Abstract
This study investigates the effects of CO2–water–rock interactions on shale oil reservoirs, specifically focusing on the mineral dissolution and pore structure alterations in shale samples from the second section of the Permian Fengcheng Formation in the Mahu Depression, Junggar Basin. Core soaking experiments were conducted under high-temperature and high-pressure conditions to simulate reservoir environments. Mineral evolution, ion migration, and microstructural changes were qualitatively and quantitatively analyzed using X-ray Diffraction (XRD), Inductively Coupled Plasma (ICP) spectroscopy, and Scanning Electron Microscopy (SEM). The findings indicate that CO2-induced mineral dissolution follows a distinct sequence: calcite > dolomite > potassium feldspar > sodium feldspar, which is directly reflected in the concentration of ions (Ca2+ > Mg2+ > K+ > Na+) in the solution. The dissolution rate and pore structure enhancement are significantly influenced by lamina density, with dolomitic rocks with high lamina density showing greater dissolution and porosity increase, and the lamina area greater than the matrix area. This study demonstrates that the dynamic changes of rock minerals are the core mechanism for controlling the pore structure of reservoirs, showing how CO2–water–rock reaction enhances the porosity and connectivity of shale reservoirs, thereby improving oil recovery potential.
1. Introduction
Against the backdrop of global energy transition and sustainable development, the exploitation of shale oil and gas resources is becoming an important part of the energy strategies of various countries [1,2,3]. As a pioneer in shale oil and gas development, the United States has achieved energy independence and rapid economic growth by virtue of its abundant resource reserves and mature mining technology [4,5]. In contrast, although China’s continental shale oil and gas resources have huge potential, the geological conditions are complex, the physical properties of the reservoirs are poor, and the maturity of crude oil is low, which significantly increases the difficulty of development [6,7,8]. The rock and mineral composition of the reservoir in the Mabei Fengcheng Formation of the Junggar Basin of Xinjiang is diverse and highly heterogeneous, mainly composed of quartz, feldspar, and carbonate. The local million-centimeter-level interlayers include various minerals such as dolomite, quartz, and clay. Affected by the reservoir lamination, the longitudinal propagation of hydraulic fractures is generally controlled [9,10,11]. Therefore, to achieve long-term stable production of shale oil reservoirs, higher requirements need to be put forward in three aspects: optimizing fracture diversion capacity, increasing the complexity of fracture networks, and replenishing formation energy. Due to the physical property advantages of CO2 fracturing fluid, such as low viscosity, high diffusivity, zero surface tension and strong solvent capacity, as well as the increasingly prominent environmental benefits of reducing water consumption and achieving carbon sequestration, supercritical CO2 fracturing has gradually become a key technology in the production increase—emission reduction coordinated strategy in the field of unconventional oil and gas development [12,13].
The core mechanism of CO2 pre-fracturing technology lies in the phase change—mechanical coupling effect of the multi-phase synergy of supercritical CO2 and the dynamic regulation of reservoir energy [14,15]. The interaction between CO2 fracturing fluid and reservoir crude oil and rock triggers a complex temperature-percolation-mechanical-chemical (T−H−M−C) coupling process, thereby reducing the viscosity of crude oil, enhancing fluidity, driving the evolution of shale microstructure, and ultimately leading to changes in its macroscopic mechanical properties [16]. At the same time, the inorganic cations produced have an inhibitory effect on the hydration expansion of clay minerals, which can reduce reservoir damage [17]. By integrating various means such as rock mechanics tests, true triaxial fracturing experiments and numerical simulations, it was systematically revealed that the water-rock reaction during the CO2 pre-fracturing process can effectively reduce the fracture pressure of the reservoir, replenish the formation energy, enhance the diversion capacity, strengthen the physical properties of the reservoir, promote the complexity of the fracture network, improve the overall oil displacement efficiency, and significantly increase the volume of fracturing modification [18,19,20,21]. Based on field experiments, the application of CO2 pre-fracturing technology can increase the post-fracturing flowback rate by more than three times, boost daily oil production, extend the stable production period, and achieve an input–output ratio of 1:2.5, thereby generating good economic benefits [22,23]. In addition, the CO2–water–rock interaction is the core issue of CO2 geological storage. Enhancing oil recovery by injecting CO2 into oil reservoirs (CCUS-EOR) is the primary application of CO2 capture, utilization, and storage (CCUS). Jilin Oilfield, Xinjiang Oilfield, and others have formed a full industrial chain demonstration project of CCUS-EOR, with an annual storage capacity of 80 × 104 tons and a cumulative storage capacity of over 320 × 104 tons [24,25]. Both laboratory and field experiments have verified that the CO2 pre-fracturing technology has positive effects, such as increasing the modified volume and enhancing energy for drainage assistance. However, the specific mechanism of action for deep mixed shale oil reservoirs on the continental surface remains unclear.
This work studies the shale reservoir of the second section of the Permian Fengcheng Formation in the Mahu Depression, Junggar Basin, Xinjiang. By conducting a series of core immersion experiments under simulated reservoir conditions, the changes in mineral composition, solution ions, and surface pore structure of rocks of different lithologies after immersion in CO2 aqueous solution are analyzed. The mineral evolution, ion migration behavior, and microscopic morphology changes under the interaction of CO2–water–rock were qualitatively and quantitatively characterized by comprehensive utilization of X-ray diffraction analysis (XRD), aqueous solution ion analysis (ICP), and electron microscopy scanning test (SEM), providing microscopic dynamic basis for revealing the efficiency enhancement mechanism of CO2 fracturing modification and the long-term stability of geological storage.
2. Materials and Methods
2.1. Sample Processing
The samples for this experiment were taken from the core of Well Xia 207 in the second section of the Fengcheng Formation of the Permian, Mahu Depression, Xinjiang Oilfield. The core sampling layer was P1f2, and the core sampling well section was 4914.81 to 5002.15 m. It belongs to a typical deep continental mixed accumulation type shale oil reservoir. The second section of the Fengcheng Formation (P1f2) is the main oil-bearing layer. This layer is mainly composed of dolomitic mudstone and dolomitic siltstone, interspersed with siliceous bands and alkaline minerals, forming a typical mixed shale oil reservoir [26,27,28]. The second section of the Fengcheng Formation (P1f2) shale oil reservoir has advantages such as large thickness, high pressure, low viscosity, well-developed fractures, and high brittleness. However, its overall physical properties are poor, with a porosity of less than 5% and a permeability of less than 0.01 mD, making it an extremely tight reservoir [29,30,31].
According to the analysis of logging data, it can be known that the core well section contains two different lithologies, namely dolomitic mudstone and dolomitic sandstone. The core contains a high proportion of oil and gas, and the oil-bearing area is mainly composed of oil spots and oil traces. Therefore, based on the logging information, the core is classified into four lithologies: dolomitic mudstone with high lamina density, dolomitic mudstone with low lamina density, dolomitic sandstone with high lamina density, and dolomitic sandstone with low lamina density (Figure 1).

Figure 1.
Four different lithology diagrams: (a) dolomitic mudstone with high lamina density; (b) dolomitic mudstone with low lamina density; (c) dolomitic sandstone with high lamina density; and (d) dolomitic sandstone with low lamina density. The red dots lines shows the untextured layer, and the red dots circle indicates the mineral-filled area.
The core samples were first processed into cylindrical thin sheets with a diameter of 25 mm and a height of 10 mm along the direction perpendicular to the grain layer using high-precision wire cutting equipment (Novick Digital Equipment Co., Ltd., Beijing, China) and a cooling medium of 99.7% anhydrous ethanol. The cutting parameters were set to a feed speed of 0.1 mm/min, a wire tension of 12 N, and full liquid cooling to prevent hydration expansion and structural damage of clay minerals. Then, one end face of the cylindrical sheet perpendicular to the direction of the lamina was mechanically polished several times, and the Gatan Ilion + II broadband argon ion polishing instrument (Gatan, Inc., Pleasanton, CA, USA) was used for secondary polishing to ensure that the final surface roughness Ra of the sample was less than 0.1 μm, meeting the requirements of high-precision electron microscopy analysis (Figure 2).
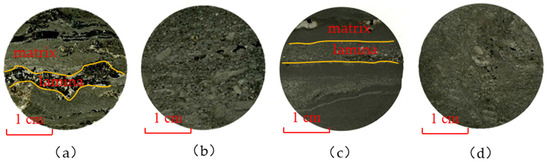
Figure 2.
SEM sample flakes. (a) dolomitic mudstone with high lamina density; (b) dolomitic mudstone with low lamina density; (c) dolomitic sandstone with high lamina density; and (d) dolomitic sandstone with low lamina density. The yellow curve indicates the boundary line between the striatum and the matrix.
To ensure the consistency of lithology and strengthen the correlation between experiments, the mineral composition test samples were also obtained by the above wire cutting method on the core column adjacent to the electron microscope scanning samples, and the samples were ground into powders smaller than 320 mesh (about 45 μm), as shown in Figure 3.
Figure 3.
The XRD test sample powder.
2.2. Experimental Equipment and Steps
To accurately simulate the CO2–water–rock interaction in the reservoir temperature–pressure–fluid coupling environment, experiments were carried out using an integrated high-temperature and high-pressure immersion system. This system consists of a high-purity gas source unit (Yongan Special Equipment Co., Ltd., Linyi, Shandong, China), a high-precision constant-speed constant-pressure pump(Jiangsu Lianyou Scientific Research Instrument Co., Ltd., Haian, Jiangsu, China), a buffer intermediate container (Weihai Borui Chemical Machinery Co., Ltd., Weihai, Shandong, China), a CO2 preheating module, a Hastelloy alloy reactor(Weihai Borui Chemical Machinery Co., Ltd., Weihai, Shandong, China), a constant temperature box (Kington Technology Co., Ltd., Chongqing, China), a drying box (Kington Technology Co., Ltd., Chongqing, China), and a temperature and pressure sensing system [32] (Figure 4).
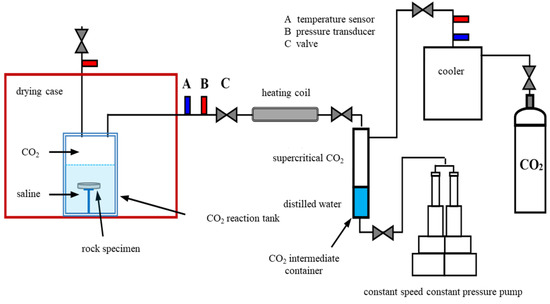
Figure 4.
High temperature and high pressure soaking experimental device (modified according to Reference [32]).
The basic steps of the CO2–water–rock reaction are as follows: (1) Washing and drying the shale samples to eliminate the interference of crude oil components on the reaction. (2) The reactor was pressurized to 25 MPa with no load, and the pressure was kept for 30 min to detect the sealing performance of the reaction system. (3) Place the electron microscope scanning sample (with the polishing side facing up) and the powder sample (7 g) in the reaction vessel, avoiding contact with the vessel wall. Then raise the temperature inside the reaction vessel to 110 °C, the reservoir temperature, and keep it constant. (4) Under the conditions of 110 °C and 20 MPa (reservoir temperature and pressure), the solubility of CO2 in water is 1.11 mol/kg. To make the dissolution of CO2 in water reach a supersaturated state, we calculate that in a 100 mL reactor with a solubility of 5 mol/kg, the volume of water to be injected is 62 mL, and the volume of CO2 is 38 mL. A constant-speed and constant-pressure displacement pump was used to inject 62 mL of water and 38 mL of CO2 into a 100 mL reactor at an injection rate of 100 mL/min, respectively, until the pressure reached 20 MPa. When injecting CO2, the CO2 in the gas cylinder needs to be converted into a supercritical state (critical point temperature 31.1 °C, pressure 7.4 MPa) through a temperature and pressure control system. (5) During the reaction process, it is necessary to monitor the temperature and pressure inside the reactor in real time to maintain the stability of the reaction environment. (6) After the reaction is completed, the pressure in the reactor is slowly released to normal pressure. After the reactor is cooled to room temperature, the sample is taken out and dried.
2.3. Experimental Scheme
To clarify the special reactions of CO2 and the changes in rock mineral composition and pore structure before and after the CO2–water–rock reaction in different lithologies and regions, we divided the areas where bedding is concentrated into laminar regions and the areas where almost no laminar exists into matrix regions. The textured part of the textured dolomitic mudstone was subjected to high-temperature and high-pressure immersion experiments under pure water solution and supersaturated CO2 conditions. The textured part of the textured dolomitic mudstone, the textured part, and the matrix part of the textured dolomitic sandstone. High-temperature and high-pressure immersion experiments were conducted on dolomitic rocks with undeveloped lamina without distinguishing between lamina regions and matrix regions under supersaturated CO2 conditions. The experimental parameter designs are shown in Table 1.

Table 1.
Experimental scheme design.
To systematically characterize the evolution law of rock physical properties in shale reservoirs under the chemical dissolution of CO2 aqueous solution, the SU8010 cold field emission scanning electron microscope was used to observe the microscopic pore structure on the rock surface, the morphology of micro-fractures, and the mineral filling of matrix and grain layers before and after the immersion experiment, respectively. The changes in mineral composition before and after the reaction were quantitatively characterized by using the Rigaku TTR-III X-ray diffractometer (the scanning step size is 0.02° and the scanning rate is 0.4°/min). And use an ion chromatograph to detect the types and concentrations of ions in the solution after the reaction. Based on the three tests, comprehensively analyze the mechanism of the CO2–water–rock reaction on reservoir rocks from both macroscopic scale (solution ion concentration) and microscopic scale (mineral composition, pore structure).
3. Results
3.1. Lithology-Reservoir Characteristics of the Shale of the Fengcheng Formation in the Mahu Depression
3.1.1. The Mineral Composition of Different Lithologies
The mineral composition of the cores from four different lithologies and different regions before participating in the CO2–water–rock reaction is shown in Figure 5, and the error range is approximately ±2%. The dolomitic mudstone with high lamina density is mainly dominated by carbonate minerals (calcite, dolomite) and terrigenous clastic rocks (plagioclase, quartz), containing a small amount of pyrite, clay minerals, and trace amounts of amphibole and anatase. The dominant mineral in the lamina area is calcite (50.2%), followed by dolomite (14.9%), plagioclase (12.6%), quartz (12.1%), and a small amount of pyrite (8.3%) and clay minerals (1.9%). The mineral distribution in the matrix area is balanced. The contents of calcite, dolomite, plagioclase, and quartz are relatively close (16.4–22%), and the content of clay minerals is slightly higher (6.6%). The K-feldspar, hornblende, and anatase are completely distributed in the matrix area. The main minerals of dolomitic mudstone with low lamina density are dolomite (29.2%) and plagioclase (28.8%), and the secondary mineral is quartz (19.8%), containing a small amount of K-feldspar, pyrite, and hornblende. The content of calcite and clay minerals is very small, accounting for 1.6%, respectively. The dolomitic sandstone with high lamina density is mainly composed of quartz and dolomite, with their relative contents both reaching over 30%. It is followed by plagioclase and contains small amounts of calcite, potassium feldspar, pyrite, and clay minerals (all with contents around 5%). Compared with the matrix area, the content of quartz and dolomite in the lamina area is lower than that in the matrix area, and the content of plagioclase and clay minerals is relatively high, while calcite is almost all enriched in the lamina, and the matrix area contains only 0.5%. The dolomitic sandstone with low lamina density contains a large amount of quartz and plagioclases, which account for 58.6% of the total. It is followed by dolomite, pyrite, potassium feldspar, and clay minerals, with calcite content being relatively low, accounting for only 2.8%. By comparing the contents of mineral components in four different lithologies, it is found that the carbonated mineral content of dolomite rock with high lamina density is higher than that of dolomite rock with low lamina density, and the content of quartz and silicate minerals is lower than that of dolomite rock with low lamina density. The mineral composition contents of different lithologies and regions indicate that lithology determines the dominant mineral types, and the density of the lamina controls the mineral distribution. The lamina region exhibits targeted enrichment of dolomite, whereas the matrix region has a relatively higher content of quartz minerals.
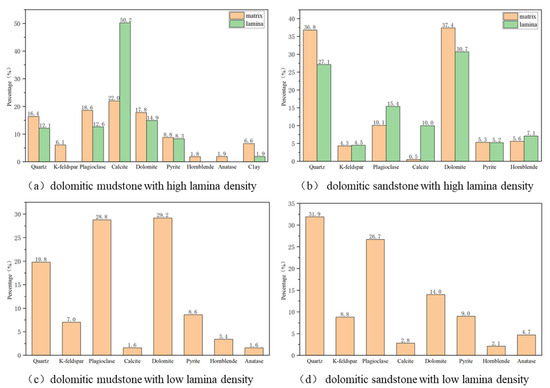
Figure 5.
Mineral composition of different lithologies.
By analyzing the relative contents of mineral compositions in the four different lithologies and different regions, the characteristics of diverse rock mineral compositions and strong heterogeneity in the shale reservoirs of the Fengcheng Formation in the Mahu Depression were revealed. Reservoir rocks are mainly composed of carbonate minerals (calcite, dolomite) and terrigenous clastic minerals (plagioclase, quartz), with pyrite and clay minerals as secondary mineral components. At the same time, the mineral composition of the lamina and the matrix varies greatly. The lamina area is rich in carbonate minerals (mainly calcite), while the content of terrigenous clastic rock minerals in the matrix area is relatively high (primarily quartz).
3.1.2. Microscopic Pore Strucapproximately Theologies
The microstructure of different lithologies and regions observed under a scanning electron microscope is shown in Figure 6. The initial pore structure distribution of rocks is affected by lithology (mineral composition) and lamina structure (mineral distribution). The dolomitic rock with high lamina density presents a layered structure, with the lamina and the matrix alternately arranged, and the microscopic pore structure is centered on the layered heterogeneity (Figure 6a,c). The lamina region is rich in carbonate minerals, and its pore structure is mainly composed of intergranular pores of carbonate minerals and interlamellar pores within the lamellar region [33,34]. The intergranular pores of carbonate minerals present as regular polygons, formed in the gaps between crystals that have not been filled or retained in the gaps after slight deformation of the crystals. The interlayer pores within the lamina are parallel to the direction of the lamina. They are formed when the minerals within the lamina are arranged directionally and are not completely compacted between layers [35]. The surface of the dolomitic mudstone with high lamina density (Figure 6a) clearly shows a distinct boundary between the lamina area and the matrix area. The surface of the matrix is dense and smooth, without obvious pores or cracks. There are tiny pores with a pore radius of approximately 3 to 100 µm and a coordination number of about 2 to 3 within the lamina region. The micro-pores of dolomitic sandstone with high lamina density mainly exist in the distribution areas of some carbonate minerals in the lamina area (Figure 6c). The mineral particles of dolomitic rocks with low lamina density are evenly distributed. The microscopic pore structure is mainly intergranular pores, and there are no obvious micro-cracks (Figure 6b,d). In the carbonate-rich areas, there are intergranular connected pores with a pore radius of 2 to 50 µm in the dolomitic mudstone with low lamina density. In the quartz and feldspar mineral crystals, there are a small number of intragranular pores with a radius less than 20 µm. The overall pore distribution is relatively dispersed. The dolomitic sandstone with low lamina density forms large intergranular pores (with a radius of approximately 30 µm) at the junctions of different mineral particles, while smaller intergranular and intragranular pores form between the same mineral particles, and the connectivity between the pores is poor.
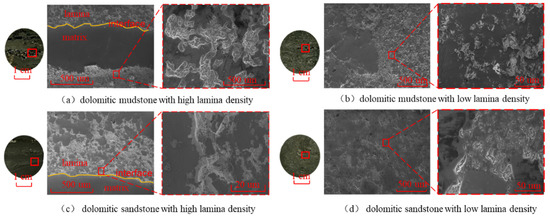
Figure 6.
Microstructure of different lithologies. The red box is an enlarged view of the observation area presented in the left figure, and the yellow curve is the interface between the lamina and the matrix.
A comprehensive analysis of the microscopic pore structures on the surfaces of different lithologies reveals that the rock pore density in the shale reservoirs of the Fengcheng Formation in the Mahu Depression is low and has poor connectivity, with extremely low overall porosity and permeability. Meanwhile, the microscopic pore structure is influenced by the lamina structure. Within the lamina region, due to the filling of different minerals and the layered arrangement structure, a pore structure with a relatively high density is formed, resulting in micro-cracks. The mineral distribution in the matrix area is relatively uniform, only generating relatively dispersed, isolated pore structures. The overall pore structure of rocks with high lamina density is superior to that of rocks with low lamina density.
The microstructure and elemental characteristics of the main minerals in the rock were observed under a scanning electron microscope, as shown in Figure 7. Calcite presents a regular polyhedral crystal form (Figure 7a), with a smooth and dense crystal surface. It is a typical hexagonal crystal system of carbonate minerals and has high symmetry. In the crystal structure, Ca2+ and CO32− are combined by ionic bonds to form a layered structure. The crystals are intergranular, and the boundaries between the particles are clear. The pores formed are mainly intergranular pores caused by insufficient diagenetic cementation or slight compaction deformation [36]. Dolomite has a rhombohedral shape and belongs to the trigonal crystal system. Its crystals are relatively small and may mix with other minerals. The crystal structure of dolomite is composed of alternating Ca2+ and Mg2+, forming a layered structure. The bonding strength of Mg2+ is higher than that of calcium ions, making the crystal structure more stable [37,38]. Potassium feldspar appears in columnar or plate-like shapes, usually with smooth crystal planes and distinct edges. It belongs to the monocline crystal system and has a relatively dense overall structure, with intragranular pores mainly formed by crystal growth defects and intergranular pores as a supplement. Its main element identification feature is potassium. Sodium feldspar and potassium feldspar have similar crystal shapes, presenting as plate-like or slender columnar forms. They belong to the triclinic crystal system. Their pore structure is mainly intergranular pores and supplemented by intragranular pores. The main element identification feature is sodium. Based on the analysis of the crystal structure and physical properties of the four minerals, under high-temperature, high-pressure, and acidic environmental conditions, the stability of the four minerals is sodium feldspar > potassium feldspar > dolomite > calcite.
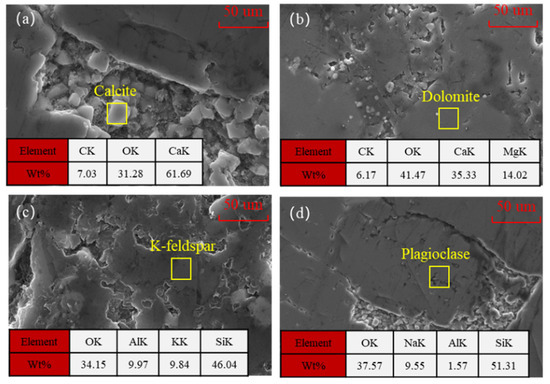
Figure 7.
The micro-morphology and element content of the four different minerals: (a) calcite; (b) dolomite; (c) K-feldspar; and (d) plagioclase.
3.2. Effect of CO2-Water-Rock Reaction on Physical Properties of Shale
After CO2 is injected into an aqueous solution, it will rapidly dissolve in the solution following Henry’s law to form carbonic acid and trigger a proton release reaction, making the solution acidic. Using the batch reaction module in the geochemical software PHREEQC (Version 3), without considering reaction rate and time evolution, the pH value of the solution in the equilibrium state is simulated if the system has reached equilibrium. By simulating the pH value changes of supersaturated CO2 dissolved in aqueous solution under different temperature and pressure conditions, it was found that under the experimental conditions of this study (110 °C, 20 MPa), the pH of the solution varied between 3.02 and 3.34 [39]. The pH in the solution was measured with a pH meter within the range of 3.1 to 3.2, which was consistent with the simulated situation. This acidic environment drives the dissolution of unstable minerals (such as carbonate rocks and silicate rocks), the formation of quartz and clay minerals, and the changes in the concentrations of ions such as Ca2+ and Mg2+ in the solution [40]. The specific reaction equation is as follows:
After the above reactions, the surface micro-pore structure and mineral composition of the cores with four different lithologies of dolomitic mudstone with high lamina density, dolomitic mudstone with low lamina density, dolomitic sandstone with high lamina density, and dolomitic sandstone with low lamina density changed significantly.
3.2.1. The Key Role of CO2
The lamellar parts of dolomitic mudstone with high lamina density were respectively placed in pure water solution and supersaturated CO2 water solution for high-temperature and high-pressure immersion experiments under the conditions of 110 °C temperature and 20 MPa pressure. After 24 h of reaction, they were taken out and dried. Then, XRD tests and SEM scans were conducted on them, and the water solution samples after reaction were taken for ion analysis tests. Comprehensively analyze the key role of CO2 in the reaction.
The mineral composition changes of core powder in the lamellar region of dolomitic mudstone with high lamina density before and after reaction in pure water solution and supersaturated CO2 aqueous solution are shown in Figure 8. The change in mineral composition after the reaction in the supersaturated CO2 aqueous solution is significantly greater than the reaction result in the pure water solution. After the reaction in the supersaturated CO2 aqueous solution, the relative content of calcite decreased from 50.2% before the reaction to 43.5%, and the relative content of dolomite decreased by 1.1%. In contrast, the relative content of calcite in the pure water solution decreased by only 2.2%, and that of dolomite decreased by only 0.9%. The corresponding ion concentrations produced are shown in Figure 9. After the reaction, the ion concentrations in the supersaturated CO2 aqueous solution are all higher than those in the pure water solution, among which the concentrations of Ca2+ and Mg2+ differ the most. The concentration of Ca2+ produced after the reaction in the supersaturated CO2 aqueous solution reached 117.9 mg/L, and the concentration of Mg2+ reached 47.8 mg/L, while in pure water solution, the concentration of Ca2+ produced after the reaction was only 50 mg/L, and the concentration of Mg2+ reached 19 mg/L. In contrast, the concentrations of Ca2+ and Mg2+ produced after the reaction in pure water solution decreased by 57.6% and 60.3%, respectively. At the same time, the surface micro-morphologies after the reaction between the original state and the pure water solution and the supersaturated CO2 aqueous solution were compared and observed as shown in Figure 10. After the reaction in the pure water solution, only some slight hydration corrosion occurred on the core surface, the overall structure was nearly complete, and the pore changes were relatively small. After reacting in a CO2 aqueous solution, the mineral particles on the core surface underwent significant dissolution and corrosion, making the surface rough and uneven.

Figure 8.
The mineral composition changes of core powder before the reaction and after reacting with aqueous solution and supersaturated CO2 aqueous solution.
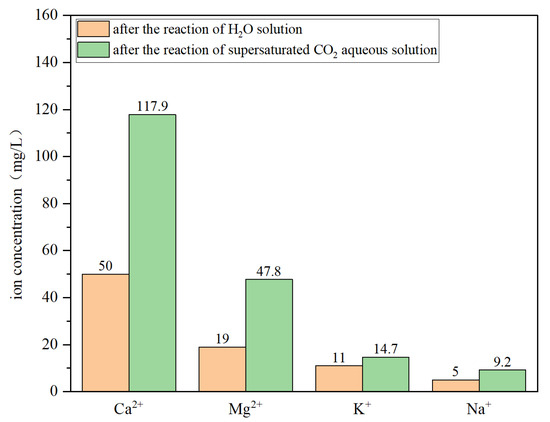
Figure 9.
The ion concentration of the core powder after reaction in aqueous solution and supersaturated CO2 aqueous solution.
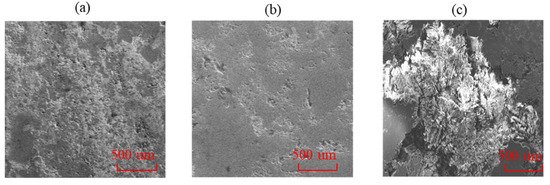
Figure 10.
The Microscopic morphology of the core surface before and after the reaction: (a) original state; (b) after reaction in pure water solution; and (c) after reaction in supersaturated CO2 aqueous solution.
Overall, a supersaturated CO2 aqueous solution plays a key role in accelerating mineral dissolution, ion release, and pore structure changes compared with pure water. Especially for carbonate minerals such as calcite and dolomite, the dissolution strength is significantly enhanced with the participation of CO2. Therefore, the acidic environment endowed by CO2 to the system is a key factor driving mineral dissolution reactions, especially the dissolution of carbonates.
3.2.2. Changes in Mineral Composition
The core powder of different lithologies and different regions was placed in the reaction kettle, filled with enough CO2 to make it a supersaturated state. The reaction was carried out at a temperature of 110 °C and a pressure of 20 MPa for 24 h. After drying, the XRD test was carried out, and the aqueous solution sample after the reaction was taken for an ion analysis test. The two test results were combined to comprehensively analyze the mineral composition changes before and after the CO2–water–rock reaction.
For the relative content changes of mineral components (Figure 11) and ion concentration changes (Figure 12) of core powders of different lithology and different regions before and after the CO2–water–rock interaction, multiple experiments were conducted to ensure reliability. Finally, the data dispersion was expressed by the average value and error, with the error of mineral content being ±3% and the error of ion concentration being ±5%. After the CO2–water–rock reaction, a large amount of Ca2+ and Mg2+ were produced in the solution. Among them, the Ca2+ concentration produced by the dissolution of the four lithologies was greater than 100 mg/L except for the individual matrix area, and the Mg2+ concentration fluctuated around 50 mg/L. Meanwhile, the relative contents of calcite and dolomite before the reaction were compared. It can be found that its relative content has decreased significantly, indicating that a large amount of carbonate minerals (calcite, dolomite) has been dissolved. The concentrations of K+ and Na+ produced in the solution are relatively low; that is, potassium feldspar and sodium feldspar are dissolved in small amounts, and the overall reaction degree is small. Because the total mass of minerals decreases after the reaction, their relative contents increase slightly. Quartz and pyrite have relatively stable chemical properties, hardly react, or react very slowly, and their relative contents increase. The relative content of clay minerals increased slightly (0.2–0.6%). After the reaction, the concentrations of different types of ions in the solution generally showed Ca2+ > Mg2+ > K+ > Na+. At the same time, considering the degree of change in the relative content of minerals before and after the reaction, calcite > dolomite > potassium feldspar > sodium feldspar, it was found that the priority of reaction between different types of minerals and the CO2 aqueous solution was different, and the reaction sequence was calcite > dolomite > potassium feldspar > sodium feldspar, respectively. The main reaction minerals of the CO2–water–rock reaction are calcite and dolomite. The concentrations of Ca2+ and Mg2+ produced by the reaction of dolomite mudstone and dolomite sandstone are in the order of lamina area > dolomitic rock with low lamina density > matrix area. The relative content of calcite in the matrix area of dolomitic mudstone with high lamina density, the lamina area of dolomitic mudstone with high lamina density, and the dolomitic mudstone with low lamina density decreased by 2.6%, 6.7%, and 0.2%, respectively, and the relative content of dolomite decreased by 1.2%, 1.1%, and 1.6%, respectively. The relative content of calcite in the matrix area of dolomitic mudstone with high lamina density, the lamina area of dolomitic mudstone with high lamina density, and the dolomitic mudstone with low lamina density decreased by 0.05%, 1.5%, and 0.4%, respectively, and the relative content of dolomite decreased by 2.8%, 2.4%, and 0.8%, respectively. Based on the change of ion concentration and the decrease in relative content, it is found that the lamina area is mainly filled with calcite and dolomite, and the CO2–water–rock reaction of carbonate rocks in the lamina area is more intense, among which calcite dissolution is the most serious.
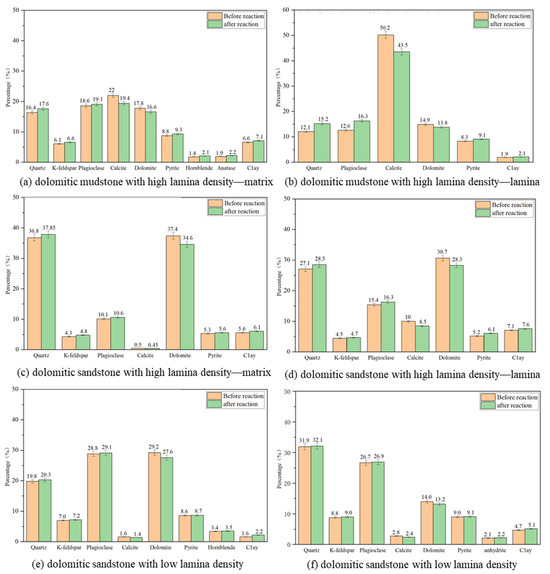
Figure 11.
Changes of mineral composition in different lithologies and different regions before and after CO2–water–rock interaction. Further details can be found in the Supplementary Materials (Supplementary File Sheet S3).
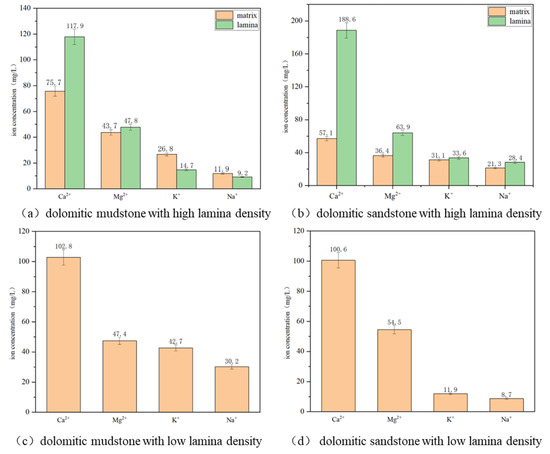
Figure 12.
Ion concentration produced by the dissolution of different lithologies and different regions after CO2–water–rock interaction. Further details can be found in the Supplementary Materials (Supplementary File Sheet S2).
3.2.3. Microscopic Pore Structure Changes
The core slices of different lithologies were placed under the SU8010 cold field emission scanning electron microscope to observe the microscopic pore structure of the surface, and then placed in the reactor filled with enough CO2 to make it a supersaturated state. After the reaction at a temperature of 110 °C and a pressure of 20 MPa for 24 h, the samples were taken out and dried, and the samples after the reaction were scanned by electron microscopy to observe the changes in the microscopic pore structure of the surface.
The dissolution morphology of four different minerals, calcite, dolomite, potassium feldspar, and albite, is shown in Figure 13. Calcite has poor stability and high sensitivity to the CO2–water system. Calcite has a large degree of dissolution, forming a needle-like residual crystal structure. The dissolution pits are long or banded, and the edges are straight and regular. Dolomite enhances lattice stability due to the polarization of Mg 2+, and its dissolution rate is relatively slower than calcite, and the overall reaction degree is not as good as calcite. After dissolution, the dolomite is mainly characterized by intragranular dissolution, and the pore edges are serrated. Several small dissolution pores are connected to form a honeycomb pore network. The coordination number reaches three to four, and the overall connectivity is greatly enhanced. The K-feldspar has high stability and weak sensitivity to the CO2–water system. It only slowly dissolves inward along the edge of the particles to form tiny dissolution pores. At the same time, scaly secondary mineral kaolinite can be observed in the dissolution pores. The stability of albite is higher, and the reaction is more difficult. After the reaction, only the edge is corroded, and the overall structure is relatively complete.
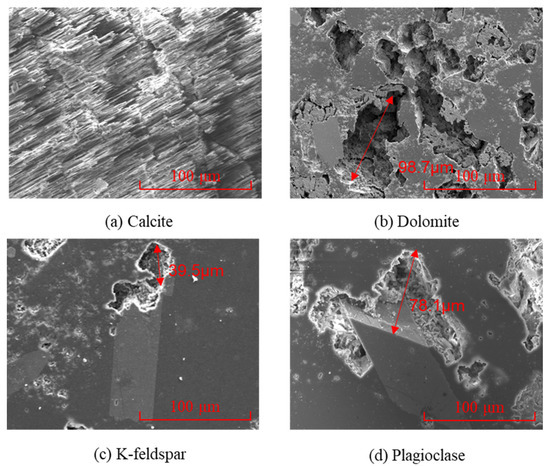
Figure 13.
Dissolution morphology of four different minerals after CO2–water–rock interaction: (a) calcite; (b) dolomite; (c) K-feldspar; and (d) plagioclase.
Under the condition of high temperature and high pressure, and supersaturated CO2, after 24 h of CO2–water–rock reaction, the surface microstructure changes and pore size distribution of four different lithologies are shown in Figure 14. The radius of the observed erosion pores was statistically analyzed, and the overall pore radius distribution was estimated with an error of 5% as shown in Figure 15. Under the condition of the original state, the number of pores on the rock surface is small, the radius is small, the distribution is scattered, the overall pores are not developed, the connectivity is poor, and the porosity and permeability are extremely low. After 24 h of CO2–water–rock reaction, the surface microstructure of four different lithologies changed. There are only 5–20 µm tiny pores scattered in the observation area of dolomitic mudstone with high lamina density in the original state. After the CO2–water–rock reaction, the calcite and dolomite in the mineral-filled area of the grayish-white part were largely eroded, and their relative contents decreased by 6.7% and 1.1%, respectively. After the dissolution of minerals, needle-like residual crystal structures are formed and distributed in a hierarchical manner, generating erosion fissures and mineral erosion holes at the interstitial interface, and they have a certain directionality, that is, they are consistent with the direction of the interstitial. The radius of the dissolved pores generated by dissolution is distributed within the range of 5 to 200 μm, among which 40.4% of the dissolved pores are mainly distributed in the range of 60 to 110 μm. In the original state, the pore radius of dolomitic mudstone with low lamina density is only 2–10 μm, and the number is small, and the mineral filling area is more dispersed. After the reaction, the mineral filling area is dissolved, resulting in many isolated pores with a small size and high density, which are not directional and have poor connectivity. The reaction degree in the matrix area is small, and only the mineral boundary is slightly dissolved. After the reaction, only the mineral boundaries of calcite and dolomite were slightly eroded, with their relative contents decreasing by only 0.2% and 1.6%, respectively. Therefore, the size of the eroded pores was relatively small, and the overall eroded pore size distribution was between 5 and 145 μm. About 65.2% of the main eroded pore sizes were between 40 and 100 μm, and the pore coordination number was mostly one, with poor connectivity. The dolomitic sandstone with high lamina density has tiny pores ranging from 5 to 15 μm in its initial state. After the reaction, the relative content of carbonate minerals decreases by 3.9%, generating honeycomb-like dissolved pores ranging from 5 to 130 μm. The dissolution expands unevenly along the interface, making the pore edge serrated, the local pore coordination number reaches four, and the pore connectivity is greatly enhanced. Under the original condition, the surface of dolomitic sandstone with low lamina density is extremely dense, and the pore structure is less. After the CO2–water–rock reaction, the relative content of carbonate minerals decreased by 1.2% in total, generating many intrallocular dissolved pores. The pore radius was within the range of 5 to 120 μm. Among them, 64.4% of the dissolved pores had a radius of 40 to 80 μm, and the pore coordination number was at most five. The local connectivity was greatly improved.

Figure 14.
Changes of rock surface microstructure in different lithologies and different regions before and after CO2–water–rock interaction.
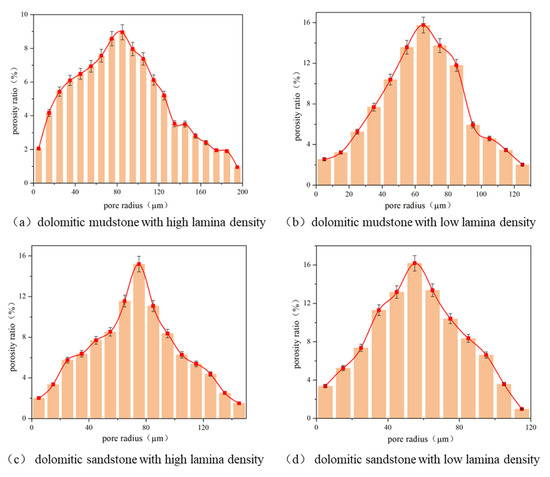
Figure 15.
Distribution of dissolution pore size on the surface of different lithology rocks after CO2–water–rock interaction. Further details can be found in the Supplementary Materials (Supplementary File Sheet S1).
By comparing the surface microstructure of the four different lithologies in their original state before the reaction, it was found that the surface of the rock was eroded by the CO2–water–rock reaction to dissolve unstable minerals (calcite, dolomite, potassium feldspar, and sodium feldspar) to form dissolution pores, increasing the pore size and connectivity. At the same time, because calcite and dolomite are enriched in the lamina area, the overall dissolution degree is greater, so the dissolution degree of the CO2–water–rock reaction shows that the dolomitic rock with low lamina density < dolomitic rock with high lamina density, matrix area < lamina area.
As shown in Figure 16, when the CO2–water–rock reaction occurs in a supersaturated CO2 aqueous solution, the higher the density of the rock laminate, the greater the relative content of carbonate minerals decreases, the greater the concentrations of Ca2+ and Mg2+ produced in the solution, and the erosion pore size gradually increases.
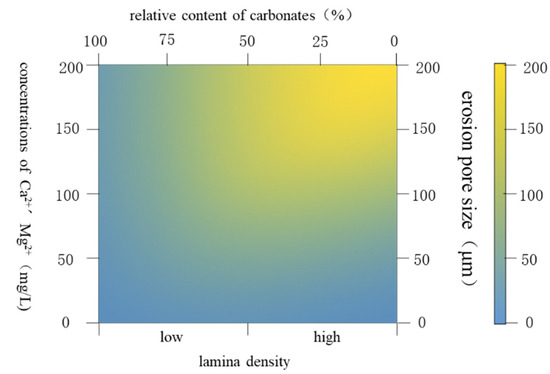
Figure 16.
Mechanism of mineral dissolution under the action of CO2–water–rock.
4. Conclusions
The key findings of this research are as the following:
(1) Rocks of different lithologies in the shale oil reservoir of the Mabei Fengcheng Formation were dissolved after CO2–water–rock reaction, and the relative content of mineral components changed. The kinetic process of mineral dissolution was controlled by both mineral species and rock structure.
(2) Due to the significant differences in the inherent chemical stability of different minerals and their reactivity in the CO2-aqueous solution system, the order of dissolution reactions is different, which is calcite > dolomite > K-feldspar > albite. The dissolution kinetic sequence is directly reflected in the concentration of characteristic ions in the reaction solution, showing Ca2+ > Mg2+ > K+ > Na+.
(3) The lamina density affects the degree of CO2–water–rock reaction. The overall mineral dissolution effect is as follows: dolomitic rock with high lamina density > dolomitic rock with low lamina density, and lamina area is greater than matrix area.
(4) In the process of CO2–water–rock reaction, the morphology of dissolution pores produced by different minerals is different. Calcite dissolution forms a needle-like residual crystal structure, dolomite produces a honeycomb pore network, potassium feldspar gradually dissolves from mineral boundary to inside, and albite only dissolves the mineral edge.
(5) The change of rock mineral dynamics is the core mechanism to control the pore structure of the reservoir. The dissolution of minerals generates dissolution pores, with the radius of the dissolution pores reaching up to 200 μm and the coordination number of dissolution pores reaching five, which greatly enhances the pore connectivity.
5. Knowledge Gaps and Future Perspectives
Under conditions of high temperature, high pressure, and super-saturated CO2 aqueous solution, the core undergoes a CO2–water–rock reaction, which leads to mineral dissolution and the formation of erosion pores. This can, to a certain extent, increase porosity and permeability and improve the physical properties of the reservoir. However, this study only provided information on the impact of carbon dioxide (CO2) injection on shale reservoirs at a laboratory scale. Future research should focus more on the field practice of CO2 injection in shale reservoirs. Laboratory research is usually conducted under controlled conditions, which may differ from the complex environment of actual reservoirs. Therefore, on-site monitoring is the key to verifying laboratory results and deriving more practical conclusions. The real-time monitoring of the CO2 injection process, especially under high-temperature and high-pressure conditions, can provide a deep understanding of the long-term reaction mechanism between CO2 and shale minerals, including the evolution of pore structure, the dissolution of minerals, and their impact on porosity and permeability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13123985/s1, Sheet S1: Pore size distribution after dissolution after reaction; Sheet S2: Solution ion analysis; Sheet S3: The mineral composition changes after the reaction.
Author Contributions
Conceptualization, J.L. and S.Z.; validation, K.L.; investigation, M.W.; supervision, J.D.; writing—original draft, L.Y.; formal analysis, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Jianmin Li, Kaixin Liu, Mingxing Wang and Jingfeng Dong are affiliated with Engineering Technology Institute, Petro China Xinjiang Oilfield Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liang, X.; Feng, L.; Cao, Y.; Guo, C.; Jia, J.; Chai, H. Critical Thinking of Some Important Indexes of Shale Oil UnconventionalDevelopment Model. Drill. Prod. Technol. 2025, 48, 92–99. [Google Scholar] [CrossRef]
- Zou, C.; Pan, S.; Jing, Z.; Gao, J.; Yang, Z.; Wu, S.; Zhao, Q. Shale oil and gas revolution and its impact. Acta Pet. Sin. 2020, 41, 1–12. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, S.; Xu, S.; Wang, X. Practice, challenges and prospects of oil and gas field development in China. China Pet. Explor. 2024, 29, 1–11. [Google Scholar] [CrossRef]
- Mcmahon, T.P.; Larson, T.E.; Zhang, T.; Shuster, M. Geologic characteristics, exploration and production progress of shale oil and gas in the United States: An overview. Pet. Explor. Dev. 2024, 51, 925–948. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, J.; Wang, Z.; He, Z.; Song, C.; Chen, R.; Liu, X.; Ji, T.; Li, Z. Analysis of the current status of global oil, gas, and associated resources exploration in 2023. Pet. Explor. Dev. 2024, 51, 1465–1479. [Google Scholar] [CrossRef]
- Lei, Q.; Weng, D.; Guan, B.; Shi, J.; Cai, B.; He, C.; Sun, Q.; Huang, R. Shale oil and gas exploitation in China: Technical comparison with US and development suggestions. Pet. Explor. Dev. 2023, 50, 944–954. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, J.; Jin, S.; Liu, C.; Abula, A.; Hou, J.; Ma, L. The occurrences and mobility of shale oil in the pore space of terrestrial shale. Fuel 2024, 374, 132377. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Li, J.; Li, J.; Yi, X.; Wang, M.; Tian, G.; Wang, J. Status, Experience, and Inspiration of Unconventional Resources Hydraulic Fracturing Technology. In Proceedings of the GOTECH, Dubai, United Arab Emirates, 7–9 May 2024. [Google Scholar]
- Li, P.; Xiong, J.; Yan, Q.; Zhu, Z.; Liu, X.; Wu, J.; Wang, Z.; Zhang, L. Lithological influences to rock mechanical properties of Permian Fengcheng Formation in Mahu Sag, Junggar Basin. Pet. Geol. Exp. 2022, 44, 569–578. [Google Scholar] [CrossRef]
- Ma, C.-F.; Huang, W.-J.; Zhou, J.; Yu, H.-Z.; Song, M.-Y. The genetic mechanism of salt minerals in Fengcheng Formation in Hashan area, northwestern margin of Junggar Basin. Pet. Sci. 2025, 22, 3991–4014. [Google Scholar] [CrossRef]
- Hao, L.; Gan, R.; Pan, L.; Ruan, D.; Liu, C. Key Technology of Volumetric Fracturing in Vertical Wells of Hugely Thick Shale Oil Reservoirs in the Fengcheng Formation of the Mahu Sag. Pet. Drill. Tech. 2021, 49, 99–105. [Google Scholar] [CrossRef]
- Teng, W. Development Path of Integration of Oil and Gas with New Energy in Xinjiang Oilfield under the Background of “Dual Carbon”. Xinjiang Oil Gas 2025, 21, 14–19. [Google Scholar] [CrossRef]
- Zhang, K.; Tao, L.; Chen, W.; Li, B.; Pan, Y.; Zhang, S.; Li, G. Fracturing mechanism, phase transformation, and energy enhancement effect of CO2 fracturing fluids in tight reservoirs. J. Eng. Geol. 2025, 33, 1647–1658. [Google Scholar] [CrossRef]
- Wu, S.; Ge, H.; Li, T.; Wang, X.; Li, N.; Zou, Y.; Gao, K. Characteristics of fractures stimulated by supercritical carbon dioxide fracturing in shale based on acoustic emission monitoring. Int. J. Rock Mech. Min. Sci. 2022, 152, 105065. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Sharma, M. Research progress and prospects of CO2 fracturing for developing unconventional energy sources. Geoenergy Sci. Eng. 2024, 241, 213137. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Wang, X.-L.; Meng, Y.-Z.; Tian, J.-L.; Lu, C.-H. Fracture propagation mechanism and application of supercritical CO2 fracturing in shale: A review. Pet. Sci. 2025, 22, 1625–1652. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, Q.; Guo, J.; Zeng, B.; Zhang, Y.; He, S. Mechanisms of shale hydration and water block removal. Pet. Explor. Dev. 2021, 48, 646–653. [Google Scholar] [CrossRef]
- Tang, W.; Zhou, F.; Sheng, J.J.; Wang, X.; Jiang, T. Further investigation of CO2 energization fracturing in shale reservoir- from microscopic mechanism to field application. Fuel 2025, 385, 134156. [Google Scholar] [CrossRef]
- Zhong, P.; Liu, C.; Wei, C.; Zhou, W.; Tian, S.; Wu, J. Experimental Study on Etched Fracture Conductivity in Carbonate Rocks for Supercritical CO2 Prepad Acid Fracturing#br#. Xinjiang Oil Gas 2024, 20, 60–69. Available online: http://www.zgxjog.com/CN/Y2024/V20/I4/60 (accessed on 4 November 2025).
- Xiao, W.; Chen, S.; Yi, Y.; Chen, H.; Ren, J. NMR-Based Experiments of Fracturing Fluid Assisted CO2 Huff-n-Puff for Enhancing Shale Oil Recovery. Xinjiang Oil Gas 2024, 20, 83–90. Available online: http://www.zgxjog.com/CN/Y2024/V20/I3/83 (accessed on 4 November 2025).
- Zou, Y.; Li, Y.; Li, S. Influence of CO2 pre-injection on fracture mophology and the petrophysical properties in shale fracturing. Nat. Gas Ind. 2021, 41, 83–94. [Google Scholar] [CrossRef]
- Dai, X.; Wei, J.; Li, Y.; Yang, Y. Study on parameters influencing fracture propagation and the influence of sensitive parameters on CO2 enhanced oil reservoirs in Gulong shale oil. Geoenergy Sci. Eng. 2025, 257, 214197. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Q.; Yang, Y.; Zhang, F.; Cao, G.; Zhang, L.; Yang, F.; Lu, M. Study and application of CO2 pre-fracturing technology of continental shale oil in Shengli Oilfield. Fault-Block Oil Gas Field 2024, 31, 945–954. [Google Scholar]
- Yang, S.; Cai, M.; Zhang, K.; Cao, D.; Zhao, X.; Liu, S. Research progress and prospect of CO2-water-rock interaction on petrophysi cal properties of CO2 geological sequestration. Pet. Geol. Recovery Effic. 2023, 30, 80–91. [Google Scholar]
- Zhang, L.; Wei, Q.; Liao, G.; Lv, W.; Yang, Y.; Zhao, Y.; Tian, Y. Current Status, Reflections and Prospects of CCUS-EOR Development in China Under the Dual-Carbon Goals. Drill. Prod. Technol. 2025, 48, 10–20. [Google Scholar]
- Tang, Y.; Jia, C.; Chen, F.; He, W.; Zhi, D.; Shan, X.; You, X.; Jiang, L.; Zou, Y.; Wu, T.; et al. Relationship between pore throat structure and crude oil mobility of full particle sequence reservoirs in Permian Fengcheng Formation, Mahu Sag, Junggar Basin, NW China. Pet. Explor. Dev. 2025, 52, 99–111. [Google Scholar] [CrossRef]
- He, W.; Wang, G.; Chen, F.; Ren, H.; Gao, G.; Zou, Y.; Han, Y.; Liu, X.; Yu, X. Pore-throat structures and lower limits of fluid-movable pore-throat radii in conventional and unconventional reservoirs of Fengcheng Formation in Mahu sag, Junggar Basin. Acta Pet. Sin. 2025, 46, 1327–1341. [Google Scholar]
- Feng, Y.; Tang, Y.; Qin, Z.; Yang, S.; Zhou, Y.; Ablimit, Y.; Wang, W.; Bai, Y. Sedimentary characteristics and palaoenvironmental evolution of Fengcheng Formation in the northern slope zone of Mahu sag, Junggar Basin. Acta Pet. Sin. 2025, 46, 1308–1326. [Google Scholar]
- Wang, S.; Wang, G.-W.; Wang, H.-Z.; Liu, M.-J.; Huang, L.-L.; Huang, Y.-Y.; Wang, Z.-S.; Li, S.-Q. Logging evaluation of acoustic anisotropy and its relationship with “sweet spots” in lacustrine shale oil reservoirs: The Fengcheng Formation of the Mahu Sag, China. Pet. Sci. 2025, 22, 3133–3151. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, S.; Deng, Y.; Liu, L.; Lei, X.; Niu, Y. Pore Throat Structures and Fluid Occurrences of Reservoirs in Fengcheng Formation, Mahu Sag. Xinjiang Pet. Geol. 2024, 45, 286–295. [Google Scholar]
- Lv, J.-H.; Hu, T.; Zhang, W.; Jiang, F.-J.; Xue, J.; Zhang, C.-X.; Qi, Z.-G.; Huang, R.-D.; Hu, M.-L.; Jiang, S. Microscopic oil occurrence in the Permian alkaline lacustrine shales: Fengcheng formation, Mahu Sag, Junggar basin. Pet. Sci. 2025, 22, 1407–1427. [Google Scholar] [CrossRef]
- Li, N.; Jin, Z.; Zhang, S.; Wang, H.; Yang, P.; Zou, Y.; Zhou, T. Micro-mechanical properties of shale due to water/supercritical carbon dioxide-rock interaction. Pet. Explor. Dev. 2023, 50, 872–882. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Li, Y.; Huo, Y.; Li, C.; Zhang, D.; Lin, K.; Yi, C.; Xue, Y. Combining pore structure types and reservoir forming limits to determine the grading evaluation criteria of Chang 7 tight oil reservoirs in Jiyuan Area, Ordos Basin. Desalination Water Treat. 2023, 314, 339–351. [Google Scholar] [CrossRef]
- Wang, R.; Shi, W.; Xie, X.; Zhang, W.; Qin, S.; Liu, K.; Busbey, A.B. Clay mineral content, type, and their effects on pore throat structure and reservoir properties: Insight from the Permian tight sandstones in the Hangjinqi area, north Ordos Basin, China. Mar. Pet. Geol. 2020, 115, 104281. [Google Scholar] [CrossRef]
- Zhang, M.-B.; Pan, H.-J.; Wang, Y.-G.; Du, M.; Cai, S.-J.; Liu, F.; Ju, M.-X. Seismic pore-type characterization in tight carbonate reservoirs: A case study of the fourth member of Ordovician Majiagou Formation in Ordos Basin, China. Pet. Sci. 2025, 22, 3583–3598. [Google Scholar] [CrossRef]
- Xie, R.; Wang, X.; Zhao, Z.; Zhou, W. First-Principles Calculation of the Electronic Structure and (104) Surface Properties of Calcite Crystals. Met. Mine 2023, 12, 93–98. [Google Scholar] [CrossRef]
- Ning, M.; Liang, Z.; Feng, Y.; Xia, P.; Shen, B.; Wen, H. Current Methodologies and Emerging Trends in Dolomite Research: Review and Perspectives. Acta Sedimentol. Sin. 2025, 43, 1814–1856. [Google Scholar] [CrossRef]
- Gao, H.; Li, X.; Zhu, G.; Li, S.; Wang, R.; Hou, J.; Zhang, J.; Zheng, K. A review of dolomite genesis analysis based on crystal nucleation-growth thermodynamic and kinetic. Earth Sci. Front. 2025, 32, 165–189. [Google Scholar] [CrossRef]
- Zou, Y.; Li, S.; Ma, X.; Zhang, S.; Li, N.; Chen, M. Effects of CO2–brine–rock interaction on porosity/permeability and mechanical properties during supercritical-CO2 fracturing in shale reservoirs. J. Nat. Gas Sci. Eng. 2018, 49, 157–168. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, P.; Huang, T.; Yan, C.; Liu, J.; Wang, B.; Zhang, B.; Zhang, Y. Study on the influence of CO2-water-rock reactions under reservoir conditions on geochemical properties of sandstone reservoirs. Pet. Reserv. Eval. Dev. 2025, 15, 545–553. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).