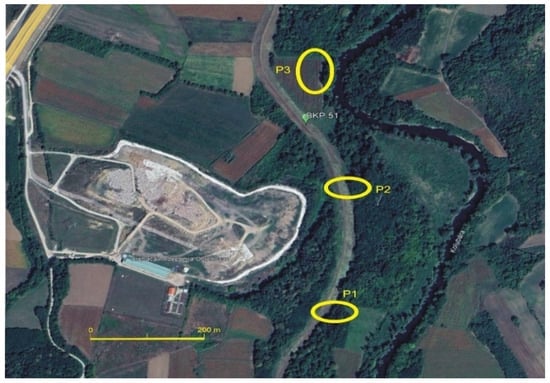
Figure 2.
Piezometer locations P1, P2 and P3.
The process of drilling exploratory boreholes and installing piezometers serves to collect data on the geological, hydrogeological, and engineering characteristics of the soil and groundwater.
The objective of drilling observation piezometers was twofold: first, to confirm the lithological profile, which was not entirely known at the time of the experiment’s setup, and second, to determine the depth of the groundwater table and assess the qualitative impact of the landfill on the groundwater system.
For this purpose, the following piezometer arrangement was selected: (1) Piezometer P-1: positioned upstream of the landfill, outside its influence on the Kolubara River watercourse; (2) Piezometer P-2: located within the landfill zone, near oxbow lake, and (3) Piezometer P-3: placed downstream of the landfill, in an area where the landfill’s impact on the river watercourse has been visually observed.
Hydrogeological mapping has identified the following general lithological profile: humus, sandy silt (0.0–1.0 m); clayey sand (1.0–2.5 m); fine- to medium-grained sand (2.5–5.0 m); coarse-grained gravel, moderately sandy, clayey, with pebble inclusions up to 3 cm in diameter (5.0–5.5 m); medium-grained, sandy gravel (5.5–6.5 m), and gray, marl-like clay (6.5–8.0 m).
Based on the mapping of the drilled material, a prospective layer for water capture was identified, and the piezometer construction was defined and installed within the exploratory borehole as follows: solid PVC pipe Ø 80 mm (non-filtered upper section; +0.50–3.0 m); filtered structure Ø 80 mm (perforated PVC pipe wrapped with mesh, 3.0–7.50 m), and solid PVC pipe Ø 80 mm (sediment trap, 7.50–8.0 m).
Following the installation of the piezometer structure, a gravel filter pack with a grain size of 1–3 mm was placed within the filter zone, covering the 3.0–8.0 m interval. A clay-based sealing layer was installed in the 0.0–3.0 m interval to ensure proper isolation. The piezometer structure was secured with a concrete base at the ground surface, measuring 0.5 × 0.5 × 0.3 m, and fitted with a lockable protective cap to prevent unauthorized access.
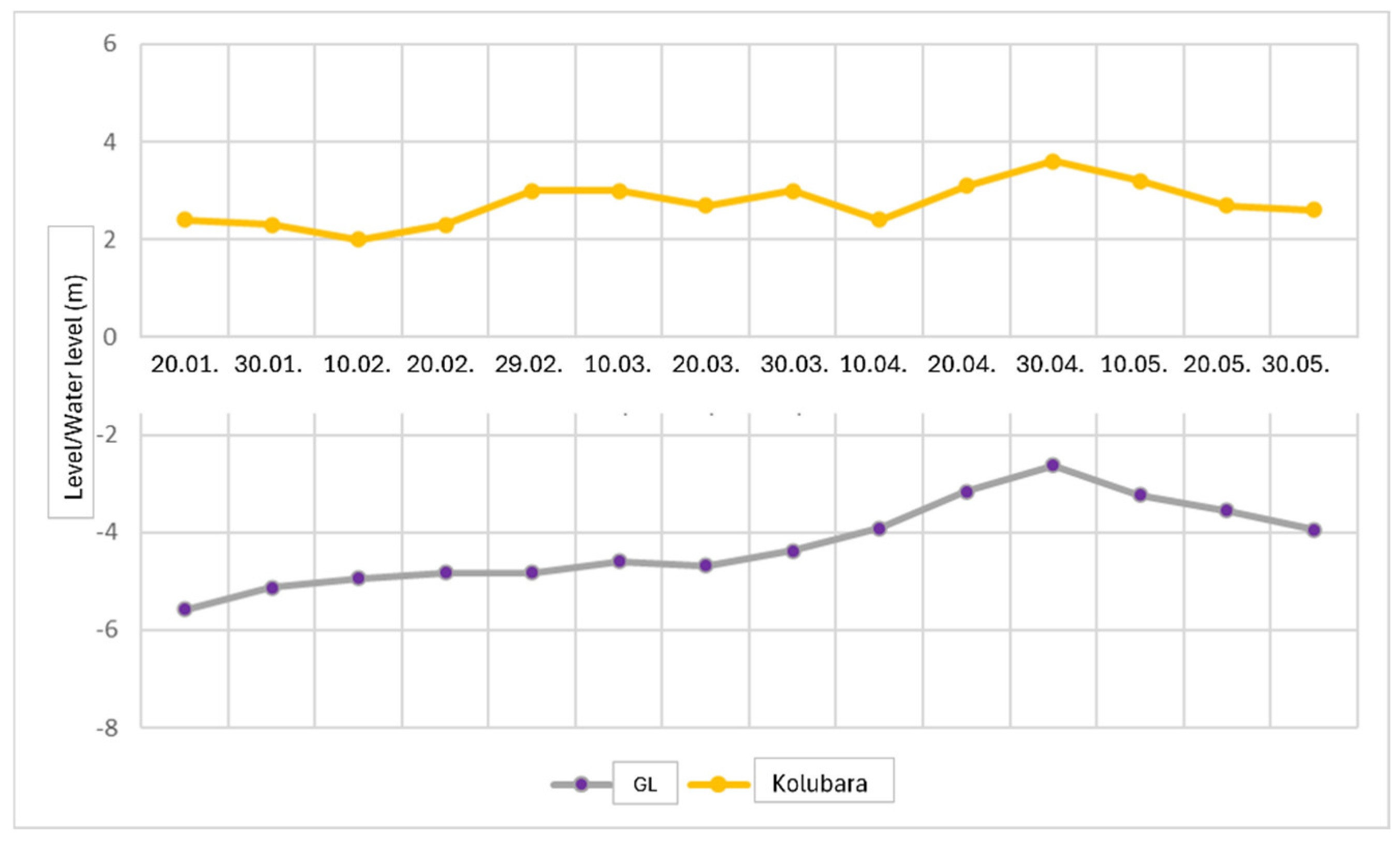
For the water level graph analysis (Figure 3), data from previous studies were utilized, confirming a general trend of interdependence. The purpose of the water level graph is to determine the hydraulic interaction between the groundwater system and the Kolubara River. The groundwater system in question is a phreatic aquifer that primarily recharges through precipitation and maintains a water level that corresponds with the river level.
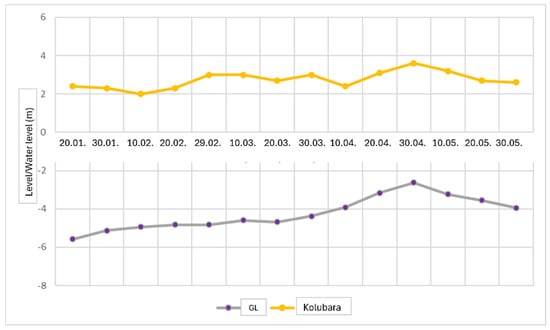
Figure 3.
Formation of the water level graph. GL—groundwater level.
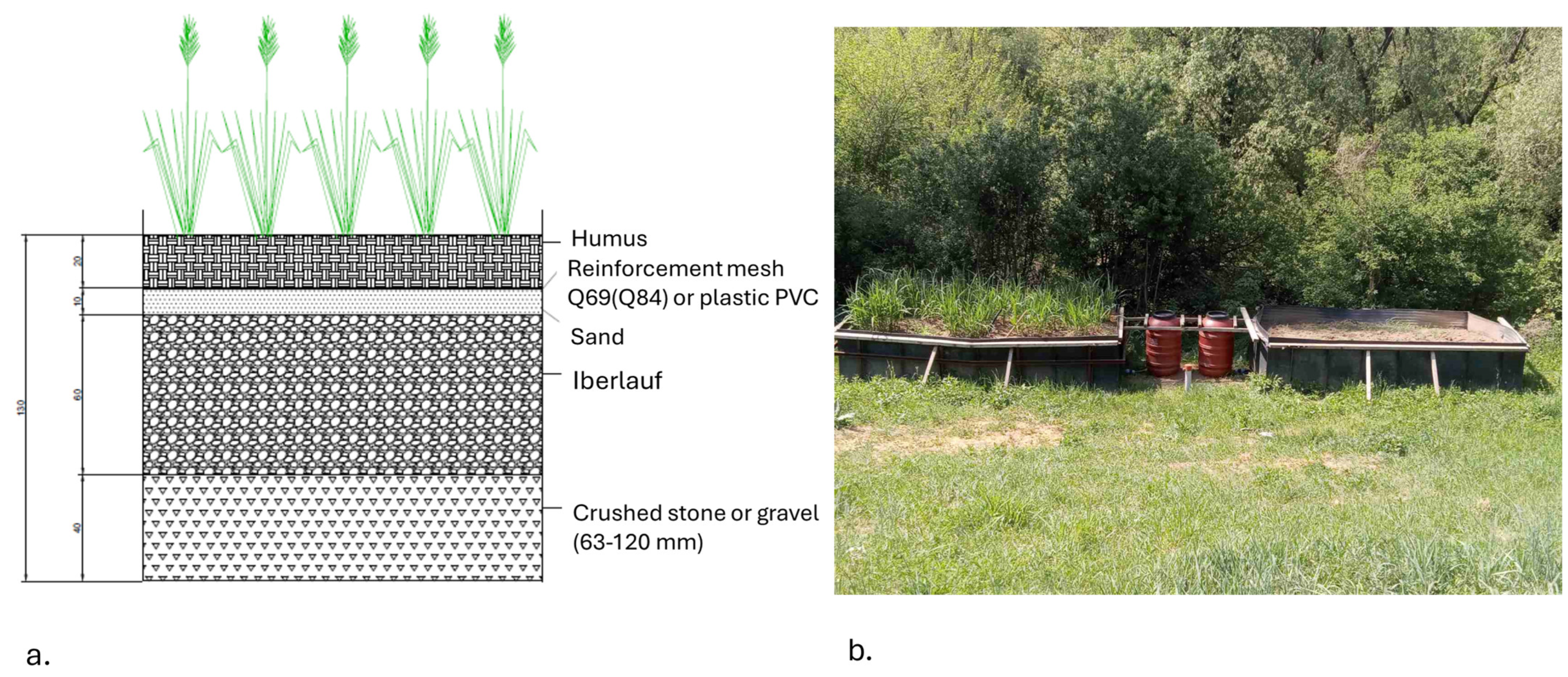
Pilot setup. Two basins, measuring 4 × 2 × 1.5 m, were filled in layers to simulate a pond ecosystem with a specific area requirement of 0.87 m2 per m3 of leachate. To monitor the water flow within the system, each basin was connected to a 200 L tank through a system of interconnected vessels. The tank served as a water level regulator for the pools. The first layer in both basins consisted of crushed stone (granulation 68–120 mm), reaching a height of 40 cm, with a total material consumption of approximately 7 cubic meters. The second layer, known as iberlauf, was added up to 60 cm in height, with around 10 cubic meters used. A 10 cm layer of sand was then placed, requiring roughly 2 cubic meters for both pools. Next, a Q69 reinforcement mesh (4 × 2 m) was installed on both basins, followed by a 20 cm layer of humus, totaling about 3.2 cubic meters (Figure 4). One of the basins was designated as a control (etalon) and remained devoid of plants, while the other was planted with Miscanthus x giganteus, a sterile triploid hybrid, fast-growing, and high-energy perennial grass (Figure 4). The plant was transplanted from an experimental field near the village of Noćaj during the third year of the vegetation period, to optimize its potential for heavy metal adsorption, particularly zinc (Zn), nickel (Ni), and copper (Cu)—the predominant metals found in landfill leachates. The transplantation aimed to simulate the plant’s natural habitat. After planting, each Miscanthus plant was covered with humus and surrounded by soil from the immediate vicinity of the newly established phytoremediation system to replicate natural conditions. Subsequently, a solution of heavy metals was introduced into the system, with 10 cubic meters of water extracted from the piezometer and pumped to match the water level in the basin. Once equilibrium was reached, the system was considered stable. This process was repeated for the basin without Miscanthus.
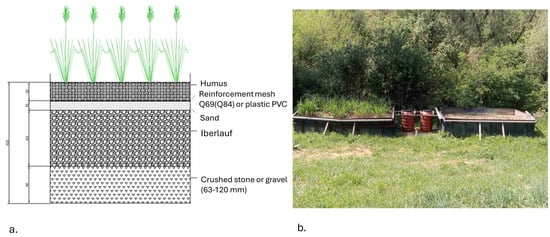
Figure 4.
Construction of wetlands conceptual design (a) and as-built condition with and without Miscanthus x giganteus (b).
Analysis of the leachate from the piezometer indicated variable concentrations of some heavy metals, likely due to the rapid flow of leachate, which caused fluctuations in concentrations at the sampling point [34]. In the laboratory, a solution was prepared to triple the concentration of these metals in the tank, resulting in contamination of the phytoremediation system. Soil, root, and plant samples in triplicate were systematically collected every seven days during a two-month period. Prior to each sampling, the contaminated water was recirculated within the system to maintain a consistent balance. The pump circulated water from the tank to the pools, temporarily disrupting the equilibrium, after which the system was allowed to stabilize before the next sampling. This circulation mimicked natural water dynamics, ensuring the accuracy of the sampling process.
For the analysis of heavy metal content in soil samples, 1 g of soil was combined with 9 mL of concentrated HNO3 (Merck, Darmstadt, Germany) and 3 mL of concentrated HCl Merck, Darmstadt, Germany, then digested at 170 °C using a Start E/0610030141 microwave system (Milestone, Sorisole, Italy) following the EPA 3051A method [35]. After digestion, the samples were filtered, diluted to 50 mL with deionized water, and analyzed for heavy metal content using an ICP-MS 7700 (Agilent Technologies, Tokyo, Japan) according to EPA 6020B [36].
For plant sample analysis, 0.5 g of material was mixed with 7 mL of concentrated HNO3 and 2 mL of H2O2 (Merck, Darmstadt, Germany), then subjected to microwave digestion using a temperature program as follows: 85 °C for 4 min, 145 °C for 9 min, 200 °C for 4 min, followed by 14 min at 200 °C. After digestion, the samples were filtered, diluted to 50 mL with deionized water, and analyzed using an ICP-MS 7700 (Agilent Technologies, Tokyo, Japan) according to EPA 6020B [36].
The bioavailability of heavy metals was assessed through BCR sequential extraction as described by Arain et al. [37], classifying metals into four fractions: acid-soluble (F1), reducible (F2), oxidizable (F3), and residual (F4). Following microwave digestion, heavy metal concentrations in each fraction were determined using an ICP-MS 7700 (Agilent Technologies, Tokyo, Japan) according to EPA 6020B.
Soil pH was measured using a glass electrode in a 1:5 (w/w) sediment–water suspension, in accordance with ISO 10390:2005 [38].
Soil texture analysis was performed following ISO 11277:2009 [39], using sieves ranging from 2 mm to 0.063 mm. Particles smaller than 0.063 mm were further classified using the sedimentation method, involving withdrawal of the suspension at specific times and depths.
3. Results
The Grebača landfill in Obrenovac has a significant impact on the water body of the Kolubara River, primarily through leachate generated by the infiltration of rainfall into the deposited waste (Figure 1). In Serbia, many landfills lack adequate systems for purifying these waters, which increases the risk of contamination of both groundwater and surface water. The flow and volume of leachate vary depending on seasonal rainfall and the hydrogeological characteristics of the area. Without an appropriate collection system, these waters first accumulate in the leachate pool at the landfill’s edge and eventually enter the Kolubara River, carrying pollutants that can negatively affect water quality and the river’s ecosystem. Field observations suggest that, during periods of heavy rainfall, excess leachate is discharged into nearby drainage channels, which ultimately lead to the Kolubara River. This confirms previous studies highlighting landfill leachate as a significant source of heavy metal contamination in surface waters [40,41]. Surface waters downstream from the landfill exhibit a significant increase in concentrations of suspended solids and heavy metals compared to upstream samples. The suspended solids downstream exceed the permissible limits for ecologically acceptable waters, reducing transparency and negatively affecting photosynthetic processes. The analysis of downstream water samples revealed an increase in heavy metal concentrations, with Zn and Ni levels exceeding EU environmental quality standards [42]. Notably, the background concentrations in upstream samples were within acceptable limits, indicating that the landfill acts as a primary contamination source. The observed values aligned with findings from similar landfill sites where leachate-driven pollution has been documented [43].
This can lead to the accumulation of toxins in sediments and bioaccumulation in aquatic organisms, which threatens biodiversity and the health of aquatic ecosystems [44,45,46]. High concentrations of nitrogen (higher than 15 mg/L) and phosphorus (higher than 2 mg/L), which originate from leachate [33], promote excessive algal blooms in the downstream sections of the Kolubara River. This reduces oxygen levels in the water, causing fish and other organisms sensitive to water quality changes to perish [4,47]. The presence of Escherichia coli and fecal streptococci [48] in downstream samples indicates significant fecal contamination, which can have direct health implications on the local population using the water for irrigation or recreation. The results for the soil characteristics at the beginning and end of the experiment are shown in Table 2.

Table 2.
The heavy metal concentrations and soil characteristics used in the experiment (at the start and the end of the experiment).
The measured parameters included pH, granulometric composition, humus content, clay percentage, and concentrations of selected heavy metals (Zn, Ni, and Cu). The pH values showed slight variations, with an increase from 7.26 ± 1.24 to 7.96 ± 1.05 in the control group, while in the presence of Miscanthus x giganteus, the initial pH of 7.95 ± 1.06 slightly decreased to 7.87 ± 1.18. These changes suggest a relatively stable pH environment in both scenarios.
The granulometric analysis revealed notable shifts in particle size distribution. In the setup with Miscanthus x giganteus, finer fractions (0–0.04 mm and 0.08–0.10 mm) increased significantly by the end of the experiment, whereas coarser fractions, such as 0.15–0.5 mm and 0.5–1.2 mm, decreased compared to their initial values. This redistribution suggests a potential influence of plant root activity on soil texture.
Contrary to the initial expectations, the control setup exhibited a greater increase in humus content compared to the Miscanthus-treated soil. One possible explanation is that Miscanthus’ high nutrient demand may have led to the increased microbial decomposition of organic matter, reducing humus accumulation in the rhizosphere [49]. This suggests that while Miscanthus contributes to organic carbon cycling, its effect on humus formation may depend on soil conditions and microbial activity.
Clay content exhibited a more pronounced increase in the experimental setup with Miscanthus x giganteus, rising from 3.25 ± 0.73% to 5.00 ± 0.51%, whereas in the control group, it increased from 2.50 ± 0.10% to 3.75 ± 0.97%.
Regarding heavy metal concentrations, zinc (Zn) levels decreased slightly in both cases, with a reduction from 170.8 ± 10.7 mg/kg to 162.1 ± 11.54 mg/kg in the control and from 135.8 ± 2.54 mg/kg to 136.3 ± 2.67 mg/kg in the presence of Miscanthus x giganteus. Nickel (Ni) showed an increase in both scenarios, with a more substantial rise in the control setup (from 147.1 ± 5.09 mg/kg to 194.7 ± 9.82 mg/kg) compared to the Miscanthus x giganteus setup (from 106.8 ± 3.07 mg/kg to 112.7 ± 3.96 mg/kg). Copper (Cu) concentrations showed a contrasting trend, significantly increasing in the control (from 31.9 ± 4.94 mg/kg to 58.94 ± 8.73 mg/kg), while decreasing slightly in the experimental group (from 15.0 ± 2.16 mg/kg to 14.04 ± 2.04 mg/kg).
These findings indicate that Miscanthus x giganteus influences soil composition by modifying granulometric distribution and affecting the accumulation of organic matter and clay content. Additionally, its presence appears to regulate heavy metal concentrations differently compared to the control group. Further research is needed to explore the mechanisms behind these changes and their implications for soil remediation and phytoremediation potential.
The primary objective of this study was to assess the ability of Miscanthus x giganteus (giant miscanthus) to treat landfill leachate, with a specific focus on the mobilization and behavior of heavy metals, such as zinc (Zn), nickel (Ni), and copper (Cu), which are common contaminants in landfill runoff due to industrial and municipal waste. The results offer significant insights into the potential of Miscanthus x giganteus as a sustainable phytoremediation tool.
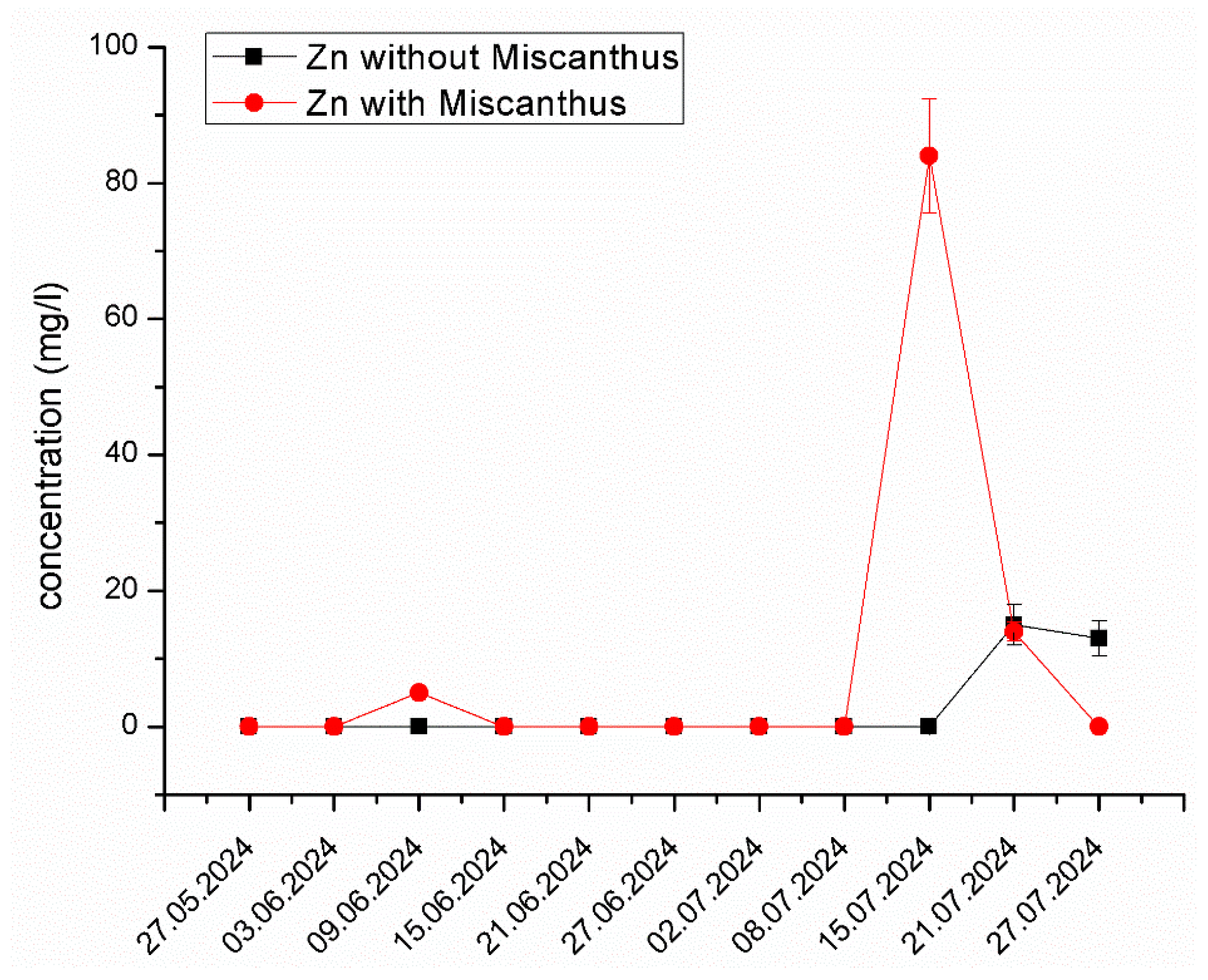
Zn Mobilization and Behavior in the System. Figure 5 illustrates the changes in Zn concentration in the experimental system containing Miscanthus x giganteus and the control system without the plant. A significant increase in Zn concentration was observed on 15 July 2024, reaching 84.19 mg/L, a value considerably higher than in the control system. This suggests that Zn was initially mobilized due to root exudates enhancing bioavailability, but subsequently stabilized, potentially due to plant uptake and rhizosphere interactions. This dual effect of temporary solubilization followed by stabilization is consistent with other phytoremediation studies [50,51]. The exudation of organic acids and chelating agents from the plant roots alters the pH and redox potential of the medium, shifting Zn from solid-phase adsorption sites into the aqueous phase, making it more bioavailable. The formation of Zn–humus complexes and carbonate dissociation reactions may further contribute to this mobilization. These findings align with previous studies by Zadel et al. [52], which highlighted the role of Miscanthus x giganteus in enhancing rhizospheric microbial activity, thus facilitating metal solubilization. Furthermore, it is well-documented that root exudates, such as organic acids and phytosiderophores, play a crucial role in metal solubilization in contaminated soils [53,54].
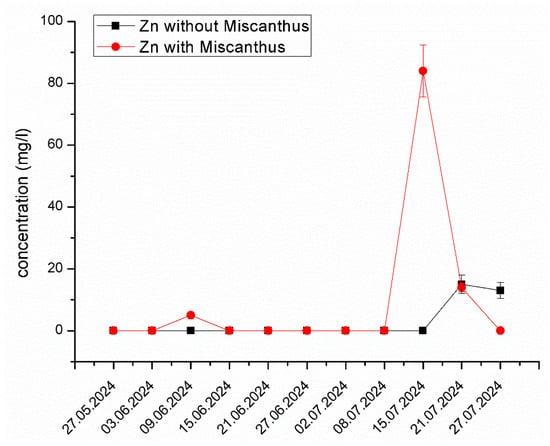
Figure 5.
The changes in Zn concentration in the experimental system containing Miscanthus x giganteus and the control system without the plant at different dates of sampling. The standard deviation represents the value of triplicates.
The leachate sampled from the Grebača landfill exhibited high concentrations of Zn, which is typical of landfill runoff [55]. Over the course of the study, the concentration of Zn significantly decreased in the system with Miscanthus x giganteus, particularly during the latter stages of the experiment. This suggests that Miscanthus x giganteus plays a significant role in Zn removal through both bioaccumulation and mobilization, making it an effective tool for phytoremediation in polluted environments. The ability of Miscanthus to enhance the bioavailability of Zn through rhizospheric activities may be an important factor in its overall effectiveness for phytoremediation [56].
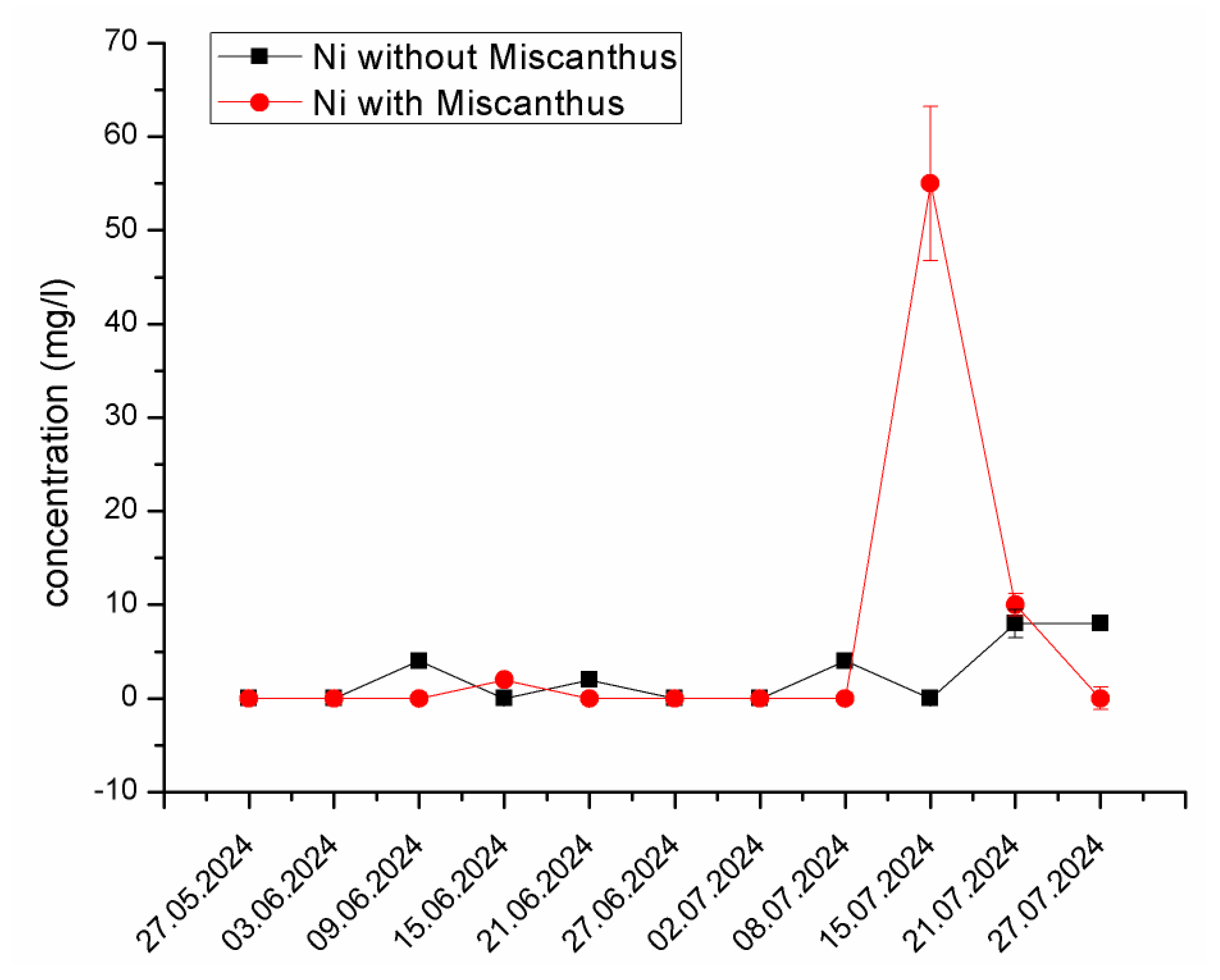
Nickel (Ni) Mobilization and Interaction. A similar trend was observed for Ni, with a peak concentration of 55.62 mg/L recorded on 15 July 2024, in the experimental system containing Miscanthus x giganteus, while the control system showed minimal fluctuation (Figure 6). Ni is particularly sensitive to pH fluctuations, and its solubility increases under acidic conditions. The reaction responsible for this mobilization can be attributed to the exudation of organic acids from the plant roots, which enhance Ni solubility in the leachate. Additionally, microbial activity and the formation of organic Ni complexes likely contributed to its increased mobility.
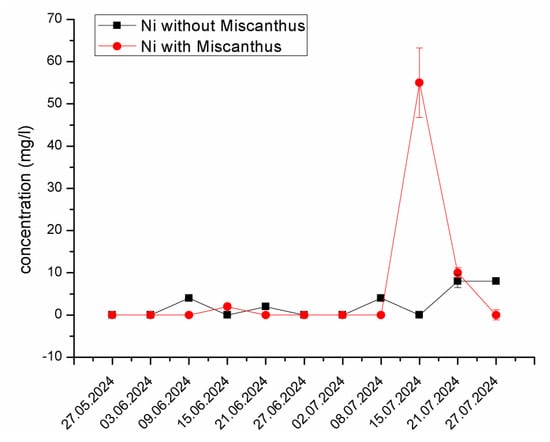
Figure 6.
The changes in Ni concentration in the experimental system containing Miscanthus x giganteus and the control system without the plant at different dates of sampling. The standard deviation represents the value of triplicates.
The sequential extraction results also support these findings, showing that the presence of Miscanthus x giganteus significantly reduced the mobile fraction of Ni in the soil, likely due to the enhanced metal retention in stable organic complexes. Similar findings reported by previous research papers [57,58] demonstrated that acidic exudates from plant roots significantly enhanced nickel solubility in contaminated environments. Moreover, studies have shown that Ni solubility can also be influenced by interactions with organic materials, including humic substances, which may enhance their mobility under appropriate conditions [59].
The reduction in Ni mobility in the Miscanthus x giganteus system suggests that the plant could play a significant role in stabilizing Ni in contaminated environments, limiting its bioavailability and preventing further environmental contamination. This is consistent with findings from other studies on metal phytoremediation, where plants have been shown to reduce metal mobility by altering soil chemistry and forming stable metal–organic complexes [12,60,61].
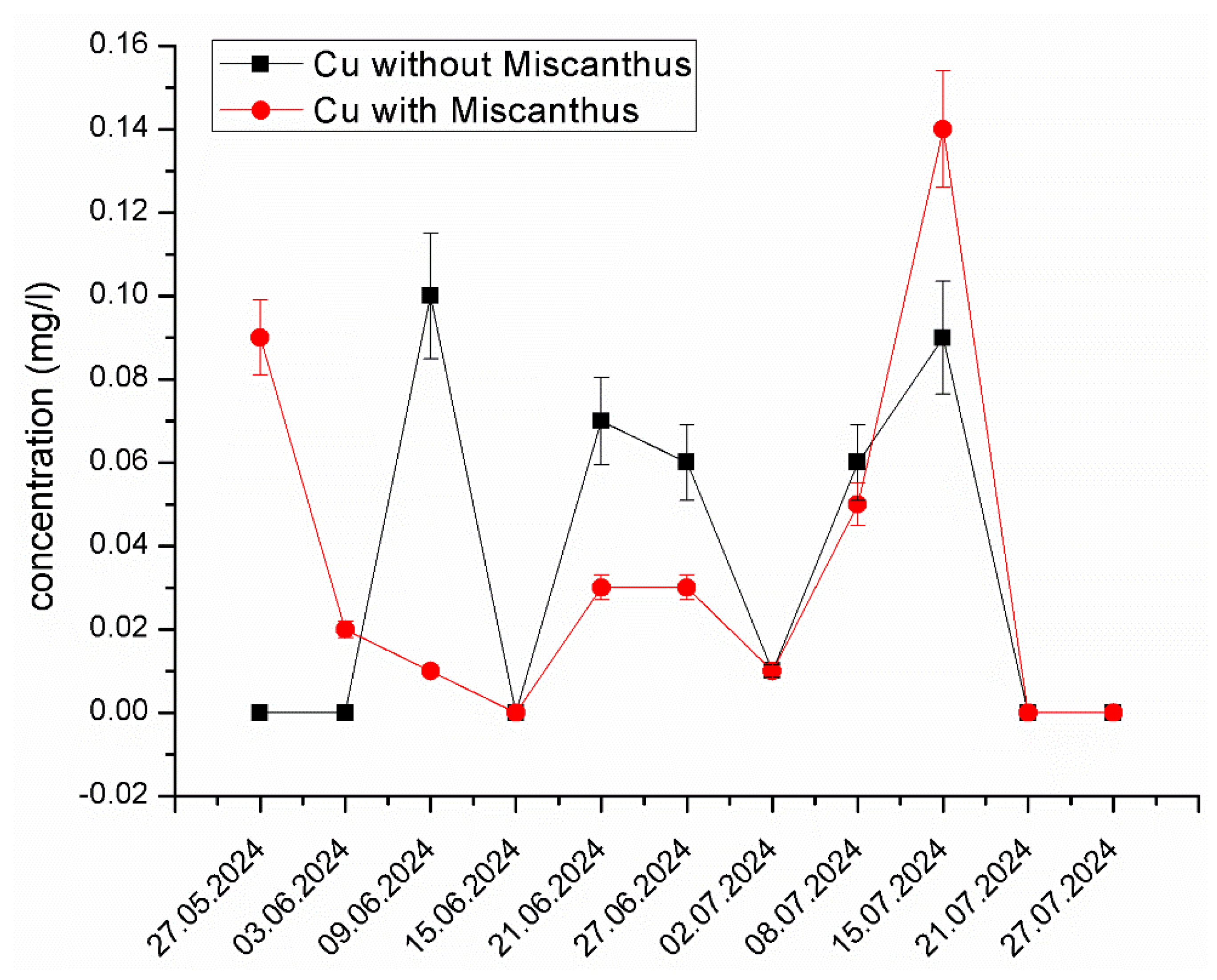
Copper (Cu) Stability and Limited Mobility. Unlike Zn and Ni, Cu exhibited relatively stable concentrations in both the experimental and control systems (Figure 7). This stability suggests that Cu remained strongly bound to organic matter and mineral particles. The dominant reaction restricting its mobility is the complexation with humic substances, which is supported by the sequential extraction analysis [62]. Cu’s strong adsorption to substrate mineral surfaces contributed to its immobilization, preventing its bioavailability in the leachate. These results align with studies by Martínez-Alcalá et al. [63], which indicated that Cu is less bioavailable due to its strong association with organic fractions in soil.
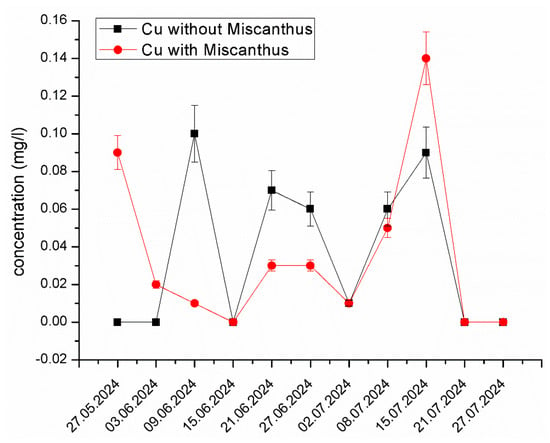
Figure 7.
The changes in Cu concentration in the experimental system containing Miscanthus x giganteus and the control system without the plant in different dates of sampling. The standard deviation represents the value of triplicates.
The sequential extraction data also revealed that the mobile fraction of Cu was significantly reduced in the presence of Miscanthus x giganteus, confirming its role as a phytostabilizer for Cu. This finding suggests that Miscanthus x giganteus can effectively limit Cu mobility, making it less available for leaching into groundwater and other environmental media. The limited mobility of Cu in the system can also be attributed to the strong affinity of Cu for binding sites on soil organic matter, reducing the metal’s leaching potential [64].
The observed peak in the Cu concentration could be due to temporary changes in pH, redox conditions, or microbial activity, which may have released Cu from its stable complexes. Additionally, organic matter decomposition, root exudates, or seasonal plant stress could have influenced Cu mobility in the system. External factors such as variations in leachate composition, rainfall, or sampling conditions might have also contributed to this fluctuation. Despite this peak, the overall trend suggests that Miscanthus x giganteus plays a significant role in Cu stabilization, reducing its long-term mobility and leaching potential.
Recent studies by Bastia et al. [14] and Al Souki et al. [11,60] suggest that Miscanthus has potential for mitigating the mobility of metals like Cu, which are typically bound to soil components, thus preventing the metals from contaminating water sources. This highlights the effectiveness of Miscanthus x giganteus as a phytostabilizer, particularly in environments where the bioavailability of metals needs to be minimized.
Sequential Extraction Analysis of Heavy Metals in Soil. The sequential extraction analysis provided further insights into the fractionation of Zn, Ni, and Cu in soil samples with and without Miscanthus x giganteus. The data indicated that the presence of Miscanthus x giganteus significantly reduced the mobile fraction of Zn and Ni, likely due to enhanced metal retention in stable organic complexes (Table 3). For Cu, the presence of the plant also led to a reduction in its mobile fraction, reinforcing the role of Miscanthus in phytostabilization.

Table 3.
Sequential extraction analysis of soil and detected metal concentrations in different phases.
The results suggest that Miscanthus x giganteus influences metal mobility through multiple mechanisms, including pH modification, the exudation of chelating compounds, and interactions with soil organic matter. The observed reduction in mobile Zn and Ni fractions highlights the plant’s potential for phytostabilization in contaminated environments such as landfill sites. By stabilizing these metals in the soil, Miscanthus x giganteus helps mitigate heavy metal contamination risks and promotes sustainable environmental management. These findings reinforce the role of Miscanthus x giganteus as a viable candidate for landfill leachate treatment, providing a cost-effective and sustainable solution for reducing metal contamination. Moreover, the integration of Miscanthus in wetland treatment systems could further enhance overall treatment efficiency by combining the plant’s bioaccumulation capabilities with microbial activity and soil filtration. This integrated approach could address a broader range of pollutants typically found in leachate, including organic contaminants and suspended solids [24,25,44]. The sustainable nature of Miscanthus x giganteus also supports the concept of using plants for bioremediation in landfill leachate treatment. As suggested by Ghosh and Singh [55], phytoremediation can be a more cost-effective and environmentally friendly alternative to traditional physical and chemical treatment methods, particularly in long-term applications
Plant analysis at the start and the end of experiment. At the start of the experiment, a composite sample of Miscanthus x giganteus was analyzed for heavy metal concentrations, yielding values of 3.28 mg/kg for Ni, 10.89 mg/kg for Zn, and 4.99 mg/kg for Cu. The decision to conduct an analysis on a composite sample rather than separate plant fractions (leaves, stems, and roots) was based on the need to obtain a representative baseline concentration. Given the inherent variability in metal accumulation across different plant parts, composite sampling ensured a more generalized overview of the initial heavy metal content in the biomass. This approach minimizes the potential discrepancies arising from variations in plant age, growth stage, or localized environmental influences. After a two-month experimental period, during which wastewater was introduced into the system, a differentiated analysis of plant tissues (leaves, stems, and roots) was conducted to assess the heavy metal distribution. The results revealed significant changes in metal concentrations across different plant compartments, highlighting the uptake and translocation dynamics of Zn, Ni, and Cu. Root analysis confirmed that Zn and Ni were predominantly retained within root tissues, suggesting phytostabilization rather than phytoextraction as the dominant remediation pathway [65]. This supports the hypothesis that Miscanthus primarily mitigates metal mobility rather than promoting their removal from soil.
Analysis of Experimental Findings for Miscanthus x giganteus analysis. The final Zn concentrations were 19.44 mg/kg in leaves, 2.16 mg/kg in stems, and 25.05 mg/kg in roots. Compared to the initial average concentration (10.89 mg/kg), Zn exhibited notable accumulation, particularly in leaves and roots. This suggests an efficient uptake from the wastewater and possible translocation within the plant. The root Ni concentration increased substantially from an initial 3.28 mg/kg to 44.35 mg/kg, whereas leaves and stems showed relatively low accumulation (0.47 mg/kg and <0.4 mg/kg, respectively). This indicates a strong retention of Ni within the root system, suggesting the limited mobility of Ni within plant tissues. The Cu concentrations increased across all plant parts, reaching 7.49 mg/kg in leaves, 1.66 mg/kg in stems, and 13.97 mg/kg in roots. This suggests that Cu was absorbed and distributed within the plant, but with preferential accumulation in underground biomass. These results indicate that Miscanthus x giganteus exhibits selective metal uptake and retention mechanisms, with Zn and Cu being more mobile within the plant compared to Ni, which remained predominantly confined to the roots. The observed differences between the initial composite sample and final differentiated measurements underscore the importance of analyzing individual plant parts for a more detailed understanding of heavy metal dynamics in phytoremediation studies. The microbial community plays a crucial role in enhancing the phytoremediation of heavy metals when utilizing Miscanthus x giganteus. This plant not only accumulates heavy metals but also interacts with soil microbes, significantly influencing its remediation efficiency. Although this study does not focus on microbial interactions, it is important to highlight key aspects of this relationship.
Miscanthus x giganteus has demonstrated an average biomass yield of 16.96 t/ha, effectively removing heavy metals from contaminated soils [66]. The plant’s metal uptake capacity increases with biomass production over time, suggesting a positive correlation between growth and remediation efficiency [66]. The root exudates from Miscanthus x giganteus modify the rhizosphere microbial community, promoting soil aggregation and heavy metal immobilization [67]. Dominant microbial phyla, such as Acidobacteriota and Chloroflexi, enhance metal stabilization through complex formation with root exudates [67]. Additionally, the rhizobacteria associated with Miscanthus x giganteus facilitate metal extraction and improve phytoremediation efficiency by upregulating the genes related to stress response and biomass production [68]. The interaction between Miscanthus x giganteus and its microbial partners not only enhances heavy metal remediation but also contributes to soil health and biodiversity [69]. However, the overall efficiency of this process can be influenced by various factors, including soil conditions and the specific types of heavy metals present. Understanding these dynamics is essential for optimizing the use of Miscanthus x giganteus in phytoremediation strategies.
4. Conclusions
This study highlights the potential of Miscanthus x giganteus (giant miscanthus) as an effective tool for the phytoremediation of landfill leachate, particularly in the removal and stabilization of heavy metals such as zinc (Zn), nickel (Ni), and copper (Cu). The findings demonstrate that Miscanthus can significantly reduce the bioavailability of these metals through bioaccumulation and phytostabilization mechanisms, particularly by modifying soil pH and promoting the formation of stable metal–organic complexes. The plant’s high resistance to environmental stress and ability to thrive under minimal nutrient conditions further support its application in contaminated environments. The experimental data underscore the promise of integrating Miscanthus into wetland treatment systems, leveraging both plant-driven bioremediation and microbial activity to enhance landfill leachate treatment efficiency. This approach offers a sustainable and cost-effective alternative to traditional methods, contributing to long-term environmental management and reducing heavy metal pollution in water systems.
While the results are promising, several limitations must be addressed in future research. One key challenge is the variability in heavy metal concentrations within leachate from the Grebača landfill, which may have influenced the consistency of the findings. Future studies should focus on standardizing pollutant concentration levels to better evaluate the plant’s effectiveness under different wastewater conditions. Additionally, the long-term sustainability of Miscanthus x giganteus in landfill leachate treatment systems requires further exploration, particularly regarding metal bioaccumulation rates, potential phytotoxicity, and the fate of accumulated metals over time.
Moreover, scaling up this approach from a pilot-scale experiment to full-scale applications presents another critical research gap. Investigating the feasibility of large-scale Miscanthus-based phytoremediation systems, along with their economic viability, would provide valuable insights for industrial and municipal wastewater treatment. Future research should also explore the synergistic effects of Miscanthus with other bioremediation strategies, such as microbial-assisted phytoremediation and biochar amendments, to further enhance pollutant removal efficiency.
Addressing these challenges will be essential to fully realize the potential of Miscanthus x giganteus as a scalable, nature-based solution for landfill leachate management, offering a resilient and environmentally friendly alternative to conventional remediation methods.
Author Contributions
S.A.—conceptualization, investigation, methodology, data curation, validation, writing—original draft preparation; G.K.—methodology, data curation, writing—original draft preparation; S.M.—conceptualization, methodology, writing—review and editing; S.R.—conceptualization, methodology, writing—review and editing; M.K.I.—conceptualization, methodology, writing—review and editing; T.Z.—investigation, methodology, data curation, validation; J.B.—conceptualization, investigation, methodology, data curation, validation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Grants Nos. 451-03-137/2025-03/200125 & 451-03-136/2025-03/200125).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, B.J.; Chakraborty, A.; Sehgal, R. A systematic review of industrial wastewater management: Evaluating challenges and enablers. J. Environ. Manag. 2023, 348, 119230. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Hu, P.; Lang-Yona, N.; Xu, M.; Guo, C.; Gu, J.D. Global landfill leachate characteristics: Occurrences and abundances of environmental contaminants and the microbiome. J. Hazard. Mater. 2024, 461, 132446. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Wołejko, E.; Wydro, U.; Leszczyński, J.; Wasil, M.; Kiełtyka-Dadasiewicz, A. Chemical Composition and Toxicological Evaluation of Landfill Leachate from Białystok. Poland. Sustainability 2023, 15, 16497. [Google Scholar] [CrossRef]
- Jamrah, A.; AL-Zghoul, T.M.; Al-Qodah, Z. An Extensive Analysis of Combined Processes for Landfill Leachate Treatment. Water 2024, 16, 1640. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Elbl, J.; Koda, E.; Adamcová, D.; Bilgin, A.; Lukas, V.; Podlasek, A.; Kintl, A.; Wdowska, M.; Brtnický, M.; et al. Chemical Composition and Hazardous Effects of Leachate from the Active Municipal Solid Waste Landfill Surrounded by Farmlands. Sustainability 2020, 12, 4531. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Kowalczyk-Juśko, A.; Mazur, A.; Pochwatka, P.; Janczak, D.; Dach, J. Evaluation of the Effects of Using the Giant Miscanthus (Miscanthus x giganteus) Biomass in Various Energy Conversion Processes. Energies 2022, 15, 3486. [Google Scholar] [CrossRef]
- Voća, N.; Leto, J.; Karažija, T.; Bilandžija, N.; Peter, A.; Kutnjak, H.; Šurić, J.; Poljak, M. Energy Properties and Biomass Yield of Miscanthus x giganteus Fertilized by Municipal Sewage Sludge. Molecules 2021, 26, 4371. [Google Scholar] [CrossRef]
- Singh, D.; Goswami, R.K.; Agrawal, K.; Chaturvedi, V.; Verma, P. Bio-inspired remediation of wastewater: A contemporary approach for environmental clean-up. Curr. Res. Green Sustain. Chem. 2022, 5, 100261. [Google Scholar] [CrossRef]
- Mulabagal, V.; Baah, D.A.; Egiebor, N.O.; Sajjadi, B.; Chen, W.Y.; Viticoski, R.L.; Hayworth, J.S. Biochar from Biomass: A Strategy for Carbon Dioxide Sequestration, Soil Amendment, Power Generation, CO2 Utilization, and Removal of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in the Environment. In Handbook of Climate Change Mitigation and Adaptation; Lackner, M., Sajjadi, B., Chen, W.Y., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Burdová, H.; Mamirova, A.; Kuráň, P.; Kříženecká, S.; Oravová, L.; Tolaszová, J.; Nebeská, D.; Popelka, J.; Ustak, S.; et al. Evaluation of the Miscanthus x giganteus short term impacts on enhancing the quality of agricultural soils affected by single and/or multiple contaminants. Environ. Technol. Innov. 2021, 24, 101890. [Google Scholar] [CrossRef]
- Nebeská, D.; Trögl, J.; Ševců, A.; Špánek, R.; Marková, K.; Davis, L.; Burdová, H.; Pidlisnyuk, V. Miscanthus x giganteus role in phytodegradation and changes in bacterial community of soil contaminated by petroleum industry. Ecotoxicol. Environ. Saf. 2021, 224, 112630. [Google Scholar] [CrossRef] [PubMed]
- Romantschuk, L.; Matviichuk, N.; Mozharivska, I.; Matviichuk, B.; Ustymenko, V.; Tryboi, O. Phytoremediation of Soils by Cultivation Miscanthus x giganteus L. and Phalaris arundinacea L. Ecol. Eng. Environ. Technol. 2024, 6, 137–147. [Google Scholar] [CrossRef]
- Bastia, G.; Al Souki, K.S.; Pourrut, B. Evaluation of Miscanthus x giganteus Tolerance to Trace Element Stress: Field Experiment with Soils Possessing Gradient Cd, Pb, and Zn Concentrations. Plants 2023, 12, 1560. [Google Scholar] [CrossRef]
- Nurzhanova, A.; Pidlisnyuk, V.; Abit, K.; Nurzhanov, C.; Kenessov, B.; Stefanovska, T.; Erickson, L. Comparative assessment of using Miscanthus x giganteus for remediation of soils contaminated by heavy metals: A case of military and mining sites. Environ. Sci. Pollut. Res. 2019, 26, 13320–13333. [Google Scholar] [CrossRef]
- Silva, J.A. Wastewater Treatment and Reuse for Sustainable Water Resources Management: A Systematic Literature Review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, C.; Pan, J.; Yang, G.; Sun, C.; Liu, Y.; Chen, X.; Zhao, Z. Leachate from municipal solid waste landfills in a global perspective: Characteristics, influential factors and environmental risks. J. Clean. Prod. 2022, 333, 130234. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Ibrahim, A.M.; Al-Sulaiman, A.M.; Okasha, R.A. Landfill leachate: Sources, nature, organic composition, and treatment: An environmental overview. Ain Shams Eng. J. 2024, 15, 102293. [Google Scholar] [CrossRef]
- Kumar, R.N.; Sadaf, S.; Verma, M.; Chakraborty, S.; Kumari, S.; Polisetti, V.; Kallem, P.; Iqbal, J.; Banat, F. Old Landfill Leachate and Municipal Wastewater Co-Treatment by Sequencing Batch Reactor Combined with Coagulation–Flocculation Using Novel Flocculant. Sustainability 2023, 15, 8205. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef]
- Kuppan, N.; Padman, M.; Mahadeva, M.; Srinivasan, S.; Devarajan, R. A comprehensive review of sustainable bioremediation techniques: Eco friendly solutions for waste and pollution management. Waste Manag. Bull. 2024, 2, 154–171. [Google Scholar] [CrossRef]
- Ebsa, G.; Gizaw, B.; Admassie, M.; Degu, T.; Alemu, T. The role and mechanisms of microbes in dichlorodiphenyltrichloroethane (DDT) and its residues bioremediation. Biotechnol. Rep. 2024, 42, e00835. [Google Scholar] [CrossRef] [PubMed]
- Kakde, P.; Sharma, J. Microbial Bioremediation of Petroleum Contaminated Soil: Structural Complexity, Degradation Dynamics and Advanced Remediation Techniques. J. Pure Appl. Microbiol. 2024, 18, 2244–2261. [Google Scholar] [CrossRef]
- Abdelaal, M.; Mashaly, I.A.; Srour, D.S.; Dakhil, M.A.; El-Liethy, M.A.; El-Keblawy, A.; El-Barougy, R.F.; Halmy, M.W.A.; El-Sherbeny, G.A. Phytoremediation Perspectives of Seven Aquatic Macrophytes for Removal of Heavy Metals from Polluted Drains in the Nile Delta of Egypt. Biology 2021, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.M.; Galal, T.M.; Sewelam, N.A.; Talha, N.I.; Abdallah, S.M. Phytoremediation of heavy metals by four aquatic macrophytes and their potential use as contamination indicators: A comparative assessment. Environ. Sci. Pollut. Res. Int. 2020, 27, 12138–12151. [Google Scholar] [CrossRef]
- Benavides, L.C.L.; Pinilla, L.A.C.; Serrezuela, R.R.; Serrezuela, W.F.R. Extraction in Laboratory of Heavy Metals Through Rhizofiltration using the Plant Zea mays (maize). Int. J. Appl. Environ. Sci. 2018, 13, 9–26. [Google Scholar]
- Robinson, T.; Robinson, H. The use of reed beds for treatment of landfill leachets. Detritus 2018, 3, 124–140. [Google Scholar] [CrossRef]
- Odinga, C.A.; Kumar, A.; Mthembu, M.S.; Bux, F.; Swalaha, F.M. Rhizofiltration system consisting of Phragmites australis and Kyllinga nemoralis: Evaluation of efficient removal of metals and pathogenic microorganisms. Desalination Water Treat. 2019, 169, 120–132. [Google Scholar] [CrossRef]
- Lutts, S.; Zhou, M.X.; Flores-Bavestrello, A.; Hainaut, P.; Dailly, H.; Debouche, G.; Foucart, G. Season-dependent physiological behavior of Miscanthus x giganteus growing on heavy-metal contaminated areas in relation to soil properties. Heliyon 2024, 10, e25943. [Google Scholar] [CrossRef]
- Grzegórska, A.; Czaplicka, N.; Antonkiewicz, J.; Rybarczyk, P.; Baran, A.; Dobrzyński, K.; Zabrocki, D.; Rogala, A. Remediation of soils on municipal rendering plant territories using Miscanthus x giganteus. Environ. Sci. Pollut. Res. Int. 2023, 30, 22305–22318. [Google Scholar] [CrossRef]
- Ranđelović, D. Reclamation methods and their outcomes in Serbian mining basins. In Proceedings of the 2nd International and 14th National Congress of Soil Science Society of Serbia, Novi Sad, Serbia, 25–28 September 2017; Available online: https://ritnms.itnms.ac.rs/bitstream/handle/123456789/1023/bitstream_2228.pdf?sequence=1&isAllowed=y (accessed on 17 January 2025).
- Nsanganwimana, F.; Al Souki, K.S.; Waterlot, C.; Douay, F.; Pelfrêne, A.; Ridošková, A.; Louvel, B.; Pourrut, B. Potentials of Miscanthus x giganteus for phytostabilization of trace element-contaminated soils: Ex situ experiment. Ecotoxicol. Environ. Saf. 2021, 214, 112125. [Google Scholar] [CrossRef]
- Institute of Public Health of Serbia. 2019. Available online: https://www.batut.org.rs/download/publikacije/pub2019a.pdf (accessed on 19 January 2025).
- Official Gazette of the RS, No. 50/2012 Regulation on the Limit Value of Pollutants in Surface and Groundwater and Sediment and Deadlines for Achieving Them. 2012. Available online: https://www.paragraf.rs/propisi/uredba-granicnim-vrednostima-zagadjujucih-materija-vodama.html (accessed on 19 January 2025).
- U.S. EPA. Method 3051A (SW-846): Microwave Assisted Acid Digestion of Sediments, Sludges and Oils; Revision 1; U.S. EPA: Washington, DC, USA, 2007.
- U.S. EPA. Method 6020B (SW-846): Inductively Coupled Plasma-Mass Spectrometry; Revision 2; U.S. EPA: Washington, DC, USA, 2014.
- Arain, M.B.; Kazi, T.G.; Jamali, M.K.; Jalbani, N.; Afridi, H.I.; Baig, J.A. Speciation of Heavy Metals in Sediment by Conventional, Ultrasound and Microwave Assisted Single Extraction Methods: A Comparison with Modified Sequential Extraction Procedure. J. Hazard. Mater. 2008, 154, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- ISO 10390:2005; Soil Quality—Determination of pH. ISO (International Organization for Standardization): Geneva, Switzerland, 2005.
- ISO 11277:2009; Determination of Particle Size Distribution in Mineral Soil Material. Method by Sieving and Sedimentation. ISO (International Organization for Standardization): Geneva, Switzerland, 2009.
- Wuave, T. Leachate migration and percolation consequences on water quality: A case study of Plateau State Nigeria. E3S Web Conf. 2024, 497, 02012. [Google Scholar] [CrossRef]
- Wijesekara, S.S.R.M.D.H.R.; Mayakaduwa, S.S.; Siriwardana, A.R.; de Silva, N.; Basnayake, B.F.A.; Kawamoto, K.; Vithanage, M. Fate and transport of pollutants through a municipal solid waste landfill leachate in Sri Lanka. Environ. Earth Sci. 2014, 72, 1707–1719. [Google Scholar] [CrossRef]
- European Union. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy; EU: Brussels, Belgium, 2013; Available online: http://data.europa.eu/eli/dir/2013/39/oj (accessed on 17 January 2025).
- EPA. Environmental Guidelines: Solid Waste Landfills, 2nd ed.; U.S. EPA: Washington, DC, USA, 2016. Available online: https://www.epa.nsw.gov.au/sites/default/files/solid-waste-landfill-guidelines-160259.pdf (accessed on 15 January 2025).
- Masoud, A.M.N.; Alfarra, A.; Sorlini, S. Constructed Wetlands as a Solution for Sustainable Sanitation: A Comprehensive Review on Integrating Climate Change Resilience and Circular Economy. Water 2022, 14, 3232. [Google Scholar] [CrossRef]
- Sossou, K.; Prasad, S.B.; Agbotsou, K.E.; Souley, H.S.; Mudigandla, R. Characteristics of landfill leachate and leachate treatment by biological and advanced coagulation process: Feasibility and effectiveness—An overview. Waste Manag. Bull. 2024, 2, 181–198. [Google Scholar] [CrossRef]
- Republic Hydrometeorological Service of Serbia. Available online: https://www.hidmet.gov.rs/eng/hidrologija/index.php (accessed on 10 January 2025).
- WHO. World Health Statistics 2017: Monitoring Health For the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2017; Available online: https://iris.who.int/bitstream/handle/10665/255336/9789241565486-eng.pdf?sequence=1 (accessed on 27 December 2024).
- Hydrogeological Institute of Serbia. 2024. Available online: https://www.hidmet.gov.rs/data/klimatologija/ciril/2023.pdf (accessed on 9 February 2025).
- Loeppmann, S.; Blagodatskaya, E.; Pausch, J.; Kuzyakov, Y. Enzyme properties down the soil profile—A matter of substrate quality in rhizosphere and detritusphere. Soil Biol. Biochem. 2016, 103, 274–283. [Google Scholar] [CrossRef]
- Duffner, A.; Hoffland, E.; Temminghoff, E.J.M. Bioavailability of zinc and phosphorus in calcareous soils as affected by citrate exudation. Plant Soil 2012, 361, 165–175. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, S.; Saavedra-Mella, F.; Nguyen, T.A.H.; Southam, G.; Chan, T.S.; Lu, Y.R.; Huang, L. Rhizosphere modifications of iron-rich minerals and forms of heavy metals encapsulated in sulfidic tailings hardpan. J. Hazard. Mater. 2020, 384, 121444. [Google Scholar] [CrossRef]
- Zadel, U.; Nesme, J.; Michalke, B.; Vestergaard, G.; Płaza, G.A.; Schröder, P.; Radl, V.; Schloter, M. Changes induced by heavy metals in the plant-associated microbiome of Miscanthus x giganteus. Sci. Total Environ. 2020, 711, 134433. [Google Scholar] [CrossRef]
- Kumar, A.; Dadhwal, M.; Mukherjee, G.; Srivastava, A.; Gupta, S.; Ahuja, V. Phytoremediation: Sustainable Approach for Heavy Metal Pollution. Scientifica 2024, 2024, 3909400. [Google Scholar] [CrossRef]
- Lavanya, M.B.; Viswanath, D.S.; Sivapullaiah, P.V. Phytoremediation: An eco-friendly approach for remediation of heavy metal-contaminated soils-A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100975. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S.P. A Review on Phytoremediation of Heavy Metals and Utilization of Its Byproducts. Appl. Ecol. Environ. Res. 2005, 3, 1–18. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Mamirova, A.; Pranaw, K.; Stadnik, V.; Kuráň, P.; Trögl, J.; Shapoval, P. Miscanthus × giganteus Phytoremediation of Soil Contaminated with Trace Elements as Influenced by the Presence of Plant Growth-Promoting Bacteria. Agronomy 2022, 12, 771. [Google Scholar] [CrossRef]
- Mench, M.; Martin, E. Mobilization of cadmium and other metals from two soils by root exudates of Zea mays L., Nicotiana tabacum L. and Nicotiana rustica L. Plant Soil 1991, 132, 187–196. [Google Scholar] [CrossRef]
- Nguyen, T.X.T.; Amyot, M.; Labrecque, M. Differential effects of plant root systems on nickel, copper and silver bioavailability in contaminated soil. Chemosphere 2017, 168, 131–138. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of heavy metals in soils and their immobilization at micro-scale interfaces among diverse soil components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Liné, C.; Louvel, B.; Waterlot, C.; Douay, F.; Pourrut, B. Miscanthus x giganteus culture on soils highly contaminated by metals: Modelling leaf decomposition impact on metal mobility and bioavailability in the soil-plant system. Ecotoxicol. Environ. Saf. 2020, 199, 110654. [Google Scholar] [CrossRef]
- Bosiacki, M. Influence of increasing nickel content in soil on Miscanthus × giganteus Greef and Deu. Yielding and on the content of nickel in above-ground biomass. Arch. Environ. Prot. 2015, 41, 72–79. [Google Scholar] [CrossRef][Green Version]
- Clemente, R.; Arco-Lázaro, E.; Pardo, T.; Martín, I.; Sánchez-Guerrero, A.; Sevilla, F.; Bernal, M.P. Combination of soil organic and inorganic amendments helps plants overcome trace element induced oxidative stress and allows phytostabilisation. Chemosphere 2019, 223, 223–231. [Google Scholar] [CrossRef]
- Martínez-Alcalá, I.; Bernal, P.M. Environmental Impact of Metals, Metalloids, and Their Toxicity. In Metalloids in Plants: Advances and Future Prospects; Deshmukh, R., Tripathi, D.K., Guerriero, G., Eds.; John and Wiley and Sons: Hoboken, NJ, USA, 2020; Chapter 21. [Google Scholar] [CrossRef]
- Luo, T.; Sheng, Z.; Chen, M.; Qin, M.; Tu, Y.; Khan, M.N.; Khan, Z.; Liu, L.; Wang, B.; Kuai, J.; et al. Phytoremediation of copper-contaminated soils by rapeseed (Brassica napus L.) and underlying molecular mechanisms for copper absorption and sequestration. Ecotoxicol. Environ. Saf. 2024, 273, 116123. [Google Scholar] [CrossRef]
- Sharma, J. Introduction to Phytoremediation—A Green Clean Technology. SSRN 2018, 3177321. [Google Scholar] [CrossRef]
- Romanchuk, L.; Matviichuk, N.; Abramova, I.; Matviichuk, B.V.; Tryboi, O. Removal of heavy metals by energy crops when grown on technologically contaminated soils. Ecol. Eng. Environ. Technol. 2024, 26, 92–102. [Google Scholar] [CrossRef]
- Wu, B.; Li, X.; Lin, S.; Jiao, R.; Yang, X.; Shi, A.; Nie, X.; Lin, Q.; Qiu, R. Miscanthus sp. root exudate alters rhizosphere microbial community to drive soil aggregation for heavy metal immobilization. Sci. Total Environ. 2024, 949, 175009. [Google Scholar] [CrossRef]
- Pešić, M.; Radović, S.; Rakić, T.; Dželetović, Ž.; Stanković, S.; Lozo, J. Insights into the response of Miscanthus x giganteus to rhizobacteria: Enhancement of metal tolerance and root development under heavy metal stress. Arch. Biol. Sci. 2024, 76, 205–221. [Google Scholar] [CrossRef]
- Khatoon, Z.; Orozco-Mosqueda, M.d.C.; Santoyo, G. Microbial Contributions to Heavy Metal Phytoremediation in Agricultural Soils: A Review. Microorganisms 2024, 12, 1945. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).