High-Pressure Extraction Techniques for Efficient Recovery of Flavonoids and Coumarins from Flower Seeds
Abstract
1. Introduction
2. High-Pressure Extraction Techniques
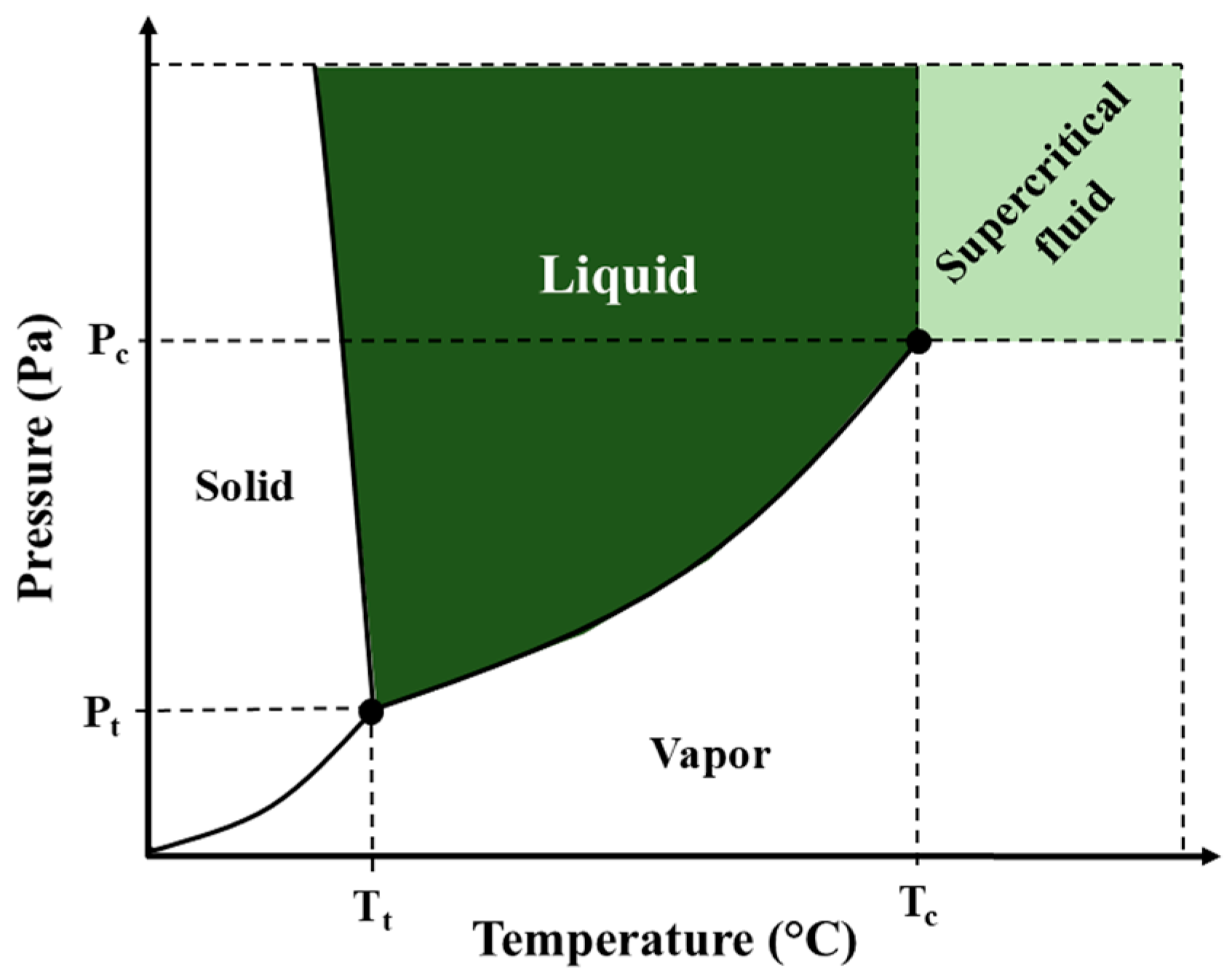
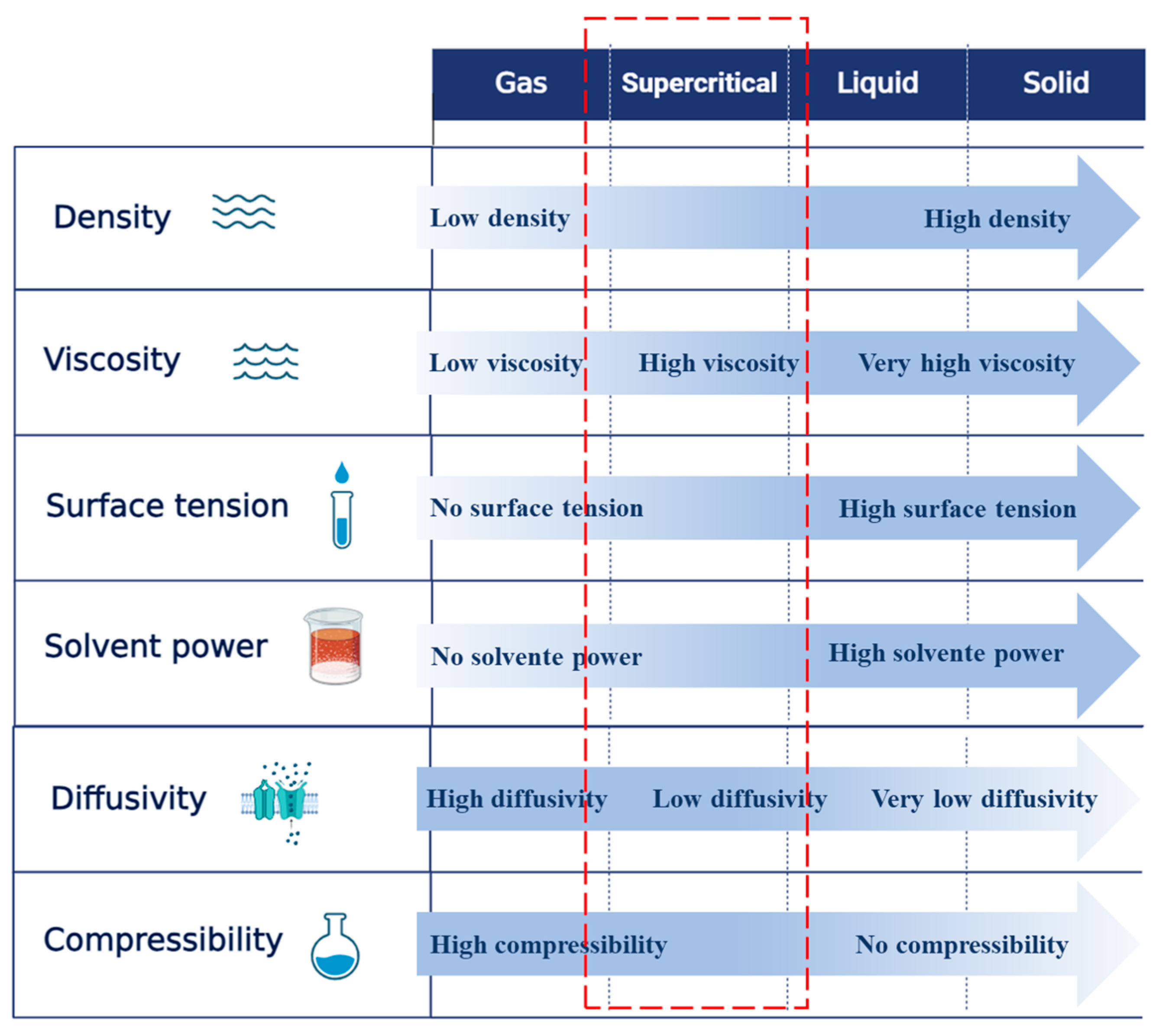
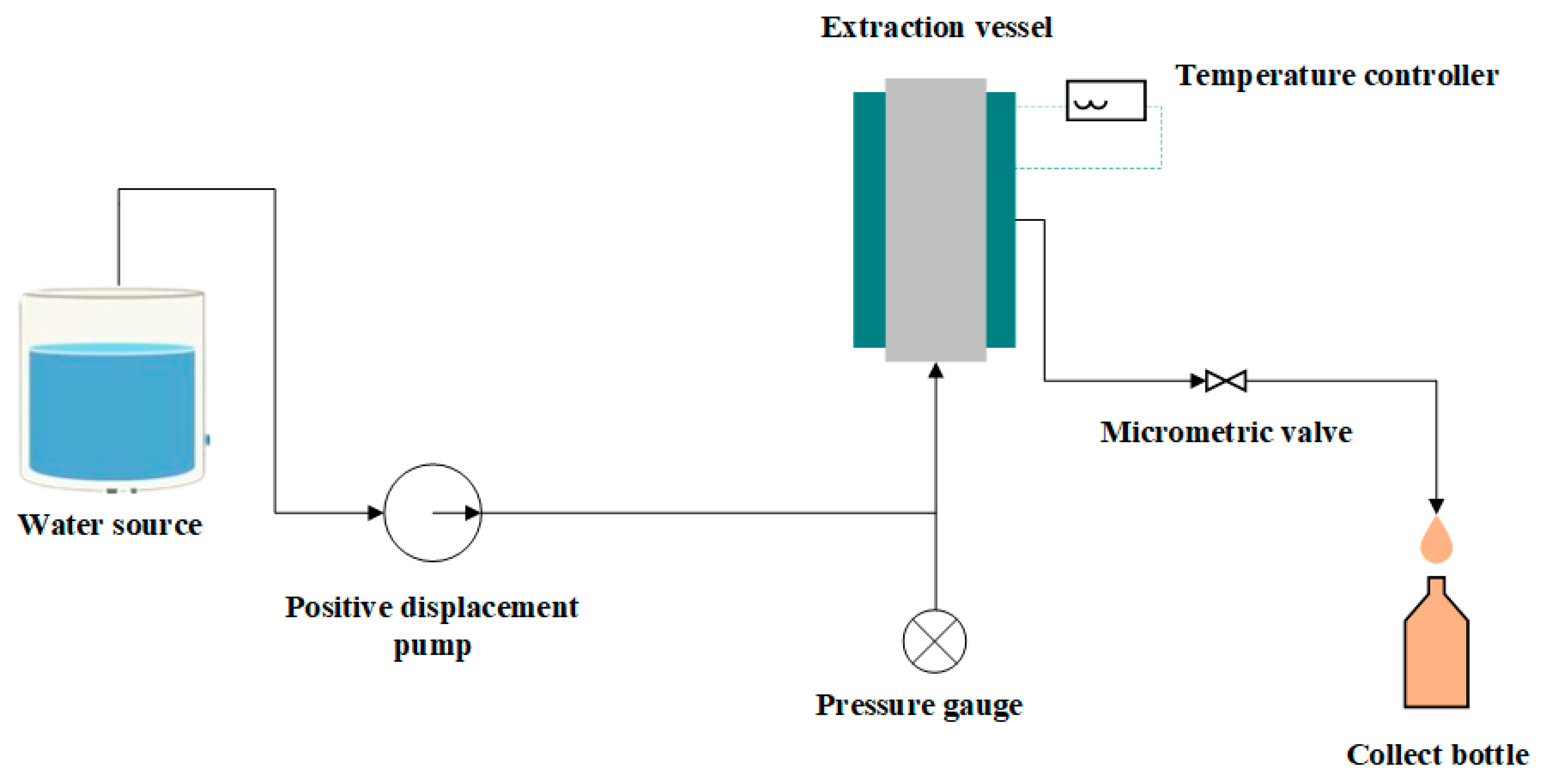
2.1. Supercritical Fluid Extraction (SFE)
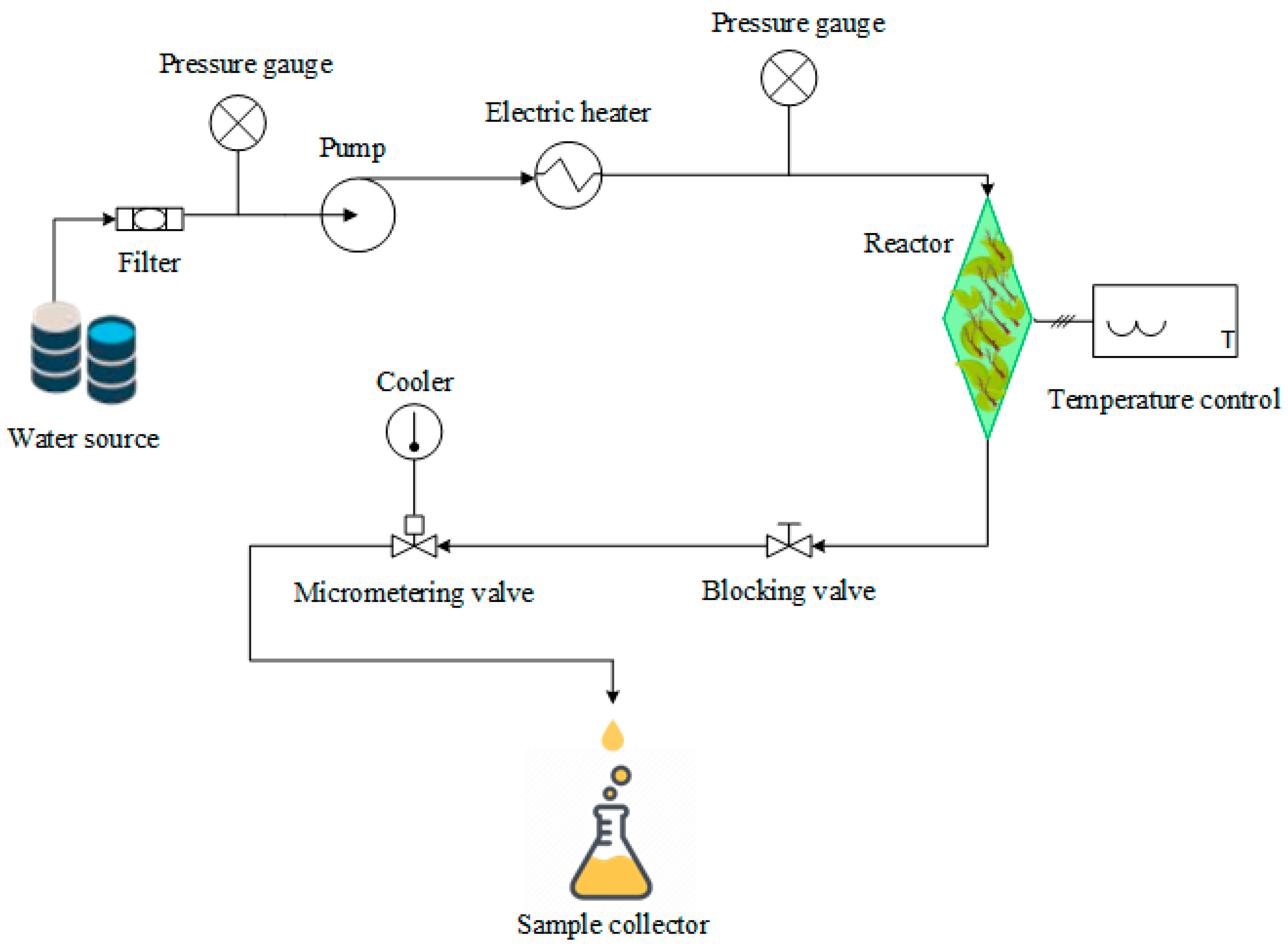
2.2. Pressurized Liquid Extraction (PLE)
3. Flower Seeds as a Source of Bioactive Compounds
4. Influence of Extraction Parameters
4.1. Temperature and Pressure
4.2. Solvent Selection and Solvent-to-Matrix Ratio
4.3. Extraction Time
5. Phenolic Compounds
6. Applications of Flavonoids and Coumarins
7. Future Directions and Challenges
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Čižmárová, B.; Hubková, B.; Tomečková, V.; Birková, A. Flavonoids as Promising Natural Compounds in the Prevention and Treatment of Selected Skin Diseases. Int. J. Mol. Sci. 2023, 24, 6324. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.F.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef] [PubMed]
- Tsivileva, O.M.; Koftin, O.V.; Evseeva, N.V. Coumarins as Fungal Metabolites with Potential Medicinal Properties. Antibiotics 2022, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Anywar, G.; Muhumuza, E. Bioactivity and toxicity of coumarins from African medicinal plants. Front. Pharmacol. 2023, 14, 1231006. [Google Scholar] [CrossRef]
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules 2023, 28, 2413. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Myrtsi, E.D.; Angelis, A.; Koulocheri, S.D.; Mitakou, S.; Haroutounian, S.A. Retrieval of high added value natural bioactive coumarins from mandarin juice-making industrial byproduct. Molecules 2021, 26, 7527. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q. Study on the Anti-Inflammatory Mechanism of Coumarins in Peucedanum decursivum Based on Spatial Metabolomics Combined with Network Pharmacology. Molecules 2024, 29, 3346. [Google Scholar] [CrossRef]
- Saadati, F.; Modarresi Chahardehi, A.; Jamshidi, N.; Jamshidi, N.; Ghasemi, D. Coumarin: A natural solution for alleviating inflammatory disorders. Curr. Res. Pharmacol. Drug Discov. 2024, 7, 100202. [Google Scholar] [CrossRef] [PubMed]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Cui, L.; Zhou, H.; Liu, Y.; Meng, L.; Chen, S.; Xi, X.; Zhang, Y.; Kang, W. Flowers: Precious food and medicine resources. Food Sci. Hum. Wellness 2023, 12, 1020–1052. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Naoum, E.; Xynopoulou, A.; Kotsou, K.; Bozinou, E.; Lalas, S.I.; Chatzimitakos, T.; Athanasiadis, V. The Value of Using Green Extraction Techniques to Enhance Polyphenol Content and Antioxidant Activity in Nasturtium officinale Leaves. Appl. Sci. 2024, 14, 10739. [Google Scholar] [CrossRef]
- Kang, H.; Kim, B. Bioactive Compounds as Inhibitors of Inflammation, Oxidative Stress and Metabolic Dysfunctions via Regulation of Cellular Redox Balance and Histone Acetylation State. Foods 2023, 12, 925. [Google Scholar] [CrossRef]
- Damergi, B.; Essid, R.; Fares, N.; Khadraoui, N.; Ageitos, L.; Ben Alaya, A.; Gharbi, D.; Abid, I.; Rashed Alothman, M.; Limam, F.; et al. Datura stramonium Flowers as a Potential Natural Resource of Bioactive Molecules: Identification of Anti-Inflammatory Agents and Molecular Docking Analysis. Molecules 2023, 28, 5195. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Ora, A.; Häkkinen, S.T.; Ritala, A.; Räisänen, R.; Kallioinen-Mänttäri, M.; Melin, K. Innovative extraction technologies of bioactive compounds from plant by-products for textile colorants and antimicrobial agents. Biomass Convers. Biorefinery 2023, 14, 24973–25002. [Google Scholar] [CrossRef]
- Ristivojević, P.; Krstić Ristivojević, M.; Stanković, D.; Cvijetić, I. Advances in Extracting Bioactive Compounds from Food and Agricultural Waste and By-Products Using Natural Deep Eutectic Solvents: A Circular Economy Perspective. Molecules 2024, 29, 4717. [Google Scholar] [CrossRef]
- López-Hortas, L.; Rodríguez, P.; Díaz-Reinoso, B.; Gaspar, M.C.; de Sousa, H.C.; Braga, M.E.M.; Domínguez, H. Supercritical fluid extraction as a suitable technology to recover bioactive compounds from flowers. J. Supercrit. Fluids 2022, 188, 105652. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Mali, P.S.; Kumar, P. Optimization of microwave assisted extraction of bioactive compounds from black bean waste and evaluation of its antioxidant and antidiabetic potential in vitro. Food Chem. Adv. 2023, 3, 100543. [Google Scholar] [CrossRef]
- Puzeryte, V.; Martusevice, P.; Sousa, S.; Balciunaitiene, A.; Viskelis, J.; Gomes, A.M.; Viskelis, P.; Cesoniene, L.; Urbonaviciene, D. Optimization of Enzyme-Assisted Extraction of Bioactive Compounds from Sea Buckthorn (Hippophae rhamnoides L.) Leaves: Evaluation of Mixed-Culture Fermentation. Microorganisms 2023, 11, 2180. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Carpena, M.; Barciela, P.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Pressurized Liquid Extraction for the Recovery of Bioactive Compounds from Seaweeds for Food Industry Application: A Review. Antioxidants 2023, 12, 612. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep eutectic solvents for the extraction of bioactive compounds from natural sources and agricultural by-products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Aili, Q.; Cui, D.; Li, Y.; Zhige, W.; Yongping, W.; Minfen, Y.; Dongbin, L.; Xiao, R.; Qiang, W. Composing functional food from agro-forest wastes: Selectively extracting bioactive compounds using supercritical fluid extraction. Food Chem. 2024, 455, 139848. [Google Scholar] [CrossRef]
- Bin Mokaizh, A.A.; Nour, A.H.; Kerboua, K. Ultrasonic-assisted extraction to enhance the recovery of bioactive phenolic compounds from Commiphora gileadensis leaves. Ultrason. Sonochem. 2024, 105, 106852. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Ferrarese, I.; Carmo, N.; Santos, D.; Rizzo, F.; Gargari, G.; Bertoli, N.; Gobbi, E.; Perosa, A.; Selva, M.; et al. Sustainable Extraction of Bioactive Compounds and Nutrients from Agri-Food Wastes: Potential Reutilization of Berry, Honey, and Chicory Byproducts. Appl. Sci. 2024, 14, 10785. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Celano, R.; Campone, L.; Rastrelli, L. Critical analysis of green extraction techniques used for botanicals: Trends, priorities, and optimization strategies-A review. TrAC—Trends Anal. Chem. 2024, 173, 117627. [Google Scholar] [CrossRef]
- Al Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef]
- Dashtian, K.; Kamalabadi, M.; Ghoorchian, A.; Ganjali, M.R.; Rahimi-Nasrabadi, M. Integrated supercritical fluid extraction of essential oils. J. Chromatogr. A 2024, 1733, 465240. [Google Scholar] [CrossRef]
- Mora, J.J.; Tavares, H.M.; Curbelo, R.; Dellacassa, E.; Cassel, E.; Apel, M.A.; von Poser, G.L.; Vargas, R.M.F. Supercritical fluid extraction of coumarins and flavonoids from citrus peel. J. Supercrit. Fluids 2025, 215, 106396. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical fluid extraction of seed oils—A short review of current trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Buszewski, B.; Rafińska, K.; Cvetanović, A.; Walczak, J.; Krakowska, A.; Rudnicka, J.; Zeković, Z. Phytochemical analysis and biological activity of Lupinus luteus seeds extracts obtained by supercritical fluid extraction. Phytochem. Lett. 2019, 30, 338–348. [Google Scholar] [CrossRef]
- Matinfard, S.; Tavakolipour, H.; Sodeifian, G.; Sajadian, S.A.; Kalvandi, R. Supercritical CO2 green solvent extraction of Nepeta crispa: Evaluation of process optimization, chemical analysis, and biological activity. J. Supercrit. Fluids 2024, 216, 106451. [Google Scholar] [CrossRef]
- Getachew, A.T.; Jacobsen, C.; Sørensen, A.-D.M. Supercritical CO2 for efficient extraction of high-quality starfish (Asterias rubens) oil. J. Supercrit. Fluids 2024, 206, 106161. [Google Scholar] [CrossRef]
- Kessler, J.C.; Martins, I.M.; Manrique, Y.A.; Rodrigues, A.E.; Barreiro, M.F.; Dias, M.M. Advancements in conventional and supercritical CO2 extraction of Moringa oleifera bioactives for cosmetic applications: A review. J. Supercrit. Fluids 2024, 214, 106388. [Google Scholar] [CrossRef]
- Gallon, R.; Draszewski, C.P.; Santos, J.A.A.; Wagner, R.; Brondani, M.; Zabot, G.L.; Tres, M.V.; Hoffmann, R.; Castilhos, F.; Abaide, E.R.; et al. Obtaining oil, fermentable sugars, and platform chemicals from Butia odorata seed using supercritical fluid extraction and subcritical water hydrolysis. J. Supercrit. Fluids 2023, 203, 106062. [Google Scholar] [CrossRef]
- Tzima, S.; Georgiopoulou, I.; Louli, V.; Magoulas, K. Recent Advances in Supercritical CO2 Extraction of Pigments, Lipids and Bioactive Compounds from Microalgae. Molecules 2023, 28, 1410. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, S.; Wang, H.; Zhang, M. Supercritical CO2 fluid extraction, physicochemical properties, antioxidant activities and hypoglycemic activity of polysaccharides derived from fallen Ginkgo leaves. Food Biosci. 2021, 42, 101153. [Google Scholar] [CrossRef]
- Tamkutė, L.; Liepuoniūtė, R.; Pukalskienė, M.; Venskutonis, P.R. Recovery of valuable lipophilic and polyphenolic fractions from cranberry pomace by consecutive supercritical CO2 and pressurized liquid extraction. J. Supercrit. Fluids 2020, 159, 104755. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Carius, B.; Simões, M.M.Q.; Portugal, I.; Saraiva, J.; Silva, C.M. Supercritical CO2 extraction of V. vinifera leaves: Influence of cosolvents and particle size on removal kinetics and selectivity to target compounds. J. Supercrit. Fluids 2020, 165, 104959. [Google Scholar] [CrossRef]
- Fetzer, D.E.L.; Kanda, L.R.S.; Xavier, L.A.; da Cruz, P.N.; Errico, M.; Corazza, M.L. Lipids and coumarin extraction from cumaru seeds (Dipteryx odorata) using sequential supercritical CO2+solvent and pressurized ethanol. J. Supercrit. Fluids 2022, 188, 105688. [Google Scholar] [CrossRef]
- Vinitha, U.G.; Sathasivam, R.; Muthuraman, M.S.; Park, S.U. Intensification of supercritical fluid in the extraction of flavonoids: A comprehensive review. Physiol. Mol. Plant Pathol. 2022, 118, 101815. [Google Scholar] [CrossRef]
- Do Dat, T.; Hai, N.D.; Nam, N.T.H.; Thanh, N.M.; Huyen, N.T.T.; Duong, L.T.T.; Nam, H.M.; Hieu, N.H. Flavonoid content and antifungal activity of Celastrus hindsii leaf extract obtained by supercritical carbon dioxide using ethanol as co-solvent. Biocatal. Agric. Biotechnol. 2023, 52, 102824. [Google Scholar] [CrossRef]
- Correa, M.d.S.; Boschen, N.L.; Rodrigues, P.R.P.; Corazza, M.L.; de Paula Scheer, A.; Ribani, R.H. Supercritical CO2 with co-solvent extraction of blackberry (Rubus spp. Xavante cultivar) seeds. J. Supercrit. Fluids 2022, 189, 105702. [Google Scholar] [CrossRef]
- Álvarez, S.A.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Cano-Dolado, M.P.; Ibáñez, E.; Cifuentes, A.; Pérez-Martínez, J.D.; Moreno-Jiménez, M.R.; González-Laredo, R.F. Pressurized liquid extraction of oak leaf polyphenols: Solvent selection via Hansen parameters, antioxidant evaluation and monoamine-oxidase-a inhibition analysis. Food Chem. 2025, 463, 141212. [Google Scholar] [CrossRef] [PubMed]
- Echenique, J.V.F.; Alvarez-Rivera, G.; Luna, V.M.A.; da Cruz Antonio, A.F.V.; Mazalli, M.R.; Ibañez, E.; Cifuentes, A.; Oliveira, A.L. de Pressurized liquid extraction with ethanol in an intermittent process for rice bran oil: Evaluation of process variables on the content of β-sitosterol and phenolic compounds, antioxidant capacity, acetylcholinesterase inhibitory activity, and oil quality. LWT 2024, 207, 116650. [Google Scholar] [CrossRef]
- Attard, T.M.; Hunt, A.J. Introduction to High-pressure Solvent Systems. In Supercritical and Other High-Pressure Solvent Systems; Hunt, A.J., Attard, T.M., Eds.; Royal Society of Chemistry: London, UK, 2018; Chapter 1; pp. 1–13. [Google Scholar]
- Barp, L.; Višnjevec, A.M.; Moret, S. Pressurized Liquid Extraction: A Powerful Tool to Implement Extraction and Purification of Food Contaminants. Foods 2023, 12, 2017. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized Liquid Extraction. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. [Google Scholar]
- Dos Santos, L.C.; De-Souza-Ribeiro, M.M.; Rodrigues, K.P.; Sanches, V.L.; Rostagno, M.A.; Martínez, J.; Viganó, J. Improving pressurized liquid extraction of passion fruit (P. edulis) bagasse: Exploring the effects of high-temperature, particle size, and extraction bed dimensions. Sustain. Chem. Pharm. 2024, 41, 101686. [Google Scholar] [CrossRef]
- de OX Machado, T.; Portugal, I.; Kodel, H.d.A.C.; Fathi, A.; Fathi, F.; Oliveira, M.B.P.P.; Dariva, C.; Souto, E.B. Pressurized liquid extraction as an innovative high-yield greener technique for phenolic compounds recovery from grape pomace. Sustain. Chem. Pharm. 2024, 40, 101635. [Google Scholar] [CrossRef]
- Višnjevec, A.M.; Barp, L.; Lucci, P.; Moret, S. Pressurized liquid extraction for the determination of bioactive compounds in plants with emphasis on phenolics. TrAC—Trends Anal. Chem. 2024, 173, 117620. [Google Scholar] [CrossRef]
- Ballesteros-Vivas, D.; Ortega-Barbosa, J.P.; del Pilar Sánchez-Camargo, A.; Rodríguez-Varela, L.I.; Parada-Alfonso, F. Pressurized Liquid Extraction of Bioactives. In Comprehensive Foodomics; Cifuentes, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 754–770. [Google Scholar]
- Raut, P.; Bhosle, D.; Janghel, A.; Deo, S.; Verma, C.; Kumar, S.S.; Agrawal, M.; Amit, N.; Sharma, M.; Giri, T.; et al. Emerging Pressurized Liquid Extraction (PLE) Techniques as an Innovative Green Technologies for the Effective Extraction of the Active Phytopharmaceuticals. Res. J. Pharm. Technol. 2015, 8, 800. [Google Scholar] [CrossRef]
- Kitryte, V.; Laurinavičiene, A.; Syrpas, M.; Pukalskas, A.; Venskutonis, P.R. Modeling and optimization of supercritical carbon dioxide extraction for isolation of valuable lipophilic constituents from elderberry (Sambucus nigra L.) pomace. J. CO2 Util. 2020, 35, 225–235. [Google Scholar] [CrossRef]
- Scopel, E.; dos Santos, L.C.; Bofinger, M.R.; Martínez, J.; Rezende, C.A. Green extractions to obtain value-added elephant grass co-products in an ethanol biorefinery. J. Clean. Prod. 2020, 274, 122769. [Google Scholar] [CrossRef]
- Chipaca-Domingos, H.S.; Ferreres, F.; Fornari, T.; Gil-Izquierdo, A.; Pessela, B.C.; Villanueva-Bermejo, D. Pressurized Liquid Extraction for the Production of Extracts with Antioxidant Activity from Borututu (Cochlospermum angolense Welw.). Foods 2023, 12, 1186. [Google Scholar] [CrossRef]
- Fraguela-Meissimilly, H.; Bastías-Monte, J.M.; Vergara, C.; Ortiz-Viedma, J.; Lemus-Mondaca, R.; Flores, M.; Toledo-Merma, P.; Alcázar-Alay, S.; Gallón-Bedoya, M. New Trends in Supercritical Fluid Technology and Pressurized Liquids for the Extraction and Recovery of Bioactive Compounds from Agro-Industrial and Marine Food Waste. Molecules 2023, 28, 4421. [Google Scholar] [CrossRef] [PubMed]
- Pilařová, V.; Al Hamimi, S.; Cunico, L.P.; Nováková, L.; Turner, C. Extending the design space in solvent extraction—From supercritical fluids to pressurized liquids using carbon dioxide, ethanol, ethyl lactate, and water in a wide range of proportions. Green Chem. 2019, 21, 5427–5436. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Grabanski, C.B.; Martin, E.; Miller, D.J. Comparisons of Soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: Recovery, selectivity and effects on sample matrix. J. Chromatogr. A 2000, 892, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nie, X.; Wang, J.; Zhao, Z.; Wang, Z.; Ju, F. Visualizing the distribution of flavonoids in litchi (Litchi chinenis) seeds through matrix-assisted laser desorption/ionization mass spectrometry imaging. Front. Plant Sci. 2023, 14, 1144449. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Potencial terapêutico de compostos fenólicos em plantas medicinais—Produtos naturais de saúde para a saúde humana. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Oubannin, S.; Bijla, L.; Nid, A.M.; Ibourki, M.; El Kharrassi, Y.; Devkota, K.; Bouyahya, A.; Maggi, F.; Caprioli, G.; Sakar, E.H.; et al. Recent advances in the extraction of bioactive compounds from plant matrices and their use as potential antioxidants for vegetable oils enrichment. J. Food Compos. Anal. 2024, 128, 105995. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. African 2023, 19, e01585. [Google Scholar] [CrossRef]
- Yıldırım, M.; Erşatır, M.; Poyraz, S.; Amangeldinova, M.; Kudrina, N.O.; Terletskaya, N.V. Green Extraction of Plant Materials Using Supercritical CO2: Insights into Methods, Analysis, and Bioactivity. Plants 2024, 13, 2295. [Google Scholar] [CrossRef]
- Lemes, A.C.; Egea, M.B.; de Oliveira Filho, J.G.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds From Agro-industrial By-products: A Review. Front. Bioeng. Biotechnol. 2022, 9, 802543. [Google Scholar] [CrossRef]
- Sławińska, N.; Olas, B. Selected Seeds as Sources of Bioactive Compounds with Diverse Biological Activities. Nutrients 2023, 15, 187. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Aguilar-Hernández, G.; Zepeda-Vallejo, L.G.; García-Magaña, M.D.L.; Vivar-Vera, M.D.L.Á.; Pérez-Larios, A.; Girón-Pérez, M.I.; Coria-Tellez, A.V.; Rodríguez-Aguayo, C.; Montalvo-González, E. Extraction of alkaloids using ultrasound from pulp and by-products of soursop fruit (Annona muricata L.). Appl. Sci. 2020, 10, 4869. [Google Scholar] [CrossRef]
- Nilmat, K.; Hunsub, P.; Ngamprasertsith, S.; Sakdasri, W.; Karnchanatat, A.; Sawangkeaw, R. Effects of Defatting Pretreatment on Polysaccharide Extraction from Rambutan Seeds Using Subcritical Water: Optimization Using the Desirability Approach. Foods 2024, 13, 1967. [Google Scholar] [CrossRef] [PubMed]
- Kultys, E.; Kurek, M.A. Green Extraction of Carotenoids from Fruit and Vegetable Byproducts: A Review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef]
- Herzyk, F.; Piłakowska-Pietras, D.; Korzeniowska, M. Supercritical Extraction Techniques for Obtaining Biologically Active Substances from a Variety of Plant Byproducts. Foods 2024, 13, 1713. [Google Scholar] [CrossRef]
- Akyüz, A.; Tekin, İ.; Aksoy, Z.; Ersus, S. Plant Protein Resources, Novel Extraction and Precipitation Methods: A Review. J. Food Process Eng. 2024, 47, e14758. [Google Scholar] [CrossRef]
- Usman, M.; Nakagawa, M.; Cheng, S. Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes 2023, 11, 3444. [Google Scholar] [CrossRef]
- Ghafoor, K.; Sarker, M.Z.I.; Al-Juhaimi, F.Y.; Babiker, E.E.; Alkaltham, M.S.; Almubarak, A.K. Extraction and Evaluation of Bioactive Compounds from Date (Phoenix dactylifera) Seed Using Supercritical and Subcritical CO2 Techniques. Foods 2022, 11, 1806. [Google Scholar] [CrossRef]
- Drăghici-Popa, A.M.; Boscornea, A.C.; Brezoiu, A.M.; Tomas, Ș.T.; Pârvulescu, O.C.; Stan, R. Effects of Extraction Process Factors on the Composition and Antioxidant Activity of Blackthorn (Prunus spinosa L.) Fruit Extracts. Antioxidants 2023, 12, 1897. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q. Ultrasonic-Assisted Efficient Extraction of Coumarins from Peucedanum decursivum (Miq.) Maxim Using Deep Eutectic Solvents Combined with an Enzyme Pretreatment. Molecules 2022, 27, 5715. [Google Scholar] [CrossRef]
- Scopel, J.M.; Medeiros-Neves, B.; Teixeira, H.F.; Brazil, N.T.; Bordignon, S.A.L.; Diz, F.M.; Morrone, F.B.; Almeida, R.N.; Cassel, E.; von Poser, G.L.; et al. Supercritical Carbon Dioxide Extraction of Coumarins from the Aerial Parts of Pterocaulon polystachyum. Molecules 2024, 29, 2741. [Google Scholar] [CrossRef] [PubMed]
- Perveen, F.; Al-Joufi, F.A.; Zeb, R.; Qamar, N.; Jabbar, A.; Naveed Umar, M.; Zahoor, M. Comparative antioxidant and antimicrobial activities of Nigella sativa flower, leaf, stem, and seed derived silver nanoparticles. Results Chem. 2024, 11, 101808. [Google Scholar] [CrossRef]
- Minh Nguyen, H.; Nguyen, K.P.; Le, A.T.P.; Nguyen, N.H.T.; Vu-Huynh, L.K.; Le, C.K.T.; Delpe Acharige, A.; Hull, K.; Romo, D. Antioxidant, anti-tyrosinase, hepatoprotective, and anti-inflammatory potential in flowers and seeds of Ochna integerrima (Lour.) Merr. Nat. Prod. Res. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Yılmaz, F.G.; Uslu, N.; Kulluk, D.A.; Dursun, N.; Yılmaz, H. Determination of bioactive compounds, phenolic contents, fatty acid and biogenic element profiles of the seeds of sunflower (Helianthus annuus L.) genotypes. Food Humanit. 2024, 2, 100222. [Google Scholar] [CrossRef]
- Machado, T.O.X.; de AC Kodel, H.; Alves dos Santos, F.; dos Santos Lima, M.; Costa, A.S.G.; Oliveira, M.B.P.P.; Dariva, C.; dos Santos Estevam, C.; Fathi, F.; Souto, E.B. Cellulase-assisted extraction followed by pressurized liquid extraction for enhanced recovery of phenolic compounds from ‘BRS Violeta’ grape pomace. Sep. Purif. Technol. 2025, 354, 129218. [Google Scholar] [CrossRef]
- Vatansever, S.; Xu, M.; Magallanes-López, A.; Chen, B.; Hall, C. Supercritical Carbon Dioxide + Ethanol Extraction to Improve Organoleptic Attributes of Pea Flour with Applications of Sensory Evaluation, HS-SPME-GC, and GC-Olfactory. Processes 2021, 9, 489. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Khantham, C.; Sringarm, K.; Sommano, S.; Jantrawut, P. Depigmented Centella asiatica Extraction by Pretreated with Supercritical Carbon Dioxide Fluid for Wound Healing Application. Processes 2020, 8, 277. [Google Scholar] [CrossRef]
- Rantaša, M.; Slaček, G.; Knez, Ž.; Knez Marevci, M. Supercritical fluid extraction of cannabinoids and their analysis by liquid chromatography and supercritical fluid chromatography: A short review. J. CO2 Util. 2024, 86, 102907. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Ray, A.; Dubey, K.K.; Marathe, S.J.; Singhal, R. Supercritical fluid extraction of bioactives from fruit waste and its therapeutic potential. Food Biosci. 2023, 52, 102418. [Google Scholar] [CrossRef]
- Pilařová, V.; Plachká, K.; Herbsová, D.; Kosturko, Š.; Svec, F.; Nováková, L. Comprehensive two-step supercritical fluid extraction for green isolation of volatiles and phenolic compounds from plant material. Green Chem. 2024, 26, 6480–6489. [Google Scholar] [CrossRef]
- Herbst, G.; Hamerski, F.; Errico, M.L.; Corazza, M. Pressurized liquid extraction of brewer’s spent grain: Kinetics and crude extracts characterization. J. Ind. Eng. Chem. 2021, 102, 370–383. [Google Scholar] [CrossRef]
- Araujo, M.N.; dos Santos, K.C.; do Carmo Diniz, N.; de Carvalho, J.C.; Corazza, M.L. A biorefinery approach for spent coffee grounds valorization using pressurized fluid extraction to produce oil and bioproducts: A systematic review. Bioresour. Technol. Reports 2022, 18, 101013. [Google Scholar] [CrossRef]
- Rodriguez, J.M.F.; Corazza, M.L.; Kruger, R.L.; Khalil, N.M.; de Campos, D.; da Silva, V.R. Pressurized liquid extraction as a strategy to recover bioactive compounds from yerba mate (Ilex paraguariensis St.-Hil.) processing waste. J. Supercrit. Fluids 2023, 203, 106088. [Google Scholar] [CrossRef]
- Vazquez-Roig, P.; Picó, Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. TrAC Trends Anal. Chem. 2015, 71, 55–64. [Google Scholar] [CrossRef]
- Wonggo, D.; Anwar, C.; Dotulong, V.; Reo, A.; Taher, N.; Syahputra, R.A.; Nurkolis, F.; Tallei, T.E.; Kim, B.; Tsopmo, A. Subcritical water extraction of mangrove fruit extract (Sonneratia alba) and its antioxidant activity, network pharmacology, and molecular connectivity studies. J. Agric. Food Res. 2024, 18, 101334. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Jurinjak Tušek, A.; Šamec, D.; Šalić, A. Modern Techniques for Flavonoid Extraction—To Optimize or Not to Optimize? Appl. Sci. 2022, 12, 11865. [Google Scholar] [CrossRef]
- Čižmek, L.; Kralj, M.B.; Čož-rakovac, R.; Mazur, D.; Ul’yanovskii, N.; Likon, M.; Trebše, P. Supercritical carbon dioxide extraction of four medicinal mediterranean plants: Investigation of chemical composition and antioxidant activity. Molecules 2021, 26, 5697. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Pellicanò, T.M.; Sicari, V.; Loizzo, M.R.; Leporini, M.; Falco, T.; Poiana, M. Optimizing the supercritical fluid extraction process of bioactive compounds from processed tomato skin by-products. Food Sci. Technol. 2020, 40, 692–697. [Google Scholar] [CrossRef]
- Avramescu, S.M.; Fierascu, I.; Fierascu, R.C.; Cudalbeanu, M. Pressurized liquid extraction of natural products. In Extraction of Natural Products from Agro-Industrial Wastes; Bhawani, S.A., Khan, A., Ahmad, F.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 53–78. [Google Scholar]
- Kauffmann, A.C.; Castro, V.S. Phenolic Compounds in Bacterial Inactivation: A Perspective from Brazil. Antibiotics 2023, 12, 645. [Google Scholar] [CrossRef] [PubMed]
- Oro, C.E.D.; Paroul, N.; Mignoni, M.L.; Zabot, G.L.; Backes, G.T.; Dallago, R.M.; Tres, M.V. Microencapsulation of Brazilian Cherokee blackberry extract by freeze-drying using maltodextrin, gum Arabic, and pectin as carrier materials. Food Sci. Technol. Int. 2023, 29, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.G.; Mtz-Enríquez, A.I.; Díaz-Jiménez, L.; Ramos-González, R.; Valdés, J.A.A.; Flores, M.E.C.; Martínez, J.L.H.; Ilyina, A. Production of fatty acid methyl esters and bioactive compounds from citrus wax. Waste Manag. 2020, 102, 48–55. [Google Scholar] [CrossRef]
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of pomegranate peel polyphenols encapsulated in orange juice industry by-product and their incorporation in cookies. Food Chem. 2020, 310, 125849. [Google Scholar] [CrossRef]
- García-Pérez, P.; Losada-Barreiro, S.; Bravo-Díaz, C.; Gallego, P.P. Exploring the use of bryophyllum as natural source of bioactive compounds with antioxidant activity to prevent lipid oxidation of fish oil-in-water emulsions. Plants 2020, 9, 1012. [Google Scholar] [CrossRef]
- Niazmand, R.; Jahani, M.; Kalantarian, S. Treatment of olive processing wastewater by electrocoagulation: An effectiveness and economic assessment. J. Environ. Manage. 2019, 248, 109262. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kim, H.Y.; Kim, J.; Son, J.E. Quantitative Analysis of UV-B Radiation Interception and Bioactive Compound Contents in Kale by Leaf Position According to Growth Progress. Front. Plant Sci. 2021, 12, 667456. [Google Scholar] [CrossRef]
- Koch, W.; Zagórska, J.; Michalak-Tomczyk, M.; Karav, S.; Wawruszak, A. Plant Phenolics in the Prevention and Therapy of Acne: A Comprehensive Review. Molecules 2024, 29, 4234. [Google Scholar] [CrossRef]
- Botella, M.Á.; Hernández, V.; Mestre, T.; Hellín, P.; García-Legaz, M.F.; Rivero, R.M.; Martínez, V.; Fenoll, J.; Flores, P. Bioactive compounds of tomato fruit in response to salinity, heat and their combination. Agric. 2021, 11, 534. [Google Scholar] [CrossRef]
- Cruz, P.N.; Pereira, T.C.S.; Guindani, C.; Oliveira, D.A.; Rossi, M.J.; Ferreira, S.R.S. Antioxidant and antibacterial potential of butia (Butia catarinensis) seed extracts obtained by supercritical fluid extraction. J. Supercrit. Fluids 2017, 119, 229–237. [Google Scholar] [CrossRef]
- Proetto, I.; Pesce, F.; Arena, E.; Grasso, A.; Parafati, L.; Fallico, B.; Palmeri, R. Use of vegetable flours obtained from artichoke by-products as a functional ingredient in gluten-free bread formulations. Int. J. Gastron. Food Sci. 2024, 38, 101015. [Google Scholar] [CrossRef]
- Voynikov, Y.; Balabanova, V.; Gevrenova, R.; Zheleva-Dimitrova, D. Chemophenetic Approach to Selected Senecioneae Species, Combining Morphometric and UHPLC-HRMS Analyses. Plants 2023, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Sena, J.d.S.; Rodrigues, S.A.; Sakumoto, K.; Inumaro, R.S.; González-Maldonado, P.; Mendez-Scolari, E.; Piau, R.; Gonçalves, D.D.; Mandim, F.; Vaz, J.; et al. Antioxidant Activity, Antiproliferative Activity, Antiviral Activity, NO Production Inhibition, and Chemical Composition of Essential Oils and Crude Extracts of Leaves, Flower Buds, and Stems of Tetradenia riparia. Pharmaceuticals 2024, 17, 888. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, R.M.; Cârâc, G.; Bahrim, G.; Camurlu, P. Utilization of enzyme extract self-encapsulated within polypyrrole in sensitive detection of catechol. Enzym. Microb. Technol. 2019, 128, 34–39. [Google Scholar] [CrossRef]
- Sobolewska, D.; Michalska, K.; Wróbel-Biedrawa, D.; Grabowska, K.; Owczarek-Januszkiewicz, A.; Olszewska, M.A.; Podolak, I. The Genus Cuphea P. Browne as a Source of Biologically Active Phytochemicals for Pharmaceutical Application and Beyond—A Review. Int. J. Mol. Sci. 2023, 24, 6614. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Sadaka, C.; Generalić Mekinić, I.; Garzoli, S.; Švarc-Gajić, J.; Rodrigues, F.; Morais, S.; Moreira, M.M.; Ferreira, E.; Spigno, G.; et al. The Chemical Variability, Nutraceutical Value, and Food-Industry and Cosmetic Applications of Citrus Plants: A Critical Review. Antioxidants 2023, 12, 481. [Google Scholar] [CrossRef]
- Afifi, S.M.; Kabbash, E.M.; Berger, R.G.; Krings, U.; Esatbeyoglu, T. Comparative Untargeted Metabolic Profiling of Different Parts of Citrus sinensis Fruits via Liquid Chromatography–Mass Spectrometry Coupled with Multivariate Data Analyses to Unravel Authenticity. Foods 2023, 12, 579. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, D.; Wang, H.; Zhu, Y.; Bi, S.; Liu, Y.; Cheng, X.; Chen, X. Effect and mechanism of fish scale extract natural hydrogel on skin protection and cell damage repair after UV irradiation. Colloids Surf. B Biointerfaces 2023, 225, 113281. [Google Scholar] [CrossRef]
- He, Y.; Yang, Y.; Chi, W.; Hu, S.; Chen, G.; Wang, Q.; Cheng, K.; Guo, C.; Liu, T.; Xia, B. Biogeochemical cycling in paddy soils controls antimony transformation: Roles of iron (oxyhydr)oxides, organic matter and sulfate. J. Hazard. Mater. 2024, 464, 132979. [Google Scholar] [CrossRef]
- Van de Velde, F.; Esposito, D.; Grace, M.H.; Pirovani, M.E.; Lila, M.A. Anti-inflammatory and wound healing properties of polyphenolic extracts from strawberry and blackberry fruits. Food Res. Int. 2019, 121, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Z.N.; Azeez, H.A.; Salih, T. Antioxidant Activity of the Prunus mahaleb Seed Oil Extracts Using n-Hexane and Petroleum Ether Solvents: In Silico and In Vitro Studies. Appl. Sci. 2023, 13, 7430. [Google Scholar] [CrossRef]
- Donadio, G.; Bellone, M.L.; Mensitieri, F.; Parisi, V.; Santoro, V.; Vitiello, M.; Dal Piaz, F.; De Tommasi, N. Characterization of Health Beneficial Components in Discarded Leaves of Three Escarole (Cichorium endivia L.) Cultivar and Study of Their Antioxidant and Anti-Inflammatory Activities. Antioxidants 2023, 12, 1402. [Google Scholar] [CrossRef]
- Chen, Y.; Cheong, L.Z.; Zhao, J.; Panpipat, W.; Wang, Z.; Li, Y.; Lu, C.; Zhou, J.; Su, X. Lipase-catalyzed selective enrichment of omega-3 polyunsaturated fatty acids in acylglycerols of cod liver and linseed oils: Modeling the binding affinity of lipases and fatty acids. Int. J. Biol. Macromol. 2019, 123, 261–268. [Google Scholar] [CrossRef]
- Bonincontro, G.; Scuderi, S.A.; Marino, A.; Simonetti, G. Synergistic Effect of Plant Compounds in Combination with Conventional Antimicrobials against Biofilm of Staphylococcus aureus, Pseudomonas aeruginosa, and Candida spp. Pharmaceuticals 2023, 16, 1531. [Google Scholar] [CrossRef]
- Gerasimova, E.; Gazizullina, E.; Radosteva, E.; Ivanova, A. Antioxidant and antiradical properties of some examples of flavonoids and coumarins—Potentiometric studies. Chemosensors 2021, 9, 112. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Chang, Y.; Kang, K.; Park, S.J.; Choi, J.C.; Kim, M.K.; Han, J. Experimental and theoretical study of polypropylene: Antioxidant migration with different food simulants and temperatures. J. Food Eng. 2019, 244, 142–149. [Google Scholar] [CrossRef]
- Lasta, H.F.B.; Lentz, L.; Mezzomo, N.; Ferreira, S.R.S. Supercritical CO2 to recover extracts enriched in antioxidant compounds from beetroot aerial parts. Biocatal. Agric. Biotechnol. 2019, 19, 101169. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; Da Mota, R.V.; Cordenunsi, B.R.; Lajolo, F.M. Physico-chemical characterization and bioactive compounds of blackberry fruits (Rubus sp.) grown in Brazil. Cienc. e Tecnol. Aliment. 2008, 28, 702–708. [Google Scholar] [CrossRef]
| Parameter | Extraction Process | Reference | |
|---|---|---|---|
| Supercritical Fluid | Pressurized Liquid | ||
| Temperature (°C) | 40–70 | 60–120 | [17,24,28,48,51,57,58,88] |
| Pressure (MPa) | 10–30 | 5–20 | |
| Vol. ratio solvent/matrix (vol vol−1) | 10–20 | 20–30 | |
| Flow (mL min−1) | 0.1–2.0 | 1.0–10.0 | |
| Time (min) | 5–20 | 30–120 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oro, C.E.D.; Wancura, J.H.C.; Santos, M.S.N.d.; Venquiaruto, L.D.; Dallago, R.M.; Tres, M.V. High-Pressure Extraction Techniques for Efficient Recovery of Flavonoids and Coumarins from Flower Seeds. Processes 2025, 13, 300. https://doi.org/10.3390/pr13020300
Oro CED, Wancura JHC, Santos MSNd, Venquiaruto LD, Dallago RM, Tres MV. High-Pressure Extraction Techniques for Efficient Recovery of Flavonoids and Coumarins from Flower Seeds. Processes. 2025; 13(2):300. https://doi.org/10.3390/pr13020300
Chicago/Turabian StyleOro, Carolina E. Demaman, João H. C. Wancura, Maicon S. N. dos Santos, Luciana D. Venquiaruto, Rogério M. Dallago, and Marcus V. Tres. 2025. "High-Pressure Extraction Techniques for Efficient Recovery of Flavonoids and Coumarins from Flower Seeds" Processes 13, no. 2: 300. https://doi.org/10.3390/pr13020300
APA StyleOro, C. E. D., Wancura, J. H. C., Santos, M. S. N. d., Venquiaruto, L. D., Dallago, R. M., & Tres, M. V. (2025). High-Pressure Extraction Techniques for Efficient Recovery of Flavonoids and Coumarins from Flower Seeds. Processes, 13(2), 300. https://doi.org/10.3390/pr13020300






