Abstract
Geothermal energy, recognized as a sustainable and clean resource, is playing an increasingly critical role in the global shift toward low-carbon energy systems. Nevertheless, the exploitation of fractured geothermal reservoirs is often impeded by severe lost circulation during drilling, where conventional plugging materials fail under high-temperature, high-salinity, and high-pressure conditions due to inadequate mechanical strength, poor thermal resistance, and lack of self-adaptive sealing behavior. In response, self-healing materials have emerged as an innovative strategy for developing intelligent lost circulation control technologies. Herein, we report a novel self-healing gel (XFFD) synthesized via inverse emulsion polymerization using acrylamide (AM), acrylic acid (AA), p-nitroblue tetrazolium (PNBT), and modified silica nanoparticles (PAS). The resulting material exhibits exceptional thermal stability, with decomposition onset above 356 °C, as determined by thermogravimetric analysis. Rheological and mechanical assessments reveal outstanding viscoelasticity, moderate swelling capacity (4.17-fold in deionized water), and a high self-recovery efficiency of 91.15%, accompanied by a bearing strength of 3.65 MPa. Mechanistic investigations indicate that the autonomous repair capability stems from dynamic non-covalent interactions—primarily hydrogen bonding and ionic associations—enabled by amide and carboxyl groups within the polymer network. Sand bed filtration tests under simulated geothermal conditions (150 °C, 8% salinity) demonstrate that XFFD forms a robust sealing barrier with significantly shallower invasion depth compared to conventional materials such as sulfonated asphalt and calcium carbonate. This work presents an effective self-healing gel system that ensures reliable wellbore strengthening and fluid loss control in challenging high-temperature, high-salinity geothermal drilling operations.
1. Introduction
Geothermal energy, as one of the most promising clean energy sources, plays a crucial role in achieving the “dual carbon” goal through efficient development [1]. China’s medium-deep fractured geothermal reservoirs (such as Ordovician and Cambrian karst fracture-type thermal reservoirs) account for 38% of the nation’s total geothermal resources [2], characterized by reservoir temperatures of 60–90 °C, fracture widths of 0.5–1.4 mm, porosity of 0.05–0.134, and mineralization levels of 4–15 g/L in SO4-Cl-Na or Cl-SO4-Na hydrochemical profiles [3]. However, drilling and completion processes often induce non-recoverable fluid losses [4,5]. Traditional sealing materials like cement slurries and chemical packings face challenges in high-temperature and high-salinity environments: unpredictable solidification time (typically >12 h) [6], poor long-term stability (permeability recovery value > 50%) [7], and potential secondary pollution [8], which hinder efficient geothermal utilization. Therefore, creating effective sealing layers around wellbore walls to prevent filtrate intrusion remains a major challenge [9,10].
The application of plugging agents [11] to seal micropores and micro-fractures, thereby forming an effective sealing layer on the wellbore wall, is instrumental in mitigating or preventing wellbore instability incidents [12]. However, traditional rigid [13] or inert plugging materials, such as sulfonated asphalt and calcium carbonate, are prone to softening, degradation, or failure under high-temperature, high-salinity, and complex stress environments. The resulting sealing bodies lack adaptability, making them inadequate for responding to the dynamic changes in fractures, which consequently constrains the safe and efficient development of geothermal resources. To overcome these limitations, flexible self-healing plugging materials [14] have emerged as a current research focus [15,16]. These materials mimic the self-healing characteristics of biological systems. Following damage, they can autonomously repair their structure and function through internal dynamic reversible bonds, such as hydrogen bonding, ionic interactions, and host–guest interactions [17,18,19,20]. This capability significantly enhances the long-term sealing reliability and durability of the plugging barrier. For instance, Han et al. [21] fabricated a self-healing hydrogel using chitosan and graphene oxide. This design enhanced the free movement between chitosan chains and graphene oxide nanosheets, resulting in a gel with excellent self-healing properties. Similarly, Liu [22] and Kai [23] et al. developed functional polymer gels capable of autonomous healing. In their systems, the hydrogen bonds formed induced a strong spontaneous attraction between polymer chains, leading to rapid self-recovery of the material. Inspired by these self-healing hydrogels or supramolecular systems, our work is grounded in the principle that the synergistic combination of hydrogen bonds and electrostatic interactions—non-covalent bonds commonly present in many polymer molecules—can provide the conditions for a dynamic transition between the cross-linking and breaking of different polymer chains. Guided by this theory, we aimed to design and develop a material with key performance metrics—thermal stability, mechanical strength, and self-healing efficiency—under simulated geothermal conditions of a high temperature and high salinity. The objective is to provide an innovative material system solution to address the challenge of fracture plugging under extreme conditions.
In this study, a novel self-healing plugging gel (denoted as XFFD) was successfully synthesized via inverse emulsion polymerization using acrylamide (AM), acrylic acid (AA), p-nitroblue tetrazolium (PNBT), and modified nano-silica (PAS) as raw materials. Comprehensive characterization confirmed its chemical structure and excellent thermal stability, with a thermal decomposition temperature exceeding 356 °C. Experimental results demonstrated that the gel possesses favorable viscoelasticity, moderate water absorption swelling capacity (a swelling ratio of 4.17 in distilled water), and remarkable self-healing efficiency (91.15%). The pressure-bearing strength of the gel reached 3.65 MPa. Crucially, it effectively formed a sealing layer under harsh conditions of 150 °C and 8% salinity, with a fluid invasion depth significantly lower than that achieved by traditional plugging agents like sulfonated asphalt and calcium carbonate. Mechanistic analysis revealed that self-healing originates from multiple dynamic non-covalent interactions formed by functional groups such as amide and carboxyl groups within the polymer network. This research provides a high-performance self-healing plugging agent and corresponding technical support for the efficient and safe drilling of high-temperature, high-salinity fractured geothermal reservoirs.
2. Experimental Part
2.1. Experimental Materials
The laboratory reagents used, including Sorbitan Oleate (Span80), azobisisobutyronitrile (NIBA), tetramethyl ethylenediamine (TEMED), acrylamide (AM), acrylic acid (AA), and Sunset Red composite dye, are all of analytical grade purchased from Sinopharm Group Chemical Reagent Co., Ltd. (Wuhan, China). The modified nano-silica (PAS), p-nitroblue tetrazole (PNBT), and distilled water were all prepared in-house. The white oil was sourced from Yiwu Enyuan Chemical Co., Ltd. (Yiwu, China).
2.2. Sample Preparation
This experiment synthesized the target gel via inverse emulsion polymerization (Figure 1). The specific procedure began with the addition of 60 g of distilled water and 0.6 g of acetic acid into a beaker to form a 1% acetic acid solution under continuous magnetic stirring. Subsequently, 0.035% modified nano-silica (PAS) was added to the solution and subjected to ultrasonic dispersion to ensure homogeneous distribution, resulting in a stable suspension. Next, reaction monomers—acrylamide (AM), acrylic acid (AA), and p-nitroblue tetrazolium (PNBT)—were sequentially added to the suspension in a molar ratio of 7:2:1, followed by continued stirring until complete dissolution to obtain a homogeneous aqueous monomer solution. Meanwhile, 2.2% white oil and the emulsifier Span 80 were added into a separate three-neck flask to form a uniform oil phase under magnetic stirring. The prepared aqueous monomer solution was then slowly added dropwise into the oil phase under continuous stirring. After complete addition, the pH of the emulsion system was adjusted to neutral (pH = 7). The mixture was transferred to a water bath and heated to the specific reaction temperature. Under nitrogen (N2) purging, the system was stirred for 20 min to remove oxygen. Finally, a solution containing 0.35% crosslinker (TEMED) and initiator (NIBA) was slowly added dropwise into the system. The polymerization was allowed to proceed for 4 h under N2 atmosphere with constant stirring at 50 °C, ensuring complete reaction. The final product was the desired emulsion-type self-healing plugging gel, XFFD. (All percentages indicated are relative to the total solution mass or volume, as appropriate).
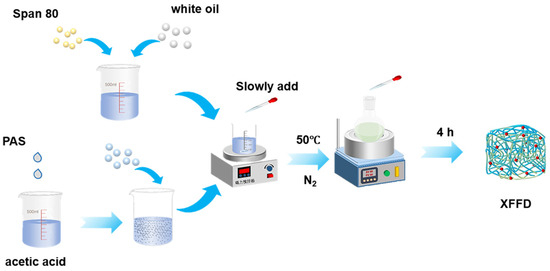
Figure 1.
Synthesis scheme of XFFD.
2.3. Sample Characterization
The characteristic functional groups of the synthesized self-healing sealing gel were determined using a 6700 Fourier Transform Infrared Spectrometer (Thermo Nicolet Corporation, Madison, WI, USA). The thermogravimetric analysis curve of the self-healing sealing gel was tested with a TG-DSC analyzer (Nicolet, Selb, Germany) to evaluate its temperature resistance. The ultra-high resolution field emission scanning electron microscope SU8600 (Hitachi, Tokyo, Japan) was used to characterize the morphological characteristics of the self-healing plugging gel. The particle size distribution of XFFD was measured using a laser particle size analyzer (masterizer 2000, Malvern, UK). The impact of XFFD on drilling fluid dispersion stability was assessed using a nano-potentiometric particle size analyzer. The sealing capability of XFFD on sand layers was determined using a visual medium-pressure sand loss meter (Hebei Tianxing Qizi Testing Equipment Co., Ltd., Cangzhou, China).
2.4. Performance Evaluation Method
2.4.1. Viscoelastic Evaluation
In order to clarify the role of each monomer in the gel system, four gel samples without any monomer in am, AA, pnbt, and PAS were prepared, and XFFD was used as the control. The contribution of each monomer to the viscoelastic properties of the gel was analyzed by rheological test. The pp20ti plate measurement system with a rotor diameter of 10 mm was used in the experiment. Under the condition of 30 °C, the frequency scanning mode was used to scan the sample in the range of 0.1–15 Hz under the fixed shear stress of 5 Pa so as to obtain the viscoelastic change curve and evaluate its viscoelasticity.
2.4.2. Water Absorption Performance Evaluation
Too high of a water absorption ratio of gel will significantly weaken its mechanical strength and sharply increase the viscosity of the drilling fluid, which will have adverse effects on drilling operations. Therefore, it is necessary to evaluate the water absorption performance of gel. By measuring the swelling equilibrium time of the gel (the time required for no significant change in the water absorption of the gel) and the maximum expansion ratio (the ratio of the gel mass to the initial dry mass at the swelling equilibrium), the samples were immersed in distilled water and NaCl solutions with different concentrations (5%, 10%, and 20%), respectively, and were taken out at the set time interval. The water absorption performance of the gel was evaluated after drying the surface water.
2.4.3. Self-Repair Performance Evaluation
Uniaxial tensile test was used to test the tensile fracture of the samples of the gel before and after repair on the universal tensile testing machine, so as to obtain the stress–strain relationship and evaluate the self-repair efficiency of the gel [24] (the ratio between the tensile stress of the gel after repair and the tensile stress of the original gel).
The calculation method of self-repair efficiency is
where σh is the tensile stress of self-healing gel, Pa. σo is the tensile stress of the original gel, Pa.
2.4.4. Sealing Performance Evaluation
The plugging performance of gel was evaluated by sand bed invasion test is as follows: compact 80–100 mesh fine sand to 350 cm3, inject drilling fluid to 250 cm3, and then record the final invasion depth of drilling fluid within 30 min at 100 psi pressure.
3. Results and Discussion
3.1. Characterization of Synthetic Samples
The infrared spectra of the PAS-free samples and XFFD (synthesized sample) are shown in Figure 2a. Its infrared spectrum (Figure 2a) shows the following characteristic absorption bands: 3440 cm−1 (N-H stretching vibration), 1620 cm−1 (C=O vibration), 1410 cm−1 (C-N stretching vibration), 1300 and 1055 cm−1 (C-O stretching vibration), and 654 cm−1 (O-H out-of-plane bending vibration) [25,26]. Compared with the control group without PAS, XFFD showed new absorption peaks at 862 cm−1 (C-H vibration) and 1410 cm−1 (silicon hydroxyl), which proved that PAS had successfully participated in the reaction and formed the target polymer. Interestingly, for XFFD without PAS, the two expected sharp, medium-intensity absorption peaks near ~3500 cm−1 and ~3400 cm−1 (assigned to non-hydrogen-bonded -NH2 groups) instead shifted to lower wavenumbers at 3440 cm−1 and 3156 cm−1, exhibiting a redshift phenomenon (Figure 2a). This shift is attributed to changes in the vibrational energy levels of the N-H bonds induced by hydrogen bonding, indicating the presence of multiple hydrogen bonding environments with varying strengths. Furthermore, after the incorporation of PAS, the characteristic peaks of -NH2 and O-H in XFFD shifted from 3440 cm−1 and 635 cm−1 to 3456 cm−1 and 665 cm−1, respectively, demonstrating a blueshift. This suggests that the strength of intermolecular hydrogen bonds within XFFD chains is somewhat reduced upon PAS addition, which likely results from a dynamic equilibrium between the formation and disruption of crosslinks among different polymer chains.
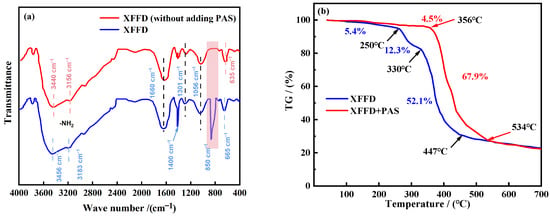
Figure 2.
FTIR spectrum (a) and thermal stability analysis diagram (b) of the synthesized product XFFD.
We propose that the PNBT structure facilitates strong π-π stacking and hydrophobic interactions with the graphitized core of the modified nano-silica (PAS). This strong association effectively anchors the polymer chains (growing from AM and AA) to the PAS nanoparticle surface, creating the “grafting core”. Compared to a simple linear polymer, this semi-interpenetrating network architecture provides key advantages for self-healing. The PAS nanoparticles act as multi-functional physical crosslinks, preventing irreversible flow upon fracture. The polymer chains grafted on the particles are rich in functional groups (from AA and PNBT), creating a high local density of hydrogen bonding sites at the interface. When two fractured surfaces make contact, these dynamic bonds can rapidly re-form, leading to efficient healing.
We conducted FTIR analysis at elevated temperatures (from 25 °C to 150 °C). As shown in Figure 3, the characteristic broad peak associated with O-H and N-H stretching significantly decreased in intensity and shifted to higher wavenumbers with increasing temperature. This is a classic signature of hydrogen bond dissociation, providing direct evidence that hydrogen bonds are a key dynamic motif in our network that respond to thermal energy.
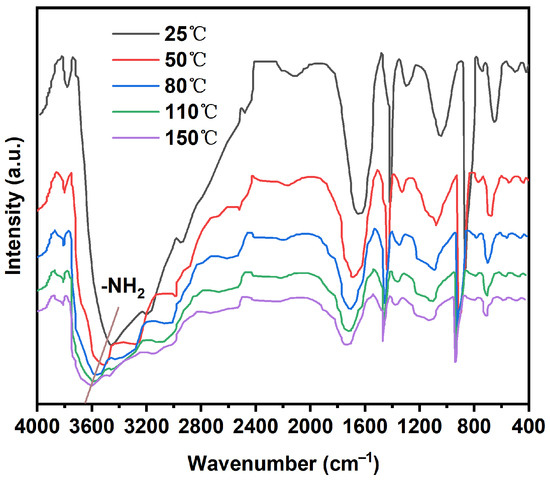
Figure 3.
FTIR spectrum of XFFD at different temperatures.
The Al2O3 crucible was first placed in the sample chamber and tared. Then, 0.4 g of the sample was weighed into the crucible, which was subsequently returned to the sample chamber. The TGA program was selected, and the temperature was raised to 700 °C at a heating rate of 10 K·min−1 under a nitrogen purge atmosphere to record the thermogravimetric (TG) curve. The thermal stability of both PCAA and PCAA without PAS was evaluated. By comparing the thermal decomposition curves of the two products, the effectiveness of PAS incorporation in enhancing the temperature resistance of PCAA was assessed. The residual mass percentage at the end of the measurement (700 °C) was found to be 22.3%. It can be seen from Figure 2b that the weight loss of XFFD without PAS is 5.4% between the normal temperature and 250 °C, which is caused by the evaporation of the free water attached to the sample. In the range of 250–330 °C, the weight loss of the sample is 12.3%, which is because the microstructure of the sample loosens with a further increase in heating temperature, and even the branch chain in the structure begins to decompose, resulting in the gradual collapse of the three-dimensional network structure. In the range of 330–447 °C, a 52.1% weight loss of the product was observed, which was due to the molecular chain breaking and decomposition at a high temperature. After 447 °C, the product was thermally degraded until complete. In conclusion, XFFD without PAS began to decompose at 270 °C, while XFFD with functional monomer PAS began to decompose after 330 °C, indicating that PAS is helpful to improve the thermal stability of XFFD. The TGA result confirms the chemical (covalent) stability of the polymer chains up to 356 °C, meaning no chain scission occurs at 150 °C. The swelling behavior is governed by the physical crosslinks (hydrogen bonds, hydrophobic interactions). At elevated temperatures, the kinetic energy increases, leading to a partial, reversible dissociation of these dynamic physical bonds. This is a well-known phenomenon in associative polymer networks. This temporary “loosening” of the network allows more water molecules to penetrate, thus increasing the equilibrium swelling ratio. Crucially, upon cooling, these bonds can readily re-form, restoring the gel’s mechanical properties. This reversible behavior is a hallmark of a robust dynamic network and is entirely consistent with excellent thermal stability, not contrary to it. We have added the FTIR spectra of the XFFD gel after aging at 150 °C for 16 h (Figure 4). The spectrum is virtually identical to that of the fresh gel, with no appearance of new degradation peaks (e.g., from oxidation) or disappearance of key functional groups. This provides direct evidence that no significant chemical degradation occurred.
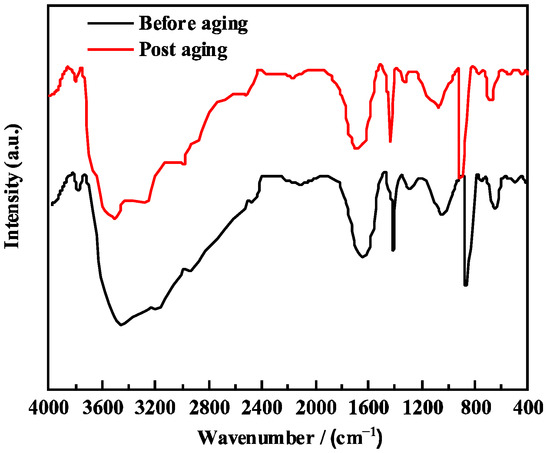
Figure 4.
Aging of XFFD at 150 °C.
3.2. Performance Evaluation of Self-Healing Sealing Gel
Viscoelastic variation. The viscoelastic variation curve of the self-healing sealing gel is shown in Figure 5a. The rheological measurements were conducted in triplicate, and the data shown represent the mean values, demonstrating good reproducibility with a standard deviation of less than 5%. We now explicitly state the physical significance of the storage modulus (G′) and loss modulus (G″) in the context of our material. Specifically, we explain that G′ > G″ across the measured frequency range indicates the dominant elastic, solid-like behavior of the gel network, which is crucial for its long-term sealing capability and structural stability. According to Figure 5a, the elastic modulus (G′) of all gel samples is greater than the viscosity modulus (G″), which proves that they are essentially elastic materials. The monomer deletion test showed that the G′ and G″ values of the gel without AA, PAS, or AM were lower than those of XFFD, which proved that these three monomers could enhance the viscoelasticity of the gel and promote the formation of a three-dimensional network structure, and the enhancement efficiency followed the order of am > AA > pas. Specifically, by extending the polymer chain and providing dynamic functional groups, am can enhance the network and endow it with a self-repairing ability. AA is electrostatically crosslinked through carboxyl groups to consolidate the network. The hydrophobic long chain of PAS has a weak effect on strength improvement. On the contrary, the gel without PNBT showed a slight increase in G′ and a slight decrease in G″, indicating that PNBT mainly contributed to the viscosity response of the system. As a multi-side chain macromolecule formed by the grafting core, PNBT can enhance the intermolecular interaction through dense hydrogen bonds [27,28].
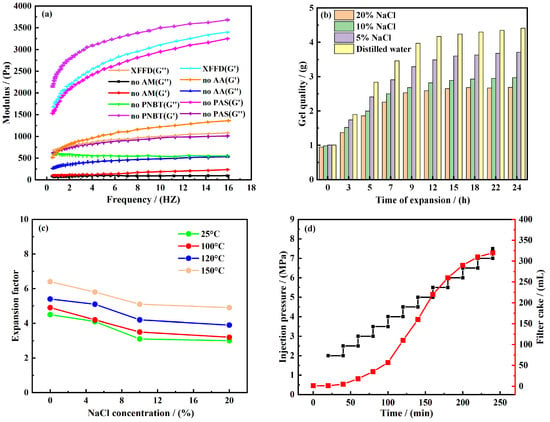
Figure 5.
Performance evaluation of self-repairing plugging gel. (a) Viscoelastic change curve of self-repairing plugging gel, (b) relationship between water absorption of XFFD and soaking time, (c) relationship between water absorption and salt concentration and temperature, and (d) results of gel sealing 0.2 mm fracture.
Evaluation of water absorption performance. As shown in Figure 5b, in distilled water and NaCl solutions with different concentrations (5%, 10%, and 20%), the water swelling behavior of the gel is positively correlated. The experimental data showed that the quality of the gel tended to be stable after soaking in distilled water for 12 h, indicating that it had basically reached the saturated state of water absorption, and its expansion ratio was 4.17 times. In NaCl solution, the time for the gel to reach the saturation equilibrium was significantly shortened with the increase in the salt concentration, but the corresponding expansion ratio showed a decreasing trend. This difference indicates that NaCl has an inhibitory effect on the water swelling of the gel. From the molecular mechanism analysis, salt ions may increase the degree of molecular chain curl by compressing the electrostatic repulsion force between polymer molecular chains and then reduce the porosity of the spatial network structure of the gel, which ultimately shows the decline of water absorption and the weakening of the swelling degree.
Evaluation of temperature resistance. To study the thermal stability and temperature resistance of gel materials, the samples were put into distilled water and NaCl solutions with different concentrations (5%, 10%, and 20%) and aged for 16 h at different temperatures. After aging, the gel was taken out, and the residual liquid on the surface was sucked dry with filter paper and weighed. The swelling ratio was calculated by comparing the mass changes before and after aging. The corresponding results are shown in Figure 5c. The data analysis showed that the expansion ratio of gel decreased with the increase in the NaCl concentration at a high temperature, which was consistent with the results at room temperature, which confirmed that NaCl solution still maintained a good anti-gel swelling performance at a high temperature. However, the gel in distilled water is not so, and its expansion ratio is positively correlated with the temperature. This temperature dependence may be attributed to two mechanisms: first, high temperature accelerates the thermal movement of water molecules and enhances the interaction between water molecules and polymer networks. Secondly, the increase in temperature promotes the extension of the polymer molecular chain, expands the porosity of the spatial network structure, and improves the water absorption capacity. It is worth noting that, compared with the traditional superabsorbent gel, the gel prepared in this study shows moderate water absorption, which helps to control the viscosity change in the drilling fluid system and maintains its good rheological properties.
Pressure-bearing strength performance. In order to verify the pressure-bearing performance of the gel in the application of formation plugging, the dynamic plugging performance evaluation device of a high-temperature and high-pressure drilling fluid was used in the experiment, and the self-repairing gel samples at room temperature were placed in the simulated fracture channel. By injecting 400 mL drilling fluid into the system and adopting the stepped pressurization method (the pressure is increased every 20 min), the leakage of drilling fluid is continuously monitored. The experimental results are shown in Figure 5d. Data analysis shows that there is a positive correlation between drilling fluid leakage and injection pressure: when the system pressure reaches 3.65 MPa, the cumulative leakage is about 100 mL. When the pressure exceeds the critical value, the leakage increases exponentially. This phenomenon shows that the gel sample treated at room temperature can effectively withstand a pressure difference of 3.65 MPa, and its pressure-bearing performance can meet the basic requirements of deep formation plugging. The determination of the pressure threshold provides a key parameter basis for the subsequent optimization of the gel formula and plugging process.
3.3. Evaluation of Self-Healing Properties of Self-Healing Sealing Gel
Self-repair performance evaluation. In order to systematically evaluate the self-healing performance and mechanical properties of the gel, the gel samples before and after self-healing were tested for stress–strain on the universal testing machine, and the results are shown in Figure 6a.
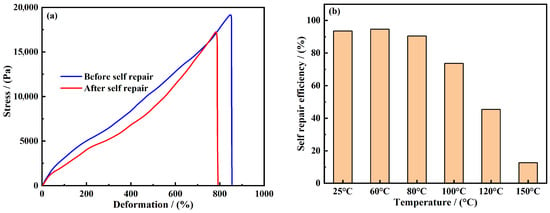
Figure 6.
Self-repairing performance evaluation of self-repairing plugging gel. (a) Tensile stress–strain relationship of gel; (b) self-repairing efficiency of gel in different temperatures.
It can be seen from the figure that the gel sample shows typical elastic deformation characteristics under the action of stress increase, which shows a linear growth trend with the strain value. When the stress reaches the critical value (19.2 KPa), the sample breaks suddenly, and the corresponding maximum deformation is 855%, indicating that the original gel has excellent tensile toughness. After self-repair treatment, the maximum tensile stress of the sample is 17.5 KPa, and the fracture deformation is 792%. According to the calculation formula of self-repair efficiency (Formula (1)), the repair efficiency is 91.15%.
This remarkable repair effect can be attributed to the synergistic mechanism of multiple non-covalent bonds in the gel system: amide groups, carboxylic groups, hydroxyl groups, and hydrophobic segments in the polymer chain form a dynamic reversible crosslinking network at the micro scale through hydrogen bonds, electrostatic interactions, and hydrophobic associations. When the gel structure is damaged, these non-covalent bonds can be re-combined through thermal movement to achieve structural self-healing, thus giving the material unique rapid repair characteristics [29].
The influence of temperature on self-healing efficiency. After the gel samples were cut into irregular blocks, they were placed at 25 °C, 60 °C, 80 °C, 100 °C, 120 °C, and 150 °C for 4 h. The results after repair are shown in Figure 6b. The experimental results show that the self-healing efficiency of the gel can be maintained above 70% in the thermal environment of 100 °C and below. The repair efficiency was 91.15% at 25 °C, 94.71% at 60 °C, 90.51% at 80 °C, and 73.67% at 100 °C. This shows that the gel system has an excellent self-healing ability in the temperature range of 100 °C and below, and its molecular segments can be effectively recombined by dynamic reversible bonds under the action of thermal excitation so as to realize the rapid recovery of structural integrity. While the macroscopic self-healing test was conducted under ambient conditions to establish a quantitative baseline, the excellent sealing performance demonstrated in the high-temperature/high-salinity sand bed tests provides strong indirect evidence that the dynamic bonding network remains functional under simulated downhole conditions. The sealing process itself likely involves a pressure-assisted healing mechanism, as gel fragments are forced together within the fracture. Future work will specifically investigate the healing kinetics under confined pressure and shear flow to more precisely simulate the downhole environment.
3.4. Evaluation of Sealing Performance of Self-Healing Sealing Gel
In order to systematically evaluate the plugging efficiency of the XFFD plugging agent under different temperatures and salt concentrations, sand bed invasion experiments were carried out on the base mud before and after aging, with drilling fluids containing different concentrations of XFFD and drilling fluids containing 2% XFFD and different concentrations of NaCl. The experimental results are shown in Figure 7.
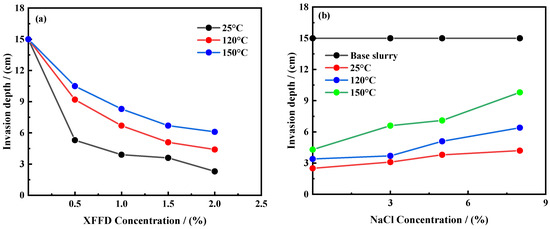
Figure 7.
Sand bed invasion depth of drilling fluids (a) XFFD, (b) NaCl + 2%XFFD.
By analyzing the data in Figure 7a, it can be seen that, under room temperature, the invasion depth of drilling fluid in the sand bed increases from 0.5% to 2% with the concentration of XFFD and significantly decreases from 3.6 cm to 1.0 cm, indicating that XFFD has an excellent plugging performance. After aging at 120 °C and 150 °C for 16 h, the invasion depth of the drilling fluid containing 2% XFFD was only 4.0 cm and 4.2 cm, respectively, while the base mud had been completely lost (15 cm), which further proved its excellent plugging performance at a high temperature.
Figure 7b shows that the invasion depth of drilling fluid increases with the increase in NaCl concentration, but when the salt concentration is 8%, the XFFD system can still maintain effective plugging, and 2% XFFD can still control the invasion depth at 25 °C, 120 °C, and 150 °C within 4.2 cm, 6.4 cm, and 8.8 cm, respectively, showing good salt resistance. It is worth noting that, although the invasion depth of drilling fluid increases slightly at a high temperature, the increase is limited, which is mainly due to the molecular chain entanglement effect of XFFD gel microspheres under the action of multiple non-covalent bonds, which makes it form a dynamic self-repairing plugging layer in the pores and micro-fractures of the sand layer, playing the role of bridging and plugging so as to effectively inhibit the invasion of drilling fluid. The above experimental results confirm that the XFFD plugging agent can withstand a 150 °C high-temperature environment and has the ability to resist 8% NaCl, which fully meets the technical requirements of deep formation micropore and micro-fracture plugging. The 16 h aging period at 150 °C was primarily selected as a standardized, accelerated screening test to allow for a rapid and comparable initial assessment of the material’s thermal stability relative to conventional sealants (e.g., sulfonated asphalt, calcium carbonate), as referenced in our study. While not representing true long-term performance, the fact that the XFFD gel successfully formed a strong seal and maintained its integrity after this aggressive thermal treatment is a strong positive indicator of its robustness. Materials that fail under such conditions are unlikely to perform well in the field.
As shown in Table 1, the effect of adding 2% XFFD on the fluid loss of drilling fluid was evaluated after aging at different temperatures. As summarized in the table, the viscosity of the drilling fluid containing XFFD gradually decreased with increasing aging temperature. Concurrently, the yield point (YP) of the drilling fluid also decreased upon heating. However, the addition of XFFD effectively increased the gel strength of the drilling fluid at elevated temperatures, indicating that XFFD promotes the formation of a three-dimensional network structure within the fluid. At 25 °C, the addition of XFFD reduced the fluid loss to 2.2 mL. After aging at 120 °C and 150 °C, the fluid loss of the base mud significantly increased to 35.6 mL and 43.0 mL, respectively. In contrast, the fluid loss of the mud containing 2% XFFD was markedly reduced to 4.0 mL and 7.4 mL under the same conditions. These results collectively demonstrate that XFFD is an effective fluid loss control additive for drilling fluids.

Table 1.
The basic performance of XFFD.
3.5. Self-Healing Blocking Mechanism Analysis
Scanning electron microscope analysis. The microstructure of the base mud obtained from the API filtration test [30] and mud cake containing 2% of the XFFD plugging agent were observed by scanning electron microscope (SEM), and the plugging efficiency of the material at a high temperature was systematically evaluated. The mud cake morphology of the base mud before and after aging and the drilling fluid containing XFFD after aging at 120 °C and 150 °C for 16 h were compared. The results are shown in Figure 8.
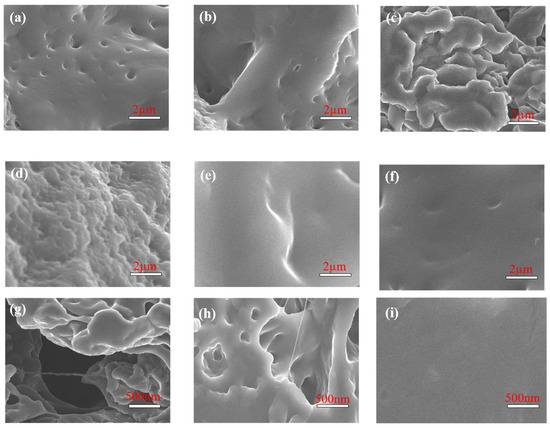
Figure 8.
SEM morphology of base mud and strong plugging drilling fluid. (a) Before base mud aging, (b) after base mud aging at 120 °C, (c) after base mud aging at 150 °C, (d) before strong plugging drilling fluid aging, (e) after strong plugging drilling fluid aging at 120 °C, and (f) after strong plugging drilling fluid aging at 150 °C. High-resolution images in (g–i) showcase the “pores”, “cracks”, and “self-healing” process, respectively.
The mud cake surface (Figure 8a) of the non-aged base slurry presents a uniform and dense structure. After high temperature treatment at 120 °C (Figure 8b) and 150 °C (Figure 8c), the mud cake surface shows significant deterioration, showing the formation of a large number of holes and cracks. This structural failure is mainly due to the aggregation effect of clay particles induced by high temperature, which leads to the loosening of the mud cake skeleton, and then causes the rapid increase in drilling fluid filtration. The mud cake containing 2% XFFD shows excellent compactness at 25 °C (Figure 8d), and there are no visible pores or cracks on the surface. This is due to the adsorption of XFFD molecules on the surface of clay particles, which promotes the polymer gel microspheres to achieve close packing through intermolecular forces, forming a dynamic sealing layer with self-healing characteristics. Under the aging condition of 120 °C (Figure 8e), the gel microspheres maintain the integrity of the plugging layer through the dynamic reorganization of the molecular chain and significantly enhance the plugging ability of the mud cake for pores and fractures. When the temperature rises to 150 °C (Figure 8f), although the bulk density of gel microspheres decreases locally, and a few microspheres have small defects on the surface, the mud cake as a whole still maintains a continuous and compact structure without penetrating pores, showing excellent high-temperature plugging resistance. The above microstructure evolution rules show that the XFFD plugging agent can effectively maintain the compactness of the mud cake in a high-temperature environment by constructing a dynamic and stable polymer network structure. The self-healing mechanism can realize the dynamic repair of defective parts through the thermal movement of molecular chains, which provides an important material support for wellbore stability in deep formation drilling. This unique temperature-responsive plugging property gives XFFD significant application advantages in a high-temperature drilling fluid system. High-resolution images showing the “pores,” “cracks,” and “self-healing” are presented in Figure 8g–i.
Particle size distribution analysis. The particle size of the final emulsion was measured using a Malvern particle size analyzer. The emulsion was diluted 100-fold with deionized water to achieve an appropriate scattering intensity for measurement. Each measurement was performed in triplicate. The data showed that the particle size of XFFD was mainly concentrated in the range of 0.6–112 μm, and the particle size parameters of D10, D50, and D90 reached 10.09 μm, 30.15 μm, and 82.93 μm, respectively (Figure 9). This micron size distribution gives it the excellent ability to seal micro-fractures and can effectively enter the formation pore structure to form a physical barrier. These micron-scale gels primarily bridge and seal major fractures (0.5–1.4 mm in width) through a mechanism known as “log-jamming” or “arch-building.” Effective bridging generally requires the gel particles to be approximately one-third to one-half the size of the fracture width. Therefore, the size of our gels theoretically enables the efficient sealing of fractures around 0.9–1.4 mm, closely matching the characteristics of the target reservoir. The drilling fluid was aged for 16 h at concentrations of a 4% base slurry and 2% XFFD with varying salt levels (120 °C and 150 °C). The particle size distribution measurements are presented in Figure 10.
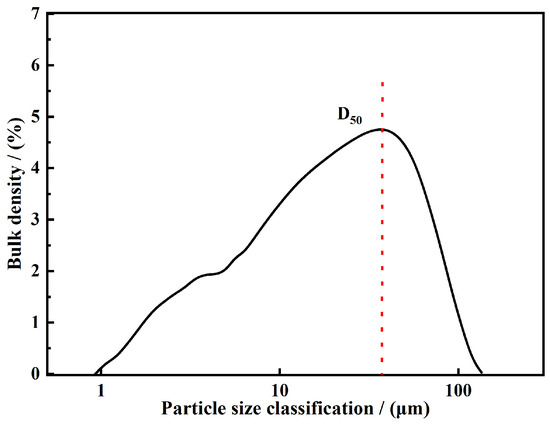
Figure 9.
Particle size cumulative distribution curve of XFFD.
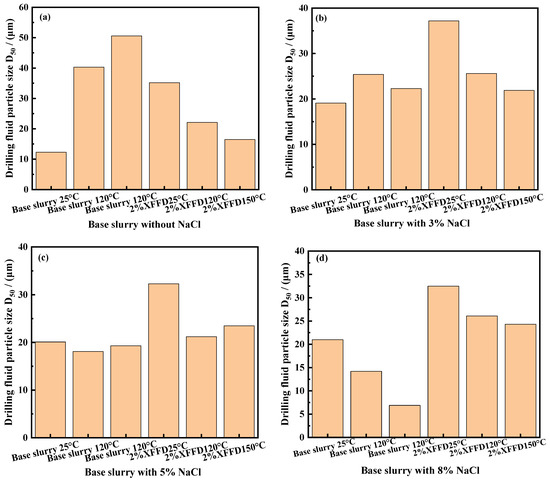
Figure 10.
Particle size cumulative distribution curve. (a) Base mud and PCAA strong plugging drilling fluid, (b) 3%NaCl, (c) 5%NaCl, and (d) 8% NaCl.
As shown in Figure 10a, at 25 °C, the particle size distribution curve of the drilling fluid containing 2% XFFD shifted significantly to the right compared with the base mud, indicating that XFFD formed a compatibilization effect on the surface of clay particles through adsorption, resulting in the increase in the average particle size of the system.
When the temperature rises to 120 °C and 150 °C, the particle size distribution curve of the system containing XFFD shifts to the left, indicating that the gel microspheres are moderately desorbed at a high temperature, and the reduction in the particle size of the system is conducive to the formation of a dense mud cake structure. The salt concentration influence experiment shows that (Figure 10b–d) the addition of NaCl has a two-way regulating effect on the particle size of the system: under low salt concentration (<5%), the particle size increase in the system containing XFFD is controlled within 8%. The particle size increased by 15% at high salt concentration (≥8%), but it still maintained a wider particle size distribution range (0.5–150 μm) than the base slurry. This unique particle size response makes the XFFD plugging agent have dual advantages: it can not only realize multi-stage pore plugging through wide particle size distribution but also optimize the compactness of the mud cake through salt-induced particle size control. The experimental results show that the system can still maintain good particle size stability under the condition of 150 °C/8% NaCl, which provides a key material guarantee for the effective plugging of high-temperature and high-salinity formations.
Zeta potential analysis. As shown in Figure 11, The zeta potential of the base slurry increased with the increase in temperature. At the same time, the zeta potential also increases with the increase in the NaCl concentration, which is attributed to the compression of the diffusion double layer on the surface of clay particles by Na+. In addition, the zeta potential of XFFD-containing drilling fluid is generally slightly higher than that of base mud at room temperature, regardless of whether the system contains salt or not. After aging at 120 °C and 150 °C, the addition of XFFD maintained the relative potential difference through adsorption, which reduced the zeta potential of the drilling fluid and promoted the dispersion of clay. A further decrease in the potential after high-temperature aging can be attributed to the weakening of the interaction between PCAA and clay. The above results confirm that the XFFD plugging agent can significantly inhibit the aggregation tendency of clay particles under high-temperature and high-salinity conditions by maintaining the charge stability of the system, thus providing effective support for the control of the rheological properties of drilling fluid in deep complex formations. As the temperature increases, the surface charge density of XFFD decreases, leading to a reduction in the absolute value of the zeta potential. This weakens the electrostatic double-layer repulsive forces, allowing van der Waals attractive forces to dominate. Consequently, the particles rapidly form a gel, achieving effective plugging. With increasing NaCl concentration, the double layer is significantly compressed, causing repulsive forces to nearly vanish. Under these conditions, the particles rapidly aggregate into a “dense fractal structure,” forming a self-healing gel via the “strong attraction” regime described in Derjaguin–Landau–Verwey–Overbeek (DLVO) theory, thereby effectively sealing the fractures. As the aging temperature increased, the zeta potential of XFFD-containing drilling fluid decreased, likely due to the reduced interaction between XFFD and clay particles under elevated temperatures.
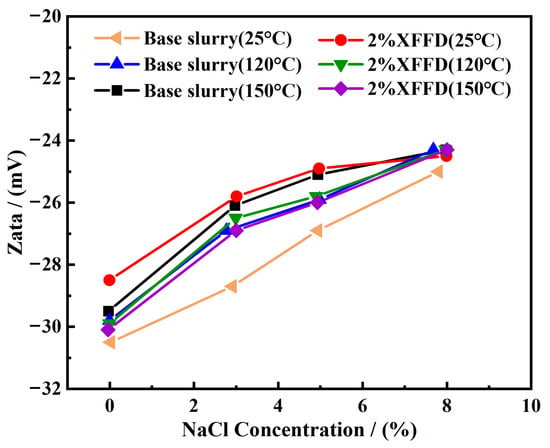
Figure 11.
Zeta potential curve of drilling fluid.
3.6. Self-Healing Blocking Mechanism
Conventional gel packers often exhibit weak particle interactions and suboptimal sealing performance. The developed packer in this study incorporates self-healing properties to enhance containment efficiency. Through reverse emulsion polymerization, we synthesized the XFFD packer where gel microspheres are suspended within a dispersed phase. When injected into formation fractures and pores via drilling fluid, these microspheres undergo migration, aggregation, filling, and compression under pressure. The self-healing process forms a cohesive gel matrix that interacts with fracture walls through adsorption, creating a robust sealing layer. This self-repairing structure maintains the integrity of a unified gel system, demonstrating high compressive strength and retention capacity. The schematic diagram illustrating the sealing mechanism is shown in Figure 12.
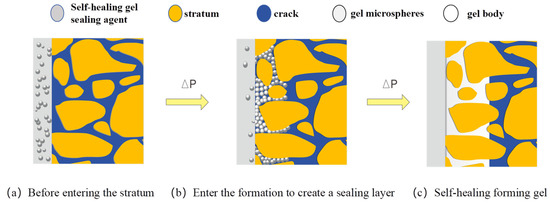
Figure 12.
Schematic diagram of self-repair and sealing mechanism.
3.7. Self-Healing Sealing Drilling Fluid System Performance Evaluation
Plugging performance evaluation. In order to evaluate the plugging performance of the self-repairing plugging agent XFFD, sulfonated asphalt and calcium carbonate commonly used in the field were selected as the contrast materials, which were, respectively, added into the drilling fluid system at a dosage of 2%, and the plugging experiment of 40–60 mesh sand bed was carried out. The results of the invasion depth of each system in the sand bed under different temperature conditions (room temperature, 120 °C, and 150 °C aging) and the environment containing 10% NaCl are summarized in Table 2. The results show that the increase in aging temperature and salt content will cause the sand bed invasion of different drilling fluid systems, but the increase in invasion depth of the XFFD system is the most limited, which is always controlled within 5 cm. Especially under the conditions of a high temperature and 10% NaCl, the invasion depth of drilling fluid containing 2% XFFD is significantly lower than that of the sulfonated asphalt and calcium carbonate system, indicating that its plugging performance is the best. Further, the drilling fluid containing 2% XFFD was aged at 150 °C for 16 h, and the plugging experiments were carried out in 60–80 mesh and 80–100 mesh sand beds, respectively (Figure 13). The results showed that the invasion depth was very low, close to zero, which fully showed that the self-healing plugging drilling fluid had an excellent adaptive plugging ability and high-temperature stability.

Table 2.
Sand bed intrusion depth of different types of drilling fluids.

Figure 13.
Penetration depth of sand bed of drilling fluid for strengthening plugging.
This low invasion depth, combined with a high breakthrough pressure, demonstrates its excellent ability to form an effective seal in narrow fracture apertures. These results collectively confirm that XFFD possesses the key properties required for high-performance sealing applications. While the sand bed is a simplified model, the demonstrated ability of XFFD to bridge and form a seal is underpinned by its key material properties—such as its specific particle size distribution relative to the sand bed pore throats and its rapid self-healing. These fundamental properties are essential for addressing the dynamic challenges of sealing real-world formations.
Inhibition performance evaluation. In order to evaluate the inhibition performance of the developed plugging drilling fluid at a high temperature, it was systematically compared with amine-based drilling fluid and silicate drilling fluid. The inhibition effect after aging treatment at 120 °C and 150 °C was investigated, with emphasis on the hydration expansion of bentonite (Figure 14a) and the recovery of shale (Figure 14b). The inhibition performance of different drilling fluid systems was evaluated, and the results are shown in Figure 14.
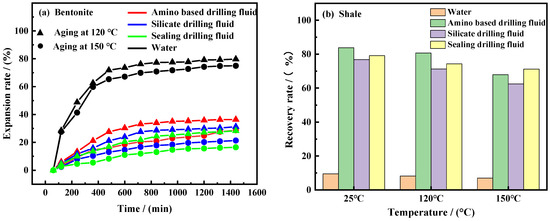
Figure 14.
Hydration expansion rate of bentonite in different drilling fluids. (a) After aging at 120 °C and 150 °C, (b).
The experimental data (Figure 14a) shows that the expansion rate of bentonite in the strong plugging drilling fluid is the lowest, regardless of whether it is at 120 °C or 150 °C. Although the expansion rate of bentonite in the three drilling fluids increased with the aging temperature rising from 120 °C to 150 °C, the growth rate of strong plugging drilling fluid was the smallest, indicating that the system can still effectively inhibit the hydration expansion of bentonite in a high-temperature environment. The experimental data (Figure 14b) shows that the shale recovery rate generally decreases with the increase in aging temperature, indicating that a high temperature intensifies the hydration dispersion. In contrast, the decline of strong plugging drilling fluid is the most limited (only 7.9%), which confirms that it can still maintain a good inhibition performance under high-temperature conditions.
3.8. Economic and Environmental Benefit Analysis of the Self-Healing Gel (XFFD)
Based on the experimental results of this study, this section provides a preliminary discussion on the potential economic benefits and environmental implications of applying the XFFD gel in drilling operations for high-temperature, high-salinity fractured geothermal reservoirs.
Economic Benefit Analysis. The potential economic benefits of the XFFD gel primarily stem from direct material costs to overall operational efficiency improvements and long-term wellbore integrity assurance. The main components of the XFFD gel, acrylamide (AM), and acrylic acid (AA) are bulk industrial chemicals with wide availability and relatively low production costs. Although the functional monomer (PNBT) and modified nanomaterial (PAS) used carry a higher unit price, their addition levels in the formulation are low (e.g., <5 wt%), and their incorporation significantly enhances the material’s key properties. Consequently, from a cost–performance perspective, the overall raw material cost remains reasonable and acceptable, indicating potential for large-scale application. The most significant economic advantage lies in its rapid autonomous self-healing capability (efficiency > 91% within 30 min at room temperature) and exceptional high-temperature stability (tolerance up to 150 °C). These attributes directly translate to a reduction in non-productive time (NPT) during drilling operations. Unlike conventional lost circulation materials that often require multiple pumping operations and long waiting times, a single injection of XFFD can form an effective seal at the fracture site and autonomously repair subsequent operational damage, significantly minimizing rig downtime caused by fluid loss. Effective wellbore sealing ensures safer drilling, enabling operators to adopt more optimized drilling parameters (e.g., increased flow rate), thereby shortening the well construction cycle. The robust sealing performance of the XFFD gel (maintaining integrity after 16 h of aging at 150 °C) ensures long-term production safety and stability for geothermal wells. A durable seal layer reduces the risk of requiring expensive workover operations due to wellbore instability during the production phase. By protecting the production zone from drilling fluid invasion damage and stress perturbations, it helps maintain the long-term productivity of geothermal wells, thereby enhancing the economic return over their entire lifecycle.
Environmental Benefit Analysis. The application of the XFFD gel offers dual environmental benefits in terms of pollution prevention, resource conservation, and promotion of clean energy development. Geothermal drilling fluids may contain chemical additives potentially harmful to aquifers. The superior plugging capacity of XFFD (breakthrough pressure > 3.6 MPa with invasion depth < 0.1 cm under conditions of 150 °C and 8% salinity) effectively prevents drilling fluid loss into aquifers or the target production zone, fundamentally mitigating the potential risk of groundwater contamination and aligning with the most stringent environmental drilling standards. The XFFD gel is synthesized and applied using water as the continuous phase, avoiding the risks of soil and groundwater pollution associated with oil-based systems. Its polymer backbone (polyacrylamide/acrylic acid) possesses the potential for biodegradation under specific conditions (e.g., presence of specific microorganisms or chemical environments). Although degradation is slow in deep geological formations, XFFD demonstrates a more promising environmental compatibility profile compared to some inert, poorly degradable plugging materials (e.g., certain polymer residues). Geothermal energy is a crucial clean baseload energy source. The application of XFFD gel technology helps address the challenge of fluid loss that hinders the efficient development of mid-deep geothermal resources, thereby directly contributing to the substitution of clean energy for fossil fuels and offering significant carbon emission reduction benefits. Owing to its high-efficiency sealing characteristics, a unit volume of XFFD gel may achieve superior plugging performance, potentially reducing the total material consumption per well depth and indirectly lowering the resource input and environmental footprint.
Preliminary analysis suggests that the XFFD gel not only exhibits excellent technical performance for lost circulation control in geothermal drilling but also demonstrates significant potential advantages in economic viability and environmental friendliness. However, a comprehensive economic and environmental assessment awaits validation through future field pilot tests and a full lifecycle assessment. This study provides a promising direction for developing drilling materials that combine technical feasibility with sustainable development characteristics.
4. Conclusions
This study successfully developed a novel self-healing sealant gel, XFFD. Its innovation lies in the molecular design that integrates dynamic non-covalent bonds (e.g., hydrogen bonds) with a rigid nano-skeleton (PAS), achieving a unique balance between rapid self-healing (efficiency > 91%) and high-strength sealing (withstand pressure > 3.65 MPa) under harsh geothermal conditions of a high temperature (150 °C) and high salinity (8% NaCl). This characteristic addresses a key bottleneck of conventional materials, which struggle to simultaneously maintain the self-healing capability and mechanical strength under such demanding environments (Table 3). Mechanistic studies reveal that the synergistic effect between PNBT, acting as a “grafting core,” and PAS facilitates the construction of a dense network rich in dynamic bonds, which constitutes the microstructural foundation for the material’s performance breakthrough. This study has certain limitations. Firstly, the homogeneous sand bed plugging model used in the laboratory cannot fully simulate the geometric complexity and mineral surface chemistry of authentic formation fractures. Secondly, the self-healing performance was evaluated on a macroscopic scale without quantifying the healing kinetics under simulated downhole confining pressure and shear flow conditions. Furthermore, while the 16 h thermal aging test provides the preliminary validation of thermal stability, it is insufficient for a comprehensive assessment of the material’s long-term durability over the entire wellbore lifecycle. Based on these considerations, future research will focus on the following: (1) employing naturally fractured core samples for core flooding experiments to more realistically evaluate sealing performance, (2) developing high-pressure/high-temperature rheological testing methods to investigate the influence of confining pressure and shear on self-healing kinetics, (3) conducting long-term aging experiments over several weeks to months to thoroughly assess the material’s long-term service performance. In summary, the XFFD gel demonstrates significant potential for addressing lost circulation challenges in mid-deep geothermal drilling. This study not only presents a high-performance material but also, by elucidating the “structure-property-mechanism” relationship, provides new insights and a theoretical foundation for designing intelligent sealants for extreme environments.

Table 3.
Comparison of sealing agent parameters.
Author Contributions
W.W., Data curation, Investigation, Methodology, Writing—original draft. Y.T., Conceptualization, Investigation, Methodology, Formal analysis, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AA | Acrylic Acid |
| AM | Acrylamide |
| API | American Petroleum Institute |
| DSC | Differential Scanning Calorimetry |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| G′ | Storage Modulus |
| G″ | Loss Modulus |
| PAS | Modified Nano-Silica |
| PNBT | p-Nitro Blue Tetrazolium |
| SEM | Scanning Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| XFFD | Self-healing Gel |
References
- Rybach, L. Geothermal energy: Sustainability and the environment. Geothermics 2003, 32, 463–470. [Google Scholar] [CrossRef]
- Zeng, L.; Song, Y.; Liu, G.; Tan, X.; Xu, X.; Yao, Y.; Mao, Z. Natural fractures in ultra-deep reservoirs of China: A review. J. Struct. Geol. 2023, 175, 104954. [Google Scholar] [CrossRef]
- Cao, R.; Duo, J.; Li, Y.; Meng, H.; Cai, Y. Deep high-temperature geothermal resources in China: Occurrence characteristics and development status. Chin. J. Eng. 2022, 44, 1623–1631. [Google Scholar] [CrossRef]
- Magzoub, M.I.; Salehi, S.; Hussein, I.A.; Nasser, M.S. Loss circulation in drilling and well construction: The significance of applications of crosslinked polymers in wellbore strengthening: A review. J. Pet. Sci. Eng. 2020, 185, 106653. [Google Scholar] [CrossRef]
- Shahri, M.P.; Oar, T.T.; Safari, R.; Karimi, M.; Mutlu, U. Advanced semianalytical geomechanical model for wellbore-strengthening applications. SPE J. 2015, 20, 1276–1286. [Google Scholar] [CrossRef]
- Chen, G.H.; He, Y.L.; Qiu, Z.S.; Guan, J.; Wang, X.J. Research and application for the fine identification method of lost circulation characteristics during drilling. Pet. Drill. Tech. 2024, 52, 26–31. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Z.; Li, C.; Sun, Q.; Wang, W.; Deng, S.; Li, W.; Xie, A. High-temperature strengthening of Portland cementitious materials by surface micro-ceramization. Cem. Concr. Res. 2025, 190, 107790. [Google Scholar] [CrossRef]
- Ahmad, M.; Khan, S.; Li, Y. Eco-friendly cadmium removal using novel modified clay/alginate floatable beads: A sustainable solution for water pollution mitigation. J. Environ. Chem. Eng. 2024, 12, 112345. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Huang, W.; Yang, X.; Liu, Z.; Zhang, X. Preparation and Performance Evaluation of a Plugging Agent with an Interpenetrating Polymer Network. Gels 2023, 9, 205. [Google Scholar] [CrossRef]
- Fan, W.; Zhuang, G.; Li, Q.; Yuan, P.; Liu, D. Review of Nanoparticles in Water-Based Drilling Fluids: Innovations, Challenges, and Future Directions. Energy Fuels 2025, 39, 8800–8826. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Zhang, S.; Liao, B.; Zhao, K.; Li, Y.; Xu, J.; Chen, L. Review of the Perspectives and Study of Thermo-Responsive Polymer Gels and Applications in Oil-Based Drilling Fluids. Gels 2023, 9, 969. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Huang, W.A.; Sun, J.S.; Jin, Z.H.; Huang, X.B. The Utilization of Self-Crosslinkable Nanoparticles as High-Temperature Plugging Agent in Water-Based Drilling Fluid. SPE J. 2022, 27, 2628–2641. [Google Scholar] [CrossRef]
- Bai, X.; Wang, M.; Chen, Y.; Wu, L.; Yu, J.; Luo, Y. Synthesis and properties of self-healing hydrogel plugging agent. J. Appl. Polym. Sci. 2023, 141, e55005. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, Y.; Sun, J.; Lv, K. Plugging mechanism of rigid and flexible composite plugging materials for millimeter-scale fractures. SPE J. 2024, 29, 1786–1801. [Google Scholar] [CrossRef]
- Suri, A.; Sharma, M.M. Strategies for sizing particles in drilling and completion fluids. SPE J. 2004, 9, 13–23. [Google Scholar] [CrossRef]
- Pan, Y.; Cui, X.; Wang, H.; Lou, X.; Yang, S.; Oluwabusuyi, F.F. Research Progress of Intelligent Polymer Plugging Materials. Molecules 2023, 28, 2975. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-healing hydrogels: The next paradigm shift in tissue engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef]
- Liu, S.; Kang, M.; Li, K.; Yao, F.; Oderinde, O.; Fu, G.; Xu, L. Polysaccharide-templated preparation of mechanically-tough, conductive and self-healing hydrogels. Chem. Eng. J. 2018, 334, 2222–2230. [Google Scholar] [CrossRef]
- Hia, I.L.; Vahedi, V.; Pasbakhsh, P. Self-healing polymer composites: Prospects, challenges, and applications. Polym. Rev. 2016, 56, 225–261. [Google Scholar] [CrossRef]
- Chakma, P.; Konkolewicz, D. Dynamic covalent bonds in polymeric materials. Angew. Chem. Int. Ed. 2019, 58, 9682–9695. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, T.; Liu, Y.; Shang, F.; Chen, B.; Hu, Y.; Wang, S.; Guo, Z. Supramolecular hydrogel of poly(vinyl alcohol)/chitosan: A dual cross-link design. Adv. Polym. Technol. 2018, 37, 2186–2194. [Google Scholar] [CrossRef]
- Liu, H.; Chung, H. Self-healing properties of lignin-containing nanocomposite: Synthesis of lignin-graft-poly(5-acetylaminopentyl acrylate) via RAFT and click chemistry. Macromolecules 2016, 49, 7246–7256. [Google Scholar] [CrossRef]
- Kai, D.; Low, Z.W.; Liow, S.S.; Karim, A.A.; Ye, H.; Jin, G.; Li, K.; Loh, X.J. Development of lignin supramolecular hydrogels with mechanically responsive and self-healing properties. ACS Sustain. Chem. Eng. 2015, 3, 2160–2169. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Z.; Deng, X.; Wang, T.; Yang, Z.; Zhou, W.; Wang, C. Dual physically cross-linked hydrogels with high stretchability, toughness, and good self-recoverability. Macromolecules 2016, 49, 5660–5668. [Google Scholar] [CrossRef]
- Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Zhou, F. Electrospun chitosan nanofibers for wound healing application. Eng. Regen. 2021, 2, 82–90. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Wan, X.; Tang, Y.; Liu, Q.; Li, W.; Liao, J. Synthesis and Characterization of a Temperature-Sensitive Microcapsule Gelling Agent for High-Temperature Acid Release. ACS Omega 2024, 9, 20849–20858. [Google Scholar] [CrossRef]
- Niu, C.; Fan, S.; Chen, X.; He, Z.; Dai, L.; Wen, Z.; Li, M. Preparation and Performance Evaluation of a Supramolecular Polymer Gel-Based Temporary Plugging Agent for Heavy Oil Reservoir. Gels 2024, 10, 536. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Modica, K.J.; Jayaraman, A. Impact of Hydrogen Bonding Interactions on Graft–Matrix Wetting and Structure in Polymer Nanocomposites. Macromolecules 2019, 52, 2725–2735. [Google Scholar] [CrossRef]
- Wu, M.; Han, L.; Yan, B.; Zeng, H. Self-healing hydrogels based on reversible noncovalent and dynamic covalent interactions: A short review. Supramol. Mater. 2023, 2, 100045. [Google Scholar] [CrossRef]
- ISO 10414-1:2008; Recommended Practice for Field Testing Water-Based Drilling Fluids. API Publishing: Washington, DC, USA, 2009.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).