An Updated Overview on the Use of the β-Carotene Bleaching Method in Assessing the Antioxidant Activity of Compounds
Abstract
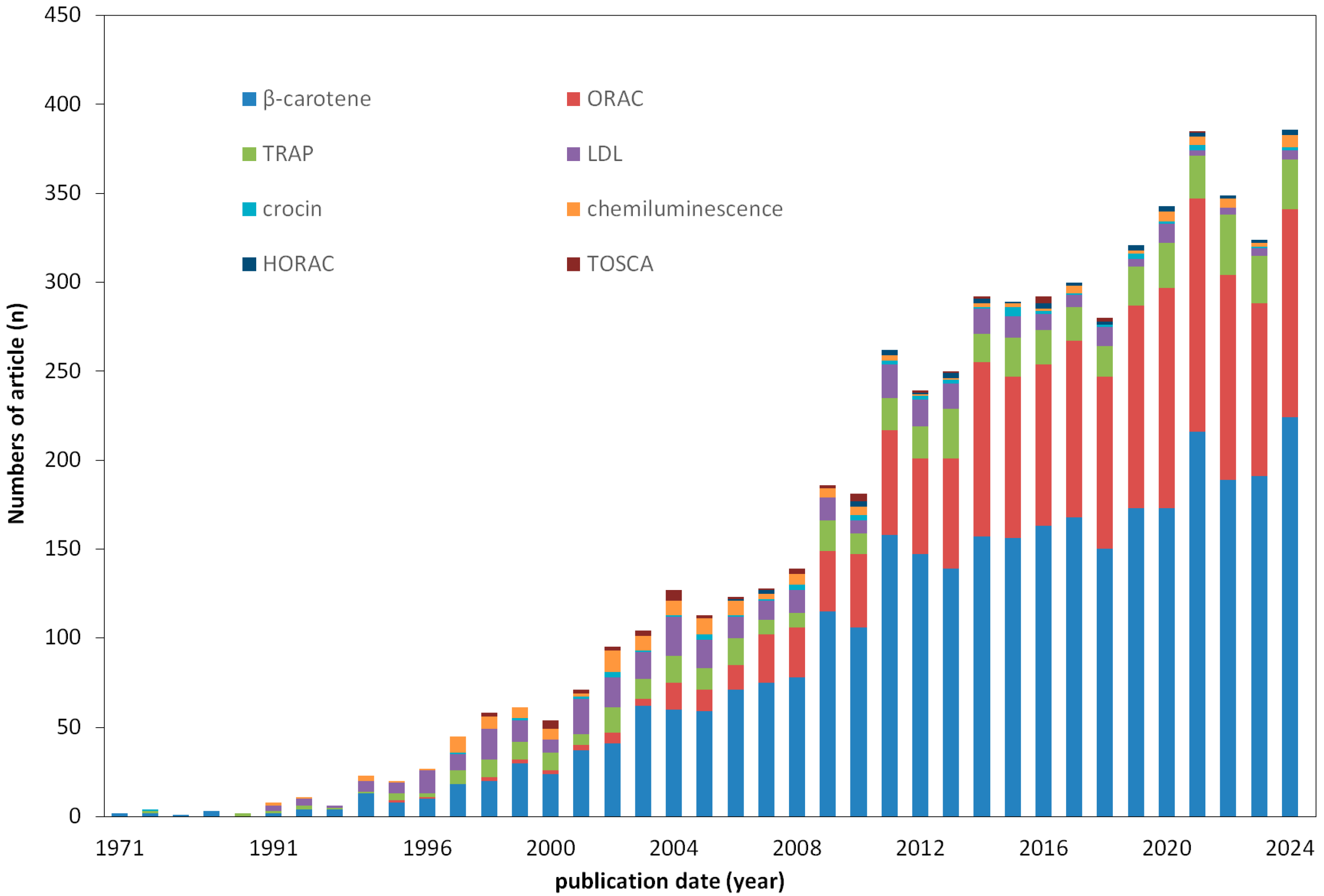
1. Introduction
- Single Electron Transfer, Abbreviated as SET;
- Hydrogen Atom Transfer, HAT.
- Oxygen radical absorbance capacity (ORAC);
- Total radical trapping antioxidant parameter (TRAP);
- Inhibition of induced low density lipoproteins (LDL) oxidation;
- Total oxyradical scavenging capacity assay (TOSCA);
- Crocin bleaching assay;
- Chemiluminescent assay;
- Hydroxyl radical antioxidant capacity (HORAC) and the title method;
- β-carotene bleaching assay.
2. β-Carotene as Molecular Probe in β-Carotene Bleaching Assay
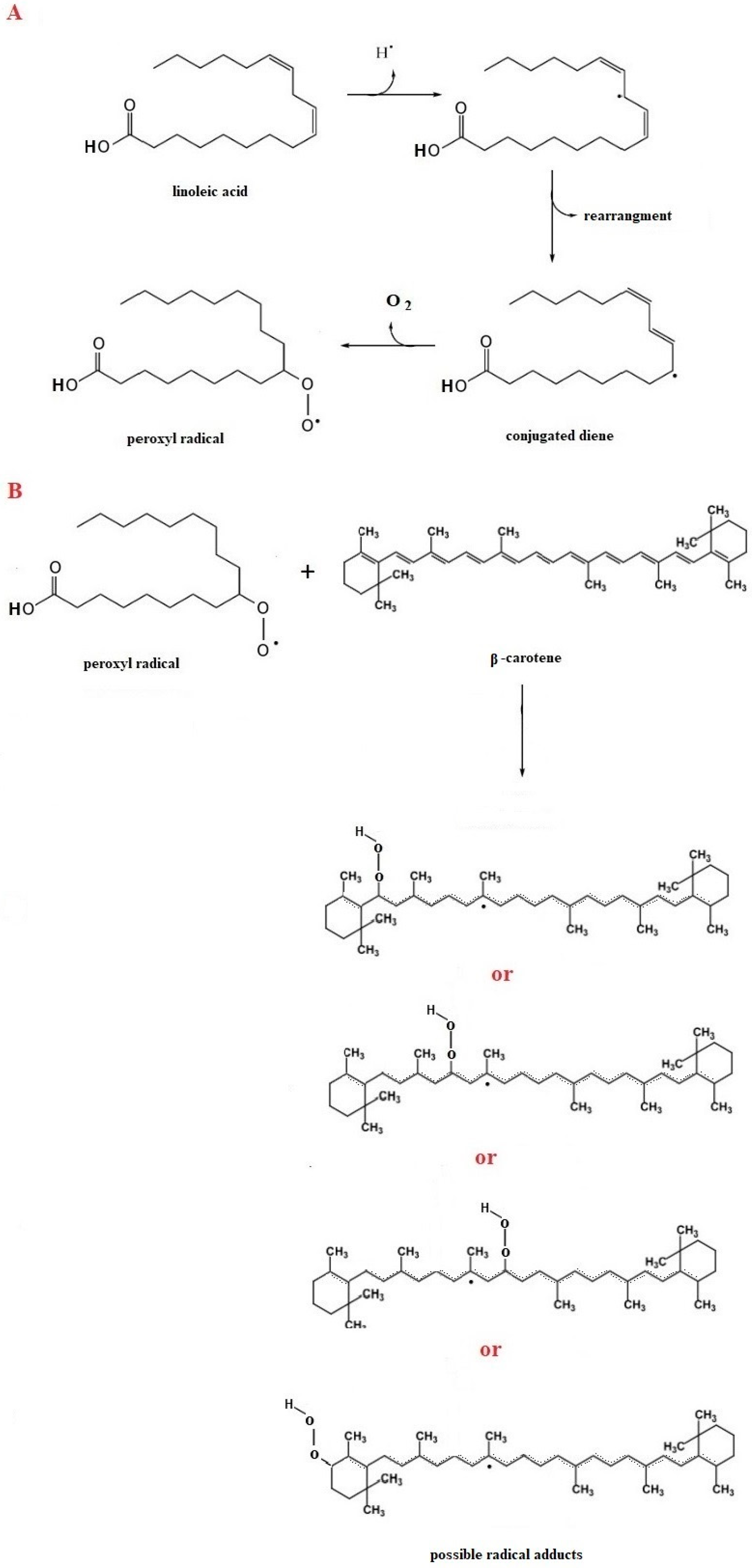
3. Principle of the Method
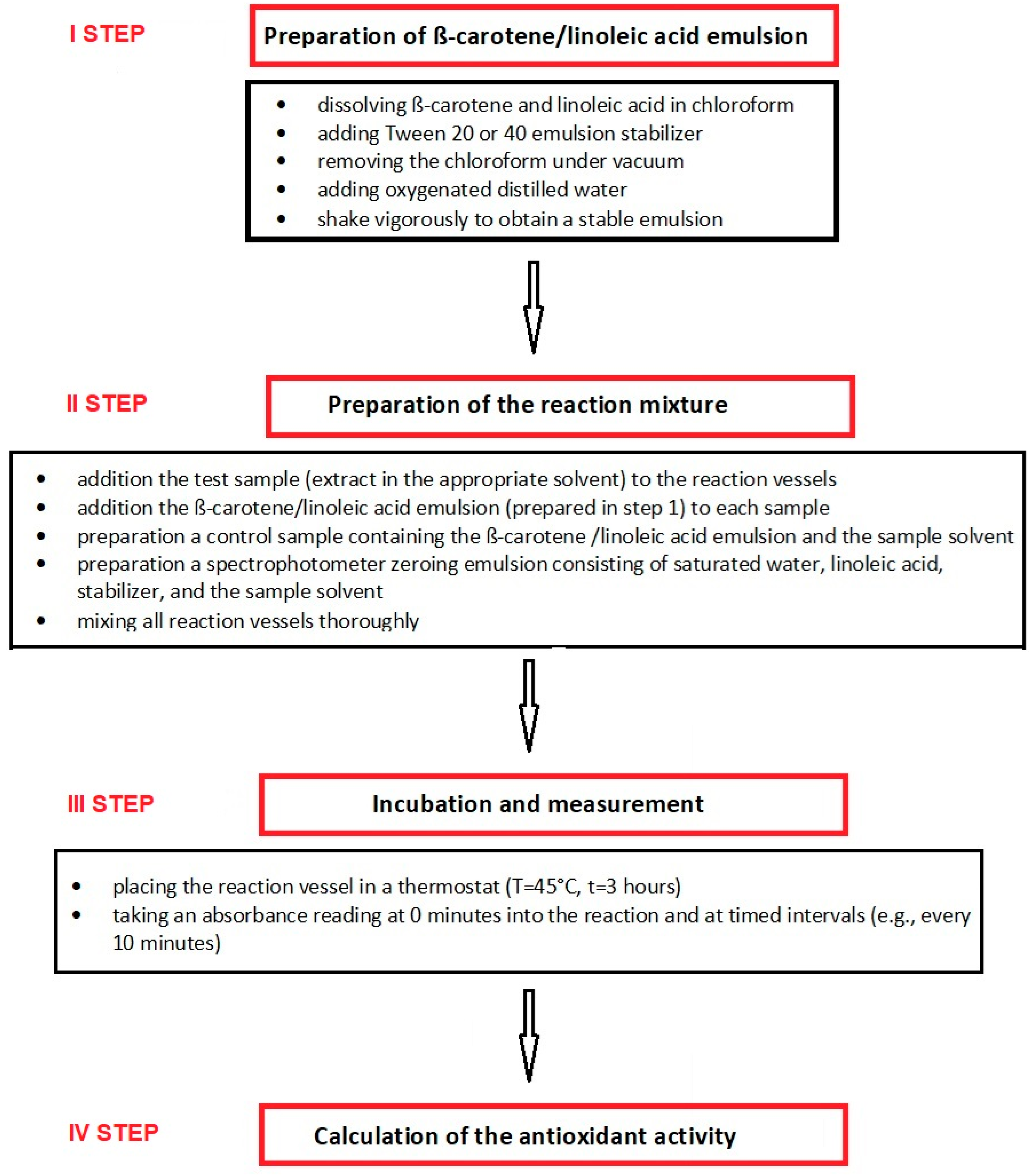
4. Procedure
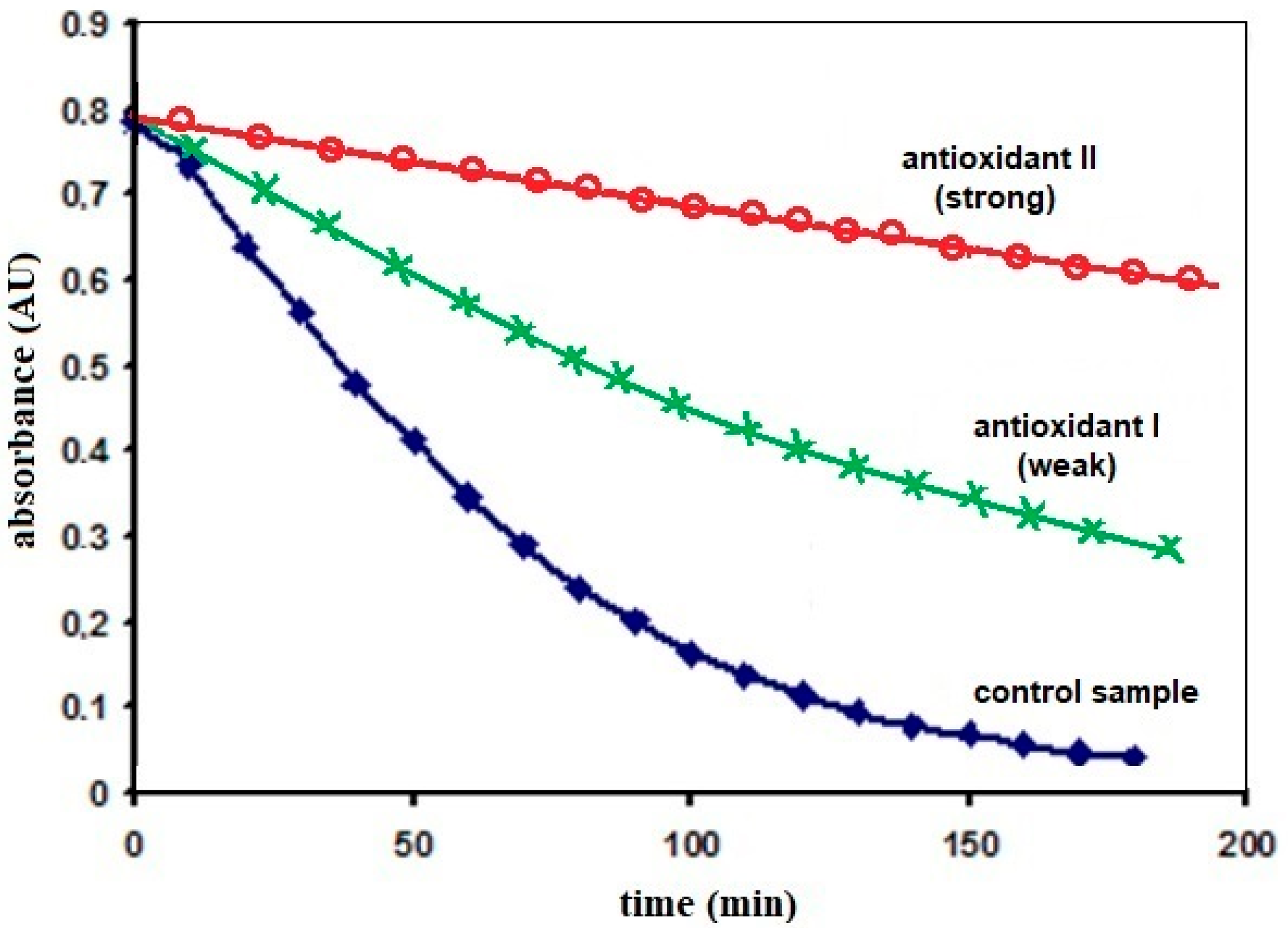
5. Presentation of the Final Results
6. Factors Influencing the Assessment of Antioxidant Properties Estimated by the β-Carotene Method
7. Application
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAC | Antioxidant Activity Coefficient |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| AH | Antioxidant |
| AOA | Antioxidant Activity |
| BDE | bond dissociation energy |
| BHA | Butylhydroxyanisole |
| BHT | Butylhydroxytoluene |
| CUPRAC | cupric ion reducing antioxidant capacity method |
| DMSO | Dimethyl sulfoxide |
| DMPD | dimethyl-p-phenylenediamine dihydrochloride |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EtOH | Ethanol |
| FRAP | ferric ion reducing antioxidant parameter method |
| GRAS | generally recognized as safe |
| HAT | Hydrogen Atom Transfer |
| HORAC | Hydroxyl Radical Antioxidant Capacity |
| IC | Inhibition Concentration |
| IP | ionization potential |
| LDL | Low Density Lipoprotein |
| MeOH | Methanol |
| ORAC | Oxygen Radical Absorbance Capacity |
| RAA | Relative Antioxidant Activities |
| ROS | Reactive Oxygen Species |
| SET | Single Electron Transfer |
| TOSCA | Total Radical Scavenging Capacity Assay |
| TRAP | Total Radical Trapping Antioxidant Parameter |
References
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of Antioxidants: Mechanisms and Pharmaceutical Applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Siddeeg, A.; Al Kehayez, N.M.; Abu-Hiamed, H.A.; Al-Sanea, E.A.; AL-Farga, A.M. Mode of action and determination of antioxidant activity in the dietary sources: An overview. Saudi J. Biol. Sci. 2021, 28, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef] [PubMed]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Boxin, O.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçclü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Jiménez-Morales, W.A.; del Pilar Cañizares-Macias, M.; Pedraza-Chaver, J. Fast ORAC-SIA method for antioxidant capacity determination in food samples. Food Chem. 2022, 384, 132524. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Evaluation of Spectrophotometric Methods for Assessing Antioxidant Potential in Plant Food Samples—A Critical Approach. Appl. Sci. 2025, 15, 5925. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçclü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar]
- Apak, R. Current Issues in Antioxidant Measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Marco, G.J. A rapid method for evaluation of antioxidants. J. Am. Oil Chem. Soc. 1968, 45, 594–598. [Google Scholar] [CrossRef]
- Amengual, J. Bioactive Properties of Carotenoids in Human Health. Nutrients 2019, 11, 2388. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Jiang, H.; Mao, X. Biotechnology advances in β-carotene production by microorganisms. Trends Food Sci. Technol. 2021, 111, 322–332. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Beta-Carotene#section=Names-and-Identifiers (accessed on 14 November 2025).
- Telegina, T.A.; Vechtomova, Y.L.; Aybush, A.V.; Buglak, A.A.; Kritsky, M.S. Isomerization of carotenoids in photosynthesis and metabolic adaptation. Biophys. Rev. 2023, 15, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Srivastava, R. Physicochemical, antioxidant properties of carotenoids and its optoelectronic and interaction studies with chlorophyll pigments. Sci. Rep. 2021, 11, 18365. [Google Scholar] [CrossRef]
- Prieto, M.A.; Vázquez, J.A.; Murado, M.A. β-Carotene Assay Revisited. Application To Characterize and Quantify Antioxidant and Prooxidant Activities in a Microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef]
- Loffredo, C.; Pires, P.A.R.; Imran, M.; El Seoud, O.A. β-Carotene: A green, inexpensive, and convenient solvatochromic probe for the determination of solvent polarizability. Dye. Pigment. 2013, 96, 16–24. [Google Scholar] [CrossRef]
- Lu, Y.; Teng-Jin, K.; Wiart, C. Phytochemical Analysis and Antioxidant Activity Determination on Crude Extracts of Melodinus eugeniifolus Barks and Leaves from Malaysia. Pharmacol. Pharm. 2014, 5, 773–780. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Miazek, K.; Beton, K.; Śliwińska, A.; Brożek-Płuska, B. The Effect of ß-Carotene, Tocopherols and Ascorbic Acid as Anti-Oxidant Molecules on Human and Animal In Vitro/In Vivo Studies: A Review of Research Design and Analytical Techniques Used. Biomolecules 2022, 12, 1087. [Google Scholar] [CrossRef]
- Nimbalkar, V.; Joshi, U.; Shinde, S.; Pawar, G. In-vivo and in-vitro evaluation of therapeutic potential of β-Carotene in diabetes. J. Diabetes Metab. Disord. 2021, 20, 1621–1630. [Google Scholar] [CrossRef]
- Ahmed, D.; Malik, W.; Maqsood, M.; Atique, I.; Qamar, M.T. Study of anti-diabetic, beta-carotene-bleaching inhibiting and iron chelating properties of Carissa opaca root extracts. Braz. J. Pharm. Sci. 2022, 58, e18628. [Google Scholar] [CrossRef]
- Roginsky, V.; Lissi, E.A. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D. Methods for determination of antioxidant capacity: A review. Int. J. Pharm. Sci. Res. 2015, 6, 546–566. [Google Scholar]
- Sadeer, N.B.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Bryshten, I.; Paprotny, Ł.; Olszowy-Tomczyk, M.; Wianowska, D. Antioxidant Properties of Green Plants with Different Vitamin K Contents. Molecules 2024, 29, 3655. [Google Scholar] [CrossRef]
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free Radical Mediated Oxidative Degradation of Carotenes and Xanthophylls. Molecules 2020, 25, 1038. [Google Scholar] [CrossRef] [PubMed]
- Miller, H. A simplified method for the evaluation of antioxidants. J. Am. Oil Chem. Soc. 1971, 48, 91. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods. Phytochem. Anal. 2022, 13, 8–17. [Google Scholar] [CrossRef]
- Gulluc, M.; Sahin, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiou, M.; Adiguzel, A.; Ozkan, H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longfolia L. ssp. longifolia. Food Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of five plantsused as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Olszowy, M. Influence of some experimental variables and matrix components in the determination of antioxidant properties by β-carotene bleaching assay: Experiments with BHT used as standard antioxidant. Eur. Food Res. Technol. 2010, 231, 835–840. [Google Scholar] [CrossRef]
- Nickavar, B.; Esbati, N. Evaluation of the Antioxidant Capacity and Phenolic Content of Three Thymus Species. J. Acupunct. Meridian Stud. 2012, 5, 119–125. [Google Scholar] [CrossRef]
- Loucif, K.; Benabdallah, H.; Benchikh, F.; Mehlous, S.; Souici, C.B.; Amira, S. Total Phenolic Contents, DPPH Radical Scavenging and β-Carotene Bleaching Activities of Aqueous Extract from Ammoides atlantica. J. Drug Deliv. Ther. 2020, 10, 196–198. [Google Scholar] [CrossRef]
- Chintong, S.; Phatvej, W.; Rerk-Am, U.; Waiprib, Y.; Klaypradit, W. In Vitro Antioxidant, Antityrosinase, and Cytotoxic Activities of Astaxanthin from Shrimp Waste. Antioxidants 2019, 8, 128. [Google Scholar] [CrossRef]
- Amiri, H. Chemical composition and antioxidant activity of essential oil and methanolic extracts of Ferula microcolea (boiss.) boiss (apiaceae). Int. J. Food Prop. 2014, 17, 722–730. [Google Scholar] [CrossRef]
- Moualek, I.; Lahcene, S.; Salem-Bekhit, M.M.; Khan, A.A.; Alanazi, A.M.; Bariz, K.; Msela, A.; Sebbane, H.; Park, H.K.; Jeon, B.H.; et al. Assessment of the Antioxidant and Anti-inflammatory Properties of Aqueous Extract of Rosa sempervirens Leaves. Cell Mol. Biol. 2023, 69, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Öğüt, K.; Özek, G.; Oztürk, N.; Yaylaci, Ö.K.; Özek, T. Chemical composition, α-amylase inhibition, and antioxidant activities of Scabiosa hololeuca Bornm. Biochem. Syst. Ecol. 2025, 122, 105028. [Google Scholar] [CrossRef]
- Soares Mateus, A.R.; Pena, A.; Sanches Silva, A. Development of functional muffins enriched with lemon by-products as sources of Bioactive compounds. Food Chem. Adv. 2025, 7, 100972. [Google Scholar] [CrossRef]
- Azzouni, D.; Alaoui Mrani, S.; Bahij, F.; Zejli, H.; Alanazi, M.M.; Fadili, D.; El Moussaoui, A.; Mahmoud, A.M.; Taleb, M. Comprehensive Phytochemical, Antioxidant, and Antibacterial Analysis of Vitex agnus-castus L. Essential Oil (VACEO): Insights from ADMET and Molecular Docking Studies. Pharmaceuticals 2025, 18, 462. [Google Scholar] [CrossRef]
- Faidi, A.; El Hédi Becheikh, M.; Ali Lassoued, M.; Stumbé, J.F.; Safta, F.; Sfar, S. Isolation of sodium alginate-like polysaccharide from Padina pavonica: Optimization, characterization and antioxidant properties. J. Mol. Struct. 2025, 1321, 139737. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Ballester, A.R.; Lopez-Rubiò, A.; Acha, V.; Aussenac, T.; Martínez-Abad, A. Antimicrobial and antioxidant properties of water-soluble lignin extracts obtained from ozonation of Miscanthus giganteus and Vitis vinifera in a pilot-scale reactor. Ind. Crops Prod. 2025, 223, 120092. [Google Scholar] [CrossRef]
- Solorzano, E.L.; Roverso, M.; Bogialli, S.; Bortoli, M.; Orian, L.; Badocco, D.; Pettenuzzo, S.; Favaro, G.; Pastore, P. Antioxidant activity of Zuccagnia-type propolis: A combined approach based on LC-HRMS analysis of bioanalyical-guided fractions and computational investigation. Food Chem. 2024, 461, 140827. [Google Scholar] [CrossRef] [PubMed]
- Prabakarans, S.; Saad, H.M.; Tan, C.H.; Rahman, S.N.S.A.; Sim, K.S. Investigation of Phytochemical Composition, Radical Scavenging Potential, Anti-Obesogenic Effects, and Anti-Diabetic Activities of Kaempferia parviflora Rhizomes. Chem. Biodivers. 2025, 22, e202401086. [Google Scholar] [CrossRef]
- Altaf, L.; Wani, S.A.; Hussain, P.R.; Suradkar, P.; Baqual, M.F.; Bhat, A.A. Bioactive compounds and antioxidant activity in various parts of Morus alba L. Cv. ichinose: A comparative analysis. Discov. Life 2024, 54, 7. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. Depletion/protection of β-carotene in estimating antioxidant activity by β-carotene bleaching assay. J. Food Sci. Technol. 2015, 52, 7321–7328. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M. How to express the antioxidant properties of substances properly? Chem. Pap. 2021, 75, 6157–6167. [Google Scholar] [CrossRef]
- Gül, A.; Pehlivan, T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Paredes, A. Antioxidant activities of Lampaya medicinalis extracts and their main chemical constituents. BMC Complement. Altern. Med. 2014, 21, 259. [Google Scholar] [CrossRef]
- Dawidowicz, A.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT-Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Gouveia-Figueira, S.C.; Gouveia, C.A.; Carvalho, M.J.; Rodrigues, A.I.; Nording, M.L.; Castilho, P.C. Antioxidant Capacity, Cytotoxicity and Antimycobacterial Activity of Madeira Archipelago Endemic Helichrysum Dietary and Medicinal Plants. Antioxidants 2014, 3, 713–729. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, A. Essential Oils Composition and Antioxidant Properties of Three Thymus Species. Evid. Based Complement. Altern. Med. 2012, 2012, 728065. [Google Scholar]
- Khouya, T.; Ramchoun, M.; Hmidani, A.; El moualij, B.; Amrani, S.; Harnafi, H.; Benlyas, M.; Zegzouti, Y.F.; Nazih, E.H.; Ouguerram, K.; et al. Acute toxicity and antiproliferative and procoagulant activities of fractions derived from Thymus satureioides of the Moroccan High Atlas. S. Afr. J. Bot. 2019, 121, 568–576. [Google Scholar] [CrossRef]
- Khouya, T.; Ramchoun, M.; Hmidani, A.; Khouyaa, T.; dine Tariq Bouhlali, E.; Alem, C. Phytochemical analysis and bioactivity evaluation of Moroccan Thymus atlanticus (Ball) fractions. Sci. Afr. 2021, 11, e00716. [Google Scholar] [CrossRef]
- Hwang, S.J.; Lee, J.H. Comparison of antioxidant activities expressed as equivalents of standard antioxidant. Food Sci. Technol. 2023, 43, e121522. [Google Scholar] [CrossRef]
- Dapkevicius, A.; Venskutonis, R.; van Beek, T.A.; Linssen, J.P.H. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J. Sci. Food Agric. 1998, 77, 140–146. [Google Scholar] [CrossRef]
- Andrade, M.A.; Ribeiro-Santos, R.; Costa Bonito, M.C.; Saraiva, M.; Sanches-Silva, A. Characterization of rosemary and thyme extracts for incorporation into a whey protein based film. LWT 2018, 92, 497–508. [Google Scholar] [CrossRef]
- Moon, J.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1660. [Google Scholar] [CrossRef]
- Valgimigli, L.; Amorati, R.; Petrucci, R.; Peduli, G.F.; Hu, D.; Hanthorn, J.J.; Pratt, D.A. Unexpected acid catalysis in reaction of peroxyl radicals with phenols. Angew. Chem. Int. Ed. 2009, 48, 8348–8351. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M.; Wianowska, D. Comparison of the Antioxidant Properties of Extracts Obtained from Walnut Husks as well as the Influence of Juglone on Their Evaluation. Appl. Sci. 2024, 14, 2972. [Google Scholar] [CrossRef]
- Marković, Z. Study of the mechanisms of antioxidative action of different antioxidants. J. Serbian Soc. Comput. Mech. 2016, 10, 135–150. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Antioxidant Behavior in Bulk Oil: Limitations of Polar Paradox Theory. J. Agric. Food Chem. 2012, 60, 4–6. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- Durand, E.; Laguerre, M.; Bourlieu-Lacanal, C.; Lecomte, J.; Villeneuve, P. Navigating the complexity of lipid oxidation and antioxidation: A review of evaluation methods and emerging approaches. Progress. Lipid Res. 2025, 97, 101317. [Google Scholar] [CrossRef]
- Chirouf, S.; Belahcene, N.; Barhouchi, B.; Zenati, N.; Daira, A.; Bensouici, C.; Bendif, H. Bioactive Metabolites from Aristolochia navicularis of Souk Ahras: Therapeutic Insights. Trop. J. Nat. Prod. Res. 2025, 9, 3140–3149. [Google Scholar] [CrossRef]
- Norhaiza, M.; Maziah, M.; Hakiman, M. Antioxidative properties of leaf extracts of a popular Malaysian herb, Labisia pumila. J. Med. Plants Res. 2009, 3, 217–223. [Google Scholar]
- Tighilet, K.; Adjebli, A.; Messis, A. Inhibition of enzymatic browning and antioxidant activities of Marrubium vulgare L extracts: A promising natural solution. Pharmacol. Res. Mod. Chin. Med. 2025, 16, 100658. [Google Scholar] [CrossRef]
- Leporini, M.; Catinella, G.; Bruno, M.; Falco, T.; Tundis, R.; Loizzo, M.R. Investigating the Antiproliferative and Antioxidant Properties of Pancratium maritimum L. (Amaryllidaceae) Stems, Flowers, Bulbs, and Fruits Extracts. Evid. Based Complement. Altern. Med. 2018, 2018, 9301247. [Google Scholar] [CrossRef]
- Lahcene, S.; Moualek, I.; Bariz, K.; Benramdane, E.; Alenazy, R.; Alqasmi, M.; Almufarriji, F.M.; Thabet, M.; Fallata, G.; Alqurainy, N.; et al. Antioxidant and Antibacterial Activities and Phytochemical Analysis of Olea europaea subsp. laperrinei Leaves Extracts. Processes 2025, 13, 1113. [Google Scholar]
- Indrianingsih, A.W.; Windarsih, A.; Noviana, E.; Suratno; Asari, S.M.; Pratiw, S.I. In vitro evaluation of antioxidant, α-glucosidase inhibitor, and antibacterial activities of frangipani flower and the principal component analysis of its constituents. Process Biochem. 2023, 130, 346–357. [Google Scholar] [CrossRef]
- Atia, A.; Atmani-Kilani, D.; Atmani, D.; Ayouni, K.; Belkhir, S.; Benloukil, M.; Saidene, N.; Moulaoui, K.; Kasmi, S.; Medjahed, Z.; et al. Wound healing potential of a formula based on Populus nigra L. flower buds extract with anti-inflammatory activity. J. Ethnopharmacol. 2024, 331, 118319. [Google Scholar] [CrossRef]
- Azam, M.; Sana, A.; Saleem, R.; Faizi, S.; Razzaq, A.; Ul-Haq, Z.; Sarwar, H. Chemical characterization, antioxidant and molecular docking studies on Ajwa pulp and seed concert extracts as potential antihemolytic agent. Pak. J. Pharm. Sci. 2025, 38, 1240–1253. [Google Scholar] [CrossRef]
- Daoudi, N.E.; Houmy, N.; Melhaoui, R.; Bnouham, M. Effect of Heating Temperatures on the Physicochemical Parameters and Antihyperglycemic Activity of Argan seeds oil In vitro and In vivo. Trop. J. Nat. Prod. Res. 2025, 9, 2543–2552. [Google Scholar] [CrossRef]
- Zitouni, H.; Hssaini, L.H.; Ouaabou, R.; Viuda-Martos, M.; Hernandez, F.; Ercisli, S.; Hachimi, H.; Zerhoune, M.; Hanine, H. Functionnal and Technological Properties of Five Strawberry (Arbutus unedo L.) Fruit as Bioactive Ingredients in Functional Foods. Int. J. Food Prop. 2021, 24, 380–399. [Google Scholar] [CrossRef]
- Djenidi, H.; Khennouf, S.; Bouaziz, A. Antioxidant activity and phenolic content of commonly consumed fruits and vegetables in Algeria. Prog. Nutr. 2020, 22, 224–235. [Google Scholar]
- Hamid, M.A.; Yeap, C.H.; Mustapha, W.A.W.; Martony, O.; Fatmawati, F. Effects of Different Solvents on the Antioxidant Activity of Several Seaweed Species from Semporna, Sabah, Malaysia. Ilmu Kelaut Indones. J. Mar. Sci. 2024, 29, 29–36. [Google Scholar] [CrossRef]
- Boghozian, A.; Shiran, H.R.; Choopani, A.; Choopani, A.; Amin, N.G. Extraction of Artemisia aucheri Essential Oils and Evaluation of their Chemical Composition and Antioxidant Activity. J. Appl. Biotechnol. Rep. 2025, 12, 1678–1685. [Google Scholar]
- Ranjbar, F.; Abedpour, M.; Abdosheikhi, M.; Ahmadi, E.; Abedi, E. An investigation of anti-oxidant properties of salvia, conducting beta-carotene bleaching assay. Sci. J. 2015, 36, 1–6. [Google Scholar]
- Budiarto, R.; Khalisha, A.; Sari, D.N.; Ujilestari, T.; Wahyono, T.; Azmi, A.F.M.; Adli, D.N.; Lusiana, E.D.; Sitaresmi, P.I.; Sholikin, M.M. Antioxidant properties of lemon essential oils: A meta-analysis of plant parts, extraction methods, dominant compounds, and antioxidant assay categories. Chem. Biol. Technol. Agric. 2024, 11, 147. [Google Scholar] [CrossRef]
- EL Moussaoui, A.; Bourhia, M.; Jawhari, F.Z.; Salamatullah, A.M.; Ullah, R.; Bari, A.; Majid Mahmood, H.; Sohaib, M.; Serhii, B.; Rozhenko, A.; et al. Chemical Profiling, Antioxidant, and Antimicrobial Activity against Drug-Resistant Microbes of Essential Oil from Withania frutescens L. Appl. Sci. 2021, 11, 5168. [Google Scholar]
- Smeriglio, A.; Imbesi, M.; Ingegneri, M.; Rando, R.; Mandrone, M.; Chiocchio, I.; Poli, F.; Trombetta, D. From Waste to Resource: Nutritional and Functional Potential of Borlotto Bean Pods (Phaseolus vulgaris L.). Antioxidants 2025, 14, 625. [Google Scholar] [CrossRef]
- Lakhmili, H.; Warda, K.; El-Abbassi, A.; Hafidi, A. Antioxidant and anti-glycation activity of eight Moroccan honeys from different botanical origins. Discov. Food 2024, 4, 6. [Google Scholar] [CrossRef]
- Bazaid, A.S.; Alamri, A.; Almashjary, M.N.; Qanash, H.; Almishaal, A.A.; Amin, J.; Binsaleh, N.K.; Kraiem, J.; Aldarhami, A.; Alafnan, A. Antioxidant, Anticancer, Antibacterial, Antibiofilm Properties and Gas Chromatography and Mass Spectrometry Analysis of Manuka Honey: A Nature’s Bioactive Honey. Appl. Sci. 2022, 12, 9928. [Google Scholar] [CrossRef]
- Ali Haimoud, S.; Allem, R.; Benyahla Djeffaland, K.; Lembarki, N.E. Evaluation in Vitro and in Vivo of Biological Activities of Propolis and Bee Pollen Extracts. Phytothérapie 2022, 20, 63–71. [Google Scholar] [CrossRef]
- Belmehdi, O.; Bouyahya, A.; Jekő, J.; Cziáky, Z.; Zengin, G.; Sotkó, G.; El baaboua, A.; Skali SenhajI, N.; Abrini, J. Chemical analysis, antibacterial, and antioxidant activitiesof flavonoid-rich extracts from four Moroccan propolis. J. Food Process Preserv. 2021, 45, e15816. [Google Scholar] [CrossRef]
- Byczkiewicz, S.; Szwajgier, D.; Baranowska-Wójcik, E.; Telichowska, A.; Szymandera-Buszka, K.; Wojtczak, J.; Kobus-Cisowska, J. Research on Application of Japanese Quince (Chaenomeles L.) and Pork Collagen in Dark Chocolate—Benefits in Prevention of Inflammation In Vitro Model. Nutrients 2024, 16, 1758. [Google Scholar] [CrossRef] [PubMed]
- Dasdemir, Y.; Tuba Findik, B.; Yildiz, H.; Birisci, E. Blueberry-added black tea: Effects of infusion temperature, drying method«fruit concentration on the iron-polyphenol complex formation, polyphenols profile, antioxidant activity, and sensory properties. Food Chem. 2023, 410, 135463. [Google Scholar] [CrossRef]
- dos Santos Lima, M.; Ferreira, E.T.J.; de Souza, M.E.A.O.; Pereira, G.E.; Toaldo Fedrigo, I.M. Artificial neural network: A powerful tool in associating phenolic compounds with antioxidant activity of grape juices. Food Anal. Methods 2022, 15, 527–540. [Google Scholar] [CrossRef]
- Luěs, Ȃ.; Sousȃ, S.; Duarte, A.P.; Pereira, L.; Domingues, F. Phytochemical characterization, and evaluation of rheological and antioxidant properties of commercially available juices of berries. J. Berry Res. 2018, 8, 11–23. [Google Scholar] [CrossRef]
- Imran, A.; Quispe, C.; Zeeshan, A.; Imran, M.; Nadeem, M.; Gilani, S.A.; Gondal, T.A.; Tufail, T.; Aslam, F.; Rodrigues, C.F.; et al. Development and antioxidant characterization of Ginger-Mint drink prepared through different extraction techniques. J. Food Meas. Charact. 2021, 15, 2576–2590. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M.; Garbaczewska, S.; Wianowska, D. Correlation Study of Biological Activity with Quercetin and Phenolics Content in Onion Extracts. Molecules 2022, 27, 8164. [Google Scholar] [CrossRef]
- Lasunon, P.; Phonkerd, N.; Tettawong, P.; Sengkhamparn, N. Total phenolic compound and its antioxidant activity of by-product from pineapple. Food Res. 2022, 6, 107–112. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Andrade, M.A.; Séndon, R.; Silva, A.S.; Ramos, F.; Vilarinho, F.; Khwaldia, K.; Barbosa-Pereira, L. Industrial Fruits By-Products and Their Antioxidant Profile: Can They Be Exploited for Industrial Food Applications? Foods 2021, 10, 272. [Google Scholar] [CrossRef]
- Haddar, A. Polysaccharides extracted from pistachio external hull: Characterization, antioxidant activity and potential application on meat as preservative. Ind. Crops Prod. 2020, 148, 112315. [Google Scholar]
- Samotyja, U. Potato Peel as a Sustainable Resource of Natural Antioxidants for the Food Industry. Potato Res. 2019, 62, 435–451. [Google Scholar] [CrossRef]
- Yusof, A.H.M.; Gani, S.S.A.; Zaidan, U.H.; Halmi, M.I.E. Central Composite Design as a Tools for Optimization of Antioxidant Activity on Cocoa Shell Extract. Int. J. Eng. Adv. Technol. 2019, 8, 1028. [Google Scholar] [CrossRef]
- Fawwaz, M.; Pratama, M.; Hasrawati, A.; Sukmawati; Widiastuti, H.; Rahmawati; Abidin, Z. Total carotenoids, antioxidant and anticancer effect of penaeus monodon shells extract. Biointerface Res. Appl. Chem. 2020, 11, 11293–11302. [Google Scholar]
- Ueno, H.; Yamakura, S.; Arastoo, R.S.; Oshima, T.; Kokubo, K. Systematic Evaluation and Mechanistic Investigation of Antioxidant Activity of Fullerenols Using β-Carotene Bleaching Assay. J. Nanomater. 2014, 2014, 802596. [Google Scholar] [CrossRef]
- Kop, T.J.; Bjelaković, M.S.; Živković, L.; Žekić, A.; Milić, D.R. Stable colloidal dispersions of fullerene C60, curcumin and C60-curcumin in water as potential antioxidants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129379. [Google Scholar] [CrossRef]
- Baba, Y.F.; Sert, Y.; Rodi, Y.K.; Hayani, S.; Mague, J.T.; Prim, D.; Marrot, J.; Chahdi, F.O.; Sebbar, N.K.; Essassi, E.M. Synthesis, crystal structure, spectroscopic characterization, Hirshfeld surface analysis, molecular docking studies and DFT calculations, and antioxidant activity of 2-oxo-1,2-dihydroquinoline-4-carboxylate derivatives. J. Mol. Struct. 2019, 1188, 255–268. [Google Scholar] [CrossRef]
- Shehzadi, N.; Hussain, K.; Khan, M.T.; Bukhari, N.I.; Islam, M.; Salman, M.; Qamar, S.; Iqbal, A.A.; Siddiqui, S.Z.; Rehman, A.; et al. Radical Scavenging and Endogenous Defence System Inducing Activities of 5-[(4-Chlorophenoxy)methyl]-1,3,4-oxadiazole-2-thiol: A Novel Antioxidant. Indian. J. Pharm. Sci. 2018, 80, 1125–1135. [Google Scholar] [CrossRef]
- Khan, M.A.; Kola, V.B.; Noor, B.; Acco, J. The Antioxidant Activity of Dihydropyridine Derivatives. Curr. Res. Bioorg. Org. Chem. 2020, 3, 124. [Google Scholar]
- Liu, J.; Chen, B.; Hu, Q.; Zhang, Q.; Huang, B.; Fei, P. Pectin grafted with resorcinol and 4-hexylresorcinol: Preparation, characterization and application in meat preservation. Int. J. Biol. Macromol. 2023, 237, 124212. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Souza, V.G.L.; Coelhoso, I.M.; Reboleira, J.; Bernardino, S.; Ganhão, R.; Mendes, S.; Fernando, A.L.; Vilarinho, F.; et al. Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat. Coatings 2021, 11, 229. [Google Scholar] [CrossRef]
- Mouna, S.; Hajji, S.; Tounsi, H. Waste to health: Green synthesis of Zn loaded LTA zeolite prepared from waste glass and aluminum scrap with high antioxidant and antimicrobial activities. J. Clean. Prod. 2024, 434, 139946. [Google Scholar] [CrossRef]
- Sayehi, M.; Hajji, S.; Boudjema, L.; Kazemian, H.; Nasri, M.; Tounsi, H. Using a zeolite produced from glass waste and aluminum scraps to develop a novel gelatin-based biodegradable composites films: Antibacterial and antioxidant properties of a potential food packaging material. Inorg. Chem. Commun. 2022, 140, 109415. [Google Scholar] [CrossRef]
- Khramchenkova, O.M. Spectrophotometric evaluation of photoprotective and antioxidant properties of ex-tracts from the cultured mushrooms fruit bodies. Chem. Plant Raw Mater. 2024, 4, 268–277. [Google Scholar] [CrossRef]
- Tel-Çayan, G.; Deveci, E.; Çayan, F. Study on Phenolic and Organic Acid Compositions and Antioxidant and Enzyme Inhibition Activities of Agaricomycetes Mushroom Species from Turkey. Int. J. Med. Mushrooms 2023, 25, 11–25. [Google Scholar] [CrossRef]
- Foo, S.C.; Lee, Z.S.; Yap, M.K.K.; Tan, J.W. The antioxidant, wound healing properties and proteomic analysis of water extracts from the tropical cyanobacteria, Nostoc NIES-2111_MUM004. 3 Biotech 2023, 13, 71. [Google Scholar] [CrossRef]
- Gani, S.S.A.; Halim, A.N.A.; Zaidan, U.H.; Halmi, M.I.E.; Wahab, N.A. Antioxidants and Characterization of Stability and Organoleptic Properties of Cocoa Facial Mask. J. Phys. Conf. Ser. 2021, 1860, 012023. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Func. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Maghsoudlou, E.; Raftani Amiri, Z.; Esmaeilzadeh kenari, R. Determination and correlation analysis of phytochemical compounds, antioxidant activity, and oxidative stability of different edible oils. Food Meas. 2024, 18, 714–726. [Google Scholar] [CrossRef]
- Zouari, O.; Deracinois, B.; Flahaut, C.; Przyblski, R.; Nedjar, N. Poultry Cruor Hydrolysate is a New Potential Source of Hemoglobin: Obtaining of Active Peptides. Waste Biomass Valor. 2024, 15, 3323–3337. [Google Scholar] [CrossRef]
- Hamed, F.; Elgaoud, I.; Eljoudi, S.; Deracinois, B.; Flahaut, C.; Nedjar, N.; Barkia, A. Diplodus Protein Hydrolysates: Antioxidant and Antibacterial Properties and Identification of Biopeptides. Waste Biomass Valor. 2024, 15, 4309–4323. [Google Scholar] [CrossRef]
| Reagents | Sample Type | Sample Preparation | Volume of Emulsion [mL]/Volume of Sample [mL] (Emulsion to Sample Ratio) | Measurement Conditions | Manner of Expressing Results | Ref. |
|---|---|---|---|---|---|---|
| Vegetables |
| 5:0.2 (25:1) | T = 45 °C λ = 470 nm t = 2 h | Equation (3) | [37] |
| Herbs |
| 2.5:0.35 (7:1) | T = 25 °C λ = 490 nm t = 48 h | Absorbance vs. time | [38] |
| Herbs |
| 2.5:0.3 (8.3:1) | T = 50 °C λ = 470 nm t = 2 h | Equation (4) | [39] |
| Herbs |
| 5:0.2 (25:1) | T = 50 °C λ = 470 nm t = 2 h | Equations (1) and (2) | [40] |
| Standard antioxidant (BHT) |
| 2.5:0.35 (7.1:1) | T = 45 °C λ = 470 nm t = 3 h | Equations (1) and (2) | [41] |
| Herbs |
| 4.5:0.5 (9:1) | T = 40 °C λ = 470 nm t = 2 h | Equation (4) | [42] |
| Herbs (medicinal plants) |
| 0.16:0.04 (4:1) | T = 50 °C λ = 470 nm t = 2 h | Equations (1) and (2) | [43] |
| Pigments |
| 0.1:0.1 (1:1) | T = 50 °C λ = 470 nm t = 2 h | Equation (3) | [44] |
| Herbs |
| 2.5:0.35 (7.1:1) | T = 50 °C λ = 470 nm t = 2 h | Equation (4) | [45] |
| Plants |
| (2.5:0.35) 7.1:1 | T = 40 °C λ = 470 nm t = 2 h | Equations (1) and (2) | [46] |
| Plants |
| 0.25:0.06 (4.2:1) | T = 50 °C λ = 492 nm t = 1.75 h | Equation (3) | [47] |
| Food products |
| 5:0.2 (25:1) | 50 470 2 | Equation (6) | [48] |
| Plants |
| 5:0.2 (25:1) | T = 50 °C λ = 470 nm t = 2 h | Equations (1) and (2) | [49] |
| Algae |
| 3:0.1 (30:1) | T = 40 °C λ = 470 nm t = 2 h | Equation (3) | [50] |
| Plants |
| (0.25:0.01) 25:1 | T = 45 °C λ = 470 nm t = 2 h | Equations (1) and (2) | [51] |
| Propolis |
| 4:0.04 (100:1) | T = 40 °C λ = 470 nm t = 2 h | Equations (1) and (2) | [52] |
| Plants |
| 0.2:0.05 (4:1) | T = 40 °C λ = 470 nm t = 3 h | Equation (3) | [53] |
| Plants |
| 4.5:0.3 (15:1) | T = 50 °C λ = 470 nm t = 1.7 h | Equation (3) | [54] |
| Parameters/Factors | Proposed Conditions | Reason |
|---|---|---|
| Wavelength | 450–470 nm | The given length range corresponds to the maximum in the absorption spectrum of β-carotene |
| Measuring time | 2–3 h | The measurement of the absorbance is continued until the color of β-carotene disappears [30] |
| Sample solvent | Mainly alcohols, acetone | The mentioned solvents mix well with the emulsion and also have a low ability to block hydrogen in the process of its transport from the antioxidant to the radical [41] |
| pH | 5.5–7.0 | At 45 °C, the time required to achieve a given degree of bleaching in the control sample is very long at low pH (3.0–4.0), changes rapidly from pH 4.0–5.5, remains essentially constant from pH 5.5 to 7.5, and then slowly increases from pH 7.5 to 11.0. Therefore, the range of 5.5–7.5 appears to be the best option for ensuring stable change over a reasonable time [20,55] |
| Temperature | 45 °C | This temperature has little effect on the spontaneous oxidation of β-carotene, allows for the observation of different levels of antioxidants (weaker, stronger), and produces the fewest side effects at this temperature [20] |
| Reagent ratio v of emulsion/v of sample | From 50:1 to 25:1 | Our studies have shown that changing the volume ratio of the reactants towards reducing the amount of emulsion and increasing the volume of the sample leads to worse antioxidant properties, even by more than 10% when the ratio changes from 50:1 to 5:1 (with the same amount of antioxidant dissolved in a different amount of solvent) [41]. |
| Way of expressing results | Equations (1) and (2) | These are the two equations most commonly found in the literature. They represent the changes in β-carotene absorbance in the control and test samples and relate these changes to the initial absorbance in a logarithmic manner. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszowy-Tomczyk, M.; Wianowska, D. An Updated Overview on the Use of the β-Carotene Bleaching Method in Assessing the Antioxidant Activity of Compounds. Processes 2025, 13, 3814. https://doi.org/10.3390/pr13123814
Olszowy-Tomczyk M, Wianowska D. An Updated Overview on the Use of the β-Carotene Bleaching Method in Assessing the Antioxidant Activity of Compounds. Processes. 2025; 13(12):3814. https://doi.org/10.3390/pr13123814
Chicago/Turabian StyleOlszowy-Tomczyk, Małgorzata, and Dorota Wianowska. 2025. "An Updated Overview on the Use of the β-Carotene Bleaching Method in Assessing the Antioxidant Activity of Compounds" Processes 13, no. 12: 3814. https://doi.org/10.3390/pr13123814
APA StyleOlszowy-Tomczyk, M., & Wianowska, D. (2025). An Updated Overview on the Use of the β-Carotene Bleaching Method in Assessing the Antioxidant Activity of Compounds. Processes, 13(12), 3814. https://doi.org/10.3390/pr13123814







