Abstract
This study has aimed to determine the effect of pre-treatment with enzymes, ultrasound, and fruit skin perforation on the kinetics of the freeze-drying process and selected properties of the dried blueberries. The dry matter, water activity, maximal compression force, and content of flavonoids, polyphenols, and anthocyanins after the pre-treatment and drying process were measured. The enzymatic, ultrasonic, and puncture treatments reduced the hardness of the blueberries by 2.5-fold, while the content of most bioactive compounds remained similar. The structure analysis has shown that freeze-dried blueberries without pre-treatment, but subjected to sonication, were almost hollow inside due to tissue rupture. It resulted in a decrease in the hardness of dried blueberries from 324.2 N (punctured) to 107.5 N (fresh) and 184.5 N (sonicated). The content of polyphenols ranged from 173.2 to 251.0 mg GAE/g d.m. in the fruits subjected to the enzymatic treatment and perforation, respectively. The application of pre-treatment with enzymes and puncturing may be recommended for the freeze-drying of blueberries as it reduces drying time by half. Moreover, the obtained products had a similar content of most bioactive compounds to those observed for freeze-dried blueberries without pre-treatment.
1. Introduction
The diet of contemporary consumers is often irrational, which leads to deficiencies in various micronutrients and trace elements. At the same time, an unhealthy lifestyle promotes the formation of free radicals in the body. In order to counteract that, the consumption of antioxidant substances should be increased [1,2]. One way to achieve that goal is the growing popularity of dietary supplementation and the expansion of the nutritional supplement production [3]. A dietary supplement is a concentrated source of vitamins, minerals, or other substances that have a nutritional or other physiological effects. Dietary supplements are produced using extracts from selected plant or animal substances and industrially synthesised chemical compounds [4]. Therefore, consuming entirely natural supplements, such as dried fruits, is more beneficial, especially when derived from raw materials with high nutritional density. Biologically active compounds found in their natural environment are much easier to absorb than those found in artificially composed supplements. Blueberries are fruits with exceptionally high nutritional and health-promoting value [5]. Blueberries constitute a rich source of ascorbic acid, folic acid, selected minerals, carotenoids, various polyphenols, and other biologically active compounds. There is ample evidence of their health-promoting effects due to the high content of antioxidant compounds [5]. Blueberries also have a balanced and desirable sweet–sour sensory profile. However, some studies have shown that highbush blueberries contain 75 bioactive compounds, and their antioxidant, anti-diabetic, and anti-obesity potential depends on the cultivar [6]. Dried fruits, including blueberries, are maximally concentrated biologically active substances in their natural proportions. Therefore, they constitute a product that enriches the diet with valuable nutrients [7].
Freeze-drying is the most effective method for preserving the nutritional value of raw materials [7]. Before freeze-drying, the product must be frozen, and sublimation occurs under very low pressure conditions, which means an atmosphere practically devoid of oxygen. Therefore, all chemical, biological, and physiological processes that could lead to unfavourable changes in the chemical composition of the freeze-dried material are almost impeded. After drying, the water activity in the product is very low, which prevents any reactions requiring water. In that case, it will retain its high nutritional value, similar to a fresh product, with a high degree of concentration due to the removal of water [8,9].
The blueberry fruit is composed of a thin epicarp (outer skin), a fleshy mesocarp (fleshy centre), and an endocarp (the innermost part where the seeds are located) [7]. The external cuticle layer indicates that blueberries are difficult to dry [10]. The cuticle layer covering, among others, blueberry fruits is made of a hydrophobic substance that coats the surface of the fruit, mainly consisting of cutin and wax. The cuticular wax of the fruit epidermis prevents mechanical damage and fruit cracking [11]. It is the main barrier preventing water loss. It may reduce fruit shrinkage and quality decline during shelf life storage [12]. The transpiration rates for blueberries are lower than those observed for grape berries (1–8 mg H2O berry−1 h−1) or any of the leaves [13,14].
Pre-treatment is recommended to partially damage the skin to increase water removal efficiency during drying. Various methods have been used for that purpose, the most important of which are mechanical [7] and chemical [15]. Grabowski et al. [10] found that cutting cranberries in half increased the diffusion of water from the open cutting area approximately 100 times as compared to the diffusion through the skin surface. Perforation in the structure of the grape berry caused more than a twofold increase in the effective diffusion coefficient during convective drying of grapes at 50 °C [16]. Munzenmayer et al. [17] applied carbon dioxide laser perforation of blueberry skin before freeze-drying. Their study showed that the primary drying time was reduced by 23.5% in effect of the pre-treatment with nine micro-perforations compared to whole, untreated fruits. Another method to improve blueberry drying conditions was to freeze and thaw the fruit. Freezing and thawing led to more pronounced cracks on the skin surface and improved mass exchange conditions [18,19].
The application of ultrasound is one pre-treatment method used for reducing drying time and maintaining product quality. Sonification may be less successful with tissue of low porosity and high stiffness [20]. Applying pre-treatment before the convection drying caused a decrease in the drying time of goldenberries [21] and apples [22]. However, the timing of sonification and temperature were crucial. The ultrasound pre-treatment was also applied before the vacuum freeze-drying of strawberries. The redness and content of antioxidative substances were retained in the sonicated samples that dried quicker than the control fruits without pre-treatment [23].
The presence of the cuticular wax in blueberries was found to significantly reduce the activities of cell wall-degrading enzymes (i.e., pectinase, polygalacturonase, and cellulase), thereby delaying the degradation of protopectin and cellulose and reducing the cell wall degradation, and senescence was intensified in fruit with the wax removed [24]. Pectinolytic and hemicellulolytic enzymes naturally contained in blueberries play an active role in the changes associated with blueberry ripening. Montecchiarini et al. [25] studied the changes occurring in the epicarp of two blueberry varieties during ripening. They found that the higher content of pectinolytic enzymes in the epicarp of the ‘O’Neal’ variety was involved in active cell wall reorganisation. It may have led to a weakening of its structure. After removing the wax layer, the weight loss of blueberries was about 6%, while for the control berries it was 5% during storage at 4 °C for 36 days [24]. The application of enzymes for the purpose of dewaxing is a mild drying pre-treatment method that saves energy and reduces chemical pollution. The mixed enzyme treatment of wolfberry led to a reduction in the vacuum-drying time and the higher rehydration rate. The pre-treatment accounted for the better retention of polyphenols and flavonoids [26].
The above information has led to the research hypothesis, according to which exposing whole blueberry fruits to enzymes may alter the skin structure, reducing the resistance to water diffusion during drying. Applying the mechanical puncture or the ultrasound treatment to the fruit skin may also improve the drying process of blueberries. Therefore, the study has aimed to determine the effect of pre-treatment with enzymes, ultrasound, and fruit skin perforation on the kinetics of the freeze-drying process and selected properties of the dried blueberries.
2. Materials and Methods
2.1. Materials
The highbush blueberry (Vaccinum corymbosum L.) variety Aurora originated from the Jerzy Wilczewski Horticulture Farm in Białosy (Poland). The fruits after harvest were transported under refrigerated conditions and stored at 7 °C for a maximum of 48 h until the analysis.
2.2. Pre-Treatment of Blueberries Before Freeze-Drying
2.2.1. Enzymatic Treatment
A commercial enzyme preparation, the BrenPect Berry Fruit (Brenntag, Kędzierzyn-Koźle, Poland), contained pectinolytic enzymes produced through the controlled fermentation of the selected strain of Aspergillus niger, was used. The acidified water (1M HCl) solution (130 cm3) with 5 g of the enzyme preparation was stirred in a glass beaker, and 130 g of the fruits were added. The mass of the added preparation was determined based on preliminary tests. The enzymatic processes were conducted for 30 min at 45 °C in a water bath. Then, after draining, the blueberries were immersed in cold water for about 30 s and dried on the absorbent paper.
2.2.2. Ultrasound Treatment
The blueberries were immersed in the chamber of the MKD Ultrasonic bath (Warsaw, Poland) filled with distilled water. The fruit-to-water weight ratio was 1:3. Sonification was carried out at a frequency of 21 Hz and power of 300 W for 4 h. After that process, the fruits were cooled with water and dried on the filter paper.
Preliminary studies preceded the determination of the US pre-treatment time. The expected effects (shortening the freeze-drying time) were not obtained in result of the sonification of berries after 30, 60, 120, and 180 min. The ultrasound pre-treatment was extended to 4 h. The long ultrasound treatment time may also be found in the related literature. Li et al. [27] studied the effect of the US treatment on the content of anthocyanins in young wine. The samples were sonicated using 4 cycles of 40 min each. The starch sample was ultrasound-treated for 2, 4, 8, 14, and 22 h [28].
2.2.3. Puncture Treatment
The fruit skin was manually punctured with a needle of 0.5 mm in diameter. The number of punctures was about 6–8 per 1 cm2 of the fruit surface. Thus, about 30–40 punctures per fruit were made.
2.3. Freeze-Drying
The blueberries with and without pre-treatment were frozen at −40 °C in a shock freezer (Irinox HC 51/20, Corbanese, Italy) for 1.5 h. Then, the frozen fruits were placed in a freeze-dryer (Gamma 1–16 LSC plus, Martin Christ, Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany). The freeze-drying process was carried out at the pressure of 63 Pa (corresponding to the temperature of the material during sublimation at −25 °C [9]) and a shelf temperature of 0 °C for the first five hours and then at 30 °C until a constant mass of fruits was achieved. After drying, the material was packed in moisture-barrier bags.
Kinetics of Drying Process
The changes in the blueberry mass during freeze-drying were recorded by means of a weighing system dedicated to low pressure (Mensor, Warsaw, Poland). The initial mass of the blueberries was about 100 g. Nowak and Jakubczyk [8] described the detailed measurement procedure. The mass of the dried fruits was measured every 5 min for the first 2 h and then at 15 min intervals. The moisture ratio (MR) was calculated based on the recorded mass during drying.
where uo u is the water content initially and after a specified time, and ue is the equilibrium water content (g water/g d.m.).
The regression analysis was carried out by means of the Table Curve v. 5.01 software (Systat Software Inc., Palo Alto, CA, USA) in order to describe the changes in the moisture ratio during the freeze-drying time. Moreover, the goodness of fit for the selected drying kinetics models was evaluated based on R2 (determination coefficients) and RMSE (root mean square error). The model designed by Midilli et al. was selected and used for calculating the drying rate (min−1) dMR/dt based on those results [8]. The changes in the drying rate throughout the process were also presented.
The equation formula is as follows:
where a, b, k, and n are the parameters in the model designed by Midilli et al.
2.4. Selected Properties of Blueberries (Fresh and After Pre-Treatment and Freeze-Drying)
2.4.1. Dry Matter and Water Activity
The dry matter (%) of fresh blueberries after pre-treatment and drying was determined according to the procedure applied by Jakubczyk and Jaskulska [29]. The sample was crushed and mixed with anhydrous sea sand and dried by means of the convection dryer (Wamed, SUP 65 W, Warsaw, Poland) for 24 h at a temperature of 70 °C.
The water activity of all the samples was measured at 25 °C by means of the gauge Hydrolab C1 (Rotronic AG, Bassersdorf, Switzerland) with the measurement accuracy of ±0.001.
The measurements were repeated four times.
2.4.2. Colour
Colour attributes of all the variants were determined in the CIE L* (lightness), a* (+a* redness/−a*greenness), b* (+b* yellowness/−b* blueness) system using a CM-5 chromameter (Minolta, Osaka, Japan) with illuminant D65 and 10° observer. The values of a* and b* were used for calculating the chroma C*.
The measurement was repeated 15 times.
2.4.3. Mechanical Resistance
The mechanical properties of all the samples were measured by means of the TA-HD plus texture analyser and flat-type probe of a 21 mm diameter (Stable Micro Systems, Surrey, UK). The samples were compressed up to a strain of 80% at a speed of 1 mm/s. The maximum force at the failure strain was recorded for the fruits before and after drying. Twenty repetitions were performed for all the variants.
2.4.4. Bioactive Compound Content
The flavonoid content was measured according to the procedure described by Tylewicz et al. [30] with some modifications. The dried blueberries weighing about 0.5 g were added to 25 mL of 80% ethanol and ground by means of a mechanical homogeniser (Ultra-Turrax T-10, IKA-Labortechnik, Staufen, Germany). The ethanol and ground material suspension was heated on a hot plate until boiling and then quickly cooled in a water bath. The samples were filtered through the filter paper into 50 mL flasks and filled with 80% ethanol. The ethanol extract (2 mL) was mixed with 2 mL of a 2% aluminium chloride solution. Furthermore, the mixture of 2 mL of 80% ethanol and 2 mL of 80% ethanol solution was prepared to enhance the extract solution absorbance. Incubation was carried out at room temperature for 10 min. Absorbance was measured at 430 nm in a spectrophotometer (Thermo Spectronic Helios Gamma, Thermo Fischer Scientific, Waltham, MA, USA). The flavonoid content was determined by means of the calibration curve of quercetin standard in the range of 0.075–0.20 mg/mL (Sigma-Aldrich, Steinheim, Germany). The total flavonoid content was expressed in terms of mg quercetin equivalent per g d.m. The total phenolic content (TPC) was determined according to the procedure described by Tylewicz et al. [30] using Folin–Ciocalteu. TPC was expressed in terms of mg of gallic acid equivalents (GAE) per g of dry matter. The anthocyanin content was measured using the method described by Giusti and Wrolstad [31]. The content of anthocyanins was expressed in terms of mg cyanidin-3-glucoside per g of dry matter.
The experiment was repeated three times for all the types of bioactive compounds.
2.4.5. Hygroscopicity of Dried Fruits
The dried blueberries were stored in glass jars over a calcium chloride solution (aw = 0.75). The mass of the sample was measured before and after 24 h of storage. The water uptake (g/g d.m.) was calculated based on the water content of the dried fruits and the gain of water vapour during storage. The measurement was repeated two times.
2.4.6. Structure of Dried Fruits
The dried blueberries were cut in half, and the cross-section of the structure was analysed by means of the SMZ 1500 NIKON stereoscopic microscope equipped with a Nikon DS-Fi1 camera (Nikon, Tokyo, Japan). The photos of the surface of the dried fruits were also taken by means of a digital camera (Model HDR-SR10E, Sony Corporation, Tokyo, Japan).
2.5. Statistical Analysis
The results of the measurements were statistically processed by means of the Statistica software (v 13.3 StatSoft Inc., Tulsa, OK, USA). The normality of distribution with the application of the Shapiro–Wilk test (α = 0.05) and homogeneity of variance (Levene’s test, α = 0.05) were checked. The results of those tests indicated that the one-way ANOVA analysis could be used for analysing significant differences in the obtained values. Tukey’s Honest Significant Difference method at the 95% significance level (The Statistica v 13.3 StatSoft Inc., Tulsa, OK, USA) was applied to determine the effect of the pre-treatment methods and the drying process on the selected properties of blueberries. The regression analysis of drying kinetics curves was conducted by means of the Table Curve v. 5.01 software (Systat Software Inc., Palo Alto, CA, USA). The results were presented in terms of mean ± standard deviations.
3. Results and Discussion
3.1. Selected Physical and Psychochemical Properties of Blueberries with Different Pre-Treatments and After Drying
The dry matter of the fresh blueberries, and after the diversified pre-treatment, ranged from 13.8 to 14.6% (Table 1). Most samples after the pre-treatment had a slightly higher water content than those observed for the fresh blueberries. The ultrasound and enzymatic pre-treatment were performed in the aqueous environment. After those processes, the fruits were dried with tissue paper, but water could have remained on their surface, influencing the measurement. The effect of the ultrasound pre-treatment is mainly linked to the structure of fruits, time, and frequency of sonification [32]. Other studies showed that the moisture content of raw and ultrasound-treated blueberries did not differ significantly [33].

Table 1.
Selected physical and physiochemical properties of fresh blueberries with and without different pre-treatments.
The dry matter of the fresh blueberries, and after the diversified pre-treatment, ranged from 13.8 to 14.6% (Table 1). Most samples after the pre-treatment had a slightly higher water content than those observed for the fresh blueberries. The ultrasound and enzymatic pre-treatment were performed in the aqueous environment. After those processes, the fruits were dried with tissue paper, but water could have remained on their surface, influencing the measurement. The effect of ultrasound pre-treatment can be related to many factors, e.g., the structure and composition of fruits, and the temperature, time and frequency of the sonification treatment [32]. In some studies, the use of sonification did not cause any changes. Nowak et al. [33] observed that other studies showed that the moisture content of raw and ultrasound-treated blueberries did not differ significantly.
The fruits with pre-treatment showed a higher water activity (aw) than the fresh blueberries (Table 1). The pre-treatment processes probably caused the degradation of the blueberry structure (disruption of the integrity of cell membranes), leading to better water availability and a lower degree of water binding. It led to higher water activity.
The texture of the fruits is an important quality feature. The maximum force recorded during the compression significantly decreased after the pre-treatment (Table 1). However, the values of the force obtained for the treated blueberries did not differ significantly. They ranged from 7.8 to 9.7 N. Nowak et al. [33] observed a decrease in the hardness of raw blueberries after the ultrasound treatment, from 29.1 to 16.0 N. The frozen/thawed berries were also less rigid and more susceptible to deformation than the control raw berries. All the pre-treatment methods could have affected the structure of the blueberries, reducing their mechanical strength during the compression.
Table 2 presents the selected physical properties of the freeze-dried blueberries. The drying process reduced the water content and activity to a level that guaranteed the stability and microbiological safety of the dried fruits. The dry matter content did not differ and equalled 95% for all the samples. The highest water activity was obtained for the raw dried blueberries (0.267), while the pre-treatment caused a decrease in aw for the freeze-dried fruits, reducing it to the range of 0.220–0.249. Another study has shown that the water activity and water content of freeze-dried blueberries of the cultivar Bluegold can range within 0.190 and 10.7%, respectively [34]. The water activity for most freeze-dried fruits has varied from 0.08 to 0.330 [9], which corresponds with our results. The pre-treatment affected the fruit structure changes and the degree of binding and water availability of the freeze-dried blueberries.

Table 2.
Selected physical and physiochemical properties of dried blueberries.
Figure 1 presents the photos of blueberries after freeze-drying. The appearance of the fruits without the pre-treatment and with sonification was similar (Figure 1a). The freeze-drying caused the fruit to rupture and caused partial leakage. That effect was not observed for the fruits subjected to the enzymatic treatment (Figure 1b) and puncturing (Figure 1c). Still, some changes were visible on the skin surface of blueberries (holes, lack of continuity) due to the pre-treatment. The microscopic image of the cross-section of the ultrasound-treated and intact freeze-dried fruits showed that the fruits, after drying, contained large holes and cavities, leaving only some rigid tissue walls intact (Figure 2a). The structure of the enzymatic-treated dried blueberries was different and contained many small pores in the solid matrix of tissue (Figure 2b).

Figure 1.
Photos of freeze-dried blueberries: (a) without pre-treatment; (b) enzymatic-treated; (c) puncture-treated.

Figure 2.
Microscopic images of freeze-dried blueberries: (a) without pre-treatment and (b) with enzymatic pre-treatment.
The structure of the freeze-dried blueberries affected their texture (Table 2). The freeze-dried blueberry without pre-treatment was characteristic of the lowest maximum force. The reduced mechanical strength of the fresh and freeze-dried blueberries was related to the structure of the fruits after drying (Figure 2a). The epicarp of the fruits was cracked during drying, and some of the inner parts of the blueberries leaked out (Figure 1a). After lyophilisation, a solid matrix with many large pores and thin walls remained. The creation of that structure made the material susceptible to deformation during compression. The ultrasound-treated dried fruit also had a lower hardness than the two other samples. The blueberries that were almost empty inside had a very low mechanical resistance. The enzymatic-treated and puncture freeze-dried blueberries had the higher hardness and a more rigid structure. The effect of the explosion of those fruits was not observed during drying (Figure 1b,c), and the inner structure was not damaged (Figure 2b). This resulted in higher mechanical resistance to deformation.
Additionally, the hygroscopicity of the freeze-dried blueberries after 24 of storage in the environment with water activity of 0.753 was analysed (Table 2). The water uptake by the dried fruits ranged from 0.10 to 0.15 g/g d.m. However, the differences were insignificant. It may be assumed that the impermeable dry skin determined the water vapour absorption despite differences in the structure of the dried fruits. That may account for the lack of differences in hygroscopicity of the freeze-dried blueberries.
Colour is one of the essential quality characteristics of any food product. Therefore, it is crucial to determine to what extent the pre-treatment affects colour parameters. The average values of colour parameters of the fresh blueberries after the treatment and freeze-drying are presented in Table 3.

Table 3.
Colour attributes of blueberries with and without pre-treatments and after freeze-drying.
The brightness of the fresh blueberries was 18.60. The pre-treatment did not significantly affect that colour attribute (L* = 19.17 ÷ 19.97). The brightness of the blueberries after freeze-drying did not change in response to the ultrasound and enzyme-based treatment but increased in the case of fruits without pre-treatment and punctured to 21.10 and 23.44, respectively (Table 3). Therefore, the brightness of the samples shifted toward white. It was caused by the damage to the skin, which is the essence of puncturing or cracking the fruit without pre-treatment during freeze-drying. Yemmireddy et al. [35] obtained lightness in the range of 15.40 to 16.29, depending on the drying method of the blueberries. Nemzer et al. [36] noted that air drying did not affect that parameter (L* = 18.01 ± 1.06) but freeze-drying and reflectance window drying increased the brightness to L* = 25.97 ± 2.17 and L* = 22.42 ± 3.02, respectively.
The a* parameter was the lowest for the fresh blueberries (0.11 ± 0.12) (Table 3). The enzymatic, ultrasound, and puncture pre-treatments increased said value to 0.67 ± 0.19, 0.99 ± 0.47, and 1.42 ± 0.30, respectively. Freeze-drying increased the chromatic coordinate a* from 1.44 for the untreated blueberries to 2.91 for the US-treated material. All the values were positive, which indicated a shift toward red colour. The lowest value of the b* parameter (−3.72) was noted for the fresh fruits after freeze-drying. The values of b* for the freeze-dried fruits with diversified pre-treatments were similar. Nemzer et al. [36] obtained the a* parameter ranging from 6.22 ± 0.87 for the air-dried blueberries to 9.36 ± 0.45 for the freeze-dried blueberries. Those values did not differ significantly from the values for the frozen fruits. However, the positive values of the b* parameter were obtained for the berries.
The chroma C* values before drying did not differ significantly, except for the enzyme-treated blueberries. After freeze-drying, the C* values ranged from 4.01 for the untreated fruit to 2.80 for the puncture-treated material. Yemmireddy et al. stated that the chroma values ranged from 2.10 to 4.59, depending on the drying method [35]. Those values are comparable with those obtained in this study.
Table 4 presents the content of the selected bioactive compounds in the blueberries. The pre-treatment of the fresh fruits caused a slight increase in the flavonoid content following the enzymatic process and puncturing. In contrast, those pre-treatment methods for the freeze-dried blueberries reduced the flavonoid content compared to the intact dried fruits. The ultrasound treatment did not affect the content of flavonoids and polyphenols in the fresh and freeze-dried blueberries. Pala et al. [37] did not observe significant changes in the total phenolic content in the ultrasonically treated pomegranate juice. The content of polyphenols was significantly lower only for the blueberries subjected to the enzymatic treatment in the case of the raw and freeze-dried fruits.

Table 4.
Bioactive components in blueberries with and without pre-treatments and after freeze-drying.
A decrease in anthocyanin content was observed in fresh fruits after sonication. Buckow et al. [38] observed that the degradation of anthocyanins in blueberries only led to a 10% reduction in the total anthocyanin content. The degradation of the compounds responsible for the colour and amount of anthocyanins during the ultrasonic treatment can be related to oxidation reactions, supported by the interaction with free radicals generated during sonication. After drying, most fruits had similar anthocyanin content, except for the enzymatic-treated blueberries, which contained 35% more of this compound than samples without pre-treatment. The activity of the applied pectinolytic enzymes could cause the degradation of the material tissue, allowing the release of anthocyanins and increasing their availability during measurement.
Nemzer et al. [36] observed that freeze-dried berries contained twice as many flavonoids as anthocyanins. This study also confirmed that the content of flavonoids was significantly higher than that of anthocyanins, and in the case of untreated freeze-dried fruits, the value was 4 times higher.
In all measurements of bioactive compounds, higher values were obtained for dried materials than for fresh fruits. These differences resulted from the measurement method. In the case of the dry samples, the extraction of substances and compounds is much better than that of raw plant material. Also, Ochmian et al. [39] noted that polyphenol content was several times higher for freeze-dried blueberries than observed for fresh fruits. Another study showed that the content of polyphenolic compounds in fresh herbs and spices was also significantly lower than the obtained freeze-dried. The authors stated that the total content of polyphenols was affected by extraction efficiency [40]. Anthocyanin and polyphenol contents increased after the freeze-drying of mangosteens extract compared to the fresh sample. The high level of bioactive compounds was extracted due to damage to the structure formed in the tissue during freeze-drying [41]. The facture of cell walls can be a factor responsible for the better extractability of compounds from plant material [42].
Enzymatic treatment reduced polyphenol content but increased anthocyanin content (Table 4). Siddiq et al. [43] investigated the effect of juice extraction enzymes on the content of total anthocyanins and total phenolics. The enzyme treatments increased total anthocyanin content from 9.69 to 11.49–12.90 mg/100 mL. A trend of lowering polyphenol content in the juice after enzyme treatment was observed. The authors explained a higher content of anthocyanins by the breakdown of the cell walls of blueberries, which led to the recovery of anthocyanins. Similar relationships were also observed by Lee and Wrolstad [44]. In plant tissue, the increase in anthocyanin content and decrease in polyphenols were observed during fruit maturation. The increased expression of enzymes was responsible for anthocyanin synthesis during ripening [45,46]. The decrease in polyphenol content in dried berries was related to the destruction of compounds (polyphenols, interferences, and Maillard products) capable of reacting with the Folin–Ciocalteu reagent [47].
3.2. Drying Kinetics of Blueberries with and Without Pre-Treatment
The preliminary analysis of the regression results was conducted. The selected mathematical models (Newton, Two terms, Henderson and Pabis, Midilli et al.) [8] were used for describing the experimental data. R2 and RMSE were obtained to evaluate the goodness of fit. The root mean square error (RMSE) ranged from 0.0011 to 0.0051 and R2 = 0.999 for the model designed by Midilli et al. (Table 5). In the case of other models, RMSE values were significantly higher and ranged from 0.012 to 0.084, but R2 values were lower (0.879 to 0.976). The best model should be characterised by the lowest RMSE and the highest value of R2 [48]. It indicates that the model designed by Midilli et al. most precisely described the experimental data for the freeze-dried blueberries. That model was also used for analysing the drying kinetics of the freeze-dried white mulberry fruits [49] and convective-dried whole blueberries at air temperatures of 30–50 °C [16]. Table 5 also presents the parameters of the selected model for the freeze-dried blueberries. An n parameter higher than 1 indicates the sigmoidal shape of the drying curve. n values close to 1 were obtained for the ultrasound-treated blueberries and the fruits without pre-treatment. The freeze-dried blueberries exhibited n values of 1.457 and 1.709 for sonification and puncturing, respectively.

Table 5.
Drying time and parameters of Midilli et al. kinetics model of freeze-dried blueberries with and without pre-treatment.
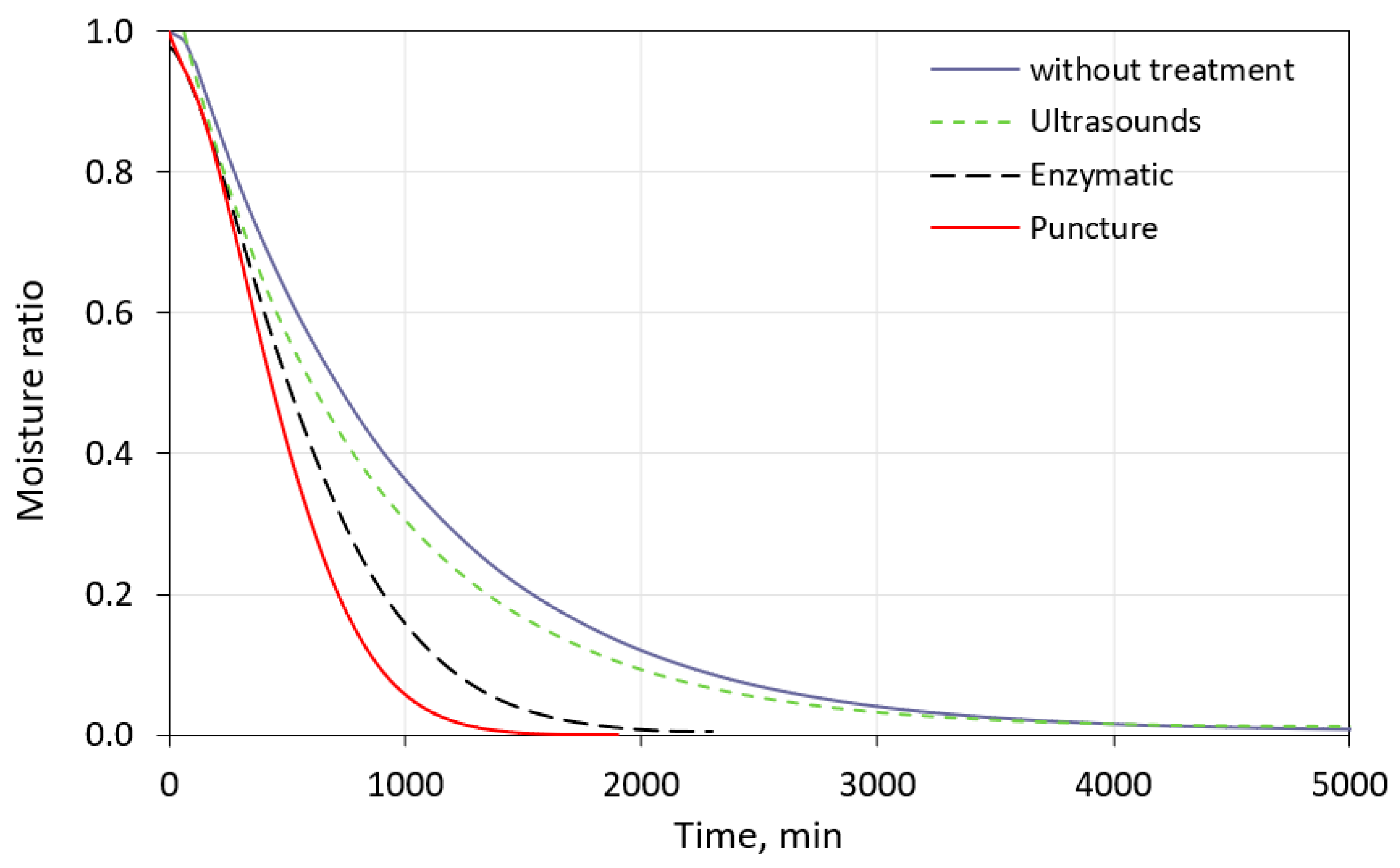
Figure 3 presents the drying curves for the freeze-dried blueberries (using the model designed by Midilli et al.). The course of the drying curves was similar. Most pre-treatment methods caused a reduction in drying time from 5010 (berries without any pre-treatment) to 1930 min for the punctured fruits. The enzyme-treated blueberries also accelerated dehydration, and the freeze-drying time decreased to 2180 min. The application of ultrasound did not affect the drying time compared to the fruits without any pre-treatment. The pre-treatment before freeze-drying may be an effective tool for decreasing the drying time. The pulsed electric field (PEF) caused the freeze-drying time to be reduced from 800 to 720 min [8]. Martín-Gómez et al. [16] noted that a two-fold reduction in the convective drying time at 30 °C was observed for the punched fruits. The perforation of the berry increased the permeability of the waxy grape skin and accelerated water evaporation [16,50]. The physical wax abrasive pre-treatment of goji berry reduced the convective drying time from 21 to 15 h [51].
Figure 3.
Drying curves of freeze-dried blueberries with and without pre-treatment.
Stratta et al. [52] calculated that the cost intensity of the primary and secondary drying accounted for 87% of the operating costs in the laboratories and 82% in the industrial devices. The operating cost of one cycle amounted to EUR 3.29 for laboratory freeze-dryers and EUR 107.27 for industrial freeze-dryers. Therefore, the operating cost of freeze-drying (without freezing and thawing) amounted to 2.85 EUR/for the laboratory cycle and 88.45 EUR/for the industrial cycle. By shortening the freeze-drying time of the blueberries subjected to the pre-treatment, the operating costs in the laboratory may be reduced to EUR 1.24 and 1.10 EUR/cycle, by means of the enzymatic treatment and puncture treatment, respectively. In industrial conditions, those costs amount to 38.49 and 34.07 EUR/cycle.
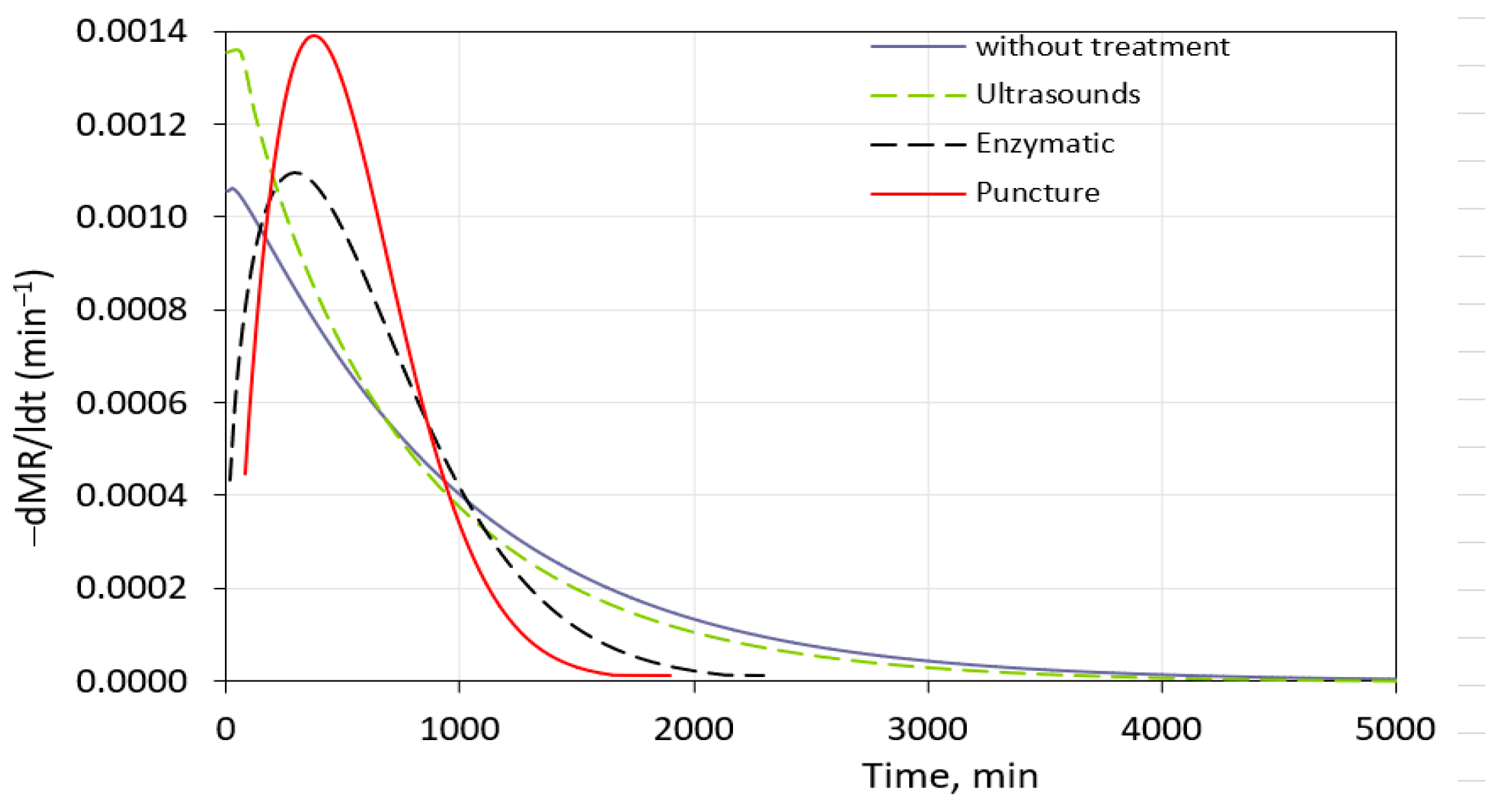
The highest drying rate (0.0014 min−1) was observed for the punched blueberries (Figure 4). A decrease in the drying rate was observed after 410 min of processing. Considerably high drying rates were also observed for the fruits with the enzymatic pre-treatment. Mechanical and enzymatic pre-treatments could have changed the permeability of the plant tissue, and the blueberries dried faster than the sample with sonification and without the pre-treatment. Knopacka and Płocharski [53] noticed that the application of enzyme preparation with pectin lyase and polygalacturonase increased the drying rate of pumpkin and apples but did not affect the drying time due to the higher water content of the processed plant before drying. It may indicate that the type of plant tissue, enzymes used, and treatment time may have a diverse impact on the water removal rate during drying. Tissue damage during the enzymatic treatment may vary.
Figure 4.
Effect of pre-treatments on drying kinetics of freeze-dried blueberries.
4. Conclusions
The enzymatic treatment and ultrasonic puncture did not change the dry matter content but reduced the hardness of the blueberries. The content of bioactive compounds was at a similar level; the enzymatic treatment significantly reduced the content of polyphenols. After freeze-drying, the dry matter content was similar, but the water activity was lower in the treated samples. After drying without pre-treatment and with sonication, the berries were almost empty due to the tissue rupture. The structure of those fruits impacted the reduction in their mechanical strength. The puncture and enzymatic treatment slightly reduced the content of polyphenols compared to the dried berries without pre-treatment. The puncture and enzymatic treatments facilitated water removal during drying and shortened its time twice as much as in the case of the fruits dried without pre-treatment. Given the results, the pre-treatment with enzymes and puncturing may be recommended for freeze-drying blueberries. Changes in the content of bioactive compounds were at a similar level as in the case of freeze-dried raw material. The retention of the structure was much better, and the drying time was considerably reduced for the samples subjected to such a pre-treatment.
Author Contributions
Conceptualization, E.J.; methodology, E.J. and E.T.-G.; software, E.T.-G.; validation, E.J. and E.T.-G.; formal analysis, E.J. and E.T.-G.; investigation, E.T.-G.; data curation, E.J. and E.T.-G.; writing—original draft preparation, E.J., A.K., A.K.-D. and D.N.; writing—review and editing, E.J., A.K.-D. and D.N.; visualisation, E.J. and E.T.-G.; supervision, E.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Uribe, E.; Vega-Galvez, A.; Pasten, A.; Ah-Hen, K.S.; Mejias, N.; Sepúlveda, L.; Poblete, J.; Gomez-Perez, L.S. Drying: A practical technology for blueberries (Vaccinium corymbosum L.)—Processes and their effects on selected health-promoting properties. Antioxidants 2024, 13, 1554. [Google Scholar] [CrossRef]
- Aoun, A.; Ghoussoub, C.; Sarieddine, M.; Aoun, M.; El Helou, K. Effectiveness of nutritional supplements (vitamins, minerals, omega-3, and probiotics) in preventing and treating COVID-19 and viral respiratory infections. Hum. Nutr. Metab. 2024, 38, 200287. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Yan, B.; Huang, L.; Zhang, H. Effects of nutritional supplementation combined with exercise training on frailty, physical function, and quality of life in chronic kidney disease: A systematic review and meta-analysis. J. Ren. Nutr. 2024; in press. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Meng, X.; Liu, S.; Mu, J.; Ning, C. Comparison of polyphenol, anthocyanin and antioxidant capacity in four varieties of Lonicera caerulea berry extracts. Food Chem. 2016, 197, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Kristo, A.S.; Klimis-Zacas, D.; Sikalidis, A.K. Protective role of dietary berries in cancer. Antioxidants 2016, 5, 37. [Google Scholar] [CrossRef]
- Lachowicz-Wiśniewska, S.; Pratap-Singh, A.; Ochmian, I.; Kapusta, I.; Kotowska, A.; Pluta, S. Biodiversity in nutrients and biological activities of 14 highbush blueberry (Vaccinium corymbosum L.) cultivars. Sci. Rep. 2024, 14, 22063. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, M.; Mujumdar, A. Berry drying: Mechanism, pretreatment, drying technology, nutrient preservation, and mathematical models. Food Eng. Rev. 2019, 11, 61–77. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. Effect of pulsed electric field pre-treatment and the freezing methods on the kinetics of the freeze-drying process of apple and its selected physical properties. Foods 2022, 11, 2407. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Jakubczyk, E. The freeze-drying of foods-the characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Grabowski, S.; Marcotte, M.; Quan, D.; Taherian, A.R.; Zareifard, M.R.; Poirier, M.; Kudra, T. Kinetics and quality aspects of canadian blueberries and cranberries dried by osmo-convective method. Dry. Technol. 2007, 25, 367–374. [Google Scholar] [CrossRef]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf life potential and the fruit cuticle: The unexpected player. Front. Plant Sci. 2019, 10, 770. [Google Scholar] [CrossRef] [PubMed]
- Ziv, C.; Zhao, Z.; Gao, Y.G.; Xia, Y. Multifunctional Roles of plant cuticle during plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Blanke, M.M.; Leyhe, A. Stomatal Activity of the grape berry cv. Riesling, Müller-Thurgau and Ehrenfelser. J. Plant Physiol. 1987, 127, 451–460. [Google Scholar] [CrossRef]
- Blanke, M. Structure and function of blueberry fruit and flowers: Stomata, transpiration and photoassimilation. Horticulturae 2024, 10, 606. [Google Scholar] [CrossRef]
- Shi, J.; Pan, Z.; McHugh, T.H.; Wood, D.; Zhu, Y.; Avena-Bustillos, R.J.; Hirschberg, E. Effect of berry size and sodium hydroxide pretreatment on the drying characteristics of blueberries under infrared radiation heating. J. Food Sci. 2008, 73, E259–E265. [Google Scholar] [CrossRef]
- Martín-Gómez, J.; Ángeles Varo, M.; Mérida, J.; Serratosa, M.P. The influence of berry perforation on grape drying kinetics and total phenolic compounds. J. Sci. Food Agric. 2019, 99, 4260–4266. [Google Scholar] [CrossRef] [PubMed]
- Munzenmayer, P.; Ulloa, J.; Pinto, M.; Ramirez, C.; Valencia, P.; Simpson, R.; Almonacid, S. Freeze-drying of blueberries: Effects of carbon dioxide (CO2) laser perforation as skin pretreatment to improve mass transfer, primary drying time, and quality. Foods 2020, 9, 211. [Google Scholar] [CrossRef]
- Zielinska, M.; Sadowski, P.; Błaszczak, W. Freezing/thawing and microwave-assisted drying of blueberries (Vaccinium corymbosum L.). LWT 2015, 62, 555–563. [Google Scholar] [CrossRef]
- López, J.; Shun Ah-Hen, K.; Vega-Gálvez, A.; Morales, A.; García-Segovia, P.; Uribe, E. Effects of drying methods on quality attributes of murta (ugni molinae turcz) berries: Bioactivity, nutritional aspects, texture profile, microstructure and functional properties. J. Food Proc. Eng. 2017, 40, e12511. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, W.; Chitrakar, B.; Fan, K. Ultrasound technology for enhancing drying efficiency and quality of fruits and vegetables: A Review. Food Bioproc. Technol. 2024, 17, 4506–4536. [Google Scholar] [CrossRef]
- Miraei Ashtiani, S.-H.; Rafiee, M.; Mohebi Morad, M.; Martynenko, A. Cold plasma pretreatment improves the quality and nutritional value of ultrasound-assisted convective drying: The case of goldenberry. Dry.Technol. 2022, 40, 1639–1657. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Rybak, K.; Witrowa-Rajchert, D.; Wiktor, A.; Rąbkowski, R.; Nowacka, M. Convective drying with the application of ultrasonic pre-treatment: The effect of applied conditions on the selected properties of dried apples. Foods 2024, 13, 3893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liao, L.; Qiao, Y.; Wang, C.; Shi, D.; An, K.; Hu, J. Effects of ultrahigh pressure and ultrasound pretreatments on properties of strawberry chips prepared by vacuum-freeze drying. Food Chem. 2020, 303, 125386. [Google Scholar] [CrossRef]
- Chu, W.; Gao, H.; Chen, H.; Fang, X.; Zheng, Y. Effects of cuticular wax on the postharvest quality of blueberry fruit. Food Chem. 2018, 239, 68–74. [Google Scholar] [CrossRef]
- Montecchiarini, M.L.; Silva-Sanzana, C.; Valderramo, L.; Alemano, S.; Gollán, A.; Rivadeneira, M.F.; Bello, F.; Vázquez, D.; Blanco-Herrera, F.; Podestá, F.E.; et al. Biochemical differences in the skin of two blueberries (Vaccinium corymbosum) varieties with contrasting firmness: Implication of ions, metabolites and cell wall related proteins in two developmental stages. Plant Physiol. Biochem. 2021, 162, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Zhang, D.; Li, X.; Xu, P.; Zhang, Z.; Yang, Y.; Yang, J.; He, Y.; ElGamal, R. Effect of biological enzyme pretreatment on the kinetics, microstructure, and quality of vacuum drying of wolfberry. LWT 2025, 117455. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Peng, Z.; Zhao, Y.; Wu, K.; Zhou, N.; Yan, Y.; Ramaswamy, H.S.; Sun, J.; Bai, W. The impact of ultrasonic treatment on blueberry wine anthocyanin color and its In-vitro anti-oxidant capacity. Food Chem. X 2020, 333, 127455. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, G.; Zhu, F. Impact of long-term ultrasound treatment on structural and physicochemical properties of starches differing in granule size. Carbohydrate Polym. 2023, 320, 121195. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Jaskulska, A. The effect of freeze-drying on the properties of Polish vegetable soups. Appl. Sci. 2021, 11, 654. [Google Scholar] [CrossRef]
- Tylewicz, U.; Nowacka, M.; Rybak, K.; Drozdzal, K.; Dalla Rosa, M.; Mozzon, M. Design of healthy snack based on kiwifruit. Molecules 2020, 25, 3309. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Fijalkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. The physical, optical and reconstitution properties of apples subjected to ultrasound before drying. Ital. J. Food Sci. 2017, 29, 343–356. [Google Scholar] [CrossRef]
- Nowak, K.W.; Zielinska, M.; Waszkielis, K.M. The effect of ultrasound and freezing/thawing treatment on the physical properties of blueberries. Food Sci. Biotechnol. 2019, 28, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Antal, T. The effect of refrigeration and room temperature storage conditions on the physico-chemical characteristics of hybrid and freeze-dried blueberries. J. Agri. Food Res. 2024, 16, 101083. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Chinnan, M.S.; Kerr, W.L.; Hung, Y.-C. Effect of drying method on drying time and physico-chemical properties of dried rabbiteye blueberries. LWT 2013, 50, 739–745. [Google Scholar] [CrossRef]
- Nemzer, B.; Vargas, L.; Xia, X.; Sintara, M.; Feng, H. Phytochemical and physical properties of blueberries, tart cherries, strawberries, and cranberries as affected by different drying methods. Food Chem. 2018, 262, 242–250. [Google Scholar] [CrossRef]
- Pala, Ç.U.; Zorba, N.N.; Özcan, G. Microbial inactivation and physicochemical properties of ultrasound processed pomegranate juice. J. Food Prot. 2015, 78, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Buckow, R.; Kastell, A.; Terefe, N.S.; Versteeg, C. Pressure and temperature effects on degradation kinetics and storage stability of total anthocyanins in blueberry juice. J. Agric. Food Chem. 2010, 58, 10076–10084. [Google Scholar] [CrossRef] [PubMed]
- Ochmian, I.; Figiel-Kroczyńska, M.; Lachowicz, S. The quality of freeze-dried and rehydrated blueberries depending on their size and preparation for freeze-drying. Acta Univ. Cibiniensis Ser. E Food Technol. 2020, 24, 61–78. [Google Scholar] [CrossRef]
- Bieżanowska-Kopeć, R.; Piątkowska, E. Total polyphenols and antioxidant properties of selected fresh and dried herbs and spices. Appl. Sci. 2022, 12, 4876. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Dolan, K.D. Total phenolics, carotenoids and antioxidant properties of Tommy Atkin mango cubes as affected by drying techniques. LWT 2015, 62, 564–568. [Google Scholar] [CrossRef]
- Capanoglu, E. Investigating the antioxidant potential of Turkish dried fruits. Int. J. Food Prop. 2014, 17, 690–702. [Google Scholar] [CrossRef]
- Siddiq, M.; Dolan, K.D.; Perkins-Veazie, P.; Collins, J.K. Effect of pectinolytic and cellulytic enzymes on the physical, chemical, and antioxidant properties of blueberry (Vaccinium corymbosum L.) juice. LWT 2018, 92, 127–132. [Google Scholar] [CrossRef]
- Lee, J.; Wrolstad, R.E. Extraction of anthocyanins and polyphenolics from blueberry processing waste. J. Food Sci. 2004, 69, 564–573. [Google Scholar] [CrossRef]
- Esquivel-Alvarado, D.; Munõz-Arrieta, R.; Alfaro-Viquez, E.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Composition of anthocyanins and proanthocyanidins in three tropical Vaccinium species from Costa Rica. J. Agric. Food Chem. 2020, 68, 2872–2879. [Google Scholar] [CrossRef]
- Kobori, R.; Yakami, S.; Kawasaki, T.; Saito, A. Changes in the polyphenol content of red raspberry fruits during ripening. Horticulturae 2021, 7, 569. [Google Scholar] [CrossRef]
- Bustos, M.C.; Rocha-Parra, D.; Sampedro, I.; de Pascual-Teresa, S.; León, A.E. The influence of different air-drying conditions on bioactive compounds and antioxidant activity of berries. J. Agric. Food Chem. 2018, 66, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Calín-Sánchez, Á.; Kharaghani, A.; Lech, K.; Figiel, A.; Carbonell-Barrachina, Á.A.; Tsotsas, E. Drying kinetics and microstructural and sensory properties of black chokeberry (Aronia melanocarpa) as affected by drying method. Food Bioproc. Technol. 2015, 8, 63–74. [Google Scholar] [CrossRef]
- Krzykowski, A.; Dziki, D.; Rudy, S.; Polak, R.; Biernacka, B.; Gawlik-Dziki, U.; Janiszewska-Turak, E. Effect of air-drying and freeze-drying temperature on the process kinetics and physicochemical characteristics of white mulberry fruits (Morus alba L.). Processes 2023, 11, 750. [Google Scholar] [CrossRef]
- Saravacos, G.D.; Tsiourvas, D.A.; Tsami, E. Effect of temperature on the water adsorption isotherms of sultana raisins. J. Food Sci. 1986, 51, 381–383. [Google Scholar] [CrossRef]
- Adiletta, G.; Alam, M.R.; Cinquanta, L.; Russo, P.; Albanese, D.; Di Matteo, M. Effect of abrasive pretreatment on hot dried goji berry. Chem. Eng. Trans. 2015, 44, 127–132. [Google Scholar] [CrossRef]
- Stratta, L.; Capozzi, L.C.; Franzino, S.; Pisano, R. Economic analysis of a freeze-drying cycle. Processes 2020, 8, 1399. [Google Scholar] [CrossRef]
- Konopacka, D.; Płocharski, W. The effect of enzymaztic treatment on convective drying kinetics and dehydration properties of dried pumpkin, carrot and apples. Acta Agroph. 2008, 12, 699–711. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).