Abstract
Rotor cooling water is a pivotal element for the safe operation of a synchronous condenser in an ultrahigh-voltage grid. To decrease the dissolved oxygen and carbon dioxide contents, tremendous efforts have been dedicated to regulating the solution pH and conductivity. The traditional chemical pH adjustment and resin regeneration methods for rotor cooling water alkalization have the disadvantages of high chemical consumption and high operation and maintenance costs. Here, we propose an electrochemical method for alkalizing the rotor cooling water of a synchronous condenser by taking advantage of the accelerating water dissociation feature in bipolar membranes. The experiments with carbon dioxide injected deionized water revealed that water dissociation in bipolar membrane is capable of increasing the solution pH from 4.6 to 5.6 and decreasing the conductivity from 9.5 μS/cm to less than 2.0 μS/cm. It is convenient to increase the solution pH from 6.5 to even 10.0 when real rotor cooling water is used. BP-A-BP is more competitive than BP-C-A-BP for alkalization purposes. The present study also provides a cost-effective and chemical-free technique to precisely control the water quality of the rotor cooling water in a synchronous condenser.
1. Introduction
At present, China’s ultrahigh voltage (UHV) construction is booming, and the most typical representative is the Qing–Henan project, which has a total line length of 1587 km and a transmission capacity of 8 GW [1,2,3]. However, in the UHV grid, the direct current (DC) transmission sending-end grid is weak, and the receiving-end grid is insufficient in terms of reactive power, which affects the stable operation of the grid. A synchronous condenser is used to automatically and rapidly regulate the power system without a mechanical load, thus improving the stability of the power system and the quality of the system power supply. To cool the coils of the synchronous condenser, deionized freshwater is introduced to ensure the safe operation of the condenser. There are two major requirements for rotor water collection in ultrahigh-voltage power transmission plants. On the one hand, the recirculating cooling water must have sufficient insulation to prevent the generator winding from being grounded through the insulation water pipe at the coil end. On the other hand, the recirculating cooling water should not contain substances that are corrosive to the hollow copper conductors or can deposit and scale in the hollow copper conductors [4,5]. Therefore, strict water quality is required to monitor the water conductivity, pH, copper ion content, and dissolved oxygen content. Specifically, the conductivity of the recirculating cooling water should be maintained below 5 μS/cm, and the water pH should be controlled between 7 and 9.
The water used for rotor cooling is desalted water from a reverse osmosis plant [6]. However, during the cooling operation of the synchronous condenser, the recirculating water directly contacts the air. If no special treatment is conducted, the dissolved oxygen and carbon dioxide contents of the rotor cooling water increase, and the pH decreases, resulting in the corrosion of the hollow copper wires. Numerous studies have indicated that copper erosion is exacerbated when the solution pH is lower than 8. The long-term use of unqualified rotor cooling water in the synchronous condenser could lead to short circuits, scaling, erosion, overheating, etc. Notably, copper is in the stable region of its potential–pH diagram when the pH value of the rotor cooling water is between 7 and 10. Therefore, the pH of the rotor cooling water should be controlled such that it is between 7 and 9 [7,8].
At present, methods for alkalizing rotor cooling water usually include periodic alternation with freshwater, small-flow ion exchange mixed bed resins [9,10], and ion exchange–base alkalization systems. For the latter method, the acidic rotor cooling water is adjusted with 0.1–0.5% NaOH or aqueous NH3·H2O solution with a dosing pump. The alkali dosing is terminated when the solution pH approaches 8.5 or when the conductivity reaches 1.5 μS/cm. These water alternation and chemical alkalinization methods can only control the quality of the rotor cooling water within the standard range for a short time, and frequent water changes are required to waste large amounts of desalinated water. In addition, frequent resin replacement and unstable pH control also have the disadvantages of high chemical consumption and high operation and maintenance costs. It is necessary to develop a more technically viable and environmentally friendly technique for alkalizing rotor cooling water [11,12,13].
A bipolar membrane (BPM) is a composited membrane that is typically composed of a negatively charged layer, a positively charged layer, and a water-splitting catalyst layer [14,15,16,17]. Accelerated water dissociation (H2O → H+ + OH−) occurs when a sufficient voltage bias (>0.8 V) is applied across a BPM [18]. By taking advantage of their ability to accelerate water dissociation, BPMs have multiple applications, such as acid–base production [19,20,21], environmental remediation [22,23], traditional manufacturing process re-engineering [24], water (photo)-electrolysis [25,26,27], and carbon and ammonia reduction [28,29]. This particular water dissociation in the BPM makes acidification or alkalization of the aqueous solution possible without the introduction of external acids or bases that are used with anion and cation exchange membranes. In this study, we propose an electrochemical method for alkalizing the rotor cooling water of a synchronous condenser. Unlike chemical methods, alkali is directly generated by the water dissociation of the BPM to remove carbonate ions and bicarbonate ions and to decrease the conductivity in the rotor cooling water. The present study also provides a cost-effective technique to precisely control the water quality of the rotor cooling water of a synchronous condenser.
2. Materials and Methods
2.1. Materials
The bipolar membrane (Neosepta BP-1E), cation-exchange membrane (Neosepta CEM), and anion-exchange membrane (Neosepta AEM) were purchased from Astom Co., Ltd., Tokyo, Japan. The main physicochemical properties of the membranes used in the experiment are listed in Table 1. Both simulated and real rotor cooling water were used as feed for the experiment. The simulated rotor cooling water was prepared from deionized water saturated with carbon dioxide. The rotor cooling water was provided by the State Grid Anhui Electric Power Research Institute.

Table 1.
Basic physiochemical properties of the membranes used in the experiments [30,31].
2.2. Experimental Setup
The custom-made BPM stack used for the experiment was supplied by Hefei Chemjoy Polymer Materials Co. Ltd., Hefei, China. The stack was composed of two acrylonitrile butadiene styrene plates (15 cm × 31 cm), with each effective area of the membrane being 22 cm2. A pair of electrodes that were made of titanium and coated with ruthenium was assembled at each end of the plate. A direct current supply (WYL1703, Hangzhou Siling Electrical Instrument Co. Ltd., Hangzhou, China) was connected to the electrodes to maintain a potential voltage drop in the range of 50–200 V.
Two kinds of cell configurations, i.e., BP-A-BP and BP-A-C-BP, were chosen for the experiment, as shown in Figure 1. Two adjacent membranes were separated by a 0.8 mm spacer to create a circulating chamber. In the BP-A-BP cell configuration, the cooling water was fed into the chamber between the bipolar membrane and the anion-exchange membrane. In the BP-A-C-BP cell configuration, the cooling water was fed into the chamber between the anion-exchange membrane and the cation-exchange membrane. The membrane stacks were all equipped as a single unit, which means BPM-AEM-BPM was assembled sequentially between the electrodes in BP-A-BP configuration, and BPM-AEM-CEM-BPM was assembled sequentially between the electrodes in BP-A-C-BP configuration. Between the BPM and the AEM is the alkaline chamber, which is fed with the rotor cooling water of the power plant, in BP-A configuration. In BP-A-C-BP, between the CEM and the AEM is the feed chamber, which is fed with the rotor cooling water from the power plant. A 500 mL 0.01 mol/L Na2SO4 solution was added to the other chambers. The 0.3 M Na2SO4 solution acts as an electrolyte and circulates between the anode and cathode of the membrane stack. Each chamber was recirculated using a peristaltic pump (Baoding Lead Fluid Technology Co., Ltd., China) while maintaining a flow rate of 200 mL/min. For each batch experiment, the solution in each chamber was circulated for 30 min to eliminate visible bubbles before the current field was applied.
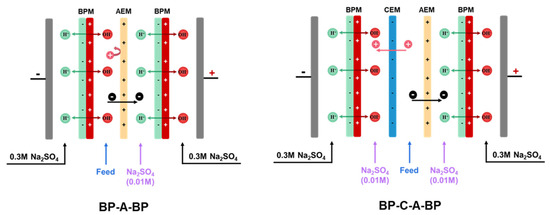
Figure 1.
A schematic diagram of the BP-A-BP and BP-C-A-BP cell configurations used for the experiment.
2.3. Analytical Methods
The changes in pH and conductivity of the cooling water in the feed chamber were monitored with a pH and conductivity meter (DDBJ-350, Shanghai INESA and Scientific Instrument Co., Ltd., Shanghai, China). The current and voltage were read directly from the power supply. The Na+ and K+ concentrations in the rotor cooling water were determined via inductively coupled plasma–optical emission spectrometry (ICP–OES, Thermo Co., Ltd., Waltham, MA, USA).
3. Results and Discussion
3.1. Alkalization of Simulated Rotor Cooling Water
To evaluate the feasibility of the bipolar membrane stack for rotor cooling water alkalization, simulated rotor cooling water was initially used. In fact, the continuous increase in conductivity in the rotor cooling water is attributed primarily to the dissolution of carbon dioxide. Thus, simulated rotor cooling is prepared by injecting CO2 into deionized water, leading to the formation of weakly acidic water with a pH of approximately 4.6–4.7 and a conductivity of 8–10 μS/cm. Owing to the low conductivity of the chamber, a potentostatic voltage drop in the range of 50–200 V was used, e.g., the conductivity of the feed solution is only 8 μs/cm–10 μs/cm, which corresponds to a high resistance of 483.64–604.55 Ω according to the resistance transformation formula. The membrane stack solution resistance can be calculated from the solution conductivity via the following equation [32]:
where represents the obstruction factor, which is equal to 1.33 (1/0.75) because the porosity of the spacers used is 75%; A represents the effective area of the membrane (22 cm2); represents the intermembrane distance of the feed chamber (0.8 mm); and represents the conductivity of the solution in the feed chamber.
Under the action of a current field, all the electrolyte ions in the interfacial layer of the bipolar membranes will be depleted. Under these circumstances, water molecules will be dissociated into H+ and OH− ions to act as current carriers [33,34,35]. In the BP-A-BP cell configuration, the dissociated OH− ions directly enter the feed chamber, leading to the alkalization of the rotor cooling water.
The voltage or current is the predominant factor affecting the desalination performance of the electrodialysis process. The effects of voltage on the alkalization of the simulated rotor cooling water are depicted in Figure 2 and Figure 3. For voltage drops of 50 and 100 V, the pH profile remains at a fixed value, and the conductivities in the rotor cooling water also do not decrease as expected. Moreover, the conductivity in the acid chamber is also maintained at approximately 1900 μS/cm, and there is no pronounced increase in the conductivity with an experimental duration of 30 min. These findings suggest that water dissociation in the BPMs does not occur. Surprisingly, water splitting does not occur even when the voltage drop applied to the stack is as high as 100 V. This phenomenon is attributed to the lower current density applied to the bipolar membrane stack. Water splitting in a bipolar membrane is directly related to the current density rather than the voltage drop [36]. As shown in Figure 3d, the applied current is fixed at 0.009 A and 0.017 A, corresponding to current densities of 0.41 mA/cm2 and 0.77 mA/cm2, respectively, when voltages of 50 V and 100 V are applied. This low current density is insufficient to drive the water dissociation reaction that occurs in the interfacial layer of the bipolar membrane.
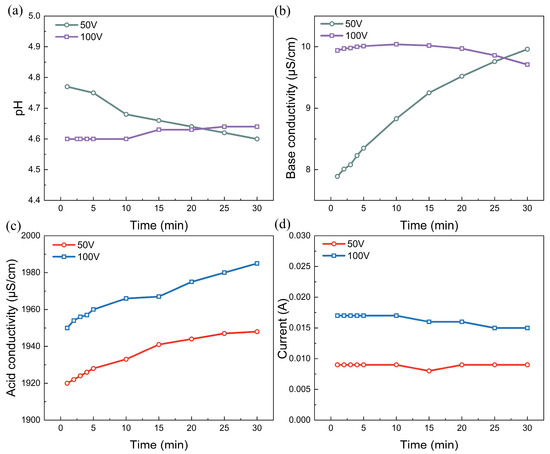
Figure 2.
The alkalization performance: (a) time dependence of pH; (b) conductivity in the feed (base) chamber; (c) acid conductivity; (d) time dependence of the current in the simulated rotor cooling water via the BMED with BP-A-BP under a constant voltage of 50 V and 100 V. The flow rate was 200 mL/min and the chamber volumes were all 500 mL.
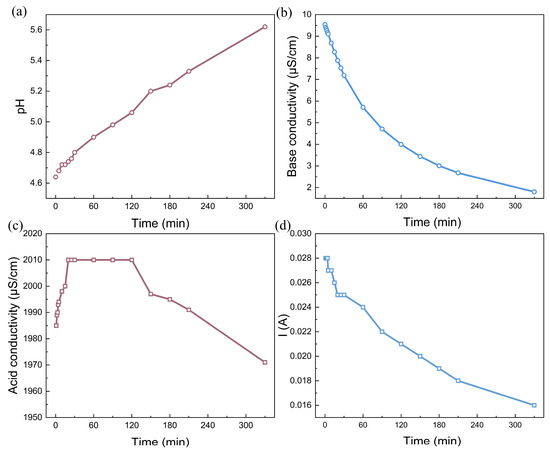
Figure 3.
The alkalization performance: (a) time dependence of pH; (b) conductivity in the feed (base) chamber; (c) acid conductivity; (d) time dependence of the current in the simulated rotor cooling water via the BMED with BP-A-BP under a constant voltage of 200 V. The flow rate was 200 mL/min and the chamber volumes were all 500 mL.
In contrast, water dissociation occurred when the voltage decreased to 200 V. Under this voltage drop, the current decreases with time, decreasing from 0.028 A (1.27 mA/cm2) to 0.016 A (0.073 mA/cm2). Figure 3a shows that the pH in the rotor cooling water increases from the initial value of 4.6 to 5.6 as the experiment progresses, providing direct evidence that it is possible to alkalize the solution without the consumption of any chemicals. Moreover, the conductivity decreases from 9.5 μS/cm to less than 2.0 μS/cm when water dissociation in bipolar membranes is activated. Under the initial pH of 4.6, most of the carbon dioxide in the water existed as carbonate acid with no small fraction of HCO3−. The hydroxide ions generated by water dissociation in the bipolar membrane shift the reaction (OH− + H2CO3 → HCO3− + H2O) forward. Under the influence of the current field, HCO3− migrates toward the anode and is transported across the anion-exchange membrane, leading to a decrease in the base conductivity. For the acid chamber, the conductivity first increased, then reached a plateau, and finally decreased over time. The increase in conductivity indicates the introduction of external ions, suggesting that the proton generated by water dissociation is generated. However, the proton further reacts with HCO3− to emit CO2 gas, leading to a slight decrease in the solution conductivity in the later period of the experiment [37]. Nevertheless, the BMED experiment with simulated rotor cooling water suggests that it is possible to alkalize the solution by an electrochemical reaction in bipolar membranes.
3.2. Alkalization of Real Rotor Cooling Water
Based on the experiment described above, the BMED is also used for the alkalization of the real rotor cooling water, as shown in Figure 4. Unlike the simulated water, this rotor cooling water has an initial pH of approximately 6.8, indicating that the majority of carbonate ions exist as HCO3− rather than carbonate acid. The quality of this water still meets the major requirement for circulation to cool the coils of the synchronous condenser. Unfortunately, in practical cases, it is difficult to collect unqualified rotor cooling water without any alkalization. The high degree of dissolution of ionized carbon dioxide also causes the conductivity of real rotor cooling water to be greater than that of simulated water. This high conductivity leads to the occurrence of water dissociation in the bipolar membrane, even when 100 V is applied to the BMED stack. As indicated in Figure 4d, the larger the voltage drop, the greater the current. This phenomenon is consistent with Ohm’s law. Moreover, the solution pH clearly initially increased logarithmically and then reached a plateau at approximately 10.0 at the end of the experiment. The pronounced increase in the solution pH also indicated that water dissociation in the bipolar membrane occurred smoothly. In addition, the base conductivity also increased during the experiment, almost increasing to 40 μS/cm for a voltage drop of 200 V, indicating the excessive alkalization of the rotor cooling water. Considering that the equivalent conductance of CO32− is much greater than that of HCO3−, the abundant OH- ions generated by water dissociation in the bipolar membrane shifts the reaction (OH− + HCO3− → CO32− + H2O) forward [38]. Considering that the equivalent conductances of OH− and CO32− are much greater than those of HCO3− ions, the continuous replenishment of OH− will cause the system to be overly alkaline. It is necessary to monitor the conductivity curve precisely to determine the termination conditions for the experiments. Interestingly, a low voltage drop can alleviate the phenomenon of excessive alkalinity. Nevertheless, considering the swift pH variation trend, the BMED technique is expected to easily alkalinize acidic rotor cooling, which is not suitable for practical use.
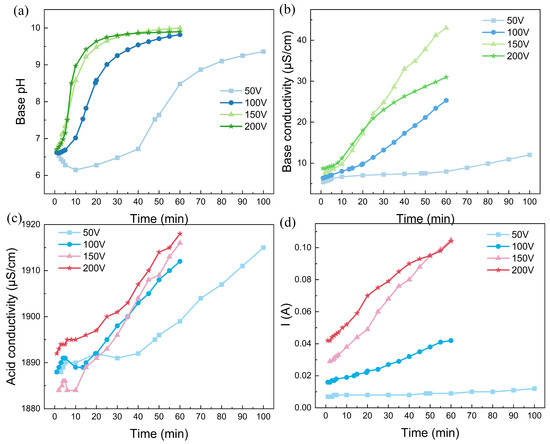
Figure 4.
The alkalization performance: (a) time dependence of pH; (b) conductivity in the feed chamber; (c) acid conductivity; (d) time dependence of the current of real rotor cooling water via the BMED with BP-A-BP under a constant voltage of 200 V. The flow rate was 200 mL/min and the chamber volumes were all 500 mL.
The fixed investment and operating costs for the industrial 40 × 80 cm bipolar membrane electrodialysis stack for the treatment of rotor cooling water were estimated. The detailed information of the membrane stack is that the effective area of a single membrane is 2800 cm2 and the number of repeating units is 20, which is 2.55 × 10 times more than the current experimental membrane stack (22 cm2). The cost is divided into fixed cost and running cost: 0.58 $/t and 0.79 $/t, respectively. The high solution resistance is finally manifested in the running cost. But this membrane stack is running in the power plant, so the running cost is also negligible. Secondly, the lifetime of the membrane stack is set at 3 years, and in practice, the high purity of the feed solution extends the lifetime of the membrane, so that the cost of bipolar membrane electrodialysis for the treatment of cooling water in a power plant is lower than 1.37 $/t (see more details in Table 2).

Table 2.
Estimation of the process cost for the industrial-scale BMED experiment.
3.3. Cell Configuration Comparison
For the application of bipolar membranes, the membrane cell configuration significantly affects the separation performance. Considering that BP-C-A-BP is the prevalent cell configuration for practical application, we also investigated the alkalization of the three-compartment rotor cooling water, and the results are depicted in Figure 5. Unlike the two-compartment BP-A-BP cell configuration, the three-compartment configuration uses one more piece of cation-exchange membrane and one more chamber. The rotor cooling water is fed into the chamber between the anion- and cation-exchange membranes. The base chamber and feed chamber are separately assembled. Owing to this special cell arrangement, all the ionized ions are depleted under the influence of the current field, as shown in Figure 5b. The conductivity of the rotor cooling water can be decreased to extremely low values (~2.0 μS/cm). However, this cell configuration cannot increase the pH of the rotor cooling water, as shown in Figure 5a. The pH even decreased when a bipolar membrane was activated. A possible reason is the leakage of H+ ions from the acid chamber into the salt chamber. Owing to the high mobility and small radius of H+, protons can be easily transported across anion-exchange membranes into the salt chamber. In the case of the BP-A-BP cell configuration, this small amount of proton leakage is neutralized by the OH− ions generated by water dissociation, leading to an overall increase in the solution pH [39]. In summary, the three-cell BP-C-A-BP configuration is not suitable for the alkalization of acidic rotor cooling water.
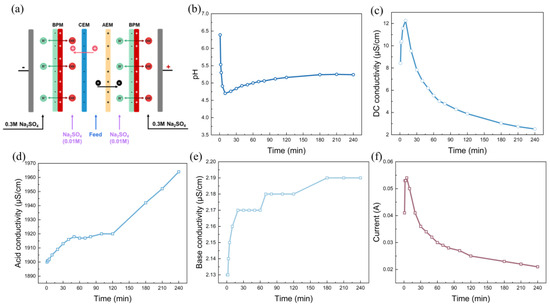
Figure 5.
The alkalization performance: (a) time dependence of pH; (b) conductivity in the feed chamber; (c) acid conductivity; (d) base conductivity; (e) time dependence of the current (f) with BP-C-A-BP under a constant voltage of 200 V. The flow rate was 200 mL/min and the chamber volumes were all 500 mL.
4. Conclusions
An electrochemical method was developed for alkalizing the rotor cooling water of a synchronous condenser by taking advantage of water dissociation in bipolar membranes. The voltage drop and cell configuration are important parameters for the alkalization performance. When simulated rotor cooling water was used, water dissociation was activated at 200 V, while many of the low voltages (50 V and 100 V) did not reach the threshold for water dissociation. Under a voltage drop of 200 V with the BP-A-BP cell configuration, the rotor cooling water conductivity decreases from 9.5 μS/cm to less than 2.0 μS/cm, and the pH increases from 4.6 to 5.6. When real rotor cooling water was used, the solution pH was increased from 6.5 to 10.0. It is necessary to precisely control the experimental duration to prevent excessive alkalization. The pronounced proton leakage in BP-C-A-BP suggests that this cell configuration is not suitable for alkalization purposes. Additional site investigations are still needed to investigate the competitiveness of this method for improving the water quality of the rotor cooling water for a synchronous condenser.
Author Contributions
Conceptualization, J.Y.; investigation, X.C. and W.L.; data curation, W.F. and D.H.; writing—original draft preparation, X.C., X.G. and Y.G.; writing—review and editing, J.Y. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the State Grid Anhui Electric Power Co., Ltd. Science and Technology Project (No.: B3120524001J) and China Postdoctoral Science Foundation (2024M763157).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no competing financial interests.
References
- Gou, H.; Ma, C.; Liu, L. Optimal wind and solar sizing in a novel hybrid power system incorporating concentrating solar power and considering ultra-high voltage transmission. J. Clean. Prod. 2024, 470, 143361. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Tanaka, K.; Ciais, P.; Penuelas, J.; Balkanski, Y.; Sardans, J.; Hauglustaine, D.; Liu, W.; Xing, X. Accelerating the energy transition towards photovoltaic and wind in China. Nature 2023, 619, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Shu, Y.; Ruan, J.; Hu, Y. Ultra high voltage transmission in China: Developments, current status and future prospects. Proc. IEEE 2009, 97, 555–583. [Google Scholar] [CrossRef]
- Wang, T.; Jia, X.; Wang, Y.; Ye, C. Corrosion and Plugging of the Hollow Copper Conductor Caused by CO2 Inleakage: Thermodynamic Analysis and Field Evidence. Ind. Eng. Chem. Res. 2023, 62, 14891–14900. [Google Scholar] [CrossRef]
- Benzarti, Z.; Arrousse, N.; Serra, R.; Cruz, S.; Bastos, A.; Tedim, J.; Salgueiro, R.; Cavaleiro, A.; Carvalho, S. Copper corrosion mechanisms, influencing factors, and mitigation strategies for water circuits of heat exchangers: Critical review and current advances. Corros. Rev. 2024. [Google Scholar] [CrossRef]
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Duffeau, F.; Aspden, D.; Coetzee, G.; Svoboda, R.; Blecken, W.-D.; Eickelbeck, U.; Fenton, R.E.; Wallis, D.; Stein, J. Guide on stator water chemistry management. Study Comm. 2010, 4, 37–41. [Google Scholar]
- Wang, T.; Wang, Y.; Jia, X.; Ye, C. Corrosion failure analysis of hollow copper coil used in generator internal cooling water system operated at low-oxygen/neutral water chemistry. Eng. Fail. Anal. 2022, 141, 106642. [Google Scholar] [CrossRef]
- Chandrasekara, N.G.N.; Pashley, R. Study of a new process for the efficient regeneration of ion exchange resins. Desalination 2015, 357, 131–139. [Google Scholar] [CrossRef]
- Guimaraes, D.; Leao, V.A. Batch and fixed-bed assessment of sulphate removal by the weak base ion exchange resin Amberlyst A21. J. Hazard. Mater. 2014, 280, 209–215. [Google Scholar] [CrossRef]
- Wilbert, F.; Corte, J.F.; do Nascimento, F.T.; Jahno, V.D.; Rodrigues, M.A.S.; Celso, F.; da Silva, S.W.; Bernardes, A.M. Cationic/Anionic Poly (p-Phenylene Oxide) Membranes: Preparation and Electrodialysis Performance for Nickel Recovery from Industrial Effluents. Membranes 2024, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, H.; Wu, Y.; Li, S.; Zhang, G.; Miao, Z. Resourceful treatment of battery recycling wastewater containing H2SO4 and NiSO4 by diffusion dialysis and electrodialysis. Membranes 2023, 13, 570. [Google Scholar] [CrossRef] [PubMed]
- Caveriviere, R.; Galier, S.; Roux-de Balmann, H. On the Use of Permselectivity to Describe the Selective Transfer of Organic Acids in Electrodialysis. Membranes 2023, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yu, W.; Wang, Z.; Wu, L.; Wang, Y.; Xu, T. Review on high-performance polymeric bipolar membrane design and novel electrochemical applications. Aggregate 2024, 5, e527. [Google Scholar] [CrossRef]
- Toh, W.L.; Dinh, H.Q.; Chu, A.T.; Sauvé, E.R.; Surendranath, Y. The role of ionic blockades in controlling the efficiency of energy recovery in forward bias bipolar membranes. Nat. Energy 2023, 8, 1405–1416. [Google Scholar] [CrossRef]
- Fu, R.; Wang, H.Y.; Yan, J.Y.; Li, R.R.; Jiang, C.X.; Wang, Y.M.; Xu, T.W. Asymmetric bipolar membrane electrodialysis for acid and base production. Aiche J. 2022, 69, e17957. [Google Scholar] [CrossRef]
- Kikuchi, S.; Hirao, S.; Kayakiri, S.; Kakihana, Y.; Higa, M. Study on Efficient Operating Conditions for Bipolar Membrane Electrodialysis Using Different Ion Species and Anion—Exchange Membranes. Membranes 2024, 14, 262. [Google Scholar] [CrossRef]
- Oener, S.Z.; Foster, M.J.; Boettcher, S.W. Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science 2020, 369, 1099–1103. [Google Scholar] [CrossRef]
- González, A.; Grágeda, M.; Ushak, S. Modeling and Validation of a LiOH Production Process by Bipolar Membrane Electrodialysis from Concentrated LiCl. Membranes 2023, 13, 187. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Rybalkina, O.; Solonchenko, K.; Butylskii, D.; Nikonenko, V. Phosphates Transfer in Pristine and Modified CJMA-2 Membrane during Electrodialysis Processing of NaxH(3−x)PO4 Solutions with pH from 4.5 to 9.9. Membranes 2023, 13, 647. [Google Scholar] [CrossRef]
- Fu, R.; Wang, H.Y.; Yan, J.Y.; Li, R.R.; Wang, B.Y.; Jiang, C.X.; Wang, Y.M.; Xu, T.W. Ion injection bipolar membrane electrodialysis realizes over 8 mol/L NaOH conversion from a brine stream. AIChE J. 2023, 70, e18345. [Google Scholar] [CrossRef]
- Miao, M.; Qiu, Y.; Yao, L.; Wu, Q.; Ruan, H.; Van der Bruggen, B.; Shen, J. Preparation of N,N,N-trimethyl-1-adamantylammonium hydroxide with high purity via bipolar membrane electrodialysis. Sep. Purif. Technol. 2018, 205, 241–250. [Google Scholar] [CrossRef]
- Szczygielda, M.; Antczak, J.; Prochaska, K. Separation and concentration of succinic acid from post-fermentation broth by bipolar membrane electrodialysis (EDBM). Sep. Purif. Technol. 2017, 181, 53–59. [Google Scholar] [CrossRef]
- He, J.; Zhou, R.; Dong, Z.; Yan, J.; Ma, X.; Liu, W.; Sun, L.; Li, C.; Yan, H.; Wang, Y. Bipolar Membrane Electrodialysis for Cleaner Production of Diprotic Malic Acid: Separation Mechanism and Performance Evaluation. Membranes 2023, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.K.; Xu, Q.C.; Oener, S.Z.; Fabrizio, K.; Boettcher, S.W. Design principles for water dissociation catalysts in high-performance bipolar membranes. Nat. Commun. 2022, 13, 3846. [Google Scholar] [CrossRef]
- Tricker, A.W.; Lee, J.K.; Babbe, F.; Shin, J.R.; Weber, A.Z.; Peng, X. Engineering Bipolar Interfaces for Water Electrolysis Using Earth-Abundant Anodes. ACS Energy Lett. 2023, 8, 5275–5280. [Google Scholar] [CrossRef]
- Blommaert, M.A.; Aili, D.; Tufa, R.A.; Li, Q.F.; Smith, W.A.; Vermaas, D.A. Insights and Challenges for Applying Bipolar Membranes in Advanced Electrochemical Energy Systems. ACS Energy Lett. 2021, 6, 2539–2548. [Google Scholar] [CrossRef]
- Yang, K.L.; Li, M.R.; Subramanian, S.; Blommaert, M.A.; Smith, W.A.; Burdyny, T. Cation-Driven Increases of CO Utilization in a Bipolar Membrane Electrode Assembly for CO Electrolysis. ACS Energy Lett. 2021, 6, 4291–4298. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, L.; Liao, Y.W.; Pang, M.B.; Xu, Q.; Wang, P.C.; Wang, B.G. Continuous ammonia electrosynthesis using physically interlocked bipolar membrane at 1000 mA cm−2. Nat. Commun. 2023, 14, 1619. [Google Scholar] [CrossRef]
- Yan, J.Y.; Yan, H.Y.; Wang, H.Y.; Li, Q.H.; Zhang, H.J.; Jiang, C.X.; Ye, B.J.; Wang, Y.M.; Xu, T.W. Bipolar membrane electrodialysis for clean production of L-10-camphorsulfonic acid: From laboratory to industrialization. AIChE J. 2022, 68, e17490. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, X.L.; Yan, H.Y.; Jiang, C.X.; Ge, L.; Xu, T.W. Bipolar membrane electrodialysis for cleaner production of methylatedglycine derivative amino acids. AIChE J. 2020, 66, e17023. [Google Scholar] [CrossRef]
- Tufa, R.A.; Pawlowski, S.; Veerman, J.; Bouzek, K.; Fontananova, E.; Di Profio, G.; Velizarov, S.; Crespo, J.G.; Nijmeijer, K.; Curcio, E. Progress and prospects in reverse electrodialysis for salinity gradient energy conversion and storage. Appl. Energy 2018, 225, 290–331. [Google Scholar] [CrossRef]
- Wang, H.Y.; Yan, J.Y.; Fu, R.; Yan, H.Y.; Jiang, C.X.; Wang, Y.M.; Xu, T.W. Bipolar Membrane Electrodialysis for Cleaner Production of Gluconic Acid: Valorization of the Regenerated Base for the Upstream Enzyme Catalysis. Ind. Eng. Chem. Res. 2022, 61, 7634–7644. [Google Scholar] [CrossRef]
- Ge, Z.J.; Shehzad, M.A.; Yang, X.Q.; Li, G.; Wang, H.J.; Yu, W.S.; Liang, X.; Ge, X.L.; Wu, L.; Xu, T.W. High-performance bipolar membrane for electrochemical water electrolysis. J. Membr. Sci. 2022, 656, 120660. [Google Scholar] [CrossRef]
- Song, K.; Chae, S.C.; Bang, J.H. Separation of sodium hydroxide from post-carbonation brines by bipolar membrane electrodialysis (BMED). Chem. Eng. J. 2021, 423, 130179. [Google Scholar] [CrossRef]
- Yan, J.; Li, R.; Wang, H.; Hou, B.; Wu, S.; Fu, W.; He, D.; Wang, Z.; Li, Q.; Wang, B.; et al. Alcohol splitting with bipolar membranes for the production of metal alkoxides: Alcohol splitting behaviour and ion transport kinetics. Chem. Eng. Sci. 2024, 286, 119657. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, J.; Wang, H.; Fu, W.; He, D.; Wang, B.; Wang, Y.; Xu, T. Separation and conversion of CO2 reduction products into high-concentration formic acid using bipolar membrane electrodialysis. J. Membr. Sci. 2024, 708, 123016. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, Y.; Yan, J.Y.; Fu, R.; Wang, B.Y.; Jiang, C.X.; Wang, Y.M.; Xu, T.W. Bipolar membrane electrodialysis: A promising paradigm for caustic soda production. AIChE J. 2023, 70, e18260. [Google Scholar] [CrossRef]
- Onishi, N.; Minagawa, M.; Tanioka, A.; Matsumoto, H. Current–voltage characteristics and solvent dissociation of bipolar membranes in organic solvents. Membranes 2022, 12, 1236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).