Production of a Microbial Biofilm and Its Application on Tomato Seeds to Improve Crop Development in a Lead-Contaminated Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture to Obtain Planktonic Cells and Biofilm
2.2. Planktonic Cells and Biofilm Production with Increasing Pb Concentrations
2.2.1. Microbial Growth in the Planktonic State
2.2.2. Biofilm Yield
2.2.3. Biofilm Stability
2.3. Experimental Site and Design and Plant Material
2.4. Seed Germination Tests
2.5. Tomato Cropping in Greenhouse Conditions
2.5.1. Commercial Substrate Composition and Addition of Excess Pb
2.5.2. Biofilm Inoculation and Experimental Setup
2.5.3. Plant Growth and Fruit Production Evaluation
2.6. Lead Concentration on Tomato Fruit
2.7. Experimental Design and Statistical Analysis
3. Results
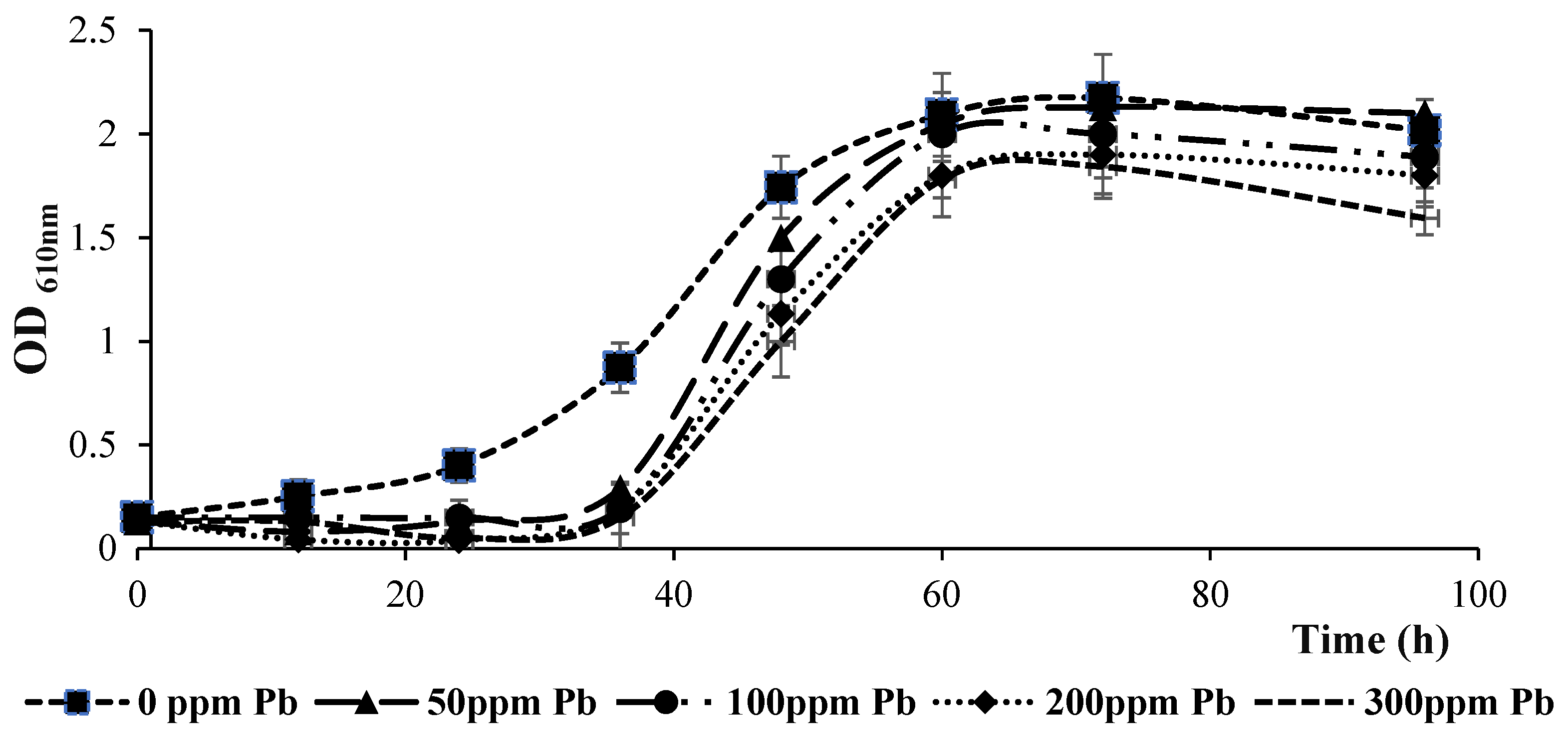
3.1. Impact of Pb Added to Liquid Medium on Bacterial Growth
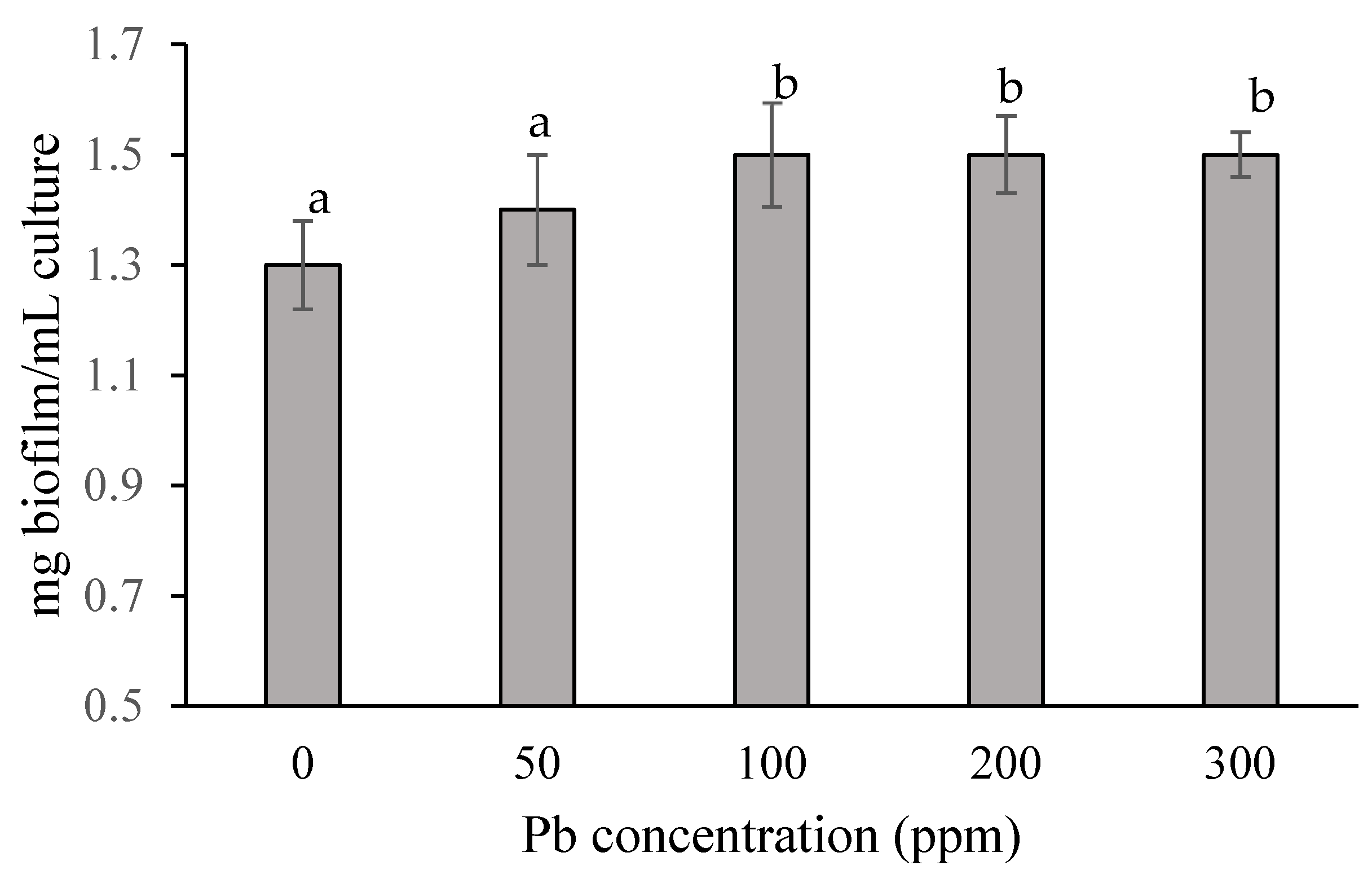
3.2. Impact of Pb on Biofilm Production
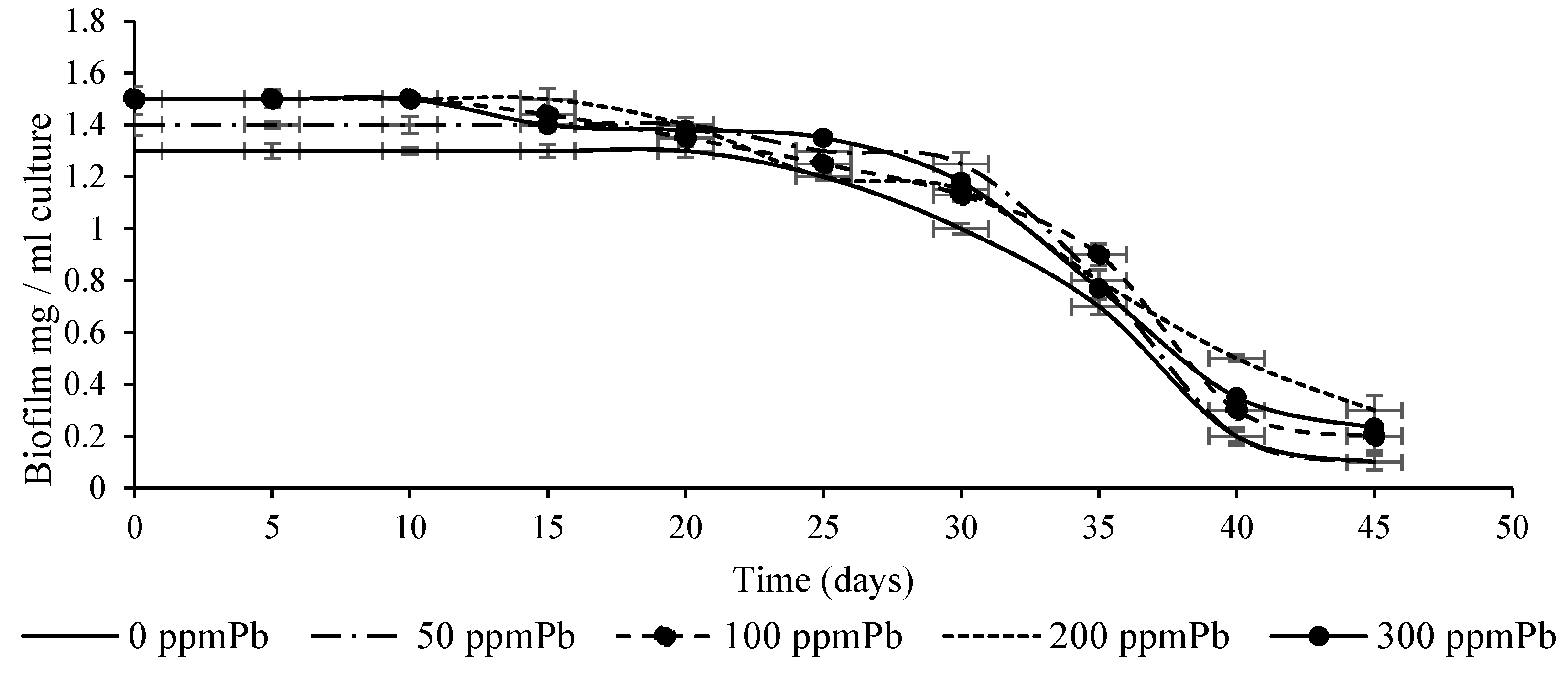
3.3. Impact of Pb and Temperature on Biofilm Stability
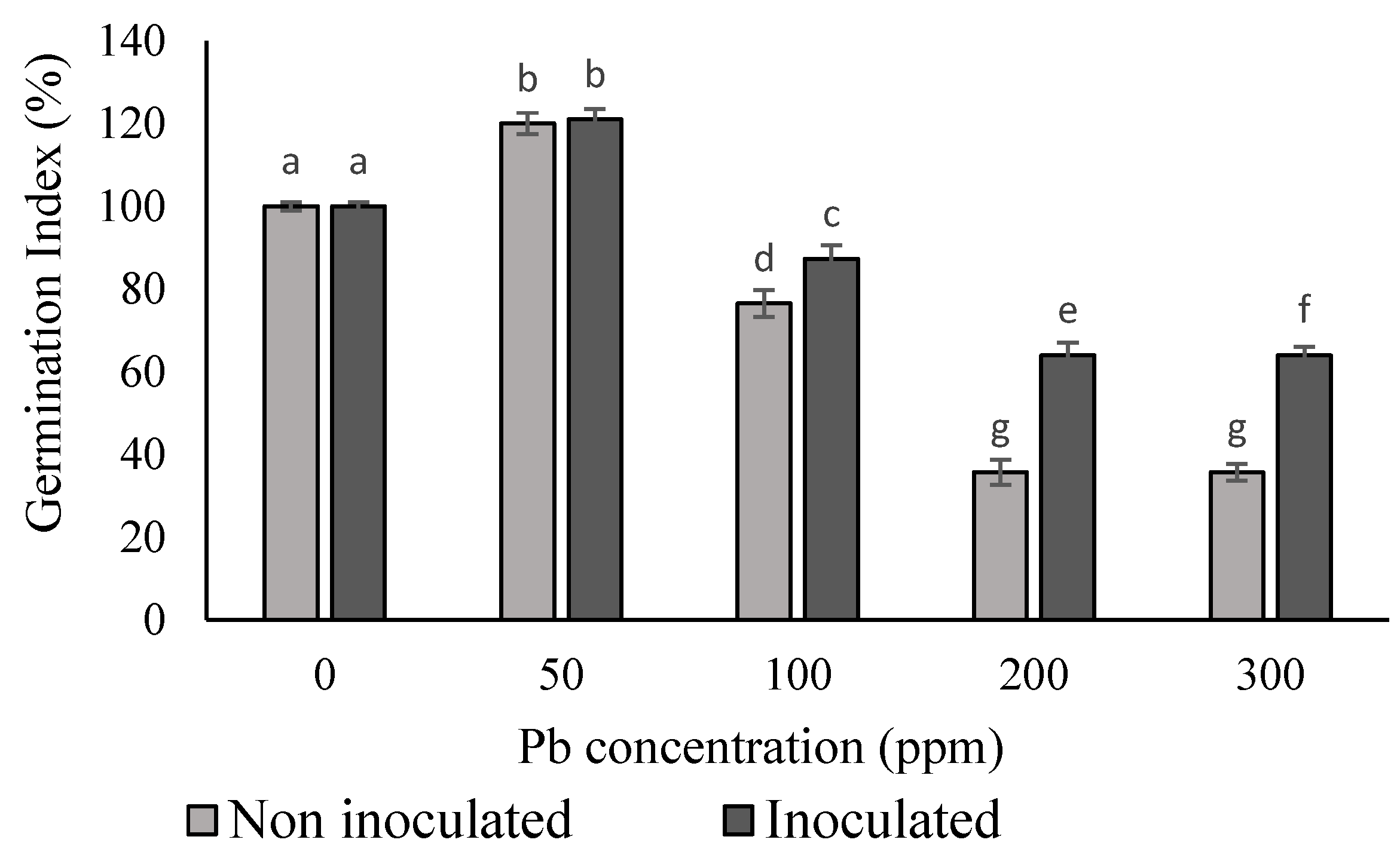
3.4. Impact of Pb and Biofilm Inoculation on Seed Germination
3.5. Tomato Plant Growth and Fruit Yield and Quality Under Greenhouse Conditions
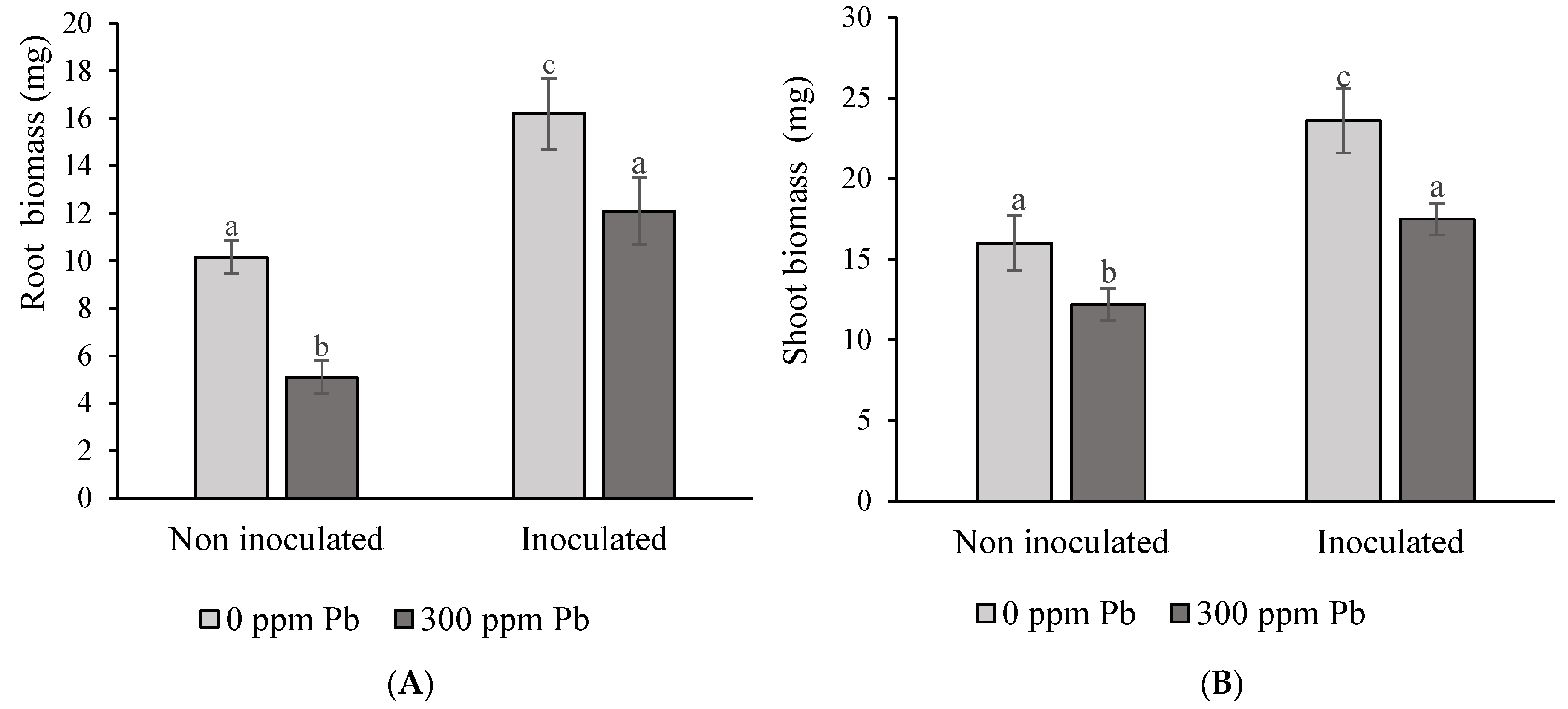
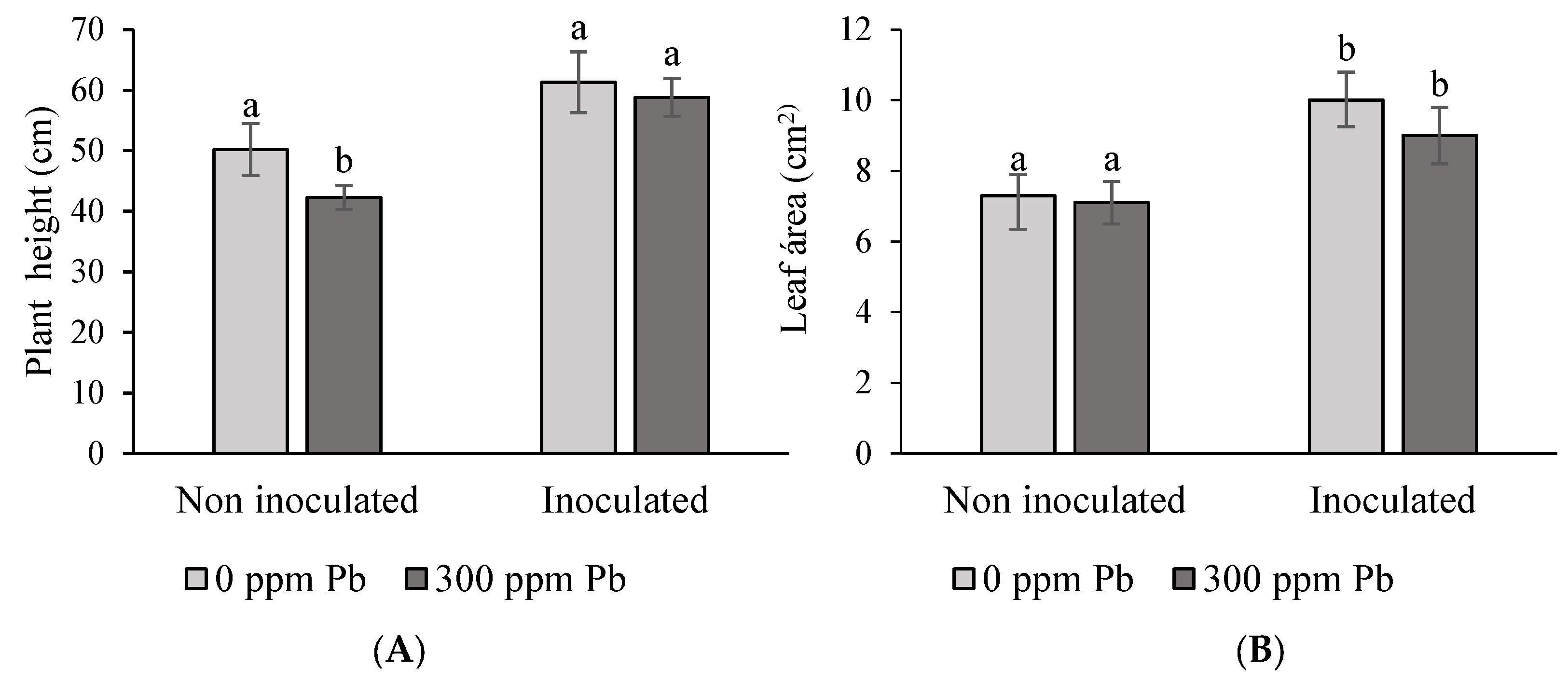
3.5.1. Plant Root and Shoot Agronomic Parameters
3.5.2. Fruit Yield and Quality
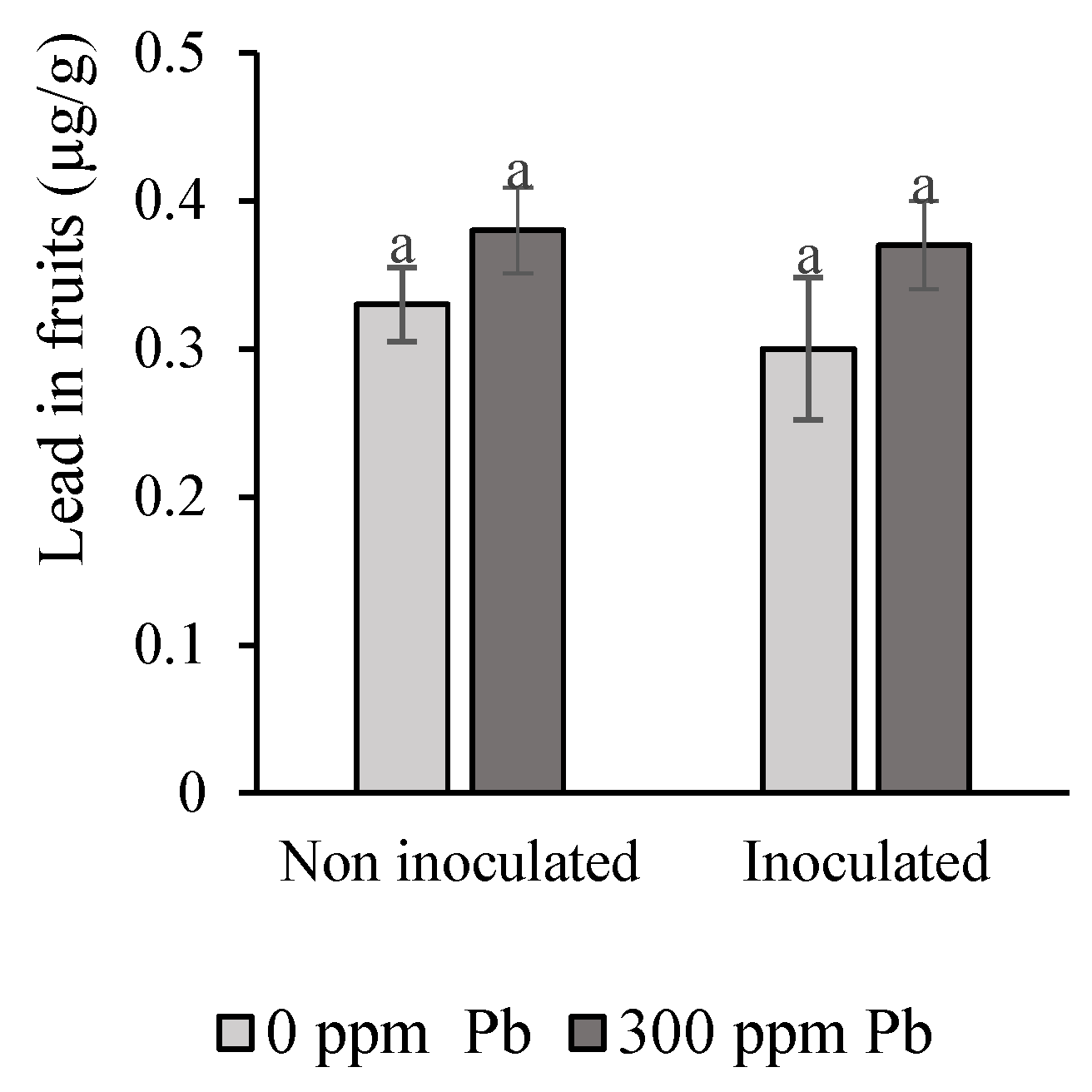
3.6. Pb Concentration in Tomato Fruits
4. Discussion
4.1. Impacts of Increasing Pb Concentrations on B. subtilis Performance
4.2. Impacts of Increasing Pb Concentrations on Seed Germination
4.3. Impacts of Pb and Seed Inoculation on Plant Growth and Fruit Quality
4.4. Lead in the Tomato Fruit
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeboah, I.B.; Tuffour, H.O.; Abubakari, A.; Melenya, C.; Bonsu, M.; Quansah, C.; Adjei-Gyapong, T. Mobility and Transport Behavior of Lead in Agricultural Soils. Sci. Afr. 2019, 5, e00117. [Google Scholar] [CrossRef]
- Hussain, M.I.; Qureshi, A.S. Health Risks of Heavy Metal Exposure and Microbial Contamination through Consumption of Vegetables Irrigated with Treated Wastewater at Dubai, UAE. Environ. Sci. Pollut. Res. 2020, 27, 11213–11226. [Google Scholar] [CrossRef]
- Collin, S.; Baskar, A.; Geevarghese, D.M.; Ali, M.N.V.S.; Bahubali, P.; Choudhary, R.; Lvov, V.; Tovar, G.I.; Senatov, F.; Koppala, S.; et al. Bioaccumulation of Lead (Pb) and Its Effects in Plants: A Review. J. Hazard. Mater. Lett. 2022, 3, 100064. [Google Scholar] [CrossRef]
- FAO; WHO. General Standard for Contaminants and Toxins in Food and Feed. Documento Debate Sobre La Revisión Del Código de Prácticas Para La Prevención y Reducción de La Presencia de Plomo En Los Alimentos (CXC 56-2004); FAO: Rome, Italy; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Hussain, M.I.; Khan, Z.I.; Ahmad, K.; Naeem, M.; Ali, M.A.; Elshikh, M.S.; uz Zaman, Q.; Iqbal, K.; Muscolo, A.; Yang, H.H. Toxicity and Bioassimilation of Lead and Nickel in Farm Ruminants Fed on Diversified Forage Crops Grown on Contaminated Soil. Ecotoxicol. Environ. Saf. 2024, 283, 116812. [Google Scholar] [CrossRef]
- Brown, S.L.; Chaney, R.L.; Hettiarachchi, G.M. Lead in Urban Soils: A Real or Perceived Concern for Urban Agriculture. J. Environ. Qual. 2016, 45, 26–36. [Google Scholar] [CrossRef]
- Hettiarachchi, G.M.; Pierzynski, G.M. In Situ Stabilization of Soil Lead Using Phosphorus and Manganese Oxide. J. Environ. Qual. 2002, 31, 564. [Google Scholar] [CrossRef]
- Prieto Méndez, J.; Ramírez González, C.; Gutiérrez Román, A.; García Prieto, F. Contaminación y Fitotoxicidad en Plantas Por Metales Pesados Provenientes De suelos y Agua. Trop. Subtrop. Agroecosyst. 2009, 10, 29–44. [Google Scholar]
- Hadi, F.; Aziz, T. A Mini Review on Lead (Pb) Toxicity in Plants. J. Biol. Life Sci. 2015, 6, 91. [Google Scholar] [CrossRef]
- Patel, M.; Surti, M.; Ashraf, S.A.; Adnan, M. Physiological and Molecular Responses to Heavy Metal Stresses in Plants. In Harsh Environment and Plant Resilience: Molecular and Functional Aspects; Springer International Publishing: Cham, Switzerland, 2021; pp. 171–202. ISBN 9783030659127. [Google Scholar]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead Toxicity: A Review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead Toxicity in Plants. Braz. J. Plant Physiol. 2005, 1, 35–52. [Google Scholar] [CrossRef]
- Jalal, A.; Júnior, E.F.; Teixeira Filho, M.C.M. Interaction of Zinc Mineral Nutrition and Plant Growth-Promoting Bacteria in Tropical Agricultural Systems: A Review. Plants 2024, 13, 571. [Google Scholar] [CrossRef] [PubMed]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Borriss, R. Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Mawarda, P.C.; Mallon, C.A.; Le Roux, X.; van Elsas, J.D.; Salles, J.F. Interactions between Bacterial Inoculants and Native Soil Bacterial Community: The Case of Spore-Forming Bacillus spp. FEMS Microbiol. Ecol. 2022, 98, fiac127. [Google Scholar] [CrossRef]
- Al-Gheethi, A.; Mohamed, R.; Noman, E.; Ismail, N.; Kadir, O.A. Removal of Heavy Metal Ions from Aqueous Solutions Using Bacillus subtilis Biomass Pre-Treated by Supercritical Carbon Dioxide. Clean-Soil Air Water 2017, 45, 1700356. [Google Scholar] [CrossRef]
- Cai, Y.; Li, X.; Liu, D.; Xu, C.; Ai, Y.; Sun, X.; Zhang, M.; Gao, Y.; Zhang, Y.; Yang, T.; et al. A Novel Pb-Resistant Bacillus subtilis Bacterium Isolate for Co-Biosorption of Hazardous Sb(III) and Pb(II): Thermodynamics and Application Strategy. Int. J. Environ. Res. Public Health 2018, 15, 702. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Khan, M.; Shamim, S. Unraveling the Underlying Heavy Metal Detoxification Mechanisms of Bacillus Species. Microorganisms 2021, 9, 1628. [Google Scholar] [CrossRef]
- Sutherland, C.; Chittoo, B.S.; Samlal, A. The Status of Scientific Development on the Application of Biosorption of Heavy Metals at Laboratory and Pilot-Scale: A Review. Desalination Water Treat. 2023, 299, 13–49. [Google Scholar] [CrossRef]
- Abdel-Monem, M.O.; Al-Zubeiry, A.H.S.; Al-Gheethi, A.A.S. Biosorption of Nickel by Pseudomonas cepacia 120S and Bacillus subtilis 117S. Water Sci. Technol. 2010, 61, 2994–3007. [Google Scholar] [CrossRef]
- Oves, M.; Khan, M.S.; Zaidi, A. Biosorption of Heavy Metals by Bacillus thuringiensis Strain OSM29 Originating from Industrial Effluent Contaminated North Indian Soil. Saudi. J. Biol. Sci. 2013, 20, 121–129. [Google Scholar] [CrossRef]
- Sarti, G.C.; Miyazaki, S.S. Actividad antifúngica de extractos crudos de Bacillus subtilis contra fitopatógenos de soja (Glycine max) y efecto de su coinoculación con Bradyrhizobium japonicum. Agrociencia 2013, 47, 373–383. [Google Scholar]
- Galelli, M.E.; Sarti, G.C.; Miyazaki, S.S. Lactuca sativa Biofertilization Using Biofilm from Bacillus with PGPR Activity. J. Appl. Hort. 2015, 17, 186–191. [Google Scholar]
- Sarti, G.C.; Galelli, M.E.; Arreghini, S.; Cristóbal-Miguez, J.A.E.; Curá, J.A.; Paz-González, A. Inoculation with Biofilm of Bacillus subtilis Promotes the Growth of Lactuca sativa. Sustainability 2023, 15, 15406. [Google Scholar] [CrossRef]
- Sarti, G.C.; Galelli, M.E.; Cristóbal-Miguez, J.A.E.; Cárdenas-Aguiar, E.; Chudil, H.D.; García, A.R.; Paz-González, A. Inoculation with Biofilm of Bacillus subtilis Is a Safe and Sustainable Alternative to Promote Tomato (Solanum lycopersicum) Growth. Environments 2024, 11, 54. [Google Scholar] [CrossRef]
- Sarti, G.C.; Cristóbal Miguez, A.E.J.; Curá, A.J. Optimización de Las Condiciones de Cultivo Para El Desarrollo de Una Biopelícula Bacteriana y Su Aplicación Como Biofertilizante En Solanum lycopersicum L. Var. Río Grande. Rev. Prot. Veg. 2019, 34, 1–13. [Google Scholar]
- Penha, R.O.; Vandenberghe, L.P.S.; Faulds, C.; Soccol, V.T.; Soccol, C.R. Bacillus Lipopeptides as Powerful Pest Control Agents for a More Sustainable and Healthy Agriculture: Recent Studies and Innovations. Planta 2020, 251, 70. [Google Scholar]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar]
- Edwards, S.J.; Kjellerup, B.V. Applications of Biofilms in Bioremediation and Biotransformation of Persistent Organic Pollutants, Pharmaceuticals/Personal Care Products, and Heavy Metals. Appl. Microbiol. Biotechnol. 2013, 97, 9909–9921. [Google Scholar]
- Daboor, S.M.; Rohde, J.R.; Cheng, Z. Disruption of the Extracellular Polymeric Network of Pseudomonas Aeruginosa Biofilms by Alginate Lyase Enhances Pathogen Eradication by Antibiotics. J. Cyst. Fibros. 2021, 20, 264–270. [Google Scholar] [CrossRef]
- Hobley, L.; Harkins, C.; MacPhee, C.E.; Stanley-Wall, N.R. Giving Structure to the Biofilm Matrix: An Overview of Individual Strategies and Emerging Common Themes. FEMS Microbiol. Rev. 2015, 39, 649–669. [Google Scholar]
- Haque, M.M.; Oliver, M.M.H.; Nahar, K.; Alam, M.Z.; Hirata, H.; Tsuyumu, S. CytR Homolog of Pectobacterium Carotovorum subsp. Carotovorum Controls Air-Liquid Biofilm Formation by Regulating Multiple Genes Involved in Cellulose Production, c-Di-GMP Signaling, Motility, and Type III Secretion System in Response to Nutritional and Environmental Signals. Front. Microbiol. 2017, 8, 972. [Google Scholar] [CrossRef]
- Haque, M.M.; Mosharaf, M.K.; Haque, M.A.; Tanvir, M.Z.H.; Alam, M.K. Biofilm Formation, Production of Matrix Compounds and Biosorption of Copper, Nickel and Lead by Different Bacterial Strains. Front. Microbiol. 2021, 12, 615113. [Google Scholar] [CrossRef]
- Sutherland, I.W. Novel and Established Applications of Microbial Polysaccharides. Trends Biotechnol. 1998, 16, 41–46. [Google Scholar] [CrossRef]
- Wei, X.; Fang, L.; Cai, P.; Huang, Q.; Chen, H.; Liang, W.; Rong, X. Influence of Extracellular Polymeric Substances (EPS) on Cd Adsorption by Bacteria. Environ. Pollut. 2011, 159, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Rebolloso-Fuentes, M.M. Nutrient Composition and Antioxidant Activity of Eight Tomato (Lycopersicon esculentum) Varieties. J. Food Compos. Anal. 2009, 22, 123–129. [Google Scholar] [CrossRef]
- Erba, D.; Casiraghi, M.C.; Ribas-Agustí, A.; Cáceres, R.; Marfà, O.; Castellari, M. Nutritional Value of Tomatoes (Solanum lycopersicum L.) Grown in Greenhouse by Different Agronomic Techniques. J. Food Compos. Anal. 2013, 31, 245–251. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, A.K.; Singh, P.P.; Kumar, A. Interaction of Plant Growth Promoting Bacteria with Tomato under Abiotic Stress: A Review. Agric. Ecosyst. Environ. 2018, 267, 129–140. [Google Scholar] [CrossRef]
- Final, I. EPA Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part A); Environmental Protection Agency, Office of Solid Waste and Emergency Response: Washington, DC, USA, 1989. [Google Scholar]
- EU. European Commission regulation No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs (Text with EEA Relevance). Off. J. Eur. Union 2006, 5–24. [Google Scholar]
- Gerhardt, P.; Murray, R.; Wood, W.; Krieg, N. Methods of General and Molecular Bacteriology; Gerhardt, P., Ed.; American Society for Microbiology: Washington, DC, USA, 1994; ISBN 10,1555810489. [Google Scholar]
- Martí, L.A. Efecto de La Salinidad y de La Temperatura En La Germinación de Semillas de Limonium Mansanetianum; Universidad Politecnica de Valencia: Valencia, Spain, 2010. [Google Scholar]
- Emino, E.R.; Warman, P.R. Biological Assay for Compost Quality. Compost. Sci. Util. 2004, 12, 342–348. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed Germination Test for Toxicity Evaluation of Compost: Its Roles, Problems and Prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Giuffré, L.; Ratto, S.; Marbán, L.; Schonwald, J.; Romaniuk, R. Heavy Metals Risk in Urban Agriculture. Cienc. Suelo 2005, 23, 101–106. [Google Scholar]
- Page, A.L. Methods of Soil Analysis. In Chemical and Microbiological Properties, 2nd ed.; Wiley: Hoboken, NJ, USA, 1982; pp. 1125–1143. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- U.S. EPA. Method 3051A (SW-846): Microwave Assisted Acid Digestion of Sediments, Sludges and Oils, Revision 1; U.S. EPA: Washington, DC, USA, 2007.
- Sharma, B.; Shukla, P. Lead Bioaccumulation Mediated by Bacillus cereus BPS-9 from an Industrial Waste Contaminated Site Encoding Heavy Metal Resistant Genes and Their Transporters. J. Hazard. Mater. 2021, 401, 123285. [Google Scholar] [CrossRef]
- Aktan, Y.; Tan, S.; Icgen, B. Characterization of Lead-Resistant River Isolate Enterococcus Faecalis and Assessment of Its Multiple Metal and Antibiotic Resistance. Environ. Monit. Assess. 2013, 185, 5285–5293. [Google Scholar] [CrossRef]
- Brown, N.L.; Stoyanov, J.V.; Kidd, S.P.; Hobman, J.L. The MerR Family of Transcriptional Regulators. FEMS Microbiol. Rev. 2003, 27, 145–163. [Google Scholar] [CrossRef]
- Etemadzadeh, S.S.; Emtiazi, G. Expression Regulation of Chitin-Binding Protein and Metal-Binding Peptide in New Bacillus Velezensis: MALDI-TOF MS/MS Analysis. J. Basic. Microbiol. 2021, 61, 982–992. [Google Scholar] [CrossRef]
- Wang, W.C.; Mao, H.; Ma, D.D.; Yang, W.X. Characteristics, Functions, and Applications of Metallothionein in Aquatic Vertebrates. Front. Mar. Sci. 2014, 1, 34. [Google Scholar] [CrossRef]
- Chandrangsu, P.; Rensing, C.; Helmann, J.D. Metal Homeostasis and Resistance in Bacteria. Nat. Rev. Microbiol. 2017, 15, 338–350. [Google Scholar] [CrossRef]
- Branda, S.S.; Vik, Å.; Friedman, L.; Kolter, R. Biofilms: The Matrix Revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Grupta, P.; Diwan, B. Bacterial Exopolysaccharide Mediated Heavy Metal Removal: A Review on Biosynthesis, Mechanism and Remediation Strategies. Biotechnol. Rep. 2017, 13, 58. [Google Scholar]
- Pagliaccia, B.; Carretti, E.; Severi, M.; Berti, D.; Lubello, C.; Lotti, T. Heavy Metal Biosorption by Extracellular Polymeric Substances (EPS) Recovered from Anammox Granular Sludge. J. Hazard Mater. 2022, 424, 126661. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, H.Q. Insight into the Roles of Microbial Extracellular Polymer Substances in Metal Biosorption. Bioresour. Technol. 2014, 160, 15–23. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, R.; Zhang, W.; Fu, Y.; Jiao, N. A Novel Exopolysaccharide with Metal Adsorption Capacity Produced by a Marine Bacterium Alteromonas sp. JL2810. Mar. Drugs 2017, 15, 175. [Google Scholar] [CrossRef]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Maiti, T.K. Alleviation of Phytotoxic Effects of Cadmium on Rice Seedlings by Cadmium Resistant PGPR Strain Enterobacter aerogenes MCC 3092. J. Hazard Mater. 2018, 351, 317–329. [Google Scholar] [CrossRef]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Soren, T.; Maiti, T.K. Characterization of Cadmium-Resistant Klebsiella Pneumoniae MCC 3091 Promoted Rice Seedling Growth by Alleviating Phytotoxicity of Cadmium. Environ. Sci. Pollut. Res. 2017, 24, 24419–24437. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Xu, R.K.; Yan, J.; Jiang, J.; Li, J.Y.; Kamran, M.A. Mechanism of Cu(II) and Cd(II) Immobilization by Extracellular Polymeric Substances (Escherichia coli) on Variable Charge Soils. Environ. Pollut. 2019, 247, 136–145. [Google Scholar] [CrossRef]
- Xu, S.; Xing, Y.; Liu, S.; Luo, X.; Chen, W.; Huang, Q. Co-Effect of Minerals and Cd(II) Promoted the Formation of Bacterial Biofilm and Consequently Enhanced the Sorption of Cd(II). Environ. Pollut. 2020, 258, 113774. [Google Scholar] [CrossRef]
- Zhu, X.; Xiang, Q.; Chen, L.; Chen, J.; Wang, L.; Jiang, N.; Hao, X.; Zhang, H.; Wang, X.; Li, Y.; et al. Engineered Bacillus subtilis Biofilm and Biochar Living Materials for In-Situ Sensing and Bioremediation of Heavy Metal Ions Pollution. J. Hazard Mater. 2024, 465, 133119. [Google Scholar] [CrossRef]
- Raklami, A.; Oufdou, K.; Tahiri, A.I.; Mateos-Naranjo, E.; Navarro-Torre, S.; Rodríguez-Llorente, I.D.; Meddich, A.; Redondo-Gómez, S.; Pajuelo, E. Safe Cultivation of Medicago Sativa in Metal-Polluted Soils from Semi-Arid Regions Assisted by Heat-and Metallo-Resistant PGPR. Microorganisms 2019, 7, 212. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Podile, A.R. Functional and Molecular Characterization of Plant Growth Promoting Bacillus Isolates from Tomato Rhizosphere. Heliyon 2020, 6, e04734. [Google Scholar] [CrossRef]
- Beauregard, P.B.; Chai, Y.; Vlamakis, H.; Losick, R.; Kolter, R. Bacillus subtilis Biofilm Induction by Plant Polysaccharides. Proc. Natl. Acad. Sci. USA 2013, 110, E1621–E1630. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, F.; Chai, Y.; Liu, H.; Kolter, R.; Losick, R.; Guo, J.H. Biocontrol of Tomato Wilt Disease by Bacillus subtilis Isolates from Natural Environments Depends on Conserved Genes Mediating Biofilm Formation. Environ. Microbiol. 2013, 15, 848–864. [Google Scholar] [CrossRef]
- Samaras, A.; Nikolaidis, M.; Antequera-Gómez, M.L.; Cámara-Almirón, J.; Romero, D.; Moschakis, T.; Amoutzias, G.D.; Karaoglanidis, G.S. Whole Genome Sequencing and Root Colonization Studies Reveal Novel Insights in the Biocontrol Potential and Growth Promotion by Bacillus subtilis MBI 600 on Cucumber. Front. Microbiol. 2021, 11, 600393. [Google Scholar] [CrossRef]
- Li, S.; Zhang, N.; Zhang, Z.; Luo, J.; Shen, B.; Zhang, R.; Shen, Q. Antagonist Bacillus subtilis HJ5 Controls Verticillium Wilt of Cotton by Root Colonization and Biofilm Formation. Biol. Fertil. Soils. 2013, 49, 295–303. [Google Scholar] [CrossRef]
- Posada, L.F.; Álvarez, J.C.; Romero-Tabarez, M.; de-Bashan, L.; Villegas-Escobar, V. Enhanced Molecular Visualization of Root Colonization and Growth Promotion by Bacillus subtilis EA-CB0575 in Different Growth Systems. Microbiol. Res. 2018, 217, 69–80. [Google Scholar] [CrossRef]
- Da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A Promising and Abundant Carbon Source for Industrial Microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef]
- Cavalheiro, J.M.; de Almeida, M.C.; Grandfils, C.; Da Fonseca, M.M. Poly(3-Hydroxybutyrate) Production by Cupriavidus Necator Using Waste Glycerol. Process Biochem. 2009, 44, 509–515. [Google Scholar] [CrossRef]
- Varrone, C.; Liberatore, R.; Crezsenci, T.; Wang, A. The Valorization of Glycerol: Economic Assessment of an Innovative Process for the Bioconversion of Crude Glycerol into Ethanol and Hydrogen. Appl. Energy. 2013, 105, 349–357. [Google Scholar] [CrossRef]
- Pinyaphong, P.; La-up, A. Optimization of 1,3-Propanediol Production from Fermentation of Crude Glycerol by Immobilized Bacillus pumilus. Heliyon 2024, 10, e35349. [Google Scholar] [CrossRef]
- Karayannis, D.; Angelou, N.; Vasilakis, G.; Charisteidis, I.; Litinas, A.; Papanikolaou, S. A Non-Aseptic Bioprocess for Production and Recovery of 2,3-Butanediol via Conversion of Crude Glycerol and Corn Steep Liquor at Pilot-Scale. Carbon Resour. Convers. 2024, 8, 100242. [Google Scholar] [CrossRef]
- Saggu, S.K.; Jha, G.; Mishra, P.C. Enzymatic Degradation of Biofilm by Metalloprotease from Microbacterium Sp. Sks10. Front. Bioeng. Biotechnol. 2019, 7, 192. [Google Scholar] [CrossRef]
- Yunus, J.; Wan Dagang, W.R.Z.; Jamaluddin, H.; Jemon, K.; Mohamad, S.E.; Jonet, M.A. Bacterial Biofilm Growth and Perturbation by Serine Protease from Bacillus sp. Arch. Microbiol. 2024, 206, 138. [Google Scholar] [CrossRef]
- Fleming, D.; Rumbaugh, K.P. Approaches to Dispersing Medical Biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef]
- Sarti, G.G. Nuevas Perspectivas Para La Horticultura Urbana: Evaluación de Un Biofilm Bacteriano Como Promotor Del Crecimiento Vegetal; Universidade A Coruña: A Coruña, Spain, 2021. [Google Scholar]
- Chhibber, S.; Nag, D.; Bansal, S. Inhibiting Biofilm Formation by Klebsiella pneumoniae B5055 Using an Iron Antagonizing Molecule and a Bacteriophage. BMC Microbiol. 2013, 13, 174. [Google Scholar] [CrossRef]
- Honsa, E.S.; Johnson, M.D.L.; Rosch, J.W. The Roles of Transition Metals in the Physiology and Pathogenesis of Streptococcus Pneumoniae. Front. Cell Infect. Microbiol. 2013, 3, 92. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA Required for Bacterial Biofilm Formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Calabrese, E.; Baldwin, A. Hormesis: The Dose-Response Revolution. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 175–179. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis Defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Lebrazi, S.; Fadil, M.; Chraibi, M.; Fikri-Benbrahim, K. Screening and Optimization of Indole-3-Acetic Acid Production by Rhizobium Sp. Strain Using Response Surface Methodology. J. Genet. Eng. Biotechnol. 2020, 18, 21. [Google Scholar] [CrossRef]
- Thakur, R.; Dhar, H.; Swarnkar, M.K.; Soni, R.; Sharma, K.C.; Singh, A.K.; Gulati, A.; Sud, R.K.; Gulati, A. Understanding the Molecular Mechanism of PGPR Strain Priestia Megaterium from Tea Rhizosphere for Stress Alleviation and Crop Growth Enhancement. Plant Stress. 2024, 12, 100494. [Google Scholar] [CrossRef]
- Pandey, S.; Gupta, K.; Mukherjee, A.K. Impact of Cadmium and Lead on Catharanthus Roseus-A Phytoremediation Study. J. Environ. Biol. 2007, 28, 655–662. [Google Scholar]
- Lamhamdi, M.; Bakrim, A.; Aarab, A.; Lafont, R.; Sayah, F. Lead Phytotoxicity on Wheat (Triticum aestivum L.) Seed Germination and Seedlings Growth. Comptes Rendus Biol. 2011, 334, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, G.I. Efecto Del Plomo Sobre La Imbibición, Germinación y Crecimiento de Phaseolus vulgaris L. y Zea mays L. Biotecnol. Veg. 2013, 13, 8. [Google Scholar]
- Tomulescu, I.M.; Radoviciu, E.M.; Merca, V.V.; Tuduce, A.D. Effect of Copper, Zinc and Lead and Their Combinations on the Germination Capacity of Two Cereals. J. Agr. Sci. 2004, 15, 39–42. [Google Scholar] [CrossRef]
- Mohamed, H.I. Molecular and Biochemical Studies on the Effect of Gamma Rays on Lead Toxicity in Cowpea (Vigna sinensis) Plants. Biol. Trace Elem. Res. 2011, 144, 1205–1218. [Google Scholar] [CrossRef]
- Aicha, B.; Yssaad, R.; Abdelhakim, H.; Hanane, H.; Tayeb, N.; Lazreg, B.; Nacer, F. Effect of Lead on Seed Germination and Seedling Growth of Cleome amblycarpa BARR. & MURB. Plant. Arch. 2020, 20, 7553–7559. [Google Scholar]
- Delatorre, C.A.; Barros, R.S. Germination of Dormant Seeds of Stylosanthes humilis as Related to Heavy Metal Ions. Biol. Plant. 1996, 38, 269–274. [Google Scholar] [CrossRef]
- Islam, E.; Yang, X.; Li, T.; Liu, D.; Jin, X.; Meng, F. Effect of Pb Toxicity on Root Morphology, Physiology and Ultrastructure in the Two Ecotypes of Elsholtzia argyi. J. Hazard Mater. 2007, 147, 806–816. [Google Scholar] [CrossRef]
- Galelli, M.E.; Cristóbal-Miguez, J.A.E.; Cárdenas-Aguiar, E.; García, A.R.; Paz-González, A.; Sarti, G.C. The Effects of Seed Inoculation with Bacterial Biofilm on the Growth and Elemental Composition of Tomato (Solanum lycopersicum L.) Cultivated on a Zinc-Contaminated Substrate. Microorganisms 2024, 12, 2237. [Google Scholar] [CrossRef]
- Baruah, N.; Mondal, S.C.; Farooq, M.; Gogoi, N. Influence of Heavy Metals on Seed Germination and Seedling Growth of Wheat, Pea, and Tomato. Water Air Soil Pollut. 2019, 230, 273. [Google Scholar] [CrossRef]
- Yahaghi, Z.; Shirvani, M.; Nourbakhsh, F.; Pueyo, J.J. Uptake and Effects of Lead and Zinc on Alfalfa (Medicago sativa L.) Seed Germination and Seedling Growth: Role of Plant Growth Promoting Bacteria. S. Afr. J. Bot. 2019, 124, 573–582. [Google Scholar] [CrossRef]
- Akinci, I.E.; Akinci, S.; Yilmaz, K. Response of Tomato (Solanum lycopersicum L.) to Lead Toxicity: Growth, Element Uptake, Chlorophyll and Water Content. Afr. J. Agric. Res. 2010, 5, 416–423. [Google Scholar]
- Zhang, J.; Qian, Y.; Chen, Z.; Amee, M.; Niu, H.; Du, D.; Yao, J.; Chen, K.; Chen, L.; Sun, J. Lead-Induced Oxidative Stress Triggers Root Cell Wall Remodeling and Increases Lead Absorption through Esterification of Cell Wall Polysaccharide. J. Hazard Mater. 2020, 385, 121524. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M.; Ahmad, M.; Anjum, M.Z. Lead Toxicity in Plants: Impacts and Remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef]
- Gupta, M.; Dwivedi, V.; Kumar, S.; Patel, A.; Niazi, P.; Yadav, V.K. Lead Toxicity in Plants: Mechanistic Insights into Toxicity, Physiological Responses of Plants and Mitigation Strategies. Plant Signal Behav. 2024, 19, 2365576. [Google Scholar] [CrossRef]
- Sinha, P.; Dube, B.K.; Srivastava, P.; Chatterjee, C. Alteration in Uptake and Translocation of Essential Nutrients in Cabbage by Excess Lead. Chemosphere 2006, 65, 651–656. [Google Scholar] [CrossRef]
- Gopal, R.; Rizvi, A.H. Excess Lead Alters Growth, Metabolism and Translocation of Certain Nutrients in Radish. Chemosphere 2008, 70, 1539–1544. [Google Scholar] [CrossRef]
- Małkowski, E.; Kita, A.; Galas, W.; Karcz, W.; Kuperberg, J.M. Lead Distribution in Corn Seedlings (Zea mays L.) and Its Effect on Growth and the Concentrations of Potassium and Calcium. Plant. Growth Regul. 2002, 37, 69–76. [Google Scholar] [CrossRef]
- Kastori, R.; Petrovic, M.; Petrovic, N. Effect of Excess Lead, Cadmium, Copper, and Zinc on Water Relations in Sunflower. J. Plant Nutr. 1992, 15, 2427–2439. [Google Scholar] [CrossRef]
- Maryam, H.; Rizwan, M.; Masood, N.; Waseem, M.; Ahmed, T.; Qayyum, M.F.; Zia-ur-Rehman, M.; Aziz, H. Mitigating Lead (Pb) Toxicity in Zea mays (L.) Plants Using Green Synthesized Iron Oxide Nanoparticles. S. Af. J. Bot. 2024, 175, 657–668. [Google Scholar] [CrossRef]
- Xia, Z.; Xue, C.; Liu, R.; Hui, Q.; Hu, B.; Rennenberg, H. Lead Accumulation and Concomitant Reactive Oxygen Species (ROS) Scavenging in Robinia pseudoacacia Are Dependent on Nitrogen Nutrition. Plant Physiol. Biochem. 2025, 219, 109388. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Abidine Triqui, Z.E.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc Hyperaccumulation in Plants: A Review. Plants 2020, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.C.; Chen, J.Y.; Lv, N.; Mi, J.D.; Chen, W.L.; Liang, J.B.; Liao, X.D. Biosorption of Lead by the Vegetative and Decay Cells and Spores of Bacilllus coagulans R11 Isolated from Lead Mine Soil. Chemosphere 2018, 211, 804–816. [Google Scholar] [CrossRef]
- Qiao, W.; Zhang, Y.; Xia, H.; Luo, Y.; Liu, S.; Wang, S.; Wang, W. Bioinmobilization of Lead by Bacillus subtilis X3 Biomass Isolated from Lead Mine. R. Soc. Open Sci. 2019, 6, 181701. [Google Scholar] [CrossRef]
- Adediran, G.A.; Ngwenya, B.T.; Mosselmans, J.F.W.; Heal, K.V.; Harvie, B.A. Mechanisms behind Bacteria Induced Plant Growth Promotion and Zn Accumulation in Brassica juncea. J. Hazard Mater. 2015, 283, 490–499. [Google Scholar] [CrossRef]
- Rajivgandhi, G.; Ramachandran, G.; Chackaravarthi, G.; Maruthupandy, M.; Quero, F.; Chelliah, C.K.; Manoharan, N.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; et al. Metal Tolerance and Biosorption of Pb Ions by Bacillus cereus RMN 1 (MK521259) Isolated from Metal Contaminated Sites. Chemosphere 2022, 308, 136270. [Google Scholar] [CrossRef]
- Pinho, S.; Ladeiro, B. Phytotoxicity by Lead as Heavy Metal Focus on Oxidative Stress. J. Bot. 2012, 2012, 369572. [Google Scholar] [CrossRef]
- Qureshi, M.I.; Abdin, M.Z.; Qadir, S.; Iqbal, M. Lead-Induced Oxidative Stress and Metabolic Alterations in Cassia Angustifolia Vahl. Biol. Plant. 2007, 51, 121–128. [Google Scholar] [CrossRef]
- Vijayarengan, P.; Mahalakshmi, G.; Words, K. Zinc Toxicity in Tomato Plants. World Appl. Sci. J. 2013, 24, 649–653. [Google Scholar]
- Seneviratne, G.; Weerasekara, M.L.M.A.W.; Seneviratne, K.A.C.N.; Zavahir, J.S.; Kecskés, M.L.; Kennedy, I.R. Importance of Biofilm Formation in Plant Growth Promoting Rhizobacterial Action. In Plant Growht and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 81–95. [Google Scholar]
- Ferreira, C.M.H.; Vilas-Boas, Â.; Sousa, C.A.; Soares, H.M.V.M.; Soares, E.V. Comparison of Five Bacterial Strains Producing Siderophores with Ability to Chelate Iron under Alkaline Conditions. AMB Express. 2019, 9, 78. [Google Scholar] [CrossRef]
- Santiago, J.; Mendoza, M.; Borrego, F. Evaluación de Tomate (Lycopersicum esculentum, MILL) En Invernadero: Criterios Fenológico y Fisiológicos. Agron. Mesoam. 1998, 9, 59–65. [Google Scholar] [CrossRef]
- Marichali, A.; Dallali, S.; Ouerghemmi, S.; Sebei, H.; Hosni, K. Germination, Morpho-Physiological and Biochemical Responses of Coriander (Coriandrum sativum L.) to Zinc Excess. Ind. Crops Prod. 2014, 55, 248–257. [Google Scholar] [CrossRef]
- Tabassam, Q.; Ahmad, M.S.A.; Alvi, A.K.; Awais, M.; Kaushik, P.; El-Sheikh, M.A. Accumulation of Different Metals in Tomato (Lycopersicon esculentum L.) Fruits Irrigated with Wastewater. Appl. Sci. 2023, 13, 9711. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc Toxicity in Plants: A Review. Planta 2021, 253, 129. [Google Scholar] [CrossRef]
- Gomes, M.A.; Hauser-Davis, R.A.; Suzuki, M.S.; Vitória, A.P. Plant Chromium Uptake and Transport, Physiological Effects and Recent Advances in Molecular Investigations. Ecotoxicol. Environ. Saf. 2017, 140, 55–64. [Google Scholar] [CrossRef]
- Murtic, S.; Zahirovic, C.; Civic, H.; Karic, L.; Jurokkovic, J. Uptake of Heavy Metals by Tomato Plants (Lycopersicum esculentum Mill.) and their distribution inside the Plant. Agric. For. 2018, 64, 251–261. [Google Scholar] [CrossRef]
- Norton, C.J.; Deacon, C.M.; Mestrot, A.; Feldmann, J.; Jenkins, P.; Baskaran, C.; Mehrag, A. Cadmium and lead in vegetable and fruit produce selected from specific regional areas of the UK. Sci. Total Environ. 2015, 533, 520–527. [Google Scholar] [CrossRef]
- Watson, G.; Andrew, P.; Margenot, J. Fruit lead concentrations of tomato (Solanum lycopersicum L.) grown in lead contaminated soils are unaffected by phosphate amendments and can vary by season, but are below risk thresholds. Sci. Total Environ. 2022, 836, 155076. [Google Scholar] [CrossRef]
- Birghila, S.; Matei, N.; Dobrinas, S.; Pospescu, V.; Soceanu, A.; Niculescu, A. Assessment of heavy metal content in soil and Lycopersicon esculentum (Tomato) and their Health Implications. Biol. Trace Elem. Res. 2023, 201, 1547–1556. [Google Scholar] [CrossRef]
- Freire de Sousa, F.; do Carmo, M.G.F.; Lima, E.S.A.; de Souza, C.C.B.; Sobrinho, N.M.B.A. Lead and Cadmium Transfer Factors and the Contamination of Tomato Fruits (Solanum lycopersicum) in a Tropical Mountain Agroecosystem. Bull. Environ. Contam. Toxicol. 2020, 105, 325–331. [Google Scholar] [CrossRef]



| Germination Index (GI%) | Toxicity Level |
|---|---|
| <50% | High |
| 50–80% | Moderate |
| >80% | No toxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarti, G.C.; Paz-González, A.; Cristóbal-Miguez, J.A.E.; García, A.R.; Galelli, M.E. Production of a Microbial Biofilm and Its Application on Tomato Seeds to Improve Crop Development in a Lead-Contaminated Substrate. Processes 2025, 13, 767. https://doi.org/10.3390/pr13030767
Sarti GC, Paz-González A, Cristóbal-Miguez JAE, García AR, Galelli ME. Production of a Microbial Biofilm and Its Application on Tomato Seeds to Improve Crop Development in a Lead-Contaminated Substrate. Processes. 2025; 13(3):767. https://doi.org/10.3390/pr13030767
Chicago/Turabian StyleSarti, Gabriela Cristina, Antonio Paz-González, Josefina Ana Eva Cristóbal-Miguez, Ana Rosa García, and Mirta Esther Galelli. 2025. "Production of a Microbial Biofilm and Its Application on Tomato Seeds to Improve Crop Development in a Lead-Contaminated Substrate" Processes 13, no. 3: 767. https://doi.org/10.3390/pr13030767
APA StyleSarti, G. C., Paz-González, A., Cristóbal-Miguez, J. A. E., García, A. R., & Galelli, M. E. (2025). Production of a Microbial Biofilm and Its Application on Tomato Seeds to Improve Crop Development in a Lead-Contaminated Substrate. Processes, 13(3), 767. https://doi.org/10.3390/pr13030767







