Boosting Hydrogen Generation with Platinum Nanoparticles Decorated on HTiNbO5 via NaBH4 Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of KTiNbO5, HTiNbO5, or Ns-HTiNbO5 Decorated with Platinum Nanoparticles
2.3. Characterization of the Materials
2.4. Application of Materials in Hydrogen Evolution from NaBH4
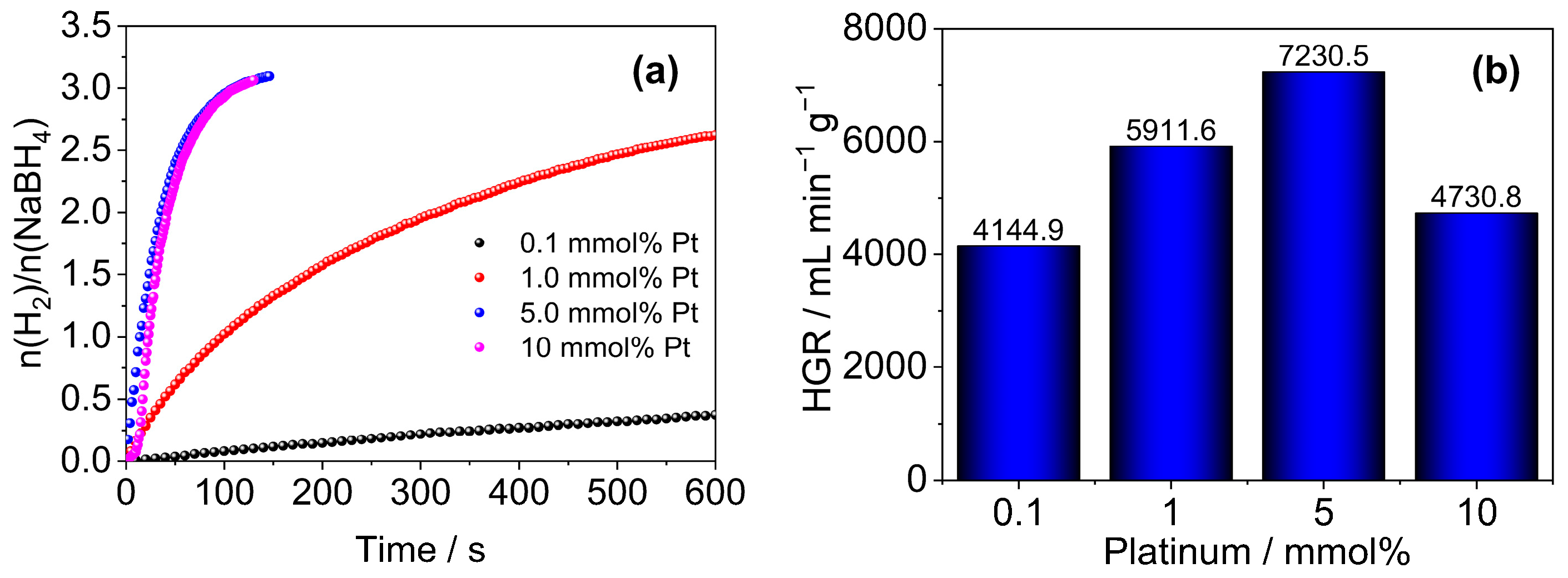
2.5. Evaluation of the Effect of Pt NPs Dose
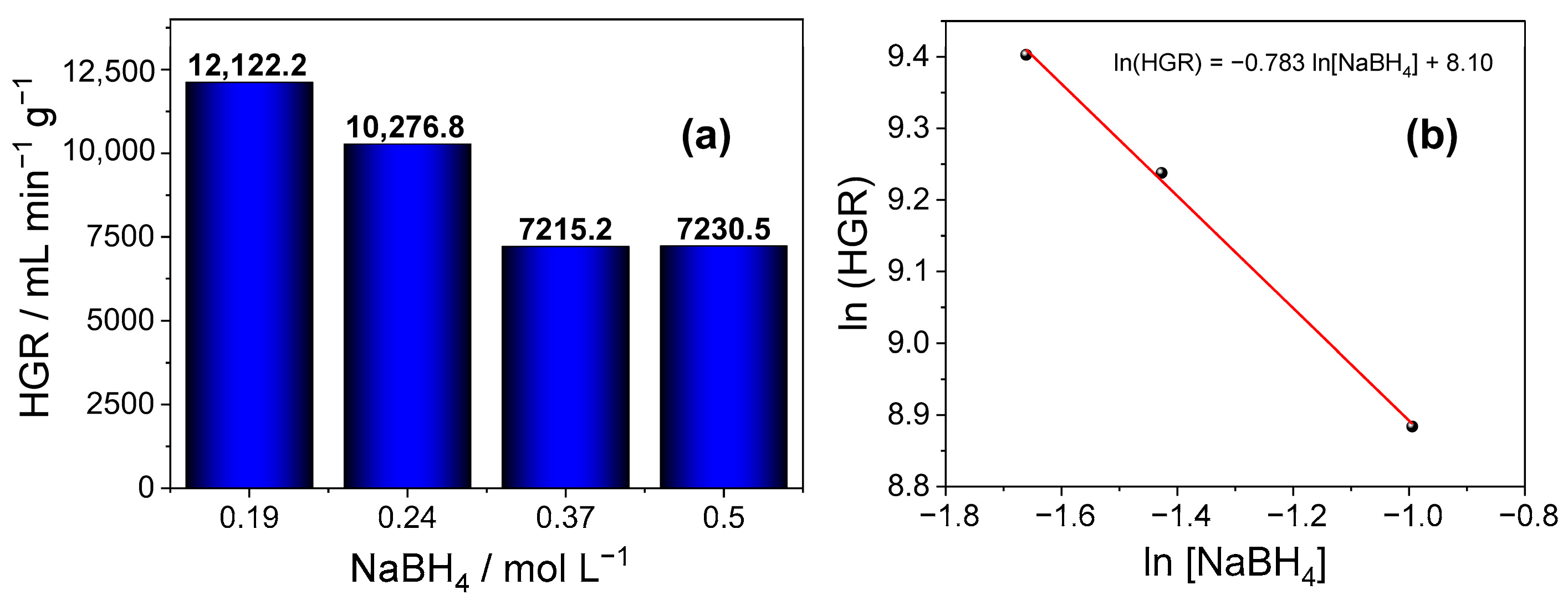
2.6. Evaluation of the Effect of NaBH4 Concentration
2.7. Evaluation of the Effect of NaOH
2.8. Kinetic Evaluation
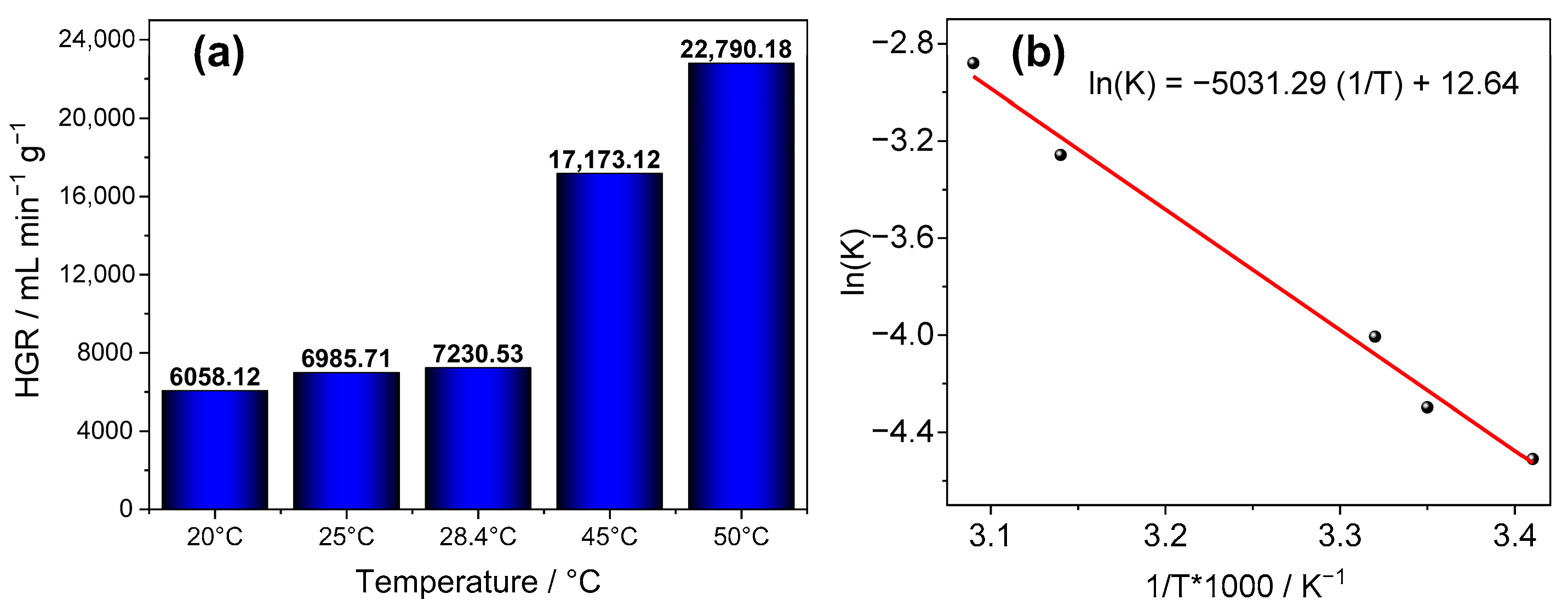
2.9. Evaluation of the Effect of Temperature
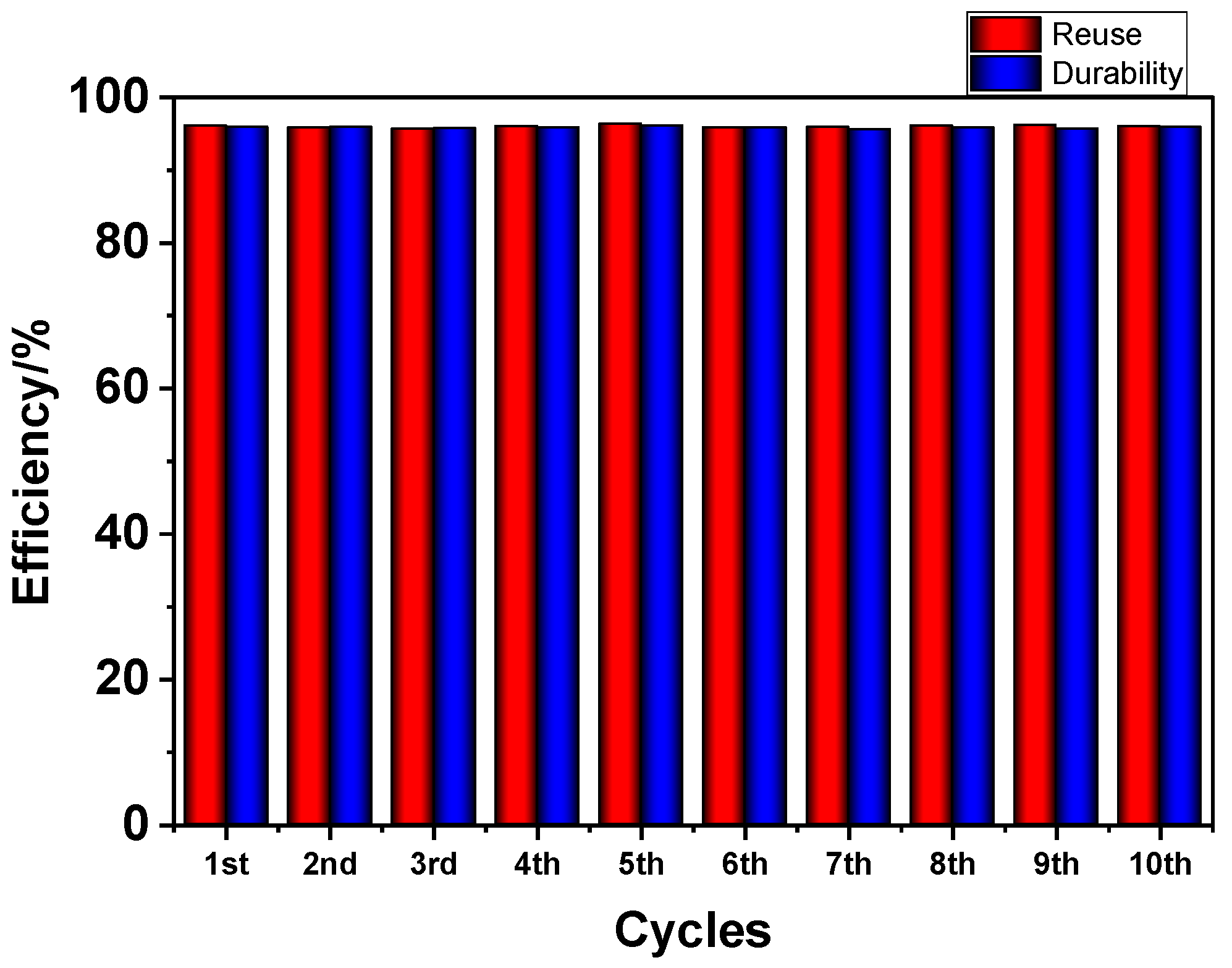
2.10. Durability and Reuse Assays
2.11. Kinetic Isotope Effect (KIE) Evaluation
3. Results
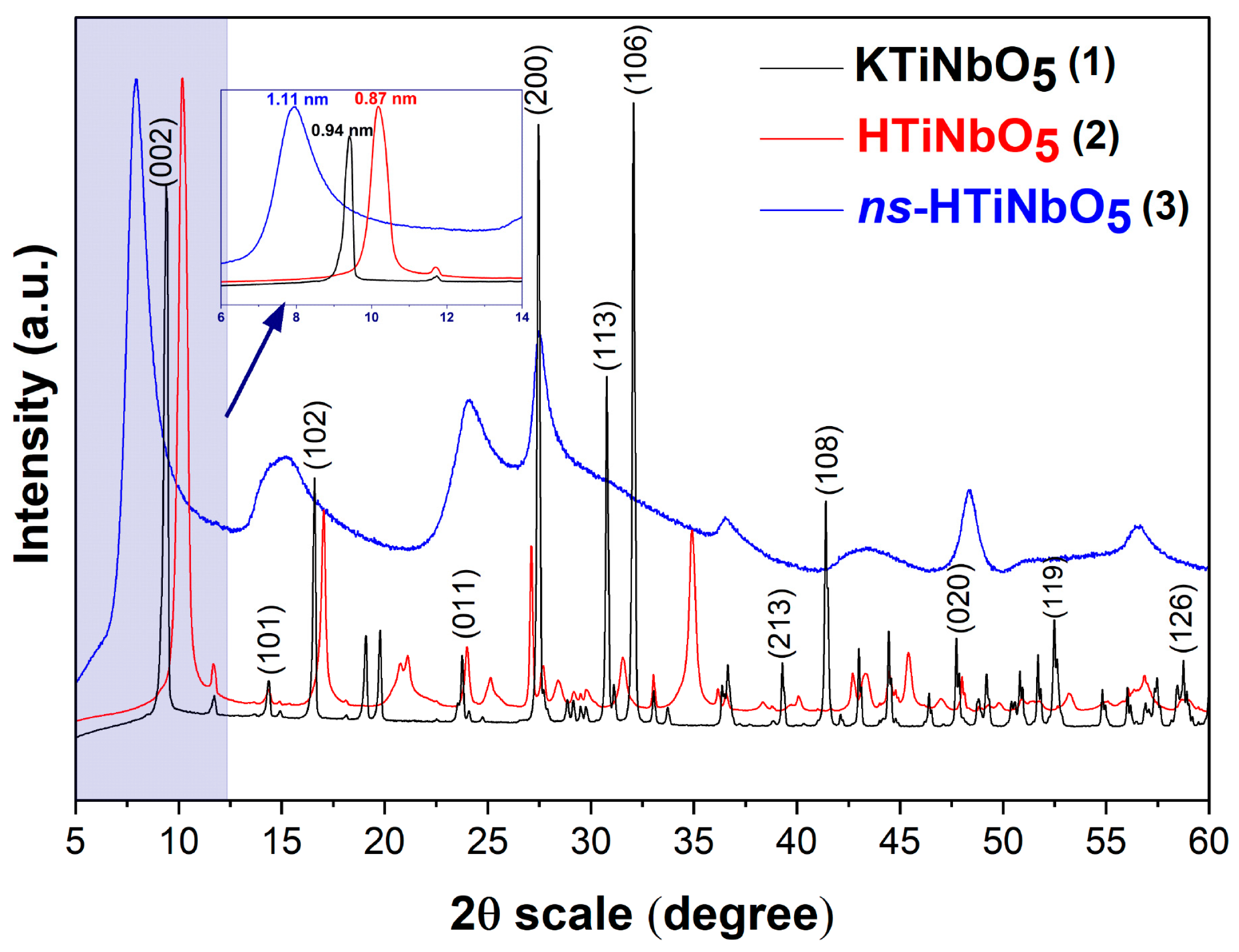
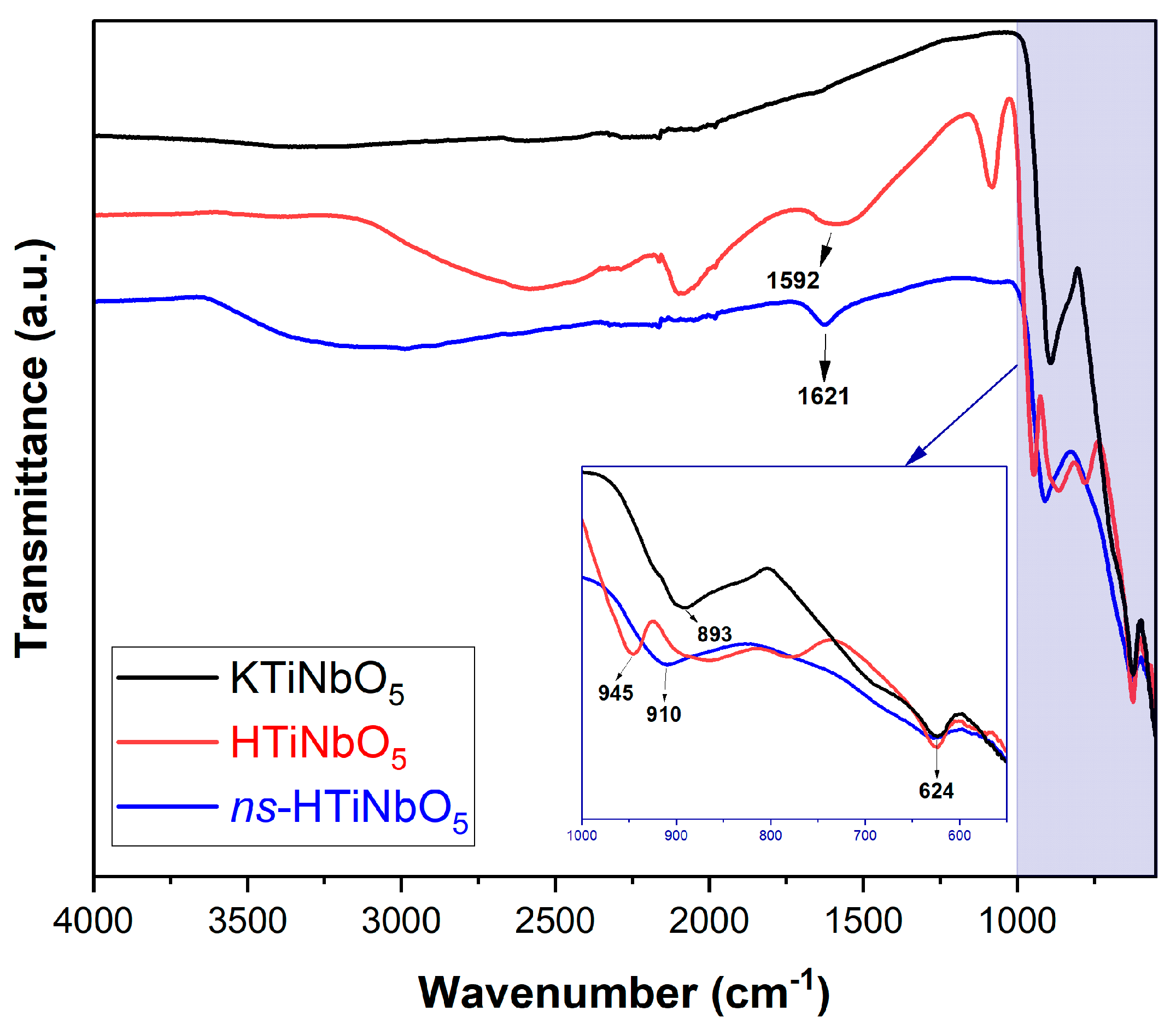
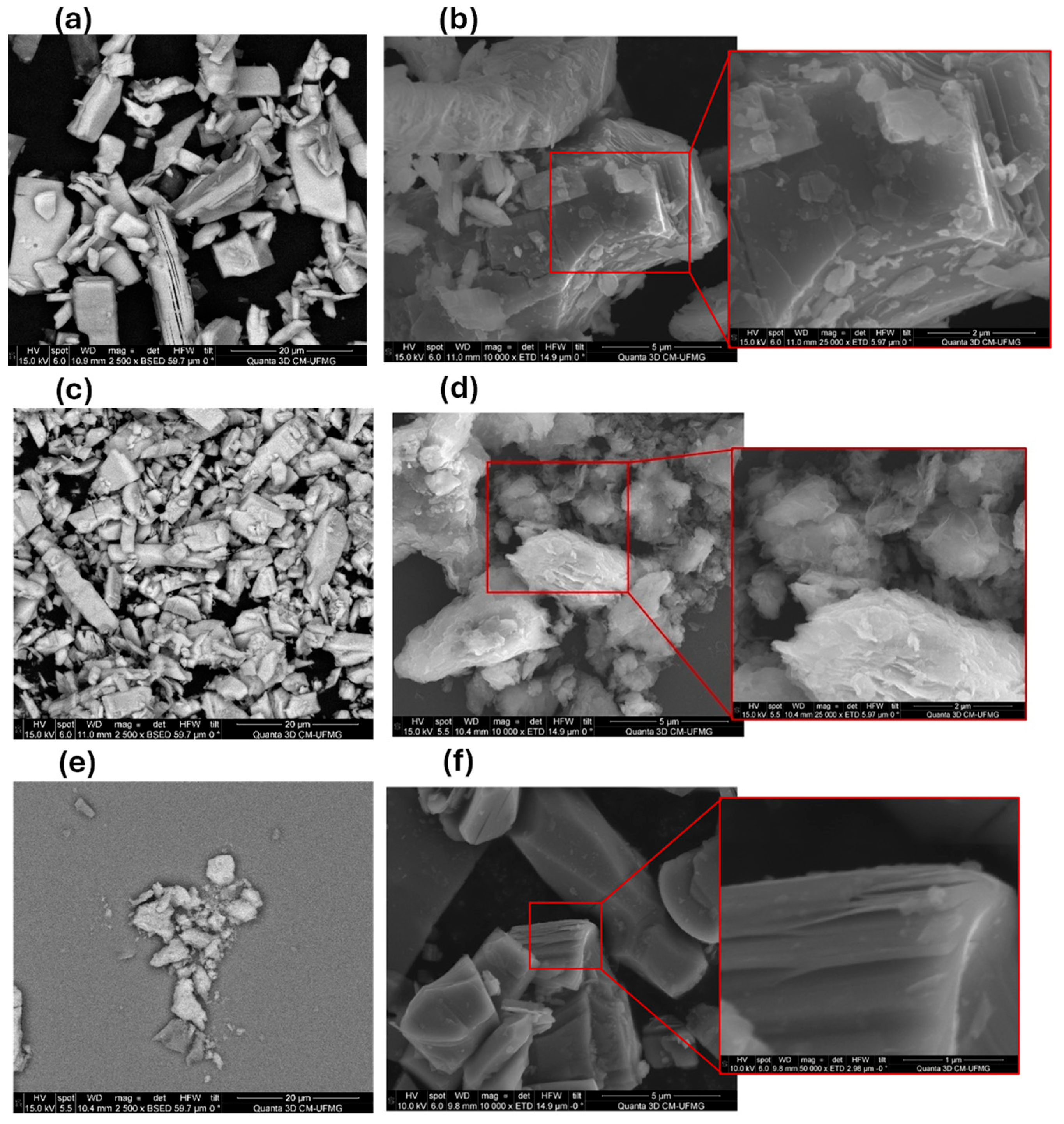
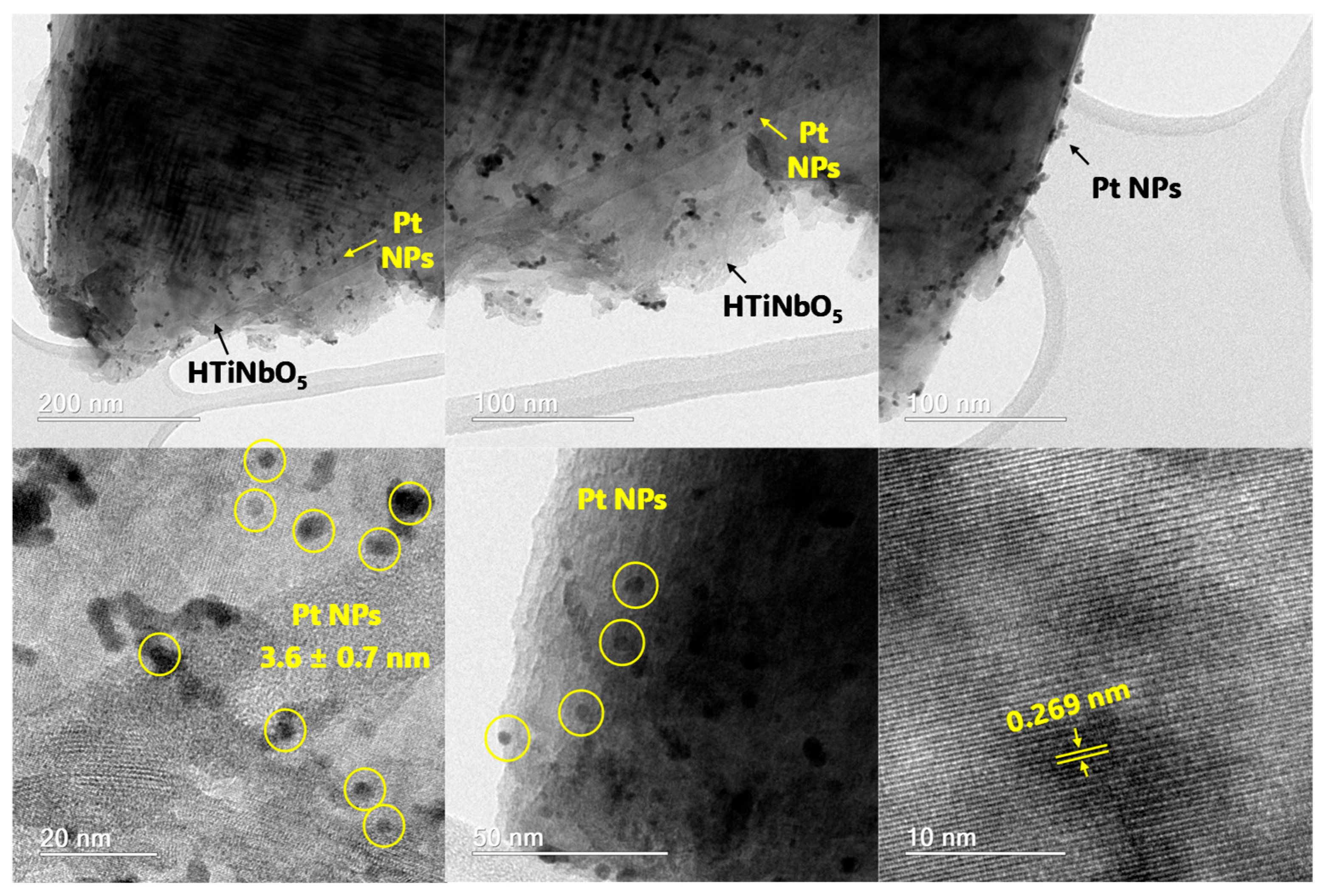
3.1. Material Characterization
3.2. Material Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sperandio, G.; Junior, I.M.; Bernardo, E.; Moreira, R. Graphene Oxide from Graphite of Spent Batteries as Support of Nanocatalysts for Fuel Hydrogen Production. Processes 2023, 11, 3250. [Google Scholar] [CrossRef]
- Bousada, G.M.; da Silva, V.; de Souza, B.; de Oliveira, R.S.; Machado Junior, I.; da Cunha, C.H.F.; Astruc, D.; Teixeira, R.R.; Lopes Moreira, R.P. Niobic Acid as a Support for Microheterogeneous Nanocatalysis of Sodium Borohydride Hydrolysis under Mild Conditions. RSC Adv. 2024, 14, 19459–19471. [Google Scholar] [CrossRef]
- Incer-Valverde, J.; Korayem, A.; Tsatsaronis, G.; Morosuk, T. “Colors” of Hydrogen: Definitions and Carbon Intensity. Energy Convers. Manag. 2023, 291, 117294. [Google Scholar] [CrossRef]
- Junior, I.M.; Sperandio, G.H.; Lopes, R.P. Efficient Hydrogen Evolution from NaBH4 Using Bimetallic Nanoparticles (Ni–Co) Supported on Recycled Zn–C Battery Electrolyte Paste. Int. J. Hydrogen Energy 2024, 53, 1323–1331. [Google Scholar] [CrossRef]
- Lopes, R.P.; Zhao, Q.; Moya, S.; Moro, M.M.; Astruc, D. Gold Nanoparticles Supported on Triazole-Functionalized Biochar as Nanocatalyst for Hydrogen Evolution from Aqueous Solution. J. Braz. Chem. Soc. 2024, 35, e-20230122. [Google Scholar] [CrossRef]
- Ahmad, S.; Ullah, A.; Samreen, A.; Qasim, M.; Nawaz, K.; Ahmad, W.; Alnaser, A.; Kannan, A.M.; Egilmez, M.J. Hydrogen production, storage, transportation and utilization for energy sector: A current status review. Energy Storage 2024, 101, 113733. [Google Scholar] [CrossRef]
- Wadsley, A.D. Alkali Titanoniobates. The Crystal Structures of KTiNbO5 and KTi3NbO9. Acta Crystallogr. 1964, 17, 623–628. [Google Scholar] [CrossRef]
- Wang, L.; Sasaki, T. Titanium Oxide Nanosheets: Graphene Analogues with Versatile Functionalities. Chem. Rev. 2014, 114, 9455–9486. [Google Scholar] [CrossRef] [PubMed]
- Marchand, R.; Brohan, L.; Tournoux, M. TiO2(B) a New Form of Titanium Dioxide and the Potassium Octatitanate K2Ti8O17. Mater. Res. Bull. 1980, 15, 1129–1133. [Google Scholar] [CrossRef]
- Akter, T.; Saupe, G.B. Exceptional Sensitizer Dye Loading via a New Porous Titanium-Niobium Metal Oxide with Tris(2,2′-Bipyridyl)Ruthenium(II) in the Structure. ACS Appl. Nano Mater. 2018, 1, 5620–5630. [Google Scholar] [CrossRef]
- Kim, Y.I.; Atherton, S.J.; Brigham, E.S.; Mallouk, T.E. Sensitized Layered Metal Oxide Semiconductor Particles for Photochemical Hydrogen Evolution from Nonsacrificial Electron Donors. J. Phys. Chem. 1993, 97, 11802–11810. [Google Scholar] [CrossRef]
- Takagaki, A. Production of 5-Hydroxymethylfurfural from Glucose in Water by Using Transition Metal-Oxide Nanosheet Aggregates. Catalysts 2019, 9, 818. [Google Scholar] [CrossRef]
- Colin, J.-F.; Pralong, V.; Caignaert, V.; Hervieu, M.; Raveau, B. A Novel Layered Titanoniobate LiTiNbO5: Topotactic Synthesis and Electrochemistry versus Lithium. Inorg. Chem. 2006, 45, 7217–7223. [Google Scholar] [CrossRef] [PubMed]
- Akatsuka, K.; Takanashi, G.; Ebina, Y.; Haga, M.; Sasaki, T. Electronic Band Structure of Exfoliated Titanium- and/or Niobium-Based Oxide Nanosheets Probed by Electrochemical and Photoelectrochemical Measurements. J. Phys. Chem. 2012, 116, 12426–12433. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chang, Y.S. Photocatalytic Water Splitting on Au/HTiNbO5 Nanosheets. Int. J. Hydrogen Energy 2014, 39, 3118–3126. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chang, Y.S. Photocatalytic Water Splitting for Hydrogen Production on Au/KTiNbO5. Int. J. Hydrogen Energy 2010, 35, 8463–8471. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Shen, C.; Xu, X. Visible Light Active Titanoniobate Nanosheets for Efficient Photocatalytic H2 Production from Water. J. Catal. 2019, 377, 409–418. [Google Scholar] [CrossRef]
- Fan, X.; Lin, B.; Liu, H.; He, L.; Chen, Y.; Gao, B. Remarkable Promotion of Photocatalytic Hydrogen Evolution from Water on TiO2-Pillared Titanoniobate. Int. J. Hydrogen Energy 2013, 38, 832–839. [Google Scholar] [CrossRef]
- Sperandio, G.H.; de Carvalho, J.P.; de Jesus, C.B.R.; Junior, I.M.; de Oliveira, K.L.A.; Puiatti, G.A.; de Jesus, J.R.; Moreira, R.P.L. Evolution from NaBH4 Using Novel Ni/Pt Nanoparticles Decorated on a Niobium-Based Composite. Int. J. Hydrogen Energy 2024, 83, 774–783. [Google Scholar] [CrossRef]
- Rebbah, H.; Desgardin, G.; Raveau, B. Les Oxydes ATiMO5: Echangeurs Cationiques. Mater. Res. Bull. 1979, 14, 1125–1131. [Google Scholar] [CrossRef]
- Thomas, C.I.; Heiska, J.; Garg, N.; Karppinen, M. Chemical Intercalation and Electrochemical Deintercalation of 2-Aminoterephthalic Acid into the Layered Titanoniobate HTiNbO5. Solid State Ion. 2021, 360, 115535. [Google Scholar] [CrossRef]
- Zhai, Z.; Hu, C.; Yang, X.; Zhang, L.; Liu, C.; Fan, Y.; Hou, W. Nitrogen-Doped Mesoporous Nanohybrids of TiO2 Nanoparticles and HTiNbO5 Nanosheets with a High Visible-Light Photocatalytic Activity and a Good Biocompatibility. J. Mater. Chem. 2012, 22, 19122–19131. [Google Scholar] [CrossRef]
- Thomas, C.I.; Karppinen, M. Intercalation of Primary Alcohols into Layered Titanoniobates. Inorg. Chem. 2017, 56, 9132–9138. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, L.; Chen, J.; Liang, J.Y.; Li, C.S.; Ji, H.M.; Hou, W.H. The Nanocomposite of Polyaniline and Nitrogen-Doped Layered HTiNbO5 with Excellent Visible-Light Photocatalytic Performance. Phys. Chem. Chem. Phys. 2014, 16, 13409–13417. [Google Scholar] [CrossRef]
- Lambert, J.F.; Deng, Z.; d’Espinose, J.B.; Fripiat, J.J. The Intercalation Process of N-Alkyl Amines or Ammoniums within the Structure of KTiNbO5. J. Colloid Interface Sci. 1989, 132, 337–351. [Google Scholar] [CrossRef]
- Du, G.H.; Yu, Y.; Chen, Q.; Wang, R.H.; Zhou, W.; Peng, L.-M. Exfoliating KTiNbO5 particles into nanosheets. Chem. Phys. Lett. 2003, 377, 445–448. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, C.; Cheng, L.; Ding, W.; Hou, W.; Chen, J. S-doped HTiNbO5 nanosheets: A novel efficient visible-light photocatalyst. Chin. J. Catal. 2013, 34, 2089–2097. [Google Scholar] [CrossRef]
- Oh, L.S.; Han, J.; Lim, E.; Kim, W.B.; Kim, H.J. PtCu Nanoparticle Catalyst for Electrocatalytic Glycerol Oxidation: How Does the PtCu Affect to Glycerol Oxidation Reaction Performance by Changing PH Conditions? Catalysts 2023, 13, 892. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Rodríguez-Pérez, I.A.; Miao, W.; Chen, K.; Wang, W.; Li, Y.; Han, S. Carbon Nanospheres Supported Bimetallic Pt-Co as an Efficient Catalyst for NaBH4 Hydrolysis. Appl. Surf. Sci. 2021, 540, 148296. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Reaction Mechanisms of the Hydrolysis of Sodium Borohydride: A Discussion Focusing on Cobalt-Based Catalysts. Comptes Rendus Chim. 2014, 17, 707–716. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Sun, X.; Ortega, J. Kinetics of Catalytic Hydrolysis of Stabilized Sodium Borohydride Solutions. J. Ind. Eng. Chem. Res. 2007, 46, 1120–1124. [Google Scholar] [CrossRef]
- Doherty, S.; Knight, J.G.; Alharbi, H.Y.; Paterson, R.; Wills, C.; Dixon, C.; Šiller, L.; Chamberlain, T.W.; Griffiths, A.; Collins, S.M.; et al. Efficient Hydrolytic Hydrogen Evolution from Sodium Borohydride Catalyzed by Polymer Immobilized Ionic Liquid-Stabilized Platinum Nanoparticles. ChemCatChem 2022, 14, e202101752. [Google Scholar] [CrossRef]
- Farrag, M.; Ali, G.A.M. Hydrogen Generation of Single Alloy Pd/Pt Quantum Dots over Co3O4 Nanoparticles via the Hydrolysis of Sodium Borohydride at Room Temperature. Sci. Rep. 2022, 12, 17040. [Google Scholar] [CrossRef] [PubMed]
- Altaf, C.T.; Minkina, V.G.; Shabunya, S.I.; Colak, T.O.; Sankir, N.D.; Sankir, M.; Kalinin, V.I. Ruthenium and Platinum-Modified Titanium Dioxide Support for NaBH4 Hydrolysis. ACS Omega 2023, 8, 36100–36108. [Google Scholar] [CrossRef]
- Uzundurukan, A.; Devrim, Y. Hydrogen Generation from Sodium Borohydride Hydrolysis by Multi-Walled Carbon Nanotube Supported Platinum Catalyst: A Kinetic Study. Int. J. Hydrogen Energy 2019, 44, 17586–17594. [Google Scholar] [CrossRef]
- Yu, Y.; Kang, L.; Sun, L.; Xu, F.; Pan, H.; Sang, Z.; Zhang, C.; Jia, X.; Sui, Q.; Bu, Y.; et al. Bimetallic Pt-Ni Nanoparticles Confined in Porous Titanium Oxide Cage for Hydrogen Generation from NaBH4 Hydrolysis. Nanomaterials 2022, 12, 2550. [Google Scholar] [CrossRef] [PubMed]
- Irum, M.; Zaheer, M.; Friedrich, M.; Kempe, R. Mesoporous Silica Nanosphere Supported Platinum Nanoparticles (Pt@MSN): One-Pot Synthesis and Catalytic Hydrogen Generation. RSC Adv. 2016, 6, 10438–10441. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, C.; Wu, F.; Yi, B. Carbon-supported platinum catalysts for on-site hydrogen generation from NaBH4 solution. Mater. Lett. 2006, 60, 2236–2239. [Google Scholar] [CrossRef]
| Catalyst | HGR/mLH2·min−1·g−1 | Activation Energy (Ea) | Reference |
|---|---|---|---|
| Niobium-based nanocomposite decorated with Ni/Pt nanoparticles. | 1782 | 23.1 | [19] |
| Carbon nanospheres (CNSs) supporting ultrafine bimetallic Pt-Co nanoparticles (CNSs@Pt0.1Co0.9) | 8943 | 38.0 | [29] |
| Pd/Pt quantum dots over CO3O4 nanoparticles | 8333 | Not informed. | [33] |
| Pt/TiO2 | 800 (50 °C) | 53.2 | [34] |
| Multi-walled carbon nanotube supported platinum catalyst (Pt/MWCNT) | 3561.6 * | 27 | [35] |
| Bimetallic Pt-Ni nanoparticles confined in porous titanium oxide cage | 10,164.3 (29 °C) | 28.7 | [36] |
| Mesoporous silica nanosphere-supported platinum nanoparticles | 19,000 (80 °C) | 40.1 | [37] |
| Carbon-supported platinum catalysts (Pt/C) | 29,600 | Not informed. | [38] |
| Platinum nanoparticles decorated on HTiNbO5 | 22,790.18 (50 °C) | 41.83 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, J.P.; de Lima, G.M.; Machado, V.E.; de Souza, N.C.S.; Ardisson, J.D.; Silva, T.A.; de Andrade, F.V.; Moreira, R.P.L. Boosting Hydrogen Generation with Platinum Nanoparticles Decorated on HTiNbO5 via NaBH4 Hydrolysis. Processes 2025, 13, 3832. https://doi.org/10.3390/pr13123832
Gómez JP, de Lima GM, Machado VE, de Souza NCS, Ardisson JD, Silva TA, de Andrade FV, Moreira RPL. Boosting Hydrogen Generation with Platinum Nanoparticles Decorated on HTiNbO5 via NaBH4 Hydrolysis. Processes. 2025; 13(12):3832. https://doi.org/10.3390/pr13123832
Chicago/Turabian StyleGómez, Juliana Peña, Geraldo Magela de Lima, Veronica Evangelista Machado, Noemí Cristina Silva de Souza, José D. Ardisson, Tiago Almeida Silva, Fabrício Vieira de Andrade, and Renata Pereira Lopes Moreira. 2025. "Boosting Hydrogen Generation with Platinum Nanoparticles Decorated on HTiNbO5 via NaBH4 Hydrolysis" Processes 13, no. 12: 3832. https://doi.org/10.3390/pr13123832
APA StyleGómez, J. P., de Lima, G. M., Machado, V. E., de Souza, N. C. S., Ardisson, J. D., Silva, T. A., de Andrade, F. V., & Moreira, R. P. L. (2025). Boosting Hydrogen Generation with Platinum Nanoparticles Decorated on HTiNbO5 via NaBH4 Hydrolysis. Processes, 13(12), 3832. https://doi.org/10.3390/pr13123832








