Mercury Adsorption/Oxidation Mechanisms on Fly Ash Under N2 Atmosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
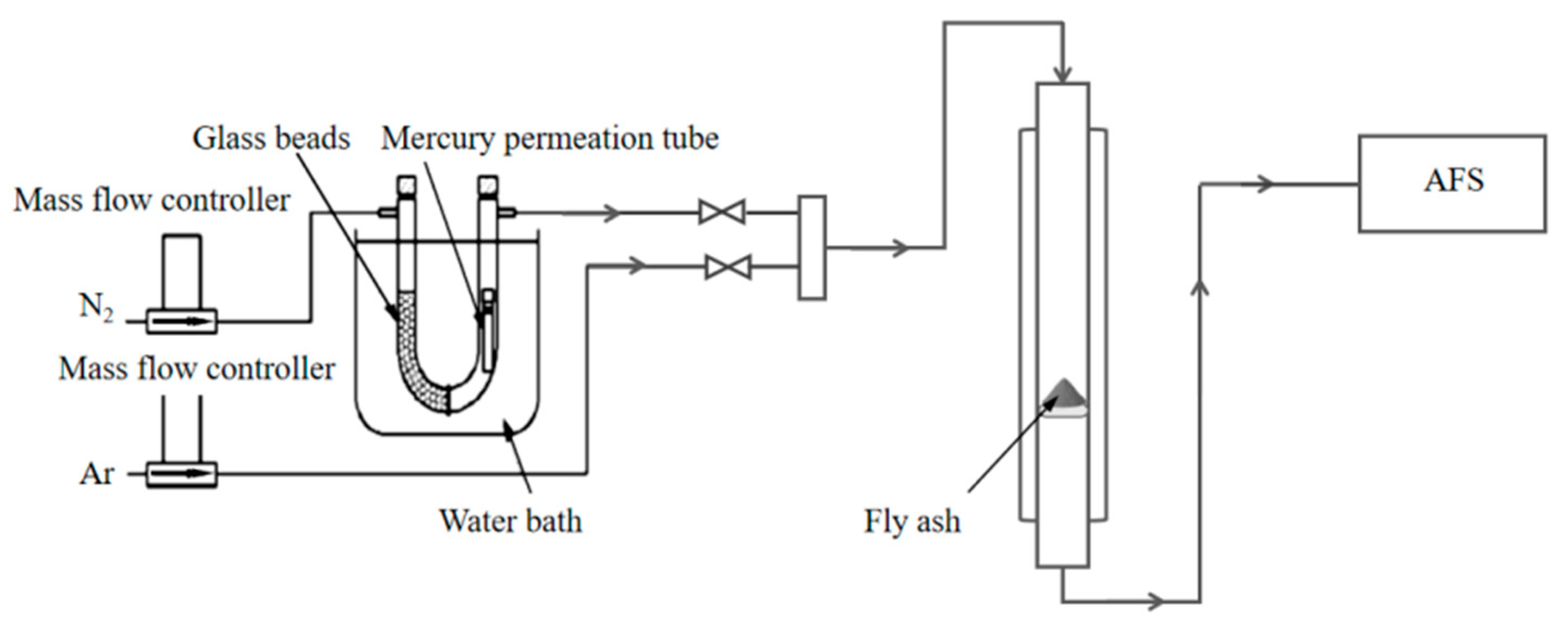
2.2. Thermal Decomposition and Mercury Adsorption Experiments
2.3. Adsorption Analysis
3. Results and Discussion
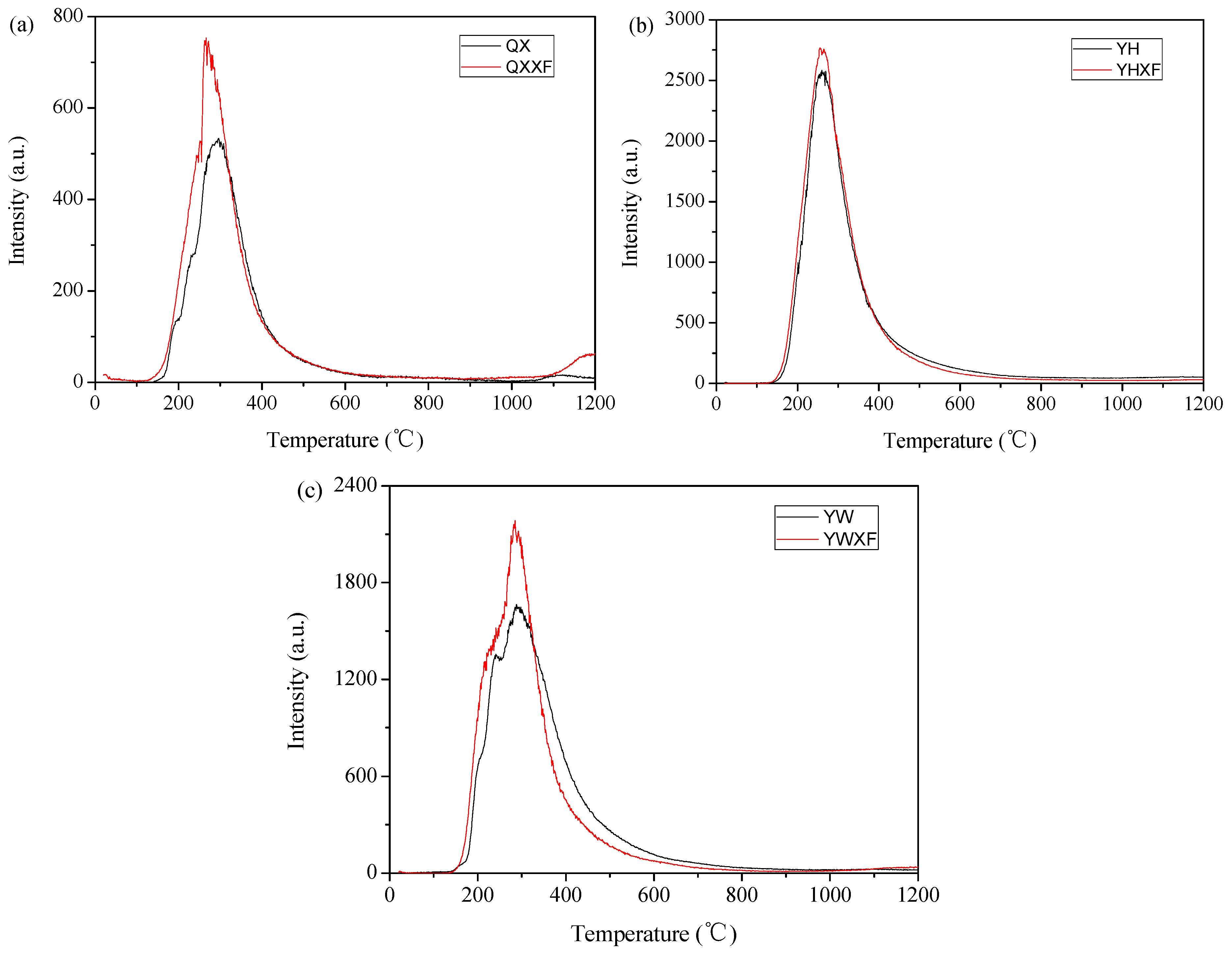
3.1. Physicochemical Properties of Fly Ash
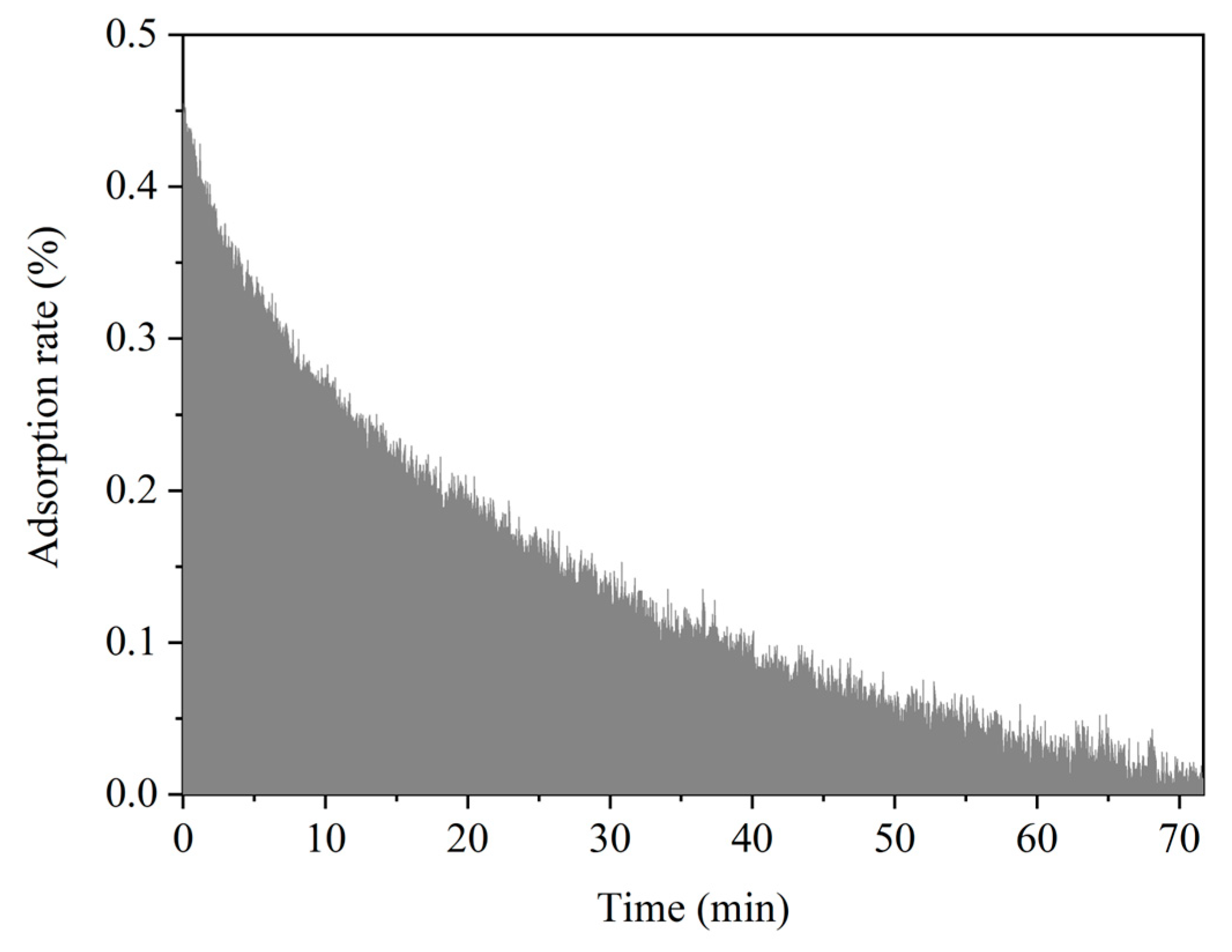
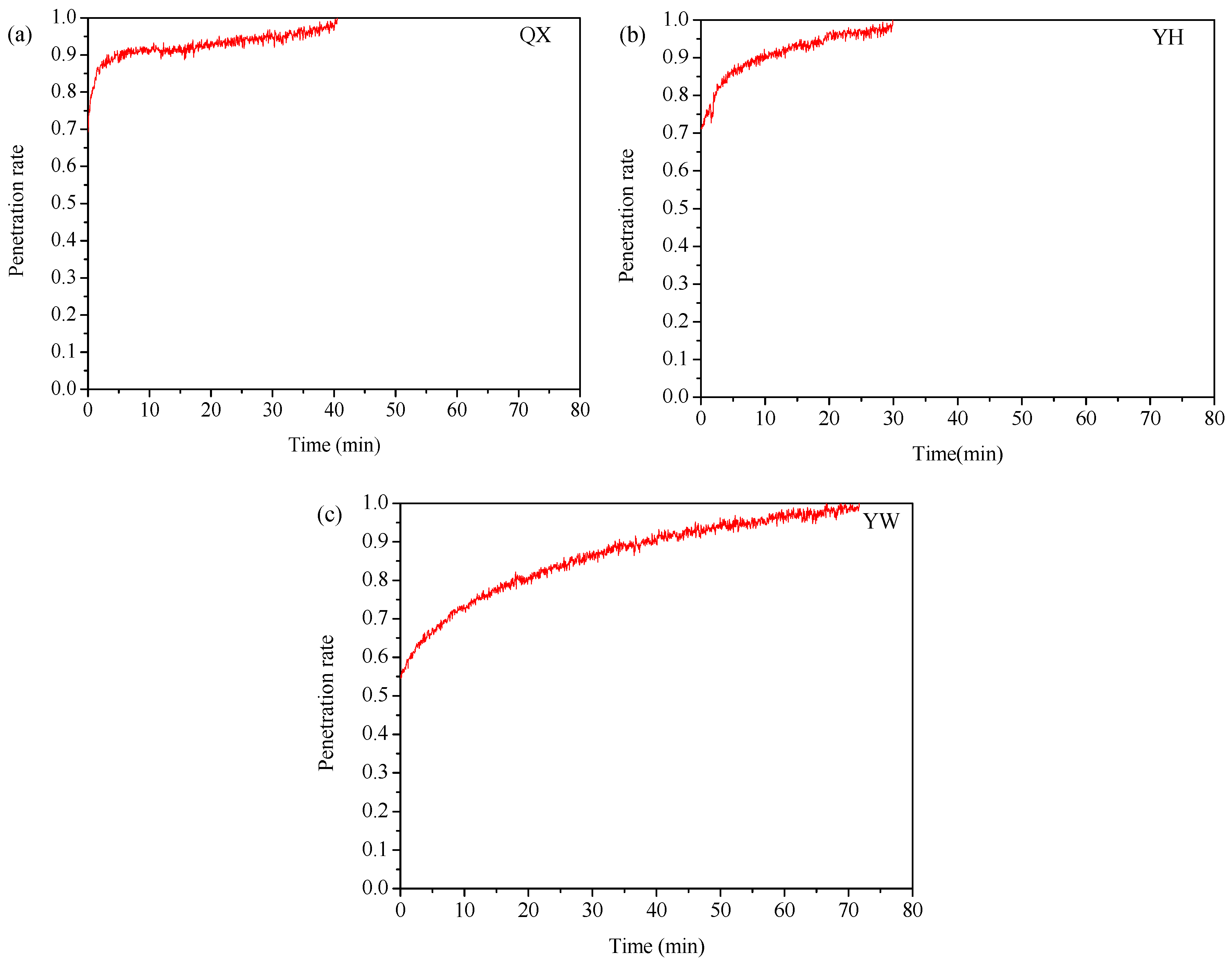
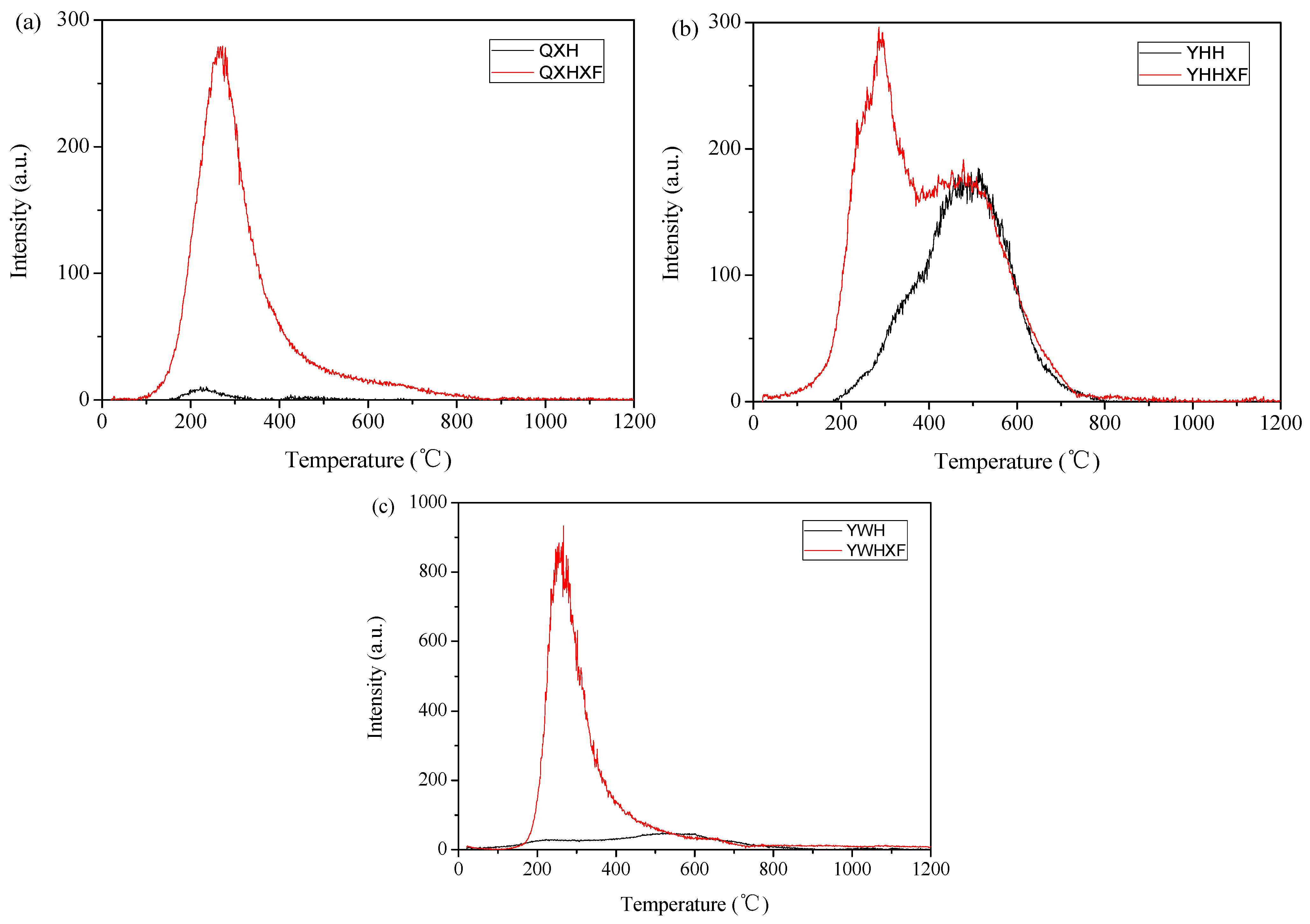
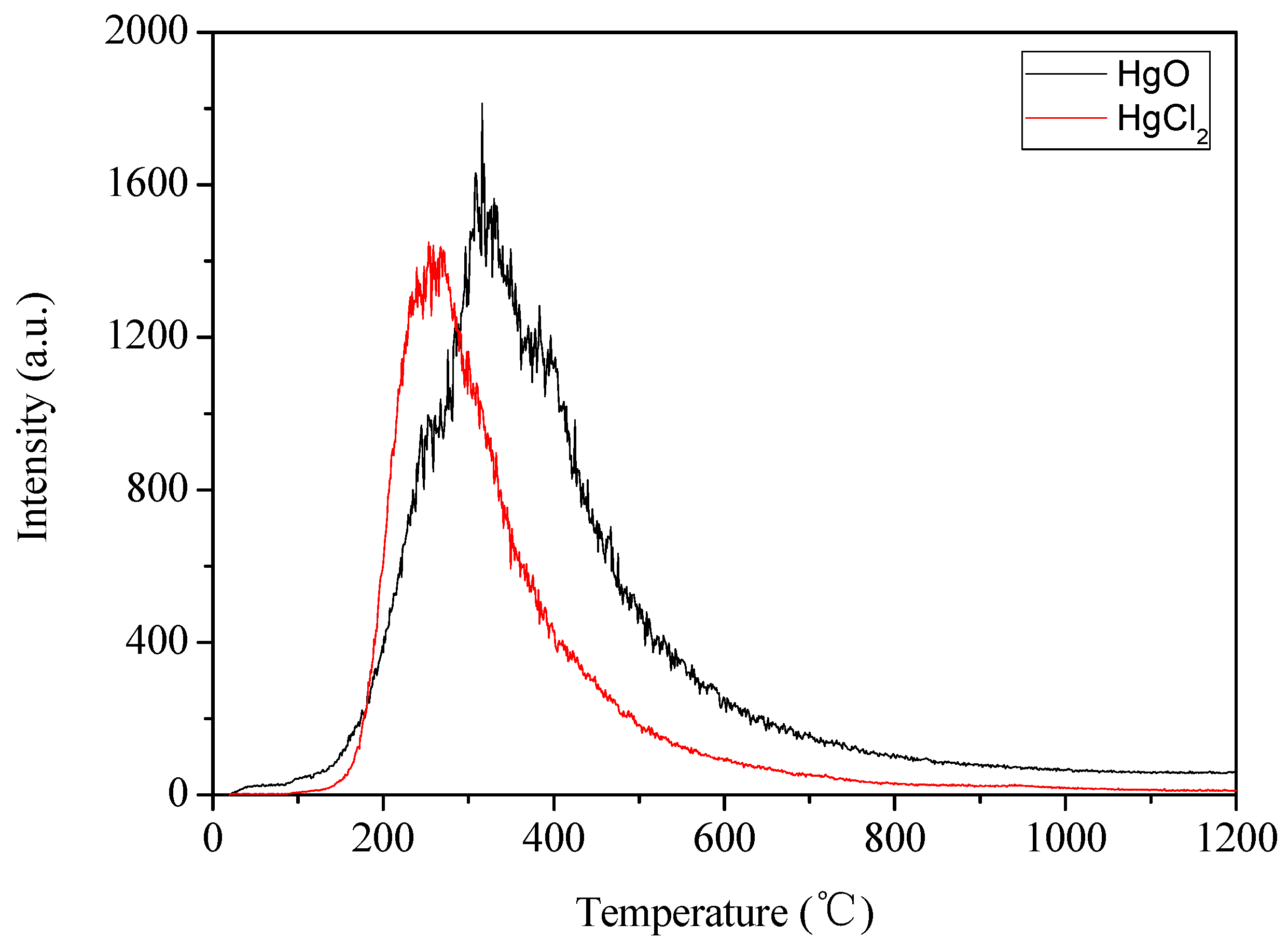
3.2. Adsorption/Oxidation Mechanisms Between Mercury and Fly Ash
3.3. Factors Affecting Mercury Adsorption/Oxidation on Fly Ash
3.3.1. Influence of Fly Ash Components
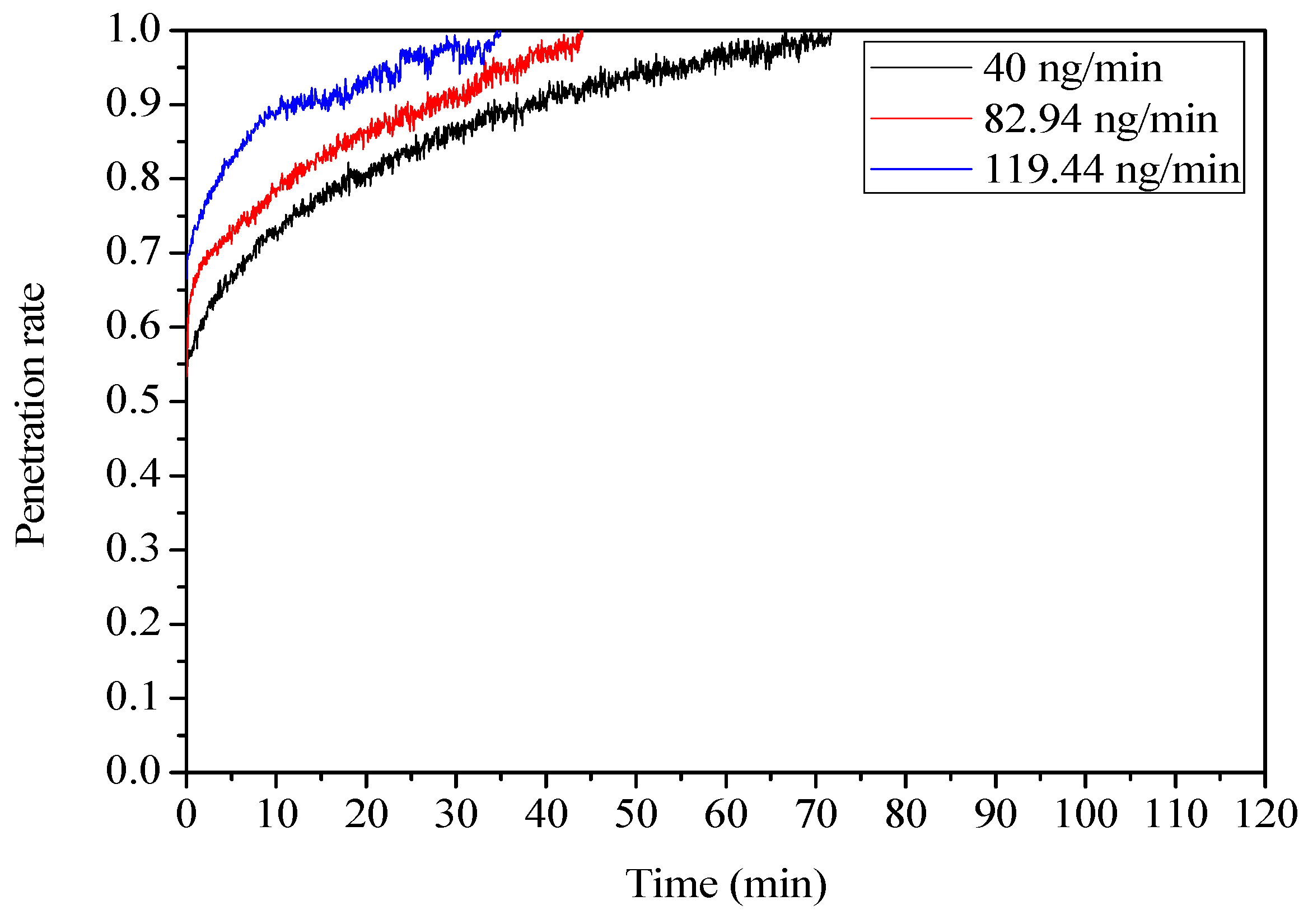
3.3.2. Influence of Mercury Inlet Concentration
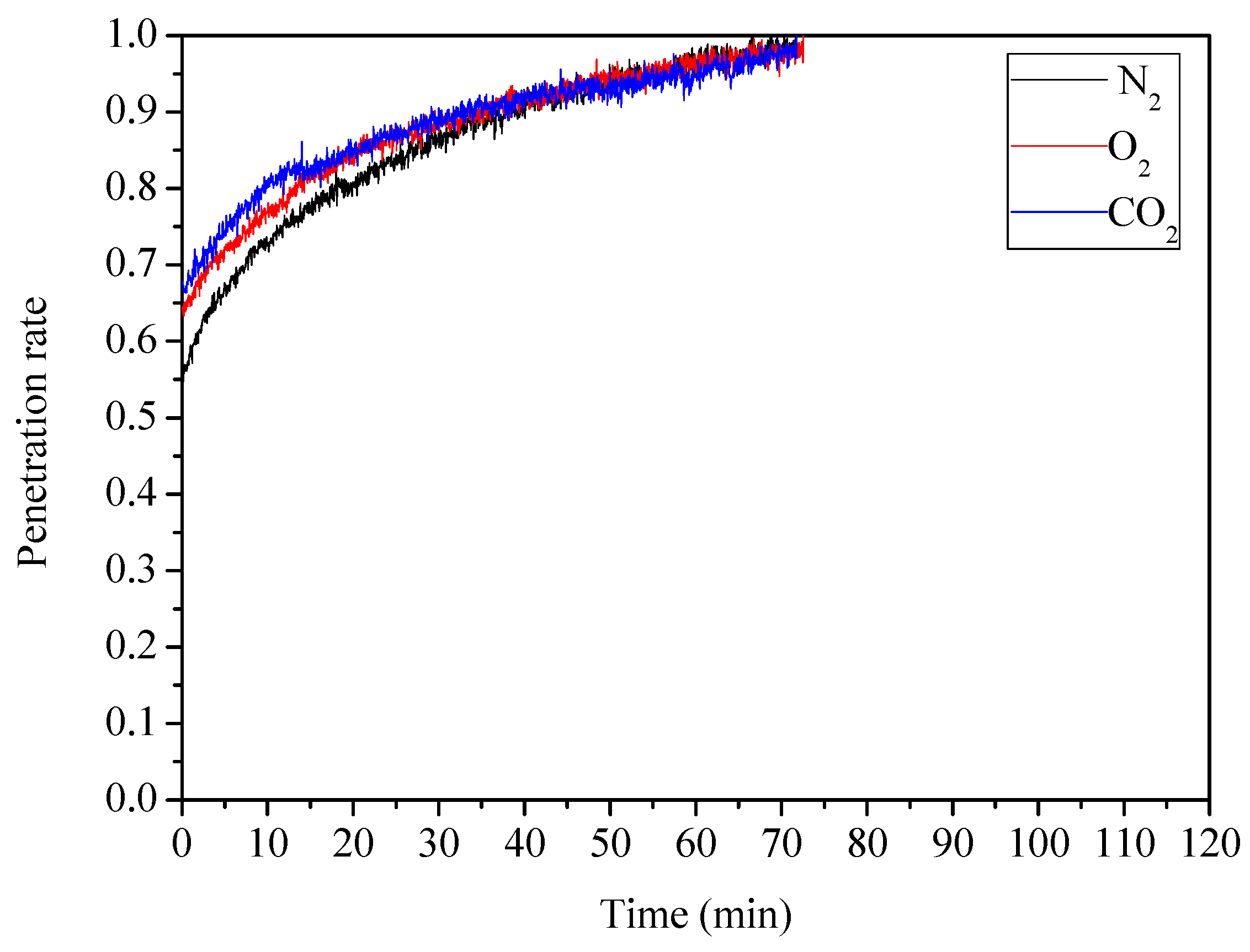
3.3.3. Influence of Adsorption Atmosphere
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TPD-AFS | temperature-programmed decomposition–atomic fluorescence spectroscopy |
| UBC | unburned carbon |
| SSA | content and the specific surface area |
| CFPPs | coal-fired power plants |
References
- Chen, Q.; Chen, L.; Li, J.; Guo, Y.; Wang, Y.; Wei, W.; Liu, C.; Wu, J.; Tou, F.; Wang, X.; et al. Increasing mercury risk of fly ash generated from coal-fired power plants in China. J. Hazard. Mater. 2022, 429, 128296. [Google Scholar] [CrossRef]
- Zhao, S.; Pudasainee, D.; Duan, Y.; Gupta, R.; Liu, M.; Lu, J. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies. Prog. Energy Combust. Sci. 2019, 73, 26–64. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, X.; Xiao, Y.D.; Wang, T.; Wang, J.; Zhang, Y. Long-term environmental stability of bromide coupled mechanical modified fly ash after mercury adsorption. J. Environ. Chem. Eng. 2023, 11, 1–12. [Google Scholar] [CrossRef]
- Gao, L.; Liu, K.; Guo, S.; Liu, K.; Liang, L.; Li, H. Release characteristics of elemental mercury during low calorific value coal combustion. R. Soc. Chem. 2022, 9, 1–10. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, T.; Gu, Y.; Wang, J.; Zhang, Y.; Pan, W. Impact of the mercury removal system using modified fly ash on particulate matter emission. Fuel 2021, 301, 121054. [Google Scholar] [CrossRef]
- Zhou, Q.; Duan, Y.; Chen, M.; Liu, M.; Lu, P.; Zhao, S. Effect of flue gas component and ash composition on elemental mercury oxidation/adsorption by NH4Br modified fly ash. Chem. Eng. J. 2018, 345, 578–585. [Google Scholar] [CrossRef]
- Ma, Z.; Qiu, Z.; Li, H.; Jiang, L.; Qian, Z.; Yuan, B.; Hao, R. Multimedia mercury recovery from coal-fired power plants utilizing N-containing conjugated polymer functionalized fly ash. Environ. Sci. Technol. 2024, 58, 2574–2583. [Google Scholar] [CrossRef]
- He, P.; Jiang, X.; Wu, J.; Pan, W.; Ren, J. Characterization of fly ash from coal-fired power plant and their properties of mercury retention. Surf. Rev. Lett. 2015, 22, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Wang, Z. Reaction mechanisms and chemical kinetics of mercury transformation during coal combustion. Prog. Energy Combust. Sci. 2020, 79, 100844. [Google Scholar] [CrossRef]
- Geng, X.; Zhong, L.; Liu, X.; Ding, X.; Liu, X.; Huang, T.; Xu, Y.; Duan, Y. Efficient Stabilization of Mercury-rich Fly Ash via Mechanochemical Method. Chem. Eng. J. 2023, 454, 140264. [Google Scholar] [CrossRef]
- Jia, L.; Fan, B.; Zheng, X.; Qiao, X.; Yao, Y.; Zhao, R.; Guo, J.; Jin, Y. Mercury emission and adsorption characteristics of fly ash in PC and CFB boilers. Front. Energy 2021, 15, 112–123. [Google Scholar] [CrossRef]
- Liu, T.; Man, C.; Guo, X.; Zheng, C. Experimental study on the mechanism of mercury removal with Fe2O3 in the presence of halogens: Role of HCl and HBr. Fuel 2016, 173, 209–216. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Hussain, A. A review on coal fly ash-based adsorbents for mercury and arsenic removal. J. Clean. Prod. 2020, 267, 122143. [Google Scholar] [CrossRef]
- Liu, L.; Chen, G.; Hu, H.; Huang, Y. Investigation of elemental mercury removal performance and mechanism of rice straw biochars from a fluidized bed pyrolysis system impregnated by NH4Br. Chem. Eng. J. 2024, 492, 152069. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Shen, F.; Liu, J.; Zhao, L.; Wang, Z.; Long, Y. Kinetic study of heterogeneous mercury oxidation by HCl on fly ash surface in coal-fired flue gas. Combust. Flame 2016, 168, 1–9. [Google Scholar] [CrossRef]
- Rumayor, M.; Svoboda, K.; Švehla, J.; Pohorely, M.; Syc, M. Mitigation of gaseous mercury emissions from waste-to-energy facilities: Homogeneous and heterogeneous Hg-oxidation pathways in presence of fly ashes. J. Environ. Manag. 2018, 206, 276–283. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Guo, R.; Wang, J.; Cao, Y.; Orndorff, W.; Pan, W.P. Influences of NO on mercury adsorption characteristics for HBr modified fly ash. Int. J. Coal Geol. 2017, 170, 77–83. [Google Scholar] [CrossRef]
- Peng, B.; Zhu, Z.L.; Hu, Z.; Xiang, L.; Peng, Y.; Peng, X.; Li, H. Removal of elemental mercury from simulated flue gas over MnOx/TiO2 adsorbent: The synergistic effect of anatase and brookite TiO2. J. Environ. Chem. Eng. 2025, 13, 115332. [Google Scholar] [CrossRef]
- Wang, F.; Wang, S.; Meng, Y.; Zhang, L.; Wu, Q.; Hao, J. Mechanisms and roles of fly ash compositions on the adsorption and oxidation of mercury in flue gas from coal combustion. Fuel 2016, 163, 232–239. [Google Scholar] [CrossRef]
- Zhai, J.; Guo, S.; Wei, X.X.; Cao, Y.; Gao, L. Characterization of the modes of occurrence of mercury and their thermal stability in coal gangues. Energy Fuels 2015, 29, 8239–8245. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, S. Research progress of mercury adsorption and oxidation mechanism on modified coal-fired fly ash. Chem. Ind. Eng. Prog. 2017, 36, 4257–4264. [Google Scholar]
- Liu, Z.; Liu, D.; Zhao, B.; Feng, L.; Ni, M.; Jin, J. Mercury removal based on adsorption and oxidation by fly ash: A review. Energy Fuels 2020, 34, 11840–11866. [Google Scholar] [CrossRef]
- Rumayor, M.; Gallego, J.R.; Rodríguez-Valdés, E.; Diaz-Somoano, M. An assessment of the environmental fate of mercury species in highly polluted brownfields by means of thermal desorption. J. Hazard. Mater. 2017, 325, 1–7. [Google Scholar]
- Li, X.; Teng, Y.; Wang, P.; Li, F.; Zhang, K. Release characteristics of mercury in fly ashes collected from coal-fired CFB power units during thermal treatment. Environmental Chemistry 2020, 39, 1375–1383. [Google Scholar]
- Lopez-Anton, M.A.; Perry, R.; Abad-Valle, P.; Diaz-Somoano, M.; Martinez-Tarazona, M.; Maroto-Valer, M. Speciation of mercury in fly ashes by temperature programmed decomposition. Fuel Process. Technol. 2011, 92, 707–711. [Google Scholar] [CrossRef]
- Zeng, H.; Jin, F.; Guo, J. Removal of elemental mercury from coal combustion flue gas by chloride-impregnated activated carbon. Fuel 2004, 83, 143–146. [Google Scholar] [CrossRef]
- Li, C.; Duan, Y.; Tang, H.; Zhu, C.; Li, Y.; Zhu, C.; Zheng, Y.; Liu, M. Study on the Hg emission and migration characteristics in coal-fired power plant of China with an ammonia desulfurization process. Fuel 2018, 211, 621–628. [Google Scholar] [CrossRef]
- Geng, X.; Liu, X.; Ding, X.; Zhou, Q.; Huang, T.; Duan, Y. Mechanochemical bromination of unburned carbon in fly ash and its mercury removal mechanism: DFT study. J. Hazard. Mater. 2022, 423, 127198. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. A retrospection on the content, association, and significance of mercury in coals and coal ashes from Bulgarian thermoelectric power stations. J. Hazard. Mater. 2023, 457, 131850. [Google Scholar] [CrossRef] [PubMed]
- Abad-Valle, P.; Lopez-Anton, M.A.; Diaz-Somoano, M.; Martinez-Tarazona, M. The role of unburned carbon concentrates from fly ashes in the oxidation and retention of mercury. Chem. Eng. J. 2011, 174, 86–92. [Google Scholar] [CrossRef]
- Hower, J.C.; Clack, H.L.; Hood, M.M.; Hopps, S.G.; Thomas, G.H. Impact of coal source changes on mercury content in fly ash: Examples from a Kentucky power plant. Int. J. Coal Geol. 2017, 170, 2–6. [Google Scholar] [CrossRef]
- Uaciquete, D.L.E.; Sakusabe, K.; Kato, T.; Okawa, H.; Kato, T.; Sugawara, K.; Nonaka, R. Influence of unburned carbon on mercury chemical forms in fly ash produced from a coal-fired power plant. Fuel 2021, 300, 120802. [Google Scholar] [CrossRef]
- Masoomi, I.; Kamata, H.; Yukimura, A.; Ohtsubo, K.; Schmid, M.; Scheffknecht, G. Investigation on the behavior of mercury across the flue gas treatment of coal combustion power plants using a lab-scale firing system. Fuel Process. Technol. 2020, 201, 106340. [Google Scholar] [CrossRef]
- Fan, B.; Jia, L.; Li, X.; Liu, J.; Zheng, X.; Jin, Y. Study on mercury adsorption by fly ash from coal-fired boilers of power plants. J. Chin. Soc. Power Eng. 2016, 36, 621–628. [Google Scholar]
- Zhu, M.; Duan, Y.; Geng, X.; Jin, Q.; Zhang, J. Effect of ball milling solution on mercury removal of mechanochemical semi-dry modified fly ash. Effect of ball milling solution on mercury removal of mechanochemical semi-dry modified fly ash. J. Cent. South Univ. Sci. Technol. 2023, 54, 3840–3851. [Google Scholar]
- Ghorishi, S.B.; Lee, C.W.; Jozewicz, W.S.; Kilgroe, J. Effects of fly ash transition metal content and flue gas HCl/SO2 ratio on mercury speciation in waste combustion. Environ. Eng. Sci. 2005, 22, 221–231. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Lu, J.; Tang, Y.; Zhang, K. Kinetics and mechanism of mercury adsorption on fly ashes from pulverized coal boiler and circulating fluidized bed boiler. CIESC J. 2019, 70, 4397–4409. [Google Scholar]
- Yin, L.B.; Gao, Z.Y.; Xu, Q.S.; Zheng, S.; Zhong, J. Analysis of species and thermal stability of particulate-bound mercury in coal-fired boiler. J. Fuel Chem. Technol. 2013, 41, 1451–1458. [Google Scholar]
- Xu, M.; Qiao, Y.; Zheng, C.; Li, L.; Liu, J. Modeling of homogeneous mercury speciation using detailed chemical kinetics. Combust. Flame 2003, 132, 208–218. [Google Scholar] [CrossRef]
| wt % | YH | YW | QX |
|---|---|---|---|
| SiO2 | 48.69 | 49.74 | 31.07 |
| Al2O3 | 39.15 | 33.35 | 17.24 |
| Fe2O3 | 3.46 | 3.14 | 5.09 |
| CaO | 4.5 | 6.61 | 32.02 |
| MgO | 0.36 | 0.82 | 1.36 |
| TiO2 | 1.13 | 1.23 | 0.59 |
| SO3 | 0.69 | 2.11 | 11.35 |
| K2O | 0.5 | 0.95 | 0.6 |
| Na2O | 0.06 | 0.68 | 0.14 |
| P2O5 | 0.2 | 0.22 | 0.17 |
| UBC % | 10.59 | 25.66 | 14.7 |
| SSA m2/g | 8.48 | 26.27 | 8.63 |
| Fly Ash | Atmosphere | Inlet Concentration (ng/min) | Adsorption Temperature (°C) | Adsorption Capacity (ng/g) |
|---|---|---|---|---|
| YW | N2 | 40 | 35 | 397.38 |
| QX | N2 | 40 | 35 | 120.65 |
| YH | N2 | 40 | 35 | 104.94 |
| YW | N2 | 109.76 | 35 | 523.43 |
| YW | N2 | 153.64 | 35 | 584.15 |
| YW | O2 | 40 | 35 | 345.09 |
| YW | CO2 | 40 | 35 | 331.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Lei, Y.; Wang, J.; Li, H.; Huo, L.; Wang, Y.; Guo, S. Mercury Adsorption/Oxidation Mechanisms on Fly Ash Under N2 Atmosphere. Processes 2025, 13, 3830. https://doi.org/10.3390/pr13123830
Gao L, Lei Y, Wang J, Li H, Huo L, Wang Y, Guo S. Mercury Adsorption/Oxidation Mechanisms on Fly Ash Under N2 Atmosphere. Processes. 2025; 13(12):3830. https://doi.org/10.3390/pr13123830
Chicago/Turabian StyleGao, Libing, Yuanzhi Lei, Jianghao Wang, Hongyan Li, Lijuan Huo, Yiping Wang, and Shaoqing Guo. 2025. "Mercury Adsorption/Oxidation Mechanisms on Fly Ash Under N2 Atmosphere" Processes 13, no. 12: 3830. https://doi.org/10.3390/pr13123830
APA StyleGao, L., Lei, Y., Wang, J., Li, H., Huo, L., Wang, Y., & Guo, S. (2025). Mercury Adsorption/Oxidation Mechanisms on Fly Ash Under N2 Atmosphere. Processes, 13(12), 3830. https://doi.org/10.3390/pr13123830





