Preparation and Performance Research of the Optimal Mix Ratio Based on the Coupling Mechanism of Dust Suppressants
Abstract
1. Introduction
2. Mechanism of Dust Suppressants
2.1. Dust Suppressant Synthesis Mechanism
2.2. The Mechanism of Action of the Dust Suppressant on Dust
2.2.1. Surface Wetting Enhancement Mechanism
2.2.2. Particle Agglomeration and Coalescence Mechanism
- Form electrostatic attraction with nitrogen-containing functional groups on the particle surface.
- Build a hydrogen bond network with oxygen-containing functional groups.
- Solidification of particles through bonding mediated by high dielectric constant water molecules.
2.2.3. Wind Resistance and Moisture Retention Synergistic Mechanism
- Bonding effect: Inducing dust particles to aggregate into larger particle size distributions, enhancing wind erosion resistance.
- Consolidation layer strengthening: After water evaporation, hydroxypropyl methylcellulose builds a three-dimensional network structure through intermolecular hydrogen bonds, significantly enhancing the mechanical strength of the consolidation layer.
- Moisture retention and crack resistance: The above rigid structure effectively inhibits the cracking of the solidified layer, reduces the rate of moisture evaporation, and ensures an extended duration of dust suppression.
3. Experimental Preparation
3.1. Raw Material Selection
3.1.1. Selection of Surfactants
3.1.2. Coagulant Selection
3.1.3. Selection of Water-Retaining Agent
- Glycerol: A colorless, viscous, hygroscopic liquid with excellent moisture retention properties and biocompatibility, which can spontaneously absorb environmental moisture.
- Polyacrylic acid: A water-soluble high-molecular-weight polymer that achieves ultra-high water absorption and retention capacity through a three-dimensional network structure.
- Triethanolamine: A chemically stable alkaline compound that combines moisturizing and surfactant synergistic effects.
3.2. Orthogonal Experimental Design
3.3. Dust Suppressant Performance Determination
3.3.1. Determination of Water Retention Rate
3.3.2. Hardness Measurement
3.3.3. Wind Erosion Rate Measurement
4. Test Results and Analysis
4.1. Orthogonal Test Results
4.2. Orthogonal Experiment Analysis
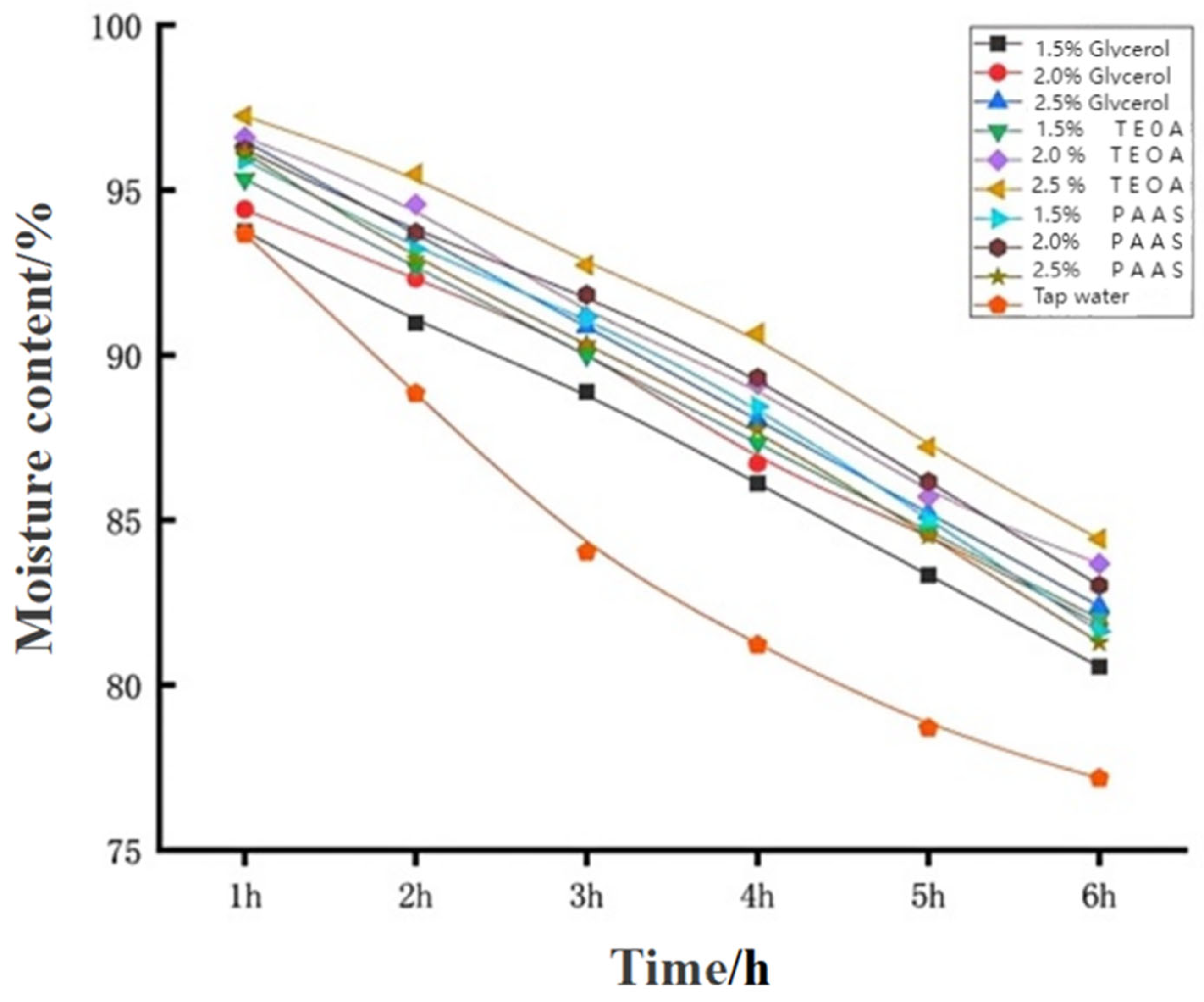
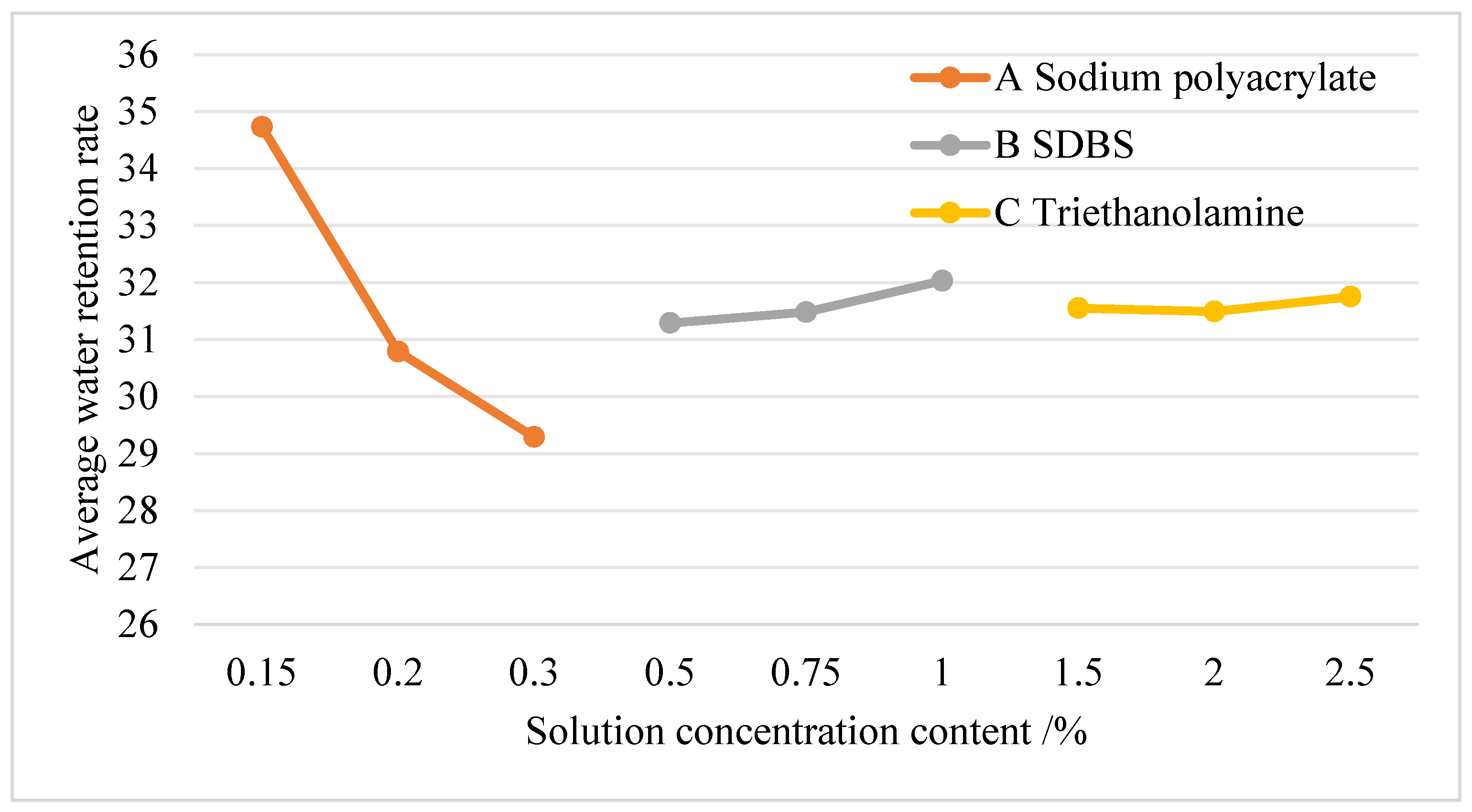
4.2.1. Analysis of Water Retention Rate in the Orthogonal Test of Dust Suppressants
4.2.2. Orthogonal Test Hardness Analysis of Dust Suppressant
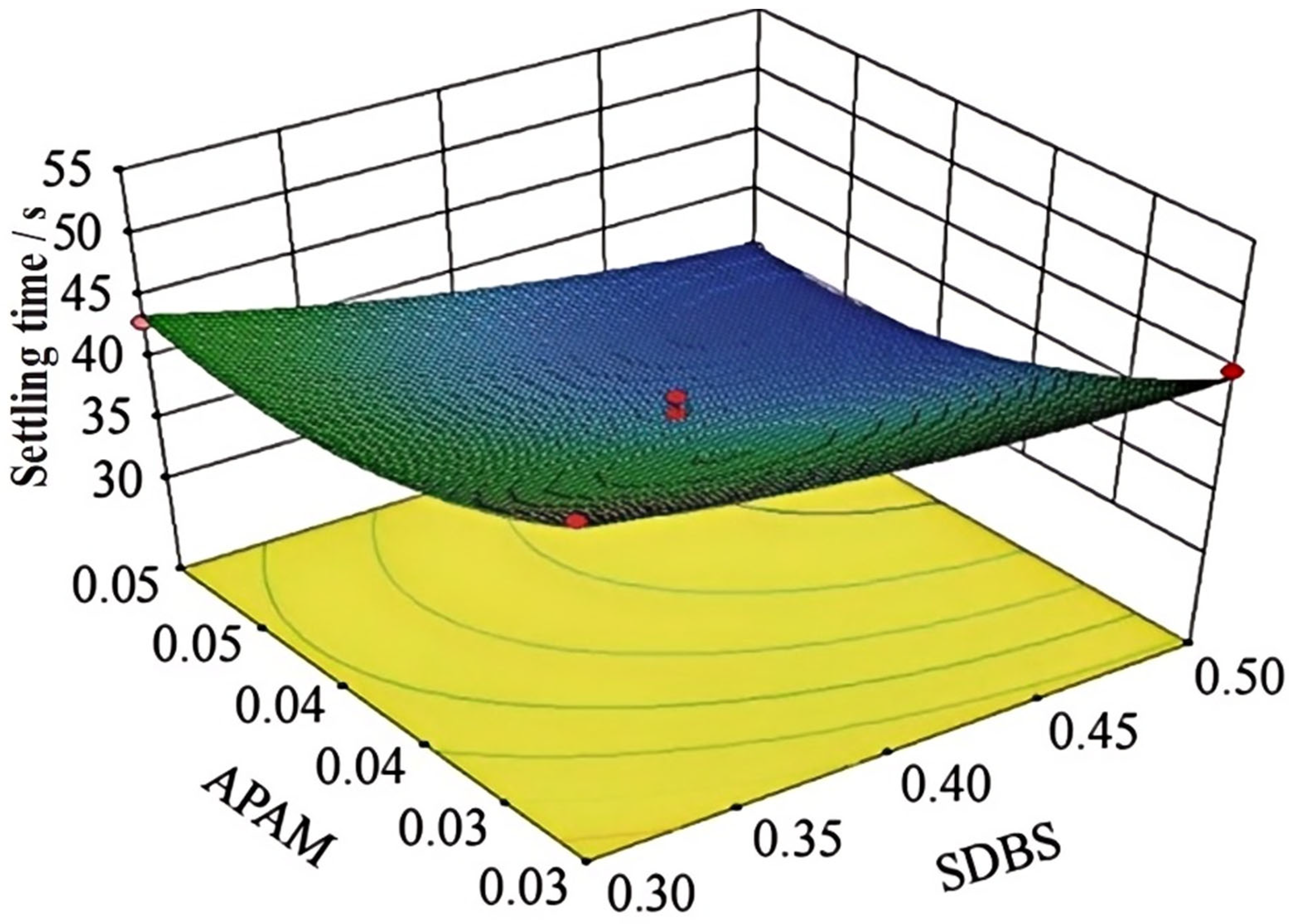
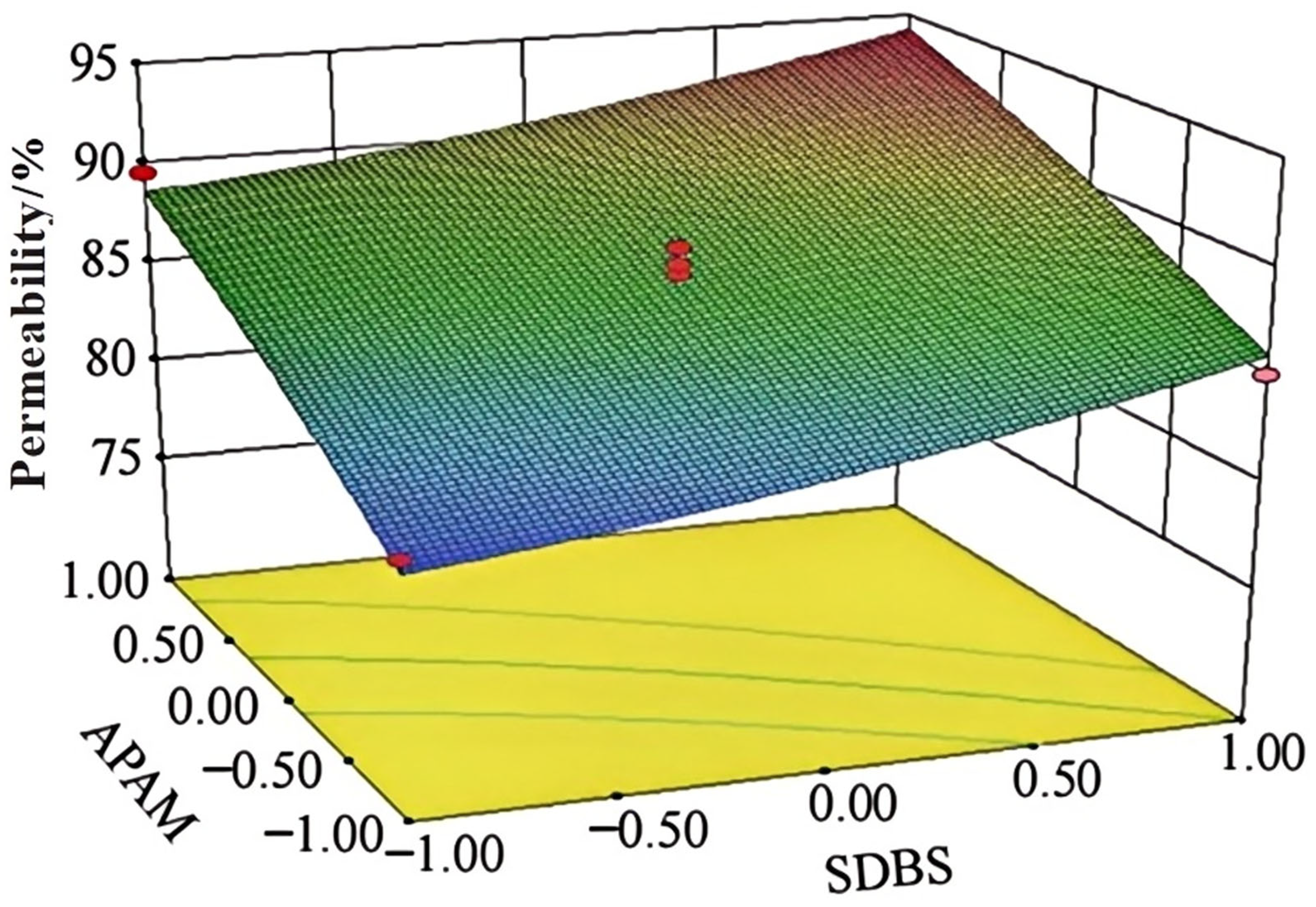
4.2.3. Dust Suppressant Orthogonal Test Wind Erosion Rate Analysis
- Sodium polyacrylate (A)
- 2.
- Sodium dodecylbenzene sulfonate (B)
- 3.
- triethanolamine (C)
5. Conclusions
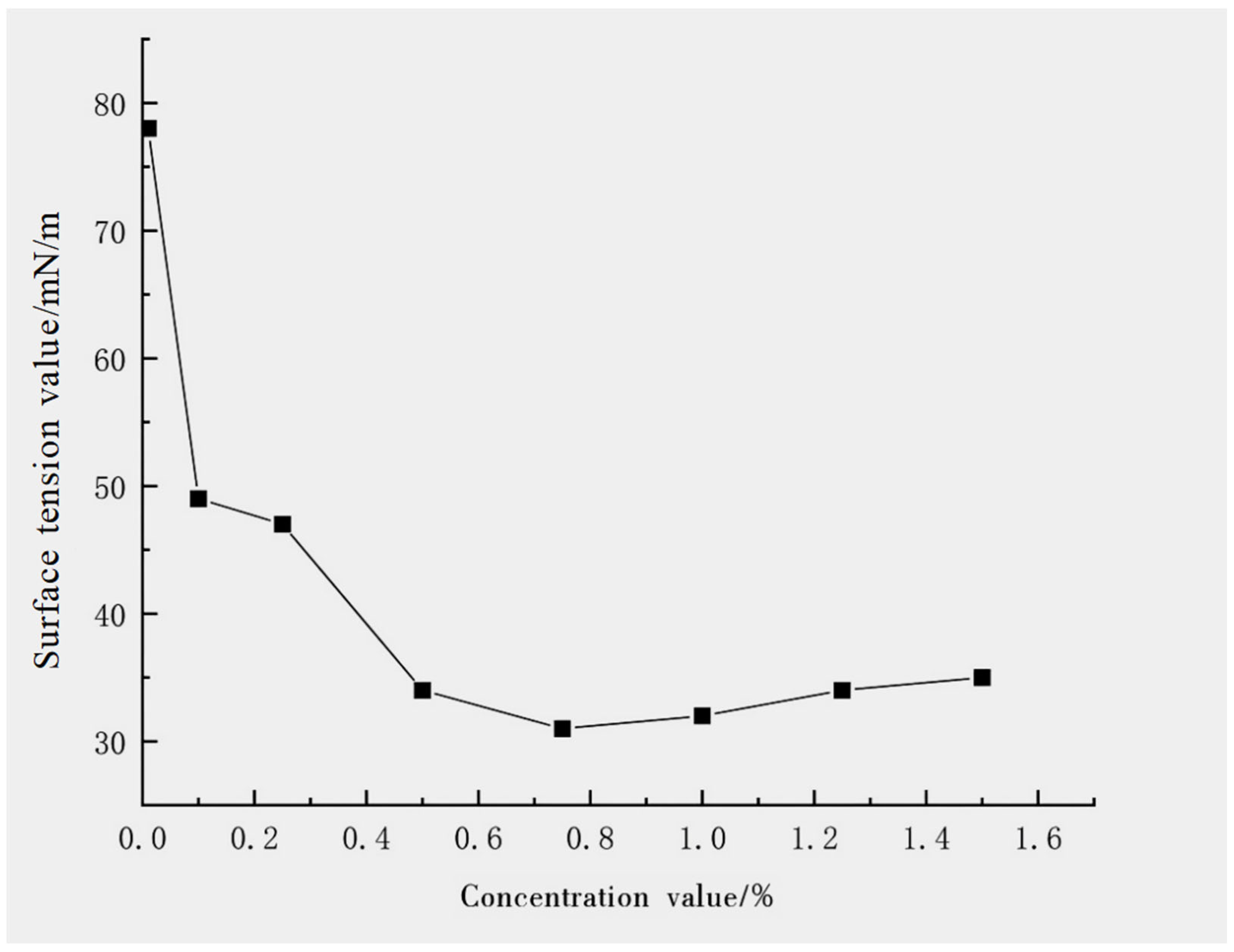
- Propose a new multi-mechanism dust suppression approach based on the synergistic action of surfactants, coagulants, and water-retaining agents, breaking through the limitations of single-function dust suppressants in terms of long-lasting performance and environmental adaptability. The “wetting—coagulation—consolidation” integrated dust suppression system was constructed by the synergy of sodium dodecylbenzene sulfonate to significantly reduce liquid surface tension (up to 27.8 mN/m), sodium polyacrylate to enhance interparticle adhesion and bridging, and triethanolamine to achieve efficient moisture retention.
- The statistical analysis revealed differentiated significance patterns: sodium polyacrylate demonstrated extremely significant effects on water retention (p = 0.003), whereas its influences on crust hardness and wind erosion resistance, while practically observable in range analysis, did not reach statistical significance (p > 0.05). Consequently, the optimal formulation A2B3C3 should be interpreted as providing practically enhanced performance in hardness and wind erosion resistance rather than statistically validated superiority. This distinction between practical optimization and statistical significance should be clearly acknowledged to maintain scientific rigor.
- Molecular mechanism analysis reveals that triethanolamine forms a hydrogen bond network with sodium polyacrylate through hydroxyl and carboxyl groups and forms electrostatic attraction and chemical bonding with oxygen/nitrogen-containing functional groups in the dust, thereby enhancing the stability of the shell layer and its resistance to evaporation. This mechanism provides a theoretical basis and structural biomimetic foundation for the design of green dust suppression materials.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PM 2.5 | Airborne fine particles ≤ 2.5 μm in aerodynamic diameter |
| PM 10 | Airborne fine particles ≤ 10 μm in aerodynamic diameter |
| TEOA | Triethanolamine (tris(2-hydroxyethyl)amine, C6H15NO3), a trihydroxy derivative of triethylamine, exhibits weak basicity from the nitrogen lone pair, enabling salt formation with inorganic and organic acids. |
| PAAS SDBS | Sodium polyacrylate, a polymeric electrolyte with both hydrophilic and hydrophobic groups (chemical formula (C3H3O2Na)n), possesses a molecular weight below 10,000. Sodium dodecylbenzene sulfonate, which is an anionic surfactant with outstanding surface activity and emulsification performance. |
References
- Li, M. Analysis of the Legal Governance Status and Improvement Measures of Smog Pollution Control in China. Nat. Environ. Pollut. Technol. 2016, 15, 123–135. [Google Scholar] [CrossRef]
- Qin, N.; Yu, H.; Ye, Y.; Xie, Y.; Li, X. Study on the influence of combined utilization of air-fog curtain on fully mechanized face. Process Saf. Environ. Prot. 2024, 192, 196–213. [Google Scholar] [CrossRef]
- Shin, H.; Hyun, M.; Jeong, S.; Ryu, H.; Lee, M.G.; Chung, W.; Hong, J.; Kwon, J.-T.; Lee, J.; Kim, Y. A correlation study of road dust pollutants, tire wear particles, air quality, and traffic conditions in the Seoul (South Korea). Atmos. Pollut. Res. 2024, 15, 102309. [Google Scholar] [CrossRef]
- Bierza, K.; Bierza, W. The effect of industrial and urban dust pollution on the ecophysiology and leaf element concentration of Tilia cordata Mill. Environ. Sci. Pollut. Res. 2024, 31, 58413–58429. [Google Scholar] [CrossRef]
- Manzhilevskaya, S. Dust Pollution in Construction Sites in Point-Pattern Housing Development. Buildings 2024, 14, 2991. [Google Scholar] [CrossRef]
- Nie, W.; Jiang, C.; Liu, Q.; Guo, L.; Zhang, H.; Cheng, C.; Zhu, Z. Study of dust pollution control effect based on orthogonal test and CFD numerical simulations. Environ. Sci. Pollut. Res. 2024, 31, 43712–43730. [Google Scholar] [CrossRef]
- Wang, W.; Liu, B.; Tian, Q.; Xu, X.; Peng, Y.; Peng, S. Predicting dust pollution from dry bulk ports in coastal cities: A hybrid approach based on data decomposition and deep learning. Environ. Pollut. 2024, 350, 124053. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Y.; Wang, Y.; Jiang, Z.; Liu, J.; Chen, J. Study on dust pollution law and chemical dust suppression technology of non-hard pavement in urban construction sites. Build. Environ. 2023, 229, 109938. [Google Scholar] [CrossRef]
- Qiu, F.; Zhao, Y.; Wang, Y. Prediction method of dust pollutant diffusion range in building demolition based on Euclidean distance transformation. Int. J. Environ. Technol. Manag. 2023, 26, 250–262. [Google Scholar] [CrossRef]
- Li, M.; Wang, R.; Li, G.; Song, X.; Yang, H.; Lai, H. Comprehensive Chemical Dust Suppressant Performance Evaluation and Optimization Method. Int. J. Environ. Res. Public Health 2022, 19, 5617. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Z.; Wei, Z.; Zhao, J.; Lu, T.; Fu, T.; Tang, S. Combined use of chemical dust suppressant and herbaceous plants for tailings dust control. Environ. Geochem. Health 2024, 46, 329. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, B.; Zhang, Y.; Fan, Y. Optimal Preparation and Performance Study of Eco-Friendly Composite Chemical Dust Suppressants: A Case Study in a Construction Site in Chengdu. Materials 2024, 17, 2346. [Google Scholar] [CrossRef]
- Li, M.; Qiu, L.; Yang, H.; Fei, X. Performance Test and Application Research of New Gemini Wetting Dust Suppressant. IOP Conf. Ser. Earth Environ. Sci. 2021, 804, 042015. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Y.; Yang, Z.; Zhang, J.; Zhang, Y.; Gao, Y.; Shao, Z.; Zhang, L. Study on the physicochemical characteristics and dust suppression performance of new type chemical dust suppressant for copper mine pavement. Environ. Sci. Pollut. Res. 2021, 28, 59640–59651. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Yang, Y.; Huang, H.; Shu, C. Inhibitory effects of three chemical dust suppressants on nitrocellulose dust cloud explosion. AIChE J. 2020, 66, e16888. [Google Scholar] [CrossRef]
- Liao, Q.; Feng, G.; Fan, Y.; Hu, S.; Shao, H.; Huang, Y. Experimental Investigations and Field Applications of Chemical Suppressants for Dust Control in Coal Mines. Adv. Mater. Sci. Eng. 2018, 2018, 6487459. [Google Scholar] [CrossRef]
- Dong, H.; Yu, H.; Xu, R.; Cheng, W.; Ye, Y.; Xie, S.; Zhao, J.; Cheng, Y. Review and prospects of mining chemical dust suppressant: Classification and mechanisms. Environ. Sci. Pollut. Res. 2022, 30, 18–35. [Google Scholar] [CrossRef]
- Liu, Y.; Du, C.; Yi, F.; Cheng, C.; Wang, M. Modified sodium alginate-based three-dimensional network hydrogel dust suppressant: Preparation, characterization, and performance. Int. J. Biol. Macromol. 2024, 274, 133408. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Y.; Yu, S.; Du, J.; Hu, X.; Bai, G.; Wang, Z. Environment-friendly dual-network hydrogel dust suppressant based on xanthan gum, polyvinyl alcohol and acrylic acid. J. Environ. Manag. 2021, 295, 113139. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.X.; Liu, Y.; Feng, Y.; Hu, X.M.; Zhao, Y.Y.; Liu, J.D.; Chen, L.; Qu, Y.L. Force mechanism analysis of composite microbial dust suppressants based on extracellular polymeric substances (EPS) mode components. J. Environ. Manag. 2024, 370, 122926. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Wu, M.; Hu, X.; Guo, Y.; Wang, J. Effectiveness and mechanism of microbial dust suppressant on coal dust with different metamorphosis degree. Environ. Sci. Pollut. Res. 2024, 31, 55437–55446. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Huang, H.; Yi, F.; Cheng, C.; Liu, Y. Preparation of an environment-friendly microbial limestone dust suppressant and its dust suppression mechanism. Environ. Geochem. Health 2024, 46, 380. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Zhao, Y.; Li, X.; Zhao, P.; Guo, Y. Effects of molasses-based microbial dust suppressant on soil dust and microbial community. Powder Technol. 2024, 441, 119831. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Z.; Zhao, Y.; Dong, Y.; Wang, C.; Zhang, L.; Yu, Y.; Wu, K.; Ren, L. Medium optimization and dust suppression performance analysis of microbial-based dust suppressant compound by response surface curve method. Environ. Sci. Pollut. Res. 2024, 31, 24525–24535. [Google Scholar] [CrossRef]
- Oh, S.Y.; Cha, S.W.; Lee, H. Biodegradable dust suppressants prepared from biomass-based materials: The role of viscosity and suppressed particles. J. Air Waste Manag. Assoc. 2024, 74, 253–260. [Google Scholar] [CrossRef]
- Yang, L.; Hong, L.; Huang, J.; Jin, J.; Wu, K.; Zhu, S. Research Progress of Bio-organic Dust Suppressants. J. Phys. Conf. Ser. 2024, 2706, 012072. [Google Scholar] [CrossRef]
- Geng, Z.; Feng, Y.; Zhao, Y.-Y.; Hu, X.-M.; Liu, J.-D.; Wang, Q.-S.; Liu, Y.; Dong, Y. Study on the timeliness and maintenance mechanism of dust suppression performance of microbial dust suppressant. Powder Technol. 2023, 426, 118618. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Hu, X.; Feng, Y.; Cheng, W.; Geng, Z. Calcium alternative on microbial dust suppressant and the mechanism of action. Int. Biodeterior. Biodegrad. 2023, 181, 105618. [Google Scholar] [CrossRef]
- Zhang, S.; Xue, W.; Liu, W.; Duan, H.; Cui, X.; Cao, X.; Cui, Z. Synthesis, performance, and adsorption mechanism of an environmentally friendly dust suppressant derived from guar gum for effective soil dust control. Int. J. Biol. Macromol. 2025, 306, 141497. [Google Scholar] [CrossRef]
- Meng, J.; Wang, C.; Chen, T. Effect of Sodium Dodecylbenzene Sulfonate on the Wetting Mechanism of Tunliu Coal. J. Surfactants Deterg. 2021, 25, 113–123. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Dong, H.; Zhang, Y.; Sun, Y.; Shi, J.; Li, R. Molecular dynamics simulations and experimental study of the effects of an ionic surfactant on the wettability of low-rank coal. Fuel 2022, 320, 123951. [Google Scholar] [CrossRef]
- Yuan, M.; Nie, W.; Zhou, W.; Yan, J.; Bao, Q.; Guo, C.; Tong, P.; Zhang, H.; Guo, L. Determining the effect of the non-ionic surfactant AEO 9 on lignite adsorption and wetting via molecular dynamics (MD) simulation and experiment comparisons. Fuel 2020, 278, 118339. [Google Scholar] [CrossRef]







| Numbering | A (Sodium Polyacrylate)/% | B (Sodium Dodecylbenzene Sulfonate)/% | C (Triethanolamine)/% |
|---|---|---|---|
| 1 | I 1 (0.15) | II 1 (0.5) | III 1 (1.5) |
| 2 | I 1 (0.15) | II 2 (0.75) | III 2 (2.0) |
| 3 | I 1 (0.15) | II 3 (1.0) | III 3 (2.5) |
| 4 | I 2 (0.2) | II 1 (0.5) | III 2 (2.0) |
| 5 | I 2 (0.2) | II 2 (0.75) | III 3 (2.5) |
| 6 | I 2 (0.2) | II 3 (1.0) | III 1 (1.5) |
| 7 | I 3 (0.3) | II 1 (0.5) | III 3 (2.5) |
| 8 | I 3 (0.3) | II 2 (0.75) | III 1 (1.5) |
| 9 | I 3 (0.3) | II 3 (1.0) | III 2 (2.0) |
| Number of Experiments | Test Indicators | ||
|---|---|---|---|
| 24 h Water Retention Rate/% | Hardness/HA | Wind Erosion Rate/% | |
| 1 | 34.42 | 26.00 | 0.29 |
| 2 | 34.62 | 30.75 | 0.70 |
| 3 | 35.14 | 30.00 | 0.25 |
| 4 | 30.20 | 34.75 | 0.10 |
| 5 | 30.87 | 44.50 | 0.05 |
| 6 | 31.29 | 51.00 | 0.10 |
| 7 | 29.25 | 44.00 | 0.05 |
| 8 | 28.95 | 33.25 | 0.20 |
| 9 | 29.66 | 33.25 | 0.05 |
| Parameters | Water Retention | Hardness | Wind Erosion Rate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | |
| k1 | 34.73 | 31.29 | 31.55 | 28.92 | 34.92 | 36.75 | 0.41 | 0.15 | 0.20 |
| k2 | 30.79 | 31.48 | 31.49 | 43.42 | 36.17 | 32.92 | 0.08 | 0.32 | 0.28 |
| k3 | 29.29 | 32.03 | 31.75 | 36.83 | 38.08 | 39.50 | 0.10 | 0.13 | 0.13 |
| R | 16.32 | 2.22 | 0.78 | 44.30 | 9.5 | 19.75 | 1.04 | 0.55 | 0.45 |
| Test Indicators | Test Factors | Deviation Sum of Squares | Degrees of Freedom | Mean Square | F Value | Significance |
|---|---|---|---|---|---|---|
| Water retention | A | 47.367 | 2 | 23.684 | 344.739 | 0.003 |
| B | 0.886 | 2 | 0.443 | 6.45 | 0.134 | |
| C | 0.111 | 2 | 0.056 | 0.809 | 0.553 | |
| Error | 0.137 | 2 | 0.069 | |||
| Hardness | A | 316.264 | 2 | 158.132 | 2.211 | 0.311 |
| B | 15.264 | 2 | 7.632 | 0.107 | 0.904 | |
| C | 65.597 | 2 | 32.799 | 0.459 | 0.686 | |
| Error | 143.014 | 2 | 71.507 | |||
| Wind erosion rate | A | 0.2074 | 2 | 0.1037 | 5.704 | 0.152 |
| B | 0.0627 | 2 | 0.0313 | 1.724 | 0.368 | |
| C | 0.0417 | 2 | 0.0208 | 1.147 | 0.459 | |
| Error | 0.0364 | 2 | 0.0182 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Zhou, L. Preparation and Performance Research of the Optimal Mix Ratio Based on the Coupling Mechanism of Dust Suppressants. Processes 2025, 13, 4061. https://doi.org/10.3390/pr13124061
Du S, Zhou L. Preparation and Performance Research of the Optimal Mix Ratio Based on the Coupling Mechanism of Dust Suppressants. Processes. 2025; 13(12):4061. https://doi.org/10.3390/pr13124061
Chicago/Turabian StyleDu, Shuncheng, and Lina Zhou. 2025. "Preparation and Performance Research of the Optimal Mix Ratio Based on the Coupling Mechanism of Dust Suppressants" Processes 13, no. 12: 4061. https://doi.org/10.3390/pr13124061
APA StyleDu, S., & Zhou, L. (2025). Preparation and Performance Research of the Optimal Mix Ratio Based on the Coupling Mechanism of Dust Suppressants. Processes, 13(12), 4061. https://doi.org/10.3390/pr13124061






